Abstract
Considering the global scientific and industrial effort to utilize ammonia as an alternative to natural gas combustion to run power plants, it is crucial to objectively assess the literature before adjusting or proposing new and advancing techniques in ammonia plants while considering a variety of factors. As a result, this paper assesses the global effort to improve existing ammonia plants and identifies progress by evaluating the currently available dataset to identify knowledge gaps and highlight aspects that have yet to be addressed. Based on the literature reviewed in this study, it was found that the majority of the efforts to advance ammonia plants mainly focus on reducing energy consumption, implementing alternative methods to extract the necessary hydrogen and nitrogen in the process, and changing the cycle arrangement and operating conditions to make the industrial plants more compact. However, regarding carbon reduction in the ammonia production process, it is clear that the effort is less significant when compared to the global scientific and industrial progress in other areas.
1. Introduction
1.1. The Importance of Ammonia
Ammonia (NH3) is a fundamental manufacturing component and the cheapest compound combining nitrogen with raw elements, utilized in more than 76% of all nitrogen-based products (Figure 1) [1]. Figure 1 shows the main categories of ammonia applications (i.e., producing ammonium bicarbonate, ammonium nitrate, ammonium sulphate, calcium ammonium nitrate, urea, urea ammonium nitrate (UAN) fertilizers, di-ammonium phosphate (DAP) fertilizers, monoammonium phosphate (MAP) fertilizers and other direct non-fertilizer uses). A translucent product with a distinctive strong odor, NH3 is mainly produced through the “harsh” reaction of N2 and H2 at high temperatures and under compression in the presence of a proper catalyst. When created by this process, it is known as synthetic ammonia. NH3 is also obtained as a byproduct in coal coking; however, this type of NH3, referred to as byproduct ammonia, is generated in considerably lower amounts than the former type (synthetic ammonia) [2]. The Haber and Bosch technique is the most commonly used worldwide; however, it is also the most expensive. van Rooij [3] devised several improvements to the Haber and Bosch technique (including the operating conditions, catalysts, hydrogen and nitrogen generation, and storage). The nitrogen required for ammonia production mainly comes from the atmosphere, whereas the required hydrogen primarily comes from natural gas through steam-methane reformations.
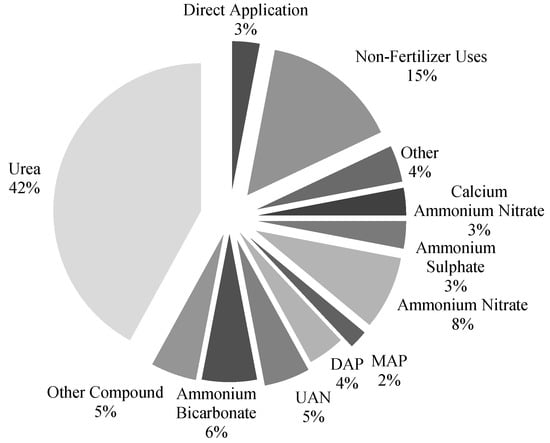
Figure 1.
The applications of ammonia, reproduced from Reference [1].
Another consideration is that ammonia facilities are constructed by converting facilities, since both CH3OH and NH3 may generate similar facilities [4]. As shown in Figure 1, NH3 is primarily employed to produce fertilizers (urea ammonium nitrate (UAN), di-ammonium phosphate (DAP), monoammonium phosphate (MAP) fertilizers). In addition, NH3 is used as an energy vector in power plants, for utilization as a zero-carbon fuel [4,5,6,7,8,9,10]. Reference [10] studied the potential to blend hydrogen with ammonia for utilization as fuel in a simple-cycle gas turbine and found that cycle efficiency could be increased compared to methane-powered gas turbines under lean combustion conditions (i.e., at an equivalence ratio of 0.75). This shows the potential to promote ammonia–hydrogen gas turbines to the power generation industry, demonstrating them to be efficient and sustainable. However, NOx emissions should be carefully monitored and mitigated using cooling and dilution approaches [10].
In the commercial refrigeration industry, liquid ammonia is the most widely used refrigerant due to its low price and high thermal performance [5]. Liquified ammonia has been used as an inexpensive alkali in the stiffening of some steel-based materials [6], and in water purification [7,8,9,10]. Given the global scientific and industrial effort to use ammonia as a fuel to run power plants instead of natural gas, it is critical to objectively assess the literature before adjusting or proposing new techniques in ammonia plants, considering a variety of factors. As a result, this paper evaluates the global effort to improve existing ammonia plants and identifies progress by evaluating the currently available datasets to identify knowledge gaps and highlight aspects that remain unresolved. This was conducted in the four sections of this paper. Section 1 provides the reader with the essential background of ammonia plants, and covers aspects related to the modern ammonia production plants and presents the latest patents in the field of ammonia production. Section 2 provides a detailed description of one of the most widely used configurations of ammonia plant (Kellogg Brown and Root (KBR)). Section 3 evaluates the efforts to advance KBR models and presents a critical comparison between KBR plants and the Linde–Ammonia-Concept (LAC) plant. Section 4 addresses the safety issues related to ammonia production.
In addition, by evaluating the global scientific effort in advancing ammonia plants in terms of its contribution to enhancing ammonia production. The effort to advance ammonia plants is classified into six main categories in this paper: (1) reducing ammonia-production-related energy consumption through renewable and sustainable approaches; (2) techno-economics of ammonia production; (3) proposing alternative approaches to supply nitrogen and hydrogen for the process; (4) advancing ammonia production catalysts; (5) altering the cycle configuration (design or/and operating conditions); (6) environmental aspects and ammonia-production-related carbon reduction. These categories were observed by evaluating the aims and the objectives of 130 research articles, published between 2015 and 2022.
1.2. Modern Ammonia Production Plants
To produce anhydrous ammonia, new NH3-producing facilities supply H2 to the process with steam-methane-reforming (SMR) methods to react with N2 under harsh temperature and pressure (730 K, 20 MPa) conditions, and is accompanied by the presence of a catalyst to synthesize the compound, as shown in Figure 2 [8,9]. The Haber–Bosch (HB) process is the concept used to describe this stage. Currently, fossil fuels, air, and water are the stream supplies required to produce ammonia. Natural gas is the most commonly used fossil-fuel energy source, accounting for about 76% of all NH3 power-generated globally. Coal-based power plants account for 24% of total capacity.
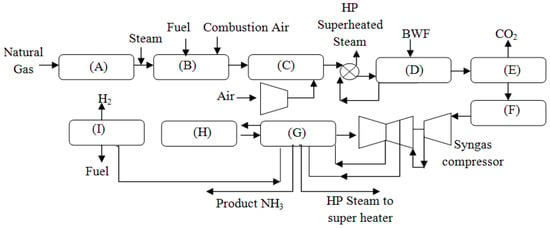
Figure 2.
Block diagram of conventional NH3 production, reproduced from the reference [8]. (A) Desulfurization (B) Primary Reformer (C) Secondary Reformer (D) CO shift (E) CO2 removal (F) Methanation (G) NH3 loop (H) Refrigeration (I) NH3 Loop.
To prepare natural gas for the SMR process, it is blended with a relatively small portion of H2~ and then preheated to approximately 730 K either within the built-in reforming furnace or within an external source of heat (heat exchangers, heaters, etc.). It is necessary to purify the preheated gas mixture of any sulfur-based contents (H2S and organic sulfur compounds) to below 1% of molar fraction in a single or double-reactor series. The first of these contains a cobalt molybdenum (Co-Mo) catalyst and the second contains zinc oxide (ZnO) adsorbent to eliminate any poisoning of the nickel-based catalyst in the latter. It is possible to divide the reformer unit into two stages. The primary reformer is a sub-unit of a methane-reforming plant, in which a heated steam–methane blend (1:4 by a molar fraction) is supplied through radiation-heated channels with a nickel-based reforming catalyst and partially converted to H2, CO, and CO2 (typically, 66% of the initial methane supply) [10]. The necessary heat for the first reformer is produced by gas-fueled burners, categorized as side-fired, top-fired, or bottom-fired burners. A convection bank is used to recycle the wasted heat produced in the furnace box (heat content of the flue gas) for use in other operations (such as supercritical steam heating and preheating process air). The partly converted gas is sent into the secondary reformer, where it is mixed with a regulated quantity of air (which has been warmed and compressed to 790 K and 4 million pounds per square inch). The temperature is increased from 1050 K to about 1490 K by partial combustion of the gas to complete the endothermic process.
Almost all CH4 is adiabatically transformed in standard plants as it passes through the catalytic material, leaving an unreacted concentration below 0.6% [10,11]. All carbon oxides must be eliminated from the mixture to fulfill the criteria for NH3 synthesis fuel. Traditionally, the water–gas-shift reaction has been used to transform CO into a form that can be removed from the atmosphere. To utilize the wasted heat used to increase the temperature of the superheated steam, the temperature of the gas products from the second reformer is reduced by heat exchange.
The superheated steam is then supplied to the high-temperature-shift reactor, filled with Fe2O3 and Cr2O3. At 600 K, the CO reacts with surplus H2O to form H2 and CO2, with a 310 K-equilibrium approach to the reaction. A sufficient amount of H2O is essential for this process to eliminate the Boudouard reaction (which is prevented using efficient Fischer–Tropsh catalysts) [11]. If a conventional plant is used, the reformer must have an S/C ratio of 3.0 at the very least to meet the high-temperature shift (HTS) requirements. A 2% or less of CO2 content is achieved at the outlet of the high-temperature-shift reactor; thus, the usage of the low-temperature-shift (LTS) reactor is necessary to transform the residual CO in the synthesis gas at 490 K. The water–gas shift (WGS) produces a large amount of CO2 as its feed output. To effectively dissolve CO2 at high pressure, the utilization of a solvent is common. Many different solvents are available at present, and they are classified as physical-based or chemical-based solvents depending on the amount of CO2 that is present in the input stream. Chemical solvents, which are mostly generated from alkanolamine, are used in the ammonia synthesis pathway because they provide a high-mass CO2 transfer while also requiring a high-energy input for regeneration.
The monoethanolamine (MEA) system consists of regeneration stripping columns and HP absorption columns, which are typically approximately 5.3 MPa, with pressure losses of 3 KPa between each stage. Whether or not a reboiler is used, the total number of steps is generally between 10 and 15 [10]. One downside to the system is that carbonate salts build in the absorption solution, which is quite caustic. Newly discovered solvent additives, such as liquid NH3- and Ca-based solvents that may prevent the production of carbonates, are being employed in the industry to reduce carbonate formation. It is necessary to perform final purification in the ammonia synthesis, since residuals range between 0.2–0.5% mol. of CO and 0.005–0.2% mol. of CO2. The copper-based scrubbing approach was extensively used in early plants but has become obsolete due to the high energy consumption. Moreover, it is deemed ecologically unfavorable when the remaining carbon is removed. Methanation is the most common approach to lowering carbon content levels to below 10 parts per million. At the same time, the exothermic process is used to recover energy and recycle it back into the system. The reaction occurs on a nickel-containing catalyst at from 2.5 to 3.5 MPa [12]. When the immediate exothermic reaction occurs, temperatures may rise to between 500 and 1040 K. The Steam Rankine Cycle (SRK) uses the rejected heat to generate electricity for power regeneration. It is necessary to remove H2 and CO2 residuals from the gas produced by methanation by running the outputs in a drying process (i.e., pressure-swing-adsorption, cryogenic separation). It should be noted that most techniques are utilized to increase the purity of the H2 and N2 required for ammonia production. The synthesis of ammonia only occurs in the last block of the reaction. At this point, N2 and H2 are routed as a set of compression stages that are powered by steam turbines to complete the process. While centrifugal-based compression has a cheap initial investment, low maintenance costs, and good dependability, it has a lower efficiency than reciprocating compressors [13], which should be considered. Preheating and increasing the synthesis gas pressure to 15–25 MPa are performed before the requisite synthesis temperature is reached.
The converter where NH3 production takes place is at the core of the synthesis system. The converter’s response rate and operational parameters impact the converter’s overall performance. When the pressure is raised, the ammonia yield dramatically rises due to the favorable equilibrium reaction and the reaction rate itself. The synthesis pressure in contemporary ammonia facilities varies between 15,000 and 25,000 kPa. In addition, maintaining the required temperature is critical because the pace of the production process varies dramatically as the temperature changes. The H2:N2 ratio in the incoming synthesis gas and the feed stream speed impact the converter’s performance when combined with the previously listed factors. The best conversion is achieved when the space velocity is high, and the H2:N2 ratio is two. On its way through the catalyst, the synthesis undergoes a partial conversion of 25–35%, according to [14]. After that, the ammonia that is created is separated from the unreacted gas before being returned to the converter. A variety of synthesis loop designs are feasible, and the position of the NH3 condensation determines which one is used. The separation of ammonia from the unreacted gas occurs in all contemporary facilities using refrigerated chilling. Ordinarily, the temperature is lowered to around 25 K, and the liquefied NH3 in the elevated-pressure separators is flashed at 2000 kPa. Then, the key individual steps involved in ammonia production are summarized.
1.3. Ammonia Synthesis Patents
Several patents were granted in ammonia synthesis, as summarized in Table 1. Haber and Bosch [15] patented the NH3 production process in 1916 (U.S. patent-1202995). Many other patents have been obtained for ammonia synthesis since then. Wright and colleagues [16] developed a set of equipment for ammonia synthesis, which consisted of two catalytic converters and was patented (U.S. patent-3721532). Several heat-exchanging means are linked to the converters’ intake and output ports on both sides of the system. The second converter’s input discharges a supply stream into the heat-exchanging means to be cooled. The patent describes the implementation of a support platform for the converters and the heat-exchanging means. To develop a new process, it is necessary to operate within a pressure of 10–31.3 MPa, while maintaining the temperature in the range of 477–320 K.

