Abstract
The [3+2] cycloaddition (32CA) reaction of an azomethine ylide (AY), derived from isatin and L-proline, with phenyl vinyl sulphone has been studied within Molecular Electron Density Theory (MEDT) at the ωB97X-D/6-311G(d,p) level. ELF topological analysis of AY classifies it as a pseudo(mono)radical species with two monosynaptic basins at the C1 carbon, integrating a total of 0.76 e. While vinyl sulphone has a strong electrophilic character, AY is a supernucleophile, suggesting a high polar character and low activation energy for the reaction. The nucleophilic Parr functions indicate that the pseudoradical C1 carbon is the most nucleophilic center. The 32CA reaction presents an activation Gibbs free energy of 13.1 kcal·mol−1 and is exergonic by −26.8 kcal·mol−1. This reaction presents high endo stereoselectivity and high meta regioselectivity. Analysis of the global electron density transfer (GEDT) at the most favorable meta/endo TS, 0.31 e, accounts for the high polar character of this 32CA reaction, classified by forward electron density flux (FEDF). A Bonding Evolution Theory (BET) study along the most favorable meta/endo reaction path characterizes this 32CA reaction, taking place through a non-concerted two-stage one-step mechanism, as a pseudo(mono)radical-type 32CA reaction, in agreement with the ELF analysis of the AY.
1. Introduction
[3+2] Cycloaddition (32CA) reactions are one of the most efficient synthetic methods for the construction of five-membered heterocyclic compounds, due to their ability to build organic cyclic motifs regio- and/or stereoselectively [1,2,3,4].
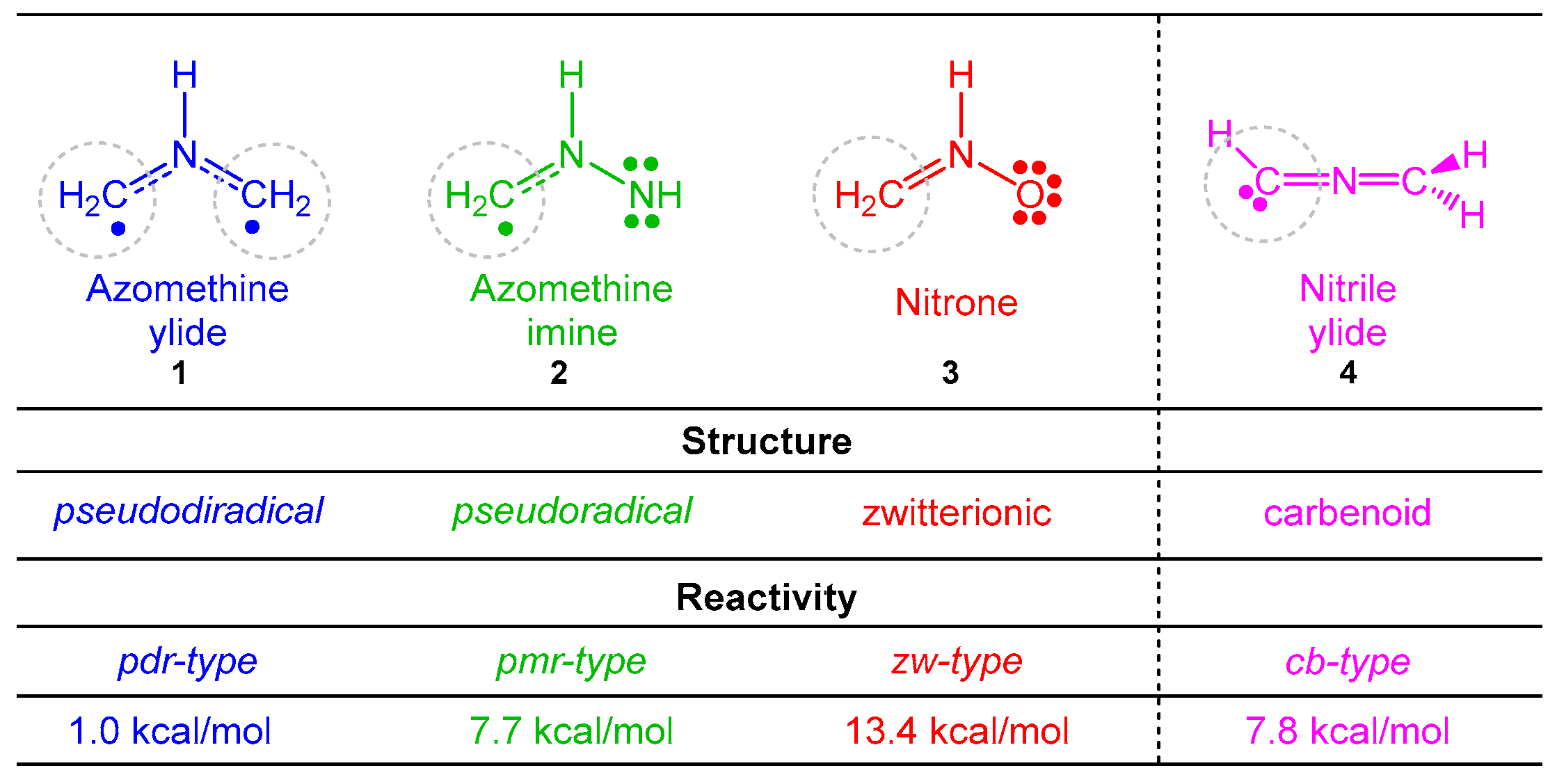
The understanding of the 32CA reactions is a challenge for organic chemists as a consequence of the chameleonic electronic structures of the three-atom-components (TACs) participating in these reactions [5,6,7,8]. Unlike the Diels–Alder reactions in which the diene is characterized by a unique Lewis structure, recent Molecular Electron Density Theory (MEDT) [9] studies of 32CA reactions have categorized four different types of TACs participating in this class of cycloaddition reactions [5,6,7,8], i.e., pseudodiradical, pseudo(mono)radical, carbenoid, and zwitterionic TACs (see Scheme 1). These MEDT studies have allowed establishing a very good correlation between the electronic structure of the simplest TACs and their reactivity towards ethylene 5 [10]. Accordingly, 32CA reactions have been classified into pseudodiradical (pdr)-, pseudo(mono)radical (pmr)-, carbenoid (cb)-, and zwitterionic (zw)-type reactions, respectively (see Scheme 1) [10], in such a manner that while pdr-type 32CA reactions take place very easily [5], zw-type 32CA reactions demand suitable nucleophilic/electrophilic activations of the reagents (see Scheme 1) [7].
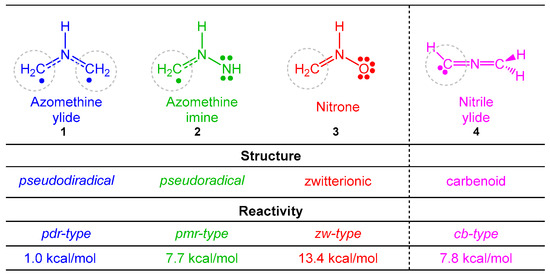
Scheme 1.
Electronic structure of simplest TACs and proposed reactivity types in 32CA reactions. MPWB1K/6-311G(d) gas phase activation energies of the non-polar 32CA reactions between the four simplest TACs 1–4 and ethylene 5, relative to the corresponding molecular complexes, are given in kcal·mol−1.
However, simplest TACs do not participate in experimental 32CA reactions. In general, TACs are very reactive intermediates which are usually generated in situ. The adequate substitution that stabilizes TACs can change their electronic structure and, consequently, their reactivity. Therefore, it is desirable to know how substitution modifies their chemical properties.
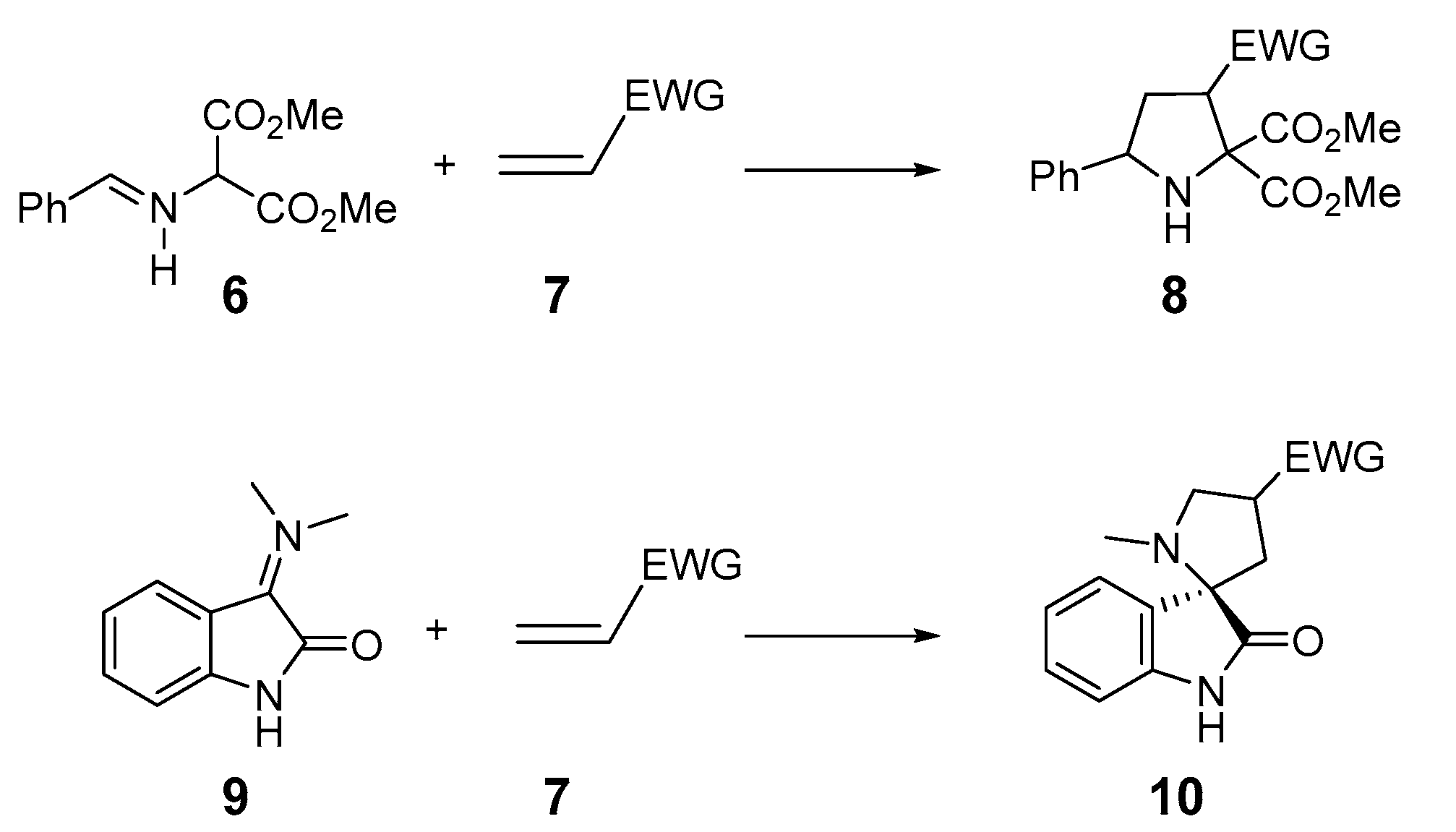
Pyrrolidines 8 are five-membered heterocyclic units with only one nitrogen and great pharmaceutical importance [11,12,13], and are easily obtained by 32CA reaction of an azomethine ylide (AY) 6 with a variety of olefins 7 (see Scheme 2). The use of AY 9, generated from isatin, permits the synthesis of spirooxindoles 10 with significant biological activities [14,15,16,17,18].
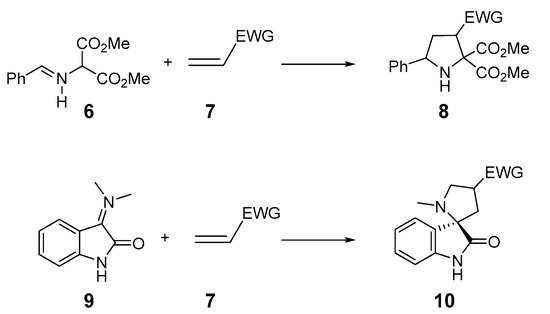
Scheme 2.
