Abstract
This study examined twenty-eight N-methylimidazolium ionic liquids (ILs) with various substituents and anions to assess their impact on the activity of Carnitine Acetyltransferase (CAT), an indispensable enzyme in human metabolism. In vitro experiments demonstrated that these compounds inhibited CAT in a concentration-dependent manner, with IC50 values ranging from 0.93 to 30.8 mM. Structural analysis of the ILs revealed the following structure–activity relationships: (i) the length of the hydrocarbon chain at N3 markedly affects CAT activity, with longer chains resulting in stronger inhibition; (ii) the degree of unsaturation and the presence of polar groups are not essential for increased activity; (iii) the effect of the anion aligns with the Hofmeister series. One of the most potent compounds, 1-decyl-3-methylimidazolium bromide [C10C1im]Br, was identified as a mixed inhibitor of CAT with a Ki of 0.77 mM. These findings raise concerns about the biocompatibility of commonly used imidazolium ILs, as they may interfere with fatty acid oxidation by inhibiting their cellular transport.
1. Introduction
Ionic liquids (ILs) are unusual salts characterized by low melting temperature (<100 °C) and negligible vapor pressure [1]. ILs have garnered significant attention from both industry and academia over the past two decades. They are valued for their adjustable properties, such as density, viscosity, polarity, miscibility with other liquids, to name a few [2,3]. This versatility enables the tuning of specific properties by choosing particular pairs of ions, which classifies ILs as “designer solvents” [4,5]. Furthermore, due to their low vapor pressure, non-flammability, minimal volatility, non-explosiveness, and high thermal stability, ILs are considered environmentally friendly substitutes for organic solvents, making them suitable media for extraction processes [6,7,8,9,10,11,12,13,14,15,16,17,18,19], and various chemical and biochemical reactions [9,20,21,22,23]. ILs are also utilized as catalysts [24,25,26,27,28], electrolytes in batteries [29,30], stationary phases in chromatography [31,32,33], in waste material recycling [34,35], and in the pharmaceutical industry, either due to their own activity [36,37,38,39] or to improve the solubility and bioavailability of different drugs [40,41,42,43]. Multiple studies also confirm that ILs can act as stabilizing agents for enzymes such as lipase (E.C. 3.1.1.3) [44,45], laccase (E.C. 1.10.3.2) [44], esterase [46], and others [47].
However, the increasing interest and use of ILs also bring questions about their short- and long-term toxicity [48,49]. Many studies have already raised environmental concerns by showing that ILs can harm various organisms, including bacteria [50], fungi [51], plants [52], aquatic species [53,54,55,56], and others [57,58]. Additionally, the lipophilicity of the ILs’ cation and anion is strongly linked to their toxicity, affecting enzymatic activity [59,60,61], membrane permeability, and causing cytotoxic effects [62,63]. Beyond a fundamental perspective, such studies are crucial because high stability, low volatility, and limited biodegradability can lead to the accumulation of ILs in certain ecosystems and, consequently, their inclusion into food chains. In this way, ILs could also enter living organisms, and any knowledge about their actions would help prevent a potential global-scale problem. Conversely, establishing activity for ILs, combined with proven low toxicity, would facilitate the development of new drug products [38].
Carnitine acyltransferases (CTs) are essential enzymes involved in bioenergetic processes in human and animal cells [64]. They play a key role in regulating fatty acid oxidation by catalyzing the reversible transfer of acid residues between L-carnitine and CoA molecules [65,66]. Additionally, CTs influence amino acid breakdown and mitochondrial energy production by managing the acetyl-CoA generated during amino acid degradation, thus preventing mitochondrial dysfunction [64]. Given their critical role in maintaining metabolic health and the connection between abnormal CTs’ activity and disorders like obesity, insulin resistance, and mitochondrial diseases, investigating how ILs affect these enzymes is valuable. To our knowledge, no such studies have been conducted yet. Based on the general structure of ILs shown in Figure 1, it is plausible to view them as “destructured” betaines similar to L-carnitine, one of the natural CAT substrates, with fragments that could interact effectively with the specific amino acids at the active site of CTs, potentially acting as inhibitors.
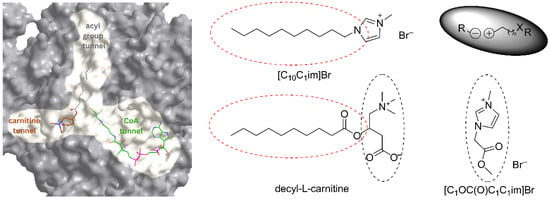
Figure 1.
The three tunnels for binding L-carnitine, CoA, and the acyl group in the active site of CTs’ representative, carnitine decyltransferase, and the position of decyl-L-carnitine in it (left); structure of two of the studied ILs (right). ILs can be considered as “destructured” betaines, which depending on the substituents in the cation, could interact with CTs, both at the L-carnitine and the fatty acid binding site.
To validate this hypothesis and develop structure–activity relationships (SARs), we conducted an in vitro study on twenty-eight N-methylimidazolium-based ILs using Carnitine Acetyltransferase (CAT) as a model enzyme. CAT is responsible for transporting short-chain fatty acids like acetate and propionate and is found within mitochondria, endoplasmic reticulum, and cytoplasmic peroxisomes [67,68,69]. Notably, because of the structural similarity among the active centers of CTs, insights gained from one representative can be applied to others [67,70].
2. Materials and Methods
2.1. General
All chemicals and CAT (isolated from pigeon breast muscle, ammonium sulfate suspension, CAS Number: 9029-90-7) used in this study were purchased from Sigma-Aldrich (FOT, Sofia, Bulgaria). The organic solvents were of analytical grade and used without further purification. Twenty-four of the studied ILs (bromides and chlorides) were synthesized and purified by us, as described elsewhere [71,72,73], and their structure and purity were confirmed by 1H and 13C NMR on a Bruker Avance III HD spectrometer (Bruker BioSpin GmbH, Rheinstetten, Germany, 500 MHz and 126 MHz for 1H and 13C, respectively) in D2O as a solvent. The NMR spectra are provided as Supplementary Materials and described for the individual ILs in the Experimental section. The chemical shifts (δ) are given in ppm, and J values are reported in Hz. ILs with different anions—[C4C1im]SCN, [C4C1im]Ac, [C4C1im]N(CN)2, and [C4C1im]Tf—were purchased from IoLiTec Ionic Liquids Technologies GmbH (Heilbronn, Germany) and tested without further purification. Biological assessment was performed using an ELISA Reader Biotek 800TS (Biotek Instruments, Inc., ELTA90, Sofia, Bulgaria).
2.2. Synthesis
2.2.1. ILs with a Varying Substituent at N3 in the Composition of the N-Methylimidazolium Ion and Bromide as an Anion (Except [HC1im]Br) Were Synthesized According to the Following General Procedure
In a round bottom flask, 1 eq. of N-methylimidazole and 1.2 eq. of the alkyl bromide were dissolved in 10 mL ACN and stirred in an inert atmosphere (nitrogen) and elevated temperature of 50 °C for 1 week. After cooling, the reaction mixture was treated with MTBE to remove the excess of alkyl bromide and the residual solvent was evaporated under reduced pressure.
N-methylimidazole hydrobromide [HC1im]Br was obtained by the reaction of N-methyl-imidazole and hydrobromic acid. In a round bottom flask 1 eq. of N-methylimidazole was stirred with 1 eq. 48% HBr for ten minutes at room temperature. The reaction mixture was evaporated under reduced pressure and [HC1im]Br was isolated (yield 77%) as light-yellow oil.
2.2.2. ILs with a Varying Substituent at N3 in the Composition of the N-Methylimidazolium Ion and Chloride as an Anion Were Synthesized According to the Following General Procedure
In a round-bottom flask, 1 eq. N-methylimidazole and 1.2 eq. of the alkyl chloride were added to 10 mL of N,N-dimethylformamide and stirred under an inert atmosphere (nitrogen) at an elevated temperature of 100 °C for 24 h. After cooling, the reaction mixture was treated with MTBE to remove the excess of alkyl chloride and the residual solvent was evaporated under reduced pressure.
