Bay Laurel of Northern Morocco: A Comprehensive Analysis of Its Phytochemical Profile, Mineralogical Composition, and Antioxidant Potential
Abstract
1. Introduction
2. Results and Discussion
2.1. Mineralogical Analysis
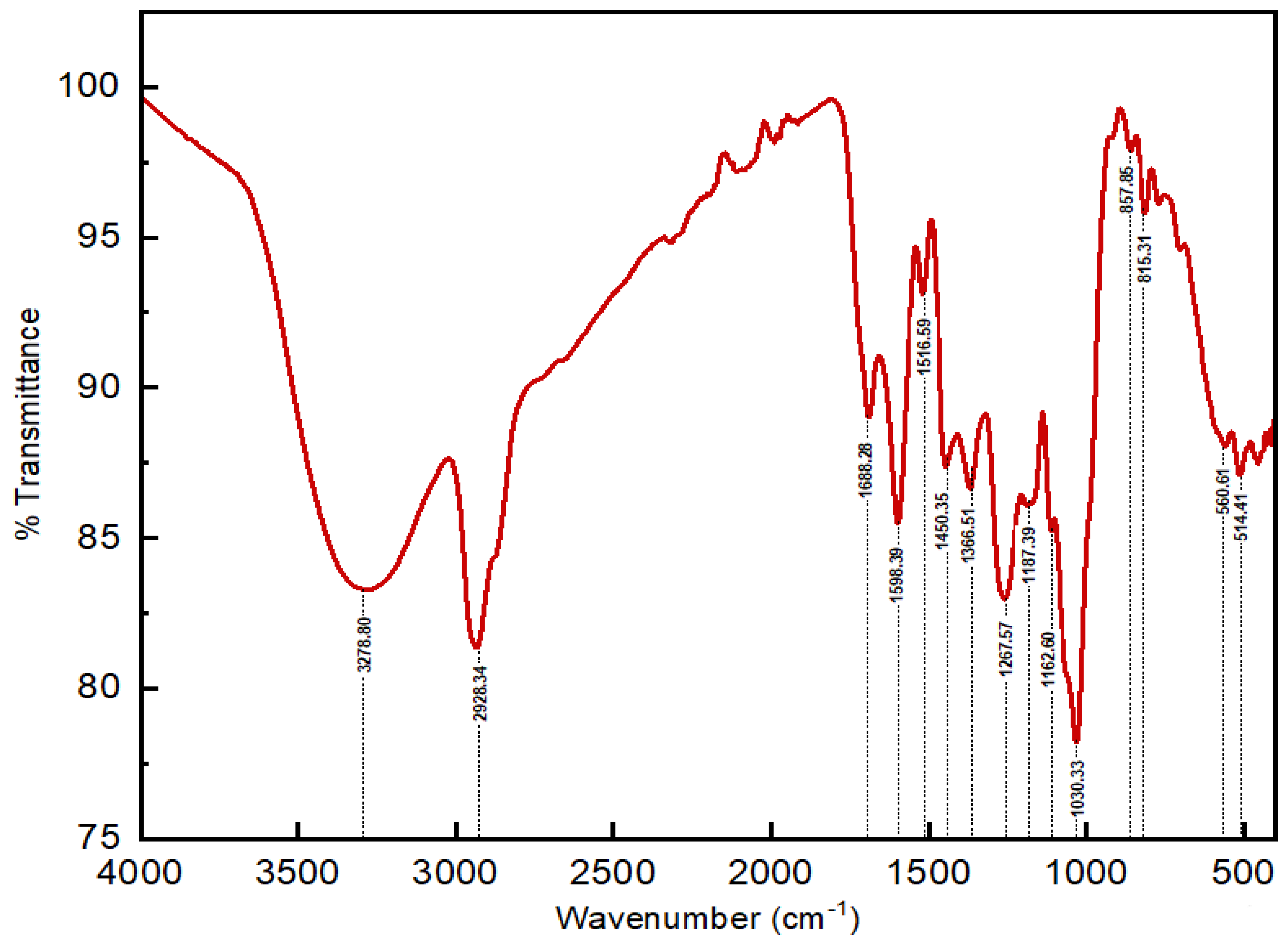
2.2. FT-IR Analysis
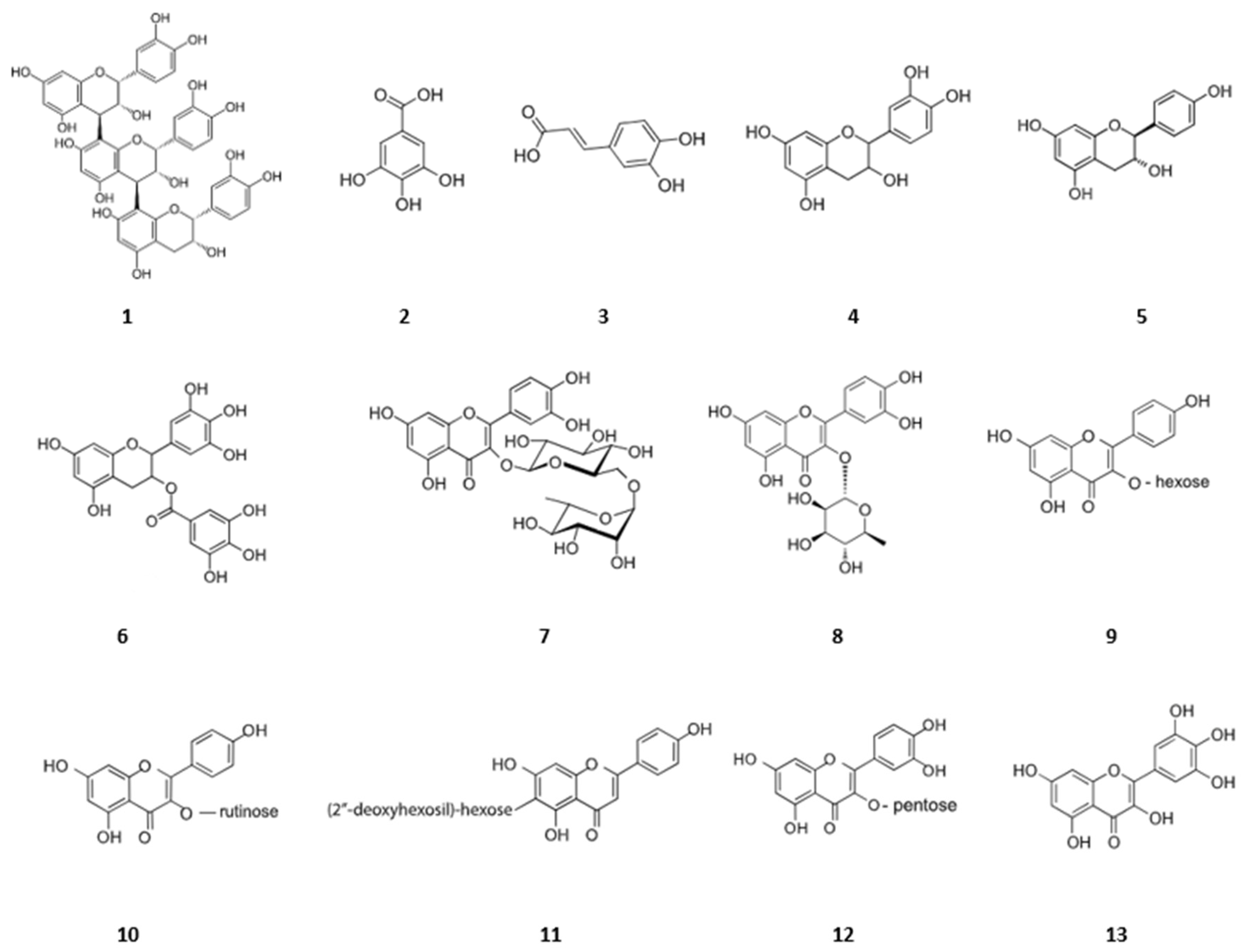
2.3. HPLC-MS-UV Analysis
- Phenolic acids
- 2.
- Flavonoids
- 3.
- Proanthocyanidins
2.4. Qualitative Phytochemical Analysis
2.5. Quantitative Phytochemical Analysis
2.6. Antioxidant Activity
3. Materials and Methods
3.1. Plant Material
3.2. Extract Preparation
3.3. ICP-AES Analysis
3.4. WD-XRF Analysis
3.5. FT-IR Analysis
3.6. HPLC-MS-UV Analysis
3.7. Phytochemical Screening
3.7.1. Flavonoids
3.7.2. Tannins
3.7.3. Alkaloids
3.7.4. Anthocyanins
3.7.5. Saponins
3.7.6. Coumarins
3.7.7. Terpenoids/Steroids
3.8. Determination of Total Phenolic Content
3.9. Determination of Total Flavonoid Content
3.10. Determination of Total Tannin Content
3.11. DPPH Radical Scavenging Assay
3.12. ABTS Radical Scavenging Assay
3.13. FRAP Assay
3.14. ORAC Assay
3.15. Statictical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bektaş, S.; Özdal, M.; Gürkök, S. Determination of Antibacterial and Antibiofilm Activities for Laurel (Laurus nobilis L.) Essential Oil Against the Fish Pathogen Pseudomonas Species. Menba Kastamonu Üniversitesi Su Ürün. Fakültesi Derg. 2023, 9, 25–33. [Google Scholar] [CrossRef]
- Khodja, Y.K.; Bachir-Bey, M.; Belmouhoub, M.; Ladjouzi, R.; Dahmoune, F.; Khettal, B. The botanical study, phytochemical composition, and biological activities of Laurus nobilis L. leaves: A review. Int. J. Second. Metab. 2023, 10, 269–296. [Google Scholar] [CrossRef]
- Ross, I.A. Medicinal Plants of the World 3; Humana Press: Totowa, NJ, USA, 2005. [Google Scholar]
- Santoyo, S.; Lloría, R.; Jaime, L.; Ibañez, E.; Señoráns, F.J.; Reglero, G. Supercritical fluid extraction of antioxidant and antimicrobial compounds from Laurus nobilis L. Chemical and functional characterization. Eur. Food Res. Technol. 2006, 222, 565–571. [Google Scholar] [CrossRef]
- El Faqer, O.; Rais, S.; Elkoraichi, I.; El Amrani, A.; Dakir, M.; Zaid, Y.; Mtairag, E.M. Phytochemical characterization and immunomodulatory effects of aqueous and ethanolic extracts and essential oil of Moroccan Laurus nobilis L. (Lauraceae) on human neutrophils. J. Herbmed Pharmacol. 2022, 12, 92–99. [Google Scholar] [CrossRef]
- Yao, R.; Heinrich, M.; Zhang, B.; Wei, X.; Qi, Y.; Gao, W. Single botanical drugs in the Ayurvedic Pharmacopoeia of India—A quantitative ethnobotanical analysis. Front. Pharmacol. 2023, 14, 1136446. [Google Scholar] [CrossRef] [PubMed]
- Ben Ayed, A.; Aissa, E.; Haouala, F. Effect of salinity on germination capacity of laurel (Laurus nobilis L.). GSC Adv. Res. Rev. 2023, 15, 304–309. [Google Scholar] [CrossRef]
- Dobroslavić, E.; Garofulić, I.E.; Ilich, J.Z. Potential of Laurel (Laurus nobilis L.) Leaf Polyphenols for Modulation of Body Composition. Appl. Sci. 2023, 13, 2275. [Google Scholar] [CrossRef]
