Abstract
4,4′-substituted-2,2′-((hexahydro-1H-benzo[d]imidazole-1,3(2H)-diyl)bis(methylene))bisphenols (1a–d) and 2,6-bis{[3-(2-hydroxy-5-substitutedbenzyl)octahydro-1H-benzimidazol-1-yl]methyl}-4-substitutedphenols (2a–b) were synthesized via microwave (MW) irradiation of aminal (2R,7R,11S,16S)-1,8,10,17-tetraazapentacyclo[8.8.1.1.8,170.2,70.11,16]icosane 2 with p-substituted phenols. Microwave (MW) irradiation improved reaction rates and yields at 80 °C. Compounds 1a–d were racemic, and 2a–b were diastereomeric. NMR spectra revealed key signals for the perhydrobenzimidazole fragment, aromatic rings, and aminal carbons. Differences in the 13C NMR spectra highlighted structural variations, such as distinct carbonyl and methoxyl signals in 2d. MW irradiation at higher temperatures (100–120 °C) reduced yields of 1, especially for phenols with methyl (Me) and methoxy (OMe) groups, suggesting a shift toward the formation of compound 2. Additionally, higher temperatures led to polymerization byproducts, emphasizing the impact of MW energy on reaction pathways. These results provide valuable insights for designing molecules with potential applications in materials science and medicinal chemistry.
1. Introduction
The Mannich reaction exemplifies the versatility, simplicity, and synthetic feasibility characteristic of key transformations in organic chemistry [1,2,3,4]. Over a century after its discovery, it remains widely used to synthesize bioactive compounds and advanced materials. Among its valuable products are imidazolidines, benzoxazines, and alkyl- or arylmethylenedialkylamines (Figure 1), which continue to attract attention for various applications [5,6,7,8,9]. Recent studies highlight their promise in advanced coatings and electronic applications, potential in flame retardancy, corrosion protection, and electronic applications, often leveraging bio-based feedstocks [10,11,12,13,14,15,16]. Imidazolidines and alkyl- or arylmethylenedialkylamines also exhibit noteworthy biological activities. A recent example includes the antimalarial amodiaquine, synthesized via a three-step route involving a Mannich reaction, substitution with 4,7-dichloroquinoline (DCQ), and rehydration [17]. Additionally, vitamin B1-modified lignin nanoparticles obtained via a Mannich reaction exhibited enhanced antibacterial, antioxidant, and wound-healing properties [18]. Sulfur-containing alcohols transformed through Mannich condensation yielded aminomethoxy derivatives with potent antimicrobial activity, outperforming ethanol and phenol, and proved effective as oil additives [19]. Cyclic aminals have been widely recognized as profitable precursors to obtain Mannich bases [20,21,22]. Aminals are particularly attractive because of their inherent stability, regioselectivity, and versatility to facilitate Mannich bases formation. Our research group has extensively explored the chemistry of cyclic aminals, which can be easily synthesized by the reaction of formaldehyde with various diamines under mild conditions. These attributes have enabled us to postulate cyclic aminals as convenient and strategically valuable precursors and intermediates for accessing complex polyfunctionalized structures. Our recent experiments have been extended to explore the potential applications of poly-Mannich bases in emerging technologies, including materials science, catalysis, and pharmaceutical development.
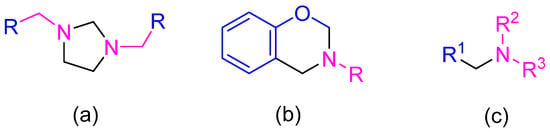
Figure 1.
Chemical structure of Mannich bases: (a) Imidazolidines, (b) Benzoxazine-type compounds; (c) alkyl- or arylmethylenedialkylamines (blue represents H active fragment precursor, and fuchsia represents amine fragment precursor).
Microwave (MW) irradiation has significantly improved Mannich reactions [23]. Compared to conventional heating, MW-assisted methods offer faster reaction times, higher selectivity and yields, and greater sustainability. In one study, MW irradiation reduced the reaction time of a terminal alkyne Mannich reaction from six hours to one minute, while maintaining yields of 70–94% [24,25]. Hydroxylated chalcones underwent regioselective C-3 Mannich substitution with higher yields under MW conditions [26]. Similarly, methylene phosphonic acid chitosan was synthesized in drastically reduced times [27], and Mannich base formation on 4-hydroxyacetophenone was reduced from 22 h to 30 min, yielding near-quantitative yields [28]. These advances underscore the transformative role of MW irradiation in accelerating synthesis, optimizing selectivity, and enabling greener, more scalable processes.
Taking into account the validity of research related to the application of Mannich bases, and expanding our knowledge regarding the synthesis of these molecules using cyclic aminals [20,21,22], this report presents the influence of MW irradiation (MW) in the synthesis of di-Mannich bases 1a–d and poly-Mannich bases 2a–d and from (2R,7R,11S,16S)-1,8,10,17-tetraazapentacyclo[8.8.1.1.8,170.2,70.11,16]icosane 3, a cyclic aminal obtained from trans-(rac)-1,2-diaminocyclohexane and formaldehyde [29]. For this purpose, a synthetic method previously reported for 4-methoxyphenol and p-cresol derivatives [22] was used, which employed a synthetic strategy combining Mannich-type Tandem reactions. The results presented here enable broadening the field of application of this class of reactions to obtain oligomeric systems that can be used to design new bioactive molecules or functional polymeric materials, as described previously. The results related to the synthesis of compounds 1a–b and 2a–d, as well as their characterization, are discussed below.
