Abstract
The art of dyeing textiles has a long history, as natural dyes have been used since prehistoric times. With the development of synthetic dyes in the 19th century, the focus shifted from natural to synthetic dyes due to their superior properties. Recently, however, interest in natural dyes has increased again due to environmental and health concerns. Among industrial dyes, heterocyclic dyes, especially azo dyes, are of great importance due to their color brilliance and fastness. This review examines the synthesis, application, and analysis of azo dyes, especially heterocyclic dyes. It deals with monoazo, diazo, and polyazo dyes and highlights their structures, synthesis methods, and fastness properties. In addition, the ecological impact of azo dyes and practical solutions for their synthesis and application are discussed.
1. Introduction
The art of textile dyeing is an activity as old as mankind, as colored textiles have been found in archeological excavations in the oldest civilizations. However, it should be emphasized that heterocyclic dyes have also been in use since prehistoric times. At that time, only natural dyes obtained from natural resources of plant, animal, mineral, and microbial origin were used [1]; many of them were, in fact, natural heterocyclic dyes such as indigo (obtained from the leaves of Indigofera tinctorial) and Tyrian purple (obtained from the shells of mollusks of the Muricidae family), which were used to dye textiles in blue and purple, respectively (Figure 1).
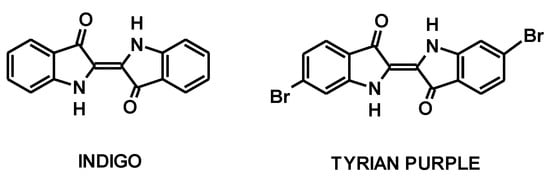
Figure 1.
Structure of indigo and Tyrian purple.
Since the second half of the 19th century, more precisely since the invention of synthetic dyes, the use of natural dyes has been almost completely replaced by synthetic dyes due to their ease of use and better fastness properties, although there has been a return to the use of natural dyes in recent decades, not only because they are environmentally friendly, but also due to the fact that many artificial textile dyes are hazardous to human health [2]. Nevertheless, the search for synthetic dyes is still very active, as the requirements of customers around the world are changing rapidly. In the field of industrial dyes, heterocyclic dyes have been increasingly used recently as they have higher color brilliance and strength as well as better color fastness compared to benzene analogous dyes. Heterocyclic dyes can be divided into different technical sections according to the type of electronic character of the dye molecule [3]. This overview reports on the most important group among them—the azo dyes. In particular, the synthesis, the applications on different textile materials, and the analyses carried out to verify the fastness properties are reported.
2. The Azo Dyes
Azo dyes are characterized by the functional group -N=N-, which connects two symmetrical or asymmetrical substituents, of which at least one, but usually both, are aromatic [4].
They are usually synthesized in a two-step reaction as follows: synthesis of an aromatic diazonium ion from an aniline derivative (diazotization) and coupling of the diazonium salt with an aromatic compound (Figure 2) [5] or by the reduction of nitro aromatic compounds in an alkaline medium and oxidation of aromatic amine with various oxidizing agents such as lead tetraacetate, permanganate, and haloxy acid [6].
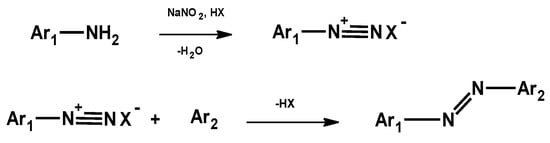
Figure 2.
Synthesis of azo compounds.
If both substituents are aromatic substituents, the dyes are referred to as carbocyclic, whereas if they contain one or more heterocyclic substituents, the dyes are referred to as heterocyclic azo dyes.
All azo compounds are classified by Griffiths [7] as donor–acceptor chromogens (D-π-A) (Figure 3), and studies have shown that replacing the benzene ring of the acceptor with a less aromatic heterocycle in these chromogens leads to a significant bathochromic shift (red shift) in the visible absorption band, which results in greater color brilliance and higher color intensity, especially in synthetic materials [8].
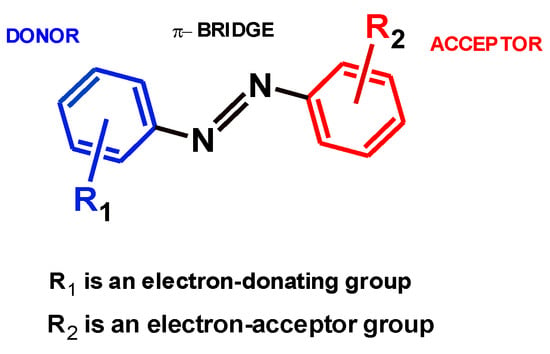
Figure 3.
Scheme of D-π-A azo compounds.
Due to the structure of azo compounds, they exhibit photochromic properties.
Photochromism is a phenomenon that occurs when two molecules (A and B) are in chemical equilibrium with each other and can be easily distinguished due to their different absorption spectra. Normally, the more stable isomer (A) can transform into the less stable, higher energy isomer (B) when excited by light. However, the B isomer can spontaneously transform into the former through a thermal or photochemical reaction (Figure 4). It follows that there are the following two types of photochromic systems: Type T, i.e., thermally reversible photochromes, and Type P, i.e., photochemically reversible photochromes [9].
