Abstract
After decades of pioneering advances and improvements, kidney transplantation is now the renal replacement therapy of choice for most patients with end-stage kidney disease (ESKD). Despite this success, the high risk of premature death and frequent occurrence of graft failure remain important clinical and research challenges. The current burst of studies and other innovative initiatives using artificial intelligence (AI) for a wide range of analytical and practical applications in biomedical areas seems to correlate with the same trend observed in publications in the kidney transplantation field, and points toward the potential of such novel approaches to address the aforementioned aim of improving long-term outcomes of kidney transplant recipients (KTR). However, at the same time, this trend underscores now more than ever the old methodological challenges and potential threats that the research and clinical community needs to be aware of and actively look after with regard to AI-driven evidence. The purpose of this narrative mini-review is to explore challenges for obtaining applicable and adequate kidney transplant data for analyses using AI techniques to develop prediction models, and to propose next steps in the field. We make a call to act toward establishing the strong collaborations needed to bring innovative synergies further augmented by AI, which have the potential to impact the long-term care of KTR. We encourage researchers and clinicians to submit their invaluable research, including original clinical and imaging studies, database studies from registries, meta-analyses, and AI research in the kidney transplantation field.
1. Introduction
After decades of pioneering advances and improvements, kidney transplantation is now the renal replacement therapy of choice for most patients with end-stage kidney disease (ESKD) because it offers higher survival rates and arguably better quality of life after transplantation. Despite this success, the high risk of premature death and frequent occurrence of graft failure requiring return to dialysis or re-transplantation remain important challenges for the research community and a constantly perceived threat for kidney transplant recipients (KTR) [1,2]. Moreover, the occurrence of graft failure imposes a huge socio-economic impact due to the higher costs for dialysis [3], decreased quality of life, and increased mortality risk [4]. Furthermore, taking into account the scarcity of organ donors, the prevention of re-transplantation through improvements in graft survival stands as an issue of paramount importance as it may translate into relief from the existing organ shortage [5]. This underscores a great need for early identification–allowing, in turn, timely management–of KTR at high risk of graft failure and other adverse long-term outcomes post-kidney transplantation, such as post-transplant diabetes, cardiovascular events, malignancy, and death.
Artificial intelligence (AI) in medicine is a developing field that promises meaningful improvements in patient care. Currently, the clinical and research community is observing a burst of studies and other innovative initiatives using AI for a wide range of analytical and practical applications in biomedical areas. This trend also holds true in the kidney transplant field, which is evident from the number studies using AI techniques for the prediction of kidney transplant outcomes that have been published over the last decades, but mostly over the last 5 years, and particularly in 2020 (Figure 1).
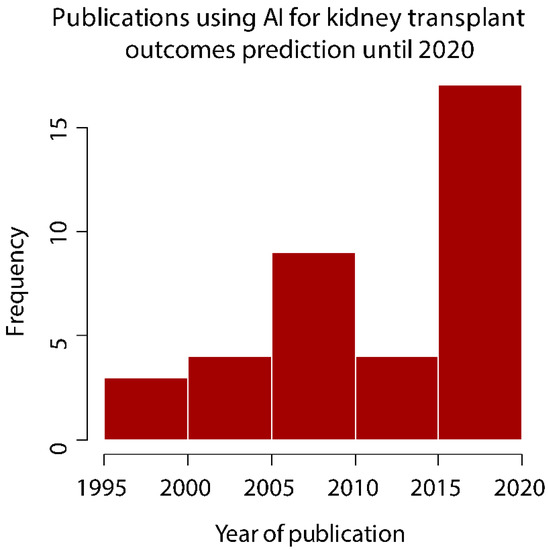
Figure 1.
Publications using artificial intelligence to develop prediction models for kidney transplant outcomes.