2. Materials and Methods
2.1. General Methods and Materials
The reagents and materials used in this study were acquired from commercial suppliers (Merck KGaA and/or Sigma-Aldrich, Darmstadt, Germany). Thin-layer chromatography (TLC) was used to monitor the progress of the reactions and product formation, on silica gel 60 F254 plates (Merck KGaA, Darmstadt, Germany) using 254 nm as the detection wavelength. All chemical reactions were carried out in a microwave reactor (Single-mode Discover System, model 908,005 series DY1030) (CEM corporation, Matthews, NC, USA), with temperature regulation in a sealed flask. FT-IR spectra were obtained using a Jasco FT/IR-6600 type A spectrophotometer (JASCO Co., Ltd., Mary’s Court, PA, USA), equipped with an ATR PRO ONE accessory. To obtain NMR spectra, a Bruker Avance AV-400 MHz spectrometer (Bruker, Billerica, MA, USA) was used, with TMS as the internal standard for chemical shifts reported in δ expressed in ppm. Low-resolution mass spectra were recorded analyzing the compounds in methanol dilutions in a UHPLC Shimadzu Nexera X2 chromatograph with a QTOF LCMS9030 spectrometer as detector (Shimadzu corporation, Kyoto, Japan), using positive and negative ion modes, with ESI+ identified as the optimal mode.
2.2. General Procedure: Reaction Between Aminal and p-Substituted Phenols
To an aminal 3 in 1,4-dioxane dilution (previously synthesized from trans-(rac)-1,2-diaminocyclohexane) [29] (1.00 mmol in 3 mL), a mixture of para-substituted phenol 4a–d (2.00 mmol) in 1,4-dioxane (3.0 mL) was added. p-substituted phenols employed in these experiments were carefully selected to represent a diverse range of electronic effects: R = Cl (an electron-withdrawing group by inductive effect), Me and MeO (electron-donating groups via inductive and resonance effects), and COOMe (a strongly electron-withdrawing group through both resonance and induction). The reaction mixture was stirred at room temperature. After 10 min, water (4.0 mL) was added. The mixture was then subjected to microwave-assisted heating under controlled conditions. All reactions were performed in a closed vessel using a single-mode Discover System microwave reactor. The system operated at a constant microwave power of 200 W, with internal pressure maintained between 0 and 10 psi. Reactions were conducted at three distinct temperatures (80 °C, 100 °C, and 120 °C) to evaluate the effect of temperature on the product yield. Each experiment was carried out in triplicate to ensure reproducibility. Upon completion of the 40-min heating period, the solvent was distilled under reduced pressure. Products were purified by column chromatography on silica gel using gradient elution with mixtures of hexanes and ethyl acetate of increasing polarity. Final products 1a–d and 2a–d were isolated and characterized by 1H and 13C NMR, high-resolution electrospray ionization mass spectrometry (HR-ESI-MS), and Fourier-transform infrared (FT-IR) spectroscopy. The obtained spectroscopic data are in agreement with literature values [22,30,31], and could be consulted in the Supplementary Material. Yields of compounds 1a–d and 2a–d were calculated based on the isolated product weights and are reported in Table 1 as the mean values ± standard deviation from the three independent experiments.

Table 1.
Yields for the synthesis of Mannich bases 1a–b and 2a–d at different temperatures.
3. Results and Discussion
To evaluate the reaction between cyclic aminal 3 and the used phenols 4a–d, mixtures of the mentioned reagents were prepared in 1,4-dioxane (Scheme 1). For this research, the stoichiometric correlation was determined based on earlier studies related to the solvent-free Mannich reaction between the macrocyclic aminal 1,3,6,8-tetraazatricyclo[4.4.1.13,8]dodecane (TATD) and different phenols. [20,21]. In these reactions, both the activation of the aromatic ring and the stoichiometric ratio of the reactants play a decisive role in determining the reaction pathway and the resulting products. According to these findings, imidazolidine is typically formed in low yields (20–30%); however, it can be efficiently converted into benzoxazines or benzylamines. When varying the molar ratio of TATD to phenol, the reaction mechanism changes significantly. For instance, at a 1:2 ratio, product formation can be prematurely halted due to solidification of the reaction medium. Increasing the ratio to 1:4 prevents this issue and promotes the formation of well-defined products. It was observed that an excess of phenol (e.g., 2-naphthol) at a 1:4 ratio facilitates a retro-Mannich reaction, resulting in the formation of 1,1′-methylenebis(2-naphthol), whereas a 1:2 ratio favors the high-yield formation (94%) of imidazolidine 3h. Additionally, the TATD-to-imidazolidine ratio influences the architecture of the resulting oligomers: a 1:1 ratio leads to heterocalixarene-type structures, while a 1:2 ratio favors the formation of linear trimeric oligomers. To avoid the retro-Mannich reaction pathway, the 1:2 ratio was employed in this study. The mixtures were heated using three different reaction temperatures under MW irradiation (Table 1). Each experiment was monitored and maintained under the described conditions until complete consumption of precursor 3 was evident. Once the reaction was complete (time of reaction of 40 min), the solvent was removed under reduced pressure. The 1a–d and 2a–d products were purified by classic column chromatography (silica gel).