Table 1.
Ammonia synthesis patents.
Da Rosa [17] used high-pressure electrolysis to produce H2 without the need for compression, which was a breakthrough in the field (U.S. patent 4107277). The concept also used oxygen at elevated pressures to liquefy ammonia in the refrigeration subsystem, which was the first in the industry. The exothermic nature of the ammonia synthesis process means that the steam recovered from the reactor may be utilized to produce energy, which can then be used to power the electrolyzer. The procedure was carried out at a pressure of 200 atm and temperatures ranging from 370 to 650 K. Becker registered a patent (U.S. 4148866) for the manufacture of ammonia with minimal energy usage, which was issued to Colman L. Becker [18].
It is necessary to initiate the ammonia production process at low pressure levels ranging between 2 and 10 MPa. A liquid–water-based combination separates the synthesized ammonia from the residual gases through absorption and stripping. A gas-based ammonia synthesis system (U.S.-patent-4479925) was designed by Shires et al. [19]. The syngas is then combined with H2O, a reforming reactor, where it undergoes an endothermic process, resulting in hydrogen production. The effluent gas is then combined with air in an autothermal reformer before being sent into the synthesis converter for further processing. High temperatures ranging from 990 to 1190 K and low pressures ranging from 2500 to 5000 kPa are necessary for this procedure. The majority of ammonia-producing facilities at present depend on fossil fuels and natural gas for their energy. These facilities have a single train of gigantic reactors. Before the nitrogen and hydrogen can be introduced into the synthesis converter, the raw gas must pass through a purification process. As a result, many patents have been issued in connection with the purifying methods used for the natural gas supply. In the patent (U.S.-4695442), the adsorption characteristics of gases that are occupied throughout an acceptable range of raw gas composition are described in detail [20]. Carbon dioxide and hydrogen are present in sufficient quantities to bring the boiling point of N2 into balance. N2 and H2 are then combined and supplied into the convertor, resulting in the production of ammonia gas with a high yield of hydrogen recovery while minimizing the need for additional adsorption bed volume and purge gas. This procedure operates at temperatures ranging from 640 to 790 K and pressures ranging from 2500 to 5000 kPa.
1.4. State-of-the-Art Ammonia Production
This section includes a survey of the literature on subjects and research related to the generation of ammonia. The section also provides an overview of the contemporary ammonia-related technologies that are used worldwide.
1.4.1. Casale Small Ammonia Plant Concepts
Casale presents two concepts for micro-NH3 plants: the A-60, which has a capacity of up to 3000 ton/month, and the A-600, with a capacity that ranges from 9000 to 30,000 ton/month. The synthesis loops of these two plant models vary to accomplish their designated production targets. The synthesizing loop operates at high pressure in the A-60 concept, which primarily reduces the number of equipment items (above 20,000 kPa). Consequently, ammonia is created in high concentrations and readily condenses using H2O or atmospheric air. As a result, the refrigeration unit is omitted. The A-600 concept envisions a low-pressure synthesizing loop instead of the A500 concept. With this design, the goal is to make the production facility simpler while maintaining the primary compression stage. When using a low-pressure synthesis loop, the mass flow rate in the compression stage increases, which allows for the employment of a more dependable centrifugal machine. As seen in Figure 3 and Figure 4, Casale’s ammonia plant designs depend on methane as an input supply to the plant. They are also applicable to alternate feedstock sources such as biomass fuel supply extracted from waste and H2 produced through electrolyzers [21].
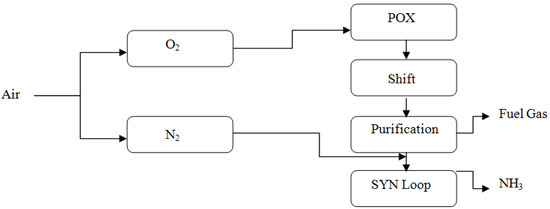
Figure 3.
Ammonia process flowsheet for Casale A-60 concept, reproduced from the reference [21].
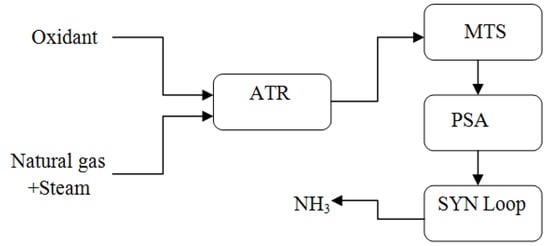
Figure 4.
Ammonia process flowsheet for Casale A-600 concept, reproduced from the reference [21].
1.4.2. Casale NH3 Plants with Biomass Feedstocks
Bio-methane is a biomass fuel supply that can be produced from renewable energy sources such as biofuel. The first-generation (organic waste) [22], second-generation (lignocellulosic biomass) [23], and third-generation (micro-algae) biofuels all depend on the organic matter used as the starting point [24]. Only a few energy-based and environmental-based research projects on NH3 synthesis from biofuel gasification have been conducted in the literature. Andersson et al. carried out a techno-economic study on NH3 synthesis by implementing a biofuel gasification of the leftover wood generated in pulp- and paper-production plants [25]. The study findings revealed that combining the pulp-production and paper-production plans improves economic sustainability and leads to a 9% boost in the performance of the cycle when compared to a standalone ammonia plant.
The price of the generated ammonia, on the other hand, is higher than the current market pricing for ammonia. Tock et al. conducted a thermo-environmental optimization evaluation of ammonia production using wood-based gasification using an energy integration approach, which they found to be effective [26]. The integration used CO2 capture and storage technology to limit CO2 emissions to the atmosphere. However, the research findings indicated that energy efficiency decreased due to the expensive CO2-compression stage. Additionally, the plant’s energy efficiency was evaluated to be 51% and 1.78 ton CO2/ton NH3 through the biofuel produced by the crop.
In contrast, the system’s energy efficiency in natural gas-based ammonia production was rated at 65% and 0.78 ton CO2/ton NH3. According to [27], the authors investigated the feasibility of NH3 synthesis using wood-based gasification for a system capable of producing 1200 tons NH3/day. According to the study’s findings, up to 66% of the gas that contributes to global warming (CO2, CO, NOx) reductions were accomplished.
The economically sustainable dry-based biomass gasification (61 USD/ton) contributed to reducing the cost of NH3 synthesis to 501 USD/ton. Biomethane may be converted to syngas in a practical manner by utilizing Casale’s A-60 or A-600 NH3 plant designs, depending on their required capacity. In the Casale A-60 design, the fuel supply is processed in a patented, partially oxidized (POX) reactor, which was developed by the company. Based on Casale’s sophisticated burner technology, Partial Oxidization (POX) enables soot-free operation at a low H2O/C ratio when the feed gas composition varied. Furthermore, POX has a meager minimum turndown ratio, allowing for steady functioning in 21% of the average load without compromising performance. With these characteristics of Casale POX, it is possible to reduce the plant’s overall size while maintaining excellent durability, performance, and an extended life cycle. After being inspired by the notion that the ammonia plant should be simplified and reduced to several units, the A-60 proposes a single shift stage (at elevated temperature levels), followed by a purification stage. The latter procedure is conducted in the Pressure Swing Adsorption (PSA) facility, which is highly automated.
Furthermore, microbes such as cyanobacteria, which generate the enzymes responsible for nitrogen fixation and ammonia production [28], should be noted. These enzymes may also be used in conjunction with a chemical reaction to react and develop electron transfer in the presence of low potential, which serves as the driving force in a biochemical reactor [29,30]. Immobilizing enzymes on the electrode surface increases the biocatalytic potential for nitrogen fixation and ammonium production.
1.4.3. ThyssenKrupp’s Green Ammonia Concept
ThyssenKrupp Industrial Solutions (TKIS) is popular in the fertilizer business due to its Uhde NH3 Production Technology [31]. Energy, transportation, agricultural sectors, and chemical industries are among the areas in which TKIS has created comprehensive and environmentally beneficial methods [32]. The alkaline–water–electrolysis (AWE) technology used by the company serves as the foundation for all these applications by supplying hydrogen to downstream technologies. TKIS’s AWE and downstream processes to “green” syngas, H2, CH3OH, and NH3 now allow for renewable energy, created from renewable sources, to be stored, addressing the primary barrier and issues associated with renewable energy: fluctuation. Ammonia may also be converted into nitrogen fertilizer solutions by additional processing. As a result, a wide range of eco-friendly technology is accessible today. Unlike a traditional NH3 plant, which generates H2 via the SMR units, the AWE generates H2 using electrolyzers. An Air-Separation Unit (ASU) generates the N2 necessary for NH3 synthesis in the concept proposed here. When developing its NH3-production approach, TKIS concluded that having a standardized and modularized concept, without spending time and resources on tailormade engineering, was a critical prerequisite for providing possible clients with optimized solutions. The modularization and standardization of the green ammonia concept are required to increase the viability of this concept and make it more feasible.
Although the AWE and ASU sections of the plant were already modularized, TKIS had to undergo additional work to modularize the NH3 synthesis section, which resulted in a comprehensive concept being developed from a single source (see Figure 5). When modularizing the green ammonia plant, it is beneficial to have both hydrogen and nitrogen accessible at the same time under the same circumstances. Furthermore, from modularization, it is helpful to retain capabilities at a lower level to maintain facilities at a compact size. According to the evaluation of the TKIS report by references [31,32], a significant proportion of wind clients from offshore and onshore farms generally have at least 20,000 kW of power. NH3 production of 1500 ton/month may be reached with a 20,000 kW input-power, according to the manufacturer. The key objective of TKIS for this 50 ton/day concept was to optimize the usage of economically sustainable equipment to avoid jeopardizing the viability of the project.
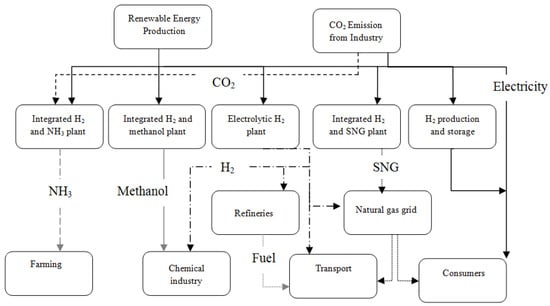
Figure 5.
The holistic concept of modularized ammonia synthesis, reproduced from reference [31].
Moreover, TKIS has created a second concept based on a 120,000 kW of input power that will produce 300 ton/day ammonia. Due to the scale of the plant, TKIS decided that, to compete with traditional approaches, somewhat more emphasis should be placed on the energy-efficiency of this particular concept. In addition, since the plant operates on a higher economic scaling level, TKIS believes that it might be a realistic upgrading option in existing NH3-production facilities, able to partially replace traditional NH3 production with green ammonia production [8].
1.4.4. Electrolysis for Ammonia Production
The electrolysis of alkaline-water is based on the well-established Chlor-alkali electrode technique [33]. Power and water are necessary for AWE-based hydrogen generation. In contrast to the direct supply of electricity to AWE via a transformer rectifier, raw water must be demineralized before it can be given to AWE.
The oxygen and hydrogen produced by the AWE process are the primary byproducts. Both goods have been thoroughly cleaned. This procedure does not need oxygen, which might be saved for use in other methods further downstream. The hydrogen produced by AWE is compressed, deoxygenated, and dried before being used. The hydrogen has now been prepared for use in the ammonia production process. To prepare the synthesis gas that is to be fed into the synthesis gas compressor, the appropriate quantity of N2 is mixed with H2 at a stoichiometric ratio. Nitrogen is created in a cryogenic air separation unit. Figure 6 illustrates how the AWE approach is well adapted to the operating circumstances of a green- NH3 plant, which might suffer from a shortage of power owing to fluctuations in the supply of renewable energy [34].
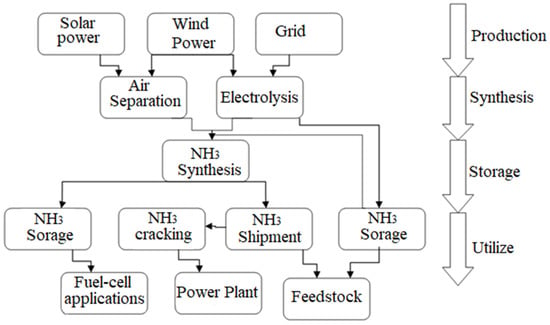
Figure 6.
Schematics of the green ammonia process from production to utilization, reproduced from Reference [33].
The AWE can be set up in minutes and responds to load fluctuations in milliseconds. It is also very cost-effective. As a result, the AWE provides the necessary flexibility from renewable energy sources. The ammonia synthesis portion is a crucial source of concern. For this reason, intermittent hydrogen storage is being constructed upstream of the synthesis gas compression to address the problem. The capacity of the intermittent storage system may be adjusted in line with the availability of power resources. With these safeguards in place, the NH3 synthesis unit will be capable of operating on a 24-h basis without interruption.
2. Kellogg Brown and Root (KBR) Ammonia Plant
The massive growth in ammonia demand between 1950 and 1980 led to the construction of bigger, more energy-saving factories. During those years, there was also a shift in the design approach. Before then, an NH3-production plant was seen as a collection of unconnected components, such as gas preparation, gas purification, gas compression, and ammonia synthesis, which were connected.
With the help of new technologies and a unified design, process units were most effectively and efficiently linked together. In the mid-1960s, the American Oil Company built a single-converter NH3-production plant in Texas City, TX, which was designed by M.W. Kellogg (MWK) and had a daily capacity of 544 metric tons (mt). Due to the single-train design concept (Figure 7), this was awarded the Kirkpatrick Chemical Engineering Achievement Award in 1967. It was necessary to increase the pressure of syngas to 153 bar using a four-case centrifugal compressor, and the final compression to an operational pressure of 323 bar was accomplished using a reciprocating-based compression stage within the plant. A centrifugal compressor system for the synthesizing loop and refrigeration units was also installed, which considerably reduced the company’s expenses.