- Bahtiti, N.H.; Orabi, F.M.A.; Kailani, M.H.; Abdel-Rahman, I.; Ismail, L.S. A Comparative LC/MS Analysis of Jordanian Bay Leaf, Branches, and Roots (Laurus nobilis L.). J. Southwest Jiaotong Univ. 2022, 57, 6. Available online: http://jsju.org/index.php/journal/article/view/1424 (accessed on 15 April 2024). [CrossRef]
- Escobedo-Monge, M.F.; Barrado, E.; Parodi-Román, J.; Escobedo-Monge, M.A.; Torres-Hinojal, M.C.; Marugán-Miguelsanz, J.M. Magnesium Status and Ca/Mg Ratios in a Series of Children and Adolescents with Chronic Diseases. Nutrients 2022, 14, 2941. [Google Scholar] [CrossRef]
- Hibler, E.A.; Zhu, X.; Shrubsole, M.J.; Hou, L.; Dai, Q. Physical activity, dietary calcium to magnesium intake and mortality in the National Health and Examination Survey 1999–2006 cohort. Int. J. Cancer 2020, 146, 2979–2986. [Google Scholar] [CrossRef]
- Appel, L.J. Potassium. In Encyclopedia of Human Nutrition, 4th ed.; Caballero, B., Ed.; Academic Press: Oxford, UK, 2023; pp. 362–367. [Google Scholar] [CrossRef]
- Aljafary, M.A.; Alshwyeh, H.; Alahmadi, N.; Shehzad, A.; Tombuloglu, H.; Gaymalov, Z.; Homieda, A.; Al-Suhaimi, E. Physiological and Cellular Functions of Vitamin K on Cardiovascular Function. In Vitamin K—Recent Topics on the Biology and Chemistry; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Manalang, M.F.; Ocampo, G.; Alonto, A.-S.; Rimocal, G. Geo-Spatial Mapping of Iron (Fe) and Manganese (Mn) Present in Groundwater of Clark, Philippines. ASEAN Eng. J. 2023, 13, 101–107. [Google Scholar] [CrossRef]
- Xu, C.; Yang, H.; Huang, C.; Lan, M.; Zou, Z.; Zhang, F.; Zhang, L. Interaction Mechanism of Fe, Mg and Mn in Karst Soil-Mango System. Land 2023, 12, 256. [Google Scholar] [CrossRef]
- Bojórquez-Quintal, E.; Escalante-Magaña, C.; Echevarría-Machado, I.; Martínez-Estévez, M. Aluminum, a Friend or Foe of Higher Plants in Acid Soils. Front. Plant Sci. 2017, 8, 1767. [Google Scholar] [CrossRef] [PubMed]
- Khvorost, O.; Posohova, I.; Fedchenkova, Y.; Skrebtsova, K. Component composition of essential oil shoots and leaves of Laurus nobilis L. Ukrainian origian. Sci. Pharm. Sci. 2021, 4, 50–58. [Google Scholar] [CrossRef]
- Guenane, H.; Gherib, A.; Carbonell-Barrachina, Á.; Cano-Lamadrid, M.; Krika, F.; Berrabah, M.; Maatallah, M.; Bakchiche, B. Minerals analysis, antioxidant and chemical composition of extracts of Laurus nobilis from southern Algeria. J. Mater. Environ. Sci. 2016, 7, 4253–4261. [Google Scholar]
- Awada, F.; Hamade, K.; Kassir, M.; Hammoud, Z.; Mesnard, F.; Rammal, H.; Fliniaux, O. Laurus nobilis Leaves and Fruits: A Review of Metabolite Composition and Interest in Human Health. Appl. Sci. 2023, 13, 4606. [Google Scholar] [CrossRef]
- de cássia Basilio, L. Óleo Laurus nobilis L. (laurel) Essential Induces Activation of Reactive Oxygen Species and Cell Signaling Pathways during Larvicidal Action; Federal University of Viçosa: Viçosa, Brazil, 2022. [Google Scholar] [CrossRef]
- Jaradat, N.; Abualhasan, M.; Hawash, M.; Qadi, M.; Al-Maharik, N.; Abdallah, S.; Mousa, A.; Zarour, A.; Arar, M.; Sobuh, S.; et al. Chromatography analysis, in light of vitro antioxidant, antidiabetic, antiobesity, anti-inflammatory, antimicrobial, anticancer, and three-dimensional cancer spheroids’ formation blocking activities of Laurus nobilis aromatic oil from Palestine. Chem. Biol. Technol. Agric. 2023, 10, 25. [Google Scholar] [CrossRef]