2. AI and Kidney Transplantation, and Modeling
In this context, the broad term AI is not a specific technology, but refers to the set of non-traditional methods and techniques that allow analyzing different types of clinical data either to find patterns or correlations within the data, or to generate predictive models with diverse applications, such as diagnosis and management of a disease or intervention [6]. Among these, machine learning (ML) refers to the set of algorithms that improve its results automatically through experience. It also includes the statistical techniques to produce models from training data without being explicitly programmed to do so, and without human intervention or command in every process [7]. This is opposed to traditional methods of statistical analysis and modeling where every step of data analysis is performed by a human. The different machine learning algorithms used for this purpose can be classified as supervised (e.g., decision trees, K-nearest neighbors), unsupervised (e.g., K-means clustering, hierarchical clustering), reinforcement learning (e.g., Q-learning, TD-Lambda), and deep learning (DL). DL is sub-field of ML that in recent years has increasingly been used in the biomedical field. It is based on multiple networks of nodes, often loosely simulating a set of neurons in a biological brain, modeling its connections, inputs, and outputs and weighing different variables of these connections to produce a prediction model [8].
Nonetheless, all AI-based approaches need data, often large sets of accurate data for training, validation, and testing. International efforts to create large datasets are expected to accelerate future advancements in the kidney transplantation field, and to pave the way toward alleviating the longstanding burden of adverse long-term outcomes post-kidney transplantation [9,10]. Such efforts require sufficient funding for setting up the architectures and communication systems that allow timely extraction and storage of the data, as well as for maintaining and potentially updating the resulting databases. An important premise to take into account is that the quality of the resulting prediction models will depend on how these data are collected, their amount, and their heterogeneity [6].
Indeed, because the great importance of collecting a large quantity of health-related data among human cohorts has increasingly been recognized, there have been many database initiatives specific to kidney diseases and kidney transplantation implementing a model of big data collection and storage [11,12,13,14], where different types of information are stored. It is relevant to point out that traditional statistics and modeling also use and analyze data gathered by these databases, generating relevant findings and the development of prediction tools and creating the need for quality data transversal for research [15]. In most countries, however, only rudimentary databases, derived from electronic health records (EHRs) or associated with the waiting-list system for organ transplantation, have been implemented. As recently shown by Thongprayoon et al. [16], the list of countries that have robust kidney transplant databases is short, and is mostly limited to the United States, Canada, China, Ireland, and a few European countries.
Table 1 provides an overview of the number, year and country of origin, aims, origin of database, sample size, and findings of the studies on kidney transplant outcomes performed with artificial intelligence. For the development of this table, a restricted literature search was performed on 4 January 2021, and included all articles up to December 2020 using the keywords “artificial intelligence”, “machine learning”, and “deep learning” paired with “kidney transplantation” through the PubMed and Google Scholar search engines, thus obtaining a total of 451 articles. A selection of 25 original articles studying kidney transplantation outcomes with AI methodologies were included. The reference lists in these articles were also searched for relevant studies on kidney transplant outcomes performed with artificial intelligence. We found 13 articles, for a total of 38 studies, which are described herein. The main finding that arises from this table is that most of the articles used data from established databases, often financed by the government, and the actual list of countries of origin of these databases is rather narrow, in agreement with recent observations by Thongprayoon et al. [16]. Moreover, studies that used established databases of this kind often included a larger number of patients than databases created for the purpose of the studies themselves. Important clinical findings and prediction models have arisen from these large and well-defined databases, yet a small number of studies have also produced and reported prediction models with acceptable performance using databases derived from small, single-center sources [17,18,19]. In relation to this, it should be noted that one of the main problems that may arise with such approaches is bias, ultimately limiting the potential and rate of success applying a model obtained from one hospital’s data or region to another hospital or region. This is of concern because it may lead to the exacerbation of health disparities as pointed out by many researchers [20,21], not only unfavorable disparities for specific groups within a country due to underrepresentation in the corresponding database, but also for whole populations belonging to countries that have not started or are lagging behind on the path to performing large data collection projects for Big Data and AI-based research. This, coupled with the increasingly higher impact of genomics in kidney transplantation [22], such as genome-wide association studies, could exclude entire populations from the benefits of advances in long-term post-kidney transplant follow-up and management. Moreover, seemingly powerful models derived from biased databases may have the counterintuitive effect of leading to a false sense of general and robust capacity for prediction and identification of high-risk patients.

