Abstract
Several fluoro phenyl triazoles were synthesized using click chemistry between fluoro phenyl azides and phenyl acetylene. Under ultrasound irradiation, this synthetic procedure was performed with Cu (I) in the presence of 1,10-phenanthroline. It is fast with high yields of target compounds. In addition, fluoro phenyl triazoles were evaluated against Candida albicans. The inhibition percentage of yeast growth was investigated using different concentrations of triazoles. Compounds containing a fluorine atom in 2, 4, 2,6, and 2,4,6 positions inhibited a higher percentage of yeast growth. All of the triazoles showed inhibition of the yeast–mycelium transition, which was related to pathogenicity of yeast strain C. albicans.
1. Introduction
Fungal diseases have become a serious public health problem. Most invasive infections are caused by five opportunistic pathogens: Candida albicans, Candida glabrata, Candida tropicalis, Candida parapsilosis, and Candida krusei [1,2]. However, C. albicans remains the most common invasive Candida infection in adult and pediatric populations, ranging from 40 to 60% [1]. C. albicans is a harmless commensal found in the oral cavity, digestive tract, and genital region of healthy individuals. Usually it does not represent a problem, but, in some cases, it can cause superficial infections [3]. The medical term for this condition is pseudomembranous candidiasis [4]. It can affect the vaginal, oropharyngeal, esophageal, and gastrointestinal organs; a prevalent infection is vulvovaginal candidiasis. Candidemia is a bloodstream infection that can develop in patients with extremely low numbers of neutrophils due to blood cancer or immunosuppressive therapy [5].
C. albicans can grow in several morphological forms: blastospore, pseudohyphae, and true hyphae [5]. Blastospores are round and grow by budding. In pseudohyphae, the buds elongate and fail to separate from the mother cell, producing filaments of elongated buds but retaining constrictions at the septal junctions. True hyphae consist of chains of tube-shaped cells without constrictions at the septal junctions [3]. The switch from the commensal to the pathogenic phase has been reported to be related to the phenotypic plasticity of C. albicans [3]. Hyphal growth is thought to be an important virulence factor. This is because filamentous cells are more invasive and penetrate tissues better, whereas blastospores are easier to transport and disseminate in the bloodstream. Modifying environmental conditions such as an increase in temperature to 37 °C as well as the presence of serum or neutral pH can induce hyphal growth. The blastospore form occurs at an environmental temperature of 30 °C and an acidic pH (pH 4.0). Pseudohyphal growth occurs at 35 °C and a pH of 5.5 [3]. Candidiasis and candidemia are truly a public health problem; in several studies, mortality rates of 30 to 50% have been reported due to this type of infection [5,6].
Triazole derivatives such as fluconazole, itraconazole, voriconazole, and posaconazole are safe and efficient for the treatment of candidiasis. However, the widespread use of these drugs, particularly fluconazole, has raised serious concerns due to the resistance of C. albicans to this type of antifungal agent [6,7,8,9]. In addition to antifungal properties, 1,2,3-triazoles are associated with other biological properties, such as antiviral, antibacterial, anticancer, anti-HIV, and antiallergic effects [10,11,12,13,14,15,16]. Fluoro phenyl 1,2,3-triazoles can form hydrogen bonds with diverse compounds, thus improving their solubility and ability to interact with biomolecular targets [17]. Due to the large number of applications of 1,2,3-triazoles and the serious concern about the development of resistance to drugs available on the market, it is important to develop efficient methodologies to synthesize them [17]. Triazoles can be obtained by a 1,3-dipolar cycloaddition between an azide and a terminal alkyne. This reaction was originally described by Huisgen [18]. In subsequent investigations, the use of Cu (I) as the catalyst for this reaction was independently reported by Sharpless and Meldal in 2002 [19,20]. In these latter conditions, Huisgen reaction was performed under very mild conditions, giving triazoles with high yields and regioselectivity. In addition, the reaction was achieved with diverse functional groups, and the purification step of the final product was relatively easy [13,19,20,21]. Since then, “click chemistry” methodology was introduced and has been extensively used in organic chemistry [19,20,21,22].
In the reaction between an azide and a terminal alkyne catalyzed by copper, several methods use Cu (I) in the reaction medium [22,23,24,25,26]. The oxidation of metallic copper (added as a wire) in the presence of Cu (II) salts represents an effective technique to obtain Cu (l). However, it has the disadvantage of requiring a long reaction time. The direct addition of soluble Cu (I) salts is also a widespread method to carry out the reaction in organic solvents. An excess of a nitrogen base (triethylamine, pyridine, 2,2-bipyridine, 2,6-lutidine) relative to the amount of copper is required to reduce the formation of secondary products [22]. Any of these bases prevents the degradation of Cu (I) by oxidation or disproportionation. Copper sulfate pentahydrate has been used as a source of copper along with a reducing agent. This in situ reduction of Cu (II) salts has the advantage of not requiring an inert atmosphere, despite the instability of Cu (I) in the presence of oxygen [26,27].
In a recent investigation by us, this latter methodology was used for the synthesis of several novel fluoro phenyl 1,2,3-triazole derivatives using microwave irradiation [28]. In this study, these derivatives were prepared using ultrasound irradiation. Initially, the corresponding fluoro phenyl azides were obtained using diazonium salts. Then, each azide was reacted with phenylacetylene using Cu (I) as a catalyst [28,29]. To have Cu (I) in the reaction medium, copper sulfate was used, and the in situ reduction was carried out with sodium ascorbate. The synthesis was performed upon stirring at room temperature and under ultrasound irradiation. In addition, the effect of a complexing agent (1,10-phenanthroline) on this reaction was also evaluated. The compounds obtained were purified and characterized [28]. Finally, they were evaluated against the opportunistic yeast C. albicans to determine the inhibition of the yeast–mycelium transition and the percentage of inhibition of yeast growth [30,31].
2. Materials and Methods
2.1. General Methods
All reagents and solvents were purchased from commercial suppliers. For the synthesis of fluorinated 1,2,3-triazoles by ultrasound, the equipment used was an Ultrasonic Processor VCX 750 with a brand probe. It was obtained from Sonics & Materials, Inc. (Newtown, CT 06470, USA). The instruments was set at 20 KHz, 750 Watts, and a temperature of 20 °C, using continuous pulses. Progress of each reaction was monitored by silica gel thin-layer chromatography (TLC), and chloroform was used as a mobile phase.
All synthesized compounds were characterized by IR spectroscopy with a Thermo Nicolet iS10 spectrophotometer using attenuated total reflectance (ATR). All triazoles prepared were characterized by melting point, UV–Vis, IR, NMR, and MS analyses [28]. Melting points were measured with a Fisher Johns apparatus. UV–Vis spectra were obtained on a Shimadzu UV-2401 PC UV–Vis spectrophotometer. MS spectra and exact mass were obtained on a JEOL MS JMS-700 station in ionization mode by electronic impact (EI). NMR spectra were obtained on a Bruker 400 MHz spectrometer with deuterated solvents.
For biological studies, an IEC CENTRA CP 812 centrifuge, New Brunswick Sci. Co. (Edison, NJ 08818, USA) incubator and a Zeiss Primostar optical microscope with a 10× objective were used. C. albicans strains were part of the strain collection of the experimental mycology laboratory at FCQ/UASLP.