2.3. NMR Spectra
2.3.1. 1-Ethyl-3-methylimidazolium Bromide—[C2C1im]Br
1H-NMR (500 MHz, D2O): δ = 7.50 (1H, d, J = 1.9 Hz, CH), 7.43 (1H, d, J = 1.8 Hz, CH), 4.24 (2H, q, J = 7.4 Hz, 1-CH2), 3.91 (3H, s, NCH3), 1.52 (3H, t, J = 7.4 Hz, 2-CH3). 13C-NMR (126 MHz, D2O): δ = 123.38 (CH), 121.81 (CH), 44.74 (CH2, 1-CH2), 35.59 (CH3, NCH3), 14.47 (CH3, 2-CH3).
2.3.2. 1-Butyl-3-methylimidazolium Bromide—[C4C1im]Br
1H-NMR (500 MHz, D2O): δ = 7.49 (1H, d, J = 1.9 Hz, CH), 7.44 (1H, d, J = 1.9 Hz, CH), 4.21 (2H, t, J = 7.2 Hz, 1-CH2), 3.90 (3H, s, NCH3), 1.94–1.81 (2H, m, 2-CH2), 1.39–1.27 (2H, m, 3-CH2), 0.93 (3H, t, J = 7.4 Hz, 4-CH3). 13C-NMR (126 MHz, D2O): δ = 123.40 (CH), 122.15 (CH), 49.24 (CH2, 1-CH2), 35.60 (CH3, NCH3), 31.23 (CH2), 18.73 (CH2), 12.61 (CH3, 4-CH3).
2.3.3. 1-Hexyl-3-methylimidazolium Bromide—[C6C1im]Br
1H-NMR (500 MHz, D2O): δ = 7.49 (1H, d, J = 1.8 Hz, CH), 7.44 (1H, d, J = 1.7 Hz, CH), 4.21 (2H, t, J = 7.1 Hz, 1-CH2), 3.91 (3H, s, NCH3), 1.96–1.72 (2H, m, 2-CH2), 1.40–1.11 (6H, m, 3–5-CH2), 0.87 (3H, t, J = 6.9 Hz, 6-CH3). 13C-NMR (126 MHz, D2O): δ = 123.42 (CH), 122.15 (CH), 49.53 (CH2, 1-CH2), 35.60 (CH3, NCH3), 30.30 (CH2), 29.12 (CH2), 24.95 (CH2), 21.74 (CH2), 13.20 (CH3, 6-CH3).
2.3.4. 1-Methyl-3-octylimidazolium Bromide—[C8C1im]Br
1H-NMR (500 MHz, D2O): δ = 7.49 (1H, d, J = 2.0 Hz, CH), 7.45 (1H, d, J = 2.0 Hz, CH), 4.21 (2H, t, J = 7.1 Hz, 1-CH2), 3.91 (3H, s, NCH3), 1.95–1.79 (2H, m, 2-CH2), 1.40–1.12 (10H, m, 3–7-CH2), 0.87 (3H, t, J = 7.0 Hz, 8-CH3). 13C-NMR (126 MHz, D2O): δ = 123.43 (CH), 122.16 (CH), 49.54 (CH2, 1-CH2), 35.61 (CH3, NCH3), 30.96 (CH2), 29.14 (CH2), 28.17 (CH2), 27.98 (CH2), 25.27 (CH2), 21.97 (CH2), 13.38 (CH3, 8-CH3).
2.3.5. 1-Decyl-3-methylimidazolium Bromide—[C10C1im]Br
1H-NMR (500 MHz, D2O): δ = 7.56 (1H, d, J = 2.0 Hz, CH), 7.54 (1H, d, J = 2.0 Hz, CH), 4.26 (2H, t, J = 7.3 Hz, 1-CH2), 3.94 (3H, s, NCH3), 1.96–1.83 (2H, m, 2-CH2), 1.43–1.17 (14H, m, 3–9-CH2), 0.84 (3H, t, J = 6.9 Hz, 10-CH3). 13C-NMR (126 MHz, D2O): δ = 123.64 (CH), 122.16 (CH), 49.53 (CH2, 1-CH2), 35.85 (CH3, NCH3), 31.64 (CH2), 29.65 (CH2), 29.25 (CH2), 29.14 (CH2), 29.03 (CH2), 28.71 (CH2), 25.82 (CH2), 22.39 (CH2), 13.69 (CH3, 10-CH3).
2.3.6. 1-Benzyl-3-methylimidazolium Bromide—[PhC1C1im]Br
1H-NMR (500 MHz, D2O): δ = 7.65–7.29 (7H, m, CH, CH, 3–7-CH), 5.39 (2H, s, 1-CH2), 3.88 (3H, s, NCH3). 13C-NMR (126 MHz, D2O): δ = 133.56 (C, 2-C), 129.31 (CH), 129.26 (CH), 128.57 (CH), 123.72 (CH), 122.21 (CH), 52.78 (CH2, 1-CH2), 35.69 (CH3, NCH3).
2.3.7. 1-Methyl-3-(2-phenylethyl)-imidazolium Bromide—[PhC2C1im]Br
1H-NMR (500 MHz, D2O): δ = 7.48–7.15 (7H, m, CH, CH, 4–8-CH), 4.51–4.44 (2H, t, J = 6.6 Hz, 1-CH2), 3.78 (3H, s, NCH3), 3.17 (2H, t, J = 6.6 Hz, 2-CH2). 13C-NMR (126 MHz, D2O): δ = 136.82 (C, 3-C), 128.95 (CH), 128.78 (CH), 127.25 (CH), 123.44 (CH), 122.12 (CH), 50.67 (CH2, 1-CH2), 35.58 (CH2, 2-CH2), 35.50 (CH3, NCH3).
2.3.8. 1-Methyl-3-(3-phenylpropyl)-imidazolium Bromide—[PhC3C1im]Br
1H-NMR (500 MHz, D2O): δ = 7.49–7.18 (7H, m, CH, CH, 5–9-CH), 4.20 (2H, t, J = 6.9 Hz, 1-CH2), 3.81 (3H, s, NCH3), 2.71 (2H, t, J = 7.3 Hz, 3-CH2), 2.24 (2H, p, J = 7.0 Hz, 2-CH2). 13C-NMR (126 MHz, D2O): δ = 140.65 (C, 4-C), 128.67 (CH), 128.44 (CH), 126.32 (CH), 123.41 (CH), 122.04 (CH), 49.05 (CH2, 1-CH2), 35.51 (CH3, NCH3), 31.81 (CH2, 2-CH2), 30.17 (CH2, 3-CH2).
2.3.9. 1-(Cyclohexylmethyl)-3-methylimidazolium Bromide—[cC6C1C1im]Br
1H-NMR (500 MHz, D2O): δ = 7.45 (2H, dd, J = 8.2, 2.0 Hz, CH, CH), 4.05 (2H, d, J = 7.2 Hz, 1-CH2), 3.91 (3H, s, NCH3), 2.07–1.57 (6H, m, CH2, CH2, CH2), 1.34–0.93 (5H, m, CH, CH2, CH2). 13C-NMR (126 MHz, D2O): δ = 123.32 (CH), 122.62 (CH), 55.34 (CH2), 37.84 (CH, 2-CH), 35.62 (CH3, NCH3), 29.44 (CH2), 25.59 (CH2), 25.02 (CH2).
2.3.10. 1-(2-Cyclohexylethyl)-3-methylimidazolium Bromide—[cC6C2C1im]Br
1H-NMR (500 MHz, D2O): δ = 8.73 (1H, s, CH), 7.49 (1H, t, J = 1.7 Hz, CH), 7.44 (1H, t, J = 1.6 Hz, CH), 4.24 (2H, t, J = 7.4 Hz, 1-CH2), 3.90 (3H, s, NCH3), 1.78 (2H, dd, J = 14.6, 7.1 Hz, 2-CH2), 1.75–1.51 (5H, m, CH, CH2, CH2), 1.33–1.10 (4H, m, CH2, CH2), 1.06–0.90 (2H, m, CH2). 13C-NMR (126 MHz, D2O): δ = 135.75 (CH), 123.44 (CH), 122.21 (CH), 47.45 (CH2), 36.60 (CH2), 35.64 (CH3, NCH3), 34.01 (CH, 3-CH), 32.26 (CH2), 25.94 (CH2), 25.60 (CH2).
