Abstract
Proton beam therapy for head and neck cancers traditionally employs a fixed relative biological effectiveness (RBE) of 1.1, which may underestimate actual biological effects in critical structures. This study evaluates how Linear Energy Transfer (LET) optimization could potentially prevent radiation-induced brachial plexopathy (RIBP). (1) Case presentation: A 65-year-old male with stage IVA p16-positive oropharyngeal squamous cell carcinoma received pencil-beam-scanning intensity-modulated proton therapy with concurrent cisplatin. Due to a right level 4 neck node, the high-risk target volume overlapped with the brachial plexus, resulting in a D0.1cc of 70.3 Gy (RBE = 1.1). Four years post-treatment, the patient developed progressive right upper extremity paresthesia, weakness, and dysesthesia. Electromyography revealed myokymia consistent with brachial plexopathy, while MRI showed hyperintensity of the right brachial plexus corresponding to the radiation field. Conservative treatment with pentoxifylline, gabapentin, and physical therapy improved his symptoms. (2) Methods: The original treatment plan was retrospectively analyzed using Monte Carlo dose algorithms and LET-dependent RBE models from McMahon and McNamara. An LET-optimized plan was created to limit LETd to 2.0 keV/µm in the brachial plexus. (3) Results: The relative biological equivalent (RBE) dose to 0.1cc of the brachial plexus was 77.8 Gy (CGE RBE), exceeding tolerance. The LET-optimized plan reduced the brachial plexus D0.1cc to 59.4 Gy (RBE = 1.1) and 63.2 Gy (CGE RBE), an 18.8% decrease, while maintaining target coverage. LETd, within the brachial plexus enhancement, decreased from 5.3 to 2.6 keV/μm. (4) Conclusion: This case highlights the potential clinical importance of LET optimization in proton therapy planning, particularly when organs-at-risk overlap with target volumes. By reducing LETd from 5.3 to 2.6 keV/μm and biological equivalent dose by 18.8%, LET optimization could potentially prevent late toxicities, like RIBP, while maintaining target coverage.
1. Introduction
Radiation-induced brachial plexopathy (RIBP) is a rare complication of radiation therapy. Dose constraints for the brachial plexus are based mainly on observational data, but Emami et al. and RTOG trials constraints recommended a maximum dose between 60 and 66 Gy [1,2,3]. A 2022 review of brachial plexus management in head and neck cancer reported a rate of RIBP ranging from 12 to 22% [4]. Proton beam radiation has the potential to reduce dose to normal tissue, such as the brachial plexus. Proton therapy treatments have traditionally been planned using a fixed relative biological effectiveness (RBE) of 1.1 compared to photons. However, many experts argue that this too simplistic and does not account for varying RBE along the beam path, particularly near the distal end of the field [5,6,7,8,9]. Linear energy transfer (LET) optimization has been put forward as a method to address this limitation. In this report, we present a case of brachial plexopathy after proton radiation therapy for oropharyngeal cancer and propose that such a complication could have been mitigated by LET optimization.
2. Case Presentation
A 65-year-old male was diagnosed with stage IVA, cT1bN2bM (AJCC 7th edition) p16-positive squamous cell carcinoma of the oropharynx after a mass was incidentally discovered on the base of tongue during an intubation for a routine procedure and was subsequently biopsied. The patient was referred to a radiation oncologist and underwent treatment with a combination of pencil-beam-scanning intensity-modulated proton therapy with a double-scatter proton boost using accelerated fractionation (six fractions per week) with concurrent cisplatin (30 mg/m2 × 6 cycles) to a dose of 70 Gy (RBE = 1.1) (high-risk volume (HR)), 60 Gy (RBE = 1.1) (intermediate-risk volume (IR)) and 54 Gy (RBE = 1.1) (standard-risk volume(SR)). The Pencil Beam Scanning (PBS) phase contributed 60 Gy (RBE = 1.1) and the double scattered (DS) phase 10 Gy (RBE = 1.1) to the total dose. The patient tolerated treatment well, experiencing no significant toxicities.
Approximately 4 years after finishing treatment, the patient developed paresthesia in the right thumb and forefinger. After conservative management failed, the patient underwent carpal tunnel release, which also did not relieve the symptoms. Several months later, the patient developed right shoulder and arm weakness and dysesthesia along the tricep, which were not improved with physical therapy. Physical exam was significant for 4/5 weakness of right supraspinatus, infraspinatus, deltoid, and biceps, as well as hyperreflexia of the right triceps and mild sensory deficits in the brachial plexus distribution. Degenerative disk disease and stenosis of the cervical spine had been noted on previous computed tomography (CT) scans. The patient had visited a neurosurgeon to discuss symptoms and considered surgery for his symptoms.