2.2. Synthesis of Fluoro Phenyl Azides
An aqueous solution of HCl (50%) was prepared. We placed 10 mL of this solution in a balloon flask, and 10 mmol of the corresponding fluoro aniline was added (Scheme 1). The flask was placed in an ice bath until a temperature of 0–5 °C was achieved while maintaining constant stirring. A solution of NaNO2 (10 mmol/1 mL) was added to the reaction mixture. After 30 min of stirring at 0–5 °C, a solution of sodium azide (12 mmol of NaN3 in 1 mL of distilled water) was added dropwise. In this stage, bubbling was observed due to the formation of N2 gas. The mixture was left stirring for 30 to 60 min and then neutralized with potassium carbonate (K2CO3). Finally, if the azide was obtained as a liquid, it was taken out with a pipette. When the azide was solid, it was filtered out and dried under vacuum. Each azide was characterized by IR spectroscopy, giving a strong characteristic band around 2100 cm−1 [28].
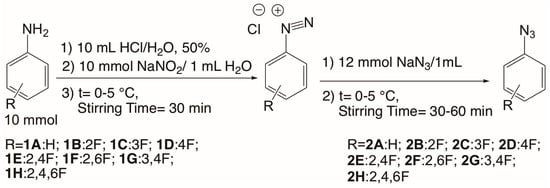
Scheme 1.
Synthesis of aromatic azides.
2.3. Procedure for the Synthesis of Fluoro Phenyl 1,2,3-Triazoles 3A-3H
All the triazoles were prepared using previously reported procedures with some modifications [28]. Two mixtures were prepared for the synthesis of triazoles (Scheme 2). To prepare the first mixture, 5 mmol of each azide was dissolved in 10 mL of ethanol, and phenylacetylene (5 mmol) was added. To prepare the second mixture, 0.2 mL of a saturated solution of copper sulfate pentahydrate (CuSO4.5H2O, 0.21 g/mL) was placed in a beaker, and an aqueous solution of sodium ascorbate (0.5 mmol/1 mL, reducing agent) was added. A color change from blue to brown was immediately observed due to the reduction of Cu (II) to Cu (I). In addition, a solution of 1,10-phenanthroline (0.25 mmol) in 2 mL of distilled water was added. The two mixtures were combined, and the resulting reaction mixture was placed under constant stirring at room temperature for 20 min to 132 min [28].
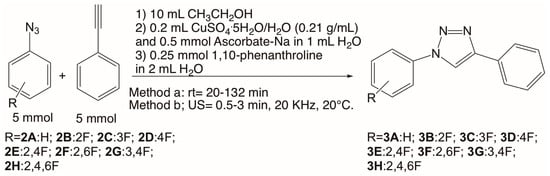
Scheme 2.
Synthesis of fluoro phenyl 1,2,3-triazoles. Some of the reactions were carried out without 1,10-phenanthroline at room temperature (RT) and under ultrasound irradiation (US).
Similar reaction mixtures were prepared, combined, and stirred under ultrasound irradiation for 0.5 to 3 min (Table 1) [32]. The instrument was set at 20 KHz, 750 Watts, temperature at 20 °C, and an amplitude of 20%. Reactions were monitored by TLC using chloroform as the mobile phase. Upon completion, the crystalline product was separated by filtration and purified by recrystallization from hexane or ethanol. Each product was characterized by UV–Vis, IR, 1H, 13C and 19F NMR, and HRMS [28]. The synthesis of some fluoro phenyl 1,2,3-triazoles was also carried out in the absence of 1,10-phenanthroline at room temperature and with ultrasound irradiation (Table 2) to evaluate the effect of a complexing agent on this reaction.

Table 1.
Synthesis of aromatic azides and fluoro phenyl 1,2,3-triazoles in the presence of 1,10-phenthroline at room temperature and ultrasound.

Table 2.
Synthesis of some phenyl 1,2,3-triazoles with and without phenanthroline.
3A. 1,4-biphenyl-1,2,3-triazole. White solid; mp:169–170 °C [11]. UV–Vis (CH3OH) λmax (nm): 248, 205. IR (ATR) (cm−1): 3097.7 (C-H aromatic), 1558.9 (C=C aromatic), 1503.4, 1480.3, 1465.7 (N-N=N), 1228.2, 1074.5 (C-N), 754.6, 688.1 (out-of-plane bending, aromatic). 1H NMR (CDCl3, 500 MHz,) δ (ppm): 8.20 (s, 1H), 7.92 (d, J = 7.6 Hz, 2H), 7.80 (d, J = 7.9 Hz, 2H), 7.56 (t, J = 7.6 Hz, 2H), 7.47 (t, J = 7.2 Hz, 3H), 7.38 (t, J = 7.4 Hz, 1H). 13C NMR (CDCl3, 200 MHz) δ (ppm): 149.35, 134.14, 133.93, 132.87, 132.09, 131.30, 129.67, 126.26, 126.08, 120.89, 120.55, 120.07, 115.82, 110.23. m/z calcd for C14H11N3: 221.10 [M]+, found: 221.00.
3B. 1-(2-fluorophenyl)-4-phenyl-1,2,3-triazole. White solid; mp: 100–101 °C [28]. UV–Vis (CH3OH) λ max (nm): 247, 205. IR (ATR) (cm−1): 3068.2 (C-H aromatic), 1598.2 (N=N), 1510.1, 1473.6, 1452.4 (N-N=N), 1243.4, 1037.9 (C-N), 1213.6 (C-F), 755.9, 689.1 (out-of-plane bending, aromatic). 1H NMR (CDCl3, 500 MHz,) δ (ppm): 8.31 (s, 1H), 8.04 (t, J = 7.7 Hz, 1H), 7.92 (d, J =7.6 Hz, 2H), 7.50–7.42 (m, 3H), 7.35 (m, 3H). 13C NMR (CDCl3, 200 MHz) δ: 154.29, 152.30, 148.22, 130.19, 130.13, 128.94, 128.47, 125.34, 125.31, 124.84, 120.70, 120.63, 117.14, 116.98. 19F NMR (CDCl3) δ: −127 ppm. HRMS (DART) m/z calcd for C14H10N3F: 239.09 [M]+, found: 239.09.
3C. 1-(3-fluorophenyl)-4-phenyl-1,2,3-triazole. White solid; mp: 185–187 °C [28]. UV–Vis (CH3OH) λ max (nm): 247.5, 206.5. IR (ATR) (cm−1): 3099.4 (C-H aromatic), 1600.9 (N=N), 1500.4, 1472.3, 1452.2 (N-N=N), 1259.8, 1038.4 (C-N), 1210.4 (C-F), 878.3, 784.1, 964.3 (out-of-plane bending, aromatic). 1H NMR (CDCl3, 500 MHz,) δ: 8.19 (s, 1H), 7.91 (d, J = 7.8 Hz, 2H), 7.6 (d, J = 8.4 Hz, 2H), 7.53 (m, 1H), 7.47 (t, J = 7.5 Hz, 2H), 7.39 (t, J = 7.3 Hz, 1H), 7.17 (t, 1H). 13C NMR (CDCl3, 200 MHz) δ: 164.16, 162.0, 131.30, 131.23, 130.01, 128.99, 128.63, 125.89, 115.83, 115.80, 115.77, 115.60, 108.40, 108.19. 19F NMR (CDCl3) δ: −110 ppm. HRMS (DART) m/z calcd for C14H10N3F: 239.09 [M]+, found: 239.06
3D. 1-(4-fluorophenyl)-4-phenyl-1,2,3-triazole. White solid; mp: 203–205 °C [28]. UV–Vis (CH3OH) λ max (nm): 247.5, 204. IR (ATR) (cm−1): 3098.1 (C-H aromatic), 1604.7 (N=N), 1575 (C=C aromatic), 1514.4, 1481.4, 1455.1 (N-N=N), 1233.4, 1040.0 (C-N), 1219.65 (C-F), 837.2, 765.3, 694.1 (out-of-plane bending, aromatic). 1H NMR (CDCl3, 500 MHz,) δ (ppm): 8.15 (s, 1H), 7.91 (d, J = 7.8 Hz, 2H), 7.78 (dd, 2H), 7.47 (t, J = 7.6 Hz, 1H), 7.38 (t, J = 7.4 Hz, 2H), 7.25 (m, 2H). 13C NMR (CDCl3, 200 MHz) δ: 163.47, 148.37, 138.00, 135.40, 130.08, 128.97, 128.56, 125.88, 122.58, 122.51, 117.76, 116.87, 116.69. 19F NMR (CDCl3) δ: −109.4 ppm. HRMS (DART) m/z calcd for C14H10N3F: 239.09 [M]+, found: 239.08.