- Obiandu, C.; Laz-Okenwa, J.O.A.; Owhorji, B.I.; Opubo, A.T.; Samuel, H.O.A. Evaluation of the Effects of Extracts of Laurus nobilis on some Biochemical Parameters of Wistar Rats. Sch. Int. J. Anat. Physiol. 2023, 6, 37–41. [Google Scholar] [CrossRef]
- Saleh, B.H.; Yahya, H.N.; Ibrahim, R.N. Study antibacterial activity of laurus nobilis leaves water extract on some isolates of pathogenic bacteria. Iraqi J. Agric. Sci. 2023, 54, 18–24. [Google Scholar] [CrossRef]
- Durocher, G.; Sandorfy, C. Anharmonicity and hydrogen bonding: Part II. Examples of moderately strong OH⋯Y bonds. J. Mol. Spectrosc. 1965, 15, 22–28. [Google Scholar] [CrossRef]
- Rizwana, H.; Al Kubaisi, N.; Al-Meghailaith, N.N.; Moubayed, N.M.; Albasher, G. Evaluation of Chemical Composition, Antibacterial, Antifungal, and Cytotoxic Activity of Laurus nobilis L Grown in Saudi Arabia. J. Pure Appl. Microbiol. 2019, 13, 2073–2085. [Google Scholar] [CrossRef]
- Deghles, A.; Hamed, O.; Azar, M.; Abu Lail, B.; Azzaoui, K.; Abu Obied, A.; Jodeh, S. Cellulose with Bidentate Chelating Functionality: An Adsorbent for Metal Ions from Wastewater. BioResources 2019, 14, 6247–6266. [Google Scholar] [CrossRef]
- Trivedi, M.K.; Tallapragada, R.M.; Branton, A.; Trivedi, D.; Nayak, G.; Km, R.; Jana, S. Biofield Treatment: A Potential Strategy for Modification of Physical and Thermal Properties of Indole. J. Environ. Anal. Chem. 2015, 6, 4. [Google Scholar] [CrossRef]
- De Lorenzi, A.; Giorgianni, S.; Bini, R. High-resolution FTIR spectroscopy of the C—Cl stretching mode of vinyl chloride. Int. J. Interface Chem. Phys. 2009, 96, 101–108. [Google Scholar] [CrossRef]
- Fabre, N.; Rustan, I.; de Hoffmann, E.; Quetin-Leclercq, J. Determination of flavone, flavonol, and flavanone aglycones by negative ion liquid chromatography electrospray ion trap mass spectrometry. J. Am. Soc. Mass Spectrom. 2001, 12, 707–715. [Google Scholar] [CrossRef]
- Rand, D.; Elizabeth, E. Identification of Digallated and Methylated Catechins Using UPLC/MS/MS and Development of a Rapid Analysis Method for Theanine in Tea (Camellia sinensis (L.) O. Kuntze) Utilizing Evaporative Light Scattering Detection. 2009. Available online: https://repository.up.ac.za/handle/2263/28932 (accessed on 2 January 2024).
- Santagati, N.A.; Salerno, L.; Attaguile, G.; Savoca, F.; Ronsisvalle, G. Simultaneous determination of catechins, rutin, and gallic acid in Cistus species extracts by HPLC with diode array detection. J. Chromatogr. Sci. 2008, 46, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Barrajón-Catalán, E.; Fernández-Arroyo, S.; Roldán, C.; Guillén, E.; Saura, D.; Segura-Carretero, A.; Micol, V. A systematic study of the polyphenolic composition of aqueous extracts deriving from several Cistus genus species: Evolutionary relationship. Phytochem. Anal. PCA 2011, 22, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Engels, C.; Gräter, D.; Esquivel, P.; Jiménez, V.M.; Gänzle, M.G.; Schieber, A. Characterization of phenolic compounds in jocote (Spondias purpurea L.) peels by ultra high-performance liquid chromatography/electrospray ionization mass spectrometry. Food Res. Int. 2012, 46, 557–562. [Google Scholar] [CrossRef]
