A Reaction of N-Substituted Succinimides with Hydroxylamine as a Novel Approach to the Synthesis of Hydroxamic Acids
Abstract
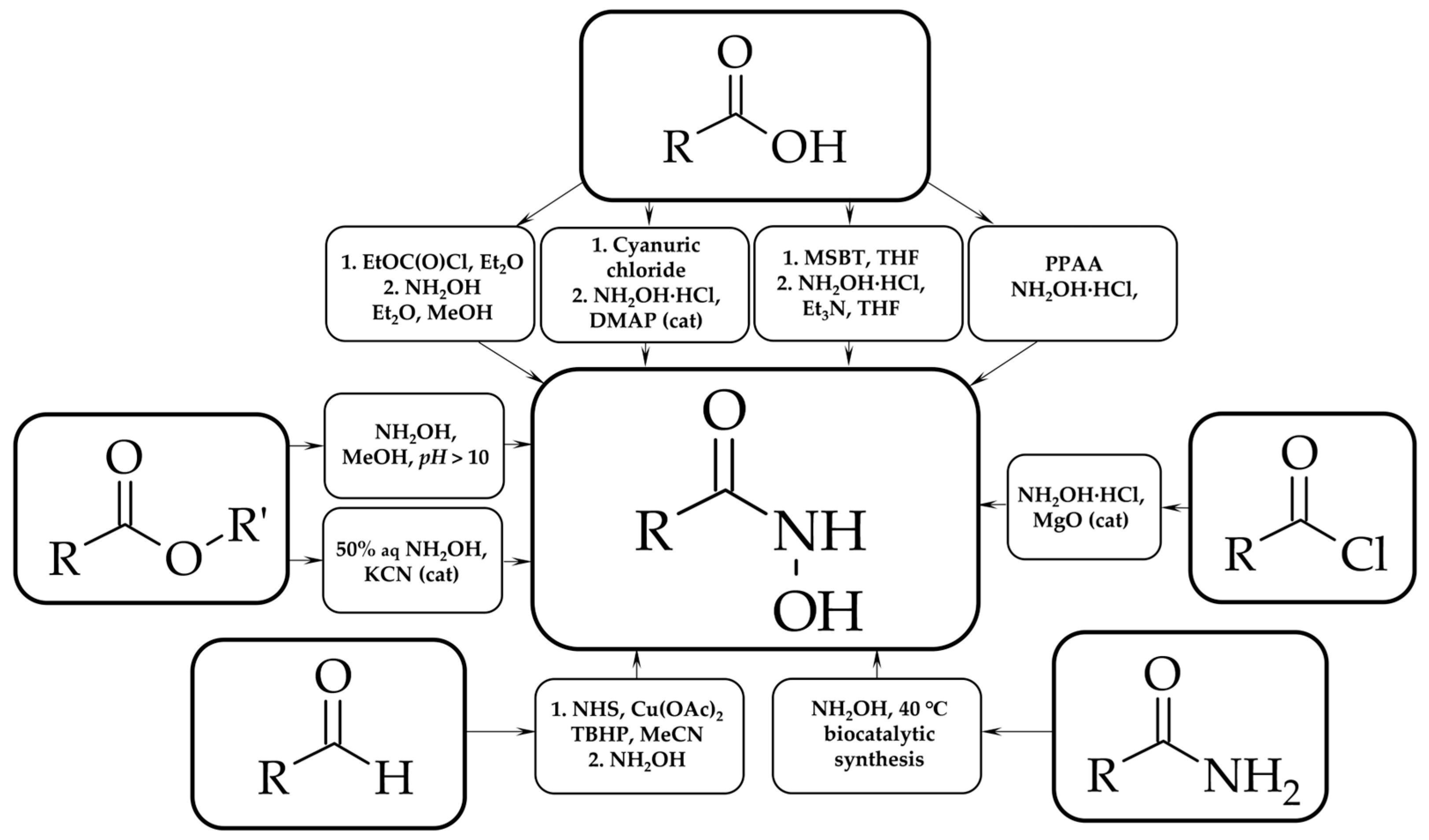
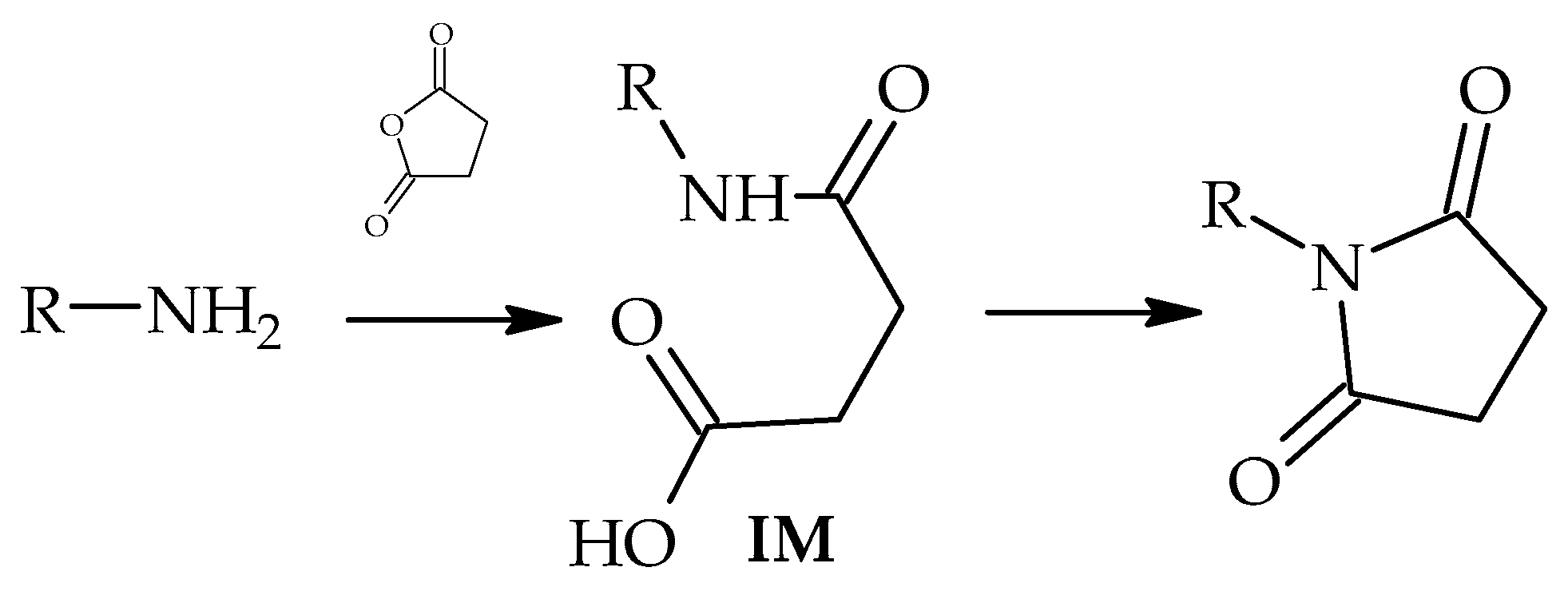
1. Introduction
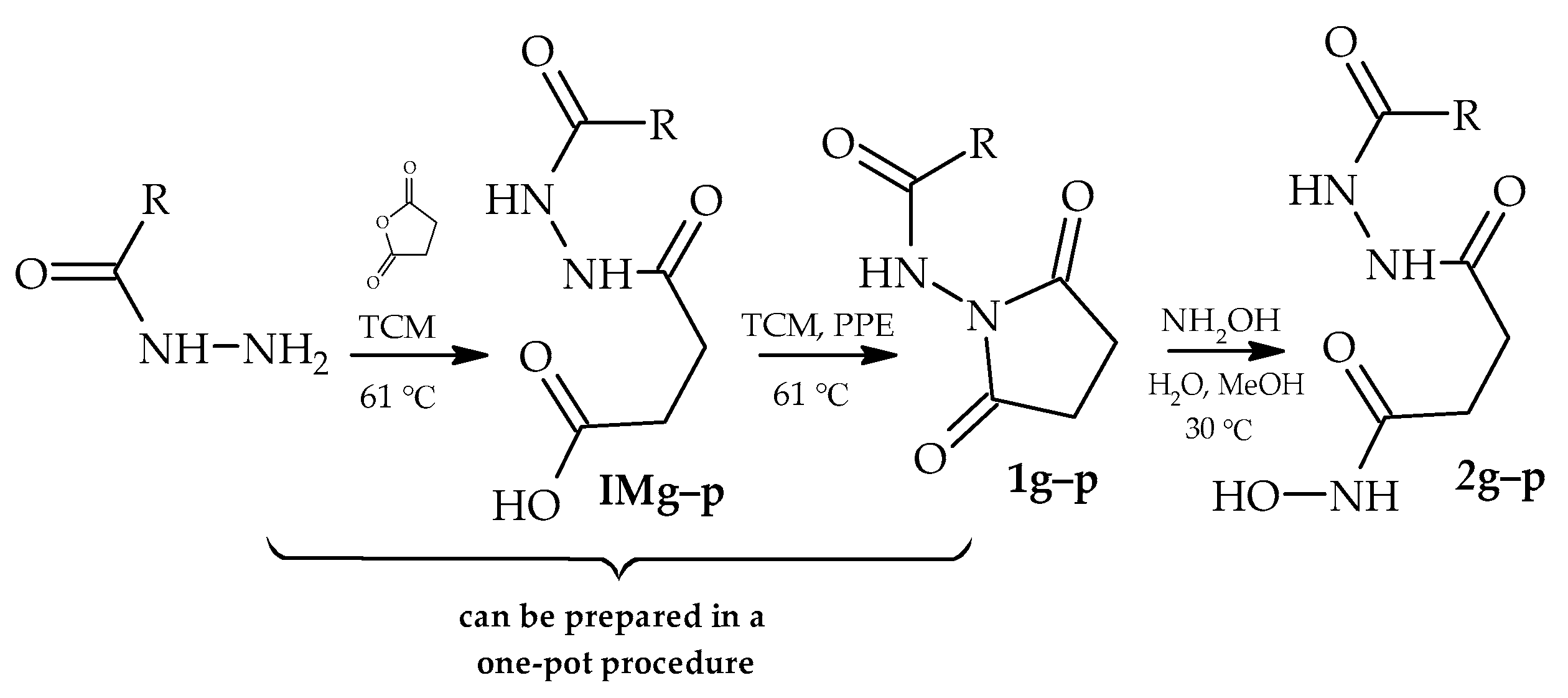
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gupta, M.K.; Singh, G.; Gupta, S. Hydroxamic Acid Derivatives as Potential Anticancer Agents. In Hydroxamic Acid: A Unique Family of Chemicals with Multiple Biological Activities; Gupta, S.P., Ed.; Springer: Heidelberg, Germany; New York, NY, USA; Dordrecht, The Netherlands; London, UK, 2013; pp. 173–204. [Google Scholar]
- Citarella, A.; Moi, D.; Pinzi, L.; Bonanni, D.; Rastelli, G. Hydroxamic Acid Derivatives: From Synthetic Strategies to Medicinal Chemistry Applications. ACS Omega 2021, 6, 21843–21849. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, P. Inside HDAC with HDAC inhibitors. Eur. J. Med. Chem. 2010, 45, 2095–2116. [Google Scholar] [CrossRef] [PubMed]
- Thaler, F.; Patil, V.M.; Gupta, S.P. Hydroxamic Acids as Histone Deacetylase Inhibitors. In Hydroxamic Acid: A Unique Family of Chemicals with Multiple Biological Activities; Gupta, S.P., Ed.; Springer: Heidelberg, Germany; New York, NY, USA; Dordrecht, The Netherlands; London, UK, 2013; pp. 99–151. [Google Scholar]
- Patil, V.M.; Gupta, S.P. Structure–Activity Relationship Studies of Hydroxamic Acids as Matrix Metalloproteinase Inhibitors. In Hydroxamic Acid: A Unique Family of Chemicals with Multiple Biological Activities; Gupta, S.P., Ed.; Springer: Heidelberg, Germany; New York, NY, USA; Dordrecht, The Netherlands; London, UK, 2013; pp. 71–98. [Google Scholar]