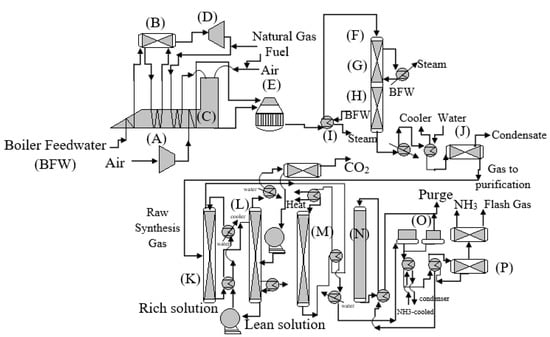
Figure 7.
KBR design of the first single-train (large-capacity ammonia plant (A) Compressor, heat recovery section (B) Steam Drum (D) Power turbine (E) Secondary reformer (F) CO convertor (G) First stage high-temperature synthesis (H) Second stage low-temperature synthesis (I) BFW heat recovery (J) Condensation (K) CO2 absorber (L) CO2 stripper (M) Methanator (N) NH3 Converter (O) Compression (P) Separator, reproduced from References [35,36].
Compared to previous ammonia plants, the MWK process incorporated the following innovations: using a centrifugal-based compression stage as part of the synthesis gas compression process, optimizing heat recovery generated by the process, and generating steam from the waste heat for the usage in steam turbines. The refrigeration compressor was also utilized for rundown and atmospheric refrigeration. An integrated system was implemented throughout the factory, which matched energy consumption with energy output, and equipment size with catalyst volumes. From 1963 to 1993, the majority of plants were massive single-train designs capable of producing synthesis gas and NH3 at 26–36 and 151–201 bar pressure, respectively. Another variant, developed by KBR, made only minor alterations to the fundamental concept. Primary reformers at low output temperatures and a high CH4 leak were used in the KBR Purifier process plants to minimize the size and expense of the reformer while maintaining the same performance. To lower the methane concentration of the primary reformer output stream to 1–3%, more air was introduced into the secondary reformer. When removing excess N2 contaminants in the methanation, taking advantage of the relatively clean synthesis gas, two axial-flow NH3 converters were utilized to obtain a high ammonia conversion rate. Some newly constructed plants’ synthesis gas-generating systems only have a single reformer (without use of a second reforming reactor), a PSA system for hydrogen purification, and an ASU to supply the necessary nitrogen. Design advancements, such as radial and horizontal catalyst beds, internal heat exchangers, and synthesis gas treatment, have assisted in increasing the NH3 contents leaving the synthesis converter from around 13% to 20%–22%. Energy consumption was further lowered due to the increased conversion per pass and more advanced compressors and turbines. Carbon-capturing techniques, such as potassium carbonate and methyldiethanolamine (MDEA), have significantly improved energy efficiency. A typical contemporary plant may create NH3 with an energy expenditure of 28,000 MJ for every metric ton of nitrogen that is produced.
Additionally, during this period, significant advancements in design, mechanical, and metallurgical aspects were achieved, and the operating pressure of the synthesis loop was significantly lowered. A high-pressure synthesis loop was installed in the first single-train facility constructed in the 1960s. A request for proposals from Imperial Chemical Industries (ICI) for a 544-metric-ton-per-day facility at their Severnside location was received by MWK in 1962, and the company responded positively. Instead of a 323-bar synthesizing loop, MWK offered a 152-bar synthesizing loop.
MWK approached HaldorTopsoe for assistance, since developing kinetic data for the NH3 reaction at 154 bar could take more time than the company can afford to reply to the ICI inquiry. HaldorTopsoe was able to provide the necessary help. Topsoe had data that covered the whole pressure range in which MWK was interested. A computer program was also available to determine the amount of catalyst that was needed at the decreased operating pressure. ICI developed a single-train ammonia plant with the help of Bechtel as the design firm. The flowsheet for a 544-metric-ton-per-day design with centrifugal compressors and a low-pressure synthesis loop was successfully developed by MWK, which was considered the most significant event in the development of the single-train ammonia plant. At 152 bar, about twice as much catalyst was needed than at 324 bar. A considerable increase seemed to be economically possible. Although the converter may need double the capacity, the reduced working pressure would minimize the thickness of the required pressure shell.
Consequently, the total weight of metal needed for the converter and catalyst remained almost the same. As a result of the lower-pressure synthesis loop, centrifugal compressors were able to replace reciprocating compressors in specific applications. The recovery of heat to create high-pressure steam for steam turbine drives was another advancement.
3. KBR Sub Technology
3.1. KBR Ammonia Plant
The developers of the most spared plants, Kellogg, Braun and Root (KBR), provided a variety of flowsheet alternatives for both the front end and the synthesis loop. Table 2 summarizes the possibilities considered in a study given in 1999 and is detailed in the following paragraphs.

Table 2.
KBR ammonia plant flow sheet options.
The conventional front-end flowsheet uses the conventional process stages of primary reforming, secondary reforming, shift conversion, carbon dioxide removal, methanation, and shift conversion, with a stoichiometric amount of process air. Dryers may or may not be included in a conventional flowsheet. A total of 50% of the extra air from the purifier front end is used in the secondary reformer. In a cryogenic purification stage that follows methanation, the excess air and methane and most of the argon are captured and removed.
The radiating part of the primary reformer is replaced by a shell and tube heat exchanger in the KBR Reforming Exchange System (KRES). It is essential to employ either oxygen-enriched process air or extra process air in the secondary reformer to generate the necessary driving power for heat transfer in the KRES exchanger. Due to the high cost of installing an air separation plant, KBR provides KRES for new plants in conjunction with their purifier technology to provide the most cost-effective design possible. The synthesis loop may be either magnetite or a KBR-Advanced-Ammonia-Processing (KAAP) loop, which are both considered viable solutions. Magnetite loop refers to the classic design that dates back to Haber and Bosch, around one hundred years ago, and employs a magnetite catalyst at relatively high pressure instead of the modern design. A KAAP loop makes use of a well-established, highly active ruthenium catalyst. Consequently, it is possible to reduce the synthesis pressure to around 90 bar. It is worth noting that, even at this lower pressure, the amount of KAAP catalyst needed is substantially lower than the amount of magnetite catalyst required at the typically greater synthesis loop pressure. Table 3 displays the number of plants that were developed, employing each component of the KBR ammonia technology.

Table 3.
KBR design experience with technology options.
KBR explored the maximum capacity of its horizontal magnetite converter while retaining the synthesis gas compressor as a two-case machine as part of ongoing efforts to improve efficiency. The maximum capacity that can be achieved is somewhat higher than 3000 metric tons per day (MTPD). The KBR developers then considered the possibility of installing a second converter downstream. The capacity may be increased to around 3500 MTPD. However, to obtain high ammonia conversions, the amount of magnetite synthesis catalyst in the second converter must be considerably increased.
The situation is significantly different in the case of a KAAP synthesis loop. The KAAP reactor, which has been shown to produce 1850 MTPD, includes four beds in a single vessel. The first layer is magnetite, and the shell has a diameter of 3.8 m. The second bed is quartz. In the lowest half of the reactor, which contains the KAAP catalyst, the diameter measures 3.4 m in circumference, scaling up to 4000 MTPD results in a reactor with diameters of 5.5 and 5.0 m, which is not a huge vessel according to nuclear reactor standards. In addition, the presence of a KAAP loop ensures that the synthesis pressure will be low, at around 90 bars. Due to the low pressure, the cost of loop equipment is reduced, and only a single-stage synthesis compressor is required. There is a reduction in both capital and operational costs. In light of these considerations, the KAAP loop was selected. As a consequence of the catalyst’s high activity, the reactor has a relatively small diameter.
A conventional purifier and KRES with purifier are the options for the front end of the ammonia plant. The purifier flow plan minimizes the size of the primary reformer by utilizing extra process air, which transfers the reforming duties to the secondary reformer, resulting in a smaller primary reformer. As the inert content of the makeup gas is much lower than that of a traditional front-end design, it also results in a reduction in the size of the synthesis loop equipment and the volume of the synthesis catalyst. Due to these considerations, the purifier technology for the front-end flow scheme was chosen.
The last alternative is to employ a steam methane reformer or KRES, KBR’s reforming exchanger system, which is currently under development. A preliminary assessment of the steam-methane reformer option found that around 375 radiant tubes would be needed for a facility with 4000 MTPD. This is around the same number of tubes as certain 1970s-vintage reformers built for 1000 MTPD, which is not enormous. In addition, KBR has constructed reformers with more than 900 radiant tubes. As seen above, an appropriate option for large-scale ammonia factories is a fired reformer. From a purely technological and economic standpoint, KRES is an option for overhauling the energy industry.
When it comes to reforming, KRES is the recommended option. It reduces construction, maintenance, and operating expenses, and is simpler to run. One of the biggest KRES units in operation is at Pacific Ammonia in Canada, where it has been in operation since 1994 and produces 350 MTPD. Another well-known KRES facility is in China, with a capacity of 1100 MTPD, which began operations in 2003. This is part of a larger project to renovate Liaohe’s ammonia factory. To establish whether KRES would be relevant to mega-ammonia plants, KBR previously created designs for the reforming exchanger with greater capacities to determine whether KRES would be appropriate for mega-ammonia plants. The outcomes of the designs for 2000 and 4500 MTPD are summarized in Table 4, and compared with the results of the designs for the two KRES projects.

Table 4.
Comparison of KRES designs for different capacities.
The maximum diameter of the tube sheet used in the fabrication of the KRES exchanger served as the fabrication limit. The maximum length of the KBR design and construction materials was 3550 mm. This implies that a single-train KRES unit with a capacity of more than 5000 MTPD is feasible. However, to maintain caution in the design of the 4000 MTPD plant, the KBR developers elected to provide a conventional reformer as the default configuration. As an alternative, KBR was willing to propose a design that included two parallel KRES exchangers, each with 2000 MTPD. Hence, it was necessary to scale up from the Liaohe design (which began operations in 2003) from two to one to achieve this alternative design; however, this was a reasonable next step in advancing KRES technology.
Figure 8 depicts the flow diagram for KBR’s proposal of an ammonia plant with a capacity of 4000 MTPD. The system shown in this figure includes a fired-reformer, a purifier, and a KAAP loop, among other components. Mild primary reforming, secondary reforming with around 50% surplus process air, cryogenic removal of the excess nitrogen, methane, and most of the argon to provide a pure synthesis makeup gas, and synthesis in an AAP loop are all features of both methods. Figure 9 illustrates the KAAPplus process, which substitutes a reforming exchanger for the fired reformer.
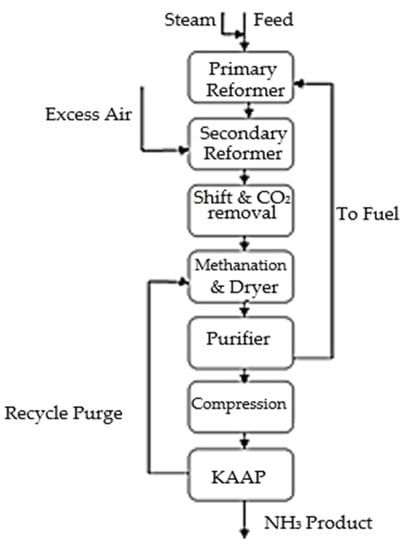
Figure 8.
Purifier and KAAP concept, reproduced from References [35,36].
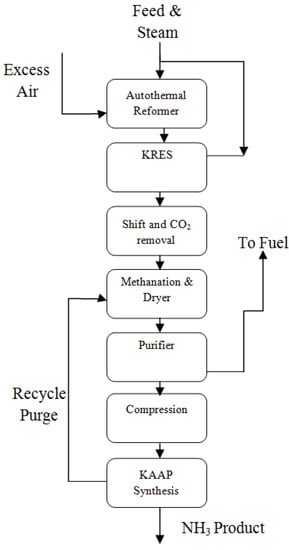
Figure 9.
The KAAPplus concept, reproduced from References [35,36].
3.2. KBR vs. Linde–Ammonia-Concept (LAC) Plant
This section compares and contrasts the LAC ammonia plant with the KBR purifier ammonia plant. As stated in the preceding section, the KBR purifier method [35] depicted in Figure 10A comprises a Fired Tubular Reformer (FTR) and an ATR unit, generating N2 via combusting syngases. A double-stage WGS unit increases hydrogen production, followed by a carbon capture phase, which results in a clean carbon dioxide stream that may be used or stored. Afterward, a cryogenic purification unit optimizes the composition of the makeup syngas and eliminates any inert elements.
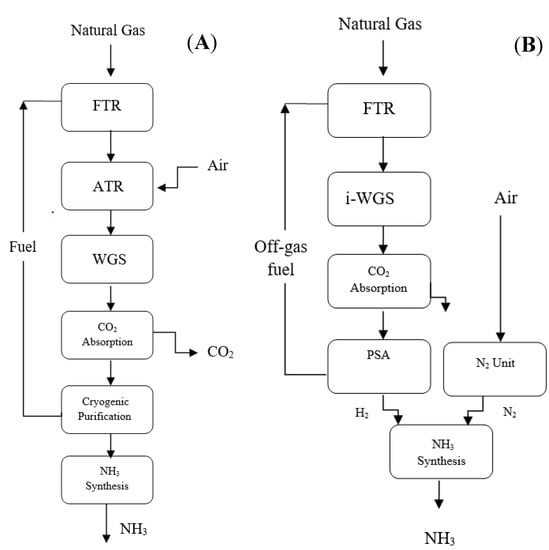
Figure 10.
