2. Materials and Methods
2.1. General Experimental Details
The experimental descriptions of seven previously uncharacterized compounds are provided here. For the corresponding spectra (
1H and
13C NMR, IR, and HRMS), please refer to the
Supplementary Materials document submitted with this work.
Commercial reagents were used as received, unless otherwise stated, from their commercial source, usually Merck. Routine monitoring of reactions was performed by thin-layer chromatography (TLC) using precoated plates of silica gel 60 F254 and visualized under ultraviolet irradiation (254 nm) and CAM (ceric ammonium molybdate) staining solution. Column chromatography was performed using silica gel 60 (0.040–0.063 mm), and EtOAc and petroleum ether (pet ether), with a boiling point range of 40–60 °C, were used. A JEOL NMR (ECX) 400 MHz instrument was operated at 400 MHz (1H) and 100 MHz (13C) for NMR analysis. Chemical shifts (δ) were reported in parts per million (ppm) relative to CHCl3 (7.26 ppm) for 1H NMR and relative to CHCl3 (77.16 ppm) for 13C NMR. Multiplicities are abbreviated as (s = singlet, d = doublet, t = triplet, q = quartet, bs = broad singlet, m = multiplet).
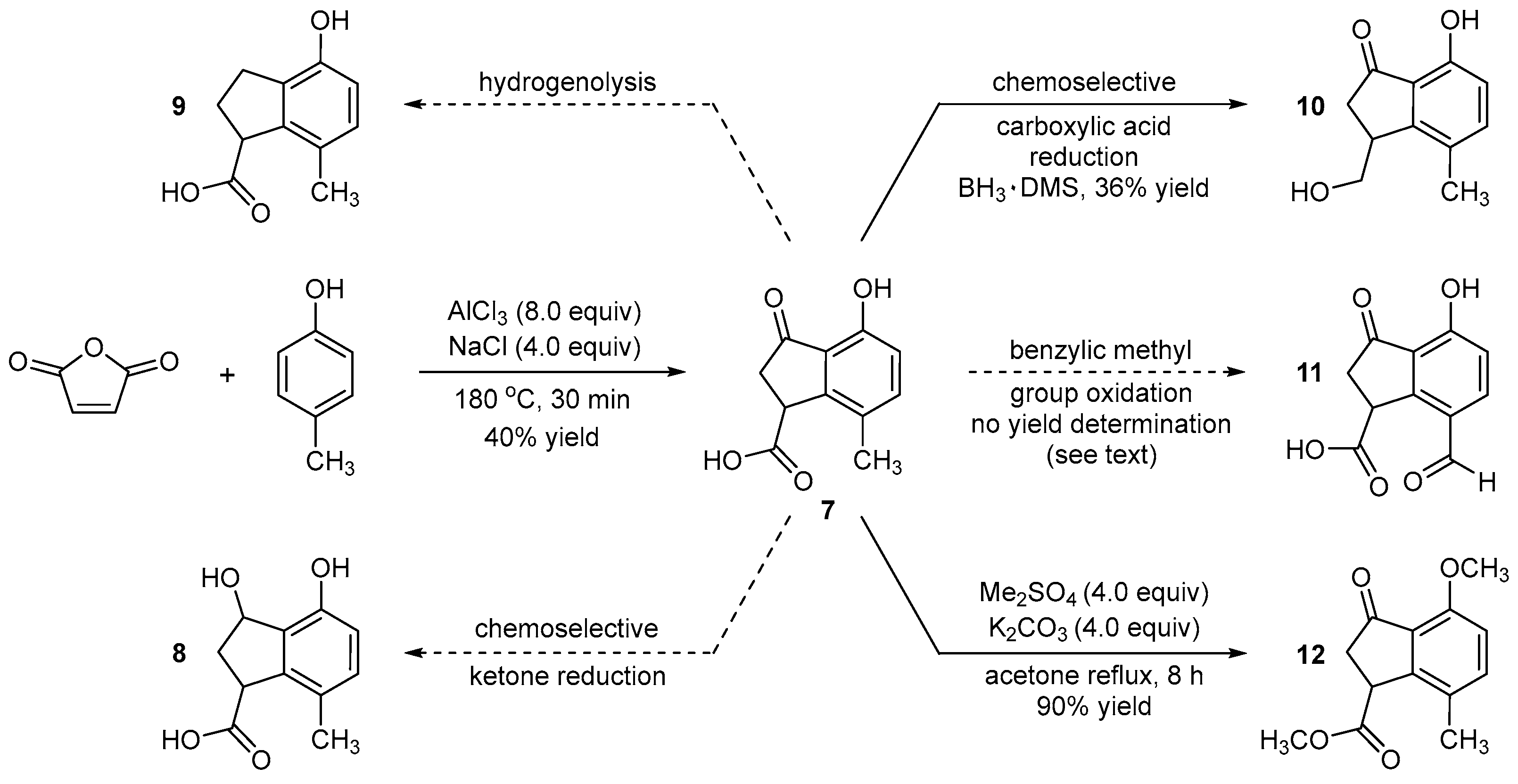
2.2. Synthesis of 4-Hydroxy-7-methyl-3-oxo-2,3-dihydro-1H-indene-1-carboxylic acid (7)
A positive flow of nitrogen was directed into one neck of a double-neck 1.0 L round-bottom flask containing a stir bar (4 × 1.5 cm). In the other neck was placed a glass funnel, and aluminum chloride (98%, MW = 133.34 g/mol, 8.00 equivalent, 740 mmol, 98.63 g) and sodium chloride (99%, MW = 58.44 g/mol, 4.00 equivalent, 370 mmol, 21.62 g) were added. The addition neck was then sealed with a glass stopper, and a positive nitrogen atmosphere was maintained while the mixture was heated to 140 °C with rapid stirring of the solids. Note 1: After ≈ 30 min at 140 °C, the aluminum chloride and sodium chloride melt to form a semi-clear, uniform, viscous liquid. The stoppered neck was then freed, the nitrogen pressure increased to maintain a good flow out of the opened neck, and a glass funnel was inserted. p-Cresol (99%, MW = 108.14 g/mol, 1.00 equivalent, 92.5 mmol, 10.00 g, d = 1.03 g/mL, 9.67 mL) was added, followed by the slow addition of maleic anhydride (≥99%, MW = 98.06 g/mol, 1.00 equivalent, 92.5 mmol, 9.07 g). Note 2: Using either maleic anhydride or p-cresol in excess, specifically 1.3 equivalent, did not result in increased yield. Note 3: Caution: Maleic anhydride was added slowly in small portions over 10 min to suppress small but erratic bursts/eruptions of material from the stirred reaction mass. The addition neck was again stoppered. The reaction mixture turned black in color and became homogenous soon after the addition of maleic anhydride. The oil bath temperature was elevated to 180 °C, and stirring was continued at that temperature for a total of 30 min. The reaction mixture continues to be homogenous and black in color. The completion of the reaction was monitored using TLC. To do that, a small portion of the reaction mixture was taken out using a disposable glass pipette, quenched in aqueous HCl (2.0 N, 2 mL), and extracted with EtOAc (≈0.1 mL). The disappearance of both starting materials was observed on TLC. The reaction was performed no less than six times at this scale and was always complete after 30 min. Note 4: Attempts to scale up the reaction, e.g., doubling or tripling the scale, resulted in approximately half the yield. We surmise this is due to inefficient stirring due to equipment limitations in larger round-bottom flasks.