Synthesis of pyrrolidines and spirooxindoles by 32CA reactions of AYs.
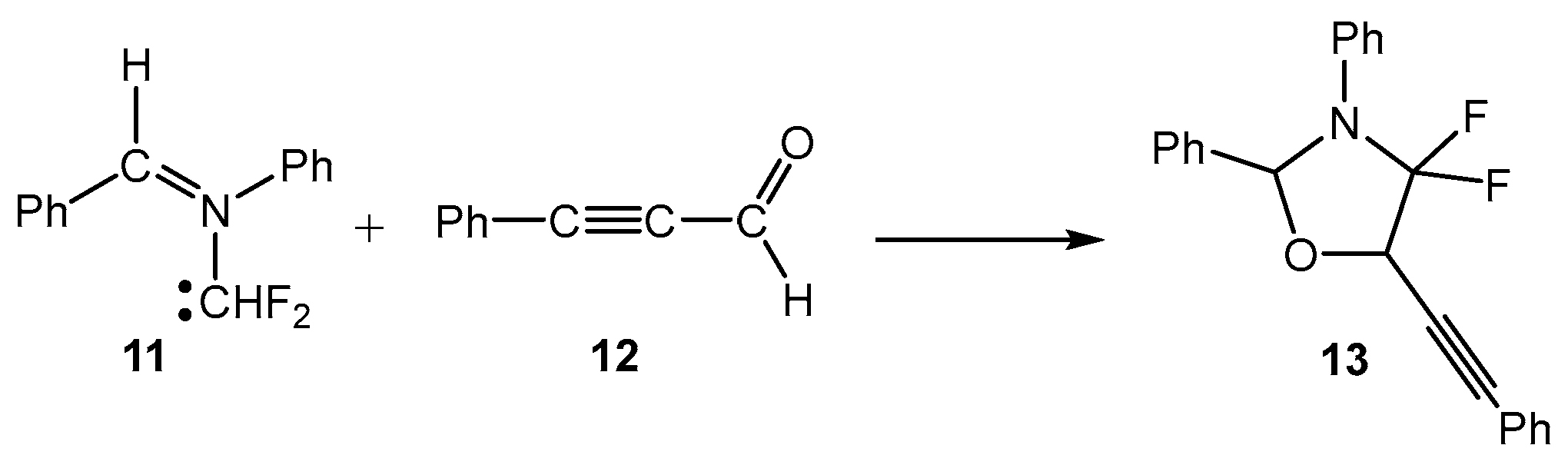
MEDT studies of the 32CA reactions of the simplest AY 1 [5] and the simplest carbonyl ylide CH2-O-CH2 [19] have shown that the presence of a pseudoradical center at each one of the two methylenes of these TACs causes the pdr-type 32CA reactions with non-activated ethylenes to have an unappreciable electronic activation energy (see Scheme 1) [5,19]. However, the presence of an electron-releasing phenyl (Ph) group and electron-withdrawing carboxyl CO2R or nitrile CN groups at the two methylenes of the simplest AY 1 stabilizes its pseudodiradical electronic structure, modifying the experimental reactivity of these substituted AYs to that of a pseudo(mono)radical TAC [20] or even a zwitterionic TAC [21]. Very recently, a MEDT study of the 32CA reactions of fluorated AY 11 with ynals has shown that the presence of two fluorine atoms in one of the two methylenes of this AY changes its structure and reactivity to that of a carbenoid TAC (see Scheme 3). Consequently, depending on the substitution on the AYs, these TACs can display one of the four types of electronic structures categorized in Scheme 1, and hence, experience a different reactivity.
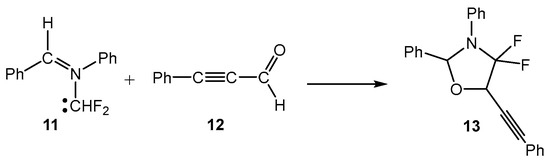
Scheme 3.
Cb-type 32CA reactions of fluorinated AY 11.
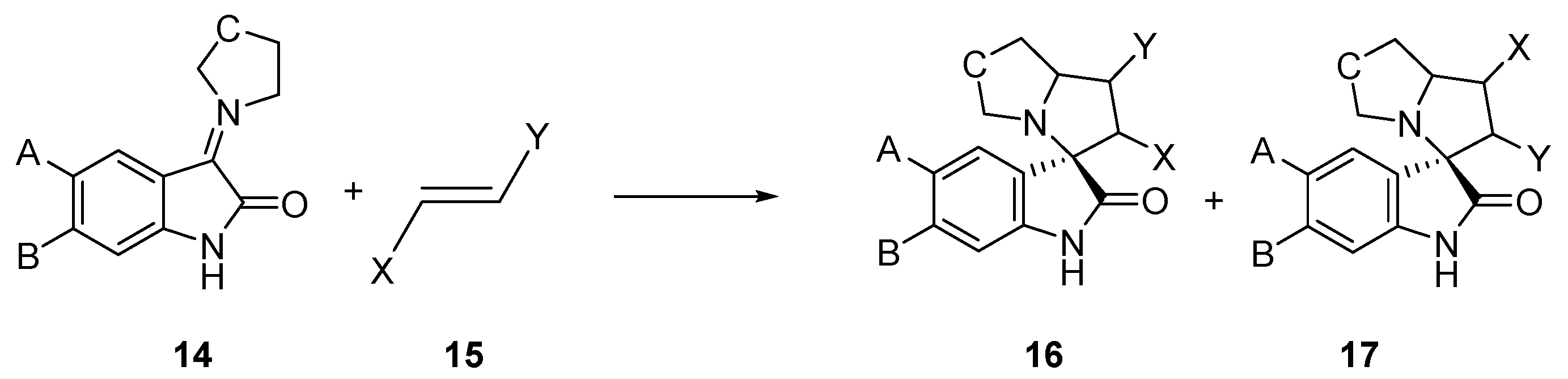
Very recently, Barakat et al. have experimentally studied a series of 32CA reactions of AYs 14, obtained in situ from isatin derivatives and secondary amines, with different ethylene derivatives 15, in the synthesis of spirooxindoles 16 and 17, with high regio- and stereoselectivity (see Scheme 4) [22,23,24]. While these 32CA reactions were endo stereoselective, the regioselective formation of spirooxindoles 16 or 17 was found to be dependent on the substitution on the ethylene derivative 15.
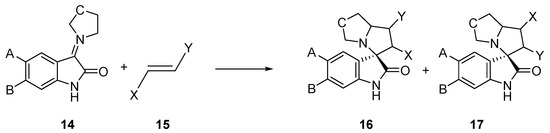
Scheme 4.
32CA reactions of AYs 14 derived from indol-2,3-diones.
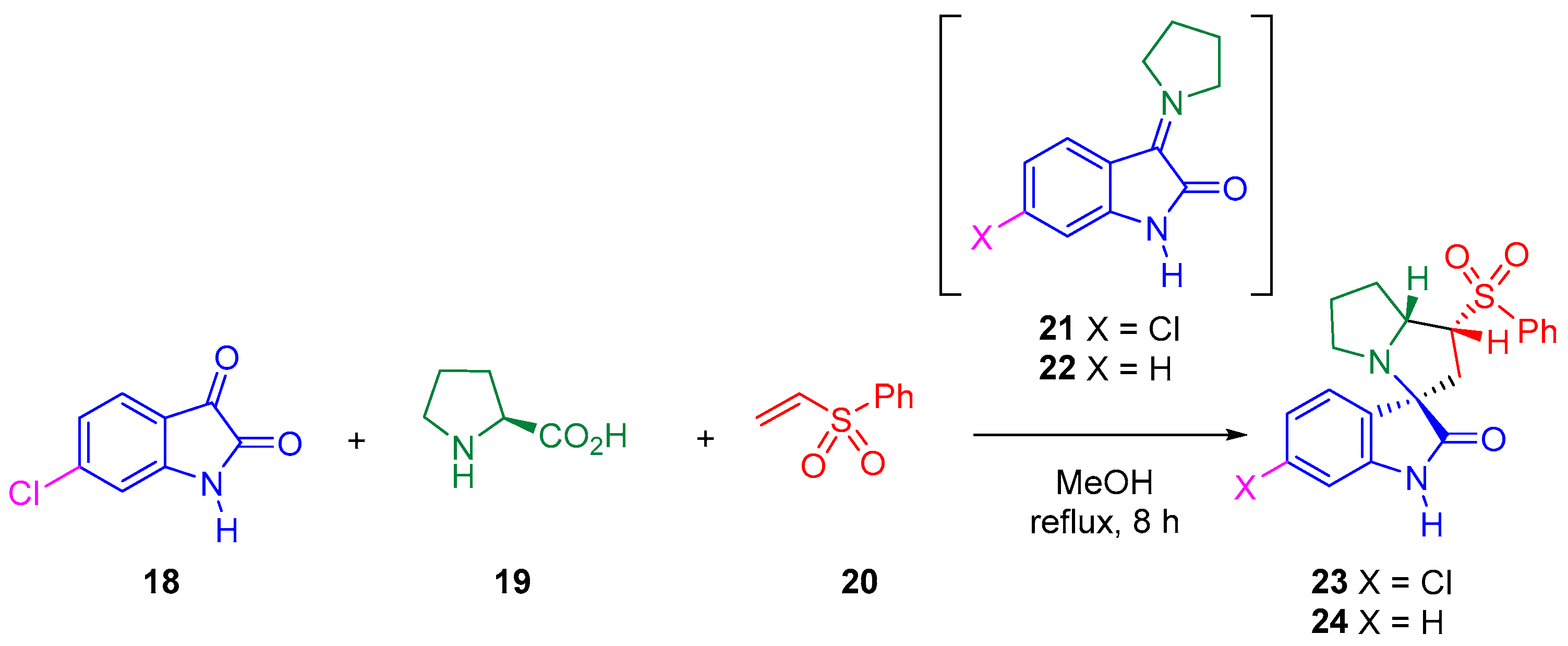
Thus, the 32CA reaction of AY 21, generated in situ from the reaction of 6-chloroisatin 18 and L-proline 19, with phenyl vinyl sulphone 20 yielded the spirooxindole 23 with total meta regio- and endo stereo-selectivities (see Scheme 5) [25].
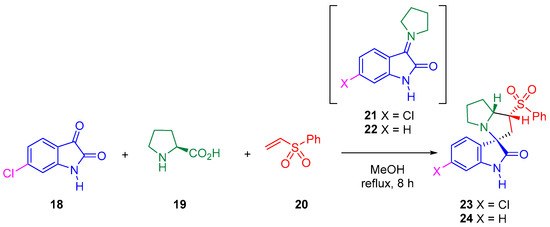
Scheme 5.
32CA reactions of AY 21 with phenyl vinyl sulphone 20 yielding spirooxindole 23 regio- and stereoselectively.
Herein, the 32CA reaction of AY 22 with phenyl vinyl sulphone 20 yielding spirooxindole 24, as a model of the 32CA reaction studied by Barakat et al., is theoretically studied within MEDT in order to understand the electronic structure of AY 21 and its reactivity in this 32CA reaction (see Scheme 5). The origin of the meta regio- and endo stereoselectivity will be analyzed.
2. Materials and Methods
The ωB97X-D [26] functional, together with the standard 6-311G(d,p) [27] basis set, which includes d-type polarization for second row elements and p-type polarization functions for hydrogen atoms, was used in this MEDT study. The TSs were characterized by the presence of only one imaginary frequency. The Berny method was used in optimizations [28,29]. The intrinsic reaction coordinate (IRC) paths [30] were obtained to establish the unique connection given between the TSs and the corresponding minima [31,32]. Solvent effects of methanol were taken into account by full optimization of the gas phase structures at the same computational level using the polarizable continuum model (PCM) [33,34] in the framework of the self-consistent reaction field (SCRF) [35,36,37]. Values of ωB97X-D/6-311G(d,p) enthalpies, entropies, and Gibbs free energies in methanol were calculated with standard statistical thermodynamics at 337. 8 K and 1 atm [27], by PCM frequency calculations at the solvent optimized structures.