- Perestrelo, R.; Lu, Y.; Santos, S.A.; Silvestre, A.J.; Neto, C.P.; Câmara, J.S.; Rocha, S.M. Phenolic profile of Sercial and Tinta Negra Vitis vinifera L. grape skins by HPLC–DAD–ESI-MSn: Novel phenolic compounds in Vitis vinifera L. grape. Food Chem. 2012, 135, 94–104. [Google Scholar] [CrossRef]
- Gattuso, G.; Barreca, D.; Gargiulli, C.; Leuzzi, U.; Caristi, C. Flavonoid Composition of Citrus Juices. Mol. J. Synth. Chem. Nat. Prod. Chem. 2007, 12, 1641–1673. [Google Scholar] [CrossRef]
- Simirgiotis, M.J.; Schmeda-Hirschmann, G.; Bórquez, J.; Kennelly, E.J. The Passiflora tripartita (Banana Passion) Fruit: A Source of Bioactive Flavonoid C-Glycosides Isolated by HSCCC and Characterized by HPLC–DAD–ESI/MS/MS. Molecules 2013, 18, 1672–1692. [Google Scholar] [CrossRef]
- Alonso-Salces, R.M.; Ndjoko, K.; Queiroz, E.F.; Ioset, J.R.; Hostettmann, K.; Berrueta, L.A.; Gallo, B.; Vicente, F. On-line characterisation of apple polyphenols by liquid chromatography coupled with mass spectrometry and ultraviolet absorbance detection. J. Chromatogr. A 2004, 1046, 89–100. [Google Scholar] [CrossRef]
- Silva, B.A.; Ferreres, F.; Malva, J.O.; Dias, A.C.P. Phytochemical and antioxidant characterization of Hypericum perforatum alcoholic extracts. Food Chem. 2005, 90, 157–167. [Google Scholar] [CrossRef]
- Ferreres, F.; Grosso, C.; Gil-Izquierdo, A.; Valentão, P.; Andrade, P.B. Phenolic compounds from Jacaranda caroba (Vell.) A. DC.: Approaches to neurodegenerative disorders. Food Chem. Toxicol. 2013, 57, 91–98. [Google Scholar] [CrossRef]
- Bravo, M.N.; Silva, S.; Coelho, A.V.; Boas, L.V.; Bronze, M.R. Analysis of phenolic compounds in Muscatel wines produced in Portugal. Anal. Chim. Acta 2006, 563, 84–92. [Google Scholar] [CrossRef]
- Borbalán, A.M.A.; Zorro, L.; Guillén, D.A.; Barroso, C.G. Study of the polyphenol content of red and white grape varieties by liquid chromatography–mass spectrometry and its relationship to antioxidant power. J. Chromatogr. A 2003, 1012, 31–38. [Google Scholar] [CrossRef]
- Benavides, A.; Montoro, P.; Bassarello, C.; Piacente, S.; Pizza, C. Catechin derivatives in Jatropha macrantha stems: Characterisation and LC/ESI/MS/MS quali–quantitative analysis. J. Pharm. Biomed. Anal. 2006, 40, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Chernonosov, A.A.; Karpova, E.A.; Lyakh, E.M. Identification of phenolic compounds in Myricaria bracteata leaves by high-performance liquid chromatography with a diode array detector and liquid chromatography with tandem mass spectrometry. Rev. Bras. Farm. 2017, 27, 576–579. [Google Scholar] [CrossRef]
- Zywicki, B.; Reemtsma, T.; Jekel, M. Analysis of commercial vegetable tanning agents by reversed-phase liquid chromatography–electrospray ionization–tandem mass spectrometry and its application to wastewater. J. Chromatogr. A 2002, 970, 191–200. [Google Scholar] [CrossRef]
- Zasheva, D.; Mladenov, P.; Rusanov, K.; Simova, S.; Zapryanova, S.; Simova-Stoilova, L.; Moyankova, D.; Djilianov, D. Fractions of Methanol Extracts from the Resurrection Plant Haberlea rhodopensis Have Anti-Breast Cancer Effects in Model Cell Systems. Separations 2023, 10, 388. [Google Scholar] [CrossRef]
