Enhanced Adsorption of Cage-Shaped Proteins on Carbon Surfaces by Carbon Nanotube (CNT)-Binding Peptide Aptamers
Abstract
1. Introduction
2. Results and Discussion
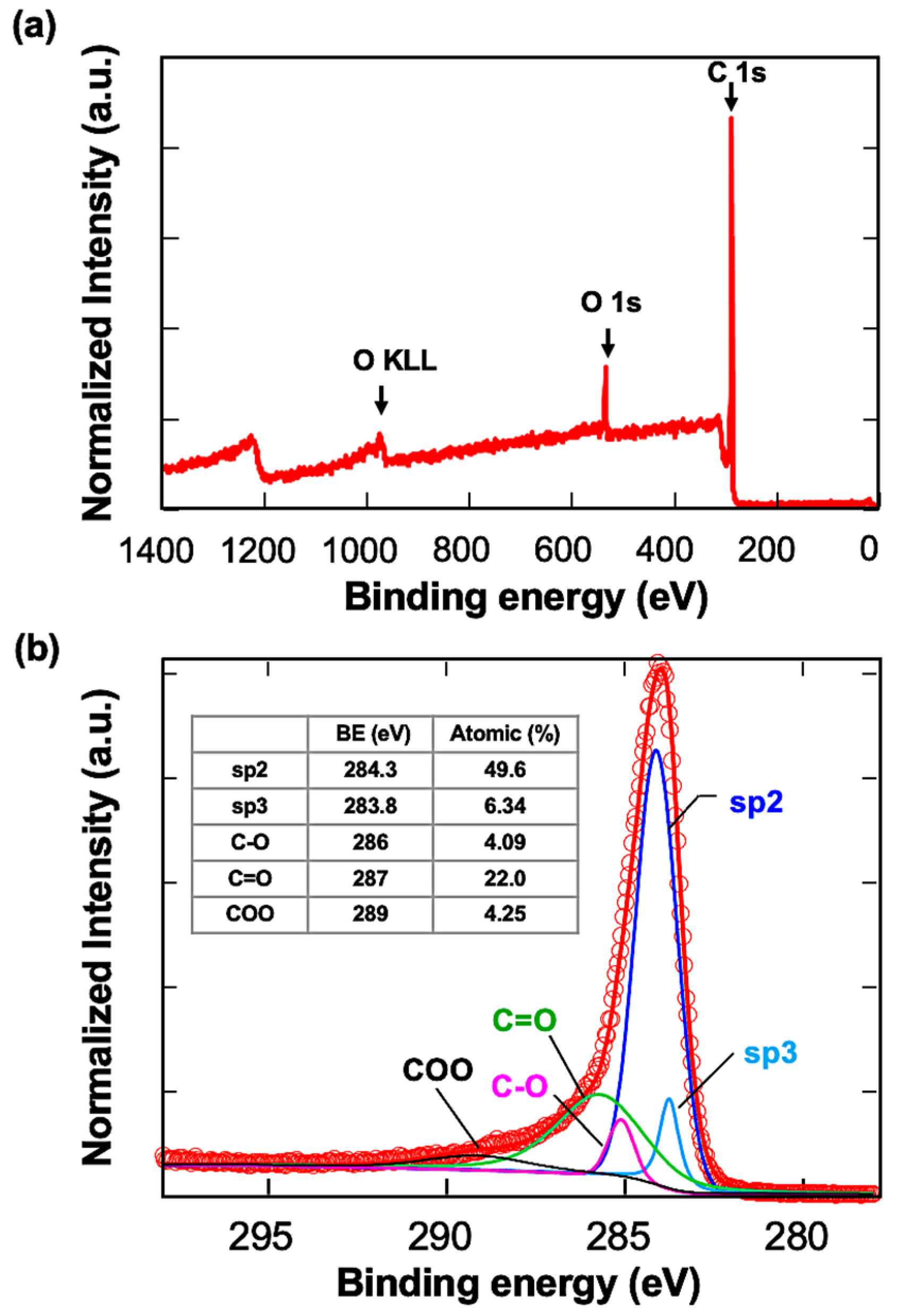
2.1. Characterization of Carbon Surface
2.2. QCM Measurements
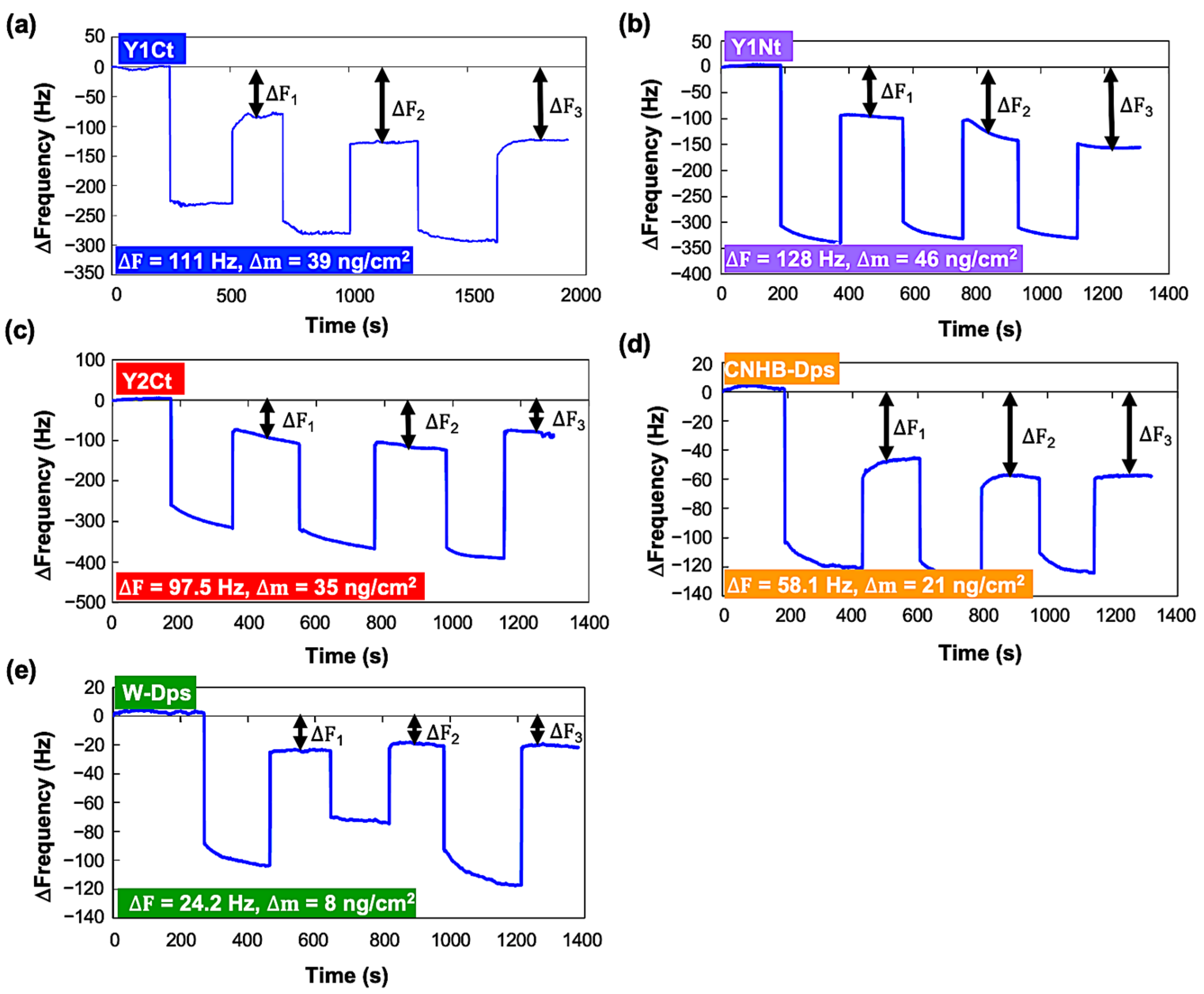
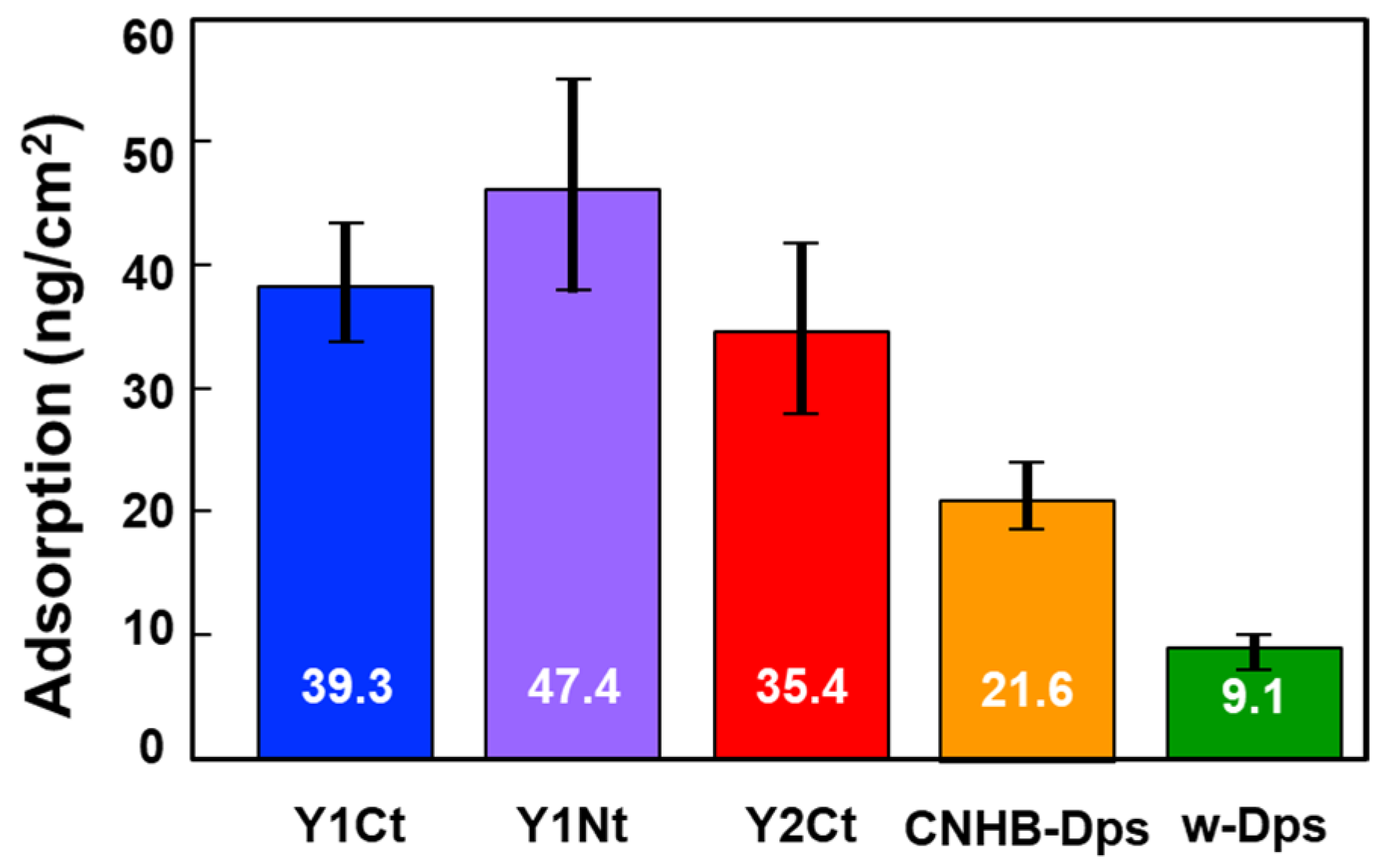
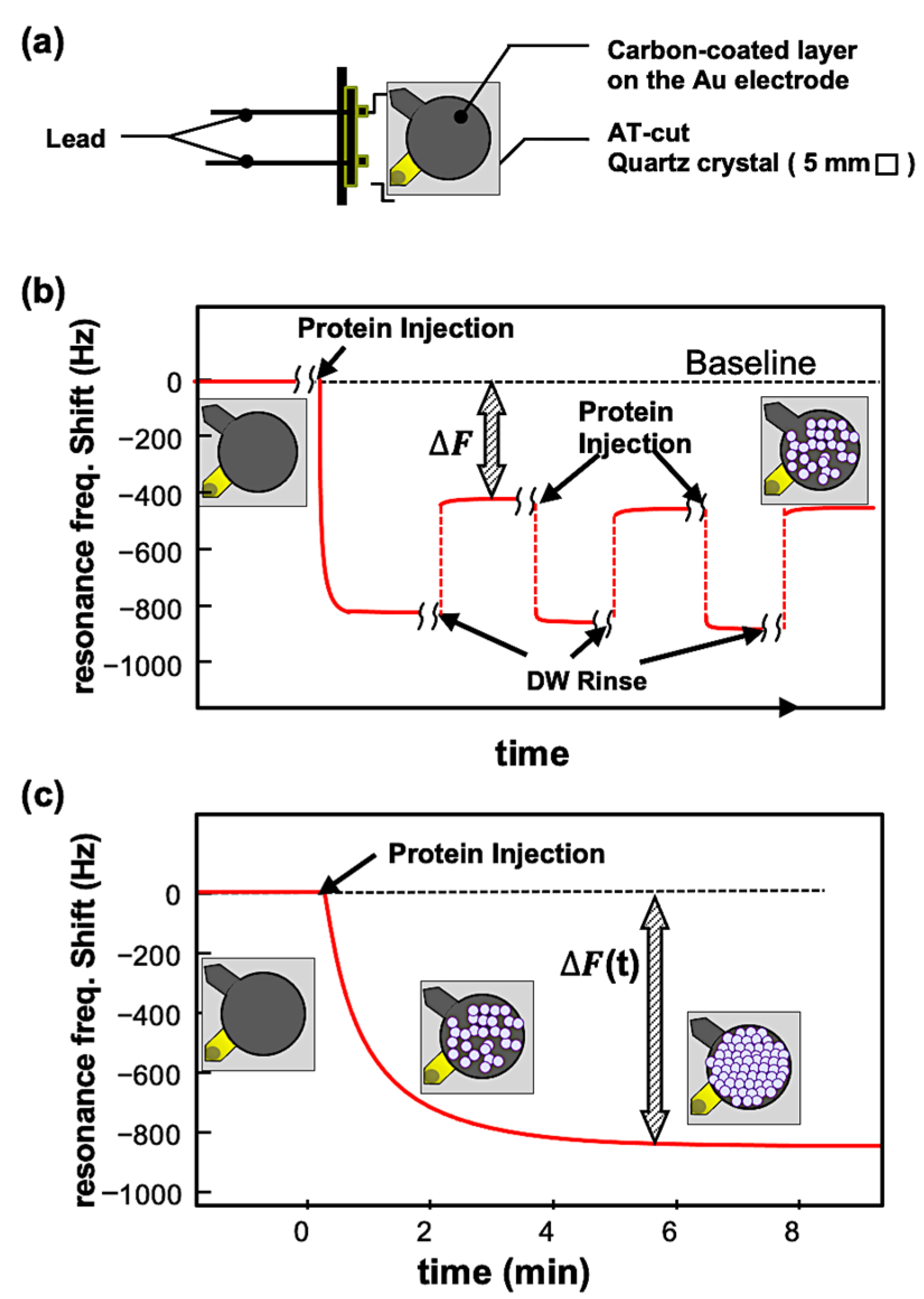
2.2.1. Batch-Mode Measurements
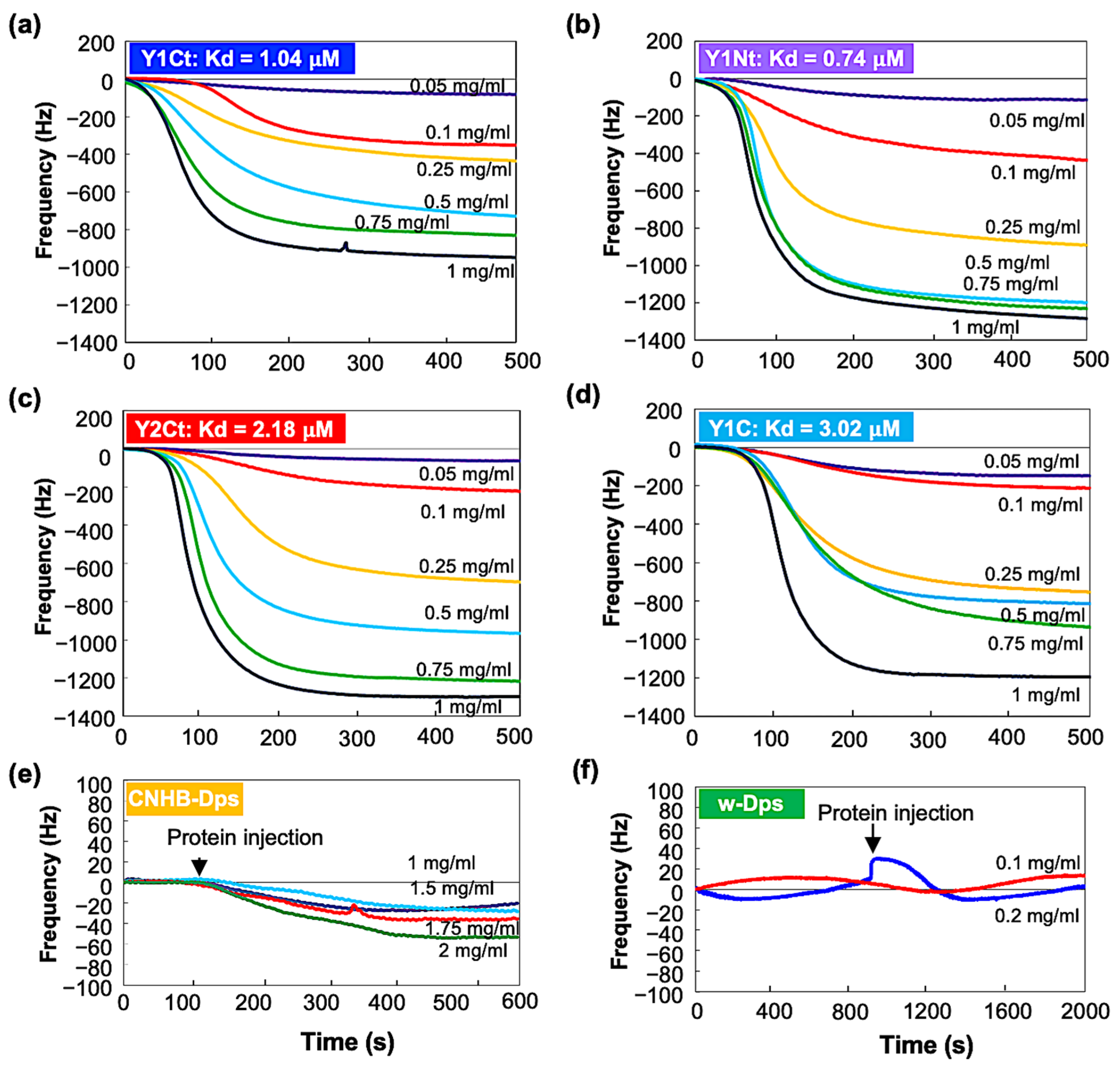
2.2.2. Open-Flow-Mode Measurement
3. Materials and Methods
3.1. Dps Protein Preparation
3.2. QCM Experiments
- ΔF: frequency change (Hz);
- F: fundamental frequency;
- Δm: mass change (g);
- ρ: density of quartz (ρ = 2.648 g/cm2);
- S: piezoelectricity active crystal area (area between electrodes, cm2);
- μ: shear modulus of quartz for AT-cut crystal (μ = 2.947 × 1011 g/cm2);
- n: overtone number (n = 3 in this work).
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ito, M.; Okamoto, N.; Abe, R.; Kojima, H.; Matsubara, R.; Yamashita, I.; Nakamura, M. Enhancement of thermoelectric properties of carbon nanotube composites by inserting biomolecules at nanotube junctions. Appl. Phys. Express 2014, 7, 065102. [Google Scholar] [CrossRef]