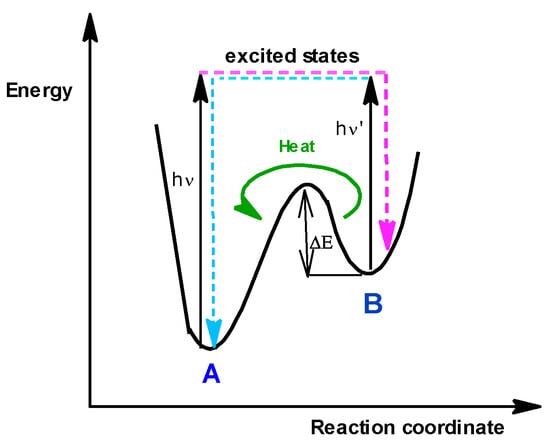
Figure 4.
Energy profile for the interconversion between the two isomers A and B [9].
In the case of azo compounds, these two isomers are E and Z isomers (Figure 5).
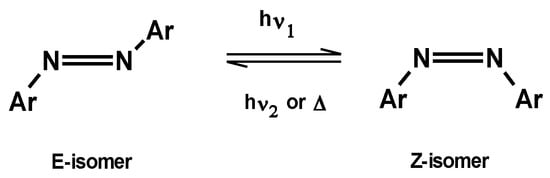
Figure 5.
Isomerization E–Z for azo compounds.
The case of isomerization of azobenzene has been extensively studied and analyzed. In this case, the photochromic system is of the T-type, and the azobenzene in the E-configuration is more stable than in the Z-form. The two isomers differ not only in their structure and properties, but they also exhibit different absorption spectra in the UV spectrum (Figure 6). The different spectral position of the bands indicates that not only is the wavelength of light required to induce the reaction different for the two isomers, but they also have different colors [9].
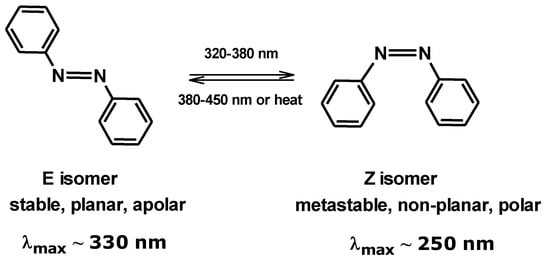
Figure 6.
The E and Z isomers of azobenzene.
Changes in the absorption spectra between the two forms are generally not very pronounced due to a small difference in electron delocalization between the two isomers, and the color change is invisible to the naked eye. In contrast, the photochromic reaction induces very significant change in the free volume [10], density, viscosity, electrical conductivity, stiffness, and porosity of the molecule [9].
As early as 1990, Gregory reported that heterocyclic azo dyes are able to “combine the brightness and high fastness properties of anthraquinone dyes with the strength and economy of azo dyes” [11]. It is therefore not surprising that numerous attempts have since been made to synthesize ever more efficient heterocyclic azo dyes.
3. Heterocyclic Azo Dyes
Heterocyclic azo dyes, like all azo dyes, can be divided into three categories according to the number of azo groups in the same molecule as monoazo, diazo, and polyazo dyes.
3.1. Heterocyclic Monoazo Dyes
In 1999, Towns gave an overview of the progress made with heterocyclic diazo components over the past decade [12]. This paper reports on the applications to textiles of heteroazo dyes consisting of five-membered rings containing a sulfur heteroatom, to which a diazotizable amino group is directly attached. These compounds are divided into the following four groups based on the heteroaromatic amine used for diazotization: Thiazoles 1 and Benzothiazoles 2, Isothiazoles 3 and Benzisothiazoles 4, Thiadiazoles 5a–5b, and Thiophenes 6 (Figure 7).
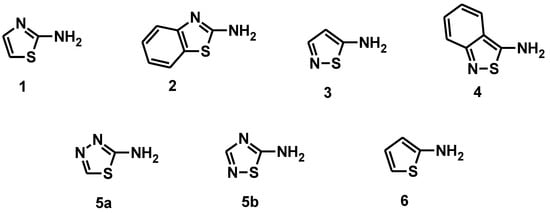
Figure 7.
Structures of heteroaromatic amines.
Towns reported that most of the numerous patents filed in the last ten years have mostly been the result of modifications and improvements to existing products, with no real innovation in molecular structures. He also said that the search for brighter and more economical dyes was still ongoing and predicted that disperse dyes from heterocyclic components would be explored in the coming years.
A few years later, the synthesis and application of novel heterocyclic dyes based on a new fused heterocyclic compound, ll-amino-3-bromo-13h-acenaphtho[l,2-e]pyridazino[3,2-b]-quinazoli-ne-13-one, was reported [13]. A series of new azo dyes (7a–k) was synthesized by diazotization of this heterocyclic amino compound and coupling with various naphthols (a–k), leading to the general structure shown in Figure 8.
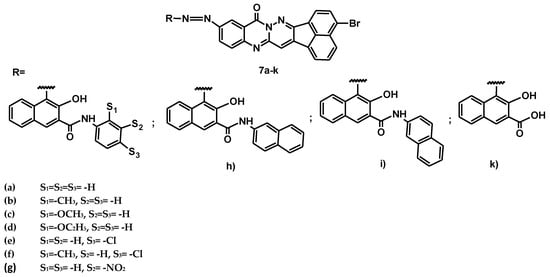
Figure 8.
General structure of dyes 7a–k.
Specifically, these dyes were synthesized by diazotization with sodium nitrite and hydrochloric acid, and coupling was carried out in a moderately alkaline medium at 0–5 °C. After characterization, the dyes were applied as azo dyes to nylon 66 and polyester fibers, resulting in a variety of shades ranging from pink to red and brown. The differences in the shades of the dyed fibers are due to the type and position of the substituent.