- Babaei-Ghaghelestany, A.; Alebrahim, M.T.; Farzaneh, S.; Mehrabi, M. The anticancer and antibacterial properties of aqueous and methanol extracts of weeds. J. Agric. Food Res. 2022, 10, 100433. [Google Scholar] [CrossRef]
- Anoor, P.K.; Yadav, A.N.; Rajkumar, K.; Kande, R.; Tripura, C.; Naik, K.S.; Burgula, S. Methanol extraction revealed anticancer compounds Quinic Acid, 2(5H)-Furanone and Phytol in Andrographis paniculata. Mol. Clin. Oncol. 2022, 17, 151. [Google Scholar] [CrossRef] [PubMed]
- Baeshen, N.A.; Almulaiky, Y.Q.; Afifi, M.; Al-Farga, A.; Ali, H.A.; Baeshen, N.N.; Abomughaid, M.M.; Abdelazim, A.M.; Baeshen, M.N. GC-MS Analysis of Bioactive Compounds Extracted from Plant Rhazya stricta Using Various Solvents. Plants 2023, 12, 960. [Google Scholar] [CrossRef] [PubMed]
- Aldughaylibi, F.S.; Raza, M.A.; Naeem, S.; Rafi, H.; Alam, M.W.; Souayeh, B.; Farhan, M.; Aamir, M.; Zaidi, N.; Mir, T.A. Extraction of Bioactive Compounds for Antioxidant, Antimicrobial, and Antidiabetic Applications. Molecules 2022, 27, 5935. [Google Scholar] [CrossRef] [PubMed]
- Souto, A.L.; Tavares, J.F.; Da Silva, M.S.; Diniz, M.d.F.F.M.; De Athayde-Filho, P.F.; Filho, J.M.B. Anti-Inflammatory Activity of Alkaloids: An Update from 2000 to 2010. Molecules 2011, 16, 8515–8534. [Google Scholar] [CrossRef] [PubMed]
- Amel, B.; Abdlouahab, Y.; Abdlhakim, B. Assessment of the antibacterial activity of crude alkaloids extracted from seeds and roots of the plant Peganum harmala L. J. Nat. Prod. Plant Resources. 2012, 2, 568–573. [Google Scholar]
- Ameyaw, Y.; Duker-Eshun, G. The alkaloid contents of the ethno-plant organs of three antimalarial medicinal plant species in the eastern region of ghana. Int. J. Chem. Sci. 2009, 7, 48–58. [Google Scholar]
- Benavente-García, O.; Castillo, J.; Marin, F.R.; Ortuño, A.; Del Río, J.A. Uses and Properties of Citrus Flavonoids. J. Agric. Food Chem. 1997, 45, 4505–4515. [Google Scholar] [CrossRef]
- Sodipo, O.A.; Akinniyi, J.A.; Ogunbameru, J.V. Studies on certain characteristics of extracts of bark of Pausinystalia johimbe and Pausinystalia macroceras (K Schum) Pierre ex Beille. Glob. J. Pure Appl. Sci. 2000, 6, 83–88. [Google Scholar] [CrossRef][Green Version]
- Akiyama, H.; Fujii, K.; Yamasaki, O.; Oono, T.; Iwatsuki, K. Antibacterial action of several tannins against Staphylococcus aureus. J. Antimicrob. Chemother. 2001, 48, 487–491. [Google Scholar] [CrossRef]
- Kumari, D.M.; Jain, S. Tannin: An Antinutrient with Positive Effect to Manage Diabetes. Res. J. Recent Sci. 2012, 2277, 2502. [Google Scholar]
- Kren, V.; Martinkova, L. Glycosides in Medicine: “The Role of Glycosidic Residue in Biological Activity”. Curr. Med. Chem. 2001, 8, 1303–1328. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and Other Phenolic Compounds from Medicinal Plants for Pharmaceutical and Medical Aspects: An Overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Li, W.; Wang, L.; Lin, T.; Poiani, G.; Wassef, A.; Hudlikar, R.; Ondar, P.; Brunetti, L.; Kong, A.