Work-up: The reaction mixture was brought to room temperature over approximately 45 min. After the mixture dipped below 100 °C, a hard, rock-like black mass ensued, and stirring ceased. The mixture was quenched at 25 °C by the slow addition of ice-cold water (250 mL). This process was strongly exothermic. After cooling to 25 °C, 2.0 N HCl (≈50 mL) was slowly added. The pH of the solution was measured to be ≈2. The solid black mass became a heterogeneous solution in which sticky chunks of black material floated or sat at the bottom of the flask when no stirring was provided. Celite (10 g) was added, which improved the stirring and reduced the sticky chunks. The mixture was further stirred for 10 min at 25 °C and was then filtered through a celite bed (2 cm in height on Whatman filter paper, 110 mm diameter, pore size = 11 μm) using a Büchner funnel (15 cm diameter). The celite bed was washed with EtOAc (500 mL) until the desired compound was fully eluted from the celite bed. This was confirmed by TLC analysis of the filtrate. After filtration, a black-colored, free-flowing solid material remained on the celite bed. The organic layer and aqueous layer were separated from the filtrate using a separatory funnel (1.0 L). The aqueous layer was again extracted with EtOAc (3 × 100 mL) until no product remained in the aqueous layer (confirmed by TLC). The combined organic extracts were dried (Na2SO4) and concentrated under rotary evaporation (bath temperature 35–40 °C) to obtain a viscous black crude product (15.6 g).
Purification: The crude product (15.6 g), with EtOAc added, was uniformly slurried with approximately two times its weight of silica gel (230–400 mesh) and concentrated using rotary evaporation (recommended bath temperature: <35 °C). The resulting free-flowing material was added on top of a silica gel (230–400 mesh) column (16 cm in height, 4.5 cm in diameter). Mobile-phase elution began with 5% EtOAc in petroleum ether doped with acetic acid (1 vol%). This solvent ratio was maintained until all colored impurities were removed from the column. Product elution began when using 10% EtOAc in petroleum ether doped with acetic acid (1 vol%). The concentration of the pure fractions provided 7 as a yellow solid (MW = 206.20 g/mol, 7.70 g, 37.3 mmol, 40% yield). Despite the low yield, the reaction was reliable.
Rf = 0.40, EtOAc/petroleum ether (30:70) doped with acetic acid.
Melting Point: 149–150 °C.
1H NMR (CDCl3, 400 MHz) δ 7.33 (d, J = 8.3 Hz, 1H), 6.81 (d, J = 8.3 Hz, 1H), 4.23 (dd, J = 7.2, 3.4 Hz, 1H), 3.05–2.90 (m, 2H), 2.29 (s, 3H).
13C NMR (CDCl3, 100 MHz) δ 207.1, 178.0, 155.8, 148.2, 139.5, 127.2, 121.8, 115.9, 43.2, 40.8, 17.2.
IR (ATR, cm−1) ν = 3400, 1686, 1603.
HRMS (ESI-QTOF) m/z: [M − H+]− Calculated for C11H9O4: 205.0495; Found: 205.0506; Error: 5.4 ppm.