Table 1.
Studies in the field of kidney transplantation that have used AI for data analysis.
Data bias is an inherent problem of AI that is exacerbated by the black-box nature of AI models, and by lack of contextual specificity. Some approaches to solve this problem include weighing data for underrepresented populations, or establishing a “human in the loop” process to monitor possible biases. This phenomenon is still present when trying to develop a “general population” AI model, as it only becomes feasible when the data reflect the vast and rich diversity of populations [23].
3. Conclusion and Future Perspectives
The conversion of rudimentary databases that most countries already have to a standard set is a reasonable goal to work toward, as it would greatly benefit AI findings by increasing representation. As shown in Table 1, when countries develop and maintain a database, studies over those populations have access to a much larger number of patients, generating better and more reliable findings. It seems compelling to describe a standard database for the development of ethnic, gender, socioeconomic, and cross-border proof kidney transplant models, establishing a common minimum of epidemiological, clinical, laboratory, genomic, and imaging data, on both donors and recipients, with the collection of well-established and relevant long-term follow-up outcomes.
Kidney transplant databases should be one of the first widespread worldwide implementations of databases to take advantage of the growing field of AI in medicine, as the availability of more and more diverse datasets will enable better AI model generation, reducing biases derived from limited populations, without restricting findings that may be particular to one population. Still, many challenges plague this adoption as a standard, and future research will require broad inter-disciplinary initiatives to take full advantage of AI in the kidney transplantation field. Whether currently used and novel imaging modalities to study kidney transplant function prior to and post-kidney transplant may be enhanced by AI remains unexplored, yet huge potential is expected in upcoming years for the evaluation and follow-up of kidney transplant recipients [59]. It should be emphasized that the success envisioned by combining imaging and AI in kidney transplantation will largely depend on strong and long-lasting collaborations between fields. In this regard, Benjamens et al. recently made a call to encourage transplant organizations to aim for partnerships with diagnostic imaging societies [59]. We support this call, as it may lead to fruitful and innovative synergies further augmented by AI, with the potential to impact the long-term care of KTR.
Thus, we encourage researchers and clinicians to submit their invaluable research, including original clinical and imaging studies, database studies from registries, meta-analyses, and AI research in the kidney transplantation field.
Author Contributions
Conceptualization, C.G.S.; formal analysis, C.G.S.; investigation, R.C.-A. and C.G.S.; data curation, R.C.-A.; writing—original draft preparation, R.C.-A. and C.G.S.; writing—review and editing, C.G.S.; visualization, C.G.S.; and supervision, C.G.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nilsson, M.; Forsberg, A.; Lennerling, A.; Persson, L.-O. Coping in relation to perceived threat of the risk of graft rejection and Health-Related Quality of Life of organ transplant recipients. Scand. J. Caring Sci. 2013, 27, 935–944. [Google Scholar] [CrossRef]