- Cathcart, J.M.; Cao, J. MMP Inhibitors: Past, present and future. Front. Biosci. Landmark Ed. 2015, 20, 1164–1178. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, V.A.; Alexanian, E.J. Metal-Free Oxyaminations of Alkenes Using Hydroxamic Acids. J. Am. Chem. Soc. 2011, 133, 11402–11405. [Google Scholar] [CrossRef] [PubMed]
- Giglio, B.C.; Alexanian, E.J. Alkene Hydrofunctionalization Using Hydroxamic Acids: A Radical-Mediated Approach to Alkene Hydration. Org. Lett. 2014, 16, 4304–4307. [Google Scholar] [CrossRef]
- Krylov, I.B.; Paveliev, S.A.; Budnikov, A.S.; Segida, O.O.; Merkulova, V.M.; Vil’, V.A.; Nikishin, G.I.; Terent’ev, A.O. Hidden Reactivity of Barbituric and Meldrum’s Acids: Atom-Efficient Free-Radical C–O Coupling with N-Hydroxy Compounds. Synthesis 2022, 54, 506–516. [Google Scholar] [CrossRef]
- Porcheddu, A.; Giacomelli, G. Synthesis of oximes and hydroxamic acids. In The Chemistry of Hydroxylamines, Oximes and Hydroxamic Acids; Rappoport, Z., Liebmann, J.F., Eds.; Wiley: An Interscience Publication: Chichester, UK, 2009; pp. 163–232. [Google Scholar]
- Alam, M.A. Methods for Hydroxamic Acid Synthesis. Curr. Org. Chem. 2019, 23, 978–993. [Google Scholar] [CrossRef]
- Hearn, M.T.W.; Ward, A.D. Hydroxamic Acids. VI The Synthesis, Properties and Reactions of Amidic Hydroxamic Acid and Dihydroxamic Acid Derivatives. Aust. J. Chem. 1977, 30, 2031–2043. [Google Scholar] [CrossRef]
- Devlin, J.P.; Ollis, W.D.; Thorpe, J.E.; Wood, R.J.; Broughton, B.J.; Warren, P.J.; Wooldridge, K.R.H.; Wright, D.E. Studies concerning the antibiotic actinonin. Part III. Synthesis of structural analogues of actinonin by the anhydride–imide method. J. Chem. Soc. Perkin Trans. 1 1975, 9, 830–841. [Google Scholar] [CrossRef]