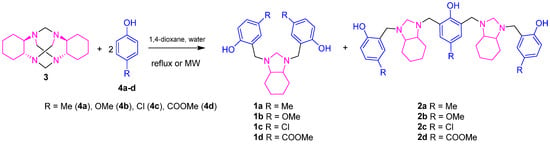
Scheme 1.
MW-assisted synthesis of Mannich bases 1a–d and 2a–d.
Structural elucidation of compounds 1a–d and 2a–d was performed using FT-IR, NMR, and HR-ESI-MS techniques. The obtained results align with those previously discussed by Quiroga et al. [31] for poly-Mannich bases 2, where the para substituents were methyl and methoxy groups, respectively. This report described the structural elucidation for this class of compounds in greater depth, which allowed the easy characterization of compounds 2c–d. These results are discussed below. The FT-IR spectra of 2c (Figure 2) allow for explaining the absence of distinct vibration modes for the respective vibrations and stretching bands, which are described below: a broad absorption band is observed in the range of 3400–2350 cm−1, assigned to the stretching vibration of the phenolic O–H bond, attributed to the presence of OH···N hydrogen bond interactions [32,33,34,35], presumably intramolecular interactions. This suggests the proton remains covalently bound to the hydroxyl group without a proton transfer to the amino group. The absorption band corresponding to the C-O stretching of the phenol functional group, located at 1253 cm−1, is shifted concerning the value reported in the literature for the infrared spectrum of phenol (1237 cm−1), indicating a shortening of the C-O bond length due to the influence of the hydrogen bond interaction mentioned [35]. Moreover, the observed signal at 1253 cm−1 suggests the presence of two distinct C-O frequencies. These bands could be attributed to the central hydroxyl group, and the terminal hydroxyl groups, indicating a possible differentiation between the two types of OH groups. The characteristic absorption bands of the aromatic rings and fused rings in the perhydrobenzimidazolidine fragment are observed in the typical ranges, highlighting a band associated with the in-plane deformation of the C-H bond in the aromatic ring with average intensity at 1037 cm−1. The absorption bands expected to stretch the Csp2-H bond are not observed since they overlap with the higher-intensity stretching vibration of the O-H bond. In addition, high-intensity bands can be observed in the ranges of 1620–1585 cm−1 and 1500–1400 cm−1, which are assigned to the symmetric and asymmetric stretching vibrations of the C-C bond within the aromatic ring. Regarding the cyclohexane ring fused to the heterocyclic ring, the C-H bending vibrations of the aliphatic ring around 1467 cm−1, of medium intensity, and the stretching bands of the Csp3-H bond stand out, highlighting a high-intensity band at 2854 cm−1.
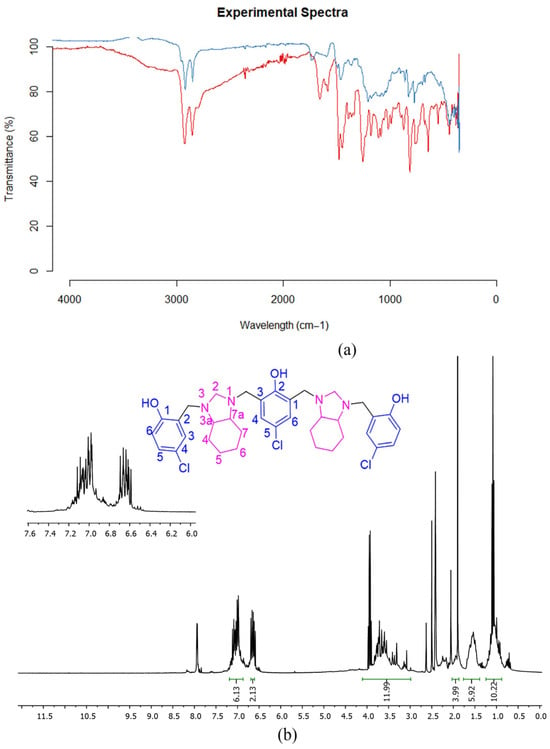
Figure 2.
Characterization data of compounds 2c–d: (a) FT-IR-ATR spectra of compounds 2c–d (red line for compound 2c and blue line for compound 2d); (b) 400 MHz 1H NMR spectrum of 2c in DMSO-d6.