3E. 1-(2,4-difluorophenyl)-4-phenyl-1,2,3-triazole. White solid; mp: 116–118 °C [28]. UV–Vis (CH3OH) λ max (nm): 206, 247. IR (ATR): 3054.9 (C-H aromatic), 1611.4 (N=N), 1557.7 (C=C aromatic), 1515.1, 1479.6, 1456.7 (N-N=N), 1241.2, 1034.6 (C-N), 1220.1 (C-F), 853.4, 766.5, 694.1 (out-of-plane bending, aromatic). 1H NMR (CDCl3, 500 MHz,) δ (ppm): 8.15 (s, 1H), 7.91 (d, J = 7.7 Hz, 2H), 7.78 (dd, J = 8.1, 4.7 Hz, 2H), 7.47 (t, J = 7.5 Hz, 2H), 7.38 (t, J = 7.4 Hz, 1H), 7.28–7.23 (m, 1H). 13C NMR (CDCl3, 200 MHz) δ (ppm): 169.31, 159.76, 129.99, 128.97, 128.57, 126.22, 126.14, 125.93, 120.58, 112.76, 112.61,105.63, 105.44. 19F NMR (CDCl3) δ: −108, −119 ppm. HRMS (DART) m/z calcd for C14H9N3F2: 257.08 [M]+, found: 257.00
3F. 1-(2,6-difluorophenyl)-4-phenyl-1,2,3-triazole. White solid; mp: 76–79 °C [28]. UV–Vis (CH3OH) λ max (nm): 204, 247. IR (ATR) (cm−1): 3097.6 (C-H aromatic), 1617.1 (N=N), 1598.6 (C=C aromatic), 1524.0, 1478.0, 1453.3 (N-N=N), 1242.4, 1038.4 (C-N), 1081.6 (C-F), 763.7, 753.7, 693.8 (out-of-plane bending, aromatic). 1H NMR (CDCl3, 500 MHz,) δ (ppm): 8.06 (s, 1H), 7.95 (dd, J = 7.9, 1.1, 2H), 7.56–7.48 (m, 3H), 7.41 (t, J = 7.9 Hz, 1H), 7.19 (m, 2H). 13C NMR (CDCl3, 200 MHz) δ (ppm): 166.20, 164.20, 151.62, 135.27, 133.23, 132.64, 131.67, 131.41, 130.04, 128.96, 128.56, 125.98, 122.10, 112.49. 19F NMR (CDCl3) δ: −119 (2F) ppm. HRMS (DART) m/z calcd for C14H9N3F2: 257.08 [M]+, found 257.05.
3G. 1-(3,4-difluorophenyl)-4-phenyl-1,2,3-triazole. White solid; mp: 198–200 °C [28]. UV–Vis (CH3OH) λ max (nm): 204, 247. IR (ATR) (cm−1): 3098.9 (C-H aromatic), 1617.7 (N=N), 1600.0 (C=C aromatic), 1515.9, 1477.6, 1451.3, (N-N=N), 1235.1, 1038.5 (C-N), 1219.0 (C-F), 878.9, 817.7, 765.7. 693.4 (out-of-plane bending, aromatic). 1H NMR (CDCl3, 500 MHz,) δ (ppm): 8.15 (s, 1H), 7.90 (d, J = 7.9 Hz, 2H), 7.76–7.69 (td, 1H), 7.55 (d, J = 8.7 Hz, 1H), 7.48 (t, J = 7.6 Hz, 2H), 7.42–7.33 (m, 2H). 13C NMR (CDCl3, 200 MHz) δ (ppm): 159.1, 157.8, 153.2, 139.6, 134.95, 132.84, 131.93, 129.01, 128.72, 125.90, 118.37, 110.54, 110.74. 19F NMR (CDCl3) δ: −134, −136 ppm. HRMS (DART) m/z calcd for C14H9N3F2: 257.08 [M]+, found: 257.08
3H. 1-(2,4,6-trifluorophenyl)-4-phenyl-1,2,3-triazole. White solid; mp: 108–110 °C. [28]. UV–Vis (CH3OH) λ max (nm): 204, 247. IR (ATR) (cm−1): 3071.9 (C-H aromatic), 1607.9 (N=N), 1526.2, (C=C aromatic), 1481.9, 1456.6, 1411.6 (N-N=N), 1224.2, 1022.7 (C-N), 1182.8 (C-F), 861.5, 763.2, 691.6 (out-of-plane bending, aromatic). 1H NMR (CDCl3, 500 MHz,) δ (ppm): 8.02 (s, 1H), 7.93 (dd, J = 7.9 Hz, 2H), 7.50 (t, J = 7.70 Hz, 2H), 7.41 (t, J = 7.8 Hz, 1H), 6.97 (t, J = 8.9 Hz, 2H). 13C NMR (CDCl3, 200 MHz) δ (ppm): 170.38, 166.9, 164.9, 151.92, 147.91, 129.77, 128.98, 128.65, 125.97, 122.12, 101.8, 101.58, 101.36. 19F NMR (CDCl3) d: −115 (2F) −103 ppm. HRMS (DART) m/z calcd for C14H9N3F2: 275.07 [M]+, found: 275.00.
2.4. Evaluation of Fluoro Phenyl 1,2,3-Triazoles Against C. albicans
The strain of C. albicans used in this work was isolated from a male HIV-positive patient. It was part of the strain collection of the experimental mycology laboratory at FCQ/UASLP. To evaluate the biological activity of phenyl 1,2,3-triazoles against the yeast C. albicans, Lee’s liquid medium was used. Its composition in grams per liter of water is described below: 2.5 g of K2PO4H (anhydrous), 0.2 g of MgSO4.7H20, 5 g of (NH4)2SO4 (anhydrous), 5 g of NaCl, 12 g of glucose, 0.5 g of proline, and 0.001 g of biotin. Finally, the pH was adjusted to 6.8 with a solution of H2SO4 (1:1) or NaOH (1 N).
2.5. Evaluation of Effect of Fluoro Phenyl 1,2,3-Triazoles on Inhibition of Yeast Growth
A stock C. albicans solution was prepared. A plate of a recent culture of C. albicans on Sabouraund Dextroxe Agar was taken under sterile conditions and dissolved in 1 mL of sterile saline solution. An aliquot (10 µL) was taken and placed in a Neu Bauer chamber and placed under a microscope, and the number of yeast cells/mL was counted under a microscope (10× objective). Subsequently, aliquots of 1 × 106 yeasts/mL were taken and seeded in Petri dishes for subsequent studies [30].
To determine the percentage of growth inhibition of C. albicans, two controls were prepared by placing 50 mL of Lee’s medium in two 125 mL Erlenmeyer flasks, and then 1 mL of dimethylsulfoxide (DMSO) was added to each one. In two other Erlenmeyer flasks, 50 mL of Lee’s medium and 100 µL of the C. albicans solution (1 × 106 yeasts/mL) were placed [30].