Block flow diagrams of different ammonia production technologies: (A) KBR (B) LAC, reproduced from the references [35].
The 2nd reference process is the Linde–Ammonia-Concept (LAC) [36,37,38,39,40], which is briefly represented in Figure 10B. The method differs from the KBR process because it only uses a single reformer, while the nitrogen required for the process is yielded in a specialized cryogenic-generating unit. A PSA unit purifies the syngas produced by the reformer after undergoing an isothermal water–gas shift (i-WGS) and after carbon dioxide is removed by absorption in the reformer. Consequently, the new syngas used for the ammonia loop in the LAC concept is highly filtered.
3.2.1. KBR Purifier NH3 Process
The necessary energy for the reforming reaction is supplied by an external source by the combustion of hydrocarbon fuels at a stoichiometric condition in ammonia plants, composed of H2-generation units that include desulphurization steps followed by an FTR, maintaining an H2O/C ratio of 2.8 to prevent CO2 buildup in the nickel-based catalyst inside the reformer’s tubes. Nitrogen is injected with air in the succeeding ATR, resulting in a CH4 fraction of approximately 4 as an output in the KBR Purifier ammonia process. The compression of the air supply is driven by a turbine, whose high-temperature flue gas is fed into the primary reformer’s furnace, resulting in the highest possible heat integration. As air is injected at higher rate than the ATR’s capacity, it is supplied to the second reformer, resulting in an FTR temperature output of around 1000 K. Following heat recovery from the syngas stream exit of the ATR, which results in the generation of a high-pressure (HP) steam, a downstream water–gas-shift train optimizes the amount of hydrogen that is produced. The heat generated by the exothermic WGS process produces additional steam and reduces the amount of water that is consumed. This is optimized for integration with the NH3 loop heat-exchanging means. The superheated steam, of 840 K and 11,000 kPa at the production time, is expanded in the steam turbine to generate power. Both intermediate-pressure (IP) and low-pressure (LP) steam extractions from the turbine are present: an IP of 3.4 MPa is used to achieve the C/S inlet to the SMR, and the other is used under low-pressure conditiosn (i.e., 0.3 MPa) to provide thermal energy for amine regeneration. The stripper’s columns elevated to high-pressure condition in a 4-stage internally cooled compression (i.e., 15 MPa).
Around 99% of the carbon dioxide is absorbed in the methyldiethanolamine phase of absorption. After that, it is sent to a methanation reactor, where the residual carbon monoxide and carbon dioxide convert to methane, lowering the hazardous contents. It is necessary to route the reactor effluent through a cryogenic purification unit before compressing and adding syngas to the loop. In this unit, the N2/H2 proportion is calibrated to the stoichiometry. Meanwhile, CH4 and Ar residuals are lowered to 0.3% molecular weight. This is utilized as fuel for both the primary SMR and the ATR, which contains the majority of methane, surplus N2, and a small amount of hydrogen. This undergoes compression to 150 bar before being combined with the recycling from the ammonia production loop, which will be discussed in more depth in the following sections.
3.2.2. Linde–Ammonia Concept
Compared to the standard Kellogg Brown and Root concept, the Linde–Ammonia Concept is devoid of an ATR, which would have provided N2 for the synthesis step. A Fired Tubular Reformer with a steam-to-carbon ratio of 2.8 is used to produce hydrogen; after that, IS is used, where an intermediate-pressure (IP) steam is produced and fed to the reformer with the natural gas feedstock. A high recovery ratio is achieved after cooling down the water from the shifted syngas stream in the absorption column with MDEA. The hydrogen-rich syngas is supplied to a pressure swing adsorption unit, resulting in a hydrogen stream with low level of residual for use in the synthesis loop after removing 99% of the CO2. The off-gas from the PSA, which contains unconverted methane, carbon dioxide, and a small amount of hydrogen, is utilized to run the reformation process. The reaction requires nitrogen, provided under stoichiometric conditions using a nitrogen-generating facility. The application range of this facility may change based on whether producing pure oxygen is necessary; then, a complete-scope air separation unit (ASU) is incorporated into the NH3-production process. If an application for pure O2 output is available, the scope of this unit might change. If only nitrogen is needed, the cryogenic unit may significantly reduce in complexity. This method is followed in the present design. The scope consists of a single-stage air compressor and distillation columns, with the ability to recover around 60% of the nitrogen with a low level of residuals. The remainder of the enhanced air (38%) is utilized in the PRF to produce molten carbon. A very tiny purge percentage is needed because the hydrogen and nitrogen streams’ high purity comprises the makeup syngas to the synthesis loop. This slight purge and the off-gas from the pressure swing adsorption unit meet the main reformer duty requirements. When the syngas cooling from the reformer is combined with the heat recovery from the reactor intercoolers, it results in a highly integrated system that delivers HP steam at 840 K and 11 MPa to a steam-power cycle. This is a similar setup to the Kellogg Brown and Root design in that two steam extractions from the steam turbine are provided to achieve a high steam-to-carbon ratio in the supply and facilitate amine regeneration at IP and LP levels.
3.2.3. Linde Ammonia Concept Subcategories
Using a nitrogen production loop that is free of inertion, Linde’s Ammonia Concept (LAC) minimizes loop size while eliminating recovery and cleaning facilities for purge gas, resulting in a smaller overall system footprint. It is theoretically possible to feed the ammonia loop a mixture combination consisting of pure N2 and H2.
The production of hydrogen is conducted through SMR. Alternatively, hydrogen is produced from heavy feedstocks through gasification. If carbon dioxide is necessary, the process may be modified to meet the requisite ammonia and carbon dioxide portions [34]. Producing NH3 from H-rich off-gas feeds (LAC.L3) in Figure 11, which arise from chemical plants, is the most straightforward approach to ammonia production since it involves the use and adaptation of key stages of the Linde ammonia concept categories.
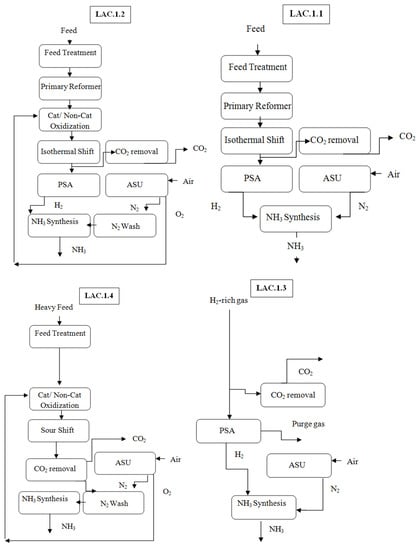
Figure 11.
Linde–Ammonia Concept categories, reproduced from Reference [34].
This technique has been executed and achieved the highest possible technoeconomic figures since it did not require the use of a separate syngas-producing unit. In Figure 11, a simplified comparison of the primary process stages of the Linde–Ammonia Concept for light feedstocks is presented, as well as the process setup of an average ammonia plant.
The LAC for light-hydrocarbon feeds (LAC.L.1) mainly consists of an H2-production unit with a one-stage ISR, PSA, conventional N2-production unit, and NH3 loop, as depicted in Figure 11. Following the treatment of the feed, a highly efficient, top-fired Linde PR is used to convert hydrocarbons into syngas, which is mainly composed of N2 and CO. Compared to a traditional ammonia process, the concept eliminates the introduction of air to the process; however, the ability to limit the inert level to a bare minimum is solely dependent on the supply feed composition. This minimizes the size of the syngas-cooling facility. It is preferable to supply pure N2 from the nitrogen-generating unit straight upstream of the ammonia syngas loop rather than via the syngas generation plant to prevent overloading [34].
A one-step reactor, Linde’s coil-wrapped IR, is used to boost the hydrogen production by isothermally shifting the CO in the syngas. This reduces the catalyst volume, which results in a higher hydrogen yield. This eliminates the necessity of using a process condensate treatment unit.
An adsorption system that uses pressure swings to remove the O2 contents and methane from the system eliminates the need for energy-extensive carbon dioxide removal and catalysis-based methanation, which is less time-consuming and more economically sustainable. Afterward, the pure hydrogen is combined with pure nitrogen upstream the NH3 loop to produce ammonia. A purge-free loop may be used, since the purity has been altered; therefore, the need for a specific purge gas separation unit for the recovery of H2 is no longer necessary. It is easy to adjust the ammonia synthesis loop to various feedstocks based on what is available at a particular location, since only clean hydrogen and nitrogen are required. As inert-free gas generation allows for the production of additional products from syngas, polygeneration schemes are intrinsically simple to implement.
4. Ammonia Health and Safety Considerations
The corrosive and toxic nature of ammonia makes it potentially dangerous to life. Although it is a colourless gas (and liquid), making it difficult to detect visually, ammonia has a strong pungent odour that is detectable by humans at concentrations between 5 and 53 ppm (depending on the individual), helping to mitigate dangerous exposure levels [41].
4.1. Exposure Limits
The reference [41] outlines the acute exposure guideline levels (AEGLs) for a variety of concentrations and durations, along with their respective consequences to health, Table 5.

Table 5.
Ammonia acute exposure guideline levels—effects on health [41].
Ammonia is hygroscopic, reacting exothermically with moisture to produce a caustic solution on moist areas of the body, such as the eyes, nose, throat, and skin, resulting in severe chemical burns at high concentrations. Workplace exposure limits in the UK are 25 ppm and 35 ppm of NH3 for 8 h and 15 min, respectively [42].
4.2. Behavior on Release to the Environment
Although the density of ammonia vapour is approximately half that of air (under ambient conditions), in the event of spillage, dispersal of the toxic gas is hindered by its high vaporization heat. As the liquid evaporates, it tends to ‘hug the ground’, so its dispersal is not as rapid as its gaseous density would suggest.
Once in the atmosphere, NH3 can rapidly return to the ground as NH3 (dry deposition) or react with acid gases to form NH4+, a fine inorganic aerosol that can persist across international boundaries. This aerosol contributes to PM 2.5 (particulate matter <2.5 μm diameter) concentrations, with negative consequences for respiratory health. Like NH3, the aerosol also eventually returns to the ground via precipitation (wet deposition). Once returned to the ground (as NH3 or NH4+), it can cause eutrophication in water bodies, threatening aquatic life and impacting biodiversity [43]. Deposited NH3 (and NH4+) is transformed by microbes to a range of other compounds, including NO, N2O (a greenhouse gas) and molecular nitrogen, depending on soil conditions [44].
4.3. Materials Selection
Ammonia is especially corrosive towards copper and zinc, necessitating the careful selection of materials [45]. For metals’ selection, both stainless steel (type 304), cast iron and aluminum have excellent corrosion resistance, although aluminum’s rating is restricted to <22 °C [46]. For seals etc., ethylene propylene diene monomer (EDPM) rubber and polytetrafluoroethylene (PTFE) are also rated as excellent, but both natural and fluorocarbon rubber (i.e., FKM, commonly known as Viton) perform poorly, and so must be avoided [46].
4.4. Flammability Risk
Ammonia has recently gained significant interest as a potential low-carbon fuel; however, it carries a far lower flammability risk than other fuels. It has a relatively high minimum ignition energy (MIE) and high autoignition temperature. Its MIE is ~8 mJ [47], compared with 0.28 mJ [48] for methane, and its autoignition temperature is 130 K higher than that of methane [49]. Ammonia’s flammability range is from 15 to 29% fuel in air, compared with from 5 to 15% for methane and 4 to 75% for hydrogen (all values are for 298 K and 1 atm [50].
5. Discussion
The literature review shows that the patented efforts to advance ammonia plants were initiated in 1916 with Haber and Bosch’s NH3 production process (U.S. Patent 1202995) [15]. Since then, many more ammonia synthesis patents have been issued. Wright and colleagues [16] developed an ammonia synthesis equipment with two catalytic converters (U.S. patent 3721532). On both sides of the system, heat exchangers connected the converters’ intake and output ports. A supply stream is discharged into this to cool the second converter’s input. The patent described a support platform for converters and heat exchangers.
The reference [17] used high-pressure electrolysis to produce H2 without compression in what was considered the first field implementation (U.S patent 4107277). The concept used high-pressure oxygen to liquefy ammonia in the refrigeration subsystem that was first used in the industry.
At present, most ammonia plants run on fossil fuels and natural gas. The raw gas must be purified before being fed into the synthesis converter with nitrogen and hydrogen. As a result, many patents have been issued for the purifying processes used for raw gas supply. The adsorption characteristics of gases occupying an acceptable range of raw gas composition [20] are detailed in the patent (US-4695442).
Finally, by evaluating the global scientific effort to advance ammonia plants in terms of its contribution to enhancing ammonia production, it could be concluded that the effort can be categorized into six main categories: (1) reducing ammonia-production-related energy consumption through renewable and sustainable approaches; (2) techno-economics of ammonia production; (3) proposing alternative approaches to supply nitrogen and hydrogen to the process; (4) advancing ammonia production catalysts; (5) altering the cycle configuration (design or/and operating conditions); (6) environmental aspects and ammonia-production-related carbon reduction. Those categories were observed by evaluating the aims and the objectives of 130 research articles [51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176,177,178,179,180] published between 2015 and 2022, as summarized in Table 6 and Figure 12.

Table 6.
Categories of advancing ammonia plants.
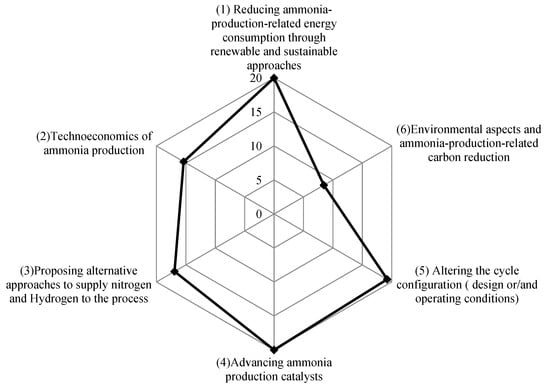
Figure 12.