The analysis showed that the dyes on both substrates had fairly good to good light fastness and very good to excellent fastness to washing, rubbing, perspiration, and sublimation, while the percentage exhaustion of the dye bath was better on nylon 66 than on polyester, probably due to the better accessibility of the open structure.
In 2010, the same authors reported the synthesis, characterization, and application of another series of heterocyclic monoazo reactive dyes (8a–g), which were synthesized by coupling diazotized 2-phenyl-3{4′-[(4″-aminophenyl)sulfonyl]phenyl}-quinazolin-4(3H)-one-6-sulfonic acid with various 2-chloro-4-nitro-anilino-cyanurate coupling components [14], with the general structure shown in Figure 9.
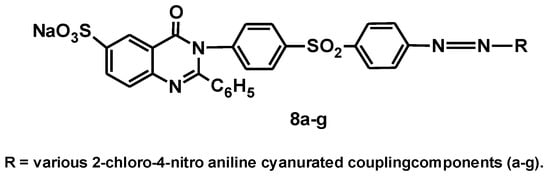
Figure 9.
The general structure of dyes 8a–g.
The 2-chloro-4-nitro anilino cyanurated coupling components used are as follows: H-acid (a), Gamma acid (b), J-acid (c), N-methyl-J-acid (d), N-phenyl-J-acid (e), Chicago acid (f), and Laurant acid (g).
When applied to silk, wool, and cotton fabrics, the dyes obtained covered almost the entire visible range and generally produced yellow to violet shades. The differences in the color shades of the dyed fabric result from the change in the coupling components.
All the dyes (8a–g) generally show moderate to good light fastness properties, while the fastness to washing and rubbing is very good to excellent. The presence of a quinazolinone structure in all the dyes results in high color strength, as the quinazolinone structure exhibits intrinsic conjugation, leading to excellent color strength. The heteroatoms in the dye structure lead to bathochromaticity and brightness of shades. The exhaustion and fixation properties are good for all the dyes, although the introduction of a triazine group improves both properties.
The same authors have published many articles on this type of research, in which they have tested not only the fastness property but also the antimicrobial activity against bacteria and fungi [15,16], which is a new trend in this field, as people’s lives have improved and consumers are aware of a hygienic lifestyle and tend to ask for textile products that do not cause toxicity, allergy, or irritation to the users [17,18,19].
For example, a recent paper reported on the synthesis of new antibacterial dyes for fabric printing [20]. In this work [21], three new azo dyes (9a–c) with sulfonamide chromophores were used to dye polyester fibers via azo coupling with nucleophilic precursors (Figure 10).
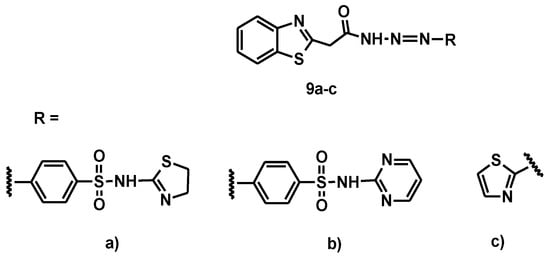
Figure 10.
The general structure of dyes 9a–c.
The analytical results showed that the fastness properties and color evaluation of the printed samples exhibited moderate to excellent results in washing, rubbing, perspiration, sublimation fastness, and light fastness. Antibacterial activity was tested against four bacterial species (Pseudomonas aeruginosa, Escherichia coli, Staphylococcus aureus and Bacillus subtilis) for the new dyes, but also for the printed fabrics after washing, using ampicillin as a reference, which is normally considered a standard antibacterial agent [22]. The diameter of the inhibition zone (mm/mg sample) as a measure of antibacterial activities was measured for all samples, and the results are shown in Table 1 and Table 2. From the results, it can be seen that the dyes 9a and 9b containing sulfate groups showed higher antibacterial activity against all types of bacteria. On the other hand, the dyed polyester fabrics showed moderate antibacterial activity, which, in this case, was also higher in the samples printed with 9a and 9b, showing that the antibacterial properties of the dyes were transferred to the corresponding dyed textile samples.

Table 1.
Inhibition zone of 9a–c as a measure of the antibacterial activities (mm/mg samples).

Table 2.
The inhibition zone of the fabrics dyed with after 4 washes 9a–c as a measure of the antibacterial activities (mm/mg samples).
These inexpensive, easy-to-synthesize, biologically active dyes could be used to sterilize textiles for future applications.
3.2. Heterocyclic Diazo Dyes
Diazo dyes, which contain two groups -N=N-, are divided into the following three groups depending on the synthesis method used for their production [23]:
3.2.1. Primary Diazo Dyes
This type of dye is synthesized by a coupling reaction of two moles of diazoic acid with the same coupling term. An example, a brown dye used for dyeing wool, is shown in Figure 11a.
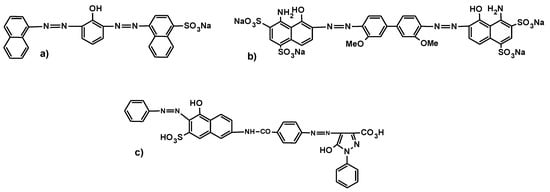
Figure 11.
Structure of: (a) a brown dye, (b) a blue dye, (c) an orange dye.
3.2.2. Secondary Symmetrical Diazo Dyes
This type of dye is derived from a diamine that is diazotized twice and coupled with the same or different terms. An example, a blue direct dye with a benzidine function, is shown in Figure 11b.
3.2.3. Secondary Asymmetrical Diazo Dyes
This type of dye is synthesized by the coupling of an amino azoic acid with a phenolic coupler. Figure 11c shows an example, an orange direct dye.