2.3. Synthesis of Methyl 4-Methoxy-7-methyl-3-oxo-2,3-dihydro-1H-indene-1-carboxylate (12)
A positive flow of nitrogen was directed into one neck of a double-neck round-bottom flask (500 mL) containing a stir bar (3 × 1.5 cm). In the other neck, a glass funnel was inserted, and compound 7 (MW = 206.20 g/mol, 1.00 equiv, 33.9 mmol, 7.00 g) and acetone (≥99.5%, 0.1 M, 339 mL), via a graduated cylinder, were added to the flask. Note that while anhydrous methods were not used, e.g., no precautions were taken to keep the acetone anhydrous (screw cap storage), there were also no extended periods of atmospheric air exposure. The addition neck was then closed using a natural rubber septum. After stirring for 2 min at 25 °C, the mixture became a clear, brown-colored solution. Through the addition neck, potassium carbonate (≥99.0%, MW = 138.21 g/mol, 4.00 equiv, 136 mmol, 18.74 g) was added to the flask while stirring, followed by the addition of dimethyl sulfate (≥99.5%, MW = 126.13 g/mol, d = 1.33 g/mL, 4.00 equiv, 136 mmol, 12.9 mL) at 25 °C. The addition neck was sealed. The reaction mixture was brown in color and heterogeneous in nature. A reflux condenser was added, and gentle reflux (55–60 °C) was maintained for 8 h. For TLC reaction monitoring, we recommend adding one drop (disposal glass pipette) of the reaction medium to a vial (2 mL), followed by evaporation of the acetone with pressurized nitrogen. EtOAc (≈0.1 mL) and water (≈1.0 mL) were then added. TLC of the EtOAc showed the reaction was only complete at 8 h. The reaction was performed no less than six times at this scale. This reaction was performed once at double the scale (15 g), and the yield and reaction time remained the same.
Work-up: The reaction mixture was brought to 25 °C and concentrated using rotary evaporation (recommended bath temperature: 25 °C or lower) until the acetone was completely removed. Water (100 mL) was added to the mixture to dissolve the potassium carbonate and stirred for 10 min. The aqueous phase was extracted with EtOAc (2 × 100 mL). The combined organic extracts were dried over Na2SO4, filtered, and concentrated using rotary evaporation to give the crude product (12.0 g) as a viscous dark brown oil.
Purification: The crude product (12.0 g), with EtOAc added, was uniformly slurried with approximately two times its weight of silica gel (230–400 mesh) and concentrated using rotary evaporation (recommended bath temperature <35 °C). The resulting free-flowing material was added on top of a silica gel (230–400 mesh) column (16 cm in height, 4.5 cm in diameter). Mobile phase elution began with 10% EtOAc in petroleum ether and was gradually increased up to 40% EtOAc/petroleum ether, at which point the product started eluting out. The concentration of the pure fractions provided 12 as a yellow solid (MW = 234.25 g/mol, 7.16 g, 30.6 mmol, 90% yield).
Rf = 0.30, EtOAc/petroleum ether (60:40)
Melting Point: 103–104 °C
1H NMR (CDCl3, 400 MHz) δ 7.35 (d, J = 8.4 Hz, 1H), 6.81 (d, J = 8.4 Hz, 1H), 4.17 (dd, J = 8.4, 3.0 Hz, 1H), 3.93 (s, 3H), 3.69 (s, 3H), 2.93 (dd, J = 18.6, 8.4 Hz, 1H), 2.80 (dd, J = 18.6, 3.0 Hz, 1H), 2.25 (s, 3H).
13C NMR (CDCl3, 100 MHz) δ 202.5, 173.5, 156.9, 152.5, 138.3, 128.0, 125.0, 111.2, 56.4, 53.0, 43.4, 42.1, 17.6.
IR (ATR, cm−1) ν = 2962, 1732, 1697, 1605, 1582.
HRMS (ESI-QTOF) m/z: [M + H+]+ Calculated for C13H15O4: 235.096485; Found: 235.095968; Error: 2.2 ppm.
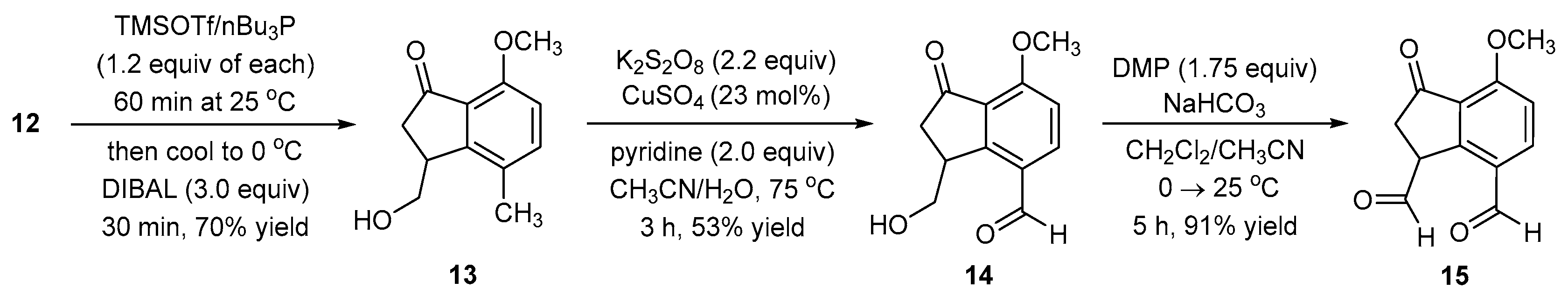
2.4. Synthesis of 3-(Hydroxymethyl)-7-methoxy-4-methyl-2,3-dihydro-1H-inden-1-one (13)