The global electron density transfer (GEDT) [38] values were computed by using the equation GEDT(f) = Σqf, where q are the natural charges [39,40] of the atoms belonging to one of the two frameworks (f) at the TS geometries. Global and local Conceptual Density Functional Theory (CDFT) indices [41,42] were calculated by using the equations given in reference [42].
The Gaussian 16 suite of programs was used to perform the calculations [43]. Electron Localization Function (ELF) [44] analyses of the ωB97X-D/6-311G(d,p) monodeterminantal wavefunctions were done by using the TopMod [45] package with a cubical grid of step size of 0.1 Bohr. Molecular geometries and ELF basin attractors were visualized by using the GaussView program [46]. The topological analysis of the non-covalent interactions (NCI) [47] was performed with the NCIplot program [48].
3. Results and Discussion
3.1. ELF Topological Analysis at the Ground State of AY 22 and Phenyl Vinyl Sulphone 20
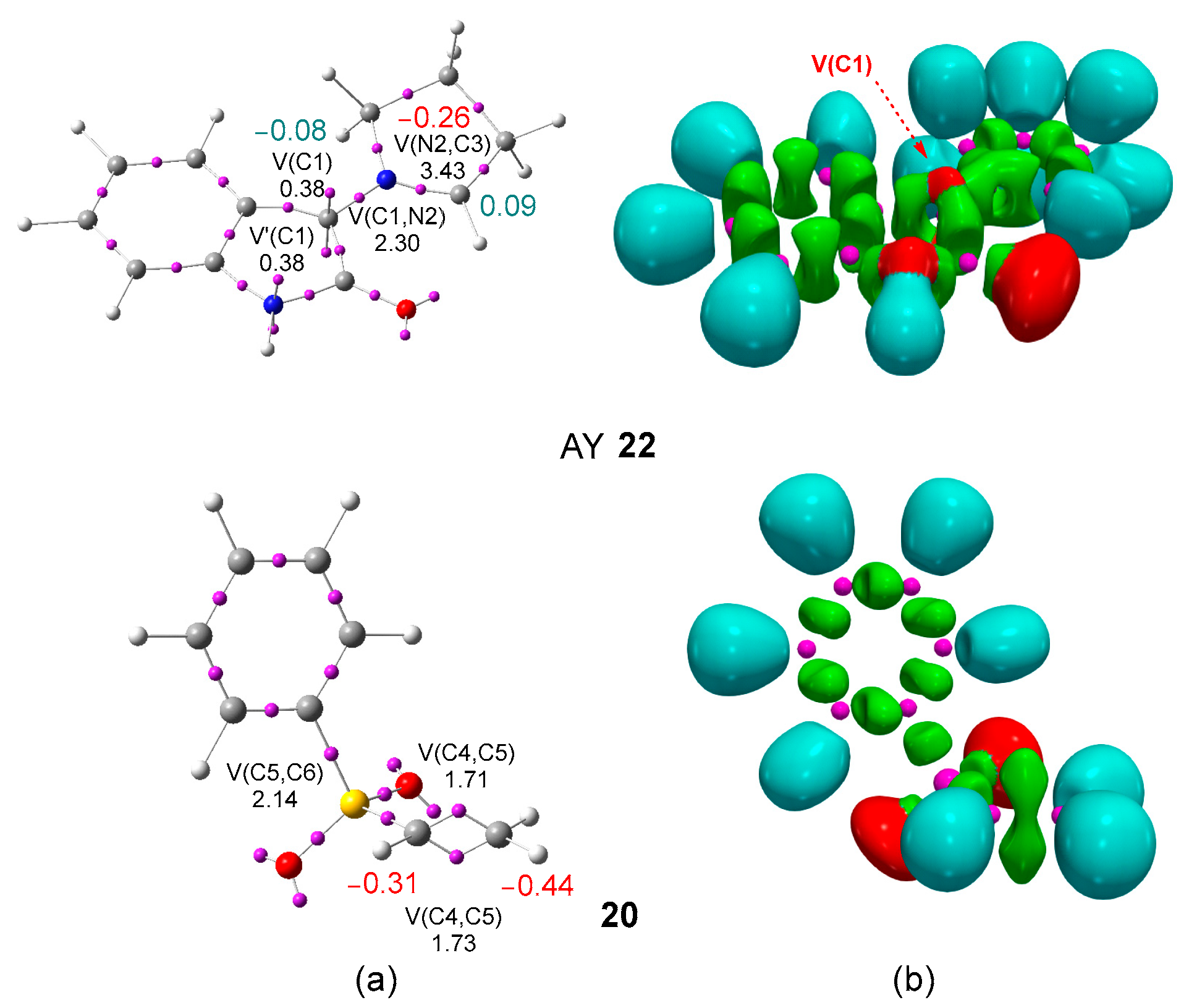
The topological analysis of the ELF [44] allows a quantitative and qualitative description of the electronic structure of molecules [49]. Given the structure–reactivity relationship in TACs [10], an ELF topological analysis of AY 22 was first performed in order to characterize its electronic structure and gain some insight about its reactivity. The most significant ELF basin attractor positions and ELF basins of AY 22 and phenyl vinyl sulphone 20 are given in Figure 1, while those for the experimental AY 21 are given in Figure S1 in the Supporting Information.
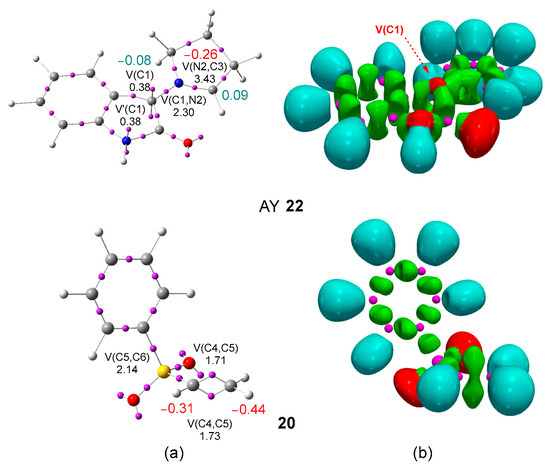
Figure 1.
ωB97X-D/6-311G(d,p) (a) ELF basin attractor positions together with the most relevant valence basin populations, and (b) ELF localization domains represented at an isosurface value of ELF = 0.70, of AY 22 and phenyl vinyl sulphone 20. Valence basin populations and natural atomic charges are given in average number of electrons, e. Negative charges are colored in red and negligible charges in green.
The ELF of AY 22 shows the presence of two monosynaptic basins, V(C1) and V′(C1), integrating a total of 0.76 e, one V(C1,N2) disynaptic basin, integrating 2.30 e, and one V(N2,C3) disynaptic basin, integrating 3.43 e. While the V(C1,N2) disynaptic basin is associated to a C1–N2 single bond, the V(N2,C3) disynaptic basin is associated to an underpopulated N2–C3 double bond. The presence of the two monosynaptic basins at the C1 carbon, which is associated with a pseudoradical carbon [19], allows the classification of this AY as a pseudo(mono)radical TAC. A comparative analysis of the ELF of the experimental AY 21 given in Figure S1 in Supporting Information and that of AY 22 indicates that the substitution of the chlorine atom by the hydrogen one in the model does not produce any electronic change in the AY core.
On the other hand, ELF of phenyl vinyl sulphone 20 shows the electronic structures of ethylene and benzene separately. The vinyl framework of vinyl sulphone 20 is characterized by the presence of two disynaptic basins, V(C4,C5) and V′(C4,C5), integrating a total of 3.44 e, and a V(C5,S6) disynaptic basin, integrating 2.14 e. The populations of these disynaptic basins indicate some delocalization of the electron density of the C4–C5 double bond at the sulfur S6 atom.
The analysis of the natural atomic charges [39,40] shows that the two carbons of AY 22 are negligibly charged by less than ±0.1 e. Interestingly, the natural charges indicate that the C4 and C5 carbons of the ethylene framework of vinyl sulphone 20 are negatively charged by −0.44 and −0.31 e, respectively, the methylene CH2 carbon being the more negatively charged. Thus, according to charges, AY 22 would have no tendency for a supposed nucleophilic attack of the C4 carbon of vinyl sulphone 20.
3.2. Conceptual DFT Analysis at the Ground State of the Reagents
The reactivity indices defined within Conceptual DFT (CDFT) [41,42] have shown to be powerful tools to understand the reactivity in polar reactions. The global reactivity indices, namely, the electronic chemical potential μ, chemical hardness η, electrophilicity ω and nucleophilicity N, for AYs 21 and 22, and phenyl vinyl sulphone 20 are gathered in Table 1.

Table 1.
B3LYP/6-31G(d) electronic chemical potential μ, chemical hardness η, electrophilicity ω, and nucleophilicity N indices, in eV, of AYs 21 and 22 and phenyl vinyl sulphone 20.
The electronic chemical potentials [50] of AYs 21 and 22, μ = −2.97 and −2.76 eV, respectively, are higher than that of vinyl sulphone 20, μ = −4.39 eV, indicating that along a polar 32CA reaction the global electron density transfer (GEDT) [38] will take place from AYs 21 and 22 to the vinyl sulphone 20, the reaction being classified as of forward electron density flux (FEDF) [51].
AYs 21 and 22 present an electrophilicity ω index [52] of 1.32 and 1.14 eV, respectively, classified as strong electrophiles within the electrophilicity scale [42], and a nucleophilicity N index [53] of 4.48 and 4.68 eV, respectively, classified as strong nucleophiles within the nucleophilicity scale [42]. The strong nucleophilic character of AY 21 and 22, higher than 4.0 eV, allows their classification as supernucleophiles [54]. Substitution of the chlorine atom in the experimental AY 21 by a hydrogen atom in the model AY 22 slightly decreases the electrophilicity and slightly increases the nucleophilicity of the latter.
Vinyl sulphone 20 presents an electrophilicity ω index of 1.61 eV, classified as a strong electrophile within the electrophilicity scale. On the other hand, it presents a nucleophilicity N index of 1.75 eV, classified as marginal nucleophile within the nucleophilicity scale.
The supernucleophilic character of AY 21 and 22 together with the electrophilic character of vinyl sulphone 20 indicate that the corresponding 32CA reactions will have a high polar character, classified as FEDF [51].