Furthermore, to evaluate the effect of each triazole at different concentrations, a stock solution of each of them was prepared at a concentration of 1000 µg/mL in DMSO. Subsequently, dilutions were made to obtain solutions with the following concentrations: 750, 500 and 250 µg/mL.
In each Erlenmeyer flask, 50 mL of Lee’s medium, 100 µL of the yeast solution, and 1 mL of the solution of each triazole (concentration: 1000, 750, 500 and 250 µg/mL) were placed.
They were incubated at 28 °C for 7 days at 100 rpm. Subsequently, the mixtures were transferred to 50 mL centrifuge (plastic) tubes that were previously weighed, and they were centrifuged at 3000 rpm/10 min. The supernatant was discarded, and the precipitate was incubated at 80 °C for 48–72 h. After this time, each tube was weighed, and, for weight difference, the growth of yeast was determined by dry weight in the presence of each triazole. Each experiment was carried out in duplicate [30].
2.6. Evaluation of Effect of Fluoro Phenyl 1,2,3-Triazoles on the Yeast–Mycelium Transition of C. albicans
To evaluate the effect of the triazoles on the yeast–mycelium transition of C. albicans, a solution of each triazole was prepared at a concentration of 100 µg/mL DMSO. Then, 1 mL of Lee’s medium was placed in test tubes, and a pellet of a young colony of C. albicans and 100 µL of the solution of the triazole (Scheme 2, 3A–3H) were resuspended. The test tubes were incubated at 37 °C for 3 h. Afterward, they were observed under a microscope. This test was positive for C. albicans and C. dubliniensis if a germ tube approximately 5 to 15 µm long was obtained from the yeast, which did not present constriction at the point of origin. After 3 h of incubation, all Candida species formed germ cell tubes, except Candida glabrata [31]. All of these tests were performed in triplicate.
In addition, the effect of the solvent was evaluated using a negative control and a positive control. For a negative control, 1 mL of Lee’s medium was placed in a test tube, and a C. albicans pellet was resuspended. It was left at room temperature for three hours and observed under a microscope [31]. For a positive control, 1 mL of Lee’s medium was placed in a test tube, and a C. albicans pellet was resuspended. It was left to incubate at 37 °C for three hours and observed under a microscope to determine the development of germinal tubes (mycelia).
Evaluation of the effect of the solvent was conducted by placing 1 mL of Lee medium in a test tube, a C. albicans pellet was resuspended, and 100 µL of DMSO was added. It was left to incubate at 37 °C for three hours and observed under a microscope.
3. Results and Discussion
3.1. Synthesis of Fluoro Phenyl 1,2,3-Triazoles
Initially, triazoles were synthesized employing a 1,3-dipolar thermal cycloaddition between a terminal alkyne and an azide; this methodology was described by Huisgen [18]. However, long heating periods were required, and a mixture of 1,2,3-triazole-1,5-disubstituted (Scheme 3, Route A) and 1,2,3-triazole-1,4-disubstituted (Scheme 3, Route B) products was generated. These two routes are feasible since the (negatively charged) nitrogen of the azide can induce a nucleophilic attack on any of the two sp-hybridized carbons of the alkyne [32,33,34,35,36].
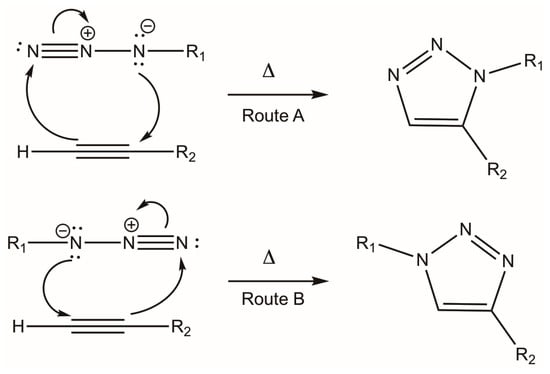
Scheme 3.
Products obtained in the 1,3-dipolar terminal cycloaddition between an azide and a terminal alkyne.
Due to the above reactions, this methodology is not very convenient for the synthesis of triazoles. In 2002, Meldal and Sharpless simultaneously reported that a small addition of Cu (I) to this particular reaction induced regioselectivity, and only 1,4-disubstituted triazole was obtained [19,20]. In addition, the reaction proceeded very fast at room temperature. The mechanism for this cycloaddition (Scheme 4) has been extensively investigated and elucidated [28,35,37,38]. In the first step, a π-Cu-alkyne complex is generated (4a), and then a copper acetylide-type intermediate is formed (4b). This later intermediate is generated upon deprotonation of the alkyne. Then, there is a subsequent cyclization through the activated azide (N3) with the generation of a metallocycle (4c). In this cyclic intermediate, the position of the azide favors ring contraction through a transannular association of the lone pair of N1 electrons with the C5-π orbital, giving rise to a 3-triazolyl copper complex (4d). Finally, in a protonation step, the 1,2,3-triazole (4e) is formed, and the catalyst is regenerated [35,36,37,38,39].
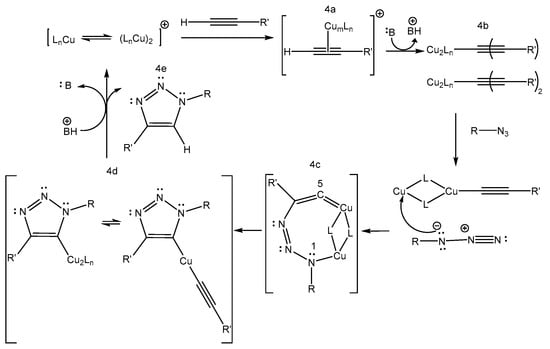
Scheme 4.
Mechanisms in catalytic cycle of the reaction of azides with terminal alkynes, catalyzed by Cu (I) [39]. Copyright 2006 Wiley-VCH Verlag GmbH & CO. KgaA, Weinheim, Germany.
Cu (I) has an extremely important role in the previously mentioned mechanism for the synthesis of triazoles [40]. Therefore, it is necessary to implement a methodology that provides the necessary conditions to maintain a good concentration of Cu (I). This will favor the cycloaddition of the alkyne with the azide, increasing yields and reducing reaction times. In this work, several fluoro phenyl 1,2,3-triazoles were synthesized using click chemistry under homogeneous Cu (I) catalyst conditions.
Initially, the synthesis of the corresponding phenyl azide (2A–2H, Table 1) was carried using the standard procedure described in Section 2.2 [28]. All the azides were synthesized within an hour with very good yields (85 to 95%). The procedure to prepare several triazoles has been previously reported, and it is described in Section 2.3 [28]. In this methodology, the corresponding azide was separated from the reaction mixture and reacted with phenyl acetylene. A solution of copper sulfate pentahydrate was used as a source of Cu (II) and sodium ascorbate (reducing agent) to reduce Cu (II) to Cu (I). The reaction was monitored by TLC using chloroform as the mobile phase. The crystalline products obtained were purified by recrystallization with ethanol or hexane and were characterized by different spectroscopic techniques [28]. Under room temperature, this reaction takes place within two hours with very good yields (84–98%), with the exception of 2,4,6-trifluoro phenyl triazole, which takes a longer time (132 min). This effect is most likely due to steric, inductive, and resonance effects of the fluorine substituent that inhibits click reactions.