An electromyography (EMG) demonstrated myokymia in multiple muscles, consistent with brachial plexopathy. Magnetic resonance imaging (MRI) of the brachial plexus revealed diffuse visualized thickening of the components of the right brachial plexus which were significantly hyperintense compared to the contralateral side (Figure 1). Severe stenosis at the level of C6 was also noted. Since the hyperintensity corresponded closely with the radiation field, RIBP was considered the most likely etiology (Figure 2).
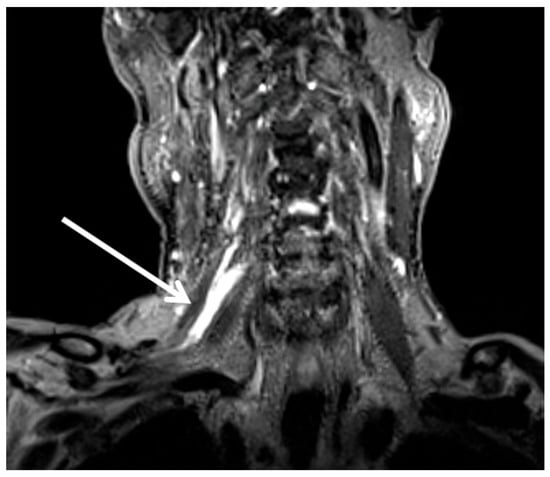
Figure 1.
A coronal T2 weighted MRI with an arrow illustrating the hyperintense right brachial plexus.
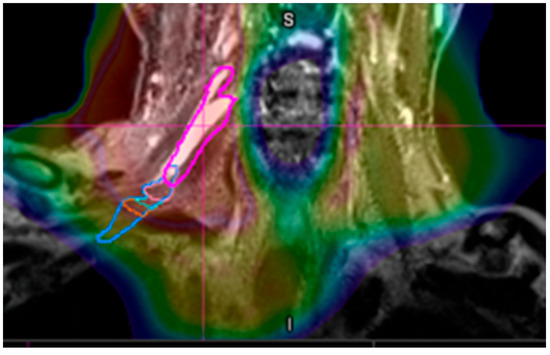
Figure 2.
The coronal MRI with the dose color-wash superimposed. The pink line is the brachial plexus. The red volume boundary indicates the 70 Gy (RB1.1) isodose line and corresponds with the hyperintense brachial plexus.
In light of this, conservative treatment of oral pentoxifylline (3 × 400 mg daily tablets with meals), gabapentin (3 × 300 mg daily oral capsules), and a structured physical therapy regimen were prescribed. The oral pentoxifylline and gabapentin were started 4 years post proton therapy and the patient reported significant improvement in right upper extremity paresthesia after 10 months on the combined oral treatment. Gabapentin was continued but pentoxifylline was discontinued after the patient became more functionally capable. While follow-up imaging was not performed after treatment initiation, the clinical improvement in symptoms provided sufficient evidence to continue with conservative management rather than pursuing surgical intervention.
3. Materials and Methods
In order to model the total dose-weighted LET (LETd) and the resulting Cobalt Gray Equivalent RBE (CGE RBE) dose, both the 60 Gy (RBE = 1.1) and 10 Gy (RBE = 1.1) phases of the treatment plan were calculated with the Monte Carlo dose algorithm and then reoptimized with LET optimization. The original 60 Gy (RBE = 1.1) was initially treated with pencil-beam-scanning (PBS) but planned with a pencil beam dose algorithm. This plan was recalculated with the Monte Carlo dose algorithm and then reoptimized using LET optimization in RayStation. The double-scatter (DS) phase of the plan was recreated with PBS, using the same beam angles and Single Field Optimization (SFO), resulting in equivalent isodose lines to the original DS plan and allowing an approximation of the LETd distribution in the DS phase. This approach was necessary because DS plan calculation with Monte Carlo was not possible in RayStation (RayStation v14 Research Non-Clinical, RaySearch Laboratories, Stockholm, Sweden) at this time, and the 10 Gy (RBE = 1.1) phase was a small portion of the total RBE dose of the composite plan, resulting in any slight differences in the PBS vs. DS LET distribution having minimal impact on the composite RBE dose.