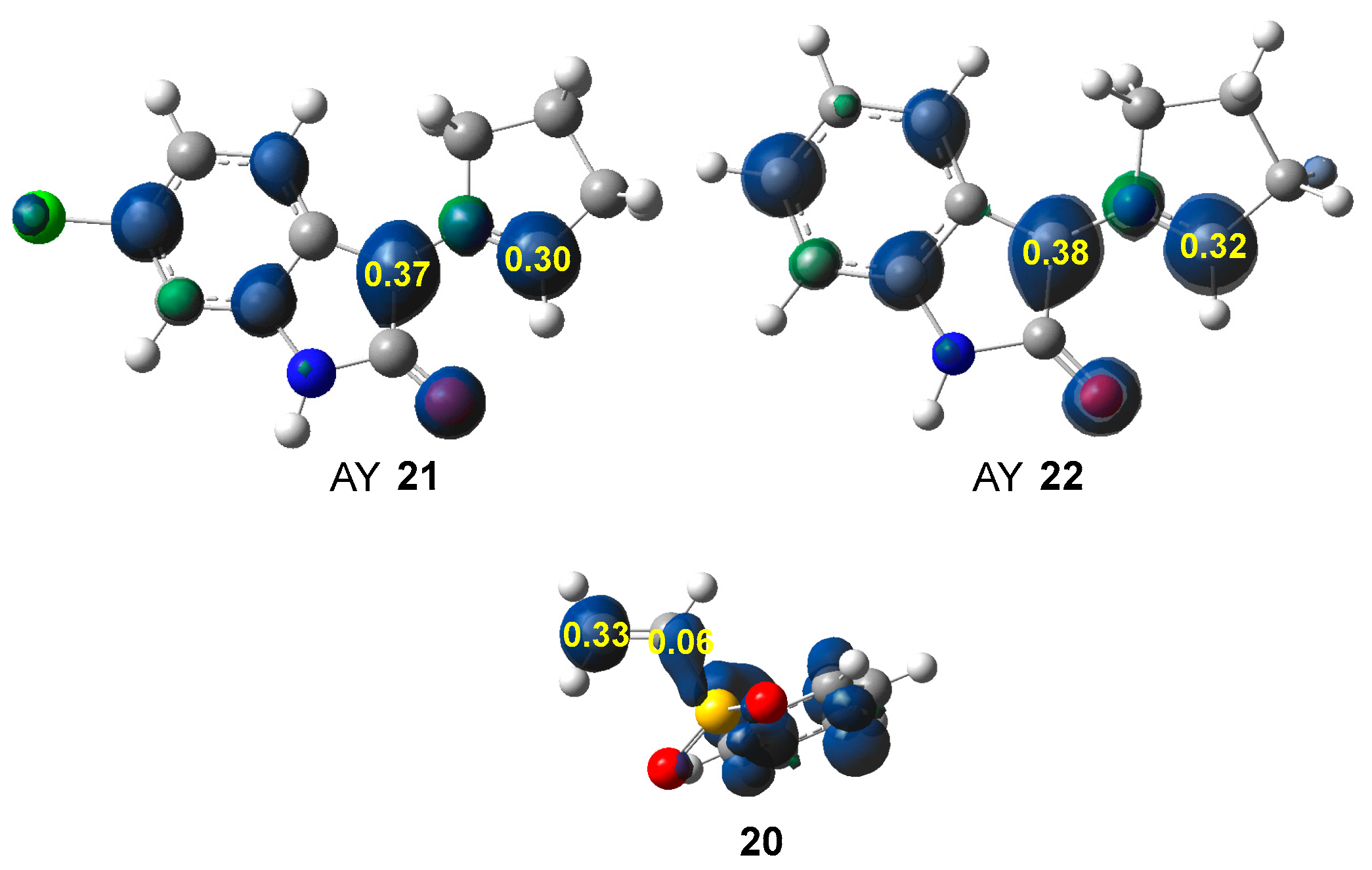
Along a polar reaction involving non-symmetric species, the most favorable reaction path involves the two-center interaction between the most electrophilic and the most nucleophilic centers [55]. Many studies have shown that the analysis of the electrophilic and nucleophilic Parr functions [56], resulting from the excess of spin electron density gathered via the GEDT [38] is one of the most accurate tools for the analysis of the local reactivity in polar and ionic processes. Hence, according to the characteristics of the reagents, the nucleophilic Parr functions of AYs 21 and 22, and the electrophilic Parr functions of vinyl sulphone 20 were analyzed (see Figure 2).
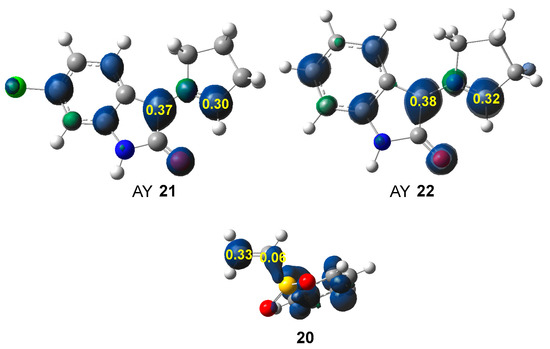
Figure 2.
B3LYP/6-31G(d) nucleophilic Parr functions of AYs 21 and 22, and electrophilic Parr functions of vinyl sulphone 20.
The two C1 and C3 carbons of AYs 21 and 21 are nucleophilically activated by = 0.37 and 0.38 (C1), and 0.30 and 0.32 (C3), respectively, the exocyclic C1 carbon being slightly more activated. Note that the nitrogen atom is deactivated. Both AYs show a similar nucleophilic activation. On the other hand, the conjugated C4 carbon of vinyl sulphone 20 is the most electrophilically activated center, = 0.33. Consequently, the most favorable two-center interaction along the corresponding polar 32CA reaction will take place between the C1 carbon of AYs 21 and 22 and the conjugated C4 carbon of vinyl sulphone 20. Note that the most electrophilic C4 carbon of vinyl sulphone 20 is negatively charged by −0.44 e (see Figure 1). This finding supports Domingo’s proposal made in 2012 that the local electrophilic/nucleophilic behaviors of organic molecules are not simply caused by charges [57]. The most electrophilic center of a molecule is the one that accepts the highest amount of electron density resulting from the nucleophilic/electrophilic interactions taking place along the approach of the two reagents [57]. Interestingly, in some cases, such as in vinyl sulphone 20, the most electrophilic C4 carbon is negatively charged [57].
3.3. Study of the Reaction Paths Associated with the 32CA Reaction of AY 22 with Phenyl Vinyl Sulphone 20
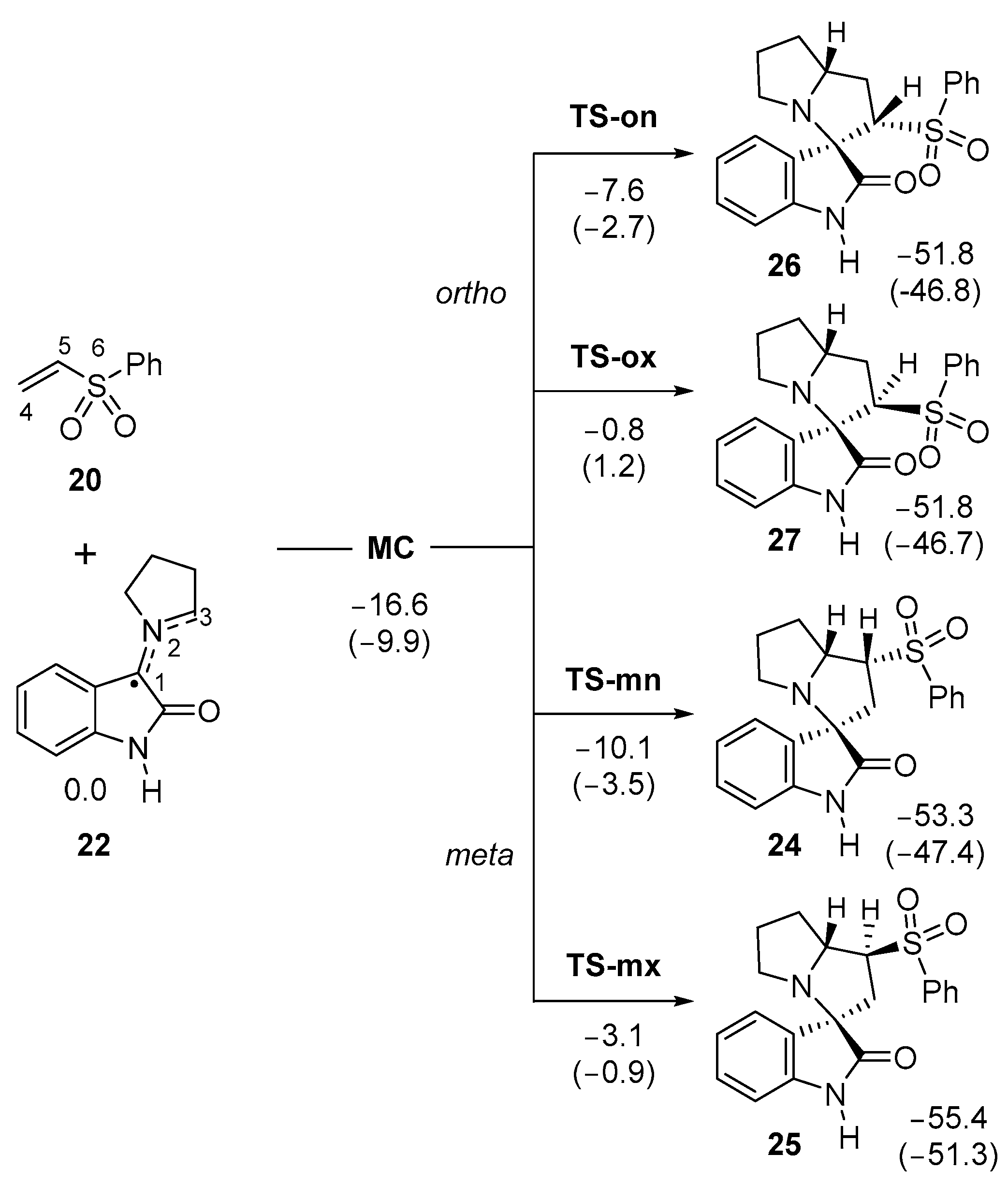
Due to the non-symmetry of both reagents, two pairs of endo and exo stereoisomeric, as well as ortho and meta regioisomeric, reaction paths are feasible. The words ortho and meta account for the relative position of the sulphone group with respect to the spiro C1 carbon at spirooxindoles 24–27. The four competitive reaction paths were studied (see Scheme 6). Analysis of the stationary points found in these reaction paths indicates that this 32CA reaction takes place though a one-step mechanism. The ωB97X-D/6-311G(d,p) relative energies in gas phase and in methanol are given in Scheme 6.
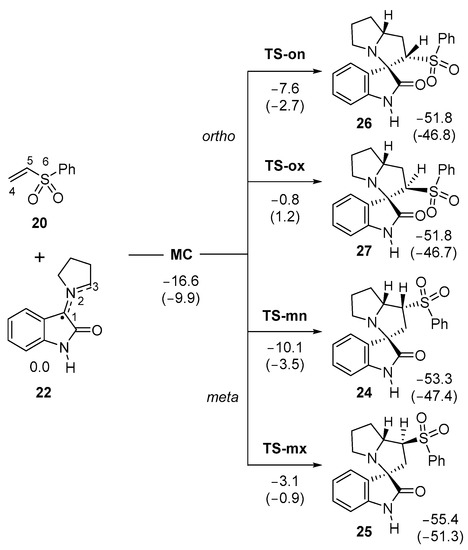
Scheme 6.
32CA reaction of AY 22 with phenyl vinyl sulphone 20. Relative energies, with respect to the separated reagents, are given in kcal·mol−1. ωB97X-D/6-311G(d,p) relative energies in methanol are given in parentheses.
A series of molecular complexes (MCs) in which the two reagents are already linked by weak intermolecular interactions were found. Only the most stable of them, MC, was selected as the energy reference. The distance between the two frameworks at this MC is ca. 3.26 Å (the geometry of MC is given in Figure S1 in the Supporting Information); in the gas phase, MC is found 16.6 kcal·mol−1 below the separated reagents (see Scheme 2). The most favorable TS-mn is found 10.1 kcal·mol−1 below the separated reagents, the corresponding reaction path being strongly exothermic by 53.3 kcal·mol−1. Some appealing conclusions can be obtained from the relative energies given in Scheme 6: (i) the most favorable TS-mn is found energetically below the separated reagents, but if the formation of MC is considered, the activation energy becomes positive by 6.5 kcal·mol−1; (ii) in gas phase, this 32CA reaction is highly meta regioselective as TS-on is found 2.5 kcal·mol−1 above TS-mn; (iii) this 32CA reaction is totally endo stereoselective as TS-mx is found 7.0 kcal·mol−1 above TS-mn; and (iv) the high exothermic character of the formation of spirooxindole 24, −53.3 kcal·mol−1, makes this 32CA reaction irreversible. Consequently, spirooxindole 24 is formed by kinetic control.
Inclusion of the solvent effects of methanol decreases the relative energies of the stationary points involved in the 32CA reaction of AY 22 with vinyl sulphone 20 by between 2.2 and 6.7 kcal mol−1 as a consequence of a better solvation of the reagents than the other species (see Scheme 6) [58]. In methanol, the regioselectivity strongly decreases to 0.8 kcal·mol−1, as a consequence of a stronger solvation of TS-on than TS-mn.
The thermodynamic data of the 32CA reaction of AY 22 with vinyl sulphone 20 were subsequently analyzed. The relative enthalpies, entropies, and Gibbs free energies, computed at 65 °C, are given in Table 2. Addition of the thermal corrections to the electronic energies in methanol increases the relative enthalpies by between 1.6 and 4.4 kcal·mol−1. Those of the TSs are only increased by less than 1.6 kcal·mol−1. Inclusion of entropies to enthalpies increases the relative Gibbs free energies by between 11.1 and 17.3 kcal·mol−1 due to the unfavorable activation entropies associated with this bimolecular reaction, which are found in the range −32.8 and −51.2 cal·mol−1·K−1. The activation Gibbs free energy associated with this 32CA reaction via TS-mn rises to 13.1 kcal·mol−1, while formation of 24 remains exergonic by −26.8 kcal·mol−1. Consequently, spirooxindole 24 is formed by kinetic control.