- Roushani, M.; Rahmati, Z.; Hoseini, S.J.; Hashemi Fath, R. Impedimetric ultrasensitive detection of chloramphenicol based on aptamer MIP using a glassy carbon electrode modified by 3-ampy-RGO and silver nanoparticle. Colloids Surf. B Biointerfaces 2019, 183, 110451. [Google Scholar] [CrossRef] [PubMed]
- Charbgoo, F.; Soltani, F.; Taghdisi, S.M.; Abnous, K.; Ramezani, M. Nanoparticles application in high sensitive aptasensor design. TrAC Trends Anal. Chem. 2016, 85, 85–97. [Google Scholar] [CrossRef]
- Turner, A.P.F.; Karube, I.; Wilson, G.S. Biosensors: Fundamentals and Applications; Oxford University Press: Oxford, UK, 1987. [Google Scholar]
- Barnes, L.M.; Lo, M.F.; Adams, M.R.; Chamberlain, A.H. Effect of Milk Proteins on Adhesion of Bacteria to Stainless Steel Surfaces. Appl. Environ. Microbiol. 1999, 65, 4543–4548. [Google Scholar] [CrossRef] [PubMed]
- Yagati, A.K.; Choi, J.-W. Protein based electrochemical biosensors for H2O2 detection towards clinical diagnostics. Electroanalysis 2014, 26, 1259–1276. [Google Scholar] [CrossRef]