-N. Pharmacokinetics, Pharmacodynamics, and PKPD Modeling of Curcumin in Regulating Antioxidant and Epigenetic Gene Expression in Healthy Human Volunteers. Mol. Pharm. 2019, 16, 1881–1889. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The Chemistry behind Antioxidant Capacity Assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Letelier, M.E.; Molina-Berríos, A.; Cortés-Troncoso, J.; Jara-Sandoval, J.; Holst, M.; Palma, K.; Montoya, M.; Miranda, D.; González-Lira, V. DPPH and oxygen free radicals as pro-oxidant of biomolecules. Toxicol. Vitr. 2008, 22, 279–286. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Chen, Y.; Xie, M.-Y.; Nie, S.-P.; Li, C.; Wang, Y.-X. Purification, composition analysis and antioxidant activity of a polysaccharide from the fruiting bodies of Ganoderma atrum. Food Chem. 2008, 107, 231–241. [Google Scholar] [CrossRef]
- Ereifej, K.I.; Feng, H.; Rababah, T.M.; Tashtoush, S.H.; Al-U’datt, M.H.; Gammoh, S.; Al-Rabadi, G.J. Effect of Extractant and Temperature on Phenolic Compounds and Antioxidant Activity of Selected Spices. Food Nutr. Sci. 2016, 07, 362–370. [Google Scholar] [CrossRef]
- Ünver, A.; Arslan, D.; Özcan, M.M.; Akbulut, M. Phenolic Content and Antioxidant Activity of Some Spices. World Appl. Sci. J. 2009, 6, 373–377. [Google Scholar]
- Gülçin, İ. Antioxidant and antiradical activities of l-carnitine. Life Sci. 2006, 78, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Yuan, B.; Zeng, M.; Chen, J. Antioxidant capacity and major phenolic compounds of spices commonly consumed in China. Food Res. Int. 2011, 44, 530–536. [Google Scholar] [CrossRef]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and Validation of an Improved Oxygen Radical Absorbance Capacity Assay Using Fluorescein as the Fluorescent Probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef] [PubMed]
- Kratchanova, M.; Denev, P.; Ciz, M.; Lojek, A.; Mihailov, A. Evaluation of antioxidant activity of medicinal plants containing polyphenol compounds. Comparison of two extraction systems. Acta Biochim. Pol. 2010, 57, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Ben Mrid, R.; Bouchmaa, N.; Bouargalne, Y.; Ramdan, B.; Karrouchi, K.; Kabach, I.; El Karbane, M.; Idir, A.; Zyad, A.; Nhiri, M. Phytochemical Characterization, Antioxidant and In Vitro Cytotoxic Activity Evaluation of Juniperus oxycedrus Subsp. oxycedrus Needles and Berries. Molecules 2019, 24, 502. [Google Scholar] [CrossRef] [PubMed]
- Ghazali, A.; Azhar, N.H. Inscribing the Compositional Changes of Heterogeneous Bio-system through FTIR Spectroscopy—Demonstration of Guideline to Sound Interpretation. J. Adv. Res. Appl. Sci. Eng. Technol. 2023, 29, 276–290. [Google Scholar] [CrossRef]
- Gaweł-Bęben, K.; Kukula-Koch, W.; Hoian, U.; Czop, M.; Strzępek-Gomółka, M.; Antosiewicz, B. Characterization of Cistus × incanus L. and Cistus ladanifer L. Extracts as Potential Multifunctional Antioxidant Ingredients for Skin Protecting Cosmetics. Antioxidants 2020, 9, 202. [Google Scholar] [CrossRef] [PubMed]
- Effect of Crataegus Azarolus on Blood Glucose, Lipid Profile and Antioxidant Status in Streptozotocin Diabetic Rats Fed Zinc Deficient Diet. Available online: https://www.researchsquare.com (accessed on 31 July 2023).