Table 2.
ωB97X-D/6-311G(d,p) relative enthalpies (∆H, kcal·mol−1), entropies (∆S, cal·mol−1·K−1) and Gibbs free energies (∆G, kcal·mol−1) of the stationary points, computed at 65 °C in methanol, involved in the 32CA reaction of AY 22 with phenyl vinyl sulphone 20.
Considering the activation Gibbs free energies of the four TSs and the Eyring–Polanyi equation [59], a reaction mixture of 87.9 (24), 3.4 (25), 8.2 (26), and 0.1 (27) is expected, in reasonable agreement with the experimental results in which only spirooxindole 24 was isolated [25].
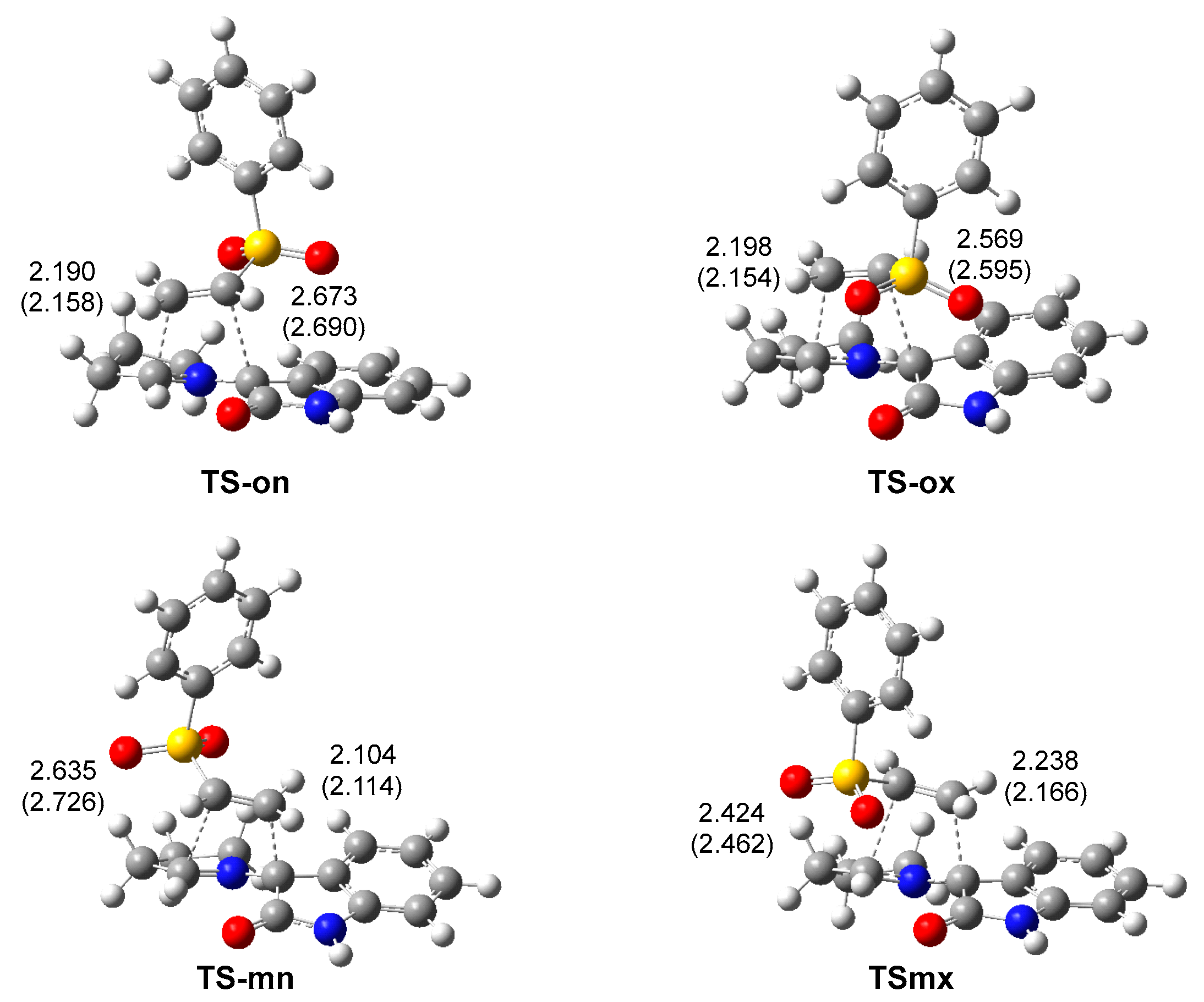
The geometries of the four TSs are given in Figure 3. The C–C distances between the four interacting carbons at the four TSs indicates that they correspond with high asynchronous C–C single bond formation processes, in which the shorter C–C distance corresponds to that involving the most electrophilic β-conjugated carbon of the vinyl sulphone 20. At the most favorable TS-mn, the C–C distances between the two pairs of interacting carbons, 2.104 and 2.635 Å, indicate that this TS is associated with a highly asynchronous C–C single bond formation process, in which the shorter C–C distance also involves the pseudoradical C1 carbon of AY 22. Considering that C–C single bond formation takes place in the short range of 2.0–1.9 Å [38], the C–C distances at the four TSs indicate that they correspond to early processes in which the formation of the new C–C single bond has not yet begun (see later). The inclusion of the solvent effects of methanol does not substantially modify the optimized geometries (see Figure 3); in methanol, the TSs are only slightly more asynchronous.
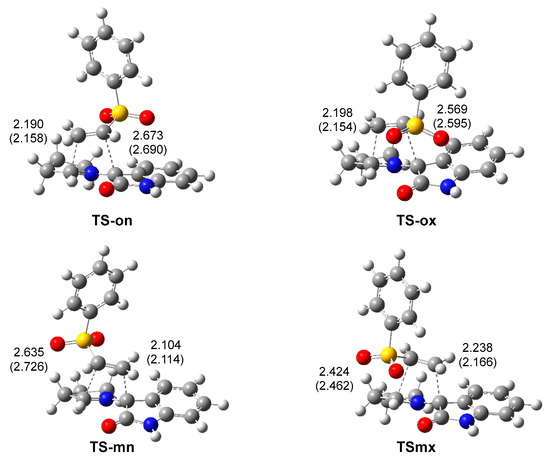
Figure 3.
ωB97X-D/6-311G(d,p) geometries of the TSs involved in the 32CA reaction of AY 22 with phenyl vinyl sulphone 20. Distances are given in angstroms, Å. Distances in methanol are given in parentheses.
Finally, the analysis of GEDT [38] at the TSs permits the assessment of the polar character of these 32CA reactions. GEDT values lower than 0.05 e correspond with non-polar processes, while values higher than 0.20 e correspond with polar processes. The GEDT value at the TSs are 0.22 e (TS-on), 0.20 e (TS-ox), 0.31 e (TS-mn), and 0.24 e (TS-mx). The high value found at the most favorable TS-mn, which is a consequence of the supernucleophilic character of AY 22 and the strong nucleophilic character of phenyl vinyl sulphone 20, indicates that this 32CA reaction has a high polar character and accounts for its low activation energy, 6.5 kcal·mol−1. The flux of the electron density, which flows from AY 22 to vinyl sulphone 20, classifies this 32CA reaction as FEDF [51], in clear agreement with the analysis of the CDFT indices.
3.4. BET Analysis along the Most Favorable Meta/Endo Reaction Path. Characterization of the Pmr-Type Mechanism
In order to characterize the C–C bond formation along the 32CA reaction between AY 22 with vinyl sulphone 20, and thus to characterize the type of 32CA reaction, a Bonding Evolution Theory (BET) [60] study along the most favorable meta/endo reaction path was performed. BET allows for a full description of the bonding changes along a reaction path by analyzing the changes in the topology of the ELF at all the structures of the corresponding IRC [30] path and selecting those chemically meaningful. As ELF valence basins and their populations can be related to the Lewis bonding model [49], a molecular mechanism can be elegantly represented by rigorous Lewis-like structures based on the quantum chemical topology framework. Complete BET data for the meta/endo reaction path of 32CA reaction between AY 22 with vinyl sulphone 20 are given in Table S3, while the representation of the molecular mechanism of this pmr-type 32CA reaction by Lewis-like structures based on the topological analysis of the ELF along the reaction path is given in Scheme S1 in Supporting Information. ELF basin attractor positions are represented in Figure 4.
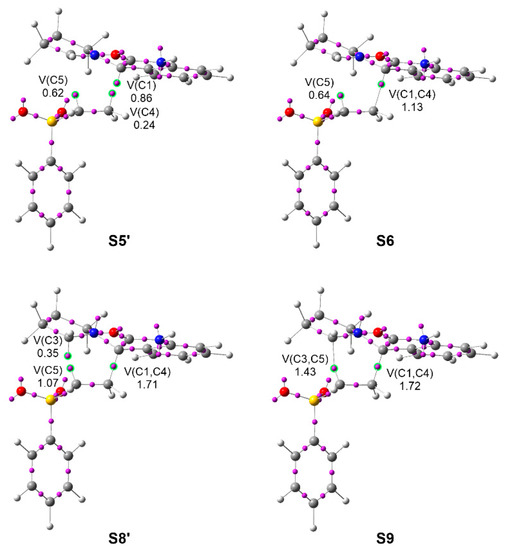
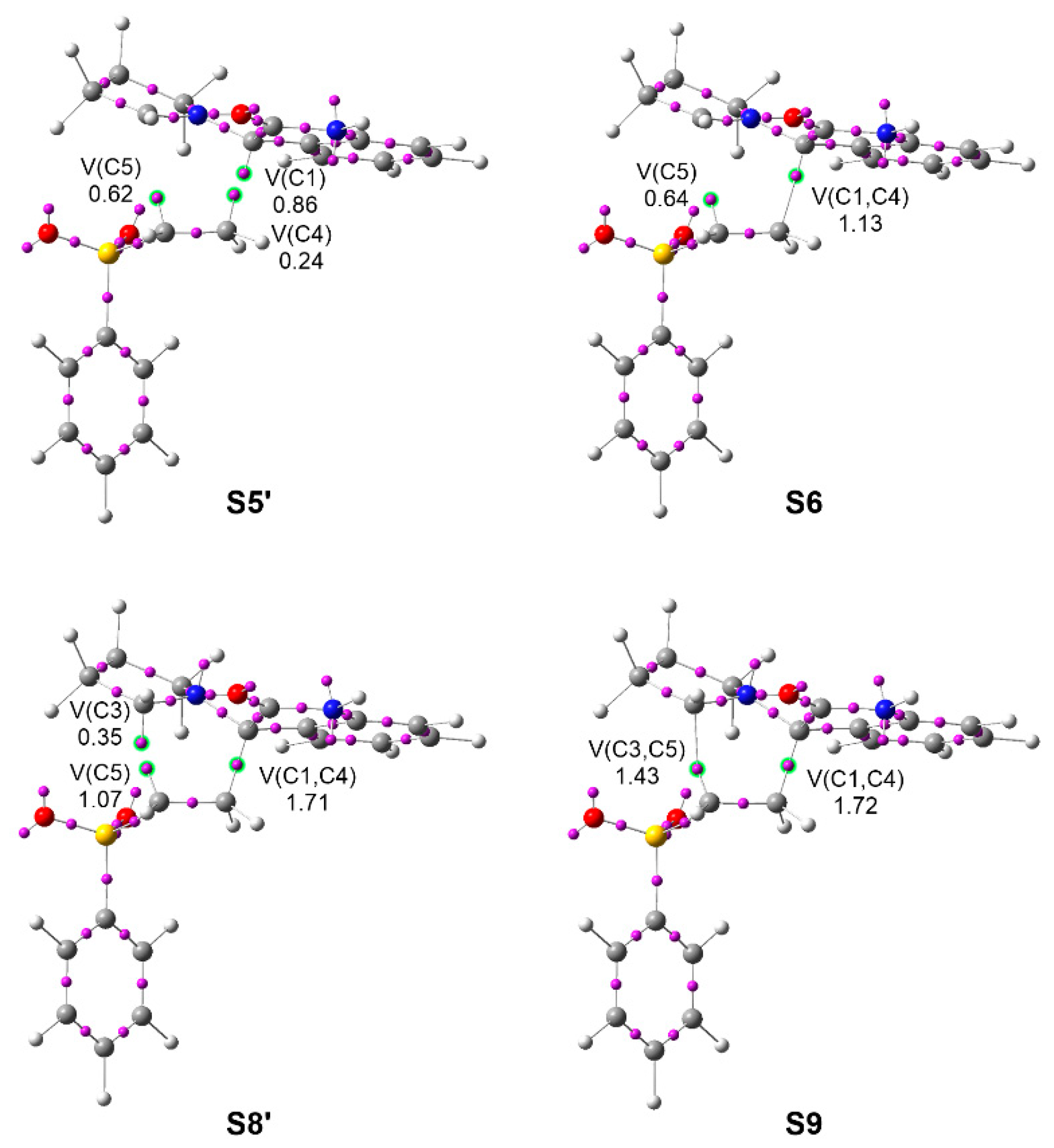
Figure 4.