- Nakanishi, K.; Sakiyama, T.; Imamura, K.J. On the adsorption of proteins on solid surfaces, a common but very complicated phenomenon. Biosci. Bioeng. 2001, 91, 233–244. [Google Scholar] [CrossRef]
- Roach, P.; Farrar, D.; Perry, C.C. Interpretation of Protein Adsorption: Surface-Induced Conformational Changes. J. Am. Chem. Soc. 2005, 127, 8168–8173. [Google Scholar] [CrossRef]
- Barnthip, N.; Parhi, P.; Golas, A.; Vogler, E.A. Volumetric interpretation of protein adsorption: Kinetics of protein-adsorption competition from binary solution. Biomaterials 2009, 30, 6495–6513. [Google Scholar] [CrossRef]
- Uchida, M.; Klem, M.T.; Allen, M.; Suci, P.; Flenniken, M.; Gillitzer, E.; Varpness, Z.; Liepold, L.O.; Young, M.; Douglas, T. Biological Containers: Protein Cages as Multifunctional Nanoplatforms. Adv. Mater. 2007, 19, 1025–1042. [Google Scholar] [CrossRef]
- Yamashita, I. Biological path for functional nanostructure fabrication and nanodevices. Surf. Innov. 2016, 4, 111–120. [Google Scholar] [CrossRef]