The results of the synthesis of aromatic azides and fluoro phenyl 1,2,3-triazoles are given in Table 1. Triazoles were prepared in the presence of phenanthroline with stirring at room temperature and with ultrasound irradiation. It can be observed that under these later conditions reaction times are considerably shorter (0.5 to 3 min), and yields are higher (91–98). Therefore, this method of synthesis is better than conventional methods. Various investigations in organic chemistry have shown that some reactions are improved using sound waves triggered by ultrasound [41,42,43,44]. When ultrasound is used as a synthesis method, cavitation bubbles are generated in the reaction mixture. The bubbles increase in size over time. However, when the pressure reaches a certain limit, these cavitation bubbles collapse violently after growing rapidly. This facilitates the formation of an emulsion between the raw materials and raises the temperature in the reaction mixture [45,46,47,48].
Comparing the results obtained for the synthesis of 1,2,3-triazoles in the presence and absence of 1,10-phenanthroline (Table 2), it can be observed that the use of 1,10-phenanthroline favors the reaction since, on average, the reaction times are shorter by up to six times, and yields increase considerably from 20–79% to 94–99%. 1,10-Phenanthroline is a bidentate ligand (Scheme 5) that forms a complex with Cu (I), providing it with greater stability and preventing it from being oxidized to Cu (II). This was also verified experimentally. After two hours, the mixture without phenanthroline returned to the blue color (characteristic color of Cu (II)), while the mixture containing phenanthroline remained brown (characteristic color of Cu (I). Indeed, it has been previously reported that the presence of an organic base in this click reaction reduces the formation of secondary products stabilizing Cu (I) [22].
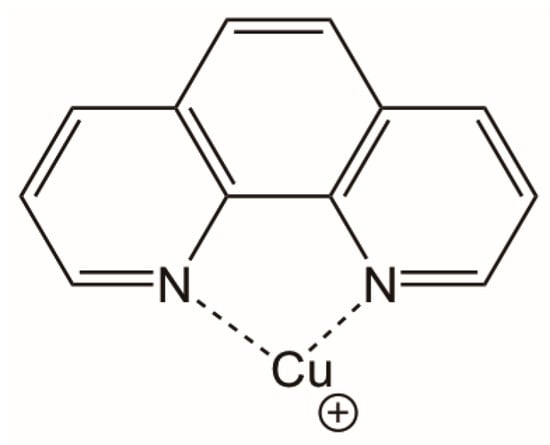
Scheme 5.
Complex formed between 1,10-phenanthroline and Cu (I).
3.2. Effect of Fluoro Phenyl 1,2,3-Triazoles on Inhibition of Yeast Growth
To evaluate the effect of triazoles on yeast growth, a solution of the compound in DMSO was prepared at a concentration of 1000 µg/mL. Subsequently, serial dilutions were prepared at concentrations of 750, 500 and 250 µg/mL [48]. Erlenmeyer flasks (125 mL), containing 50 mL of Lee’s medium, were inoculated with 1 × 106 yeasts/mL and 1 mL of each compound to be analyzed.
An Erlenmeyer flask was also prepared that only contained Lee’s medium and the yeast solution. To evaluate the effect of DMSO on yeast growth, Lee’s medium, DMSO, and the yeast solution were placed in another Erlenmeyer flask. All flasks were incubated at 28 °C, for 7 days at 100 rpm. Subsequently, the contents of each flask were placed in a previously weighed conical tube and centrifuged. When they were dry, the tubes with the biomass were weighed, and the weight of the biomass was obtained by difference. Taking the flask that only contained Lee’s medium and the yeast solution as a reference, the percentage of inhibition was obtained [30].
No significant difference was observed in the amount of biomass contained in the tube where there was only yeast and yeast medium and between the tube that also contained DMSO, so the solvent did not have a significant effect on yeast growth (inhibition = 2%). Table 3 shows the results obtained from the evaluation of the effect of triazoles on the percentage of inhibition of yeast growth. In the case of compound 3A, the percentage of inhibition improved as the concentration increased; however, at 1000 µg/L there was a decrease in this percentage. In compound 3B at 250 and 500 µg/mL, the percentage of inhibition was the same, but there was a decrease at 750 µg/mL and a slight increase of two units at 1000 µg/L. In compounds 3C, 3D, 3E, and 3H, the percentage of inhibition increased with concentration. In compounds 3F and 3G, a similar behavior was observed. In both cases, at concentrations of 250, 500, and 750 µg/L, the inhibition percentage remained at 24 and 19%, but at 1000 µg/L it increased to 22 and 58%, respectively.

Table 3.
Percentage of inhibition of yeast growth at different concentrations of fluorinated 1,2,3-triazoles (concentration: 250, 500, 750, and 1000 µg/L DMSO).
3.3. Effect of Fluoro Phenyl 1,2,3-Triazoles on the Yeast–Mycelium Transition of C. albicans
This test evaluated the effect of triazoles on the inhibition of the yeast–mycelium transition, a process that is directly related to the pathogenicity of C. albicans. To perform this, a solution of each triazole in DMSO was prepared at a concentration of 1000 µg/mL. A pellet of a young colony of C. albicans was placed in 0.5 mL of Lee’s medium and 100 µL of the solution of each triazole and incubated at 37 °C for three hours. Tubes were also prepared with Lee’s medium and the yeast, subjecting one of them to a temperature of 37 °C and the other to 28 °C. On the other hand, to evaluate the effect of the solvent (DMSO), Lee’s medium, yeast, and DMSO were placed in another tube [31].
After three hours, a drop of the solution from each tube was placed on a slide and observed under a microscope. In the sample that only contained Lee’s medium and the yeast solution, the yeast–mycelium transition was observed (Figure 1a). In all the other cases, inhibition of the yeast–mycelium transition was observed (Figure 1b). The results are presented in Table 4.
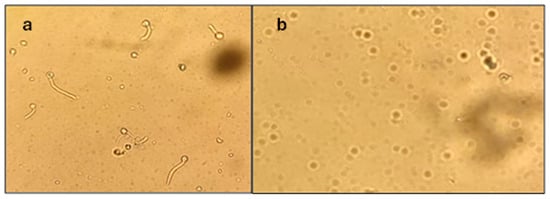
Figure 1.
Observation under the microscope. (a) Tube containing Lee’s medium and yeast solution, subjected to a temperature of 38 °C for three hours, where there was no inhibition of the yeast–mycelium transition. (b) Tube containing Lee’s medium, yeast solution, and solution of triazole 3A subjected to a temperature of 38 °C for three hours; there was inhibition of the yeast–mycelium transition.

Table 4.
Results obtained in the evaluation of the effect of fluorinated 1,2,3-triazoles on the yeast–mycelium transition of C. albicans.
Antifungal triazole agents present several molecular mechanisms of action [49]. The primary mechanism consists of inhibition of ergosterol biosynthesis, with the resulting accumulation of its precursors. At molecular level, one of the nitrogens in the triazole ring binds to the heme iron on cytochrome P-450, thus inhibiting cytochrome activation and enzyme function. High concentrations of triazoles have been associated with rapid fungicidal action. This effect could not be explained by this mechanism. This antifungal effect can be explained in terms of direct membrane damage by means of primary attack of drug on membrane phospholipids. Furthermore, triazole compounds also inhibit cytochrome c oxidative and peroxidative enzymes. In general, fluorination of a pharmaceutical compound improves its bioactivity and pharmacokinetic properties [50]. It improves its solubility and ability to interact with biological targets. In this particular case, fluorine in certain positions (2, 4, 2,6, and 2,4,6) improves physicochemical properties. Due to strong inductive, resonance, and electrostatic effects, fluorine modifies the lipophilicity/hydrophilicity balance of parent triazole, improving membrane permeation and bioavailability. Thus, better antifungal activity is achieved with some fluoro phenyl 1,2,3-triazoles.