- Reichelt, A.; Gaul, C.; Frey, R.R.; Kennedy, A.; Martin, S.F. Design, Synthesis, and Evaluation of Matrix Metalloprotease Inhibitors Bearing Cyclopropane-Derived Peptidomimetics as P1′ and P2′ Replacements. J. Org. Chem. 2002, 67, 4062–4075. [Google Scholar] [CrossRef]
- Tretyakov, B.A.; Gadomsky, S.Y.; Terentiev, A.A. Method for Producing Derivatives of N-Hydroxybutanamide. Russian Federation Patent RU2769320 C1, 30 March 2022. [Google Scholar]
- Dixon, L.A. Polyphosphate Ester. In Encyclopedia of Reagents for Organic Synthesis; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2001. [Google Scholar] [CrossRef]
- Yale, H.L.; Losee, K.; Martins, J.; Holsing, M.; Perry, F.M.; Bernstein, J. Chemotherapy of Experimental Tuberculosis. VIII. The Synthesis of Acid Hydrazides, their Derivatives and Related Compounds. J. Am. Chem. Soc. 1953, 75, 1933–1942. [Google Scholar] [CrossRef]
- Lea, Z.-G.; Chen, Z.-C.; Hu, Y.; Zheng, Q.-G. Organic Reactions in Ionic Liquids: Ionic Liquid-Promoted Efficient Synthesis of N-Alkyl and N-Arylimides. Synthesis 2004, 7, 995–998. [Google Scholar] [CrossRef]
- Lee, H.-S.; Yu, J.-S.; Lee, C.-K. Use of Correlation of 1H and 13C Chemical Shifts of N-Arylsuccinanilic Acids, N-Arylsuccinimides, N-Arylmaleanilic Acids, and N-Arylmaleimides with the Hammett Substituent Constants for the Studies of Electronic Effects. Bull. Korean Chem. Soc. 2009, 30, 2351–2354. [Google Scholar] [CrossRef]
- Garad, D.N.; Tanpure, S.D.; Mhaske, S.B. Radical-mediated dehydrative preparation of cyclic imides using (NH4)2S2O8–DMSO: Application to the synthesis of vernakalant. Beilstein J. Org. Chem. 2015, 11, 1008–1016. [Google Scholar] [CrossRef]