A positive flow of nitrogen was delivered via one neck of a three-neck round-bottom flask (250 mL) containing a stir bar (3 × 1.5 cm). In another neck, a graduated addition funnel (closed with a natural rubber septum) was added, and the joint was sealed with Teflon tape. In the third neck, a glass funnel was inserted, and compound 12 (MW = 234.25 g/mol, 1.00 equiv, 17.1 mmol, 4.00 g) was added. The neck was stoppered using a natural rubber septum. CH2Cl2 (≥99.5%, purchased with ≤0.02% water content (no sure-seal cap was present, the bottle was capped with a natural rubber septum and stored under nitrogen), 0.4 M, 42.75 mL) was added via glass syringe through the third neck. Within 2 min of stirring at 25 °C, the starting material dissolved. Dry THF (sodium and benzophenone distilled, 0.8 M, 21.4 mL) was added via a glass syringe through the third neck. The mixture was stirred for 2 min at 25 °C. Tributylphosphine (nBu3P) (≥93.5%, neat, MW = 202.32 g/mol, 1.20 equiv, 20.52 mmol, d = 0.82 g/mL, 5.13 mL) was slowly added over 1 min via syringe at 25 °C. Approximately 2 min after the complete addition of the nBu3P, a homogenous brown-colored solution ensued. Trimethylsilyl trifluoromethanesulfonate (TMSOTf) (≥98.0%, MW = 222.26 g/mol, 1.20 equiv, 20.5 mmol, d = 1.228 g/mL, 3.71 mL) was slowly added via glass syringe over 2 min due to the exothermic nature of the addition at 25 °C. White fumes were observed during TMSOTf addition. The reaction mixture was stirred for 1 h at 25 °C. Diisobutylaluminium hydride (DIBAL-H, 1.0 M in hexanes, MW = 142.22 g/mol, 3.00 equiv, 51.3 mmol, 51.3 mL) was transferred to the graduated addition funnel via cannula. The reaction flask was first cooled in an ice-water bath, and only then was DIBAL-H added dropwise from the graduated addition funnel over 20 min. The reaction was a brown-colored homogenous solution. To monitor consumption of the starting material (12), a small aliquot (≈50 μL) was taken by disposable syringe into a sample vial (2.0 mL), 2 drops of tetrabutylammonium fluoride (TBAF, 1.0 M in THF) were added, and this mixture was directly spotted onto the TLC plate. After complete DIBAL-H addition, the reaction was stirred for 30 min at 0 °C and then worked up. This reaction was also performed on a larger scale (10 g) and was complete at the same time, with a very similar yield.
Work-up: Aqueous NaHCO3 (saturated solution, 40 mL) was slowly added to the reaction; effervescence was observed and ceased after the complete addition of the aqueous NaHCO3. The mixture became a heterogeneous solution in which sticky chunks of white material floated or sat at the bottom of the flask when no stirring was provided. Celite (10 g) was added to improve stirring and reduce the agglomeration of sticky material into balls. Methanol (20 mL) was added, and after adding a reflux condenser, the mixture was heated at 40 °C for 2 h. After 2 h, the mixture was cooled down to 25 °C and filtered through a celite bed (2 cm height on Whatman filter paper, 110 mm diameter, pore size = 11 μm) using a Büchner funnel (diameter 15 cm). The celite bed was washed portion-wise with EtOAc (≈200 mL) until the product was fully eluted from the celite bed (confirmed by TLC analysis of the last celite bed rinse). After filtration, a white-colored, free-flowing solid remained on the celite bed. The filtrated aqueous layer was extracted with EtOAc (4 × 50 mL). The combined organic phase was dried over Na2SO4, filtered, and rotary evaporated (recommended bath temperature <35 °C). Crude product weight: 6.25 g.
Purification: The crude product (6.25 g), with added EtOAc, was uniformly slurried with approximately two times its weight of silica gel (230–400 mesh) under a rotary evaporator (recommended bath temperature < 35 °C). The resulting free-flowing material was added on top of a silica gel (230–400 mesh) column (16 cm in height, 4.5 cm in diameter). The mobile phase elution began with 10% EtOAc in petroleum ether and was gradually increased up to 60% EtOAc/petroleum ether, at which point the product started eluting out. The concentration of the pure fractions provided 13 as a white solid (MW = 206.24 g/mol, 2.46 g, 11.93 mmol, 70% yield).
Rf = 0.20, EtOAc/petroleum ether (80:20)
Melting Point: 133–134 °C
1H NMR (CDCl3, 400 MHz) δ 7.34 (d, J = 8.4 Hz, 1H), 6.76 (d, J = 8.4 Hz, 1H), 3.98 (m, 1H), 3.91 (s, 3H), 3.64 (m, 1H), 3.55 (m, 1H), 2.80 (dd, J = 18.5, 7.4 Hz, 1H), 2.68 (dd, J = 18.5, 1.8 Hz, 1H), 2.35 (s, 3H).
13C NMR (CDCl3, 100 MHz) δ 204.2, 156.2, 154.7, 137.7, 126.9, 125.4, 110.0, 64.8, 55.7, 41.9, 40.2, 17.3.
IR (ATR, cm−1) ν = 3360, 2932, 1677, 1585, 1493.
HRMS (ESI-QTOF) m/z: [M+H+]+ Calculated for C12H15O3: 207.101571; Found: 207.101448; Error: 0.6 ppm.