4. Conclusions
A fast and efficient synthesis of several fluoro phenyl triazoles from the corresponding fluoro phenyl azides was achieved. Several aryl azides were prepared using diazonium salts from commercial anilines. Each azide reacted with phenyl acetylene and a Cu (I) catalyst solution obtained combining copper sulfate and sodium ascorbate. To stabilize Cu (I), a complexing agent (phenanthroline) was added. Performing the reactions under ultrasound irradiation, crystalline triazoles were obtained within minutes.
In the biological tests, for the evaluation of yeast growth inhibition, DMSO was used as the solvent in the preparation of triazole solutions, so the effect of this on the percentage of inhibition of C. albicans growth was also evaluated. It was verified that the inhibition was due to the compound under study and not to the solvent. Triazoles with a higher percentage of inhibition of yeast growth (3B: 250 and 500 µg/mL, 57%; 3D 1000 µg/L, 39%; 3F: 1000 µg/mL, 54%; 3H: 1000 µg/mL, 58%) contain a fluorine in either the 2, 4, 2,6, or 2,4,6 position. It was observed that at a concentration of 1000 µg/L, triazole 3H (R = 2,4,6-F) gave the highest percentage of inhibition.
All compounds showed inhibition of the yeast–mycelium transition. This property is related to the pathogenicity of C. albicans.
In this particular investigation, fluorine in certain positions (2, 4, 2,6, and 2,4,6) improved the physicochemical properties of triazoles. Thus, better antifungal activity is achieved with several fluoro phenyl 1,2,3-triazoles.
Supplementary Materials
The following contributions supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/org6030042/s1 [28,32,51], Figure S1: 1,4-diphenyl-1,2,3-triazole (3A) UV-Vis spectrum; Figure S2: 1,4-diphenyl-1,2,3-triazole (3A) IR spectrum; Figure S3: 1,4-diphenyl-1,2,3-triazole (3A) 1H NMR spectrum; Figure S4: 1,4-diphenyl-1,2,3-triazole (3A) 13C NMR spectrum; Figure S5: 1-(2-fluorophenyl)-4-phenyl-1,2,3-triazole (3B) UV-Vis spectrum; Figure S6: 1-(2-fluorophenyl)-4-phenyl-1,2,3-triazole (3B) IR spectrum; Figure S7: 1-(2-fluorophenyl)-4-phenyl-1,2,3-triazole (3B) NMR-1H spectrum; Figure S8: 1-(2-fluorophenyl)-4-phenyl-1,2,3-triazole (3B) NMR-13C spectrum; Figure S9: 1-(2-fluorophenyl)-4-phenyl-1,2,3-triazole (3B) NMR-19F spectrum; Figure S10: 1-(3-fluorophenyl)-4-phenyl-1,2,3-triazole (3C) UV-Vis spectrum; Figure S11: 1-(3-fluorophenyl)-4-phenyl-1,2,3-triazole (3C) IR spectrum; Figure S12: 1-(3-fluorophenyl)-4-phenyl-1,2,3-triazole (3C) NMR-1H spectrum; Figure S13: 1-(3-fluorophenyl)-4-phenyl-1,2,3-triazole (3C) NMR-13C spectrum; Figure S14: 1-(3-fluorophenyl)-4-phenyl-1,2,3-triazole (3C) NMR-19F spectrum; Figure S15: 1-(4-fluorophenyl)-4-phenyl-1,2,3-triazole (3D) UV-Vis spectrum; Figure S16: 1-(4-fluorophenyl)-4-phenyl-1,2,3-triazole (3D) IR spectrum; Figure S17: 1-(4-fluorophenyl)-4-phenyl-1,2,3-triazole (3D) NMR-1H spectrum; Figure S18: 1-(4-fluorophenyl)-4-phenyl-1,2,3-triazole (3D) NMR-13C spectrum; Figure S19: 1-(4-fluorophenyl)-4-phenyl-1,2,3-triazole (3D) NMR-19F spectrum; Figure S20: 1-(2,4-difluorophenyl)-4-phenyl-1,2,3-triazole (3E) UV-Vis spectrum Figure S21: 1-(2,4-difluorophenyl)-4-phenyl-1,2,3-triazole (3E) IR spectrum Figure S22: 1-(2,4-difluorophenyl)-4-phenyl-1,2,3-triazole (3E) NMR-1H spectrum Figure S23: 1-(2,4-difluorophenyl)-4-phenyl-1,2,3-triazole (3E) NMR-13C spectrum; Figure S24: 1-(2,4-difluorophenyl)-4-phenyl-1,2,3-triazole (3E) NMR-19F spectrum; Figure S25: 1-(2,6-difluorophenyl)-4-phenyl-1,2,3-triazole (3F) UV-Vis spectrum Figure S26: 1-(2,6-difluorophenyl)-4-phenyl-1,2,3-triazole (3F) IR spectrum Figure S27: 1-(2,6-difluorophenyl)-4-phenyl-1,2,3-triazole (3F) NMR-1H spectrum; Figure S28: 1-(2,6-difluorophenyl)-4-phenyl-1,2,3-triazole (3F) NMR-13C spectrum; Figure S29: 1-(2,6-difluorophenyl)-4-phenyl-1,2,3-triazole (3F) NMR-19F spectrum; Figure S30: 1-(3,4-difluorophenyl)-4-phenyl-1,2,3-triazole (3G) UV-Vis spectrum; Figure S31: 1-(3,4-difluorophenyl)-4-phenyl-1,2,3-triazole (3G) IR spectrum; Figure S32: 1-(3,4-difluorophenyl)-4-phenyl-1,2,3-triazole (3G) IR spectrum; Figure S33: 1-(3,4-difluorophenyl)-4-phenyl-1,2,3-triazole (3G) NMR-1H spectrum; Figure S34: 1-(3,4-difluorophenyl)-4-phenyl-1,2,3-triazole (3G) NMR-13C spectrum; Figure S35: 1-(3,4-difluorophenyl)-4-phenyl-1,2,3-triazole (3G) NMR-19F spectrum; Figure S36: 1-(2,4,6-trifluorophenyl)-4-phenyl-1,2,3-triazole (3H) UV-Vis spectrum; Figure S37: 1-(2,4,6-trifluorophenyl)-4-phenyl-1,2,3-triazole (3H) IR spectrum; Figure S38: 1-(2,4,6-trifluorophenyl)-4-phenyl-1,2,3-triazole (3H) NMR-1H spectrum; Figure S39: 1-(2,4,6-trifluorophenyl)-4-phenyl-1,2,3-triazole (3H) NMR-13C spectrum; Figure S40: 1-(2,4,6-trifluorophenyl)-4-phenyl-1,2,3-triazole (3H) NMR-19F spectrum.
Author Contributions
Conceptualization, E.L. and J.A.; methodology, J.A. and I.A.-R.; writing, E.L. and J.A.; editing, S.E.L.-C. All authors have read and agreed to the published version of the manuscript.
Funding
Research was funded by L’Oréal/UNESCO/Mexican Academy of Science (grant G2022). M.Sc. Johana Aguilar acknowledges CONAHCYT for a scholarship (CVU 1007105).