- Okuda, M.; Suzumoto, Y.K.; Iwahori, K.; Kang, S.; Uchida, M.; Dougles, T.; Yamashita, I. Bio-templated CdSe nanoparticle synthesis in a cage shaped protein, Listeria-Dps, and their two dimensional ordered array self- assembly. Chem. Commun. 2010, 46, 8797–8799. [Google Scholar] [CrossRef] [PubMed]
- Iwahori, K.; Enomoto, T.; Furusho, H.; Miura, A.; Nishio, K.; Mishima, Y.; Yamashita, I. CadmiumSulfide Nanoparticle Synthesis in Dps Protein from Listeria innocua. Chem. Mater. 2007, 19, 3105–3111. [Google Scholar] [CrossRef]
- Iwahori, K.; Yamashita, I. Fabrication of CdS nanoparticles in the bio-template, apoferritin cavity by a slow chemical reaction system. J. Phys. Conf. Ser. 2007, 61, 492–496. [Google Scholar] [CrossRef]
- Higo, A.; Kiba, T.; Chen, S.; Chen, Y.; Tanikawa, T.; Thomas, C.; Lee, C.Y.; Lai, Y.-C.; Ozaki, T.; Takayama, J.; et al. Optical Study of Sub-10 nm In0.3Ga0.7N Quantum Nanodisks in GaN Nanopillars. ACS Photon. 2017, 4, 1851–1857. [Google Scholar] [CrossRef]
- Huang, C.-H.; Wang, X.-Y.; Igarashi, M.; Murayama, A.; Okada, Y.; Yamashita, I.; Samukawa, S. Optical absorption characteristic of highly ordered and dense two-dimensional array of silicon nanodiscs. Nanotechnology 2011, 22, 105301. [Google Scholar] [CrossRef] [PubMed]