ELF attractor positions and populations of the ELF valence basins involved in the C1–C4 and C3–C5 bond formation at S6 and S9, respectively, and the structures immediately before, along the meta/endo path of the pmr-type 32CA reaction between AY 22 with vinyl sulphone 20. Populations are given in average number of electrons, e.
The most relevant mechanistic aspects obtained from the BET analysis can be summarized as follows: (i) the pseudoradical C1 carbon present at AY 22, which characterizes this TAC as pseudo(mono)radical (see Figure 4), is initially delocalized into the indolinone C1–CO region due to the presence of vinyl sulphone 20, reappearing at a very early stage of the reaction with an unappreciable energy cost of only 0.4 kcal·mol−1; (ii) the other three pseudoradical carbons participating in the formation of the two new C–C single bonds [38] are created by the depopulation of the double bond regions along the reaction path; (iii) given the insignificant energy cost demanded for the reappearance of the delocalized pseudoradical C1 carbon, the low activation energy associated with TS-mn, 5.8 kcal·mol−1 from the first structure of the IRC, can mainly be associated with the depopulation of the C4–C5 double bond of vinyl sulphone 20; (v) formation of the C1–C4 single bond at the S6 structure, and that of the C3–C5 single bond at the S9 structure, takes place at C–C distances of 2.02 and 2.16 Å, with initial populations of 1.13 and 1.43 e, respectively, through the C-to-C coupling of the two C1 and C4, and C3 and C5 pairs of pseudoradical centers present at the structures S5′ and S8′ (see Figure 4) [38]; (vi) while the pseudoradical C1 carbon present at AY 22 contributes 78% to the formation of the first C1–C4 single bond, the ethylene pseudoradical C5 carbon contributes 75% to the formation of the second C3–C5 single bond. Consequently, formation of both single bonds can be considered to take place mainly by donation of the electron density of one of the two pseudoradical centers to the other one; (vii) the participation of the pseudoradical C1 carbon present at the pseudo(mono)radical AY 22 in the formation of the first C3–C5 single bond makes it possible to characterize this 32CA reaction as a pmr-type reaction; and finally, (viii) formation of the second C3–C5 bond takes places when the first C1–C4 bond has reached 91% of its population at spirooxindole 24, and with a synchronicity value of 0.12. Consequently, the pmr-type 32CA reaction between AY 22 with vinyl sulphone 20 takes place through a non-concerted two-stage one-step mechanism [61].
3.5. Analysis of the Origin of the Meta Regio- and Endo Stereo-Selectivities
Both analysis of the TS geometry and BET study of the meta/endo reaction path indicate that the highly asynchronous TS-mn is associated with the most favorable two-center interaction taking place between the pseudoradical C1 carbon of AY 22, which corresponds to the most nucleophilic center of this TAC, and the β-conjugated C4 carbon of vinyl sulphone 20, which corresponds to the most electrophilic center of this ethylene derivative (see the values of the Parr functions in Figure 2). These favorable electronic interactions account for the fact that the ortho TS-on will be located 2.5 kcal·mol−1 above TS-mn, and consequently, these favorable electronic interactions are responsible for the meta regioselectivity found in this pmr-type 32CA reaction [10]. Note that at the ortho TSs, the pseudoradical C1 carbon present at AY 22 does not participate in the initial C3–C4 single bond formation.
Endo stereoselectivity in cycloaddition reactions has been explained within the outdated Frontier Molecular Orbital (FMO) theory [62] by “secondary orbital interactions” (SOI) present at the endo TSs, a concept introduced in 1983 by Gleiter and Bohm to explain the regio- and stereoselectivity in Diels–Alder reactions [63]. Today, SOI continues being used to explain endo stereoselectivity in cycloaddition reactions [64]. However, molecular orbitals, which are only mathematical artefacts without any physical reality used in the approximation to the molecular wave function, cannot control anything [9]. In 2000, Salvatella et al. proposed that a combination of well-known mechanisms, such as solvent effects, steric interactions, hydrogen bonds (HBs), electrostatic forces, and others, can be invoked instead of SOI in the endo/exo selectivity of Diels–Alder reactions [65].
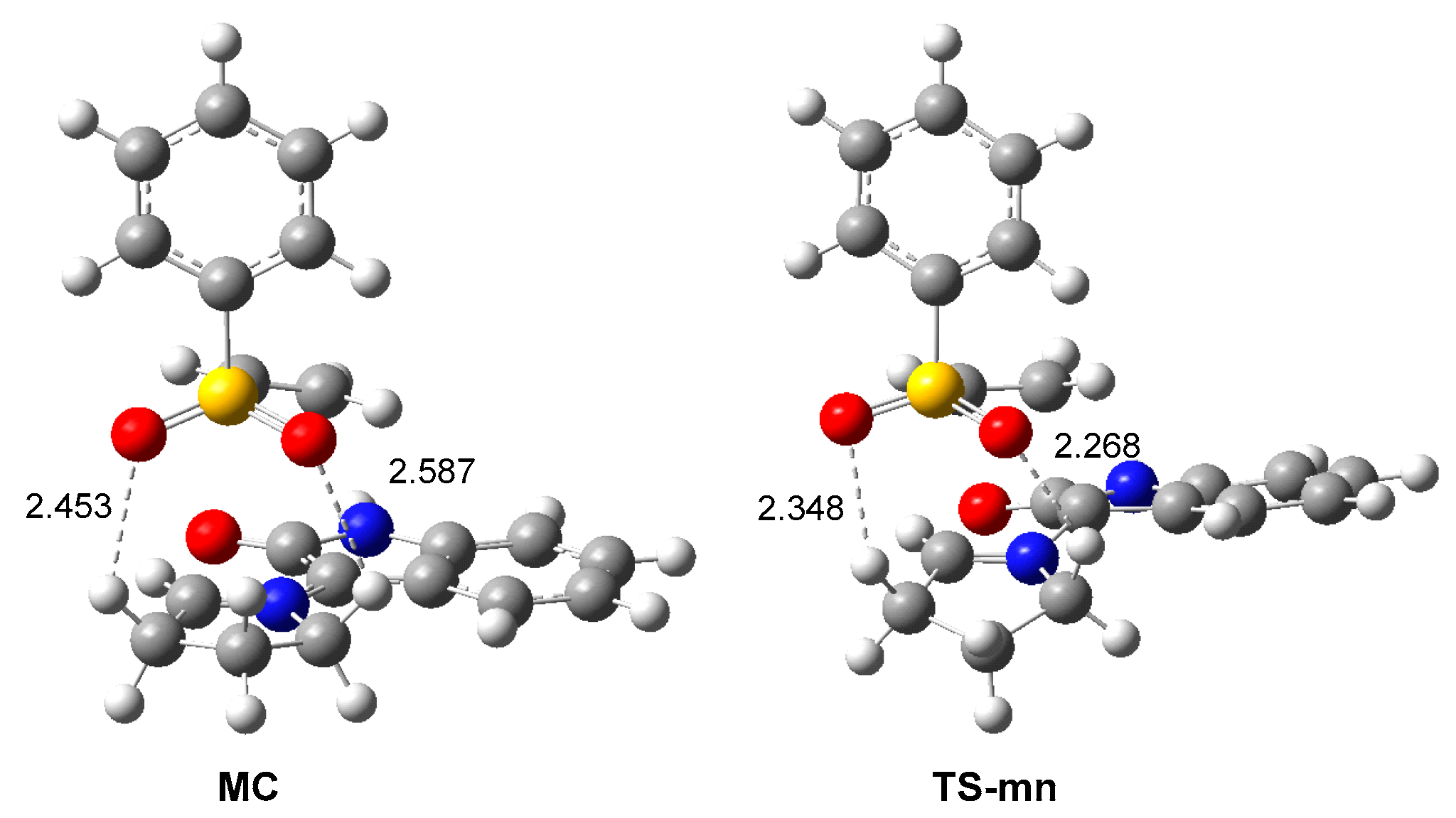
Analysis of the geometry of the most favorable TS-mn shows that the two oxygen atoms of the sulfonyl group of the vinyl sulphone 20 are located at 2.27 and 2.35 Å from two methylene hydrogens of the proline residue (see Figure 5). These short distances suggest the presence of two HBs at endo TS-mn, which are not at the exo TS-mx. These HBs, which are already present at the most favorable MC, can be responsible for the high stabilization in gas phase of MC with respect to the separated reagents; ca. 16 kcal·mol−1 (see Scheme 5).
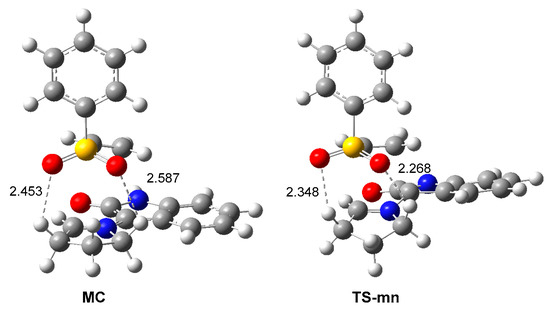
Figure 5.
ωB97X-D/6-311G(d,p) geometries of MC and TS-mn showing the distances of the two O–H HBs. Distances are given in angstroms, Å.