The analysis of the 1H NMR spectrum in CDCl3 for compound 2c showed that the compound exists as a mixture of different stereoisomers, specifically two main types: a pair of enantiomers and a meso isomer. Although their spectra are quite similar, subtle differences allow them to be distinguished. The enantiomeric forms exhibited distinct signals corresponding to various protons in the molecule, including axial and equatorial protons on the perhydrobenzimidazole ring, as well as protons on the chiral carbons. The benzylic CH2 protons appear as separate signals due to their different environments caused by the molecule’s chirality. Additionally, signals from the animal group and the aromatic protons in the trisubstituted benzene rings were identified by their specific chemical shifts and splitting patterns, which helped confirm the structure and stereochemistry of the compound. For the meso isomer of the compound, the protons on the central tetrasubstituted aromatic ring show up as a singlet at 7.00 ppm. The signals from the perhydrobenzimidazole part are very similar to those seen in the enantiomeric pair, with axial, equatorial, and chiral protons appearing at roughly the same chemical shifts. However, the benzylic protons showed slight differences in their chemical shifts compared to the enantiomers, though their coupling constants remain the same. The aminal protons also appear as a singlet. The aromatic protons in both the trisubstituted and central rings have signals very close to those found in the enantiomeric forms, confirming the similarity between these isomers with only subtle spectral differences. Trying to improve the resolution of the 1H NMR spectrum, it was recorded in DMSO-d6 (Figure 2b), where it is evident that the signals appear in the chemical shifts discussed previously; however, the nature of the solvent caused overlapping of the signals, especially those corresponding to the benzylic and aminalic hydrogen atoms.
Finally, the protons of the central tetrasubstituted aromatic ring appear as a singlet at 7.00 ppm. For the meso isomer, the signals from the perhydrobenzimidazole fragment are almost identical to those observed for the enantiomeric pair, with values at 1.27, 1.81, 2.05, and 2.33 ppm for the axial, equatorial, and chiral center-attached protons, respectively. However, the benzylic protons show slight differences in chemical shifts, appearing as double signals at 3.44 ppm, 3.48 ppm, 4.00 ppm, and 4.12 ppm, although with the same coupling (2J of 14.0 Hz). The aminal protons are also detected as a singlet but slightly shifted towards 3.52 ppm. The signals of the aromatic protons in both the 1,2,4-trisubstituted rings and the central tetrasubstituted ring in the meso isomer are like those of the enantiomeric pair, located at 6.72 ppm, 6.92 ppm, 7.09 ppm, and 7.02 ppm.
The 13C NMR spectrum in CDCl3 or DMSO-d6 (Figure 3b) showed that the diastereomers of the compound have many similar carbon signals, but slight differences in their chemical shifts help distinguish them. For the enantiomeric pair, specific signals correspond to carbons in the perhydrobenzimidazole ring, reflecting the symmetry of this part of the molecule. The aminal carbons appear at around 76 ppm, highlighting their connection to electronegative nitrogen atoms. Benzyl and aromatic carbons each show characteristic signals at various chemical shifts. The meso isomer exhibits very similar carbon signals overall, with only slight differences in the exact chemical shifts, which make it distinguishable from the enantiomers. Mass spectrometry (ESI-MS) results in positive mode confirm the molecular composition of the compound 2c (Figure 3a). The [M+H]+ ion exhibited an experimental value of m/z 685, with characteristic chlorine isotopically ions at m/z 686, 687, and 688.
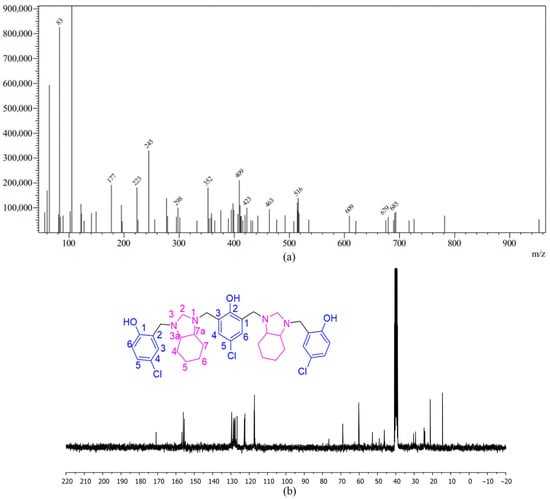
Figure 3.
Characterization data of compound 2c: (a) ESI-MS spectra of compound 2c; (b) 100 MHz 13C NMR spectrum of compound 2c in DMSO-d6.