Data Availability Statement
The data supporting the findings of this study are provided in the article and Supplementary Material. Additional inquiries should be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Mareković, I.; Pleško, S.; Vranješ, V.R.; Herljević, Z.; Kuliš, T.; Jandrlić, M. Epidemiology of Candidemia: Three-Year Results from a Croatian Tertiary Care Hospital. J. Fungi 2021, 7, 267. [Google Scholar] [CrossRef]
- Talapko, J.; Juzbasic, M.; Matijevic, T.; Pustijanac, E.; Bekic, S.; Kotris, I.; Skrlec, I. Candida albicans—The Virulence Factors and Clinical Manifestations of Infection. J. Fungi 2021, 7, 79. [Google Scholar] [CrossRef]
- Huang, G. Regulation of phenotypic transitions in the fungal pathogen Candida albicans. Virulence 2012, 3, 251–261. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, R.; Luan, Z.; Ma, X. Risk of invasive candidiasis with prolonged duration of ICU stay: A systematic review and meta-analysis. BMJ Open 2020, 10, e036452. [Google Scholar] [CrossRef]
- Kim, J.; Sudbery, P. Candida albicans, a major human fungal pathogen. Res. Microbiol 2011, 49, 171–177. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Diekema, D.J. Epidemiology of Invasive Candidiasis: A Persistent Public Health Problem. Clin. Microbiol. Rev. 2007, 20, 133–163. [Google Scholar] [CrossRef]
- Pristov, K.E.; Ghannoum, M.A. Resistance of Candida to azoles and echinocandins worldwide. Clin. Microbiol. Infect. 2019, 25, 792–798. [Google Scholar] [CrossRef]
- Sullivan, D.J.; Moran, G.P.; Pinjon, E.; Al-Mosaid, A.; Stokes, C.; Vaughan, C.; Coleman, D.C. Comparison of epidemiology, drug resistance mechanisms, and virulence of Candida dubliniensis and Candida albicans. FEMS Yeast Rev. 2004, 4, 369–376. [Google Scholar] [CrossRef]
- Sanguinetti, M.; Posteraro, B.; Lass-Flörl, C. Antifungal drug resistance among Candida species: Mechanisms and clinical impact. Mycoses 2015, 58, 2–13. [Google Scholar] [CrossRef]
- Buckle, D.R.; Smith, H.; Spicer, B.A.; Tedder, J.M. Studies on v-Triazoles. Antiallergic 4,9-Dihydro-4,9-dioxo-1H-naphtho [2,3-d}-v-triazoles. J. Med. Chem. 1983, 26, 714–719. [Google Scholar] [CrossRef]
- Mani, G.S.; Donthiboina, K.; Shaik, S.P.; Shankaraiah, N.; Kamal, A. Iodine-mediated C–N and N–N bond formation: A facile one-pot synthetic approach to 1,2,3-triazoles under metal-free and azide-free conditions. RSC Adv. 2019, 9, 27021–27031. [Google Scholar] [CrossRef]
- Sharghi, H.; Ebrahimpourmoghaddam, S.; Doroodmand, M.M.; Purkhosrow, A. Synthesis of Vasorelaxaing 1,4-Disubstituted 1,2,3-Triazoles Catalyzed by a 4′-Phenyl-2,2′:6′,2″-Terpyridine Copper(II) Complex Immobilized on Activated Multiwalled Carbon Nanotubes. Asian J. Org. Chem. 2012, 1, 377–388. [Google Scholar] [CrossRef]
- Chavan, P.V.; Pandit, K.S.; Desai, U.V.; Wadgaonkar, P.P.; Nawale, L.; Bhansali, S.; Sarkar, D. Click-chemistry-based multicomponent condensation approach for design and synthesis of spirochromene-tethered 1,2,3-triazoles as potential antitubercular agents. Res. Chem. Intermed. 2017, 43, 5675–5690. [Google Scholar] [CrossRef]
- Abdel-Wahab, B.F.; Abdel-Latif, E.; Mohamed, H.A.; Awad, G.E. Design and synthesis of new 4-pyrazolin-3-yl-1, 2, 3-triazoles and 1,2,3-triazol-4-yl-pyrazolin-1-ylthiazoles as potential antimicrobial agents. Eur. J. Med. Chem. 2012, 52, 263–268. [Google Scholar] [CrossRef]
- Bukowski, K.; Kciuk, M.; Kontek, R. Mechanisms of Multidrug Resistance in Cancer Chemotherapy. Int. J. Mol. Sci. 2020, 21, 3233. [Google Scholar] [CrossRef]
- Nagesh, H.N.; Suresh, N.; Prakash, G.V.S.B.; Gupta, S.; Rao, J.V.; Sekhar, K.V.G.C. Synthesis and biological evaluation of novel phenanthridinyl piperazine triazoles via click chemistry as antiproliferative agents. Med. Chem. Res. 2015, 24, 523–532. [Google Scholar] [CrossRef]
- Dixit, D.; Verma, P.K.; Marwaha, R.K. A review on ‘triazoles’: Their chemistry, synthesis and pharmacological potentials. J. Indian Chem. Soc. 2021, 18, 2535–2565. [Google Scholar] [CrossRef]
- Huisgen, R. Kinetics and reaction mechanisms: Selected examples from the experience of forty years. Pure Appl. Chem. 1989, 61, 613–628. [Google Scholar] [CrossRef]
- Tornoe, C.W.; Christensen, C.; Meldal, M. Peptidotriazoles on Solid Phase: [1,2,3]-Triazoles by Regiospecific Copper(I)-Catalyzed 1,3-Dipolar Cycloadditions of Terminal alkynes to Azides. J. Org. Chem. 2002, 67, 3057–3063. [Google Scholar] [CrossRef]
- Rostovtsev, V.V.; Green, L.G.; Fokin, V.V.; Sharpless, K.B. A Stepwise Huisgen Cycloaddition Process: Copper(I)-Catalyzed Regioselective “Ligation” of Azides and Terminal Alkynes. Angew. Chem. Int. Ed. 2002, 42, 2596–2599. [Google Scholar] [CrossRef]
- Aguilar-Morales, C.M.; de Loera, D.; Contreras-Celedón, C.; CortésGarcía, C.J.; Chacón-García, L. Synthesis of 1,5-disubstituted tetrazole-1,2,3 triazoles hybrids via Ugi-azide/CuAAC. Synth. Commun. 2019, 49, 2086–2209. [Google Scholar] [CrossRef]
- Noriega, S.; Leyva, E.; Moctezuma, E.; Flores, L.; Loredo-Carrillo, S. Recent Catalysts Used in the Synthesis of 1,4-Disubstituted 1,2,3-Triazoles by Heterogeneous and Homogeneous Methods. Curr. Org. Chem. 2020, 24, 536–549. [Google Scholar] [CrossRef]
- Horne, W.S.; Stout, C.D.; Ghadiri, M.R. A heterocyclic peptide nanotube. J. Am. Chem. Soc. 2003, 125, 9372–9376. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Kumar, V. Copper (I) catalyzed azide-alkyne click reaction: Synthesis and metal-ion binding studies of some 1,2,3-triazole derivatives. Asian J. Chem. 2016, 28, 613–616. [Google Scholar] [CrossRef]
- Yoo, E.J.; Ahlquist, M.; Kim, S.H.; Bae, I.; Fokin, V.V.; Sharpless, K.B.; Chang, S. Copper-Catalyzed Synthesis of N-Sulfonyl-1,2,3-triazoles: Controlling Selectivity. Angew. Chem. Int. Ed. 2007, 46, 1730–1733. [Google Scholar] [CrossRef]
- Dai, J.; Tian, S.; Yang, X.; Liu, Z. Synthesis methods of 1,2,3-/1,2,4-triazoles: A review. Front Chem. 2022, 10, 891484. [Google Scholar] [CrossRef]
- Meldal, M.; Diness, F. Recent fascinating aspects of the CuAAC click reaction. Trends Chem. 2020, 2, 569–584. [Google Scholar] [CrossRef]