Although a patent [24] for the use of heterocyclic diazo dyes was granted as early as 1954, only a few studies [25,26] were conducted in the literature regarding the comparison with monoazo compounds until 2005. In that year, Karci reported the synthesis of ten novel diazo dyes derived from various heterocyclic coupling components and aminomethylphenylazo pyrazoles [27]. The entire synthesis process begins with the preparation of nitrile 10, then pyrazoles 11a–b, which are finally used for the synthesis of diazo dyes 12a–j (Figure 12).
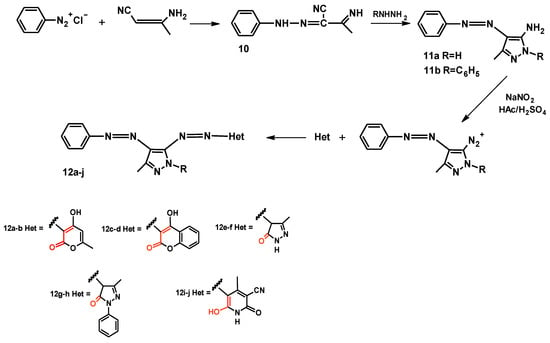
Figure 12.
Synthesis process of diazo dyes 12a–j.
All diazo compounds 12a–j may exhibit keto–enol tautomerism due to the functional groups highlighted in red in Figure 12. The tautomeric forms for compounds 12a–b are shown in Figure 13 as an example. In the particular case of dyes, tautomerism is important not only because the two tautomeric forms can have different colors but also different tinctorial strengths and properties [28,29,30]. From the data of the infrared spectra of compounds 12a–j, it appears that some of them are predominantly in the hydrazone–keto form in the solid state, while other in the azo–enol form.
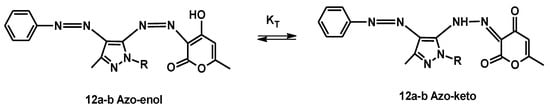
Figure 13.
Tautomeric forms ketone–enol for diazo compounds 12a–b.
Instead, the 1H NMR spectra in dimethyl sulfoxide (DMSO) show that compounds 12a–d are present as a mixture of tautomeric forms, whereas in 12e–j the single tautomeric form predominates.
The effect of solvent and acid or base on the wavelength of the absorption maximum (λmax) was also investigated. It was found that the absorption spectra of dyes 12i–j show a maximum at a longer wavelength than those of dyes 12a–h in all the solvents used. In addition, a bathochromic shift was observed for dyes 12c and 12e compared to the analogous dyes 12d and 12f in all the solvents tested, while the other analogs showed very similar λmax but only in DMSO and dimethylformamide (DMF). These results led us to conclude that for the dyes 12a, 12c, 12e, 12g, and 12i, there could be a tautomeric equilibrium between the pyrazole ring and the phenylazo group at 1-H (Figure 14).
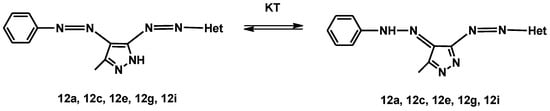
Figure 14.
Tautomeric forms enamine–imine for diazo compounds 12a, 12c, 12e, 12g, and 12i.
In conclusion, the authors report that all dyes 12a–j can be applied to polyester and/or polyamide fibers as disperse dyes.
Another important aspect studied over the years is the color yields obtainable by modifying the position of the substituents on the heteroatomic rings. In particular, for example, in a 2016 article, a study on six novel pyrazole diazo disperse dyes (13a–c, 14a–c, Figure 15), substituted with methyl (-CH3) group at their o-, m-, p-position, was reported [31]. Their synthesis was carried out according to the literature procedures [27,32,33] (Figure 12 and Figure 15), and the studies of their application to three different synthetic fibers at certain dyeing conditions are described.
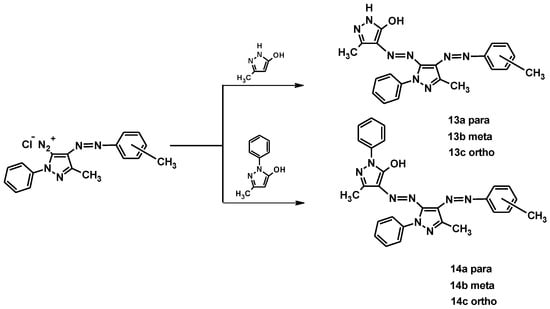
Figure 15.
Synthesis and molecular structures of pyrazole disperse dyes 13a–c and 14a–c.
All synthesized dyes were characterized by elemental analysis and spectral methods and then tested on different fibers. The fibers used for the evaluation were polylactic acid (PLA), polyethylene terephthalate (PET) and polyamide 6.6 (PA 6.6) fibers.
The colorimetric properties, yield, and fastness parameters of all fiber samples dyed with the novel synthesized disperse dyes were described and compared. Yellow and orange shades were obtained on PLA, PET, and PA 6.6 fibers by applying 2% of all dyes. With the dyes 13a–c (K/S > 10), predominantly darker shades were achieved, and with the dyes 14a–c (K/S < 10), medium-dark shades were obtained. In addition, due to the different molecular weights, the exhaustion and color yield of the 13a–c dyes (lower molecular weight) were higher than those of the 14a–c dyes. The following differences between the dyes resulting from the position of the methyl group were also observed: The color yield of all tissues dyed with 13b and 14b dyes (meta-methyl auxochrome) had the darkest hue compared to the other dyes (ortho- and para-methyl auxochrome). The darkest shade was observed in the PLA tissue dyed with 14a (p-CH3). The yield of all dyes was over 76%, with the exception of the PET fiber fabric dyed with 14c, where the yield was 67%.