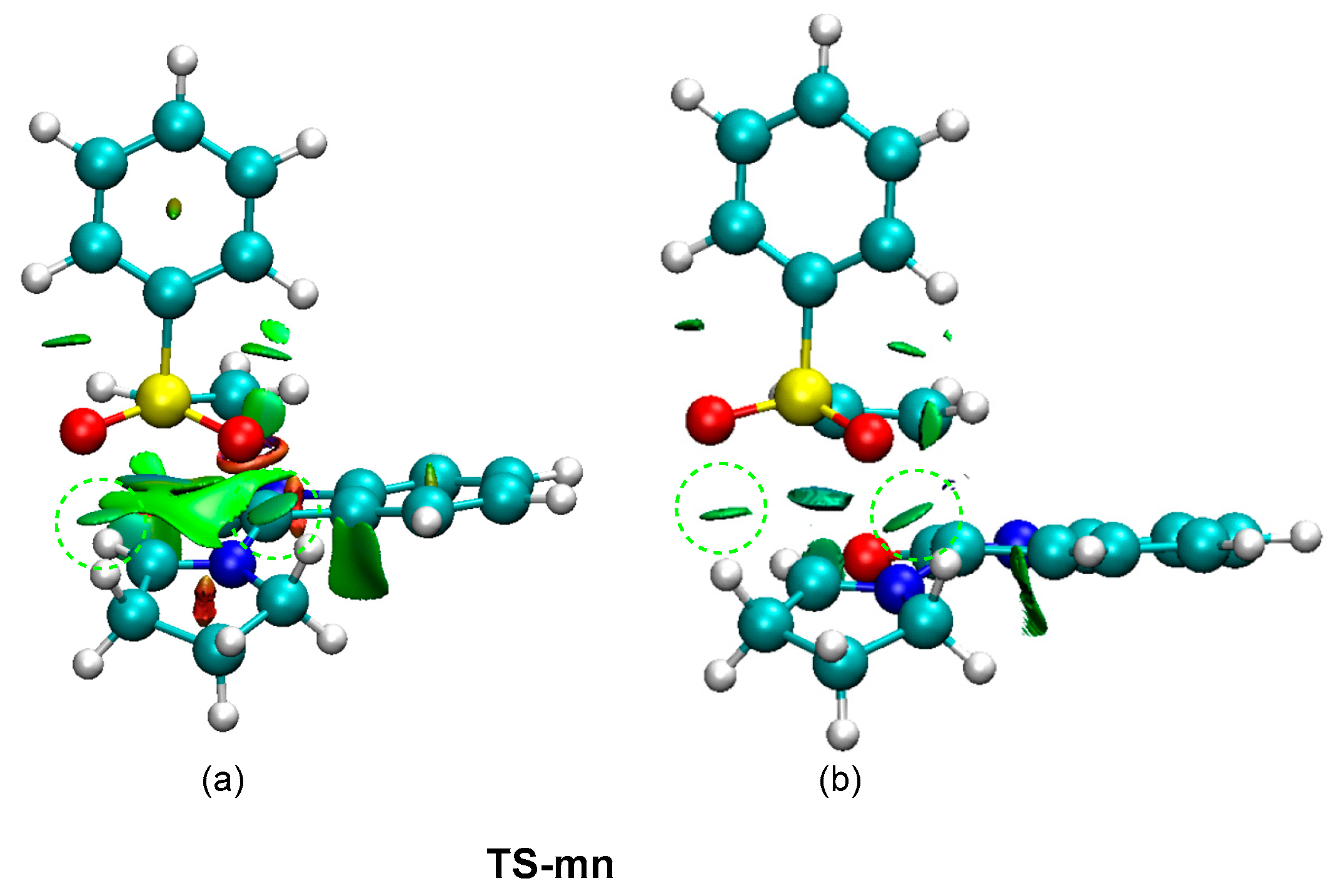
In order to characterize the presence of the two HBs at the most favorable TS-mn, a topological analysis of the non-covalent interactions (NCI) [47] taking place at this TS was performed. The NCI surfaces are shown in Figure 6. The topological analysis of the NCI at TS-mn shows the presence of two green surfaces at the interatomic separation between the two oxygen atoms of the sulfonyl group and the two methylene hydrogens of the proline residue. In order to characterize the rather attractive nature of these surfaces, the NCI interactions at TS-mn were decomposed into their attractive (sign(λ2)ρ(r) < 0) counterpart (see Figure 6b). As can be observed, after removing the repulsive interactions, two green surfaces at the two HBs regions are observed. Consequently, formation of two HBs at MC and TS-on along the meta/endo reaction path may be responsible for the high stabilization of MC with respect to the separated reagents, and hence for the endo stereoselectivity found in this pmr-type 32CA reaction.
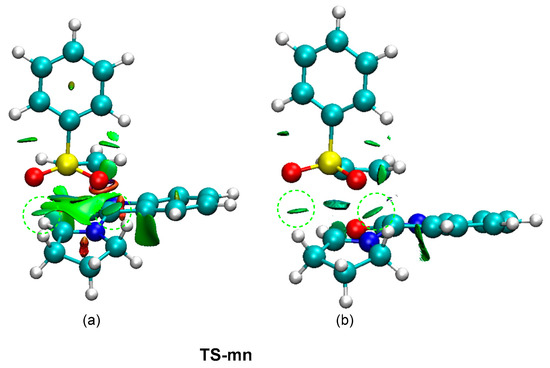
Figure 6.
(a) NCI isosurfaces associated with the density overlap at TS-mn from −0.08 < sign(λ2)ρ(r) < 0.08 a.u, and (b) attractive contribution from −0.08 < sign(λ2)ρ(r) < 0.00 a.u. The attractive NCI surfaces associated with the two HBs are highlighted by a line-dashed green circle.
4. Conclusions
The pmr-type 32CA reaction of AY 22, derived from isatin and L-proline, with phenyl vinyl sulphone 20 has been studied within MEDT at the ωB97X-D/6-311G(d,p) computational level.
Analysis of the ELF topology of AY 22 indicates that this TAC has a pseudo(mono)radical structure characterized by the presence of two monosynaptic basins, integrating a total of 0.76 e, at the C1 carbon. Analysis of the CDFT reactivity indices indicates while vinyl sulphone 20 has a strong electrophilic character, AY 22 is a supernucleophile, suggesting that the corresponding 32CA reaction will have high polar character. Analysis of the nucleophilic Parr functions indicates that the pseudoradical C1 carbon of AY 22 is the most nucleophilic center of this TAC, while analysis of the electrophilic Parr functions of vinyl sulphone 20 indicates that the β-conjugated C4 carbon is the most electrophilic center.
The most favorable reaction path via the meta/endo TS-mn presents an activation Gibbs-free energy of 13.1 kcal·mol−1, the 32CA reaction being exergonic by −26.8 kcal·mol−1. This reaction presents high endo stereoselectivity and high meta regioselectivity. While the meta regioselectivity is determined by the most favorable two-center interaction between the most nucleophilic center of AY 22, the pseudoradical C1 carbon, and the most electrophilic center of vinyl sulphone 20, the β-conjugated C4 carbon, the endo stereoselectivity is determined by the formation of two intramolecular HBs between the two oxygens of sulphone 20 and two methylene hydrogens of the proline residue. Analysis of the GEDT at the most favorable TS-mn, 0.31 e, accounts for the high polar character of this 32CA reaction, classified as FEDF, in complete agreement with the analysis of the CDFT indices at the ground state of the reagents.
A BET analysis along the most favorable meta/endo reaction path characterized this 32CA reaction, which takes place through a non-concerted two-stage one-step mechanism, as a pmr-type 32CA reaction, in agreement with the pseudo(mono)radical structure of AY 22.
The present MEDT study shows that substitution on the simplest AY 1 can modify its pseudodiradical structure and reactivity to that of a pseudo(mono)radical, carbenoid, or zwitterionic TAC, but in any case, substituted AYs are found in one of the four types of TACs, characterizing their chemical reactivity.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/org3020010/s1, Figure S1: ELF basin attractor positions of AY 21; Figure S2: geometries of MC; Table S1: ωB97X-D/6-311G(d,p) total electronic energies of the stationary points involved in the 32CA reaction of AY 22 with phenyl vinyl sulphone 20 in gas phase and in methanol; Table S2: ωB97X-D/6-311G(d,p) total enthalpies, entropies, and Gibbs-free energies of the stationary points involved in the 32CA reaction of AY 22 with phenyl vinyl sulphone 20; Table S3: most relevant ELF valence basins and their total populations, C–C bond formation distances, IRC values, and relative energies, for the structures of the IRC in which there is a relevant chemical change along the meta/endo path of the pmr-type 32CA reaction between AY 22 with phenyl vinyl sulphone 20; Scheme S1: representation of the molecular mechanism of the pmr-type 32CA reaction between AY 22 with phenyl vinyl sulphone 20 by Lewis-like structures based on the topological analysis of the ELF along the meta/endo reaction path; ωB97X-D/6-311G(d,p) gas phase Cartesian coordinates of the stationary points involved in the 32CA reaction between AY 22 with phenyl vinyl sulphone 20.
Author Contributions
Conceptualization, L.R.D. and M.R.-G.; methodology, L.R.D. and M.R.-G.; investigation, L.R.D., A.B. and M.R.-G.; resources, L.R.D. and M.R.-G.; writing—original draft preparation, L.R.D.; writing—review and editing, M.R.-G. and A.B.; supervision, L.R.D.; funding acquisition, L.R.D., M.R.-G. and A.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Spain Ministry of Science and Innovation (MICINN), project PID2019-110776GB-I00 (AEI/FEDER, UE), by the European Union’s Horizon 2020 research and innovation program under the Marie Skłodowska-Curie grant agreement No. 846181 (MRG), and by the Researchers Supporting Project (RSP-2021/64) of the King Saud University, Riyadh, Saudi Arabia.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Carruthers, W. Some Modern Methods of Organic Synthesis, 2nd ed.; Cambridge University Press: Cambridge, UK, 1978. [Google Scholar]
- Padwa, A. 1,3-Dipolar Cycloaddition Chemistry; Wiley-Interscience: New York, NY, USA, 1984; Volumes 1–2. [Google Scholar]
- Carruthers, W. Cycloaddition Reactions in Organic Synthesis; Pergamon: Oxford, UK, 1990. [Google Scholar]
- Padwa, A.; Pearson, W.H. Synthetic Applications of 1,3-Dipolar Cycloaddition Chemistry Toward Heterocycles and Natural Products; John Wiley & Sons, Inc.: New York, NY, USA, 2002; Volume 59. [Google Scholar]
- Domingo, L.R.; Chamorro, E.; Pérez, P. Understanding the High Reactivity of the Azomethine Ylides in [3+2] Cycloaddition Reactions. Lett. Org. Chem. 2010, 7, 432–439. [Google Scholar] [CrossRef]
- Domingo, L.R.; Ríos-Gutiérrez, M. A Molecular Electron Density Theory Study of the Reactivity of Azomethine Imine in [3+2] Cycloaddition Reactions. Molecules 2017, 22, 750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domingo, L.R.; Ríos-Gutiérrez, M.; Pérez, P. A Molecular Electron Density Theory Study of the Reactivity and Selectivities in [3+2] Cycloaddition Reactions of C,N-Dialkyl Nitrones with Ethylene Derivatives. J. Org. Chem. 2018, 83, 2182–2197. [Google Scholar] [CrossRef]
- Domingo, L.R.; Ríos-Gutiérrez, M.; Pérez, P. An MEDT study of the carbenoid-type [3+2] cycloaddition reactions of nitrile ylides with electron-deficient chiral oxazolidinones. Org. Biomol. Chem. 2016, 14, 10427–10436. [Google Scholar] [CrossRef]
- Domingo, L.R. Molecular Electron Density Theory: A Modern View of Reactivity in Organic Chemistry. Molecules 2016, 21, 1319. [Google Scholar] [CrossRef]
- Ríos-Gutiérrez, M.; Domingo, L.R. Unravelling the Mysteries of the [3+2] Cycloaddition Reactions. Eur. J. Org. Chem. 2019, 2019, 267–282. [Google Scholar] [CrossRef]
- Bailly, C. Lamellarins, from A to Z: A family of anticancer marine pyrrole alkaloids. Curr. Med. Chem. Anti-Cancer Agents 2004, 4, 363–378. [Google Scholar] [CrossRef]
- Bellina, F.; Rossi, R. Synthesis and biological activity of pyrrole, pyrroline and pyrrolidine derivatives with two aryl groups on adjacent positions. Tetrahedron 2006, 62, 7213–7256. [Google Scholar] [CrossRef]
- Narayan, R.; Potowski, M.; Jia, Z.-J.; Antonchick, A.P.; Waldmann, H. Catalytic Enantioselective 1,3-Dipolar Cycloadditions of Azomethine Ylides for Biology-Oriented Synthesis. Acc. Chem. Res. 2014, 47, 1296–1310. [Google Scholar] [CrossRef]
- Barakat, A.; Islam, M.S.; Ali, M.; Al-Majid, A.M.; Alshahrani, S.; Alamary, A.S.; Yousuf, S.; Choudhary, M.I. Regio- and Stereoselective Synthesis of a New Series of Spirooxindole Pyrrolidine Grafted Thiochromene Scaffolds as Potential Anticancer Agents. Symmetry 2021, 13, 1426. [Google Scholar] [CrossRef]
- Boudriga, S.; Haddad, S.; Murugaiyah, V.; Askri, M.; Knorr, M.; Strohmann, C.; Golz, C. Three-Component Access to Functionalized Spiropyrrolidine Heterocyclic Scaffolds and Their Cholinesterase Inhibitory Activity. Molecules 2020, 25, 1963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barakat, A.; Soliman, S.M.; Alshahrani, S.; Islam, M.S.; Ali, M.; Al-Majid, A.M.; Yousuf, S. Synthesis, X-ray Single Crystal, Conformational Analysis and Cholinesterase Inhibitory Activity of a New Spiropyrrolidine Scaffold Tethered Benzo [b] Thiophene Analogue. Crystals 2020, 10, 120. [Google Scholar] [CrossRef] [Green Version]
- Barakat, A.; Alshahrani, S.; Al-Majid, A.M.; Ali, M.; Altowyan, M.S.; Islam, M.S.; Alamary, A.S.; Ashraf, S.; Ul-Haq, Z. Synthesis of a New Class of Spirooxindole–Benzo [b] Thiophene-Based Molecules as Acetylcholinesterase Inhibitors. Molecules 2020, 25, 4671. [Google Scholar] [CrossRef] [PubMed]