- Mythili, K.; Reddy, C.U.; Chamundeeswari, D.; Manna, P.K. Determination of Total Phenol, Alkaloid, Flavonoid and Tannin in Different Extracts of Calanthe Triplicata. Res. Rev. J. Pharmacogn. Phytochem. 2014, 2, 40–44. [Google Scholar]
- Kancherla, N.; Dhakshinamoothi, A.; Chitra, K.; Komaram, R.B. Preliminary Analysis of Phytoconstituents and Evaluation of Anthelminthic Property of Cayratia auriculata (In Vitro). Mædica 2019, 14, 350–356. [Google Scholar]
- Mouissi, S.; Bouchelaghem, S.; Djabali, N. Phytochemical study of two medicinal plants (Rosmarinus officinalis and Anthémis nobili) from the Haddada region (El Tarf-Algeria). Ukr. J. Ecol. 2022, 12, 7–14. [Google Scholar]
- Angelina, M.; Mardhiyah, A.; Dewi, R.T.; Fajriah, S.; Muthiah, N.; Ekapratiwi, Y.; Dewijanti, I.D.; Sukirno, S.; Jamilah, J.; Hartati, S. Physicochemical and phytochemical standardization, and antibacterial evaluation of Cassia alata leaves from different locations in Indonesia. Pharmacia 2021, 68, 947–956. [Google Scholar] [CrossRef]
- Boufellous, M.; Lrhorfi, L.A.; Berrani, A.; Haoud, H.E.; Zaher, A.; Bouhaddioui, B.; Bengueddour, R. Phytochemical screening of a medicinal plant: Lavandula stoechas (Lamiaceae). J. Pharmacogn. Phytochem. 2017, 6, 56–62. [Google Scholar]
- Meharie, B.G.; Tunta, T.A. Evaluation of Diuretic Activity and Phytochemical Contents of Aqueous Extract of the Shoot Apex of Podocarpus falcactus. J. Exp. Pharmacol. 2020, 12, 629–641. [Google Scholar] [CrossRef] [PubMed]
- Ennoury, A.; BenMrid, R.; Nhhala, N.; Roussi, Z.; Latique, S.; Zouaoui, Z.; Nhiri, M. River’s Ulva intestinalis L. extract protects common bean plants (Phaseolus vulgaris L.) against salt stress. S. Afr. J. Bot. 2022, 150, 334–341. [Google Scholar] [CrossRef]
- Olatunji, O.J.; Zuo, J.; Olatunde, O.O. Securidaca inappendiculata stem extract confers robust antioxidant and antidiabetic effects against high fructose/streptozotocin induced type 2 diabetes in rats. Exploration of bioactive compounds using UHPLC-ESI-QTOF-MS. Arch. Physiol. Biochem. 2021, 129, 1187–1199. [Google Scholar] [CrossRef]
- Escribano, J.; Cabanes, J.; Jiménez-Atiénzar, M.; Ibañez-Tremolada, M.; Gómez-Pando, L.R.; García-Carmona, F.; Gandía-Herrero, F. Characterization of betalains, saponins and antioxidant power in differently colored quinoa (Chenopodium quinoa) varieties. Food Chem. 2017, 234, 285–294. [Google Scholar] [CrossRef]

| Element | Cr | Zn | Ni | Mn | Fe | Mg | Ca | Cu | Al | K |
|---|---|---|---|---|---|---|---|---|---|---|
| Value (mg/kg) | - | - | - | 72.25 | 124.275 | 1635 | 10,012.75 | 3.475 | 144.45 | 4645.5 |
| Element | CaO | K2O | SiO2 | SO3 | P2O5 | Al2O3 | MgO |
|---|---|---|---|---|---|---|---|
| Concentration % | 1.362 | 0.763 | 0.255 | 0.255 | 0.162 | 0.084 | 0.074 |
| Element | Fe2O3 | MnO | Na2O | ZnO | SrO | CuO | NiO |
| Concentration % | 0.061 | 0.022 | 0.019 | 0.007 | 0.006 | 0.003 | 0.003 |
| Peak No. | Wavenumber (cm−1) | Vibration Type | Functional Group Assignment | Intensity |
|---|---|---|---|---|
| 1 | 3278.80 | O-H stretching vibration | Alcohols or phenols | Strong sharp |
| 2 | 2928.34 | C-H stretching vibration | Aliphatic hydrocarbons | Strong |
| 3 | 1688.28 | C=O stretching vibration | Ketones or aldehydes | Medium |
| 4 | 1598.39 | C=C stretching | Aromatic rings | Strong |
| 5 | 1516.59 | C=C stretching | Aromatic compounds | Weak |
| 6 | 1450.35 | C-H (CH2) bending | Alkanes | Medium |
| 7 | 1366.51 | C-H bending | Methyl groups | Medium |
| 8 | 1267.57 | C-N stretching | Amines | Medium |
| 9 | 1187.39 | C-N stretching | Nitro compounds or amines | Weak |
| 10 | 1162.60 | C-N stretching | Amines | Weak |
| 11 | 1116.62 | C-O stretching vibration | Ethers | Weak |
| 12 | 1030.33 | C-H bending | Alkanes | Strong |