The scientific trend of advancing ammonia plants (in% with respect to the total number of the evaluated literature [51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176,177,178,179,180]).
As shown in Figure 12, driven by the industrial support for enhancing techno-economic sustainability, increasing profitability, reducing energy consumption and accelerating the ammonia production process, the research categories (1–5) received and attracted the most scientific effort and interest. Within the evaluated references [77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96], the highest potential to enhance the techno-economic sustainability of producing ammonia could be found in green-ammonia production processes using green hydrogen and integrating biomass gasification to ammonia plants (namely biomass- and power-to-ammonia). As reported by Reference [181], the bio-mass-to-ammonia ratio reached above 450 USD/ton ammonia production cost with a payback time of over 6 years, higher than those of methane-to-ammonia (400 USD/ton, 5 years).
On the other hand, advances in ammonia in category 6 (the environmental aspects and ammonia-production-related carbon reduction) received the least interest.
6. Conclusions
As a preliminary stage when adjusting or proposing new techniques for ammonia plants, it is crucial to critically assess the literature while considering a variety of aspects (including energy consumption, economically sustainable methods of extracting the necessary hydrogen and nitrogen, the plant’s arrangement and operating conditions and efforts to reduce the process-related carbon emissions). Hence, this paper evaluates the global effort to enhance ammonia plants and looks at the progress, particularly regarding these aspects. This paper assesses the currently available datasets to find the gap in the knowledge and highlight aspects which have not yet been addressed. Within the literature evaluated in this study, the majority of the efforts to advance ammonia plants focused on reducing the energy consumption, implementing alternative methods to extract the necessary hydrogen and nitrogen, and altering the cycle arrangement and operating conditions to increase the plants’ compactness and lifetime. However, regarding carbon reductions within the ammonia production process, efforts remain minimal compared to the global scientific and industrial efforts in other operating aspects.
Author Contributions
Conceptualization, A.I.A., S.S.Q., S.H., A.S., Y.A., R.J.I. and O.F.A.; methodology, A.I.A.; S.H.; A.S.; Y.A. and O.F.A.; software, A.I.A.; S.H.; A.S.; Y.A. and O.F.A.; validation, A.I.A.; S.H.; A.S.; Y.A. and O.F.A.; formal analysis, A.I.A.; S.H.; A.S.; Y.A. and O.F.A.; investigation, A.I.A.; S.H.; A.S.; Y.A. and O.F.A.; resources, A.I.A.; S.H.; A.S.; Y.A. and O.F.A.; data curation, A.I.A.; S.H.; A.S.; Y.A. and O.F.A.; writing—original draft preparation, A.I.A.; S.H.; A.S.; Y.A. and O.F.A.; writing—review and editing, A.I.A.; S.H.; A.S.; Y.A.; O.F.A. and R.J.I. visualization, A.I.A.; S.H.; A.S.; Y.A. and O.F.A.; supervision, A.I.A.; S.H.; A.S.; Y.A. and O.F.A.; project administration, A.I.A.; S.H.; A.S.; Y.A. and O.F.A.; funding acquisition, A.I.A.; S.H.; A.S.; Y.A. and O.F.A. All authors have read and agreed to the published version of the manuscript.
Funding
This publication was made possible by NPRP 12 grant # (NPRP12C-0821-190017) from the Qatar National Research Fund (a member of the Qatar Foundation). The findings herein reflect the work and are solely the responsibility of the authors. Open Access funding provided by the Qatar National Library.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Nomenclature
| ASU | Air Separation Unit |
| ATR | Autothermal Reformer |
| AWE | Alkaline–water electrolysis |
| BWF | Boiler Water Feed |
| FTR | Fired Tubular Reformer |
| HB | Haber–Bosch |
| HP | High-Pressure |
| HTS | High-Temperature-Shift |
| ICI | Imperial Chemical Industries |
| IP | Intermediate pressure |
| IS | Isothermal Shift |
| i-WGS | Isothermal water gas shift |
| KAAP | KBR Advanced Ammonia Processing |
| KBR | Kellogg Brown and Root |
| KRES | KBR Reforming Exchanger System |
| LAC | Linde–Ammonia Concept |
| LTS | Low-Temperature Shift |
| MDEA | Methyldiethanolamine |
| MEA | Monoethanolamine |
| MTPD | Metric Tons Per Day |
| MTS | Medium-Temperature Shift |
| MWK | M.W. Kellogg |
| POX | Partial oxidation |
| PRF | Primary reformer furnace |
| PSA | Pressure swing adsorption |
| SMR | Steam–Methane-Reforming |
| SNG | Syngas |
| SRKC | Steam Rankine Cycle |
| SYN | Synthesis |
| TKIS | ThyssenKrupp Industrial Solutions |
| WGS | Water Gas Shift |
| SMR | Steam–Methane-Reforming |
| HP | High-Pressure |
| BWF | Boiler Water Feed |
| HB | Haber–Bosch |
| HTS | High-Temperature Shift |
| LTS | Low-Temperature Shift |
| WGS | Water–Gas Shift |
| MDEA | Methyldiethanolamine |
| MTPD | Metric Tons Per Day |
| MEA | Monoethanolamine |
| SRKC | Steam Rankine Cycle |
| POX | Partial oxidation |
| SYN | Synthesis |
| ATR | Autothermal Reformer |
| MTS | Medium-Temperature Shift |
| PSA | Pressure swing adsorption |
| TKIS | ThyssenKrupp Industrial Solutions |
| AWE | Alkaline–water electrolysis |
| ASU | Air Separation Unit |
| SNG | Syngas |
| KBR | Kellogg Brown and Root |
| MWK | M.W. Kellogg |
| ICI | Imperial Chemical Industries |
| KRES | KBR Reforming Exchanger System |
| KAAP | KBR Advanced Ammonia Processing |
| MTPD | Metric Tons Per Day |
| LAC | Linde–Ammonia Concept |
| FTR | Fired Tubular Reformer |
| i-WGS | Isothermal water gas shift |
| IP | Intermediate pressure |
| IS | Isothermal Shift |
| PRF | Primary reformer furnace |
References
- Cheremisinoff, N.P.; Rosenfeld, P. Industry and Products. In Handbook of Pollution Prevention and Cleaner Production; Elsevier: Amsterdam, The Netherlands, 2011; pp. 1–24. [Google Scholar] [CrossRef]
- Modak, J.M. Haber process for ammonia synthesis. Resonance 2002, 7, 69–77. [Google Scholar] [CrossRef]
- van Rooij, A. Engineering contractors in the chemical industry. the development of ammonia processes, 1910–1940. HistTechnol 2005, 21, 345–366. [Google Scholar] [CrossRef]
- Tso, W.W.; Demirhan, C.D.; Powell, J.B.; Pistikopoulos, E.N. Toward Optimal Synthesis of Renewable Ammonia and Methanol Processes (RAMP). Comput. Aided Chem. Eng. 2018, 44, 1705–1710. [Google Scholar] [CrossRef]
- Pearson, A. Refrigeration with ammonia. Int. J. Refrig. 2008, 31, 545–551. [Google Scholar] [CrossRef]
- Kusmanov, S.A.; Smirnov, A.A.; Kusmanova, Y.V.; Belkin, P.N. Anode plasma electrolytic nitrohardening of medium carbon steel. Surf. Coat. Technol. 2015, 269, 308–313. [Google Scholar] [CrossRef]
- Kristiana, I.; Lethorn, A.; Joll, C.; Heitz, A. To add or not to add: The use of quenching agents for the analysis of disinfection by-products in water samples. Water Res. 2014, 59, 90–98. [Google Scholar] [CrossRef]
- Brightling, J. Ammonia and the Fertiliser Industry: The Development of Ammonia at Billingham. Johns. Matthey Technol. Rev. 2018, 62, 32–47. [Google Scholar] [CrossRef]
- Rafiqul, I.; Weber, C.; Lehmann, B.; Voss, A. Energy efficiency improvements in ammonia production—Perspectives and uncertainties. Energy 2005, 30, 2487–2504. [Google Scholar] [CrossRef]
- Aalrebei, O.F.; Al Assaf, A.H.; Amhamed, A.; Swaminathan, N.; Hewlett, S. Ammonia-hydrogen-air gas turbine cycle and control analyses. Int. J. Hydrogen Energy 2022, 47, 8603–8620. [Google Scholar] [CrossRef]
- Ratnasamy, C.; Wagner, J.P. Water Gas Shift Catalysis. Catal. Rev. 2009, 51, 325–440. [Google Scholar] [CrossRef]
- Gao, J.; Wang, Y.; Ping, Y.; Hu, D.; Xu, G.; Gu, F.; Su, F. A thermodynamic analysis of methanation reactions of carbon oxides for the production of synthetic natural gas. RSC Adv. 2012, 2, 2358. [Google Scholar] [CrossRef]
- Sanchez, A.; Martín, M. Scale up and scale down issues of renewable ammonia plants: Towards modular design. Sustain. Prod. Consum. 2018, 16, 176–192. [Google Scholar] [CrossRef]
- Kandemir, T.; Schuster, M.E.; Senyshyn, A.; Behrens, M.; Schlögl, R. The Haber-Bosch Process Revisited: On the Real Structure and Stability of “Ammonia Iron” under Working Conditions. Angew. Chem. Int. Ed. 2013, 52, 12723–12726. [Google Scholar] [CrossRef] [PubMed]
- Haber, F.; Le Rossignol, R. Production of Ammonia. U.S. Patent 1202995A, 13 August 1909. Available online: https://patents.google.com/patent/US1202995A/en?oq=(U.S+patent+1202995) (accessed on 16 January 2021).
- Wright, L.; Pickford, A. Ammonia Synthesis System. U.S. Patent 3721532A, 8 February 1971. Available online: https://patents.google.com/patent/US3721532A/en (accessed on 27 January 2021).
- Da Rosa, A.V. Process for Production of Ammonia. U.S. Patent 4107277A, 15 August 1978. Available online: https://patents.google.com/patent/US4107277A/en (accessed on 16 January 2022).
- Becker, C.L. Low Energy Ammonia Synthesis Process. U.S. Patent 4148866, 4 October 1976. Available online: https://patents.google.com/patent/US4153673A/en (accessed on 16 January 2022).
- Shires, P.J.; Cassata, J.R.; Mandelik, B.G.; Dijk, C.P. Preparation of Ammonia Synthesis Gas. U.S. Patent 4479925, 30 October 1984. [Google Scholar]
- Pinto, A.; Johnson, J.B.H. Ammonia Synthesis Process. U.S. Patent 4695442A, 20 February 1985. Available online: https://patents.google.com/patent/US4695442A/en (accessed on 1 February 2022).
- Francesco, B. Ammonia Process A60TM 2019. Available online: https://www.casale.ch/downloads/ammonia (accessed on 30 October 2020).
- Molino, A.; Nanna, F.; Ding, Y.; Bikson, B.; Braccio, G. Biomethane production by anaerobic digestion of organic waste. Fuel 2013, 103, 1003–1009. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhao, J.; Xu, F.; Li, Y. Pretreatment of lignocellulosic biomass for enhanced biogas production. Prog. Energy Combust. Sci. 2014, 42, 35–53. [Google Scholar] [CrossRef]
- Ding, L.; Cheng, J.; Xia, A.; Jacob, A.; Voelklein, M.; Murphy, J.D. Co-generation of biohydrogen and biomethane through two-stage batch co-fermentation of macro-and micro-algal biomass. BioresourTechnol 2016, 218, 224–231. [Google Scholar] [CrossRef]
- Andersson, J.; Lundgren, J. Techno-economic analysis of ammonia production via integrated biomass gasification. Appl Energy 2014, 130, 484–940. [Google Scholar] [CrossRef] [Green Version]
- Tock, L.; Mar´echal, F.; Perrenoud, M. Thermo-environomic evaluation of the ammonia production. Can. J. ChemEng. 2015, 93, 356–362. [Google Scholar] [CrossRef]
- Gilbert, P.; Alexander, S.; Thornley, P.; Brammer, J. Assessing economically viable carbon reductions for the production of ammonia from biomass gasification. J. Clean. Prod. 2014, 64, 581–589. [Google Scholar] [CrossRef]
- Hoffman, B.M.; Lukoyanov, D.; Dean, D.R.; Seefeldt, L.C. Nitrogenase: A draft mechanism. AccChem. Res. 2013, 46, 87–95. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Li, Y.; Wang, B.; Luo, Y.; Yang, D.; Tong, P.; Zhao, J.; Luo, L.; Zhou, Y.; Chen, S.; et al. Ammonia formation by a thiolate-bridged diiron amide complex as a nitrogenase mimic. Nat. Chem. 2013, 5, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.S.; Rittle, J.; Peters, J.C. Catalytic conversion of nitrogen to ammonia by an iron model complex. Nature 2013, 501, 84–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larsen, J.S.; Lippmann, D. The Uhde Dual Pressure Process-Reliability Issues and Scale-Up Considerations The Uhde Dual Pressure Process-Reliability Issues and Scale-Up Considerations. In Proceedings of the 47th Annual Safety in Ammonia Plants and Related Facilities Symposium, San Diego, CA, USA, 16–September 2002. [Google Scholar]
- Thyssenkrupp Industrial Solutions. Small-Scale Green Ammonia Plants Open up New Storage Possibilities for Wind and Solar Power. n.d. Available online: https://insights.thyssenkrupp-industrial-solutions.com/story/small-scale-green-ammonia-plants-open-up-new-storage-possibilities-for-wind-and-solar-power/ (accessed on 30 October 2020).