2.3.11. 1-Allyl-3-methylimidazolium Bromide—[AllylC1im]Br
1H-NMR (500 MHz, D2O): δ = 7.48 (2H, dd, J = 7.7, 1.9 Hz, CH, CH), 6.13–6.01 (1H, m, 2-CH), 5.50–5.34 (2H, m, 1-CH2), 4.83 (2H, dt, J = 6.1, 1.2 Hz, 3-CH2), 3.92 (3H, s, NCH3). 13C-NMR (126 MHz, D2O): δ = 130.42 (CH), 123.54 (CH), 122.24 (CH), 121.00 (3-CH2), 51.45 (1-CH2), 35.70 (CH3, NCH3).
2.3.12. 1-Isobutyl-3-methyl-imidazolium Bromide—[i-C4C1im]Br
1H-NMR (500 MHz, D2O): δ = 7.46 (2H, dd, J = 12.5, 1.9 Hz, CH, CH), 4.04 (2H, d, J = 7.2 Hz, 1-CH2), 3.92 (3H, s, NCH3), 2.21–2.10 (1H, m, 2-CH), 1.04–0.84 (6H, m, 3-CH3, 4-CH3). 13C-NMR (126 MHz, D2O): δ = 123.39 (CH), 122.58 (CH), 56.40 (CH2, 1-CH2), 35.66 (CH3, NCH3), 28.85 (CH, 2-CH), 18.53 (CH3, 3-CH3, 4-CH3).
2.3.13. 1-Isopentyl-3-methyl-imidazolium Bromide—[i-C5C1im]Br
1H-NMR (500 MHz, D2O): δ = 7.50 (1H, d, J = 2.0 Hz, CH), 7.45 (1H, d, J = 1.9 Hz, CH), 4.28–4.14 (2H, m, 1-CH2), 3.91 (3H, s, CH3), 1.79 (2H, dd, J = 14.8, 7.1 Hz, 2-CH2), 1.64–1.53 (1H, m, 3-CH), 0.96 (3H, s, 4-CH3), 0.95 (3H, s, 5-CH3). 13C-NMR (126 MHz, D2O): δ = 123.41 (CH), 122.15 (CH), 47.91 (CH2, 1-CH2), 38.00 (CH2, 2-CH2), 35.63 (CH3, NCH3), 24.68 (CH, 3-CH), 21.34 (CH3, 4-CH3, 5-CH3).
2.3.14. 1-(3-Hydroxypropyl)-3-methyl-imidazolium Bromide—[HOC3C1im]Br
1H-NMR (500 MHz, D2O): δ = 8.76 (1H, s, CH), 7.52 (1H, t, J = 1.8 Hz, CH), 7.46 (1H, t, J = 1.7 Hz, CH), 4.32 (2H, t, J = 7.1 Hz, 1-CH2), 3.92 (CH3, NCH3), 3.65 (2H, t, J = 6.1 Hz, 3-CH2), 2.17–2.09 (2H, m, 2-CH2). 13C NMR (126 MHz, D2O): δ = 136.08 (CH), 123.60 (CH), 122.29 (CH), 57.86 (CH2, 3-CH2), 46.44 (CH2, 1-CH2), 35.71 (CH3, NCH3), 31.59 (CH2, 2-CH2).
2.3.15. 1-(3-Cyanopropyl)-3-methyl-imidazolium Bromide—[NCC3C1im]Br
1H-NMR (500 MHz, D2O): δ = 7.57 (1H, d, J = 2.0 Hz, CH), 7.51 (1H, d, J = 2.0 Hz, CH), 4.39 (2H, t, J = 7.0 Hz, 1-CH2), 3.94 (3H, s, NCH3), 2.63 (2H, t, J = 7.0 Hz, 3-CH2), 2.31 (2H, m, 2-CH2). 13C-NMR (126 MHz, D2O): δ = 123.92 (CH), 122.21 (CH), 120.04 (C, CN), 48.01 (CH2, 1-CH2), 35.78 (CH3, NCH3), 25.07 (3-CH2), 13.75 (2-CH2).
2.3.16. 1-(Methoxymethyl)-3-methylimidazolium Bromide—[C1OC1C1im]Br
1H-NMR (500 MHz, D2O): δ = 8.98 (1H, s, CH), 7.65 (1H, t, J = 1.8 Hz, CH), 7.55 (1H, t, J = 1.7 Hz, CH), 5.57 (2H, s, 1-CH2), 3.97 (3H, s, NCH3), 3.44 (3H, s, 3-CH3). 13C-NMR (126 MHz, D2O): δ = 136.57 (CH), 124.13 (CH), 121.67 (CH), 79.78 (CH2), 56.97 (CH), 35.95 (CH3, NCH3).
2.3.17. 1-(2-Methoxy-2-oxoethyl)-3-methylimidazolium Bromide—[C1OC(O)C1C1im]Br
1H-NMR (500 MHz, D2O): δ = 8.84 (1H, s, CH), 7.55–7.52 (2H, m, CH, CH), 5.21 (2H, s, 1-CH2), 3.97 (3H, s, NCH3), 3.86 (3H, s, OCH3). 13C-NMR (126 MHz, D2O): δ = 168.68 (C=O), 137.42 (CH), 123.54 (CH), 123.48 (CH), 53.56 (OCH3), 49.76 (CH2), 35.96 (CH3, NCH3).
2.3.18. 1-(3-Methoxy-3-oxopropyl)-3-methylimidazolium Bromide—[C1OC(O)C2C1im]Br
Isolated as mixture (50:50) with [HC1im]Br
1H-NMR (500 MHz, D2O): δ = 8.82 (1H, s, CH), 7.49–7.47 (2H, m, CH, CH), 4.54 (2H, t, J = 6.3 Hz, 1-CH2), 3.93 (3H, s, NCH3), 3.73 (3H, s, OCH3), 3.07 (2H, t, J = 6.3 Hz, 2-CH2). 13C NMR (126 MHz, D2O): δ = 172.92 (C=O), 134.99 (CH), 123.00 (CH), 119.47 (CH), 52.57 (OCH3), 44.77 (CH2, 1-CH2), 35.54 (CH3, NCH3), 33.99 (CH2, 2-CH2).
2.3.19. 1-(Sec-butyl)-3-methylimidazolium Bromide—[sec-C4C1im]Br
1H-NMR (500 MHz, D2O): δ = 8.79 (1H, s, CH), 7.56 (1H, t, J = 1.8 Hz, CH), 7.47 (1H, t, J = 1.8 Hz, CH), 4.48–4.37 (1H, m, 1-CH), 3.91 (3H, s, NCH3), 1.96–1.79 (2H, m, 2-CH2), 1.55 (3H, d, J = 6.8 Hz, 1′-CH3), 0.85 (3H, t, J = 7.4 Hz, 3-CH3). 13C NMR (126 MHz, D2O): δ = 134.90 (CH), 123.58 (CH), 120.37 (CH), 58.77 (CH, 1-CH), 35.68 (CH3, NCH3), 29.39 (CH2, 2-CH2), 19.97 (CH3, 1′-CH3), 9.34 (CH3, 3-CH3).
2.3.20. N-Methylimidazole hydrobromide—[HC1im]Br
1H-NMR (500 MHz, D2O): δ = 8.29 (1H, s, CH), 7.32 (1H, t, J = 1.5 Hz, CH), 7.28 (1H, t, J = 1.4 Hz, CH), 3.84 (3H, s, NCH3). 13C NMR (126 MHz, D2O): δ = 136.17 (CH), 122.41 (CH), 122.19 (CH), 34.65 (CH3, NCH3).