- Short, F.W.; Long, L.M. Synthesis of 5-aryl-2-oxazolepropionic acids and analogs. Antiinflammatory agents. J. Heterocycl. Chem. 1969, 6, 707–712. [Google Scholar] [CrossRef]
- Itsuo, M.; Kohya, N.; Masateru, M. Reaction of benzaldoxime with N-phenylmaleimide. Kobunshi Ronbunshu 1988, 45, 605–608. [Google Scholar] [CrossRef]
- Hermant, P.; Bosc, D.; Piveteau, C.; Gealageas, R.; Lam, B.; Ronco, C.; Roignant, M.; Tolojanahary, H.; Jean, L.; Renard, O.-Y.; et al. Controlling Plasma Stability of Hydroxamic Acids: A MedChem Toolbox. J. Med. Chem. 2017, 60, 9067–9089. [Google Scholar] [CrossRef]
- Brown, D.A.; Glass, W.K.; Mageswaran, R.; Girmay, B. cis-trans Isomerism in monoalkylhydroxamic acids by 1H, 13C and 15N NMR spectroscopy. Magn. Reson. Chem. 1988, 26, 970–973. [Google Scholar] [CrossRef]
- Kar, A.; Argade, N.P. A Simple Key for Benzylic Mono- and gem-Dibromination of Primary Aromatic Amine Derivatives Using Molecular Bromine. Synthesis 2002, 2, 221–224. [Google Scholar] [CrossRef]
- Liang, J.; Lv, J.; Fan, J.-C.; Shang, Z.-C. Polyethylene Glycol as a Nonionic Liquid Solvent for the Synthesis of N-Alkyl and N-Arylimides. Synth. Commun. 2009, 39, 2822–2828. [Google Scholar] [CrossRef]
- Abdel-Aziz, A.A.-M.; Eltahir, K.E.H.; Asiri, Y.A. Synthesis, anti-inflammatory activity and COX-1/COX-2 inhibition of novel substituted cyclic imides. Part 1: Molecular docking study. Eur. J. Med. Chem. 2011, 46, 1648–1655. [Google Scholar] [CrossRef] [PubMed]
- Makurina, V.I.; Chuvurin, A.V.; Karnozhitskaya, T.M.; Chernykh, V.P. Kinetics and Mechanism of the Hydrazinolysis of the Imides of 4-Substituted Succinanilic Acids in Dimethylformamide. J. Org. Chem. USSR Engl. Transl. 1990, 26, 1978–1980. [Google Scholar]