The patient’s original treatment plan using a proton RBE of 1.1 was reviewed. Due to the level 4 neck node, the high-risk target volume overlapped slightly with the brachial plexus, resulting in a brachial plexus D0.1cc of 70.3 Gy (RBE = 1.1). Retrospectively, LET-dependent RBE dose was calculated using the McMahon and McNamara models to account for the biological impact of LETd variation [5,6]. These models are derived from a comprehensive collection of proton LET and RBE experimental cell survival data. The McMahon model is a linear model with the form RBE = (1 + 0.055 × LETd), while the McNamara model is dependent on tissue-specific α/β and dose per fraction. For the brachial plexus, α/β = 3 was used, and α/β = 10 for the target volumes [10]. Using this method, the biological equivalent dose delivered to the D0.1cc of the right brachial plexus was determined to be 76.7 Gy (CGE RBE) and 77.8 Gy (CGE RBE) using the McMahon and McNamara models, respectively.
To limit CGE RBE dose to within clinical tolerances [2,3], an LET-optimized plan was created. The LET optimization objectives limited the LETd to 2.0 keV/µm to the right brachial plexus with a dose threshold of 54.4 Gy (RBE = 1.1) for the initial phase and 9.7 Gy (RBE = 1.1) for the boost phase. Maximum dose objectives were used to limit the right brachial plexus to 54 Gy (RBE = 1.1) for the initial phase and 10 Gy (RBE = 1.1) for the boost, with a relative weight of 10 compared to 100 for the targets. LET optimization planning objectives for the brachial plexus from each phase of the treatment plan are shown in Figure 3. For both phases, standard-risk (SR), intermediate-risk (IR) and high-risk (HR) CTV coverage was robustly optimized with 3 mm isotropic setup uncertainty for all beams independently and with 3.5% range uncertainty.
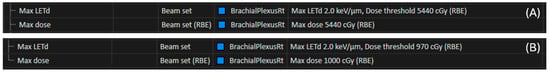
Figure 3.
Planning objectives added for the right brachial plexus during LET optimization for the initial 60 Gy (RBE = 1.1) phase (A). Planning objectives added for the right brachial plexus during LET optimization for the boost 10 Gy (RBE = 1.1) phase recreated using PBS (B).
4. Results
After LET optimization, the brachial plexus dose to 0.1cc was reduced to 59.4 Gy (RBE = 1.1), 62.4 Gy (CGE RBE) when calculated with the McMahon model and 63.2 Gy (CGE RBE) when calculated with the McNamara model, while still meeting target coverage goals to the CTV, Table 1. This represents a ~19% decrease from the delivered plan, using either RBE model. The LETd from the initial 60 Gy (RBE = 1.1) phase of the original and LET-optimized plans are compared in Figure 4A,B. The CGE RBE dose comparison using the McMahon model is shown in Figure 4C–F.

Table 1.
McMahon and McNamara LET Dependent RBE of the composite plan. The brachial plexus (BP) D0.1cc increased from 70.3 Gy (RBE = 1.1) to 77.8 Gy (CGE RBE) in the McNamara model, an increase of 10.7% relative to the original reference plan. The LET optimization plan reduced both the dose and LETd, resulting in a reduction of LETd in the MRI BP enhancement from 5.3 to 2.6 keV/μm and a reduction in the BP McNamara modeled dose from 77.8 Gy (CGE RBE) to 63.2 Gy (CGE RBE), resulting in a reduction of 18.8%. The mean LETd in the CTV increased from 2.6 to 3.1 keV/um, resulting in an increase of 2.2% in the McNamara modeled dose from the original plan compared to the LET-optimized plan.
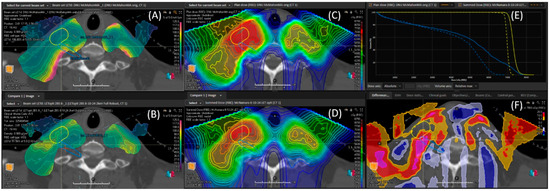
Figure 4.