- Forsberg, A.; Lennerling, A.; Fridh, I.; Karlsson, V.; Nilsson, M. Understanding the Perceived Threat of the Risk of Graft Rejections. Glob. Qual. Nurs. Res. 2015, 2, 233339361456382. [Google Scholar] [CrossRef] [PubMed]
- Mohnen, S.M.; van Oosten, M.J.M.; Los, J.; Leegte, M.J.H.; Jager, K.J.; Hemmelder, M.H.; Logtenberg, S.J.J.; Stel, S.V.; Roijen, H.L.; de Wit, G.A. Healthcare costs of patients on different renal replacement modalities—Analysis of Dutch health insurance claims data. PLoS ONE 2019, 14, e0220800. [Google Scholar] [CrossRef]
- Brar, A.; Markell, M.; Stefanov, D.G.; Timpo, E.; Jindal, R.M.; Nee, R.; Sumrani, N.; John, D.; Tedla, F.; Salifu, M.O. Mortality after Renal Allograft Failure and Return to Dialysis. Am. J. Nephrol. 2017, 45, 180–186. [Google Scholar] [CrossRef]
- United States Renal Data System. 2020 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States; National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health: Bethesda, MD, USA, 2020. [Google Scholar]
- Wang, F.; Preininger, A. AI in Health: State of the Art, Challenges, and Future Directions. Yearb. Med. Inform. 2019, 28, 16–26. [Google Scholar] [CrossRef]
- Watkins, C.J.C.H.; Dayan, P. Q-Learning. Mach. Learn. 1992, 8, 279–292. [Google Scholar] [CrossRef]
- Davenport, T.; Kalakota, R. The potential for artificial intelligence in healthcare. Futur. Healthc. J. 2019, 6, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Rashidi, P.; Bihorac, A. Artificial intelligence approaches to improve kidney care. Nat. Rev. Nephrol. 2020, 16, 71–72. [Google Scholar] [CrossRef] [PubMed]
- Díez-Sanmartín, C.; Sarasa Cabezuelo, A. Application of Artificial Intelligence Techniques to Predict Survival in Kidney Transplantation: A Review. J. Clin. Med. 2020, 9, 572. [Google Scholar] [CrossRef]
- United States Renal Data System. USRDS 2018 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States; National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health: Bethesda, MD, USA, 2018; USRDS Home Page; Available online: https://www.usrds.org/Default.aspx (accessed on 8 September 2019).
- OPTN: Organ Procurement and Transplantation Network—OPTN. Available online: https://optn.transplant.hrsa.gov/ (accessed on 1 February 2021).
- Find and Compare Transplant Programs. Available online: https://www.srtr.org/ (accessed on 1 February 2021).
- CKDdb. Available online: http://www.padb.org/ckddb/ (accessed on 1 February 2021).
- Loupy, A.; Aubert, O.; Orandi, B.J.; Naesens, M.; Bouatou, Y.; Raynaud, M.; Divard, G.; Jackson, A.M.; Viglietti, D.; Giral, M.; et al. Prediction system for risk of allograft loss in patients receiving kidney transplants: International derivation and validation study. BMJ 2019, 366, 14923. [Google Scholar] [CrossRef]
- Thongprayoon, C.; Kaewput, W.; Kovvuru, K.; Hansrivijit, P.; Kanduri, S.R.; Bathini, T.; Chewcharat, A.; Leeaphorn, N.; Gonzalez-Suarez, M.L.; Cheungpasitporn, W. Promises of Big Data and Artificial Intelligence in Nephrology and Transplantation. J. Clin. Med. 2020, 9, 1107. [Google Scholar] [CrossRef] [PubMed]
- DonYoo, K.; Noh, J.; Lee, H.; Kim, D.K.; Lim, C.S.; Kim, Y.H.; Lee, J.P.; Kim, G.; Kim, Y.S. A Machine Learning Approach Using Survival Statistics to Predict Graft Survival in Kidney Transplant Recipients: A Multicenter Cohort Study. Sci. Rep. 2017, 7, 8904. [Google Scholar]