- Correa-Basurto, J.; Flores-Sandoval, C.; Marin-Cruz, J.; Rojo-Dominguez, A.; Espinoza-Fonseca, L.M.; Trujillo-Ferrara, J.G. Docking and quantum mechanic studies on cholinesterases and their inhibitors. Eur. J. Med. Chem. 2007, 42, 10–19. [Google Scholar] [CrossRef]
- Sortino, M.; Garibotto, F.; Cechinel Filho, V.; Gupta, M.; Enriz, R.; Zacchino, S. Antifungal, cytotoxic and SAR studies of a series of N-alkyl, N-aryl and N-alkylphenyl-1,4-pyrrolediones and related compounds. Bioorg. Med. Chem. 2011, 19, 2823–2834. [Google Scholar] [CrossRef] [PubMed]
- Araghi, M.; Mirkhani, V.; Moghadam, M.; Tangestaninejad, S.; Mohammadpoor-Baltork, I. New porphyrin–polyoxometalate hybrid materials: Synthesis, characterization and investigation of catalytic activity in acetylation reactions. Dalton Trans. 2012, 41, 11745–11752. [Google Scholar] [CrossRef]
- Moghadam, M.; Tangestaninejad, S.; Mirkhani, V.; Mohammadpoor-Baltork, I.; Gharaati, S. Polystyrene-bound electron-deficient tin(IV) porphyrin: A new, highly efficient, robust and reusable catalyst for acetylation of alcohols and phenols with acetic anhydride. C. R. Chim. 2011, 14, 1080–1087. [Google Scholar] [CrossRef]
- Yakuschenko, I.; Pozdeeva, N.N.; Gadomsky, S.Y. A novel one-pot synthesis method of 3,4,5-triaryl-substituted 1,2,4-triazoles. Chem. Heterocycl. Compd. 2019, 55, 834–838. [Google Scholar] [CrossRef]
- Kokovina, T.S.; Gadomsky, S.Y.; Terentiev, A.A.; Sanina, N.A. A Novel Approach to the Synthesis of 1,3,4-Thiadiazole-2-Amine Derivatives. Molecules 2021, 26, 5159. [Google Scholar] [CrossRef]
- Lide, D.R. (Ed.) CRC Handbook of Chemistry and Physics, 84th ed.; CRC Press LLC: Boca Raton, FL, USA, 2003; p. 2616. [Google Scholar]
- Available online: https://scifinder-n.cas.org (accessed on 15 November 2022).