2.3.21. 1-Butyl-3-methylimidazolium Chloride—[C4C1im]Cl
1H NMR (500 MHz, D2O): δ = 8.72 (1H, s, CH), 7.49 (1H, t, J = 1.7 Hz, CH), 7.44 (1H, t, J = 1.7 Hz, CH), 4.20 (2H, t, J = 7.1 Hz, 1-CH2), 3.90 (3H, s, NCH3), 1.90–1.82 (2H, m, 2-CH2), 1.38–1.27 (2H, m, 3-CH2), 0.86 (3H, t, J = 7.0 Hz, 4-CH2). 13C NMR (126 MHz, D2O): δ = 135.81 (CH), 123.44 (CH), 122.18 (CH), 49.25 (CH2, 1-CH2), 35.59 (CH3, NCH3), 31.23 (CH2, 2-CH2), 18.71 (CH2, 3-CH2), 12.59 (CH3, 4-CH3).
2.3.22. 1-Hexyl-3-methylimidazolium Chloride—[C6C1im]Cl
1H-NMR (500 MHz, D2O): δ = 8.72 (1H, s, CH), 7.49 (1H, t, J = 1.8 Hz, CH), 7.44 (1H, t, J = 1.8 Hz, CH), 4.20 (2H, t, J = 7.1 Hz, 1-CH2), 3.90 (3H, s, NCH3), 1.87 (2H, p, J = 7.2 Hz, 2-CH2), 1.36–1.24 (6H, m, 3–5-CH2), 0.81 (3H, t, J = 7.0 Hz, 6-CH3). 13C NMR (126 MHz, D2O): δ = 135.78 (CH), 123.45 (CH), 122.19 (CH), 49.54 (1-CH2), 35.60 (CH3, NCH3), 30.29 (CH2), 29.12 (CH2), 24.94 (CH2), 21.73 (CH2), 13.19 (CH3, 6-CH3).
2.3.23. 1-Methyl-3-octylimidazolium Chloride—[C8C1im]Cl
1H-NMR (500 MHz, D2O): δ = 8.74 (1H, s, CH), 7.50 (1H, t, J = 1.8 Hz, CH), 7.46 (1H, t, J = 1.7 Hz, CH), 4.21 (2H, t, J = 7.1 Hz, 1-CH2), 3.91 (3H, s, NCH3), 1.93–1.83 (2H, m, 2-CH2), 1.36–1.20 (10H, m, 3–7-CH2), 0.86 (3H, t, J = 7.0 Hz, 8-CH3). 13C NMR (126 MHz, D2O): δ = 135.78 (CH), 123.49 (CH), 122.21 (CH), 49.56 (1-CH2), 35.63 (CH3, NCH3), 30.98 (CH2), 29.17 (CH2), 28.20 (CH2), 28.01 (CH2), 25.29 (CH2), 21.98 (CH2), 13.40 (CH3, 8-CH3).
2.3.24. 1-Decyl-3-methylimidazolium Chloride—[C10C1im]Cl
1H-NMR (500 MHz, D2O): δ = 7.53 (1H, d, J = 2.0 Hz, CH), 7.52 (1H, d, J = 2.0 Hz, CH), 4.24 (2H, t, J = 7.2 Hz, 1-CH2), 3.94 (3H, s, NCH3), 1.92–1.83 (2H, m, 2-CH2), 1.36–1.18 (14H, m, 3–9-CH2), 0.84 (3H, t, J = 6.9 Hz, 10-CH3). 13C NMR (126 MHz, D2O): δ = 135.92 (CH), 123.66 (CH), 122.09 (CH), 49.49 (1-CH2), 35.76 (CH3, NCH3), 31.60 (CH2), 29.57 (CH2), 29.20 (CH2), 29.08 (CH2), 28.98 (CH2), 28.65 (CH2), 25.76 (CH2), 22.36 (CH2), 13.67 (CH3, 10-CH3).
2.4. In Vitro Studies
A modified method for spectrophotometric measurement of L-carnitine using Ellman’s reagent [74] was used to study the effect of the targeted compounds on CAT activity. An enzyme with an activity of 71 U/mg protein that was isolated from pigeon breast muscle (Sigma Aldrich, FOT, Sofia, Bulgaria) was utilized in all analyses in the form of an ammonium sulfate suspension. One μmol of L-carnitine and acetyl-CoA can be converted into acetylcarnitine and free CoA in one minute by one unit of enzyme. The enzyme is diluted using phosphate buffer (0.5 M, pH = 7.6) to produce a stock solution with a concentration of 24 U/mL.
Ellman’s reagent was prepared immediately before each measurement by dissolving 25 mg DTNB in 5 mL 1 mM solution of Na2EDTA in phosphate buffer (0.5 M, pH = 7.6), the final concentration of DTNB in this solution is 12.8 mM. Stock solutions of acetyl-CoA and L-carnitine were prepared in deionized water with concentration 348.0 μM and 303.4 μM, respectively. An aqueous solution of tris(hydroxymethyl)aminomethane (TRIS) with pH = 7.6 and 1 M concentration was used as buffer solution. Test compounds were dissolved and diluted with deionized water to the desired stock concentration. The working volume of the reaction is 300 μL. It contains 50 μL of each of the six components (TRIS, DTNB, CAT, acetyl-CoA, L-carnitine and the inhibitor) solutions with concentrations as described below. Water is used in place of the inhibitor solution in the control samples. All components without L-carnitine are incubated for 5 min at 37 °C. L-carnitine was added to start the reaction, and the change in absorbance at 405 nm in kinetic mode was used to monitor its progress. The final concentrations of the components were respectively: c (TRIS) = 100 mM, c (DTNB) = 114 μM, c (CAT) = 4 U/mL, c (acetyl-CoA) = 58 μM, c (L-carnitine) = 50.56 μM. The time for reading the results was the first minute after starting the reaction. Inhibition activity was assessed by calculating the reaction rate during the initial minute, within the linear range of A/t, utilizing the following formula:
%Inh = 100 × ((∆A/∆t)control − (∆A/∆t) Inh)/((∆A/∆t) control).
The response (A) was plotted against the inhibitor’s concentration (c) to determine the half-maximal inhibitory concentration (IC50). All analyses were performed in triplicate over three consecutive days, and the corresponding IC50 values were averaged.
Extensive kinetic studies were conducted in order to determine the mechanism of inhibition of one of the most active compounds. The v0 vs. [S] (Michaelis–Menten) and 1/v0 vs. 1/[S] (Lineweaver–Burk) relationships were constructed using the data on the starting rate of the reaction, v0. Solutions of the inhibitor and L-carnitine were prepared in five different concentrations, respectively. The concentrations of the remaining components in the final volume were c (TRIS) = 100 mM, c (DTNB) = 114 μM, c (CAT) = 4 U/mL and c (acetyl-CoA) = 58 μM, respectively. The time for reading the results is every six seconds for three minutes after the start of the reaction, which corresponds to the end of the linear interval. By computing the cut-off of the v vs. t (time) dependency for the linear interval of each reaction, the initial velocity v0 was ascertained using the technique put out by Baici [75]. The experiments enabled identification of the inhibition mechanism alongside calculations of Km, Vmax, and Ki. We utilized SigmaPlot version 12.5 (Systat Software Inc. San Jose, CA, USA), which incorporates modules for regression analysis and various inhibition models.
3. Results and Discussion
To assess how ILs influence CAT activity and explore structure–activity relationships, we tested twenty-eight ILs divided into three main groups (see Figure 2). Our main focus was on the most common N-methylimidazolium ion, {[RC1im]+}, which has different substituents at the N3 position and various anions. Full names, abbreviations, and IC50 values are listed in Table 1. For better understanding of the following discussion, the IC50 values are also presented in Figure 3. The chosen structural fragments allow us to examine the following factors: (i) chain length; (ii) saturated vs. unsaturated residues; (iii) aromatic vs. aliphatic residues; (iv) nonpolar vs. polar residues; (v) branched vs. normal residues; (vi) type of anion (Figure 2).