- Bae, S.; Massie, A.B.; Thomas, A.G.; Bahn, G.; Luo, X.; Jackson, K.R.; Ottmann, S.E.; Brennan, D.C.; Desai, N.M.; Coresh, J.; et al. Who can tolerate a marginal kidney? Predicting survival after deceased donor kidney transplant by donor–recipient combination. Am. J. Transplant. 2019, 19, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Mark, E.; Goldsman, D.; Gurbaxani, B.; Keskinocakid, P.; Sokol, J.; Stewart, H.M. Using machine learning and an ensemble of methods to predict kidney transplant survival. PloS ONE 2019, 14, e0209068. [Google Scholar] [CrossRef]
- Gianfrancesco, M.A.; Tamang, S.; Yazdany, J.; Schmajuk, G. Potential Biases in ML Algorithms Using EHR Data. JAMA Intern Med. 2018, 178, 1544–1547. [Google Scholar] [CrossRef] [PubMed]
- Wyber, R.; Vaillancourt, S.; Perry, W.; Mannava, P.; Folaranmi, T.; Celi, L.A. Big data in global health: Improving health in low- and middle-income countries. Bull. World Health Organ. 2015, 93, 203–208. [Google Scholar] [CrossRef]
- Oetting, W.S.; Dorr, C.; Remmel, R.P.; Matas, A.J.; Israni, A.K.; Jacobson, P.A. Concepts of Genomics in Kidney Transplantation. Curr. Transplant. Rep. 2017, 4, 116–123. [Google Scholar] [CrossRef]
- Panch, T.; Mattie, H.; Atun, R. Artificial intelligence and algorithmic bias: Implications for health systems. J. Glob. Health 2019, 9, 1–5. [Google Scholar] [CrossRef]
- Abdolmaleki, P.; Movhead, M.; Taniguchi, R.-I.; Masuda, K.; Buadu, L.D. Evaluation of complications of kidney transplantation using artificial neural networks. Nucl. Med. Commun. 1997, 18, 623–630. [Google Scholar] [CrossRef]
- Shoskes, D.A.; Ty, R.; Barba, L.; Sender, M. Prediction of early graft function in renal transplantation using a computer neural network. Transplant. Proc. 1998, 30, 1316–1317. [Google Scholar] [CrossRef]
- Ahn, J.-H.; Kwon, J.-W.; Lee, Y.-S. Prediction of 1-year Graft Survival Rates in Kidney Transplantation: A Bayesian Network Model. Korean Oper. Res. Manag. Sci. Soc. 2000, 25, 505–513. [Google Scholar]
- Petrovsky, N.; Khum Tam, S.; Brusic, V.; Russ, G.; Socha, L.; Bajic, V.B. Use of artificial neural networks in improving renal transplantation outcomes. Graft 2002, 5, 6–13. [Google Scholar] [CrossRef]
- Brier, M.E.; Ray, P.C.; Klein, J.B. Prediction of delayed renal allograft function using an artificial neural network. Nephrol. Dial. Transplant. 2003, 18, 2655–2659. [Google Scholar] [CrossRef] [PubMed]
- Shadabi, F.; Cox, R.; Sharma, D.; Petrovsky, N. Use of artificial neural networks in the prediction of kidney transplant outcomes. In Proceedings of the Lecture Notes in Computer Science (Including Subseries Lecture Notes in Artificial Intelligence and Lecture Notes in Bioinformatics); Springer: Berlin/Heidelberg, Germany, 2004; Volume 3215, pp. 566–572. [Google Scholar]
- Fritsche, L.; Hoerstrup, J.; Budde, K.; Reinke, P.; Neumayer, H.H.; Frei, U.; Schlaefer, A. Accurate prediction of kidney allograft outcome based on creatinine course in the first 6 months posttransplant. In Proceedings of the Transplantation Proceedings; Elsevier: Frisco, CO, USA, 2005; Volume 37, pp. 731–733. [Google Scholar]
- Santori, G.; Fontana, I.; Valente, U. Application of an Artificial Neural Network Model to Predict Delayed Decrease of Serum Creatinine in Pediatric Patients After Kidney Transplantation. Transpl. Proc. 2007, 39, 1813–1819. [Google Scholar] [CrossRef]
- Krikov, S.; Khan, A.; Baird, B.C.; Barenbaum, L.L.; Leviatov, A.; Koford, J.K.; Goldfarb-Rumyantzev, A.S. Predicting Kidney Transplant Survival Using Tree-Based Modeling. ASAIO J. 2007, 53, 592–600. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.S.; Horn, S.D.; Hurdle, J.F.; Goldfarb-Rumyantzev, A.S. Single and multiple time-point prediction models in kidney transplant outcomes. J. Biomed. Inform. 2008, 41, 944–952. [Google Scholar] [CrossRef]