- Rothenburg, R. Saureimide und Hydrazinhydrat. Chem. Berich. 1894, 27, 691. [Google Scholar] [CrossRef]
- Ing, H.R.; Manske, R.H.F. CCCXII.—A modification of the Gabriel synthesis of amines. J. Chem. Soc. 1926, 129, 2348–2351. [Google Scholar] [CrossRef]
- Sanz, D.; Pérez-Torralba, M.; Alarcón, S.H.; Claramunt, R.M.; Foces-Foces, C.; Elguero, J. Tautomerism in the Solid State and in Solution of a Series of 6-Aminofulvene-1-aldimines. J. Org. Chem. 2002, 67, 1462–1471. [Google Scholar] [CrossRef] [PubMed]
- Dey, S.K.; Lightner, D.A. 1,1′-Bipyrroles: Synthesis and Stereochemistry. J. Org. Chem. 2007, 72, 9395–9397. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.X.; Mekelburga, T.; Hyland, C. Unusual (Z)-selective palladium(ii)-catalysed addition of aryl boronic acids to vinylaziridines. Org. Biomol. Chem. 2014, 12, 9113. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.-Y.; Tseng, C.-C.; Li, S.-M.; Tsai, S.-E.; Lin, H.-Y.; Wong, F.F. Structural Identification between Phthalazine-1,4-Diones and N-Aminophthalimides via Vilsmeier Reaction: Nitrogen Cyclization and Tautomerization Study. Molecules 2021, 26, 2907. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.N. Kinetic Evidence for the Occurrence of a Stepwise Mechanism in the Hydrazinolysis of Phthalimide. J. Org. Chem. 1995, 60, 4536–4541. [Google Scholar] [CrossRef]
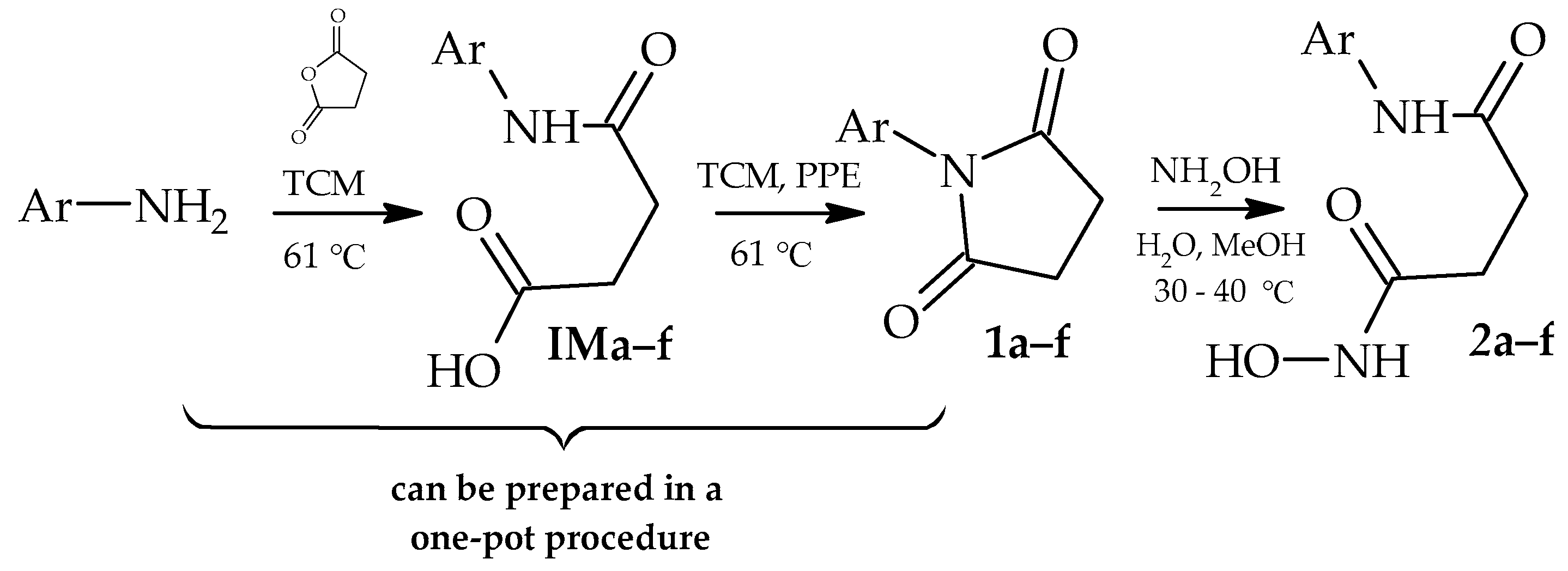

| Ar | Yield of 1, % | Yield of 2, % | ||
|---|---|---|---|---|
| One-Pot Approach | Two-Step Approach | |||
| a | 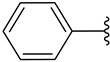 | 15 | 68 | 73 |
| b | 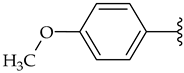 | 35 | 54 | 66 |
| c |  | 52 | 65 | 53 |
| d |  | 31 | 44 | 64 |
| e |  | 42 | 64 | 38 |
| f |  | 33 | 48 | 34 |
| Amine | pKa [36] | |
| Ammonia | 9.25 | |
| Hydroxylamine | 5.94 | |
| a | Aniline | 4.87 |
| b | 4-Methoxyaniline | 5.36 |