- Aguilar, J.; Leyva, E.; Loredo-Carrillo, S.E.; Cárdenas-Chaparro, A.; Martínez-Richa, A.; Hernández-López, H.; Araujo-Huitrado, J.G.; Granados-López, A.J.; López-Hernández, Y.; López, J.A. Synthesis of Novel Fluoro Phenyl Triazoles Via Click Chemistry with or without Microwave Irradiation and their Evaluation as Anti-proliferative Agents in SiHa Cells. Curr. Org. Synth. 2024, 21, 559–570. [Google Scholar] [CrossRef] [PubMed]
- Díaz, D.D.; Finn, M.G.; Sharpless, K.B.; Fokin, V.V.; Hawker, C.J. Cicloadición 1, 3-dipolares de azidas y alquinos: I Principales aspectos sintéticos. An. De La Real Soc. Esp. De Quim. 2008, 3, 173–180. [Google Scholar]
- Acosta, I.; Contreras, D.; Tovar, J.; Turrubiartes, E.A.; Pacheco, N.C.; Rodríguez, A.; Cárdenas, J.F.; Martínez, V.M. Properties and Effect of Fresh Concentrated Extract of Garlic on Different Bacteria and Fungi. Asian J. Res. Biochem. 2022, 10, 15–32. [Google Scholar] [CrossRef]
- Lee, K.L.; Buckley, H.R.; Campbell, C.C. An amino acid liquid synthetic medium for the development of mycelial and yeast forms of Candida albicans. Sabouraudia 1975, 13, 148–153. [Google Scholar] [CrossRef]
- Castillo, J.C.; Bravo, N.-F.; Tamayo, L.V.; Mestizo, P.D.; Hurtado, J.; Macías, M.; Portilla, J. Water-Compatible Synthesis of 1,2,3-Triazoles under Ultrasonic Conditions by a Cu(I) Complex-Mediated Click Reaction. ACS Omega 2020, 5, 30148–30159. [Google Scholar] [CrossRef]
- Kategaonkar, A.H.; Shinde, P.V.; Kategaonkar, A.H.; Pasale, S.K.; Shingate, B.B.; Shingarea, M.S. Synthesis and biological evaluation of new 2-chloro-3-((4-phenyl-1H-1,2,3-triazol-1-yl)methyl)quinoline derivatives via click chemistry approach. Eur. J. Med. Chem. 2010, 45, 3142–3146. [Google Scholar] [CrossRef]
- Appukkuttan, P.; Dehaen, W.; Fokin, V.V.; Van der Eycken, E. A Microwave-Assisted Click Chemistry Synthesis of 1,4-Disubstituted 1,2,3-Triazoles via a Copper(I)-Catalyzed Three-Component Reaction. Org. Lett. 2004, 6, 4223–4225. [Google Scholar] [CrossRef]
- Suárez, A. Reacciones de cicloadición 1,3-dipolares a alquinos catalizadas por cobre. An. De La Real Soc. Esp. De Quim. 2012, 108, 306–313. [Google Scholar]
- Hajipour, A.R.; Karimzadeh, M.; Fakhari, F.; Karimi, H. CuFeO2/tetrabutylammonium bromide catalyzes selective synthesis of 1,4-disubstituted 1,2,3-triazoles in neat water at room temperature. Appl. Organomet. Chem. 2016, 30, 946–948. [Google Scholar] [CrossRef]
- Leyva, E.; Rodríguez-Gutiérrez, I.R.; Moctezuma, E.; Noriega, S. Mechanisms, Copper Catalysts, and Ligands Involved in the Synthesis of 1,2,3-Triazoles Using Click Chemistry. Curr. Org. Chem. 2022, 26, 2098–2121. [Google Scholar] [CrossRef]
- Ye, W.; Xiao, X.; Wang, L.; Hou, S.; Hu, C. Synthesis of mono and binuclear Cu(II) complexes bearing unsymmetrical bipyridinepyrazole-amine ligand and their applications in azide-alkyne cycloaddition. Organometallics 2017, 36, 2116–2125. [Google Scholar] [CrossRef]
- Bock, V.D.; Hiemstra, H.; van Maarseveen, J.H. CuI-Catalyzed Alkyne–Azide “Click” Cycloadditions from a Mechanistic and Synthetic Perspective. Eur. J. Org. Chem. 2006, 1, 51–68. [Google Scholar] [CrossRef]
- Devaraj, N.K.; Finn, M.G. Introduction: Click chemistry. Chem. Rev. 2021, 121, 6697–6698. [Google Scholar] [CrossRef] [PubMed]
- Mason, T.J. Ultrasound in synthetic organic chemistry. Chem. Soc. Rev. 1997, 26, 443. [Google Scholar] [CrossRef]
- Jaiswal, P.K.; Sharma, V.; Prikhodko, J.; Mashevskaya, I.V.; Chaudhary, S. “On water” ultrasound-assisted one pot efficient synthesis of functionalized 2-oxo-benzo[1,4]oxazines: First application to the synthesis of anticancer indole alkaloid, Cephalandole A. Tetrahedron Lett. 2017, 58, 2077–2083. [Google Scholar] [CrossRef]
- Saranya, S.; Radhika, S.; Afsina Abdulla, C.M.; Anilkumar, G. Ultrasound irradiation in heterocycle synthesis: An overview. J Heterocycl. Chem. 2021, 58, 1570–1580. [Google Scholar] [CrossRef]
- Talha, A.; Tachallait, H.; Benhida, R.; Bougrin, K. Green one-pot four-component synthesis of 3,5-disubstituted isoxazoles- sulfonates and sulfonamides using a combination of NaDCC as metal-free catalyst and ultrasonic activation in water. Tetrahedron Lett. 2021, 81, 153366. [Google Scholar] [CrossRef]
- Borah, B.; Chowhan, L.R. Ultrasound-assisted transition-metal-free catalysis:a sustainable route towards the synthesis of bioactive heterocycles. RSC Adv. 2022, 12, 14022–14051. [Google Scholar] [CrossRef] [PubMed]
- Asgharzadehahmadi, S.; Abdul Raman, A.A.; Parthasarathy, R.; Sajjadi, B. Sonochemical reactors: Review on features, advantages and limitations. Renew. Sustain. Energy Rev. 2016, 63, 302–314. [Google Scholar] [CrossRef]
- Phakhodee, W.; Duangkamol, C.; Wiriya, N.; Pattarawarapan, M. Ultrasound-assisted synthesis of substituted 2-aminobenzimidazoles, 2-aminobenzoxazoles, and related heterocycles. Tetrahedron Lett. 2016, 57, 5290–5293. [Google Scholar] [CrossRef]
- Fu, N.; Wang, S.; Zhang, Y.; Zhang, C.; Yang, D.; Weng, L.; Zhao, B.; Wang, L. Efficient click chemistry towards fatty acids containing 1,2,3-triazole: Design and synthesis as potential antifungal drugs for Candida albicans. Eur. J. Med. Chem. 2017, 136, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Peyton, L.R.; Gallagher, S.; Hashemzadeh, M. Triazole antifungals: A review. Drugs Today 2015, 51, 705–718. [Google Scholar] [CrossRef] [PubMed]
- Sheikhi, N.; Bahraminejad, M.; Saeedi, M.; Mirfazli, S.S. A review: DFA-approved fluorine-containing small molecules from 2015–2022. Eur. J. Med. Chem. 2023, 260, 115758. [Google Scholar] [CrossRef]
- Leyva, E.; Aguilar, J.; González-Balderas, R.M.; Vega-Rodríguez, S.; Loredo-Carrillo, S.E. Synthesis of nitrophenyl and fluorophenyl azides and diazides by SNAr under phase transfer or microwave irradiation: Fast and mild methodologies to prepare photoaffinity labeling, crosslinking, and click chemistry reagents. J. Phys. Org. Chem. 2020, 34, e4171. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).