The colorimetric properties, yield, and fastness parameters of all the fiber samples dyed with the novel synthesized disperse dyes were described and compared. Yellow and orange shades were obtained on PLA, PET, and PA 6.6 fibers by applying 2% of all dyes. With the dyes 13a–c (K/S > 10), predominantly darker shades were achieved, and with the dyes 14a–c (K/S < 10), medium-dark shades were obtained. In addition, due to the different molecular weights, the exhaustion and color yield of the 13a–c dyes (lower molecular weight) were higher than those of 14a–c dyes. The following differences between the dyes resulting from the position of the methyl group were also observed: The color yield of all tissues dyed with 13b and 14b dyes (meta-methyl auxochrome) had the darkest hue compared to the other dyes (ortho- and para-methyl auxochrome). The darkest shade was observed in the PLA tissue dyed with 14a (p-CH3). The yield of all dyes was over 76%, with the exception of the PET fiber fabric dyed with 14c, where the yield was 67%.
The fastness values (wash fastness, acid and alkaline perspiration fastness, wet and dry rubbing fastness, sublimation fastness, light fastness, water fastness, and seawater fastness) were in the commercially acceptable range, which means that the dyeing of PLA, PET, and PA 6.6 with the dyes 13a–c and 14a–c is suitable for the textile industry.
3.3. Heterocyclic Polyazo Dyes
Polyazo dyes are complex dyes characterized by the fact that the azo group is repeated three or more times in the same molecule. They are intended for dyeing leather in the colors red, brown, and dark black [23]. The presence of multiple -N=N- groups increases conjugation and enhances the delocalization of electrons. They also appear to be more stable, as the more azo groups present in the dye, the less likely it is to degrade; in general, monoazo or diazo dyes are more degradable than polyazo dyes [34].
There are few studies in the literature on the use of heterocyclic polyazo dyes in general but especially with regard to their use for dyeing textiles.
In 2022, a paper was published on the synthesis and characterization of a novel heterocyclic polyazo dye, but the authors referred to its application as a universal acid–base indicator [35]. The authors also reported that the polyazo dyes could find numerous practical applications, but there was no mention of their use as dyes for tissues [35,36,37,38].
In any case, an article published in 2011 [39] reported on the synthesis of various natural trisazo dyes. Arylazopyridone dyes have been intensively synthesized as disperse dyes since the 1970s (the 20th century). One of them, the trisazo pyridone dye shown in Figure 16, is used to dye polyamide materials such as nylon 6.
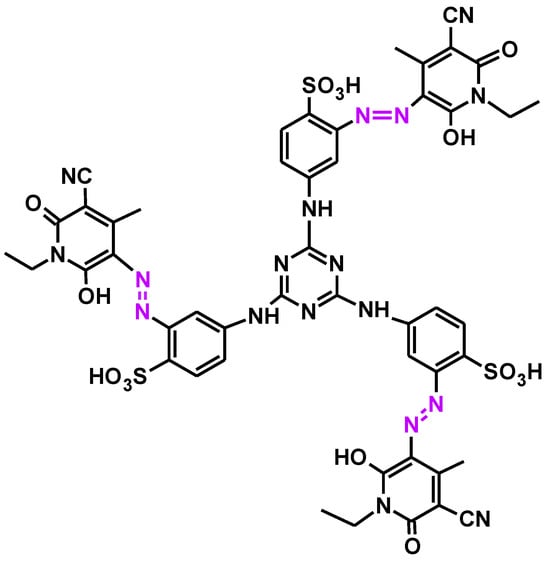
Figure 16.
Structure of a trisazo pyridone dye.
4. Ecological Impacts and Sustainable Solutions for Heterocyclic Azo Dyes
Approximately 70% of the dyes used in the textile industry are azo dyes. During the dyeing process, 15–20% of these dyes are not absorbed and end up in wastewater, causing environmental pollution due to their toxicity. The industry is trying to mitigate these effects by improving dye binding or promoting degradation through biological or physico-chemical methods [40]. Wastewater also contains toxic substances such as heavy metals, which pose health risks such as cancer and chronic diseases [41] and damage ecosystems by interfering with aquatic life and plant growth [17]. Conventional treatment methods (filtration, adsorption, coagulation/flocculation) [42] have disadvantages such as secondary sludge formation, limited effectiveness, and high costs [40]. Therefore, environmentally friendly approaches and methods are being researched not only for azo dyes in general but also for those containing heterocycles.
In an article published in 2021 [43], for example, the authors reported on an environmentally friendly approach for the synthesis of an azocoumarin dye using cation exchange resins. The azo dye with coumarin moieties 17 was prepared by diazotization of 4-nitroaniline 15 and the subsequent coupling of the resulting diazonium salt with coumarin 16 (Figure 17). Amberlyst-15 was used as the acidic catalyst for the diazotization (Figure 18). Amberlyst-15, which consists of brown-gray granules, is a reticulated polystyrene-based ion exchange resin with strong acidic sulfonic groups. It is an effective heterogeneous acid catalyst for reactions in acidic media. It is safe to use and can be easily separated from the reaction mixture after completion of the reaction. Amberlyst-15 is also environmentally friendly as it can be renewed and reused up to four times.