The LETd of the original plan (A), LET-optimized (B). The McMahon RBE modeled dose comparison of the original plan (C), LET-optimized (D). The brachial plexus (BP) can be seen out-lined in blue. The dose-volume histogram comparisons (E) are shown, with the original plan being the solid line and the LET optimized plan being the dotted line. The primary dose CTV HR is in yellow and brachial plexus is in blue. The percentage difference between the two plans (F) is shown. The BP % difference frame reveals an increase in dose of nearly 20%, highlighted in bright magenta within the blue brachial plexus contour. The LETd, in the area of brachial plexus enhancement, was reduced from 5.3 keV/μm to 2.6 keV/μm.
In the original plan, the LETd in the area of brachial plexus enhancement was as high as 5.3 keV/μm. After LET optimization, this was reduced to 2.6 keV/μm. Table 1 summarizes the comparison between the original and LET-optimized plans using both the McMahon and McNamara models. The mean LETd in the CTV increased from 2.6 to 3.1 keV/um with the LET-optimized plan, resulting in an increase of 2.2% in the McNamara modeled dose compared to the original plan.
5. Discussion
RIBP is an uncommon late toxicity, mainly found after radiotherapy for head and neck, breast, and lung cancers. It may occur as a result of neural and soft tissue fibrosis leading to ischemic damage of nerve cells [11]. Our patient was able to recover sensation and strength, but in certain cases the damage may be progressive and irreversible [12]. Due to the rarity of RIBP, estimates vary on the latency to development but it generally occurs approximately 7–45 months after completing radiotherapy [4,13,14]. Given the extended time it takes to develop and the tendency to mimic cervical radiculopathy, it can make diagnosis challenging, as was the case with our patient. The total dose, volume treated, concurrent systemic therapies, and preceding neck surgeries can increase the risk of RIBP [12]. Certainly, our patient was at higher risk given the proximity of the positive distal lymph node to the brachial plexus. Our institution generally does not compromise target coverage in order to meet brachial plexus constraints.
Proton beam radiotherapy can minimize dose to normal tissue; however, radiobiologic uncertainty remains a one of the challenges with proton beam planning. LET optimization has the potential to help mitigate this uncertainty [6]. Proton therapy treatments have traditionally been planned using an RBE of 1.1 compared to photons, which is based on expert consensus review of various experimental data available. However, this assumes a stable spatial distribution of RBE along the path of the proton. The RBE can significantly exceed 1.1, particularly in the distal portion of the Bragg Peak [15], which can lead to underestimating the true biologic effect on normal tissue.
Since LET, similar to dose, is a calculable quantity and high RBE is mainly a consequence of high LET [16], LET optimization has been proposed as a potential tool to mitigate high RBE in healthy tissue [17]. The potential benefit would be minimizing high RBE in normal tissue by shifting out areas of high LET. Although experimental evidence supports this [18], LET optimization is a relatively new technique, necessitating further research to validate its effectiveness and optimize application in a clinical setting. The heterogeneity of LET optimization, the uncertainty in proton RBE models, and the entanglement between distal edge robustness and LET reduction introduce great complexity to LET optimization and its adoption. As was illustrated in the case of this patient, LET optimization can be especially important in instances where the organs-at-risk (OAR) overlap with the target volumes. The reduction in brachial plexus RBE dose using the LET-optimized plan had the potential to prevent RIBP in this patient.
One of the limitations of this study is that the DS Single Field Uniform Dose (SFUD) 10 Gy (RBE = 1.1) boost plan was recreated with PBS and Monte Carlo in order to calculate LETd; this is not a clinically significant difference but it is a limitation as DS plans only have a pencil beam dose algorithm and do not offer LETd calculation. From publication, the LETd difference between DS and PBS is about 0.26 keV/µm [19] (3.43 keV/µm with DS vs. 3.17 keV/µm with PBS) showing recreation of the DS plan with PBS Monte Carlo provides a conservative estimate of the LETd and CGE RBE contributed by the boost phase. LET optimization creates much more significant changes (5.3 keV/µm without LET optimization vs. 2.6 keV/µm with LET optimization). The difference in LETd created by approximating the DS boost phase with an SFUD PBS plan would have little to no impact on the findings with the McNamara model or McMahon model, i.e., ~1.4% difference in CGE RBE on just the 10 Gy (RBE = 1.1) phase out of 70 Gy (RBE = 1.1) and there would be a 0.2% difference in the total dose delivered.
6. Conclusions
This case report demonstrates that LET optimization has the potential to significantly reduce the biological dose to critical structures in proton therapy while maintaining adequate target coverage. In our patient, the LET-optimized plan reduced the biological dose to the brachial plexus by 18.8% compared to conventional planning, potentially preventing radiation-induced brachial plexopathy. The decrease in LETd from 5.3 to 2.6 keV/μm in the area of brachial plexus enhancement substantiates the clinical relevance of considering variable RBE effects along the proton beam path.