The reactivity of cyclic aminal 3 against 4a–d has been previously reported under conventional heating conditions [31]. Chemical reactivity tests were performed using aminal 3 in solvent mixtures of 1,4-dioxane:water and ethanol:water, varying the solvent ratios and maintaining a temperature of 90 °C for 40 h. The findings indicated that raising the ratio of water compared to ethanol or 1,4-dioxane resulted in a reduction in the recovery yield of 3, hitting a lowest point at a composition of 60:40 (1,4-dioxane:water) and 1:1 (ethanol:water), after which the recovery began to rise once more. Notably, the decrease in recovery of 3 did not follow a linear trend with increasing water content. The reaction between phenol and 3 proceeded with high conversion in the 60:40 mixture, favoring the formation of products 1a–d. However, extending the reaction time to 20 h in some reactions between aminal 3 and 4a–d—intended to improve yields of products 1—resulted in a 15–30% decrease in these yields, while the concentration of compound 2a–d gradually increased by 12–17%. The formation of compound 2a–d is explained as a side reaction stemming from type 1a–d compounds. Compounds 1a–d have two free ortho positions on the external aromatic rings; they can engage in hydrogen-bonding interactions with aminal 3, facilitating a secondary aminomethylation reaction between 3 and the phenol ring. Computational calculations supported this hypothesis by revealing the free energy changes (∆G°) for these reactions: –150.63 kcal/mol for the meso isomers (3aR,7aR,3a’S,7a’S)-2a–d, and –134.74 kcal/mol for the stereoisomers (3aS,7aS,3a’S,7a’S)- and (3aR,7aR,3a’R,7a’R)-2a–d. These negative ∆G° values indicate that the formation of 2a–d is thermodynamically spontaneous and favored by prolonged reaction times. When the reactions were conducted under microwave (MW) irradiation, the yields of compounds 1a–d and 2a–d (Table 1) demonstrated that the substituent nature on the phenols significantly influences the reaction outcome. The methyl (Me), methoxy (OMe), chloro (Cl), and methoxycarbonyl (COOMe) groups each exert inductive and resonance effects on the aromatic ring, thereby modifying phenol nucleophilicity and affecting product distribution. For methyl (Me) and methoxy (OMe) substituents—both electron-donating groups via +I and +M effects—the electron density on the aromatic ring increases, enhancing phenol nucleophilicity. Consequently, yields of compound 1 were higher than those of compound 2a–d for these substituents, suggesting they favor the pathway leading to compound 1a–d. Specifically, the conventional reflux protocol at 80 °C yielded compound 1a–d in 45% (R = Me) and 34% (R = OMe), indicating that these groups facilitate interaction with cyclic aminal 3 at relatively low temperatures. In contrast, chlorine, a deactivating substituent with an adverse inductive effect (–I), reduces electron density on the aromatic ring and decreases phenol nucleophilicity. Accordingly, yields of compound 1a–d were lower than those for R = Me and R = OMe, and further reduced at higher MW irradiation temperatures (31% at 100 °C and 24% at 120 °C). This trend suggests that the –I effect of chlorine impedes the formation of compound 1a–d. For the methoxycarbonyl group (COOMe), despite the +M effect of the oxygen, the carbonyl exerts a strong –I effect, resulting in a net decrease in phenol nucleophilicity. Consequently, yields of compound 1a–d were lower compared to phenols with methyl or methoxy substituents, while yields of compound 2a–d were higher under MW conditions. This indicates that the COOMe group has a balancing influence, favoring the formation of compound 2a–d, which may be more stable under microwave irradiation.
The use of microwaves (MW) as an energy source in the reaction influenced the reaction time for the formation of compounds 1a–d and 2a–d. Although the yields obtained for these compounds may be comparable in both protocols, one of the most significant and widely recognized advantages of microwave irradiation in synthetic chemistry is the drastic reduction in reaction times, which are often shortened by orders of magnitude without sacrificing yield. Numerous studies demonstrate that reactions that usually require hours or even days with conventional heating can be completed in minutes under microwave irradiation. In the reactions discussed, the conventional heating methodology involves a reaction time of greater than 48 h, which is reduced to approximately 40 min, representing a significant advantage that enhances the synthetic process of the Mannich bases 1a–d and 2a–d. These advantages have been previously reported in Mannich reactions, where starting from 4-hydroxyacetophenone and applying MW irradiation allowed for control over the selectivity of the final product by adjusting the molar ratios of formaldehyde and secondary amine. Notably, the reaction time was significantly reduced from 22 h under conventional thermal conditions to just 30 min using microwave irradiation at 300 W and 120 °C in a sealed vessel. This approach led to the formation of mono- and disubstituted products in high yields, primarily when 1,4-dioxane was used as the solvent [28]. Additionally, the microwave-assisted Mannich reaction has enabled the efficient synthesis of Mannich bases of 2-hydroxy-chalcones, achieving good to excellent yields, with regioselectivity observed preferentially at the C-3 position of the 2-hydroxy-chalcone backbone.
Compared to conventional heating, microwave-assisted reactions provide higher yields in significantly shorter times [26]. In this study, at higher temperatures (100 °C and 120 °C), MW-assisted protocols generally generated lower yields of compound 1a–d than the conventional reflux protocol, particularly for phenols with the Me and OMe substituents. These results suggest that MW irradiation may favor the formation of compounds 2a–d over compounds 1a–d. Furthermore, the formation of a greater amount of highly polar resins was detected, which remained retained in the silica gel during the purification process. As the temperature increases, the MW reaction appears to favor a different pathway than the conventional one, leading to polymerization reactions, possibly due to the more efficient and homogeneous distribution of heat in the system, facilitating the formation of alternative products. The yields of 1a–d decreased considerably at 120 °C in the presence of MWs, suggesting that electronic activation via MWs could modify the reaction mechanism, favoring the formation of 2a–d, which could continue to react with the cyclic aminal 3a–d until its complete consumption (Scheme 2). This mechanistic purpose is based on the previously reported results obtained for the reaction between the aminal TATD [21], where the key mechanism governing the formation of both cyclic and linear products relies on the preliminary arrangement of intermolecular hydrogen type interactions. Under solvent-free conditions and at high temperatures, these hydrogen bonds break the strong intramolecular interactions of the Mannich bases, allowing new interactions with cyclic aminal. If both hydrogen bonds are formed with nitrogens from the same ethylenediamine unit (1,2-diamine), a preorganization leading to macrocyclic cyclization is favored. In contrast, if the bonds involve nitrogens from different ethylenediamine units, cyclic aminal opens, favoring the formation of linear oligomers. Likewise, the simultaneous interaction of a TATD or 3 molecule with Mannich bases 1 can generate other linear oligomers, depending on the type of diamine involved (1,2 or 1,1). Regarding the stereochemistry of the products, this proposal explains the formation of racemic mixtures for products 1 and diastereomeric mixtures for products 2. In this sense, when the phenol used reacts with the aminal in the first aminomethylation step, it has the same probability of forming both the (R, R)- and (S, S)-1 stereoisomers, since no symmetry element can alter the chemical environment over the reactive amine centers, generating any selectivity. When this occurs, any of the enantiomers can react with a second molecule of the aminal, forming compounds 2, such that if the (R, R)-1a–d isomer reacts, it will form the (R, R, R, R)- or (R, R, S, S)-2 diastereomers. The same will occur with the enantiomer (S, S)-1, which will lead to the formation of the diastereoisomers (S, S, R, R)- or (S, S, S, S)-2.