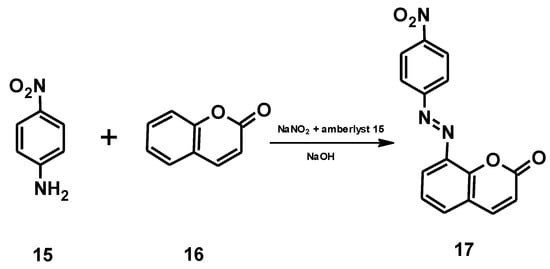
Figure 17.
Synthesis of the azocoumarin 17.
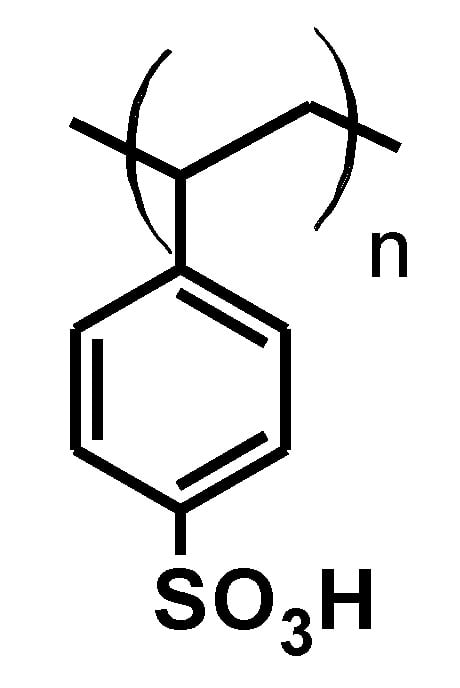
Figure 18.
Structure of amberlyst-15.
The described synthesis has shown that it is an effective method for organic synthesis and that it could be suitable and economical for industrial and applied fields, possibly including textile dyeing as the applications of some coumarin-based azo dyes on polyester fibers [44] are already known.
Research into the removal of azo dyes from factory wastewater is constantly evolving. The activated carbon method has been extensively studied [45]. Unfortunately, the activated carbon currently available comes from non-renewable sources and is therefore not cheap. Research into the possibility of producing activated carbon from renewable, environmentally friendly and economically advantageous sources has shown that biomass is a very effective alternative. The effectiveness of activated carbon for the adsorption of azo dyes depends on several factors as follows: Type of biomass, activating agent, pH value, activation temperature, contact time, initial concentration of the dye and dosage of the adsorbent. Future research could focus on optimizing the absorptive capacity of activated carbon for specific types of azo dyes [46].
Another research group has developed a bioprocess that utilizes the complementary catalytic properties of azoreductases and laccases to convert various azo dyes into valuable aromatic compounds that are precursors of biologically active molecules. The enzymes in question, azoreductases and laccases, are supplied by free and immobilized E. coli cells. The reaction mixtures produced in this way are less contaminated and have a final yield of up to 90%. The result is that these optimized biocatalytic systems are not only an effective method for purifying wastewater but are also a source of valuable chemicals for various applications in the chemical industry [47].
Another alternative method recently reported consists in the biosorption of some azo dyes using activated biochar sewage sludge adsorbent, demonstrating high absorption percentages of the tested colors [48].
5. Conclusions
The field of heterocyclic azo dyes continues to be dynamic, driven by the need for brighter, more durable, and environmentally friendly dyes. The synthesis and application of heterocyclic azo dyes, especially in textiles, offers significant advantages in terms of color brilliance, fastness, and versatility. The development of novel dyes, including those with antimicrobial properties, is evidence of the constant innovation in this field. However, the impact of azo dyes on the environment, particularly in terms of wastewater pollution, remains a critical issue. Innovative and sustainable methods such as enzyme-based biocatalysis and biosorption using renewable materials are promising approaches to mitigate these effects. Future research should continue to focus on improving the environmental footprint of dye synthesis and application to ensure that advances in dye technology serve both industry needs and environmental sustainability.
Author Contributions
Conceptualization, L.E. and M.D.; software, L.E. and M.D.; investigation, L.E. and M.D.; resources, L.E. and M.D; data curation, L.E. and M.D.; writing—original draft preparation, L.E. and M.D.; writing—review and editing, L.E. and M.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Saxena, S.; Raja, A.S.M. Natural Dyes: Sources, Chemistry, Application and Sustainability Issues. In Roadmap to Sustainable Textiles and Clothing. Textile Science and Clothing Technology; Muthu, S., Ed.; Springer: Singapore, 2014; pp. 37–80. [Google Scholar] [CrossRef]
- Patel, B.H. Natural Dyes. In Handbook of Textile and Industrial Dyeing: Principles, Processes and Types of Dyes; Clark, M., Ed.; Woodhead Publishing Ltd.: Cambridge, UK, 2011; Volume 1, pp. 395–421. [Google Scholar]
- Waring, D.R. Heterocyclic Dyes and Pigments. Compr. Heterocycl. Chem. 1984, 1, 317–346. [Google Scholar]
- Winkler, F. The Colour Science of Dyes and Pigments; Adam Hilger Ltd.: Bristol, UK, 1983. [Google Scholar]
- Decelles, C. The story of dyes and dyeing. J. Chem. Educ. 1949, 26, 583. [Google Scholar] [CrossRef]
- Salman, M.; Jabbar, A.; Farooq, S.; Bashir, I.; Rafiq, M.S. New heterocyclic azo-disperse dyes; their synthesis, characterization, application, photo physical properties and solvatochromic studies. J. Mol. Struct. 2023, 1287, 135664. [Google Scholar] [CrossRef]