2.5. Synthesis of 3-(Hydroxymethyl)-7-methoxy-1-oxo-2,3-dihydro-1H-indene-4-carbaldehyde (14)
In a single-neck round-bottom flask (100 mL) containing a stir bar (1 × 0.5 cm), potassium persulfate (≥99.0%, MW = 270.32 g/mol, 2.24 equiv, 5.43 mmol, 1.47 g) was added, followed by copper(II) sulfate pentahydrate (≥98.0%, MW = 249.69 g/mol, 0.23 equiv, 0.55 mmol, 139 mg). Deionized water (0.2 M, 12.1 mL) was added, and despite stirring, the materials did not fully dissolve. In another flask, compound 13 (MW = 206.24 g/mol, 1.00 equiv, 2.42 mmol, 500 mg) was dissolved in acetonitrile (≥99.8%, 0.16 M, 14.7 mL), and this solution was added over 1 min to the aqueous-containing reaction flask. The reaction mixture remained heterogeneous. Pyridine (MW = 79.10 g/mol, 2.0 equiv, 4.84 mmol, d = 0.982 g/mL, 0.39 mL) was added, and the mixture turned blue in color and remained heterogeneous. A reflux condenser was added, and the mixture was stirred for 3 h at a gentle reflux (75–80 °C) under an open atmosphere (the reflux condenser was not stoppered). As the temperature reached 75–80 °C, the mixture became a homogenous blue-colored solution. After 2 h, the reaction color turned green. TLC monitoring at 2 h showed persistence of the starting material (13), but at 3 h the homogenous solution turned yellow in color, and TLC analysis showed no 13 remained. To monitor the reaction, a small portion (≈25 μL) was taken and quenched by adding it to an aqueous NaHCO3 solution (saturated, ≈1.0 mL) with EtOAc (≈0.1 mL) present. The EtOAc was sampled and co-spotted against 13 on a TLC plate.
Work-up: Aqueous NaHCO3 (saturated solution, 10 mL) was slowly added to the reaction mixture. Effervescence was observed while adding the NaHCO3 solution and ceased after complete addition. The reaction mixture was extracted with EtOAc (4 × 50 mL). The aqueous layer was checked for residual products using TLC. If aqueous dissolved product remained, it was extracted using EtOAc (2 × 50 mL). The combined organic phase was dried over Na2SO4, filtered, and rotary evaporated (recommended bath temperature: ≤25 °C). Crude product weight: 420 mg.
Purification: The crude product (420 mg), with added EtOAc, was uniformly slurried with approximately two times its weight of silica gel (230–400 mesh). Under rotary evaporation (recommended bath temperature: 25 °C), the solvent was removed. The resulting free-flowing material was added on top of a silica gel (230–400 mesh) column (16 cm in height, 2 cm in diameter). Mobile phase elution began with 10% EtOAc in petroleum ether and was gradually increased up to 80% EtOAc/petroleum ether, at which point the product eluted out. The concentration of the pure fractions provided 14 as a light brown solid (MW = 220.22 g/mol, 283 mg, 1.29 mmol, 53% yield). This reaction was examined on a larger scale (1 g) on two occasions but resulted in a lower yield (30%). In these situations, the persistence of the starting material was noted, and extended reaction times did not overcome the problem and may have oxidized the desired aldehyde to the corresponding carboxylic acid.
Rf = 0.15, EtOAc/petroleum ether (90:10)
Melting Point: 141–142 °C
1H NMR (CDCl3, 400 MHz) δ 10.07 (s, 1H), 8.05 (d, J = 8.6 Hz, 1H), 7.01 (d, J = 8.6 Hz, 1H), 4.16 (q, J = 5.7 Hz, 1H), 4.05 (s, 3H), 3.88 (m, 1H), 3.80 (m, 1H), 2.85 (dd, J = 18.6, 7.6 Hz, 1H), 2.68 (dd, J = 18.6, 1.6 Hz, 1H), 1.78 (t, 1H).
13C NMR (CDCl3, 100 MHz) δ 202.7, 190.5, 162.2, 159.8, 141.5, 126.7, 126.3, 110.4, 66.4, 56.6, 41.7, 40.0
IR (ATR, cm−1) ν = 3389, 2964, 1677, 1574.
HRMS (ESI-QTOF) m/z: [M + H+]+ Calculated for C12H13O4: 221.0808; Found: 221.0816; Error: −3.4 ppm.
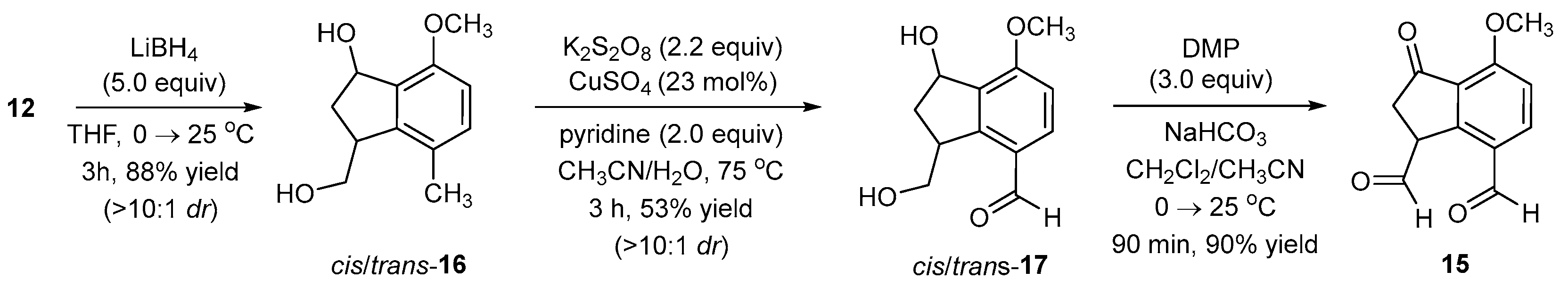
2.6. Synthesis of 4-Methoxy-3-oxo-2,3-dihydro-1H-indene-1,7-dicarbaldehyde (15)
Method A (from compound 14). A positive flow of nitrogen was directed into one neck of a double-neck round-bottom flask (50 mL) containing a stir bar (1 × 0.5 cm). In the other neck, a glass funnel was inserted, and Dess–Martin periodinane (89.99% pure by GC, 99.45% pure by titration, MW = 424.14 g/mol, 1.75 equiv, 1.59 mmol, 674 mg) and sodium hydrogen carbonate (≥99.5%, MW = 84.01 g/mol, 5.00 equiv, 4.55 mmol, 382 mg) were added. The neck was then stoppered using a natural rubber septum. CH2Cl2 (≥99.5%, purchased with ≤0.02% water content [no sure-seal cap was present, the bottle was capped with a natural rubber septum and stored under nitrogen], 0.05 M, 18.2 mL) was added via glass syringe. The mixture was cooled using an ice-water bath. The mixture was a heterogeneous, colorless solution. In another flask, compound 14 (MW = 220.22 g/mol, 1.00 equiv, 0.91 mmol, 200 mg) was fully dissolved in acetonitrile (≥99.8%, 0.5 M, 1.8 mL), and this solution was added (over 1 min) to the reaction flask. The final molarity is 0.045 M. The reaction mixture remains heterogeneous throughout the entire reaction. The ice bath was removed within 5 min of adding 14, and the reaction was complete after 5 h of stirring at 25 °C. After 1 h of stirring at 25 °C, a white suspended material was noted and stayed for the remainder of the reaction. To monitor consumption of the starting material (14), an aliquot containing the reaction liquid (≈25 μL) was removed (disposable syringe) and added to a vial (2 mL) containing CH2Cl2 (≈100 μL), an aqueous saturated sodium thiosulfate solution (≈0.2 mL), and a saturated aqueous sodium bicarbonate solution (≈0.2 mL). The capped vial was shaken vigorously. TLC of the CH2Cl2 layer was required.