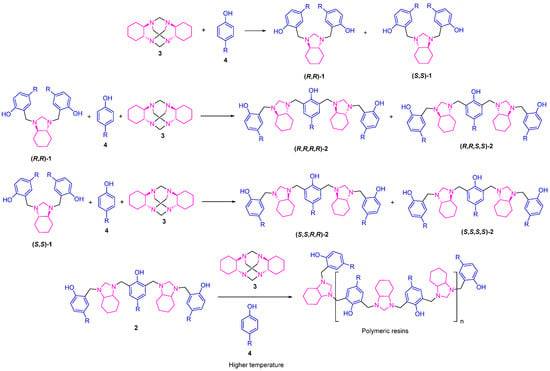
Scheme 2.
Proposed pathway for the formation of compounds 1 and 2 by the MW-assisted protocol.
4. Conclusions
The electronic nature of the phenol substituents significantly impacts the product distribution. Electron-donating groups (e.g., Me, OMe) enhance the nucleophilicity of the phenol, favoring the formation of compounds 1a–d, whereas electron-withdrawing groups (e.g., Cl, COOMe) promote the formation of compounds 2a–d. Although microwave (MW) irradiation at 80 °C favored the yields of compounds 2a–d, elevated temperatures tend to reduce the yields of 1a–d. This suggests that MW-assisted heating alters the reaction pathway, possibly by stabilizing transition states leading to alternative products, including polar resin-like byproducts. Collectively, these findings highlight the dual influence of phenol substituents and reaction conditions on the selectivity and efficiency of the synthetic route, providing valuable insight into the targeted synthesis of these complex heterocyclic systems.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/org6040044/s1. Characterization data for synthesized compounds.
Author Contributions
Conceptualization, D.Q., J.R.-M. and A.R.; Data curation, D.Q.; Formal analysis, J.R.-M. and A.R.; Funding acquisition, D.Q.; Investigation, D.Q. and A.R.; Methodology, D.Q. and J.R.-M.; Project administration, D.Q.; Resources, D.Q. and A.R.; Software, D.Q.; Supervision, A.R.; Writing—original draft, D.Q.; Writing—review and editing, D.Q., J.R.-M. and A.R. All authors have read and agreed to the published version of the manuscript.
Funding
The present work is a product derived from the project INV-CIAS-3954 funded by Universidad Militar Nueva Granada—Validity 2024.
Data Availability Statement
The data that support the findings of this study are available in the Supplementary Materials of this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bala, S.; Sharma, N.; Kajal, A.; Kamboj, S.; Saini, V. Mannich Bases: An Important Pharmacophore in Present Scenario. Int. J. Med. Chem. 2014, 2014, 1–15. [Google Scholar] [CrossRef]
- Roman, G. Mannich Bases in Medicinal Chemistry and Drug Design. Eur. J. Med. Chem. 2015, 89, 743–816. [Google Scholar] [CrossRef] [PubMed]
- Janowska, S.; Andrzejczuk, S.; Gawryś, P.; Wujec, M. Synthesis and Antimicrobial Activity of New Mannich Bases with Piperazine Moiety. Molecules 2023, 28, 5562. [Google Scholar] [CrossRef] [PubMed]
- Raju, S.K.; Settu, A.; Thiyagarajan, A.; Rama, D. Synthetic Applications of Biologically Important Mannich Bases: An Updated Review. Open Access Res. J. Biol. Pharm. 2023, 7, 001–015. [Google Scholar] [CrossRef]
- Tietze, R.; Chaudhari, M. Advanced Benzoxazine Chemistries Provide Improved Performance in a Broad Range of Applications. In Handbook of Benzoxazine Resins; Elsevier: Amsterdam, The Netherlands, 2011; pp. 595–604. [Google Scholar] [CrossRef]
- Liu, J.; Sheng, W.; Yang, R.; Liu, Y.; Lu, Y.; Zhang, K. Synthesis of Bio-diamine Derived Main-chain Type Benzoxazine Resins with Low Surface Free Energy. J. Appl. Polym. Sci. 2023, 140, e53578. [Google Scholar] [CrossRef]
- Gungor, F.S. Investigating Oxazine Exchange Reactions for Thermally Curable Naphthoxazine Synthesis. Eur. Polym. J. 2023, 196, 112285. [Google Scholar] [CrossRef]
- Appasamy, S.; Shanmugam, K.; Krishnasamy, B.; Arumugam, H.; Muthukaruppan, A. Hybrid Benzoxazines from Natural Bio-Phenolics for Enhanced Thermal Stability and Hydrophobicity: A Study on Vermiculite Reinforced Composites with Low Dielectric Constant. Int. J. Polym. Anal. Charact. 2024, 29, 226–240. [Google Scholar] [CrossRef]