- Griffiths, J. Color and Constitution of Organic Molecules; Academic Press: London, UK, 1976. [Google Scholar]
- Hallas, G.; Choi, J.-H. Synthesis and properties of novel aziridinyl azo dyes from 2-aminothiophenes—Part 2: Application of some disperse dyes to polyester fibres. Dyes Pigments 1999, 40, 119–129. [Google Scholar] [CrossRef]
- Baroncini, M.; Groppi, J.; Corra, S.S.; Silvi, S.; Credi, A. Light-Responsive (Supra)Molecular Architectures: Recent Advances. Adv. Opt. Mater. 2019, 7, 1900392. [Google Scholar] [CrossRef]
- Wu, W.; Yao, L.; Yang, T.; Yin, R.; Li, F.; Yu, Y. NIR-light-induced deformation of cross-linked liquid crystalpolymers using upconversion nanophosphors. J. Am. Chem. Soc. 2011, 133, 15810–15813. [Google Scholar] [CrossRef]
- Gregory, P. Classification of Dyes by Chemical Structure. In The Chemistry and Application of Dyes: Topics in Applied Chemistry; Waring, D.R., Hallas, G., Eds.; Springer: Boston, MA, USA, 1990; pp. 17–47. [Google Scholar]
- Towns, A.D. Developments in azo disperse dyes derived from heterocyclic diazo components. Dyes Pigments 1999, 42, 3–28. [Google Scholar] [CrossRef]
- Patel, V.J.; Patel, M.P.; Patel, R.G. Synthesis and application of novel heterocyclic dyes based on 1l-amino-3-bromo-13H-acenaphtho[l,2-e]pyridazino[3,2-b]- quinazoline-13-one. J. Serb. Chem. Soc. 2002, 67, 727–734. [Google Scholar] [CrossRef]
- Patel, D.R.; Patel, K.C. Synthesis, characterization and application of quinazolinone based reactive dyes for various fibers. Fibers Polym. 2010, 11, 537–544. [Google Scholar] [CrossRef]
- Patel, D.R.; Patel, K.C. Synthesis of Some New Thermally Stable Reactive Dyes Having 4(3H)-quinazolinone Molecule for the Dyeing of Silk, Wool, and Cotton Fibers. Fibers Polym. 2011, 12, 741–752. [Google Scholar] [CrossRef]
- Patel, D.R.; Patel, K.C. Synthesis, characterization and in vitro antimicrobial screening of some new MCT reactive dyes bearing nitro quinazolinone moiety. J. Saudi Chem. Soc. 2015, 19, 347–359. [Google Scholar] [CrossRef]
- Lellis, B.; Fávaro-Polonio, C.Z.; Pamphile, J.A.; Polonio, J.C. Effects of textile dyes on health and the environment and bioremediation potential of living organisms. Biotechnol. Res. Innov. 2019, 3, 275–290. [Google Scholar] [CrossRef]
- Malinauskiene, L.; Bruze, M.; Ryberg, K.; Zimerson, E.; Isaksson, M. Contact allergy from disperse dyes in textiles: A review. Contact Dermat. 2013, 68, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Moreau, L.; Goossens, A. Allergic contact dermatitis associated with reactive dyes in a dark garment: A case report. Contact Dermat. 2005, 53, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Miles, L.W.C. Textile Printing, 2nd ed.; Society of Dyers and Colourists: Bradford, UK, 2003. [Google Scholar]
- Abdel Zaher, K.S.; Shaban, E.; Nawwar, G.A.M. Antibacterial Azo Dyes Containing Sulfa Drug Moieties and Their Colour Assessment on Printing Polyester Fabric. ChemistrySelect 2023, 8, e202300804. [Google Scholar]
- National Center for Biotechnology Information. “PubChem Compound Summary for CID 6249, Ampicillin” PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Ampicillin (accessed on 22 February 2024).
- Benkhaya, S.; M’rabet, S.; El Harfi, A. Classifications, properties, recent synthesis and applications of azo dyes. Heliyon 2020, 6, e03271. [Google Scholar] [CrossRef] [PubMed]
- Gunst, R. Heterocyclic Disazo Dyestuffs. U.S. Patent No 2,686,178, 2 February 1953. [Google Scholar]
- Fabian, W.M.F.; Timofei, S. Comparative molecular field analysis (CoMFA) of dye-fibre affinities. Part 2. Symmetrical bisazo dyes. J. Mol. Struct. THEOCHEM 1996, 362, 155–162. [Google Scholar] [CrossRef]
- Matsui, M.; Kamino, Y.; Hayashi, M.; Funabiki, K.; Shibata, K.; Muramatsu, H.; Abe, Y.; Kaneko, M. Fluorine-containing benzothiazolyl bisazo dyes-their application to guest-host liquid crystal displays. Liq. Cryst. 1998, 25, 235–240. [Google Scholar] [CrossRef]
- Karcı, F. Synthesis of disazo dyes derived from heterocyclic components. Color. Technol. 2005, 121, 275–280. [Google Scholar] [CrossRef]
- Hadjoudis, E.; Mavridis, I.M. Photochromism and thermochromism of Schiff bases in the solid state: Structural aspects. Chem. Soc. Rev. 2004, 33, 579–588. [Google Scholar] [CrossRef]
- Raczyńska, E.D.; Kosińska, W.; Ośmiałowski, B.; Gawinecki, R. Tautomeric equilibria in relation to pi-electron delocalization. Chem. Rev. 2005, 105, 3561–3612. [Google Scholar] [CrossRef]