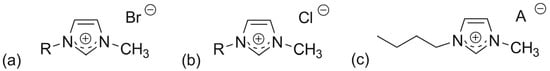
Figure 2.
General structure of the synthesized ILs based on the imidazolium ion {[RC1im]+} with: (a) a variable substituent at N3 and bromide anion, (b) an unbranched carbon chain (C4–C10) at N3 and chloride anion, (c) 1-butyl-3-methylimidazolium cation and a variable anion.

Table 1.
Abbreviation, name and IC50 values of the ILs studied.
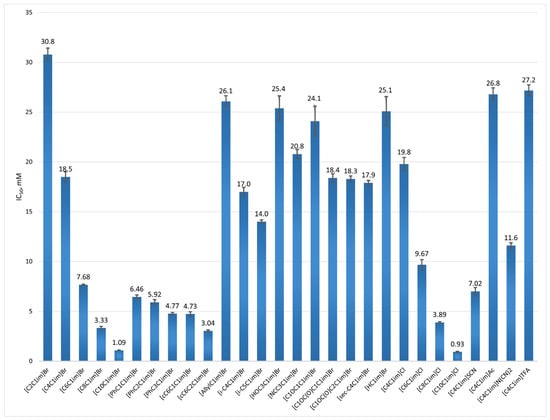
Figure 3.
IC50 values for the ILs studied.
As seen in Figure 3, all studied compounds demonstrate inhibitory activity in the low millimolar range (IC50 = 0.93–30.8 mM), comparable to that of a known betaine CAT inhibitor, Meldonium (IC50 = 1.44 mM, Ref. [76]), or its heterocyclic analogs (IC50 = 2.24–43.6 mM, Ref. [77]). These results support our initial hypothesis that ILs, due to their unique structure, can effectively interact with CAT, thus exhibiting inhibitory activity. Within this range, we can classify “conditionally” ILs as (i) strong inhibitors (IC50 < 10 mM), (ii) intermediate inhibitors (10 mM < IC50 < 20 mM), and (iii) weak inhibitors (IC50 > 20 mM). The data clearly show that the most potent ILs are the most hydrophobic, specifically those with substituents containing six or more carbon atoms. Further analysis of structure–activity relationships leads to the following general conclusions: (i) The length of the hydrocarbon chain markedly affects CAT activity, with longer chains resulting in stronger inhibition. This is best demonstrated by the IC50 values for n-alkyl bromides, which decrease in the order: [C2C1im]Br < [C4C1im]Br < [C6C1im]Br < [C8C1im]Br < [C10C1im]Br, where [C2C1im]Br has an IC50 of 30.8 ± 0.6 mM and [C10C1im]Br 1.09 ± 0.02 mM. A similar trend is observed in the chloride series {[CnC1im]Cl, n = 4, 6, 8, 10} and in other ILs with varying methylene groups, such as {[PhCnC1im]Br, n = 1, 2, 3} and {([cC6C1C1im]Br, n = 1, 2)}. A behavior similar to the one described has recently been observed by us [78] for nonionic heterocyclic inhibitors of CAT, which exhibit a mixed mode of inhibition with a preference for interacting with the enzyme-substrate complex. Similarly, we can assume that ILs interact with the hydrophobic pocket in the active site or a nearby hydrophobic region, causing allosteric inhibition. (ii) The degree of unsaturation and the presence of polar groups are not essential for increased activity. Since the active site of CAT contains aromatic amino acids—histidine (H), tyrosine (Y), and phenylalanine (F)—adding an aromatic group like a benzene ring to the alkyl chain of ILs is expected to promote additional π-π interactions between these amino acids and phenyl-containing ILs {[PhCnC1im]Br, n = 1, 2, 3} compared to their aliphatic counterparts {[cC6CnC1im]Br, n = 1, 2}. However, we observed an opposite trend: aliphatic derivatives are nearly twice as active as aromatic ones, which confirms that the main factor influencing inhibitory activity is hydrophobic interactions. This somewhat contradicts findings for ILs with polar substituents—such as terminal hydroxy, cyano, ether, or ester groups—versus nonpolar groups like n-butyl, which all fall within the higher range of intermediate inhibitors (IC50 close to 20 mM). This suggests that while polar substituents may not inherently contribute to hydrophobicity, their presence in ILs can enable specific interactions similar to those seen with more hydrophobic ILs [79,80], thus causing similar inhibitory effects. (iii) The effect of the anion on the activity of the studied ILs aligns with the Hofmeister series. Numerous studies [81,82,83] have explored how anions influence enzyme stability and activity, identifying their size and charge as key factors. Small, highly charged ions are known as kosmotropic (structure-making) because they disrupt hydrogen bonds between water molecules and form new ones, creating a structured environment and increasing overall polarity. This results in decreased solubility of non-polar compounds like proteins, thereby stabilizing enzymes. Conversely, large, monovalent ions disrupt hydrogen bonds without forming a structured network—these are chaotropic (structure-breaking) ions. They reduce solution order and allow water molecules to interact directly with enzyme amino acids. Bulky monovalent ions are also less solvated, enabling direct interactions with protein structures. The Hofmeister series summarizes the kosmotropic-chaotropic order of ions [84]. We studied six anions—SCN−, DCA−, Br−, Cl−, Ac−, and TFA−—as counterions to [C4C1im]+. The IC50 values of these compounds follow the Hofmeister series: thiocyanate, the most chaotropic, strongly inhibits CAT activity with an IC50 of 7.02 mM ± 0.37 mM. Acetate, a weakly kosmotropic ion, shows less inhibition with an IC50 of 26.8 ± 0.6 mM. While the position of DCA− in the series is not precisely known, it is considered chaotropic [85,86], with an IC50 of 11.6 ± 0.3 mM, more active than halides. Interestingly, TFA− displayed an unusual inhibitory effect, with an IC50 of 27.2 ± 0.5 mM, despite being a chaotropic ion [84]. This suggests specific interactions with the enzyme’s active or other sites, resulting in the lowest activity observed for [C4C1im]TFA in this series.
One of the most active representatives of the group, [C10C1im]Br, was further tested and its mechanism of inhibition was determined as described in the Experimental section. With the highest inhibitor concentration of 2.2 mM, no reaction was observed, indicating 100% inhibition; consequently, we excluded these values from subsequent analysis. The numerical results are summarized in Table 2, while the corresponding Lineweaver–Burk and Michaelis–Menten kinetic plots are available in the Supplementary Materials. The compound is mixed-type inhibitor with Ki = 0.77 mM, preferentially interacting with the enzyme (α > 1), Table 2.

Table 2.
Coefficient of determination (R2), Akaike Information Criterion (AIC), and standard deviation of the residuals (Sy.x) for the different types of enzyme inhibition based on kinetic measurement of CAT activity in the presence of [C10C1im]Br.
4. Conclusions
This study investigates the inhibitory effects of twenty-eight N-methylimidazolium-based ILs on CAT, a vital enzyme involved in fatty acid metabolism. The results confirm that ILs can serve as effective CAT inhibitors, with their activity significantly influenced by structural characteristics such as alkyl chain length, hydrophobicity, and the type of anion. Hydrophobic ILs with extended hydrocarbon chains demonstrated the strongest inhibitory effects, supporting the hypothesis that hydrophobic interactions predominantly contribute to binding at the enzyme’s active site or a nearby hydrophobic region. Furthermore, the inhibitory potency of ILs adheres to the Hofmeister series, with chaotropic anions, such as thiocyanate, exhibiting greater inhibitory effects than kosmotropic anions like acetate. The most potent inhibitor, [C10C1im]Br, exhibited mixed-type inhibition, with a preference for binding to the enzyme (α > 1) and a Ki value of 0.77 mM.