- Berrouane, A.; Derradji, M.; Khiari, K.; Mehelli, O.; Habes, A.; Abdous, S.; Amri, B.; Kadi, M.E.A.; Liu, W. Sustainable Synthesis of a Novel Bio-Based Low Temperature Curable Benzoxazine Monomer from Quercetin: Synthesis, Curing Reaction and Thermal Properties. High Perform. Polym. 2024, 36, 3–16. [Google Scholar] [CrossRef]
- Chinnamuthu, R.; Madesh, P.; Arumugam, H.; Krishnasamy, B.; Govindraj, L.; Jaganathan, M.; Muthukaruppan, A. Synthesis and Characterization of New Quinolinyl Phenol Based Polybenzoxazine: Thermal Stability, Hydrophobicity and Corrosion Resistant Properties. Int. J. Polym. Anal. Charact. 2023, 28, 32–47. [Google Scholar] [CrossRef]
- Madesh, P.; Arumugam, H.; Krishnasamy, B.; Muthukaruppan, A. Synthesis of Sustainable Curcumin Based Photo-Crosslinkable Benzoxazines: Thermal, Hydrophobic and Anti-Corrosion Properties. J. Mol. Struct. 2024, 1296, 136902. [Google Scholar] [CrossRef]
- Madesh, P.; Ramachandran, S.; Krishnasamy, B.; Muthukaruppan, A. Synthesis of a New Type of Bisphenol-BC-Based Benzoxazines from Sustainable Bio-Phenol for Hydrophobic, Dielectric and Corrosion-Resistant Applications. J. Macromol. Sci. Part A 2024, 61, 203–212. [Google Scholar] [CrossRef]
- Sha, X.-L.; Fei, P.; Wang, X.; Gao, Y.; Zhu, Y.; Liu, Z.; Lv, R. Large Free Volume Biobased Benzoxazine Resin: Synthesis and Characterization, Dielectric Properties, Thermal and Mechanical Properties. Eur. Polym. J. 2023, 198, 112420. [Google Scholar] [CrossRef]
- Liu, J.; Yang, R.; Sheng, W.; Zhang, K. A Study of Ortho-Phthalimide Functional Benzoxazine Resins with Additional Cross-Linkable Group. Polym. Bull. 2024, 81, 5403–5420. [Google Scholar] [CrossRef]
- Mikesell, L.D.; Livinghouse, T. Base-Catalyzed Phenol-Mannich Condensation of Preformed Cesium Iminodiacetate. The Direct Synthesis of Calcein Blue AM and Related Acyloxymethyl Esters. J. Org. Chem. 2023, 88, 12064–12068. [Google Scholar] [CrossRef] [PubMed]
- Wile, B.M.; Griffith, C.L.; Johnson, A.R. Crystal Structure of Bis(3,5-Dichloro-2-Hydroxybenzyl)(2-Methoxyethyl)Amine. Acta Crystallogr. Sect. E Crystallogr. Commun. 2023, 79, 782–785. [Google Scholar] [CrossRef]
- Gohain, M.; Malefo, M.S.; Kunyane, P.; Scholtz, C.; Baruah, S.; Zitha, A.; Klashorst, G.v.d.; Malan, H. Process Development for the Manufacture of the Antimalarial Amodiaquine Dihydrochloride Dihydrate. Org. Process Res. Dev. 2024, 28, 124–131. [Google Scholar] [CrossRef]
- Yang, Y.; Ding, N.; Du, X.; Gao, D.; Puglia, D.; Wang, F.; Yang, X.; Xu, F.; Yang, W. Grafting Vitamin B onto Lignin to Produce Highly Bioactive Materials for Wound Dressing Hydrogels. ACS Sustain. Chem. Eng. 2024, 12, 14854–14865. [Google Scholar] [CrossRef]
- Mammadbayli, E.H.; Jafarov, I.A.; Astanova, A.D.; Shakhmammadova, A.G.; Habibova, A.G. Synthesis and Properties of Aminomethoxy Derivatives of 1-(Benzylsulfanyl)Octan-2-Ol. Russ. J. Org. Chem. 2023, 59, 54–59. [Google Scholar] [CrossRef]
- Rivera, A.; Quevedo, R. Solvent-Free Mannich-Type Reaction of Tetraazatricyclododecane (TATD) with Phenols. Tetrahedron Lett. 2013, 54, 1416–1420. [Google Scholar] [CrossRef]
- Rivera, A.; Nerio, L.S.; Quevedo, R. Synthesis of Macrocyclic and Linear Benzylimidazolidine Oligomers from Solvent Free Aromatic Mannich-Type Reaction. Tetrahedron Lett. 2015, 56, 6059–6062. [Google Scholar] [CrossRef]