- David, M.; Ocampo-Martínez, C.; Sanchez-Pena, R. Advances in alkaline water electrolyzers: A review. J. Energy Storage 2019, 23, 392–403. [Google Scholar] [CrossRef] [Green Version]
- Araújo, A.; Skogestad, S. Control structure design for the ammonia synthesis process. Comput. Chem. Eng. 2008, 32, 2920–2932. [Google Scholar] [CrossRef] [Green Version]
- Gosnell, J.; Malhotra, A. New Ammonia Process. Ammon. Plant SafRelatFacil 2000, 40, 116–125. [Google Scholar]
- Eskicioglu, C.; Galvagno, G.; Cimon, C. Approaches and processes for ammonia removal from side-streams of municipal effluent treatment plants. Bioresour. Technol. 2018, 268, 797–810. [Google Scholar] [CrossRef]
- Linde Engineering, LAC™ (the Linde Ammonia Concept). Available online: https://www.linde-engineering.com/en/process-plants/hydrogen_and_synthesis_gas_plants/gas_products/ammonia/index.html (accessed on 12 January 2021).
- Lin, B.; Heng, L.; Fang, B.; Yin, H.; Ni, J.; Wang, X.; Lin, J.; Jiang, L. Ammonia Synthesis Activity of Alumina-Supported Ruthenium Catalyst Enhanced by Alumina Phase Transformation. ACS Catal. 2019, 9, 1635–1644. [Google Scholar] [CrossRef]
- Pattabathula, V.; Richardson, J. Introduction to ammonia production. Chem. Eng. Prog. 2016, 112, 69–75. [Google Scholar]
- Akpa, J.G.; Raphael, N.R. Optimization of an Ammonia Synthesis Converter. World J. Eng. Technol. 2014, 2, 305. [Google Scholar] [CrossRef] [Green Version]
- National Academies. Ammonia Final AEGL Document. 2008. Available online: http://www.nap.edu/catalog/12018.html (accessed on 14 April 2022).
- Health and Safety Executive. EH40/2005 Workplace Exposure Limits EH40, 2nd ed.; HSE Books: London, UK, 2011.
- Adam, M.R.; Othman, M.H.D.; Abu Samah, R.; Puteh, M.H.; Ismail, A.; Mustafa, A.; Rahman, M.A.; Jaafar, J. Current trends and future prospects of ammonia removal in wastewater: A comprehensive review on adsorptive membrane development. Sep. Purif. Technol. 2019, 213, 114–132. [Google Scholar] [CrossRef]
- The Royal Society. Ammonia: Zero-Carbon Fertiliser, Fuel and Energy Store. Policy Briefing. 2020. Available online: https://royalsociety.org/topics-policy/projects/low-carbon-energy-programme/green-ammonia/ (accessed on 12 July 2021).
- Health & Safety Executive. COMAH—Guidance—Technical Aspects—Measures Documents—Corrosion/Selection of Materials. Available online: http://www.hse.gov.uk/comah/sragtech/techmeasmaterial.htm (accessed on 14 November 2021).
- Cole-Parmer Instrument Company. Chemical Compatibility Database from Cole-Parmer United Kingdom. 2021. Available online: https://www.coleparmer.co.uk/Chemical-Resistance (accessed on 5 January 2022).
- Verkamp, F.J.; Hardin, M.C.; Williams, J.R. Ammonia combustion properties and performance in gas-turbine burners. Symp. Combust. 1967, 11, 985–992. [Google Scholar] [CrossRef]
- Lees, F. Electrostatic hazards: Their evaluation and control: By Heinz Haase; published by Verlag Chemie, New York, 1976; x + 125 pp.; price DM 24.00. Chem. Eng. J. 1977, 14, 229. [Google Scholar] [CrossRef]
- Kobayashi, H.; Hayakawa, A.; Somarathne, K.K.A.; Okafor, E.C. Science and technology of ammonia combustion. Proc. Combust. Inst. 2019, 37, 109–133. [Google Scholar] [CrossRef]
- McAllister, S.; Chen, J.-Y.; Fernandez-Pello, A.C. Fundamentals of Combustion Processes. In Fundamentals of Combustion Processes; Springer: New York, NY, USA, 2011; pp. 15–47. [Google Scholar] [CrossRef]
- Verleysen, K.; Coppitters, D.; Parente, A.; De Paepe, W.; Contino, F. How can power-to-ammonia be robust? Optimization of an ammonia synthesis plant powered by a wind turbine considering operational uncertainties. Fuel 2016, 266, 117049. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, Y.; Shi, R.; Waterhouse, G.I.; Zhang, T. Photocatalytic ammonia synthesis: Recent progress and future. EnergyChem 2019, 1, 100013. [Google Scholar] [CrossRef]
- Mohamed, A.M.; Bicer, Y. Development and assessment of concentrated solar energy driven ammonia synthesis from liquefied natural gas. Int. J. Hydrogen Energy 2020, 46, 10093–10103. [Google Scholar] [CrossRef]
- Siddiqui, O.; Dincer, I. Development and evaluation of a solar-based integrated ammonia synthesis and fuel cell system. J. Clean. Prod. 2020, 256, 120393. [Google Scholar] [CrossRef]
- Fasihi, M.; Weiss, R.; Savolainen, J.; Breyer, C. Global potential of green ammonia based on hybrid PV-wind power plants. Appl. Energy 2021, 294, 116170. [Google Scholar] [CrossRef]
- Ghavam, S.; Vahdati, M.; Wilson, I.A.; Styring, P. Sustainable ammonia production processes. Front. Energy Res. 2021, 9, 34. [Google Scholar] [CrossRef]
- Palys, M.J.; Wang, H.; Zhang, Q.; Daoutidis, P. Renewable ammonia for sustainable energy and agriculture: Vision and systems engineering opportunities. Curr. Opin. Chem. Eng. 2021, 31, 100667. [Google Scholar] [CrossRef]
- Armijo, J.; Philibert, C. Flexible production of green hydrogen and ammonia from variable solar and wind energy: Case study of Chile and Argentina. Int. J. Hydrogen Energy 2019, 45, 1541–1558. [Google Scholar] [CrossRef]
- Liu, X.; Elgowainy, A.; Wang, M. Life cycle energy use and greenhouse gas emissions of ammonia production from renewable resources and industrial by-products. Green Chem. 2020, 22, 5751–5761. [Google Scholar] [CrossRef]
- Cesaro, Z.; Ives, M.; Nayak-Luke, R.; Mason, M.; Bañares-Alcántara, R. Ammonia to power: Forecasting the levelized cost of electricity from green ammonia in large-scale power plants. Appl. Energy 2021, 282, 116009. [Google Scholar] [CrossRef]
- Siddiqui, O.; Dincer, I. A new solar energy system for ammonia production and utilization in fuel cells. Energy Convers. Manag. 2020, 208, 112590. [Google Scholar] [CrossRef]
- Cloete, S.; Khan, M.N.; Nazir, S.M.; Amini, S. Cost-effective clean ammonia production using membrane-assisted autothermal reforming. Chem. Eng. J. 2020, 404, 126550. [Google Scholar] [CrossRef]
- Osman, O.; Sgouridis, S.; Sleptchenko, A. Scaling the production of renewable ammonia: A techno-economic optimization applied in regions with high insolation. J. Clean. Prod. 2020, 271, 121627. [Google Scholar] [CrossRef]
- Yan, Z.; Ji, M.; Xia, J.; Zhu, H. Recent advanced materials for electrochemical and photoelectro-chemical synthesis of ammonia from dinitrogen: One step closer to a sustainable energy future. Adv. Energy Mater. 2020, 10, 1902020. [Google Scholar] [CrossRef]
- Hollevoet, L.; De Ras, M.; Roeffaers, M.; Hofkens, J.; Martens, J.A. Energy-Efficient Ammonia Production from Air and Water Using Electrocatalysts with Limited Faradaic Efficiency. ACS Energy Lett. 2020, 5, 1124–1127. [Google Scholar] [CrossRef] [Green Version]
- Salmon, N.; Bañares-Alcántara, R. Green ammonia as a spatial energy vector: A review. Sustain. Energy Fuels 2021, 5, 2814–2839. [Google Scholar] [CrossRef]
- Flórez-Orrego, D.; Sharma, S.; de Oliveira Junior, S.; Marechal, F. Combined exergy analysis, energy integration and optimization of syngas and ammonia production plants: A cogeneration and syngas purification perspective. J. Clean. Prod. 2020, 244, 118647. [Google Scholar] [CrossRef]
- Hasan, A.; Dincer, I. An ocean thermal energy conversion based system for district cooling, ammonia and power production. Int. J. Hydrogen Energy 2020, 45, 15878–15887. [Google Scholar] [CrossRef]
- Peng, X.; Liu, H.-X.; Zhang, Y.; Huang, Z.-Q.; Yang, L.; Jiang, Y.; Wang, X.; Zheng, L.; Chang, C.; Au, C.-T.; et al. Highly efficient ammonia synthesis at low temperature over a Ru–Co catalyst with dual atomically dispersed active centers. Chem. Sci. 2021, 12, 7125–7137. [Google Scholar] [CrossRef] [PubMed]
- Ishaq, H.; Dincer, I. Dynamic analysis of a new solar-wind energy-based cascaded system for hydrogen to ammonia. Int. J. Hydrogen Energy 2020, 45, 18895–18911. [Google Scholar] [CrossRef]
- Demirhan, C.D.; Tso, W.W.; Powell, J.B.; Pistikopoulos, E.N. Sustainable ammonia production through process synthesis and global optimization. AIChE J. 2018, 65, e16498. [Google Scholar] [CrossRef]
- Philibert, C. Producing Ammonia and Fertilizers: New Opportunities from Renewables; IEA Rep: Paris, France, 2017; pp. 1–6. [Google Scholar]
- Nørskov, J.; Chen, J.; Miranda, R.; Fitzsimmons, T.; Stack, R. Sustainable Ammonia Synthesis–Exploring the Scientific Challenges Associated with Discovering Alternative, Sustainable Processes for Ammonia Production; US DOE Office of Science: Washington, DC, USA, 2016.