- Budiman, M.F.; Hu, W.; Igarashi, M.; Tsukamoto, R.; Isoda, T.; Itoh, K.M.; Yamashita, I.; Murayama, A.; Okada, Y.; Samukawa, S. Control of optical bandgap energy and optical absorption coefficient by geometric parameters in sub-10 nm silicon-nanodisc array structure. Nanotechnology 2012, 23, 065302. [Google Scholar] [CrossRef][Green Version]
- Kobayashi, M.; Tomita, S.; Sawada, K.; Shiba, K.; Yanagi, H.; Yamashita, I.; Uraoka, Y. Chiral meta- molecules consisting of gold nanoparticles and genetically engineered tobacco mosaic virus. Opt. Express 2012, 20, 24856–24863. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Okamoto, N.; Yamamoto, S.; Obokata, S.; Nishioka, K.; Benten, H.; Nakamura, M. Carbon Nanotube/Biomolecule Composite Yarn for Wearable Thermoelectric Applications. ACS Appl. Energy Mater. 2022, 5, 3698–3705. [Google Scholar] [CrossRef]
- Brown, S. Metal-recognition by repeating polypeptides. Nat. Biotechnol. 1997, 15, 269–272. [Google Scholar] [CrossRef] [PubMed]
- Sarikaya, M.; Tamerler, C.; Jen, A.K.; Schulten, K.; Baneyx, F. Molecular biomimetics: Nanotechnology through biology. Nat. Mater. 2003, 2, 577–585. [Google Scholar] [CrossRef]
- Sano, K.; Shiba, K. A Hexapeptide Motif that Electrostatically Binds to the Surface of Titanium. J. Am. Chem. Soc. 2003, 125, 14234–14235. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, N.; Iwahori, K.; Yamashita, I. Silicon-Dioxide-Specific Peptides for Biological Nanofabrication. IEEE Nanotechnol. Mag. 2019, 6, 43–48. [Google Scholar] [CrossRef]
- Kase, D.; Kulp, J.L.I.; Yudasaka, M.; Evans, J.S.; Iijima, S.; Shiba, K. Affinity selection of peptide phage libraries against single-wall carbon nanohorns identifies a peptide aptamer with conformational variability. Langmuir 2004, 20, 8939–8941. [Google Scholar] [CrossRef] [PubMed]
- Han, H.-W.; Chu, T.-C.; Okamoto, N.; Nakamura, M.; Yamashita, I. CNT binding peptides selected by the phage display method. Langmuir 2023, 39, 14204. [Google Scholar] [CrossRef] [PubMed]
- Grant, R.A.; Fliman, D.J.; Finkel, S.E.; Kolter, R.; Hoglem, J.M. The crystal structure of Dps a ferritin homolog that binds and protects DNA. Nat. Struct. Biol. 1998, 4, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Somorjai, G.A.; York, R.L.; Butcher, D.; Park, J.Y. The evolution of model catalytic systems; studies of structure, bonding and dynamics from single crystal metal surfaces to nanoparticles, and from low pressure (10−3 Torr) to liquid interfaces. Phys. Chem. Chem. Phys. 2007, 9, 3500–3513. [Google Scholar] [CrossRef]
- Mermut, O.; Phillips, D.C.; York, R.L.; McCrea, K.R.; Ward, R.S.; Somorjai, G.A. In situ adsorption studies of a 14-amino acid leucine-lysine peptide onto hydrophobic polystyrene and hydrophilic silica surfaces using quartz crystal microbalance, atomic force microscopy, and sum frequency generation vibrational spectroscopy. J. Am. Chem. Soc. 2006, 128, 3598–3607. [Google Scholar] [CrossRef]
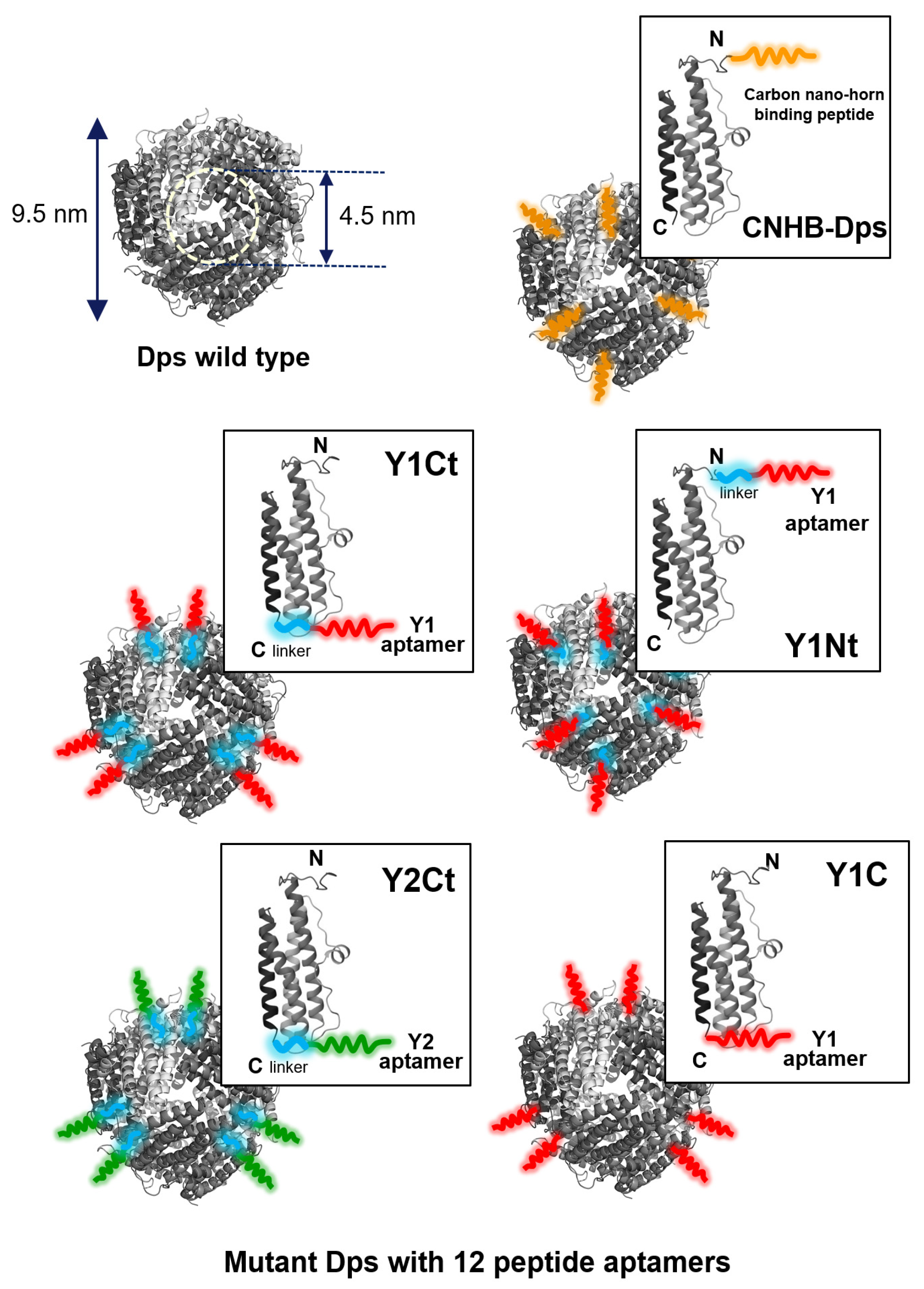
| Y1Ct | Y1Nt | Y2Ct | Y1C | CNHB-Dps | w-Dps | |
|---|---|---|---|---|---|---|
| (Batch-mode) ∆m 30 Averaged | 39.3 ng/cm2 | 47.4 ng/cm2 | 35.4 ng/cm2 | - | 21.6 ng/cm2 | 9.1 ng/cm2 |