- Akl, A.; Ismail, A.M.; Ghoneim, M. Prediction of Graft Survival of Living-Donor Kidney Transplantation: Nomograms or Artificial Neural Networks? Transplantation 2008, 86, 1401–1406. [Google Scholar] [CrossRef]
- Ashfari, M.; Hamidi Beheshti, M.; Shahidi, S.; Ashfari, F. Application of artificial neural network to predict graft survival after kidney transplantation: Reports of 22 years follow up of 316 patients in Isfahan—Tehran University Medical Journal TUMS Publications. Tehran Univ. Med. J. 2009, 67, 353–359. [Google Scholar]
- Li, J.; Serpen, G.; Selman, S.; Franchetti, M.; Riesen, M.; Schneider, C. Bayes Net Classifiers for Prediction of Renal Graft Status and Survival Period. World Acad. Sci. Eng. Technol. 2010, 1. [Google Scholar]
- Greco, R.; Papalia, T.; Lofaro, D.; Maestripieri, S.; Mancuso, D.; Bonofiglio, R. Decisional Trees in Renal Transplant Follow-up. Transpl. Proc. 2010, 42, 1134–1136. [Google Scholar] [CrossRef]
- Lofaro, D.; Maestripieri, S.; Greco, R.; Papalia, T.; Mancuso, D.; Conforti, D.; Bonofiglio, R. Prediction of Chronic Allograft Nephropathy Using Classification Trees. Transpl. Proc. 2010, 42, 1130–1133. [Google Scholar] [CrossRef]
- Hummel, A.D.; Maciel, R.F.; Rodrigues, R.G.S.; Pisa, I.T. Application of Artificial Neural Networks in Renal Transplantation: Classification of Nephrotoxicity and Acute Cellular Rejection Episodes. Transpl. Proc. 2010, 42, 471–472. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Poynton, M.R.; Hurdle, J.F.; Baird, B.C.; Koford, J.K.; Goldfarb-Rumyantzev, A.S. Predicting three-year kidney graft survival in recipients with systemic lupus erythematosus. ASAIO J. 2011, 57, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.S.; Elster, E.A.; Stevens, K.; Graybill, J.C.; Gillern, S.; Phinney, S.; Salifu, M.O.; Jindal, R.M. Bayesian modeling of pretransplant variables accurately predicts kidney graft survival. Am. J. Nephrol. 2012, 36, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Lasserre, J.; Arnold, S.; Vingron, M.; Reinke, P.; Hinrichs, C. Predicting the outcome of renal transplantation. J. Am. Med. Inform. Assoc. 2012, 19, 255–262. [Google Scholar] [CrossRef]
- Decruyenaere, A.; Decruyenaere, P.; Peeters, P.; Vermassen, F.; Dhaene, T.; Couckuyt, I. Prediction of delayed graft function after kidney transplantation: Comparison between logistic regression and machine learning methods Standards, technology, and modeling. BMC Med. Inform. Decis. Mak. 2015, 15, 1–10. [Google Scholar] [CrossRef]
- Esteban, C.; Staeck, O.; Baier, S.; Yang, Y.; Tresp, V. Predicting Clinical Events by Combining Static and Dynamic Information Using Recurrent Neural Networks. In Proceedings of the 2016 IEEE International Conference on Healthcare Informatics, ICHI 2016, Chicago, IL, USA, 4–7 October 2016; Institute of Electrical and Electronics Engineers Inc.: Piscataway, NJ, USA, 2016; pp. 93–101. [Google Scholar]
- Shahmoradi, L.; Langarizadeh, M.; Pourmand, G.; Fard, Z.A.; Borhani, A. Comparing Three Data Mining Methods to Predict Kidney Transplant Survival. Acta Inform. Med. 2016, 24, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Topuz, K.; Zengul, F.D.; Dag, A.; Almehmi, A.; Yildirim, M.B. Predicting graft survival among kidney transplant recipients: A Bayesian decision support model. Decis. Support Syst. 2018, 106, 97–109. [Google Scholar] [CrossRef]
- Luck, M.; Sylvain, T.; Cardinal, H.; Lodi, A.; Bengio, Y. Deep Learning for Patient-Specific Kidney Graft Survival Analysis. arXiv 2017, arXiv:1705.10245. [Google Scholar]