Because ILs structurally resemble endogenous molecules like carnitine, there is a potential for competitive interference with natural metabolic processes. While these findings indicate possible therapeutic uses for metabolic disorders, the potent inhibitory effects of certain ILs also raise concerns about unintended impacts on human metabolic pathways. Abnormal CAT activity is associated with conditions related to mitochondrial dysfunction, so extended exposure to ILs—whether from environmental build-up or medical use—could threaten health by disrupting vital energy metabolism. These results highlight the importance of thorough toxicological research to evaluate both the therapeutic benefits and possible health risks of ILs before advancing to clinical or industrial applications.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/org6040045/s1, Figure S1: Plot of the reaction rate versus time; Figure S2: Michaelis–Menten and Lineweaver–Burk plots for different types of inhibition; Figures S3–S74: 1H-, 13C- and DEPT-135 NMR spectra of all compounds described in the script.
Author Contributions
S.S. and M.G.B. contributed equally to this article. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in this study are included in the Supplementary Materials. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Shamshina, J.; Zavgorodnya, O.; Rogers, R. Ionic Liquids. In Encyclopedia of Analytical Science, 3rd ed.; Reedijk, J., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 218–225. [Google Scholar]
- Bogdanov, M.; Kantlehner, W. Simple Prediction of Some Physical Properties of Ionic Liquids: The Residual Volume Approach. Z. Naturforsch. B 2009, 64, 215–222. [Google Scholar] [CrossRef]
- Bogdanov, M.; Iliev, B.; Kantlehner, W. The Residual Volume Approach II: Simple Prediction of Ionic Conductivity of Ionic Liquids. Z. Naturforsch. B 2009, 64, 756–764. [Google Scholar] [CrossRef]
- Philippi, F.; Welton, T. Targeted modifications in ionic liquids—From understanding to design. Phys. Chem. Chem. Phys. 2021, 23, 6993–7021. [Google Scholar] [CrossRef] [PubMed]
- Andresová, A.; Bendová, M.; Schwarz, J.; Wagner, Z.; Feder-Kubis, J. Influence of the alkyl side chain length on the thermophysical properties of chiral ionic liquids with a (1R, 2S, 5R)-(−)-menthol substituent and data analysis by means of mathematical gnostics. J. Mol. Liq. 2017, 242, 336–348. [Google Scholar] [CrossRef]
- Passos, H.; Freire, M.; Coutinhoa, J. Ionic liquid solutions as extractive solvents for value-added compounds from biomass. Green Chem. 2014, 16, 4786–4815. [Google Scholar] [CrossRef]
- Lu, Y.; Ma, W.; Hu, R.; Dai, X.; Pan, Y. Ionic liquid-based microwave-assisted extraction of phenolic alkaloids from the medicinal plant Nelumbo nucifera Gaertn. J. Chromatogr. A 2008, 1208, 42–46. [Google Scholar] [CrossRef]
- Liu, T.; Sui, X.; Zhang, R.; Yang, L.; Zu, Y.; Zhang, L.; Zhang, Y.; Zhang, Z. Application of ionic liquids based microwave-assisted simultaneous extraction of carnosic acid, rosmarinic acid and essential oil from Rosmarinus officinalis. J. Chromatogr. A 2011, 1218, 8480–8489. [Google Scholar] [CrossRef]
- Wang, P.; Wang, R.; Matulis, V. Ionic Liquids as Green and Efficient Desulfurization Media Aiming at Clean Fuel. Int. J. Environ. Res. Public Health 2024, 21, 914. [Google Scholar] [CrossRef] [PubMed]
- Bogdanov, M. Ionic Liquids as Alternative Solvents for Extraction of Natural Products. In Alternative Solvents for Natural Products Extraction; Chemat, F., Vian, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 127–166. [Google Scholar]
- Bogdanov, M.; Svinyarov, I.; Keremedchieva, R.; Sidjimov, A. Ionic liquid-supported solid-liquid extraction of bioactive alkaloids. I. New HPLC method for quantitative determination of glaucine in Glaucium flavum Cr. (Papaveraceae). Sep. Purif. Technol. 2012, 97, 221–227. [Google Scholar] [CrossRef]
- Tonova, K.; Svinyarov, I.; Bogdanov, M. Hydrophobic 3-alkyl-1-methylimidazolium saccharinates as extractants for L-lactic acid recovery. Sep. Purif. Technol. 2014, 125, 239–246. [Google Scholar] [CrossRef]
- Kreuter, J.; Bica-Schröder, K.; Pálvölgyi, Á.; Krska, R.; Sommer, R.; Farnleitner, A.; Kolm, C.; Reischer, G. A novel ionic liquid-based approach for DNA and RNA extraction simplifies sample preparation for bacterial diagnostics. Anal. Bioanal. Chem. 2024, 416, 7109–7120. [Google Scholar] [CrossRef]
- Sprakel, L.; Schuur, B. Solvent developments for liquid-liquid extraction of carboxylic acids in perspective. Sep. Purif. Technol. 2019, 211, 935–957. [Google Scholar] [CrossRef]
- Pereira, J.; Lima, Á.; Freire, M.; Coutinho, J. Ionic liquids as adjuvants for the tailored extraction of biomolecules in aqueous biphasic systems. Green Chem. 2010, 12, 1661–1669. [Google Scholar] [CrossRef]
- Ventura, S.; Silva, F.; Quental, M.; Mondal, D.; Freire, M.; Coutinho, J. Ionic-Liquid-Mediated Extraction and Separation Processes for Bioactive Compounds: Past, Present, and Future Trends. Chem. Rev. 2017, 117, 6984–7052. [Google Scholar] [CrossRef] [PubMed]
- Freire, M.; Neves, C.; Marrucho, I.; Lopes, J.; Rebelo, L.; Coutinho, J. High-performance extraction of alkaloids using aqueous two-phase systems with ionic liquids. Green Chem. 2010, 12, 1715–1718. [Google Scholar] [CrossRef]
- Yudaev, P.; Chistyakov, E. Ionic Liquids as Components of Systems for Metal Extraction. Chem. Eng. 2022, 6, 6. [Google Scholar] [CrossRef]
- Zeng, Y.; Li, C.; Yu, P.; Qiu, B.; Okun, Z.; Chen, C.; Li, W.; Zhi, D.; Shpigelman, A.; Achmon, Y. Valorization of tea (Camellia sinensis) waste: Extraction of bioactive compounds using ionic liquids and evaluation of their stability, efficiency, and volatile profiles during the process. Food Chem. 2025, 492, 145338. [Google Scholar] [CrossRef]
- Itoh, T. Ionic Liquids as Tool to Improve Enzymatic Organic Synthesis. Chem. Rev. 2017, 117, 10567–10607. [Google Scholar] [CrossRef]
- Imam, H.T.; Krasňan, V.; Rebroš, M.; Marr, A. Applications of Ionic Liquids in Whole-Cell and Isolated Enzyme Biocatalysis. Molecules 2021, 26, 4791. [Google Scholar] [CrossRef]
- Welton, T. Room-Temperature Ionic Liquids. Solvents for Synthesis and Catalysis. Chem. Rev. 1999, 99, 2071–2084. [Google Scholar] [CrossRef]
- Eisele, L.; Bica-Schröder, K. Photocatalytic Carbon Dioxide Reduction with Imidazolium-Based Ionic Liquids. ChemSusChem 2025, 18, e202402626. [Google Scholar] [CrossRef]
- Stalpaert, M.; Janssens, K.; Marquez, C.; Henrion, M.; Bugaev, A.; Soldatov, A.; De Vos, D. Olefins from Biobased Sugar Alcohols via Selective, Ru-Mediated Reaction in Catalytic Phosphonium Ionic Liquids. ACS Catal. 2020, 10, 9401–9409. [Google Scholar] [CrossRef]