| (Flow-mode) Kd | 1.04 μM | 0.74 μM | 2.18 μM | 3.02 μM | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ganbaatar, N.; Chu, T.-C.; Okamoto, N.; Iwahori, K.; Nakamura, M.; Yamashita, I. Enhanced Adsorption of Cage-Shaped Proteins on Carbon Surfaces by Carbon Nanotube (CNT)-Binding Peptide Aptamers. Biophysica 2024, 4, 256-266. https://doi.org/10.3390/biophysica4020018
Ganbaatar N, Chu T-C, Okamoto N, Iwahori K, Nakamura M, Yamashita I. Enhanced Adsorption of Cage-Shaped Proteins on Carbon Surfaces by Carbon Nanotube (CNT)-Binding Peptide Aptamers. Biophysica. 2024; 4(2):256-266. https://doi.org/10.3390/biophysica4020018
Chicago/Turabian StyleGanbaatar, Narangerel, Ting-Chieh Chu, Naofumi Okamoto, Kenji Iwahori, Masakazu Nakamura, and Ichiro Yamashita. 2024. "Enhanced Adsorption of Cage-Shaped Proteins on Carbon Surfaces by Carbon Nanotube (CNT)-Binding Peptide Aptamers" Biophysica 4, no. 2: 256-266. https://doi.org/10.3390/biophysica4020018
APA StyleGanbaatar, N., Chu, T.-C., Okamoto, N., Iwahori, K., Nakamura, M., & Yamashita, I. (2024). Enhanced Adsorption of Cage-Shaped Proteins on Carbon Surfaces by Carbon Nanotube (CNT)-Binding Peptide Aptamers. Biophysica, 4(2), 256-266. https://doi.org/10.3390/biophysica4020018