Current proton therapy planning using a fixed RBE of 1.1 may underestimate the true biological impact, particularly in critical structures near the distal edge of the treatment field. LET optimization represents a practical approach to address this challenge by redistributing high-LET regions away from radiosensitive normal tissues. This technique appears particularly valuable in cases where organs-at-risk unavoidably overlap with target volumes, as demonstrated in our head and neck cancer case.
Further clinical research is necessary to validate these findings in larger patient cohorts and to establish standardized LET optimization protocols. Future studies should focus on identifying specific patient subgroups most likely to benefit from this approach, such as those with target volumes adjacent to critical nervous system tissues, and determining optimal LET constraints for various organs-at-risk. As proton therapy continues to evolve, incorporating variable RBE considerations into treatment planning may become essential for maximizing the therapeutic ratio and minimizing late toxicities.
Author Contributions
Conceptualization, M.E.A., J.P., H.S.G., A.C., A.H., and R.D.; methodology, M.E.A., J.P., H.S.G., A.C., and R.D.; software, M.E.A., E.T., L.G., and H.S.G.; validation, M.E.A., R.D., E.D.B., E.T., L.G., J.A.B., and H.S.G.; formal analysis, M.E.A., J.B., E.T., and H.S.G.; investigation, M.E.A., J.P., J.A.B., H.S.G., A.C., and R.D.; resources, M.E.A., P.B.J., and R.D.; data curation, A.H., M.E.A., R.D., and H.S.G.; writing—original draft preparation, M.E.A., J.P., H.S.G., Y.Z., M.S., J.A.B., T.R.W., A.C., A.H., E.D.B., E.T., L.G., J.B., and R.D.; writing—review and editing, M.E.A., J.P., H.S.G., Y.Z., M.S., J.A.B., T.R.W., A.C., A.H., E.D.B., E.T., L.G., J.B., and R.D.; visualization, M.E.A., J.B., A.H., and H.S.G.; supervision, M.E.A., P.B.J., and R.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The authors agree to share anonymized data upon reasonable request by researchers.
Conflicts of Interest
All authors report no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| RBE | Relative Biological Effectiveness |
| LET | Linear Energy Transfer |
| LETd | Dose-weighted Linear Energy Transfer |
| RIBP | Radiation Induced Brachial Plexopathy |
| CGE RBE | Cobalt Gray Equivalent Relative Biological Effectiveness |
| HR | High Risk Target Volume |
| IR | Intermediate Risk Target Volume |
| SR | Standard Risk Target Volume |
| CTV | Clinical Target Volume |
| MRI | Magnetic Resonance Imaging |
| CT | Computed Tomography |
| PBS | Pencil Beam Scanning |
| DS | Double Scatter |
| SFUD | Single Field Uniform Dose |
References
- Ang, K.K.; Zhang, Q.; Rosenthal, D.I.; Nguyen-Tan, P.F.; Sherman, E.J.; Weber, R.S.; Galvin, J.M.; Bonner, J.A.; Harris, J.; El-Naggar, A.K.; et al. Randomized phase III trial of concurrent accelerated radiation plus cisplatin with or without cetuximab for stage III to IV head and neck carcinoma: RTOG 0522. J. Clin. Oncol. 2014, 32, 2940–2950. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Kong, W.; Kerr, A.; Brundage, M. The radiation dose tolerance of the brachial plexus: A systematic review and meta-analysis. Clin. Transl. Radiat. Oncol. 2019, 18, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Emami, B.; Lyman, J.; Brown, A.; Cola, L.; Goitein, M.; Munzenrider, J.E.; Shank, B.; Solin, L.J.; Wesson, M. Tolerance of normal tissue to therapeutic irradiation. Int. J. Radiat. Oncol. Biol. Phys. 1991, 21, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Fockens, M.M.; Kraak, J.T.; Leemans, C.R.; Eerenstein, S.E.J. Management of the brachial plexus in head and neck cancer. Curr. Opin. Otolaryngol. Head Neck Surg. 2023, 31, 105–110. [Google Scholar] [CrossRef] [PubMed]