- Tao, Y.; Dong, R.; Pavlidis, I.; Chen, B.; Tan, T. Using imidazolium-based ionic liquids as dual solvent-catalysts for sustainable synthesis of vitamin esters: Inspiration from bio- and organo-catalysis. Green Chem. 2016, 18, 1240–1248. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, X.; Wang, W. Acidic ionic liquid grafted PPF membrane reactor and its catalytic esterification kinetics. Chem. Eng. J. 2020, 400, 125319. [Google Scholar] [CrossRef]
- Kukawka, R.; Pawlowska-Zygarowicz, A.; Dzialkowska, J.; Pietrowski, M.; Maciejewski, H.; Bica, K.; Smiglak, M. Highly Effective Supported Ionic Liquid-Phase (SILP) Catalysts: Characterization and Application to the Hydrosilylation Reaction. ACS Sustain. Chem. Eng. 2019, 7, 4699–4706. [Google Scholar] [CrossRef]
- Padvi, S.; Dalal, D. Task-specific Ionic Liquids as a Green Catalysts and Solvents for Organic Synthesis. Curr. Green Chem. 2020, 7, 104–118. [Google Scholar] [CrossRef]
- Ray, A.; Saruhan, B. Application of Ionic Liquids for Batteries and Supercapacitors. Materials 2021, 14, 2942. [Google Scholar] [CrossRef]
- Rana, S.; Thakur, R.; Dosanjh, H. Ionic liquids as battery electrolytes for lithium ion batteries: Recent advances and future prospects. Solid State Ionics 2023, 400, 116340. [Google Scholar] [CrossRef]
- Cagliero, C.; Bicchi, C. Ionic liquids as gas chromatographic stationary phases: How can they change food and natural product analyses? Anal. Bioanal. Chem. 2020, 412, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Santasalo, S.; Wiedmer, S. Ionic liquids in liquid chromatography. J. Chromatogr. Open 2025, 8, 100239. [Google Scholar] [CrossRef]
- Liu, H.; Chen, J.; Chen, M.; Wang, J.; Qiu, H. Recent development of chiral ionic liquids for enantioseparation in liquid chromatography and capillary electrophoresis: A review. Anal. Chim. Acta 2023, 1274, 341496. [Google Scholar] [CrossRef]
- Christoff-Tempesta, T.; Epps, T., III. Ionic-Liquid-Mediated Deconstruction of Polymers for Advanced Recycling and Upcycling. ACS Macro Lett. 2023, 12, 1058–1070. [Google Scholar] [CrossRef]
- Kamimura, A.; Kawamoto, T.; Fujii, K. Ionic Liquids for the Chemical Recycling of Polymeric Materials and Control of Their Solubility. Chem. Rec. 2023, 23, e202200269. [Google Scholar] [CrossRef] [PubMed]
- Gomes, J.; Silva, S.; Reis, R. Biocompatible ionic liquids: Fundamental behaviours and applications. Chem. Soc. Rev. 2019, 48, 4317–4335. [Google Scholar] [CrossRef] [PubMed]
- Egorova, K.; Gordeev, E.; Ananikov, V. Biological Activity of Ionic Liquids and Their Application in Pharmaceutics and Medicine. Chem. Rev. 2017, 117, 7132–7189. [Google Scholar] [CrossRef]
- Shamshina, J.; Rogers, R. Ionic Liquids: New Forms of Active Pharmaceutical Ingredients with Unique, Tunable Properties. Chem. Rev. 2023, 123, 11894–11953. [Google Scholar] [CrossRef] [PubMed]
- Pedro, S.; Freire, C.; Silvestre, A.; Freire, M. The Role of Ionic Liquids in the Pharmaceutical Field: An Overview of Relevant Applications. Int. J. Mol. Sci. 2020, 21, 8298. [Google Scholar] [CrossRef]
- Singh, O.; Kaur, R.; Aswal, V.; Mahajan, R. Composition and Concentration Gradient Induced Structural Transition from Micelles to Vesicles in the Mixed System of Ionic Liquid-Diclofenac Sodium. Langmuir 2016, 32, 6638–6647. [Google Scholar] [CrossRef]
- Viau, L.; Tourne-Peteilh, C.; Devoisselle, J.; Vioux, A. Ionogels as drug delivery system: One-step sol-gel synthesis using imidazolium ibuprofenate ionic liquid. Chem. Comm. 2010, 46, 228–230. [Google Scholar] [CrossRef]
- Shukla, M.; Tiwari, H.; Verma, R.; Dong, W.; Azizov, S.; Kumar, B.; Pandey, S.; Kumar, D. Role and Recent Advancements of Ionic Liquids in Drug Delivery Systems. Pharmaceutics 2023, 15, 702. [Google Scholar] [CrossRef]
- Jaitely, V.; Karatas, A.; Florence, A. Water-immiscible room temperature ionic liquids (RTILs) as drug reservoirs for controlled release. Int. J. Pharm. 2008, 354, 168–173. [Google Scholar] [CrossRef]
- Itoh, T.; Takagi, Y. Activation and stabilization of enzymes using ionic liquid engineering. In Biocatalysis in Green Solvents; Lozano, P., Ed.; Academic Press: Cambridge, MA, USA, 2022; pp. 57–83. [Google Scholar]
- Lou, W.; Zong, M. Efficient kinetic resolution of (R,S)-1-trimethylsilylethanol via lipase-mediated enantioselective acylation in ionic liquids. Chirality 2006, 18, 814–821. [Google Scholar] [CrossRef]
- Persson, M.; Bornscheuer, U. Increased stability of an esterase from Bacillus stearothermophilus in ionic liquids as compared to organic solvents. J. Mol. Catal. B Enzym. 2003, 22, 21–27. [Google Scholar] [CrossRef]
- Tarver, C.; Yuan, Q.; Pusey, M. Ionic Liquids as Protein Crystallization Additives. Crystals 2021, 11, 1166. [Google Scholar] [CrossRef]
- Kuroda, K. A simple overview of toxicity of ionic liquids and designs of biocompatible ionic liquids. New J. Chem. 2022, 46, 20047–20052. [Google Scholar] [CrossRef]
- Gonçalves, A.; Paredes, X.; Cristino, A.; Santos, F.; Queirós, C. Ionic Liquids—A Review of Their Toxicity to Living Organisms. Int. J. Mol. Sci. 2021, 22, 5612. [Google Scholar] [CrossRef]
- Lee, S.; Chang, W.; Choi, A.; Koo, Y. Influence of ionic liquids on the growth of Escherichia coli. Korean J. Chem. Eng. 2005, 22, 687–690. [Google Scholar] [CrossRef]
- Petkovic, M.; Ferguson, J.; Bohn, A.; Trindade, J.; Martins, I.; Carvalho, M.; Leitão, M.; Rodrigues, C.; Garcia, H.; Ferreira, R.; et al. Exploring fungal activity in the presence of ionic liquids. Green Chem. 2009, 11, 889–894. [Google Scholar] [CrossRef]
- Biczak, R.; Bałczewski, P.; Pawłowska, B.; Bachowska, B.; Rychter, P. Comparison of Phytotoxicity of Selected Phosphonium Ionic Liquid. Ecol. Chem. Eng. S 2014, 21, 281–295. [Google Scholar] [CrossRef]
- Ventura, S.; Gonçalves, A.; Sintra, T.; Pereira, J.; Gonçalves, F.; Coutinho, J. Designing ionic liquids: The chemical structure role in the toxicity. Ecotoxicology 2013, 22, 1–12. [Google Scholar] [CrossRef]
- Sadeghi, A. Toxicity and Biodegradability of Solvents: A Comparative Analysis. Preprints 2016. [Google Scholar] [CrossRef]
- Delgado-Mellado, N.; Ayuso, M.; Villar-Chavero, M.; Garcia, J.; Rodriguez, F. Ecotoxicity evaluation towards Vibrio fischeri of imidazolium- and pyridinium-based ionic liquids for their use in separation processes. SN Appl. Sci. 2019, 1, 896. [Google Scholar] [CrossRef]
- Stolte, S.; Matzke, M.; Arning, J.; Böschen, A.; Pitner, W.; Welz-Biermann, U.; Jastorff, B.; Ranke, J. Effects of different head groups and functionalised side chains on the aquatic toxicity of ionic liquids. Green Chem. 2007, 9, 1170–1179. [Google Scholar] [CrossRef]