| 13 | 857.85 | C-H bending | Aromatic compounds | Weak |
| 14 | 815.31 | C-H bending | Substituted aromatic compounds | Weak |
| 15 | 560.61 | C-Cl stretching | Alkyl chlorides | Medium |
| 16 | 514.41 | C-Cl stretching | Alkyl chlorides | Medium |
| 17 | 458.82 | C-Br stretching | Alkyl bromides | Medium |
| 18 | 446.30 | C-Cl stretching | Alkyl chlorides | Medium |
| 19 | 429.91 | C-Cl stretching | Alkyl chlorides | Medium |
| 20 | 417.58 | C-Br stretching | Alkyl bromides | Medium |
| Peak Number | Rt (min) | [M-H]−/[M+H]+ (m/z) | MS2 Ions (m/z) | Molecular Formula | Proposed Compound | Molecular Weight (g/mol) |
|---|---|---|---|---|---|---|
| 1 | 16.64 | 865.2 | 577, 289 | C45H38O18 | Procyanidin trimer | 866.772 |
| 2 | 17.93 | 169.015 | 125 | C7H6O5 | Gallic acid | 170.120 |
| 3 | 19.82 | 179.039 | 135 | C9H8O4 | Caffeic acid | 180.159 |
| 4 | 20.34 | 290.07 | 152, 139, 123 | C15H14O6 | Epicatechin | 290.27 |
| 5 | 21.77 | 289.072 | 272, 152, 139, 123 | C15H14O6 | Catechin | 290.271 |
| 6 | 24.71 | 443.09 | 289, 139 | C22H18O10 | Epicatechin gallate | 442.4 |
| 7 | 27.79 | 609.145 | 301 | C27H30O16 | Rutin | 610.521 |
| 8 | 33.40 | 447.09 | 301, 271, 255, 151 | C21H20O11 | Quercetin-3-rhamnoside | 448.38 |
| 9 | 33.57 | 447.093 | 284 | C21H20O11 | Kaempferol-3-O-hexoside | 448.4 |
| 10 | 36.33 | 593.15 | 285, 145 | C27H30O15 | Kaempferol-3-O-rutinoside | 594.526 |
| 11 | 37.23 | 463 | 268, 179, 151 | C21H19O12 | Quercetin-3-glucoside | 463.4 |
| 12 | 37.55 | 434.085 | 301, 151 | C20H18O11 | Quercetin-3-O-pentoside | 434.30 |
| 13 | 38.92 | 317.03 | 179, 151 | C15H10O8 | Myricetin | 318.24 |
| Flavonoids | Tannins | Alkaloids | Anthocyanins | Saponins | Coumarins | Terpenoids/Steroids | |
|---|---|---|---|---|---|---|---|
| Aqueous Extract | + | + | + | − | + | + | − |
| Methanolic Extract | + | + | + | − | − | + | + |
| Extraction Solvent | Total Phenolic Content (mg GAE/g dw) | Flavonoid Content (mg QE/g dw) | Tannin Content (mg TAE/g dw) |
|---|---|---|---|
| Aqueous Extract | 33.766 ± 1.701 a | 37.059 ± 1.905 a | 98.439 ± 2.581 a |
| Methanolic Extract | 46.223 ± 0.637 b | 42.386 ± 0.514 b | 300.506 ± 7.747 b |
| Extraction Solvent | DPPH Scavenging IC50 (mg/mL) | ABTS Scavenging IC50 (mg/mL) | FRAP (mg TE/g dw) | ORAC (mg TE/g dw) |
|---|---|---|---|---|
| Aqueous Extract | 0.169 ± 0.005 a | 0.221 ± 0.026 a | 46.291 ± 0.299 a | 40.754 ± 0.109 a |
| Methanolic Extract | 0.079 ± 0.002 b | 0.148 ± 0.006 b | 73.262 ± 0.535 b | 77.006 ± 2.682 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mrabet, A.; Abdelfattah, B.; El Mansouri, F.; Simou, A.; Khaddor, M. Bay Laurel of Northern Morocco: A Comprehensive Analysis of Its Phytochemical Profile, Mineralogical Composition, and Antioxidant Potential. Biophysica 2024, 4, 238-255. https://doi.org/10.3390/biophysica4020017
Mrabet A, Abdelfattah B, El Mansouri F, Simou A, Khaddor M. Bay Laurel of Northern Morocco: A Comprehensive Analysis of Its Phytochemical Profile, Mineralogical Composition, and Antioxidant Potential. Biophysica. 2024; 4(2):238-255. https://doi.org/10.3390/biophysica4020017
Chicago/Turabian StyleMrabet, Amena, Bahia Abdelfattah, Fouad El Mansouri, Ayoub Simou, and Mohamed Khaddor. 2024. "Bay Laurel of Northern Morocco: A Comprehensive Analysis of Its Phytochemical Profile, Mineralogical Composition, and Antioxidant Potential" Biophysica 4, no. 2: 238-255. https://doi.org/10.3390/biophysica4020017
APA StyleMrabet, A., Abdelfattah, B., El Mansouri, F., Simou, A., & Khaddor, M. (2024). Bay Laurel of Northern Morocco: A Comprehensive Analysis of Its Phytochemical Profile, Mineralogical Composition, and Antioxidant Potential. Biophysica, 4(2), 238-255. https://doi.org/10.3390/biophysica4020017