- Jiang, J.; Feng, X. Energy optimization of ammonia synthesis processes based on oxygen purity under different purification technologies. Energy 2019, 185, 819–828. [Google Scholar] [CrossRef]
- Sanchez, A.; Martín, M. Optimal renewable production of ammonia from water and air. J. Clean. Prod. 2018, 178, 325–342. [Google Scholar] [CrossRef]
- Beerbühl, S.S.; Fröhling, M.; Schultmann, F. Combined scheduling and capacity planning of electricity-based ammonia production to integrate renewable energies. Eur. J. Oper. Res. 2015, 241, 851–862. [Google Scholar] [CrossRef]
- Nosherwani, S.A.; Neto, R.C. Techno-economic assessment of commercial ammonia synthesis methods in coastal areas of Germany. J. Energy Storage 2021, 34, 102201. [Google Scholar] [CrossRef]
- Yoshida, M.; Ogawa, T.; Imamura, Y.; Ishihara, K.N. Economies of scale in ammonia synthesis loops embedded with iron- and ruthenium-based catalysts. Int. J. Hydrogen Energy 2021, 46, 28840–28854. [Google Scholar] [CrossRef]
- Guerra, C.F.; Reyes-Bozo, L.; Vyhmeister, E.; Caparrós, M.J.; Salazar, J.L.; Clemente-Jul, C. Technical-economic analysis for a green ammonia production plant in Chile and its subsequent transport to Japan. Renew. Energy 2020, 157, 404–414. [Google Scholar] [CrossRef]
- Ziaei, M.; Panahi, M.; Fanaei, M.A.; Rafiee, A.; Khalilpour, K.R. Maximizing the prof-itability of integrated Fischer-Tropsch GTL process with ammonia and urea synthesis using response surface methodology. J. CO2 Util. 2020, 35, 14–27. [Google Scholar] [CrossRef]
- Graça, V.C.; Loureiro, F.J.; Holz, L.I.; Mikhalev, S.M.; Araújo, A.J.; Fagg, D.P. Electrochemical ammonia synthesis: Mechanism, recent developments, and challenges in catalyst design. Heterog. Catal. 2022, 497–514. [Google Scholar]
- del Pozo, C.A.; Cloete, S. Techno-economic assessment of blue and green ammonia as energy carriers in a low-carbon future. Energy Convers. Manag. 2022, 255, 115312. [Google Scholar] [CrossRef]
- Gomez, J.R.; Baca, J.; Garzon, F. Techno-economic analysis and life cycle assessment for electrochemical ammonia production using proton conducting membrane. Int. J. Hydrogen Energy 2019, 45, 721–737. [Google Scholar] [CrossRef]
- Hochman, G.; Goldman, A.S.; Felder, F.A.; Mayer, J.M.; Miller, A.J.M.; Holland, P.L.; Goldman, L.A.; Manocha, P.; Song, Z.; Aleti, S. Potential Economic Feasibility of Direct Electrochemical Nitrogen Reduction as a Route to Ammonia. ACS Sustain. Chem. Eng. 2020, 8, 8938–8948. [Google Scholar] [CrossRef]
- Smith, C.; Torrente-Murciano, L. The potential of green ammonia for agricultural and economic development in Sierra Leone. One Earth 2021, 4, 104–113. [Google Scholar] [CrossRef]
- Morlanés, N.; Katikaneni, S.P.; Paglieri, S.N.; Harale, A.; Solami, B.; Sarathy, S.M.; Gascon, J. A technological roadmap to the ammonia energy economy: Current state and missing technologies. Chem. Eng. J. 2020, 408, 127310. [Google Scholar] [CrossRef]
- Zang, G.; Sun, P.; Elgowainy, A.; Wang, M. Technoeconomic and Life Cycle Analysis of Synthetic Methanol Production from Hydrogen and Industrial Byproduct CO2. Environ. Sci. Technol. 2021, 55, 5248–5257. [Google Scholar] [CrossRef]
- Fernandez, C.A.; Hatzell, M.C. Editors’ Choice—Economic Considerations for Low-Temperature Electrochemical Ammonia Production: Achieving Haber-Bosch Parity. J. Electrochem. Soc. 2020, 167, 143504. [Google Scholar] [CrossRef]
- MacFarlane, D.R.; Cherepanov, P.V.; Choi, J.; Suryanto, B.H.; Hodgetts, R.Y.; Bakker, J.M.; Vallana, F.M.F.; Simonov, A.N. A Roadmap to the Ammonia Economy. Joule 2020, 4, 1186–1205. [Google Scholar] [CrossRef]
- Lin, B.; Theodore, W.; Mahdi, M. Performance of a small-scale Haber process: A techno-economic analysis. ACS Sustain. Chem. Eng. 2020, 8, 15517–15531. [Google Scholar] [CrossRef]
- Bose, A.; Nikifar, L.; Michal, G.; Karthish, M.; Dharik, M. Spatial variation in cost of electricity-driven continuous ammonia production in the United States. ACS Sustain. Chem. Eng. 2022, 10, 7862–7872. [Google Scholar] [CrossRef]
- Nayak-Luke, R.M.; Bañares-Alcántara, R. Techno-economic viability of islanded green ammonia as a carbon-free energy vector and as a substitute for conventional production. Energy Environ. Sci. 2020, 13, 2957–2966. [Google Scholar] [CrossRef]
- Nayak-Luke, R.M.; Forbes, C.; Cesaro, Z.; Bañares-Alcántara, R.; Rouwenhorst KH, R. Techno-Economic Aspects of Production, Storage and Distribution of Ammonia. In Techno-Economic Challenges of Green Ammonia as Energy Vector; Bañares-Alcántara, R., Valera-Medina, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 191–208. [Google Scholar]
- Morgan, E.R.; Manwell, J.F.; McGowan, J.G. Sustainable Ammonia Production from U.S. Offshore Wind Farms: A Techno-Economic Review. ACS Sustain. Chem. Eng. 2017, 5, 9554–9567. [Google Scholar] [CrossRef]
- Demirel, Y.; Matzen, M.; Alhajji, M. Technoeconomics and Sustainability of Renewable Methanol and Ammonia Productions Using Wind Power-based Hydrogen. J. Adv. Chem. Eng. 2015, 5. [Google Scholar] [CrossRef] [Green Version]
- Akbari, M.; Oyedun, A.O.; Kumar, A. Ammonia production from black liquor gasification and co-gasification with pulp and waste sludges: A techno-economic assessment. Energy 2018, 151, 133–143. [Google Scholar] [CrossRef]
- Yáñez, M.; Relvas, F.M.; Ortiz, A.; Gorri, D.; Mendes, A.; Ortiz, I. PSA purification of waste hydrogen from ammonia plants to fuel cell grade. Sep. Purif. Technol. 2019, 240, 116334. [Google Scholar] [CrossRef]
- Valera-Medina, A.; Amer-Hatem, F.; Azad, A.K.; Dedoussi, I.C.; de Joannon, M.; Fernandes, R.X.; Glarborg, P.; Hashemi, H.; He, X.; Mashruk, S.; et al. Review on Ammonia as a Potential Fuel: From Synthesis to Economics. Energy Fuels 2021, 35, 6964–7029. [Google Scholar] [CrossRef]
- Ghavam, S.; Garcia-Garcia, G.; Styring, P. A novel approach to ammonia synthesis from hydrogen sulfide. Int. J. Hydrogen Energy 2020, 46, 4072–4086. [Google Scholar] [CrossRef]
- Sadeek, S.; Chan, T.L.; Ramdath, R.; Rajkumar, A.; Guo, M.; Ward, K. The influence of raw material availability and utility power consumption on the sustainability of the ammonia process. Chem. Eng. Res. Des. 2020, 158, 177–192. [Google Scholar] [CrossRef]
- Long, J.; Chen, S.; Zhang, Y.; Guo, C.; Fu, X.; Deng, D.; Xiao, J. Direct Electrochemical Ammonia Synthesis from Nitric Oxide. Angew. Chem. Int. Ed. 2020, 59, 9711–9718. [Google Scholar] [CrossRef] [PubMed]
- Bicer, Y.; Khalid, F. Life cycle environmental impact comparison of solid oxide fuel cells fueled by natural gas, hydrogen, ammonia and methanol for combined heat and power generation. Int. J. Hydrogen Energy 2020, 45, 3670–3685. [Google Scholar] [CrossRef]
- Al-Aboosi, F.Y.; Mahmoud, M.E.-H.; Margaux, M.; Rasmus, B.N. Renewable ammonia as an alter-native fuel for the shipping industry. Curr. Opin. Chem. Eng. 2021, 31, 100670. [Google Scholar] [CrossRef]
- Lazouski, N.; Chung, M.; Williams, K.; Gala, M.L.; Manthiram, K. Non-aqueous gas diffusion electrodes for rapid ammonia synthesis from nitrogen and water-splitting-derived hydrogen. Nat. Catal. 2020, 3, 463–469. [Google Scholar] [CrossRef]
- Sajid, M.U.; Bicer, Y. Thermodynamic assessment of chemical looping combustion and solar thermal methane cracking-based integrated system for green ammonia production. Therm. Sci. Eng. Prog. 2020, 19, 100588. [Google Scholar] [CrossRef]
- Hansson, J.; Brynolf, S.; Fridell, E.; Lehtveer, M. The Potential Role of Ammonia as Marine Fuel—Based on Energy Systems Modeling and Multi-Criteria Decision Analysis. Sustainability 2020, 12, 3265. [Google Scholar] [CrossRef] [Green Version]
- Sharma, R.K.; Patel, H.; Mushtaq, U.; Kyriakou, V.; Zafeiropoulos, G.; Peeters, F.; Welzel, S.; van de Sanden, M.C.M.; Tsampas, M.N. Plasma Activated Electrochemical Ammonia Synthesis from Nitrogen and Water. ACS Energy Lett. 2020, 6, 313–319. [Google Scholar] [CrossRef]
- Alrebei, O.F.; Al-Doboon, A.; Bowen, P.; Medina, A.V. CO2-Argon-Steam Oxy-Fuel Production for (CARSOXY) Gas Turbines. Energies 2019, 12, 3580. [Google Scholar] [CrossRef] [Green Version]
- Fawwaz Alrebei, O.I.; Amhamed, A.; Mashruk, S.; Bowen, P.; Valera Medina, A. Planar la-ser-induced fluorescence and chemiluminescence analyses of CO2-argon-steam oxyfuel (CARSOXY) combustion. Energies 2021, 15, 263. [Google Scholar] [CrossRef]
- Alrebei, O.F.; Bowen, P.; Medina, A.V. Parametric Study of Various Thermodynamic Cycles for the Use of Unconventional Blends. Energies 2020, 13, 4656. [Google Scholar] [CrossRef]
- Alrebei, O.F.; Amhamed, A.I.; El-Naas, M.H.; Hayajnh, M.; Orabi, Y.A.; Fawaz, W.; Al-Tawaha, A.S.; Medina, A.V. State of the Art in Separation Processes for Alternative Working Fluids in Clean and Efficient Power Generation. Separations 2022, 9, 14. [Google Scholar] [CrossRef]
- Chehade, G.; Dincer, I. Development and analysis of a polygenerational smart energy hub for sustainable communities. Energy Convers. Manag. 2020, 226, 113475. [Google Scholar] [CrossRef]
- Pfromm, P.H. Towards sustainable agriculture: Fossil-free ammonia. J. Renew. Sustain. Energy 2017, 9, 034702. [Google Scholar] [CrossRef] [Green Version]
- Frattini, D.; Cinti, G.; Bidini, G.; Desideri, U.; Cioffi, R.; Jannelli, E. A system approach in energy evaluation of different renewable energies sources integration in ammonia production plants. Renew. Energy 2016, 99, 472–482. [Google Scholar] [CrossRef]
- McEnaney, J.M.; Singh, A.R.; Schwalbe, J.A.; Kibsgaard, J.; Lin, J.C.; Cargnello, M.; Nørskov, J.K. Ammonia synthesis from N2 and H2O using a lithium cycling electrification strategy at atmospheric pressure. Energy Environ. Sci. 2017, 10, 1621–1630. [Google Scholar] [CrossRef] [Green Version]
- Cinti, G.; Frattini, D.; Jannelli, E.; Desideri, U.; Bidini, G. Coupling Solid Oxide Electrolyser (SOE) and ammonia production plant. Appl. Energy 2017, 192, 466–476. [Google Scholar] [CrossRef] [Green Version]
- Ghannadzadeh, A.; Sadeqzadeh, M. Diagnosis of an alternative ammonia process technology to reduce exergy losses. Energy Convers. Manag. 2016, 109, 63–70. [Google Scholar] [CrossRef]
- Bicer, Y.; Dincer, I. Life cycle assessment of nuclear-based hydrogen and ammonia production options: A comparative evaluation. Int. J. Hydrogen Energy 2017, 42, 21559–21570. [Google Scholar] [CrossRef]
- Wang, M.; Khan, M.A.; Mohsin, I.; Wicks, J.; Ip, A.H.; Sumon, K.Z.; Dinh, C.-T.; Sargent, E.H.; Gates, I.D.; Kibria, G. Can sustainable ammonia synthesis pathways compete with fossil-fuel based Haber–Bosch processes? Energy Environ. Sci. 2021, 14, 2535–2548. [Google Scholar] [CrossRef]
- Rouwenhorst, K.H.; Lefferts, L. Feasibility study of plasma-catalytic ammonia synthesis for energy storage applications. Catalysts 2020, 10, 999. [Google Scholar] [CrossRef]
- Barboun, P.M.; Hicks, J.C. Unconventional catalytic approaches to ammonia synthesis. Annu. Rev. Chem. Biomol. Eng. 2020, 11, 503–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- An, Q.; Mcdonald, M.; Fortunelli, A.; Goddard, W.A. Si-Doped Fe Catalyst for Ammonia Synthesis at Dramatically Decreased Pressures and Temperatures. J. Am. Chem. Soc. 2020, 142, 8223–8232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, B.; Ding, W.; Zhang, H.; Zhang, S. Recent progress in electrochemical synthesis of ammonia from nitrogen: Strategies to improve the catalytic activity and selectivity. Energy Environ. Sci. 2021, 14, 672–687. [Google Scholar] [CrossRef]
- Hao, D.; Chen, Z.-G.; Figiela, M.; Stepniak, I.; Wei, W.; Ni, B.-J. Emerging alternative for artificial ammonia synthesis through catalytic nitrate reduction. J. Mater. Sci. Technol. 2021, 77, 163–168. [Google Scholar] [CrossRef]
- Patil, B.S.; Cherkasov, N.; Srinath, N.V.; Lang, J.; Ibhadon, A.O.; Wang, Q.; Hessel, V. The role of heterogeneous catalysts in the plasma-catalytic ammonia synthesis. Catal. Today 2021, 362, 2–10. [Google Scholar] [CrossRef]
- Ooya, K.; Li, J.; Fukui, K.; Iimura, S.; Nakao, T.; Ogasawara, K.; Sasase, M.; Abe, H.; Niwa, Y.; Kitano, M.; et al. Ruthenium Catalysts Promoted by Lanthanide Oxyhydrides with High Hydride-Ion Mobility for Low-Temperature Ammonia Synthesis. Adv. Energy Mater. 2021, 11, 2003723. [Google Scholar] [CrossRef]
- Hollevoet, L.; Jardali, F.; Gorbanev, Y.; Creel, J.; Bogaerts, A.; Martens, J.A. Towards Green Ammonia Synthesis through Plasma-Driven Nitrogen Oxidation and Catalytic Reduction. Angew. Chem. 2020, 132, 24033–24037. [Google Scholar] [CrossRef]