- Zhao, D.; Yongcheng, L.; Zhang, Z. Toxicity of Ionic Liquids. CLEAN–Soil Air Water 2007, 35, 42–48. [Google Scholar] [CrossRef]
- Saraiva, M.; Costa, S.; Pinto, P.; Azevedo, A. Environmental impact of ionic liquids: An overview of recent (eco)toxicological and (bio)degradability literature. ChemSusChem 2017, 10, 2321–2347. [Google Scholar]
- Stock, F.; Hoffmann, J.; Ranke, J.; Störmann, R.; Ondruschka, B.; Jastorff, B. Effects of ionic liquids on the acetylcholinesterase—A structure–activity relationship consideration. Green Chem. 2004, 6, 286–290. [Google Scholar] [CrossRef]
- Fan, Y.; Dong, X.; Yan, L.; Li, D.; Hua, S.; Hu, C.; Pan, C. Evaluation of the toxicity of ionic liquids on trypsin: A mechanism study. Chemosphere 2016, 148, 241–247. [Google Scholar] [CrossRef]
- Fan, Y.; Dong, X.; Li, X.; Zhong, Y.; Kong, J.; Hua, S.; Miao, J.; Li, Y. Spectroscopic studies on the inhibitory effects of ionic liquids on lipase activity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 159, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, D.; Shimizu, K.; Siopa, F.; Leitão, M.; Afonso, C.; Lopes, J.; Pereira, S. Plasma membrane permeabilisation by ionic liquids: A matter of charge. Green Chem. 2015, 17, 4587–4598. [Google Scholar] [CrossRef]
- Kumar, V.; Malhotra, S. Study on the potential anti-cancer activity of phosphonium and ammonium-based ionic liquids. Bioorg. Med. Chem. Lett. 2009, 19, 4643–4646. [Google Scholar] [CrossRef]
- Volpicella, M.; Sgobba, M.; Laera, L.; Francavilla, A.; De Luca, D.; Guerra, L.; Pierri, C.; De Grassi, A. Carnitine O-Acetyltransferase as a Central Player in Lipid and Branched-Chain Amino Acid Metabolism, Epigenetics, Cell Plasticity, and Organelle Function. Biomolecules 2025, 15, 216. [Google Scholar] [CrossRef]
- Fritz, I.; Yue, K. Long-chain carnitine acyltransferase and the role of acylcarnitine derivatives in the catalytic increase of fatty acid oxidation induced by carnitine. J. Lipid Res. 1963, 4, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Ramsay, R.; Arduini, A. The carnitine acyltransferases and their role in modulating acyl-CoA pools. Arch. Biochem. Biophys. 1993, 302, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Govindasamy, L.; Kukar, T.; Lian, W.; Pedersen, B.; Gu, Y.; Agbandje-McKenna, M.; Jin, S.; McKenna, R.; Wu, D. Structural and mutational characterization of L-carnitine binding to human carnitine acetyltransferase. J. Struct. Biol. 2004, 146, 416–424. [Google Scholar] [CrossRef]
- Jogl, G.; Tong, L. Crystal structure of carnitine acetyltransferase and implications for the catalytic mechanism and fatty acid transport. Cell 2003, 112, 113–122. [Google Scholar] [CrossRef]
- Wu, D.; Govindasamy, L.; Lian, W.; Gu, Y.; Kukar, T.; Agbandje-McKenna, M.; McKenna, R. Structure of human carnitine acetyltransferase. Molecular basis for fatty acyl transfer. J. Biol. Chem. 2003, 278, 13159–13165. [Google Scholar] [CrossRef]
- Ramsay, R.; Gandour, R.; van der Leij, F. Molecular enzymology of carnitine transfer and transport. Biochim. Biophys. Acta 2001, 1546, 21–43. [Google Scholar] [CrossRef]
- Bogdanov, M.G.; Petkova, D.; Hristeva, D.; Svinyarov, I.; Kantlehner, W. New guanidinium-based room-temperature ionic liquids. Substituent and anion effect on density and solubility in water. Z. Naturforsch. B 2010, 65, 37. [Google Scholar] [CrossRef]
- Bogdanov, M.G.; Svinyarov, I. Distribution of N-Methylimidazole in Ionic Liquids/Organic Solvents Systems. Processes 2017, 5, 52. [Google Scholar] [CrossRef]
- Bogdanov, M.G.; Svinyarov, I. Efficient purification of halide-based ionic liquids by means of improved apparatus for continuous liquid-liquid extraction. Sep. Purif. Technol. 2018, 196, 57–60. [Google Scholar] [CrossRef]
- Marquis, N.; Fritz, I. Enzymological determination of free carnitine concentrations in rat tissues. J. Lipid. Res. 1964, 5, 184–187. [Google Scholar] [CrossRef] [PubMed]
- Baici, A. Kinetics of Enzyme-Modifier Interactions; Springer: Vienna, Austria, 2015. [Google Scholar]
- Jaudzems, K.; Kuka, J.; Gutsaits, A.; Zinovjevs, K.; Kalvinsh, I.; Liepinsh, E.; Liepinsh, E.; Dambrova, M. Inhibition of carnitine acetyltransferase by mildronate, a regulator of energy metabolism. J. Enzyme Inhib. Med. Chem. 2009, 24, 1269–1275. [Google Scholar] [CrossRef]
- Stoyanova, S.; Bogdanov, M.G. Rational Design, Synthesis, and In Vitro Activity of Heterocyclic Gamma-Butyrobetaines as Potential Carnitine Acetyltransferase Inhibitors. Molecules 2025, 30, 735. [Google Scholar] [CrossRef]
- Stoyanova, S.; Bogdanov, M.G. Rational Design, Synthesis and In Vitro Activity of Diastereomeric cis-/trans-3-substituted-3,4-dihydroisocoumarin-4-carboxylic Acids as Potential Carnitine Acetyltransferase Inhibitors. Molecules 2025, 30, 3159. [Google Scholar] [CrossRef]
- Nakamura, K.; Kudo, Y.; Takeda, Y.; Katsuta, S. Partition of Substituted Benzenes between Hydrophobic Ionic Liquids and Water: Evaluation of Interactions between Substituents and Ionic Liquids. J. Chem. Eng. Data 2011, 56, 2160–2167. [Google Scholar] [CrossRef]
- Mohajeri, A.; Ashrafi, A. Structure and Electronic Properties of Amino Acid Ionic Liquids. J. Phys. Chem. A 2011, 115, 6589–6593. [Google Scholar] [CrossRef]
- Cacace, M.; Landau, E.; Ramsden, J. The Hofmeister series: Salt and solvent effects on interfacial phenomena. Q. Rev. Biophys. 1997, 30, 241–277. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cremer, P. Interactions between macromolecules and ions: The Hofmeister series. Curr. Opin. Chem. Biol. 2006, 10, 658–663. [Google Scholar] [CrossRef]
- Gregory, K.; Elliott, G.; Robertson, H.; Kumar, A.; Wanless, E.; Webber, G.; Craig, V.; Andersson, G.; Page, A. Mitochondrial and metabolic alterations in cancer cells. Eur. J. Cell Biol. 2022, 101, 151225. [Google Scholar] [CrossRef]
- Mazzini, V.; Craig, V. What is the fundamental ion-specific series for anions and cations? Ion specificity in standard partial molar volumes of electrolytes and electrostriction in water and non-aqueous solvents. Chem. Sci. 2017, 10, 3430–3433. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Fernández, F.; Ríos, A.; Tomás-Alonso, F.; Gómez, D.; Víllora, G. Stability of hydrolase enzymes in ionic liquids. Can. J. Chem. Eng. 2009, 87, 910–914. [Google Scholar] [CrossRef]
- Hyde, A.; Zultanski, S.; Waldman, J.; Zhong, Y.; Shevlin, M.; Peng, F. General Principles and Strategies for Salting-Out Informed by the Hofmeister Series. Org. Process. Res. Dev. 2017, 21, 1355–1370. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).