| c | 4-Bromoaniline | 3.89 |
| d | 4-Nitroaniline | 1.02 |
| e | 4-Fluoroaniline | 4.65 |
| f | 3-(Trifluoromethyl)aniline | 3.49 |
| Hydrazide | pKa [37] | |
| Acetohydrazide | 3.25 | |
| Benzohydrazide | 3.06 | |
| 4-Methoxybenzohydrazide | 3.26 |
| R | Yield of 1, % | Yield of 2, % | ||
|---|---|---|---|---|
| One-Pot Approach | Two-Step Approach | |||
| g |  | 42 | 45 | 75 |
| h | 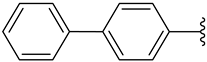 | 39 | 43 | 69 |
| i |  | 68 | 76 | 72 |
| j | 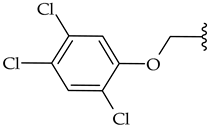 | 42 | 44 | 63 |
| k | 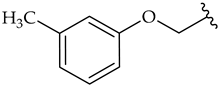 | 71 | 69 | 35 |
| l | 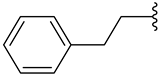 | 46 | 45 | 43 |
| m | 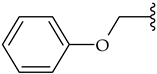 | 77 | 80 | 46 |
| n |  | 53 | 60 | 85 |
| o |  | 59 | 70 | 86 |
| p |  | 81 | 88 | 80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tretyakov, B.A.; Gadomsky, S.Y.; Terentiev, A.A. A Reaction of N-Substituted Succinimides with Hydroxylamine as a Novel Approach to the Synthesis of Hydroxamic Acids. Organics 2023, 4, 186-195. https://doi.org/10.3390/org4020015
Tretyakov BA, Gadomsky SY, Terentiev AA. A Reaction of N-Substituted Succinimides with Hydroxylamine as a Novel Approach to the Synthesis of Hydroxamic Acids. Organics. 2023; 4(2):186-195. https://doi.org/10.3390/org4020015
Chicago/Turabian StyleTretyakov, Bogdan A., Svyatoslav Y. Gadomsky, and Alexei A. Terentiev. 2023. "A Reaction of N-Substituted Succinimides with Hydroxylamine as a Novel Approach to the Synthesis of Hydroxamic Acids" Organics 4, no. 2: 186-195. https://doi.org/10.3390/org4020015
APA StyleTretyakov, B. A., Gadomsky, S. Y., & Terentiev, A. A. (2023). A Reaction of N-Substituted Succinimides with Hydroxylamine as a Novel Approach to the Synthesis of Hydroxamic Acids. Organics, 4(2), 186-195. https://doi.org/10.3390/org4020015