- Barakat, A.; Al-Majid, A.M.; Lotfy, G.; Ali, M.; Mostafa, A.; Elshaier, Y.A.; Al-Habib, S. Drug Repurposing of Lactoferrin Combination in a Nanodrug Delivery System to Combat Severe Acute Respiratory Syndrome Coronavirus-2 Infection. Med. J. 2021, 3, 104–112. [Google Scholar] [CrossRef]
- Domingo, L.R.; Sáez, J.A. Understanding the Electronic Reorganization along the Nonpolar [3+2] Cycloaddition Reactions of Carbonyl Ylides. J. Org. Chem. 2011, 76, 373–379. [Google Scholar] [CrossRef]
- Domingo, L.R.; Ríos-Gutiérrez, M.; Pérez, P. A Molecular Electron Density Theory Study of the Role of the Copper Metalation of Azomethine Ylides in [3+2] Cycloaddition Reactions. J. Org. Chem. 2018, 83, 10959–10973. [Google Scholar] [CrossRef] [PubMed]
- Domingo, L.R.; Aurell, M.J.; Pérez, P. A mechanistic study of the participation of azomethine ylides and carbonyl ylides in [3+2] cycloaddition reactions. Tetrahedron 2015, 71, 1050–1057. [Google Scholar] [CrossRef]
- Aziz, Y.M.A.; Lotfy, G.; Said, M.M.; El Ashry, E.S.H.; El Tamany, E.S.H.; Soliman, S.M.; Abu-Serie, M.M.; Teleb, M.; Yousuf, S.; Dömling, A.; et al. Design, Synthesis, Chemical and Biochemical Insights Into Novel Hybrid Spirooxindole-Based p53-MDM2 Inhibitors With Potential Bcl2 Signaling Attenuation. Front. Chem. 2021, 9, 735236. [Google Scholar] [CrossRef]
- Islam, M.S.; Al-Majid, A.M.; Azam, M.; Verma, V.P.; Barakat, A.; Haukka, M.; Domingo, L.R.; Elgazar, A.A.; Mira, A.; Badria, F.A. Synthesis of Spirooxindole Analogs Tethered Pyrazole Scaffold as Acetylcholinesterase Inhibitors. Chem. Sel. 2021, 6, 14039–14053. [Google Scholar]
- Barakat, A.; Haukka, M.; Soliman, S.M.; Ali, M.; Al-Majid, A.M.; El-Faham, A.; Domingo, L.R. Straightforward Regio- and Diastereoselective Synthesis, Molecular Structure, Intermolecular Interactions and Mechanistic Study of Spirooxindole-Engrafted Rhodanine Analogs. Molecules 2021, 26, 7276. [Google Scholar] [CrossRef]
- Al-Majid, A.M.; Soliman, S.M.; Haukka, M.; Ali, M.; Islam, M.S.; Shaik, M.R.; Barakat, A. Design, Construction, and Characterization of a New Regioisomer and Diastereomer Material Based on the Spirooxindole Scaffold Incorporating a Sulphone Function. Symmetry 2020, 12, 1337. [Google Scholar] [CrossRef]
- Chai, J.-D.; Head-Gordon, M. Long-range corrected hybrid density functionals with damped atom–atom dispersion corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615–6620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hehre, M.J.; Radom, L.; Schleyer, P.V.R.; Pople, J. Ab initio Molecular Orbital Theory; Wiley: New York, NY, USA, 1986. [Google Scholar]
- Schlegel, H.B. Optimization of equilibrium geometries and transition structures. J. Comput. Chem. 1982, 3, 214–218. [Google Scholar] [CrossRef]
- Schlegel, H.B. Modern Electronic Structure Theory; Yarkony, D.R., Ed.; World Scientific Publishing: Singapore, 1994. [Google Scholar]
- Fukui, K. Formulation of the reaction coordinate. J. Phys. Chem. 1970, 74, 4161–4163. [Google Scholar] [CrossRef]
- González, C.; Schlegel, H.B. Reaction path following in mass-weighted internal coordinates. J. Phys. Chem. 1990, 94, 5523–5527. [Google Scholar] [CrossRef]
- González, C.; Schlegel, H.B. Improved algorithms for reaction path following: Higher-order implicit algorithms. J. Chem. Phys. 1991, 95, 5853–5860. [Google Scholar] [CrossRef] [Green Version]
- Tomasi, J.; Persico, M. Molecular interactions in solution: And overview of methods based on continuous distributions of the solvent. Chem. Rev. 1994, 94, 2027–2094. [Google Scholar] [CrossRef]
- Simkin, B.Y.; Sheikhet, I.I. Quantum Chemical and Statistical Theory of Solutions–Computational Approach; Ellis Horwood: London, UK, 1995. [Google Scholar]
- Cossi, M.; Barone, V.; Cammi, R.; Tomasi, J. Ab initio study of solvated molecules: A new implementation of the polarizable continuum model. Chem. Phys. Lett. 1996, 255, 327–335. [Google Scholar] [CrossRef]
- Cances, E.; Mennucci, B.; Tomasi, J. A new integral equation formalism for the polarizable continuum model: Theoretical background and applications to isotropic and anisotropic dielectrics. J. Chem. Phys. 1997, 107, 3032–3041. [Google Scholar] [CrossRef]
- Barone, V.; Cossi, M.; Tomasi, J. Geometry optimization of molecular structures in solution by the polarizable continuum model. J. Comput. Chem. 1998, 19, 404–417. [Google Scholar] [CrossRef]
- Domingo, L.R. A new C–C bond formation model based on the quantum chemical topology of electron density. RSC Adv. 2014, 4, 32415–32428. [Google Scholar] [CrossRef] [Green Version]
- Reed, A.E.; Weinstock, R.B.; Weinhold, F. Natural population analysis. J. Chem. Phys. 1985, 83, 735–746. [Google Scholar] [CrossRef]
- Reed, A.E.; Curtiss, L.A.; Weinhold, F. Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem. Rev. 1988, 88, 899–926. [Google Scholar] [CrossRef]
- Parr, R.G.; Yang, W. Density Functional Theory of Atoms and Molecules; Oxford University Press: New York, NY, USA, 1989. [Google Scholar]
- Domingo, L.R.; Ríos-Gutiérrez, M.; Pérez, P. Applications of the conceptual density functional indices to organic chemistry reactivity. Molecules 2016, 21, 748. [Google Scholar] [CrossRef] [Green Version]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision A.03; Gaussian, Inc.: Wallingford, CT, USA, 2016.
- Becke, A.D.; Edgecombe, K.E. A simple measure of electron localization in atomic and molecular-systems. J. Chem. Phys. 1990, 92, 5397–5403. [Google Scholar] [CrossRef]
- Noury, S.; Krokidis, X.; Fuster, F.; Silvi, B. Computational tools for the electron localization function topological analysis. Comput. Chem. 1999, 23, 597–604. [Google Scholar] [CrossRef]
- Dennington, R.; Keith, T.A.; Millam, J.M. GaussView, 6th ed.; Semichem Inc.: Shawnee Mission, KS, USA, 2016. [Google Scholar]
- Johnson, E.R.; Keinan, S.; Mori-Sánchez, P.; Contreras-García, J.; Cohen, J.; Yang, W. Revealing Noncovalent Interactions. J. Am. Chem. Soc. 2010, 132, 6498–6506. [Google Scholar] [CrossRef] [Green Version]
- Contreras-García, J.; Johnson, E.R.; Keinan, S.; Chaudret, R.; Piquemal, J.-P.; Beratan, D.N.; Yang, W. NCIPLOT: A Program for Plotting Noncovalent Interaction Regions. J. Chem. Theo. Comput. 2011, 7, 625–632. [Google Scholar] [CrossRef]
- Silvi, B.; Savin, A. Classification of chemical bonds based on topological analysis of electron localization functions. Nature 1994, 371, 683–686. [Google Scholar] [CrossRef]
- Parr, R.G.; Pearson, R.G. Absolute hardness: Companion parameter to absolute electronegativity. J. Am. Chem. Soc. 1983, 105, 7512–7516. [Google Scholar] [CrossRef]
- Domingo, L.R.; Ríos-Gutiérrez, M.; Pérez, P. A Molecular Electron Density Theory Study of the Reactivity of Tetrazines in Aza- Diels-Alder Reactions. RSC Adv. 2020, 10, 15394–15405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parr, R.G.; Szentpaly, L.V.; Liu, S. Electrophilicity index. J. Am. Chem. Soc. 1999, 121, 1922–1924. [Google Scholar] [CrossRef]
- Domingo, L.R.; Chamorro, E.; Pérez, P. Understanding the reactivity of captodative ethylenes in polar cycloaddition reactions. A theoretical study. J. Org. Chem. 2008, 73, 4615–4624. [Google Scholar] [CrossRef] [PubMed]
- Chamorro, E.; Duque-Noreña, M.; Gutiérrez-Sánchez, N.; Rincón, E.; Domingo, L.R. A Close Look to the Oxaphosphetane Formation along the Wittig Reaction: A [2 + 2] Cycloaddition? J. Org. Chem. 2020, 85, 6675–6686. [Google Scholar] [CrossRef]
- Aurell, M.J.; Domingo, L.R.; Perez, P.; Contreras, R. A theoretical study on the regioselectivity of 1,3-dipolar cycloadditions using DFT-based reactivity indexes. Tetrahedron 2004, 60, 11503–11509. [Google Scholar] [CrossRef]
- Domingo, L.R.; Perez, P.; Sáez, J.A. Understanding the local reactivity in polar organic reactions through electrophilic and nucleophilic Parr functions. RSC Adv. 2013, 3, 1486–1494. [Google Scholar] [CrossRef]
- Domingo, L.R.; Aurell, M.J.; Perez, P.; Sáez, J.A. Understanding the Origin of the Asynchronicity in Bond-Formation in Polar Cycloaddition Reactions. A DFT Study of the 1,3-Dipolar Cycloaddition Reaction of Carbonyl Ylides with 1,2-Benzoquinones. RSC Adv. 2012, 2, 1334–1342. [Google Scholar] [CrossRef]
- Benchouk, W.; Mekelleche, S.M.; Silvi, B.; Aurell, M.J.; Domingo, L.R. Understanding the kinetic solvent effects on the 1,3-dipolar cycloaddition of benzonitrile N-oxide: A DFT study. J. Phys. Org. Chem. 2011, 24, 611–618. [Google Scholar] [CrossRef]
- Evans, M.G.; Polanyi, M. Some applications of the transition state method to the calculation of reaction velocities, especially in solution. Trans. Faraday Soc. 1935, 31, 875–894. [Google Scholar] [CrossRef]
- Krokidis, X.; Noury, S.; Silvi, B. Characterization of Elementary Chemical Processes by Catastrophe Theory. J. Phys. Chem. A 1997, 101, 7277–7282. [Google Scholar] [CrossRef]
- Domingo, L.R.; Sáez, J.A.; Zaragozá, R.J.; Arnó, M. Understanding the Participation of Quadricyclane as Nucleophile in Polar Cycloadditions toward Electrophilic Molecules. J. Org. Chem. 2008, 73, 8791–8799. [Google Scholar] [CrossRef] [PubMed]
- Fukui, K. Molecular Orbitals in Chemistry, Physics, and Biology; Academic Press: New York, NY, USA, 1964. [Google Scholar]
- Gleiter, R.; Bohm, M.C. Regio- and Stereoselectivity in Diels-Alder Reactions. Theoretical Considerations. Pure Appl. Chem. 1983, 55, 237–244. [Google Scholar] [CrossRef]
- Ramírez, M.; Svatunek, D.; Liu, F.; Garg, N.K.; Houk, K.N. Origins of Endo Selectivity in Diels–Alder Reactions of Cyclic Allene Dienophiles. Angew. Chem. Int. Ed. 2021, 60, 14989–14997. [Google Scholar] [CrossRef]
- García, J.I.; Mayoral, J.A.; Salvatella, L. Do Secondary Orbital Interactions Really Exist? Acc. Chem. Res. 2000, 33, 658–664. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).