- Humphreys, J.; Lan, R.; Chen, S.; Tao, S. Improved stability and activity of Fe-based catalysts through strong metal support interactions due to extrinsic oxygen vacancies in Ce0.8Sm0.2O2−δ for the efficient synthesis of ammonia. J. Mater. Chem. A 2020, 8, 16676–16689. [Google Scholar] [CrossRef]
- Nanba, T.; Nagata, Y.; Kobayashi, K.; Javaid, R.; Atsumi, R.; Nishi, M.; Mochizuki, T.; Manaka, Y.; Kojima, H.; Tsujimura, T.; et al. Explorative Study of a Ru/CeO2 Catalyst for NH3 Synthesis from Renewable Hydrogen and Demonstration of NH3 Synthesis under a Range of Reaction Conditions. J. Jpn. Pet. Inst. 2021, 64, 1–9. [Google Scholar] [CrossRef]
- Wang, J.; Feng, T.; Chen, J.; Ramalingam, V.; Li, Z.; Kabtamu, D.M.; Fang, X. Electrocatalytic nitrate/nitrite reduction to ammonia synthesis using metal nanocatalysts and bio-inspired metalloen-zymes. Nano Energy 2021, 86, 106088. [Google Scholar] [CrossRef]
- Patil, B.S.; Van Kaathoven, A.S.; Peeters, F.J.; Cherkasov, N.; Lang, J.; Wang, Q.; Hessel, V. Deciphering the synergy between plasma and catalyst support for ammonia synthesis in a packed dielectric barrier discharge reactor. J. Phys. D Appl. Phys. 2020, 53, 144003. [Google Scholar] [CrossRef]
- Utomo, W.P.; Leung, M.K.H.; Yin, Z.; Wu, H.; Ng, Y.H. Advancement of Bismuth-Based Materials for Electrocatalytic and Photo(electro)catalytic Ammonia Synthesis. Adv. Funct. Mater. 2021, 32, 2106713. [Google Scholar] [CrossRef]
- Carreon, M.L. Plasma catalytic ammonia synthesis: State of the art and future directions. J. Phys. D Appl. Phys. 2019, 52, 483001. [Google Scholar] [CrossRef]
- Akay, G.; Zhang, K. Process Intensification in Ammonia Synthesis Using Novel Coassembled Supported Microporous Catalysts Promoted by Nonthermal Plasma. Ind. Eng. Chem. Res. 2017, 56, 457–468. [Google Scholar] [CrossRef] [Green Version]
- Barboun, P.M.; Mehta, P.; Herrera, F.A.; Go, D.B.; Schneider, W.F.; Hicks, J.C. Distinguishing Plasma Contributions to Catalyst Performance in Plasma-Assisted Ammonia Synthesis. ACS Sustain. Chem. Eng. 2019, 7, 8621–8630. [Google Scholar] [CrossRef]
- Tang, Y.; Kobayashi, Y.; Masuda, N.; Uchida, Y.; Okamoto, H.; Kageyama, T.; Kageyama, H. Metal-Dependent Support Effects of Oxyhydride-Supported Ru, Fe, Co Catalysts for Ammonia Synthesis. Adv. Energy Mater. 2018, 8, 1801772. [Google Scholar] [CrossRef]
- Wang, H.; Li, Y.; Li, C.; Deng, K.; Wang, Z.; Xu, Y.; Wang, L. One-pot synthesis of bi-metallic PdRu tripods as an efficient catalyst for electrocatalytic nitrogen reduction to ammonia. J. Mater. Chem. A 2019, 7, 801–805. [Google Scholar] [CrossRef]
- Riotto, T.; Cao, G.; Luyben, W.L.; Baltrusaitis, J. Atmospheric Pressure DBD Plasma Ammonia Synthesis and Separation Process Design and Environmental Impact Assessment. ACS Sustain. Chem. Eng. 2021, 9, 13233–13244. [Google Scholar] [CrossRef]
- Hattori, M.; Mori, T.; Arai, T.; Inoue, Y.; Sasase, M.; Tada, T.; Kitano, M.; Yokoyama, T.; Hara, M.; Hosono, H. Enhanced Catalytic Ammonia Synthesis with Transformed BaO. ACS Catal. 2018, 8, 10977–10984. [Google Scholar] [CrossRef]
- Humphreys, J. The Development of Water and Oxygen Tolerant Catalysts for Ammonia Synthesis. Ph.D. Thesis, University of Warwick, Warwick, UK, 2019. [Google Scholar]
- Abghoui, Y.; Skúlason, E. Computational Predictions of Catalytic Activity of Zincblende (110) Surfaces of Metal Nitrides for Electrochemical Ammonia Synthesis. J. Phys. Chem. C 2017, 121, 6141–6151. [Google Scholar] [CrossRef]
- Hawtof, R.; Ghosh, S.; Guarr, E.; Xu, C.; Sankaran, R.M.; Renner, J.N. Catalyst-free, highly selective synthesis of ammonia from nitrogen and water by a plasma electrolytic system. Sci. Adv. 2019, 5, 5778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogura, Y.; Tsujimaru, K.; Sato, K.; Miyahara, S.I.; Toriyama, T.; Yamamoto, T.; Nagaoka, K. Ru/La0. 5Pr0. 5O1. 75 catalyst for low-temperature ammonia synthesis. ACS Sustain. Chem. Eng. 2018, 6, 17258–17266. [Google Scholar] [CrossRef]
- Rossetti, I. Reactor Design, Modelling and Process Intensification for Ammonia Synthesis. In Sustainable Ammonia Production; Springer: Cham, Switzerland, 2020; pp. 17–48. [Google Scholar]
- Ishaq, H.; Dincer, I. Design and simulation of a new cascaded ammonia synthesis system driven by renewables. Sustain. Energy Technol. Assess. 2020, 40, 100725. [Google Scholar] [CrossRef]
- Han, Z.; Xu, Y.; Wang, H.; Tian, H.; Qiu, B.; Sun, D. Synthesis of ammonia molecularly im-printed adsorbents and ammonia adsorption separation during sludge aerobic composting. Bioresour. Technol. 2020, 300, 122670. [Google Scholar] [CrossRef]
- Kyriakou, V.; Garagounis, I.; Vourros, A.; Vasileiou, E.; Stoukides, M. An electrochemical haber-bosch process. Joule 2020, 4, 142–158. [Google Scholar] [CrossRef]
- Pereira, R.J.L.; Argyris, P.A.; Spallina, V. A comparative study on clean ammonia production using chemical looping based technology. Appl. Energy 2020, 280, 115874. [Google Scholar] [CrossRef]
- Shen, H.; Choi, C.; Masa, J.; Li, X.; Qiu, J.; Jung, Y.; Sun, Z. Electrochemical ammonia synthesis: Mechanistic understanding and catalyst design. Chem 2021, 7, 1708–1754. [Google Scholar] [CrossRef]
- Ozturk, M.; Dincer, I. An integrated system for ammonia production from renewable hydrogen: A case study. Int. J. Hydrogen Energy 2021, 46, 5918–5925. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, J.; Ma, Y.; Wu, W.; Huang, K.; Jiang, L. Designing Low-Viscosity Deep Eutectic Solvents with Multiple Weak-Acidic Groups for Ammonia Separation. ACS Sustain. Chem. Eng. 2021, 9, 7352–7360. [Google Scholar] [CrossRef]
- Duan, G.; Chen, Y.; Tang, Y.; Gasem, K.A.; Wan, P.; Ding, D.; Fan, M. Advances in electrocatalytic ammonia synthesis under mild conditions. Prog. Energy Combust. Sci. 2020, 81, 100860. [Google Scholar] [CrossRef]
- Wang, J.; Chen, S.; Li, Z.; Li, G.; Liu, X. Recent Advances in Electrochemical Synthesis of Ammonia through Nitrogen Reduction under Ambient Conditions. ChemElectroChem 2020, 7, 1067–1079. [Google Scholar] [CrossRef]
- Hu, J.; Wildfire, C.; Stiegman, A.E.; Dagle, R.A.; Shekhawat, D.; Abdelsayed, V.; Bai, X.; Tian, H.; Bogle, M.B.; Hsu, C.; et al. Microwave-driven heterogeneous catalysis for activation of dinitrogen to ammonia under atmospheric pressure. Chem. Eng. J. 2020, 397, 125388. [Google Scholar] [CrossRef]
- Qin, Q.; Oschatz, M. Overcoming chemical inertness under ambient conditions: A critical view on recent devel-opments in ammonia synthesis via electrochemical N2 reduction by asking five questions. ChemElectroChem 2020, 7, 878–889. [Google Scholar] [CrossRef]
- Liu, A.; Yang, Y.; Ren, X.; Zhao, Q.; Gao, M.; Guan, W.; Ma, T. Current progress of electrocatalysts for ammonia synthesis through electrochemical nitrogen reduction under ambient conditions. ChemSusChem 2020, 13, 3766–3788. [Google Scholar] [CrossRef]
- Badescu, V. Optimal design and operation of ammonia decomposition reactors. Int. J. Energy Res. 2020, 44, 5360–5384. [Google Scholar] [CrossRef]
- Ogura, Y.; Asai, T.; Sato, K.; Miyahara, S.I.; Toriyama, T.; Yamamoto, T.; Nagaoka, K. Effect of Calcination and Reduction Temperatures on the Catalytic Activity of Ru/La0.5 Ce0.5O1.75 for Ammonia Synthesis under Mild Conditions. Energy Technol. 2020, 8, 2000264. [Google Scholar] [CrossRef]
- Bland, M.J. Optimisation of an Ammonia Synthesis Loop-Investigation of a Novel Approach for Optimisation of Integrated Plants. Master’s Thesis, NTNU, Trondheim, Norway, 2015. [Google Scholar]
- Zeinalipour-Yazdi, C.D.; Hargreaves, J.S.; Catlow, C.R.A. Low-T mechanisms of ammonia syn-thesis on Co3Mo3N. J. Phys. Chem. C 2018, 122, 6078–6082. [Google Scholar] [CrossRef] [Green Version]
- Bicer, Y.; Dincer, I.; Zamfirescu, C.; Vezina, G.; Raso, F. Comparative life cycle assessment of various ammonia production methods. J. Clean. Prod. 2016, 135, 1379–1395. [Google Scholar] [CrossRef]
- Chen, C.; Lovegrove, K.M.; Sepulveda, A.; Lavine, A.S. Design and optimization of an ammonia synthesis system for ammonia-based solar thermochemical energy storage. Sol. Energy 2018, 159, 992–1002. [Google Scholar] [CrossRef]
- Michalsky, R.; Pfromm, P.H.; Steinfeld, A. Rational design of metal nitride redox materials for solar-driven ammonia synthesis. Interface Focus 2015, 5, 20140084. [Google Scholar] [CrossRef]
- Ghani, R.; Iranshahi, D. Conceptual comparison of four configurations in the thermal coupling of ammonia synthesis and 2-butanol dehydrogenation. Appl. Therm. Eng. 2019, 154, 238–250. [Google Scholar] [CrossRef]
- Guo, W.; Liang, Z.; Zhao, J.; Zhu, B.; Cai, K.; Zou, R.; Xu, Q. Hierarchical cobalt phosphide hollow nanocages toward electrocatalytic ammonia synthesis under ambient pressure and room temperature. Small Methods 2018, 2, 1800204. [Google Scholar] [CrossRef]
- Nayak-Luke, R.; Bañares-Alcántara, R.; Wilkinson, I. Green ammonia: Impact of renewable energy inter-mittency on plant sizing and levelized cost of ammonia. Ind. Eng. Chem. Res. 2018, 57, 14607–14616. [Google Scholar] [CrossRef] [Green Version]
- Penkuhn, M.; Tsatsaronis, G. Comparison of different ammonia synthesis loop configurations with the aid of advanced exergy analysis. Energy 2017, 137, 854–864. [Google Scholar] [CrossRef]
- Li, J. An Integrated Evaluation Method with Application to a New Ammonia Synthesis Process Design. In Proceedings of the 2019 AIChE Annual Meeting, Orlando, FL, USA, 10–15 November 2019. [Google Scholar]
- Anastasopoulou, A.; Keijzer, R.; Patil, B.; Lang, J.; van Rooij, G.; Hessel, V. Environmental impact assessment of plasma-assisted and conventional ammonia synthesis routes. J. Ind. Ecol. 2020, 24, 1171–1185. [Google Scholar] [CrossRef]
- Fu, Y.; Richardson, P.; Li, K.; Yu, H.; Yu, B.; Donne, S.; Ma, T. Transition metal alu-minum boride as a new candidate for ambient-condition electrochemical ammonia synthesis. Nanomicro Lett. 2020, 12, 65. [Google Scholar]
- Chisalita, D.-A.; Petrescu, L.; Cormos, C.-C. Environmental evaluation of european ammonia production considering various hydrogen supply chains. Renew. Sustain. Energy Rev. 2020, 130, 109964. [Google Scholar] [CrossRef]
- Chehade, G.; Dincer, I. Progress in green ammonia production as potential carbon-free fuel. Fuel 2021, 299, 120845. [Google Scholar] [CrossRef]
- Smith, C.; Hill, A.K.; Torrente-Murciano, L. Current and future role of Haber–Bosch ammonia in a carbon-free energy landscape. Energy Environ. Sci. 2020, 13, 331–344. [Google Scholar] [CrossRef]
- Siddiqui, O.; Ishaq, H.; Chehade, G.; Dincer, I. Performance investigation of a new renewable energy-based carbon dioxide capturing system with aqueous ammonia. Int. J. Energy Res. 2019, 44, 2252–2263. [Google Scholar] [CrossRef]
- McPherson, I.; Zhang, J. Can Electrification of Ammonia Synthesis Decrease Its Carbon Footprint? Joule 2020, 4, 12–14. [Google Scholar] [CrossRef]
- Castellani, B.; Rinaldi, S.; Morini, E.; Nastasi, B.; Rossi, F. Flue gas treatment by power-to-gas integration for methane and ammonia synthesis—Energy and environmental analysis. Energy Convers. Manag. 2018, 171, 626–634. [Google Scholar] [CrossRef]
- Arora, P.; Hoadley, A.F.; Mahajani, S.M.; Ganesh, A. Small-Scale Ammonia Production from Biomass: A Techno-Enviro-Economic Perspective. Ind. Eng. Chem. Res. 2016, 55, 6422–6434. [Google Scholar] [CrossRef]
- Bicer, Y.; Dincer, I.; Vezina, G.; Raso, F. Impact Assessment and Environmental Evaluation of Various Ammonia Production Processes. Environ. Manag. 2017, 59, 842–855. [Google Scholar] [CrossRef] [PubMed]
- Flórez-Orrego, D.; Junior, S.D.O. On the efficiency, exergy costs and CO2 emission cost allocation for an integrated syngas and ammonia production plant. Energy 2016, 117, 341–360. [Google Scholar] [CrossRef]
- Essehli, R.; Sabri, S.; El-Mellouhi, F.; Aïssa, B.; Ben Yahia, H.; Altamash, T.; El Bali, B. Single crystal structure, vibrational spectroscopy, gas sorption and antimicrobial properties of a new inorganic acidic diphosphates material (NH4)2Mg (H2P2O7)2•2H2O. Sci. Rep. 2020, 10, 8909. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, L.; Van Herle, J.; Maréchal, F.; Desideri, U. Techno-economic comparison of green ammonia production processes. Appl. Energy 2019, 259, 114135. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).