- Bártová, K.; Císařová, I.; Lyčka, A.; Dračínský, M. Tautomerism of azo dyes in the solid state studied by 15N, 14N, 13C and 1H NMR spectroscopy, X-ray diffraction and quantum-chemical calculations. Dyes Pigments 2020, 178, 108342. [Google Scholar] [CrossRef]
- Bakan, E.; Karci, F.; Avinc, O. Synthetic Fiber Dyeing with Synthesized Novel Disperse Disazo Dyes Containing Methyl (-CH3) Group as an Auxochrome and Their Color Properties. Int. J. Eng. Appl. Sci. 2016, 10, 8269. [Google Scholar]
- Elnagdi, M.H.; Sallam, M.M.M.; Fahmy, H.M.; Ibrahim, S.A.M.; Elias, M.A.M. Reactions with the Arylhydrazones of α-Cyanoketones: The Structure of 2-Arylhydrazono-3-ketimino-nitriles. Helv. Chim. Acta 1976, 59, 551–557. [Google Scholar] [CrossRef]
- Elnagdi, M.H.; Elgemeie, G.E.; Abd-elaal, F.A.E. Recent developments in the synthesis of pyrazole derivatives. Heterocycles 1985, 23, 3121–3153. [Google Scholar]
- Naime, J.; Al Mamun, M.S.; Aly, M.A.S.; Maniruzzaman, M.; Badal, M.M.R.; Karim, K.M.R. Synthesis, characterization and application of a novel polyazo dye as a universal acid–base indicator. RSC Adv. 2022, 12, 28034–28042. [Google Scholar] [CrossRef] [PubMed]
- Çanakçı, D. Synthesis, characterisation, solvatochromic behaviour and thermal decomposition kinetics of novel polyazo dyes containing amide group and their transition metal complexes. J. Mol. Struct. 2019, 1181, 493–506. [Google Scholar] [CrossRef]
- Nath, I.; Chakraborty, J.; Abednatanzi, S.; Van Der Voort, P. A ‘Defective’ Conjugated Porous Poly-Azo as Dual Photocatalyst. Catalysts 2021, 11, 1064. [Google Scholar] [CrossRef]
- Zhang, J.; Khayatnezhad, M.; Ghadimi, N. Optimal model evaluation of the proton-exchange membrane fuel cells based on deep learning and modified African Vulture Optimization Algorithm. Energy Sources Part A 2022, 44, 287–305. [Google Scholar] [CrossRef]
- Bo, G.; Cheng, P.; Dezhi, K.; Xiping, W.; Chaodong, L.; Mingming, G.; Ghadimi, N. Optimum structure of a combined wind/photovoltaic/fuel cell-based on amended Dragon Fly optimization algorithm: A case study. Energy Sources Part A 2022, 44, 7109–7131. [Google Scholar] [CrossRef]
- Mijin, D.Ž.; Ušćumlić, G.S.; Valentić, N.V.; Marinković, A.D. Synthesis of azo pyridone dyes. Hem. Ind. 2011, 65, 517–532. [Google Scholar] [CrossRef][Green Version]
- Zouari-Mechichi, H.; Benali, J.; Alessa, A.H.; Hadrich, B.; Mechichi, T. Efficient Decolorization of the Poly-Azo Dye Sirius Grey by Coriolopsis gallica Laccase-Mediator System: Process Optimization and Toxicity Assessment. Molecules 2024, 29, 477. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.-T. Azo Dyes and Human Health: A Review. J. Environ. Sci. Health Part C 2016, 34, 233–261. [Google Scholar] [CrossRef]
- Pereira, L.; Alves, M. Dyes—Environmental impact and remediation. In Environmental Protection Strategies for Sustainable Development; Springer: Dordrecht, The Netherlands, 2012; pp. 111–162. [Google Scholar]
- Hussien, F.A.H. An eco-friendly methodology for the synthesis of azocoumarin dye using cation exchange resins. Heliyon 2021, 7, e08439. [Google Scholar] [CrossRef]
- Amjad, R.; Munawar, M.A.; Khan, S.R.; Naeem, M. Synthesis and Spectral Studies of Some Novel Coumarin Based Disperse Azo Dyes: Studies of Coumarin Based Azo Dyes. Pak. J. Sci. Ind. Res. 2009, 52, 117–121. [Google Scholar]
- Al-Harby, N.F.; Albahly, E.F.; Mohamed, N.A. Kinetics, isotherm and thermodynamic studies for efficient adsorption of Congo Red dye from aqueous solution onto novel cyanoguanidine-modified chitosan adsorbent. Polymers 2021, 13, 4446. [Google Scholar] [CrossRef]
- Ali, A.E.; Chowdhury, Z.Z.; Devnath, R.; Ahmed, M.M.; Rahman, M.M.; Khalid, K.; Wahab, Y.A.; Badruddin, I.A.; Kamangar, S.; Hussien, M.; et al. Removal of Azo Dyes from Aqueous Effluent Using Bio-Based Activated Carbons: Toxicity Aspects and Environmental Impact. Separations 2023, 10, 506. [Google Scholar] [CrossRef]
- Fernandes, A.; Pinto, B.; Bonardo, L.; Royo, B.; Robalo, M.P.; Martins, L.O. Wasteful Azo dyes as a source of biologically active building blocks. Front. Bioeng. Biotechnol. 2021, 9, 672436. [Google Scholar] [CrossRef]
- Ravindiran, G.; Sundaram, H.; Rajendran, E.M.; Ramasamy, S.; Nabil, A.Z.; Ahmed, B. Removal of azo dyes from synthetic wastewater using biochar derived from sewage sludge to prevent groundwater contamination. Urban Clim. 2023, 49, 101502. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).