Method B (from compound cis-17). A positive flow of nitrogen was directed into one neck of a double-neck round-bottom flask (50 mL) containing a stir bar (1 × 0.5 cm). In the other neck, a glass funnel was inserted, and Dess–Martin periodinane (89.99% pure by GC, 99.45% pure by titration, MW = 424.14 g/mol, 3.00 equiv, 2.69 mmol, 1.14 g) and sodium hydrogen carbonate (≥99.5%, MW = 84.01 g/mol, 5.00 equiv, 4.50 mmol, 378 mg) were added. The neck was stoppered using a natural rubber septum. CH2Cl2 (≥99.5%, purchased with ≤0.02% water content (no sure-seal cap was present, the bottle was capped with a natural rubber septum and stored under nitrogen), 0.05 M, 18.0 mL) was added via glass syringe. The mixture was cooled using an ice-water bath. The reaction mixture was a heterogeneous, colorless solution. In another flask, compound cis-17 (MW = 222.22 g/mol, 1.00 equiv, 0.90 mmol, 200 mg) was fully dissolved in acetonitrile (≥99.8%, 0.5 M, 1.8 mL), and this solution was added (over 1 min) to the reaction flask. The final molarity is 0.045 M. The reaction mixture remains heterogeneous throughout the entire reaction. The ice bath was removed within 5 min of adding cis-17, and the reaction was complete after 90 min of stirring at 25 °C. After 1 h of stirring at 25 °C, a suspended white material was noted and stayed for the remainder of the reaction. To monitor consumption of the starting material (cis-17), an aliquot containing the reaction liquid (≈25 μL) was removed (disposable syringe) and added to a vial (2 mL) containing CH2Cl2 (≈100 μL), an aqueous saturated sodium thiosulfate solution (≈0.2 mL), and a saturated aqueous sodium bicarbonate solution (≈0.2 mL). The capped vial was shaken vigorously. TLC of the CH2Cl2 layer was required.
Work-up and Purification (Methods A and B): Aqueous Na2S2O3 (saturated solution, 10 mL) and aqueous NaHCO3 (saturated solution, 10 mL) were added to the reaction mixture and stirred for 5 min at 25 °C. The solution was biphasic, and the organic phase was brown-colored while the aqueous phase was red-colored. The organic phase was separated from the aqueous phase using a separatory funnel, and the organic phase was returned to the round-bottom flask. Fresh aqueous Na2S2O3 (saturated solution, 10 mL) was added, and the biphasic solution was stirred for 5 min. The aqueous phase was slightly red in color, while the organic phase turned orange in color. The organic phase was separated from the aqueous phase. The organic phase was washed for a third time with a fresh aqueous Na2S2O3 solution (saturated solution, 10 mL) with 5 min of stirring. After phase separation, the aqueous layer was colorless, and the organic phase remained orange in color. The lack of the red color in the aqueous phase (third aqueous washing) appears to indicate when complete removal of excess DMP and/or reduced DMP by-products from the organic phase has occurred. All three aqueous phases were combined and back-extracted with CH2Cl2 (15 mL), and we recommend that this CH2Cl2 extract be washed for 5 min with fresh aqueous saturated sodium thiosulfate before adding it to the main organic extract. Lastly, the combined organic extracts were washed with water, dried over Na2SO4, filtered, and concentrated under rotary evaporation (recommended bath temperature: ≤25 °C) to give dialdehyde 15 as a yellow solid. For method A: 180 mg (MW = 218.21 g/mol, 0.83 mmol, 91% yield). For method B: 177 mg (MW = 218.21 g/mol, 0.81 mmol, 90% yield).
The purity of the produced dialdehyde (
15), after work-up (no chromatography), was high and required no further purification. The
method A material was used to collect all characterization data (see
Supplementary Materials). A
1H NMR was also collected for the
method B material (see
Supplementary Materials). The TLC examination of the dialdehyde did not provide a single spot. Furthermore, attempts at silica gel chromatography using mixtures of EtOAc/petroleum either with or without a dopant (Et
3N or AcOH) always resulted in a greatly reduced yield of the dialdehyde, still impure, and with new impurities.