- Quiroga, D.; Ríos-Motta, J.; Rivera, A. Synthesis of Diastereomeric 2,6-Bis{[3-(2-Hydroxy-5-Substitutedbenzyl)Octahydro-1H-Benzimidazol-1-Yl]Methyl}-4-Substituted Phenols (R = Me, OMe) by Mannich-Type Tandem Reactions. Molbank 2024, 2024, M1876. [Google Scholar] [CrossRef]
- Martina, K.; Cravotto, G.; Varma, R.S. Impact of Microwaves on Organic Synthesis and Strategies toward Flow Processes and Scaling Up. J. Org. Chem. 2021, 86, 13857–13872. [Google Scholar] [CrossRef] [PubMed]
- Kabalka, G.W.; Wang, L.; Pagni, R.M. A Microwave-Enhanced, Solventless Mannich Condensation on CuI-Doped Alumina. Synlett 2001, 2001, 0676–0678. [Google Scholar] [CrossRef]
- Sharifi, A.; Farhangian, H.; Mohsenzadeh, F.; Naimi-Jamal, M.R. Microwave Assisted Mannich Reaction of Terminal Alkynes on Alumina. Monatshefte Chem./Chem. Mon. 2002, 133, 199–204. [Google Scholar] [CrossRef]
- Dong, X.; Liu, T.; Chen, J.; Ying, H.; Hu, Y. Microwave-Assisted Mannich Reaction of 2-Hydroxy-Chalcones. Synth. Commun. 2009, 39, 733–742. [Google Scholar] [CrossRef]
- Dadhich, P.; Das, B.; Dhara, S. Microwave Assisted Rapid Synthesis of N-Methylene Phosphonic Chitosan via Mannich-Type Reaction. Carbohydr. Polym. 2015, 133, 345–352. [Google Scholar] [CrossRef]
- Aljohani, G.; Said, M.A.; Lentz, D.; Basar, N.; Albar, A.; Alraqa, S.Y.; Ali, A.A.-S. Microwave-Assisted Synthesis of Mono- and Disubstituted 4-Hydroxyacetophenone Derivatives via Mannich Reaction: Synthesis, XRD and HS-Analysis. Molecules 2019, 24, 590. [Google Scholar] [CrossRef]
- Glister, J.F.; Vaughan, K.; Biradha, K.; Zaworotko, M.J. (2S,7R,11S,16R)-1,8,10,17-Tetraazapentacyclo [8.8.1.18,17.02,7.011,16]Icosane and Its Enantiomer. Synthesis, NMR Analysis and X-Ray Crystal Structure. J. Mol. Struct. 2005, 749, 78–83. [Google Scholar] [CrossRef]
- Rivera, A.; Quiroga, D.; Ríos-Motta, J.; Carda, J.; Peris, G. Synthesis, Characterization and X-Ray Crystal Structure of the Di-Mannich Base 2,2′-(3aR,7aR/3aS,7aS)-Hexahydro-1H-Benzo[d]Imidazole-1,3(2H)-Diyl)Bis(Methylene)Bis(4-Methylphenol). J. Chem. Crystallogr. 2009, 39, 827–830. [Google Scholar] [CrossRef]
- Daza, Q.E.D. Estudios de La Reacción de Los Aminales (2R,7R,11S,16S)-y (2S,7R,11S,16R)-1,8,10,17-Tetrazapentaciclo[8.8.1.1.8,170.2,7011,16]Eicosano Con Nucleófilos y Electrófilos. Ph.D. Thesis, Universidad Militar Nueva Granada, Bogotá, Colombia, 2019. [Google Scholar]
- Rivera, A.; Quiroga, D.; Ríos-Motta, J.; Eigner, V.; Dušek, M. Single-Step Synthesis of a New Series of Meso Di-Mannich Bases from the Cyclic Aminal (2S,7R,11S,16R)-1,8,10,17-Tetraazapentacyclo[8.8.1.1.8,170.2,7011,16]Icosane and p-Substituted Phenols. Chem. Cent. J. 2013, 7, 100. [Google Scholar] [CrossRef]
- Brzezinski, B.; Radziejewski, P.; Rabold, A.; Zundel, G. Hydrogen Bonds and Hydrogen-Bonded Systems in Mannich Bases of 2,2′-Biphenol: An FTIR Study of the Proton Polarizability and Fermi Resonance Effects as a Function of Temperature. J. Mol. Struct. 1995, 355, 185–191. [Google Scholar] [CrossRef]
- Brzezinski, B.; Wojciechowski, G.; Urjasz, H.; Zundel, G. FT-IR Study of the Proton Polarizability of Hydrogen Bonds and of the Hydrogen-Bonded Systems in a Di-Mannich Base of 5,5′-Dimethoxy-2,2′-Biphenol. J. Mol. Struct. 1998, 470, 335–339. [Google Scholar] [CrossRef]
- Koll, A.; Melikova, S.M.; Karpfen, A.; Wolschann, P. Spectroscopic and Structural Consequences of Intramolecular Hydrogen Bond Formation in Ortho -Dimethylaminomethylphenol. J. Mol. Struct. 2001, 559, 127–145. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).