- McNamara, A.L.; Schuemann, J.; Paganetti, H. A phenomenological relative biological effectiveness (RBE) model for proton therapy based on all published in vitro cell survival data. Phys. Med. Biol. 2015, 60, 8399–8416. [Google Scholar] [CrossRef] [PubMed]
- McMahon, S.J.; Paganetti, H.; Prise, K.M. LET-weighted doses effectively reduce biological variability in proton radiotherapy planning. Phys. Med. Biol. 2018, 63, 225009. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-C.; McNamara, A.L.; Shin, J.; Schuemann, J.; Grassberger, C.; Taghian, A.G.; Jimenez, R.B.; MacDonald, S.M.; Paganetti, H. End-of-Range Radiobiological Effect on Rib Fractures in Patients Receiving Proton Therapy for Breast Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2020, 107, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Gergelis, K.R.; Shen, J.; Afzal, A.; Mullikin, T.C.; Gao, R.W.; Aziz, K.; Shumway, D.A.; Corbin, K.S.; Liu, W.; et al. Study of linear energy transfer effect on rib fracture in breast cancer patients receiving pencil-beam-scanning proton therapy. Med. Phys. 2025, 52, 3428–3438. [Google Scholar] [CrossRef] [PubMed]
- Puthenpura, V.; DeNunzio, N.J.; Zeng, X.; Giantsoudi, D.; Aboian, M.; Ebb, D.; Kahle, K.T.; Yock, T.I.; Marks, A.M. Radiation Necrosis with Proton Therapy in a Patient with Aarskog-Scott Syndrome and Medulloblastoma. Int. J. Part Ther. 2022, 8, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Sood, S.S.; McClinton, C.; Badkul, R.; Aguilera, N.; Wang, F.; Chen, A.M. Brachial plexopathy after stereotactic body radiation therapy for apical lung cancer: Dosimetric analysis and preliminary clinical outcomes. Adv. Radiat. Oncol. 2018, 3, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.; Engle, A.M.; Raggi, E.; Alter, B.; Emerick, T. Pulsed Radiofrequency Ablation: An Alternative Treatment Modality for Radiation-Induced Brachial Plexopathy. Pain Med. 2021, 22, 749–753. [Google Scholar] [CrossRef] [PubMed]
- Attard, K.A.; Vella, J.C.; Chircop, C. Late-onset radiation-induced brachial plexopathy. BMJ Case Rep. 2021, 14, e243354. [Google Scholar] [CrossRef] [PubMed]
- Shekouhi, R.; Gerhold, C.; Chim, H. The role of surgery in the management of radiation-induced brachial plexopathy: A systematic review. J. Hand Surg. Eur. 2024, 49, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Rudra, S.; Roy, A.; Brenneman, R.; Gabani, P.; Roach, M.C.; Ochoa, L.; Prather, H.; Appleton, C.; Margenthaler, J.; Peterson, L.L.; et al. Radiation-Induced Brachial Plexopathy in Patients with Breast Cancer Treated with Comprehensive Adjuvant Radiation Therapy. Adv. Radiat. Oncol. 2021, 6, 100602. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, M.; Wilson, P.; Gorayski, P.; Bezak, E. A Systematic Review of LET-Guided Treatment Plan Optimisation in Proton Therapy: Identifying the Current State and Future Needs. Cancers 2023, 15, 4268. [Google Scholar] [CrossRef] [PubMed]
- Underwood, T.S.; McMahon, S.J. Proton relative biological effectiveness (RBE): A multiscale problem. Br. J. Radiol. 2019, 92, 20180004. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Yang, Y.; Liu, C.; Bues, M.; Mohan, R.; Wong, W.W.; Foote, R.H.; Patel, S.H.; Liu, W. A Critical Review of LET-Based Intensity-Modulated Proton Therapy Plan Evaluation and Optimization for Head and Neck Cancer Management. Int. J. Part Ther. 2021, 8, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Paganetti, H. Relative biological effectiveness (RBE) values for proton beam therapy. Variations as a function of biological endpoint, dose, and linear energy transfer. Phys. Med. Biol. 2014, 59, R419–R472. [Google Scholar] [CrossRef] [PubMed]
- Michaelidesová, A.; Vachelová, J.; Klementová, J.; Urban, T.; Brabcová, K.P.; Kaczor, S.; Falk, M.; Falková, I.; Depeš, D.; Vondráček, V.; et al. In Vitro Comparison of Passive and Active Clinical Proton Beams. Int. J. Mol. Sci. 2020, 21, 5650. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).