All data and spectroscopy were collected for the Method A dialdehyde product (15).
Rf = 0.20, EtOAc/petroleum ether (90:10)
Melting Point: 75–76 °C.
1H NMR (CDCl3, 400 MHz) δ 10.00 (s, 1H), 9.94 (d, J = 1.3 Hz, 1H), 8.06 (d, J = 8.5 Hz, 1H), 7.08 (d, J = 8.6 Hz, 1H), 4.93 (dd, J = 8.5, 3.1 Hz, 1H), 4.06 (s, 3H), 2.97 (dd, J = 19.0, 3.1 Hz, 1H), 2.85 (dd, J = 19.0, 8.5 Hz, 1H).
13C NMR (CDCl3, 100 MHz) δ 200.5, 197.4, 190.3, 162.5, 153.8, 142.5, 126.2, 126.1, 111.1, 56.7, 49.5, 37.7.
IR (ATR, cm−1) ν = 3412, 2947, 2849, 1700, 1683, 1591, 1574.
HRMS (ESI-QTOF) m/z: [M − H+]− Calculated for C12H9O4: 217.0506; Found: 217.0507, Error: −0.3 ppm.
2.7. Synthesis of 3-(Hydroxymethyl)-7-methoxy-4-methyl-2,3-dihydro-1H-inden-1-ol (cis-16)
A positive flow of nitrogen was directed into one neck of a three-neck round-bottom flask (500 mL) containing a stir bar (3 × 1.5 cm). In one of the other two necks, a graduated addition funnel (closed with a natural rubber septum) was added, and the joint was sealed with teflon tape. In the third neck, a glass funnel was placed, and compound 12 (MW = 234.25 g/mol, 1.00 equiv, 21.3 mmol, 5.0 g) was added. This neck was then sealed using a natural rubber septum. Dry THF (≥99.5% and distilled from sodium and benzophenone, 0.1 M, 213.40 mL) was added to a single-neck round-bottom flask (500 mL) and subsequently transferred to the reaction flask via cannula. After stirring for 2 min at 25 °C, a light yellow-colored homogenous solution ensued. Lithium borohydride (MW = 21.78 g/mol, 5.0 equiv, 106.7 mmol, 2.0 M in THF, 53.36 mL) was transferred to the graduated addition funnel via cannula. The reaction mixture was cooled using an ice bath, and then the LiBH4 solution was added dropwise over 20 min. After complete addition, the mixture became brown in color and remained homogenous. The ice bath was removed, and the reaction mixture was allowed to stir at 25 °C for 3 h. To monitor consumption of the starting material, a reaction aliquot was taken using a disposable syringe (≈50 μL) and quenched in a vial (2 mL) containing an aqueous saturated ammonium chloride solution (≈1.0 mL) and EtOAc (≈100 μL). The organic layer was co-spotted with 12 on a TLC plate. This reaction was also performed at a larger scale (10 g) and was complete at the same reaction time with a very similar yield.
Work-up: Aqueous NH4Cl (saturated solution, 30 mL) was slowly added to the reaction; effervescence was observed and ceased after addition was complete. The solution was monophasic and colorless, but upon addition of EtOAc (≈75 mL), a biphasic solution ensued. The organic phase was removed, and the aqueous phase was further extracted with EtOAc (3 × 50 mL). The aqueous phase was checked for residual products (TLC analysis). If aqueous dissolved product remained, it was extracted using EtOAc (2 × 30 mL). The combined organic phases were dried over Na2SO4, filtered, and rotary evaporated. Crude product weight: 6.50 g.
Purification: The crude product (6.50 g), with added EtOAc, was uniformly slurried with approximately two times its weight of silica gel (230–400 mesh). Under rotary evaporation (recommended bath temperature: <30 °C), the solvent was removed. The resulting free-flowing material was then added on top of a silica gel (230–400 mesh) column (16 cm in height, 4.5 cm in diameter). Mobile phase elution was performed with EtOAc/petroleum ether and began with a 1:9 ratio, which was gradually increased to a 2:3 ratio, at which point the product started eluting out.
Cis-
16 elutes out slightly before
trans-
16. The
cis and
trans product fractions were combined and concentrated to provide
cis/
trans-
16 as a white solid (MW = 208.26 g/mol, 3.91 g, 18.77 mmol, 88% yield). A
1H NMR was recorded (see
Supplementary Materials), and a
cis/
trans ratio of 14.5:1 was recorded (see
1H NMR expansion at 5.2 to 5.6 ppm). However, other resonance comparisons imply a
cis/
trans ratio of no better than >10:1. A small portion of the
cis/
trans-
16 product was exposed to a second round of chromatography, wherein the
cis-
16 (major product) was carefully isolated free of
trans-
16. Note: We do not have definitive proof for the structure of
trans-
16 (minor product).
All data and spectroscopy were collected for cis-16 (racemic).
Rf = 0.40, EtOAc/petroleum ether (80:40).
Melting Point: 113–114 °C.
1H NMR (CDCl3, 400 MHz) δ 7.07 (d, J = 8.2 Hz, 1H), 6.68 (d, J = 8.2 Hz, 1H), 5.25 (d, J = 6.6 Hz, 1H), 3.97 (dd, J = 10.6, 3.5 Hz, 1H), 3.84 (s, 3H), 3.81 (dd, J = 10.5, 2.6 Hz, 1H), 3.41 (dt, J = 8.8, 2.9 Hz, 1H), 2.56 (ddd, J = 14.4, 8.9, 6.9 Hz, 1H), 2.27 (s, 3H), 1.96 (d, J = 14.5 Hz, 1H).