- Shaikhina, T.; Lowe, D.; Daga, S.; Briggs, D.; Higgins, R.; Khovanova, N. Decision tree and random forest models for outcome prediction in antibody incompatible kidney transplantation. Biomed. Signal Process. Control 2019, 52, 456–462. [Google Scholar] [CrossRef]
- Tapak, L.; Hamidi, O.; Amini, P.; Poorolajal, J. Prediction of Kidney Graft Rejection Using Artificial Neural Network. Healthc. Inform. Res. 2017, 23, 277–284. [Google Scholar] [CrossRef]
- Tang, J.; Liu, R.; Zhang, Y.L.; Liu, M.Z.; Hu, Y.F.; Shao, M.J.; Zhu, L.J.; Xin, H.W.; Feng, G.W.; Shang, W.J.; et al. Application of Machine-Learning Models to Predict Tacrolimus Stable Dose in Renal Transplant Recipients. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef]
- Luo, Y.; Tang, Z.; Hu, X.; Lu, S.; Miao, B.; Hong, S.; Bai, H.; Sun, C.; Qiu, J.; Liang, H.; et al. Machine learning for the prediction of severe pneumonia during posttransplant hospitalization in recipients of a deceased-donor kidney transplant. Ann. Transl. Med. 2020, 8, 82. [Google Scholar] [CrossRef] [PubMed]
- Scheffner, I.; Gietzelt, M.; Abeling, T.; Marschollek, M.; Gwinner, W. Patient Survival after Kidney Transplantation: Important Role of Graft-sustaining Factors as Determined by Predictive Modeling Using Random Survival Forest Analysis. Transplantation 2020, 104, 1095–1107. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.; Gong, H.; Tian, H.; Zhuang, Q.; Li, J.; Cheng, K.; Ming, Y. The study of the association between immune monitoring and pneumonia in kidney transplant recipients through machine learning models. J. Transl. Med. 2020, 18, 370. [Google Scholar] [CrossRef] [PubMed]
- Massie, A.B.; Boyarsky, B.J.; Werbel, W.A.; Bae, S.; Chow, E.K.H.; Avery, R.K.; Durand, C.M.; Desai, N.; Brennan, D.; Garonzik-Wang, J.M.; et al. Identifying scenarios of benefit or harm from kidney transplantation during the COVID-19 pandemic: A stochastic simulation and machine learning study. Am. J. Transplant. 2020, 20, 2997–3007. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.; Massie, A.B.; Caffo, B.S.; Jackson, K.R.; Segev, D.L. Machine learning to predict transplant outcomes: Helpful or hype? A national cohort study. Transpl. Int. 2020, 33, 1472–1480. [Google Scholar] [CrossRef]
- Costa, S.D.; de Andrade, L.G.M.; Barroso, F.V.C.; de Oliveira, C.M.C.; de Francesco Daher, E.; Fernandes, P.F.C.B.C.; de Matos Esmeraldo, R.; de Sandes-Freitas, T.V. The impact of deceased donor maintenance on delayed kidney allograft function: A machine learning analysis. PLoS ONE 2020, 15, e0228597. [Google Scholar] [CrossRef]
- Elihimas Júnior, U.F.; Couto, J.P.; Pereira, W.; Barros De Oliveira Sá, M.P.; Tenório De França, E.E.; Aguiar, F.C.; Cabral, D.B.C.; Alencar, S.B.V.; Feitosa, S.J.D.C.; Claizoni Dos Santos, T.O.; et al. Logistic Regression Model in a Machine Learning Application to Predict Elderly Kidney Transplant Recipients with Worse Renal Function One Year after Kidney Transplant: Elderly KTbot. J. Aging Res. 2020, 2020, 1–13. [Google Scholar] [CrossRef]
- Kawakita, S.; Beaumont, J.L.; Jucaud, V.; Everly, M.J. Personalized prediction of delayed graft function for recipients of deceased donor kidney transplants with machine learning. Sci. Rep. 2020, 10, 18409. [Google Scholar] [CrossRef]
- Benjamens, S.; Moers, C.; Slart, R.H.J.A.; Pol, R.A. Kidney Transplantation and Diagnostic Imaging: The Early Days and Future Advancements of Transplant Surgery. Diagnostics 2020, 11, 47. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).