13C NMR (CDCl3, 100 MHz) δ 154.3, 143.4, 133.1, 131.5, 125.9, 109.3, 71.6, 63.4, 55.3, 45.4, 39.1, 17.8.
IR (ATR, cm−1) ν = 3308, 3239, 2926, 1595, 1495.
HRMS (ESI-QTOF) m/z: [M + H+]+ Calculated for C12H15O3: 207.101571; Found: 207.101821; Error: −1.2 ppm.
2.8. Synthesis of 1-Hydroxy-3-(hydroxymethyl)-7-methoxy-2,3-dihydro-1H-indene-4-carbaldehyde (cis-17)
In a single-neck round-bottom flask (100 mL) containing a stir bar (1 × 0.5 cm), was added potassium persulfate (≥99.0%, MW = 270.32 g/mol, 2.24 equiv, 5.37 mmol, 1.45 g), followed by copper(II) sulfate pentahydrate (≥98.0%, MW = 249.69 g/mol, 0.23 equiv, 0.55 mmol, 138 mg). Deionized water (0.2 M, 12.0 mL) was added to the flask, and despite being stirred, it did not fully dissolve. In another flask, cis/trans-16 (MW = 208.26 g/mol, 1.00 equiv, 2.40 mmol, 500 mg) was fully dissolved in acetonitrile (≥99.8%, 0.17 M, 14.5 mL), and this solution was added (over 1 min) to the aqueous K2S2O8 medium. The reaction mixture was heterogeneous. Pyridine (≥99.0%, MW = 79.10 g/mol, 2.0 equiv, 4.80 mmol, d = 0.982 g/mL, 0.4 mL) was added, and the mixture turned blue and remained heterogeneous. A reflux condenser was added, and the mixture was stirred for 3 h at gentle reflux (75–80 °C) under an open atmosphere of air by not stoppering the reflux condenser. As the temperature reached 75–80 °C, the mixture became homogenous, and the color remained blue. After approximately 2 h, the color of the mixture turned green. TLC monitoring at 2 h showed persistence of the starting material (cis/trans-16), but at 3 h the homogenous solution turned yellow in color, and TLC analysis showed no more cis/trans-16. To monitor the reaction, an aliquot (≈25 μL) was taken and quenched by adding it to a vial (2 mL) containing an aqueous saturated solution of NaHCO3 (≈1.0 mL) and EtOAc (≈0.1 mL). The EtOAc was sampled and co-spotted against cis/trans-16 on a TLC plate. This reaction was examined on a larger scale (1 g) on two occasions but resulted in a lower yield. During those reactions, the persistence of the starting material was noted, and extended reaction times did not overcome the problem and may have oxidized the desired aldehyde to the corresponding carboxylic acid.
Work-up: Aqueous NaHCO3 (saturated solution, 10 mL) was slowly added to the reaction mixture. Effervescence was observed and ceased after complete addition. The reaction mixture was extracted with EtOAc (4 × 50 mL). The aqueous layer was checked for residual products using TLC. If aqueous dissolved product remained, it was extracted using EtOAc (2 × 50 mL). The combined organic phases were dried over Na2SO4, filtered, and rotary evaporated (recommended bath temperature: ≤25 °C). Crude product weight: 425 mg.
Purification: The crude product (425 mg), with added EtOAc, was uniformly slurried with approximately two times its weight of silica gel (230–400 mesh). Under rotary evaporation (recommended bath temperature: ≤25 °C), the solvent was removed. The resulting free-flowing material was added on top of a silica gel (230–400 mesh) column (16 cm in height, 2 cm in diameter). Mobile phase elution was performed with EtOAc/petroleum ether, beginning with a 10:90 ratio and gradually increasing to an 85:15 ratio, at which time the product eluted out. Concentration of the pure fractions provided cis/trans-17 (10:1 dr) as a white solid (MW = 222.24 g/mol, 283 mg, 1.27 mmol, 53% yield). A smaller portion of the isolated cis/trans-17 product was exposed to a second round of chromatography, wherein cis-17 (major diastereomer) eluted off the column before trans-17 and was isolated free of trans-17.
All data and spectroscopy were collected for cis-17 (racemic).
Rf = 0.10, EtOAc/petroleum ether (90:10)
Melting Point: 151–152 °C.
1H NMR (CDCl3, 400 MHz) δ 9.95 (s, 1H), 7.80 (d, J = 8.3 Hz, 1H), 6.92 (d, J = 8.5 Hz, 1H), 5.21 (d, J = 7.1 Hz, 1H), 4.13 (dd, J = 10.5, 2.9 Hz, 1H), 4.00 (dt, J = 9.1, 3.1 Hz, 1H), 3.97 (s, 3H), 3.86 (dd, J = 10.3, 3.1 Hz, 1H), 3.79 (bs, 1H, -OH), 2.58 (ddd, J = 14.4, 9.0, 7.0 Hz, 1H), 2.27 (s, 1H, -OH), 2.03 (d, J = 14.3 Hz, 1H).
13C NMR (CDCl3, 100 MHz) δ 191.4, 160.9, 147.8, 138.0, 135.4, 126.2, 109.5, 70.6, 64.4, 55.9, 45.3, 39.4.
IR (ATR, cm−1) ν = 3203, 2943, 2846, 2742, 1679, 1601, 1577, 1498.
HRMS (ESI-TOF) m/z: [M − H+]− Calculated for C12H14O4: 221.068014; Found: 221.068863. Error: −3.8 ppm.