Abstract
Metal–organic frameworks (MOFs) have characteristics such as a large specific surface area, distinct functional sites, and an adjustable pore size. However, the inherent low conductivity of MOFs significantly affects the charge transfer efficiency when they are used for electrocatalytic sensing. Combining MOFs with conductive materials can compensate for these deficiencies. For MOF/metal nanoparticle composites (e.g., composites with gold, silver, platinum, and bimetallic nanoparticles), the high electrical conductivity and catalytic activity of metal nanoparticles are utilized, and MOFs can inhibit the agglomeration of nanoparticles. MOF/carbon-based material composites integrate the high electrical conductivity and large specific surface area of carbon-based materials. MOF/conductive polymer composites offer good flexibility and tunability. MOF/multiple conductive material composites exhibit synergistic effects. Although MOF composites provide an ideal platform for electrocatalytic reactions, current research still suffers from several issues, including a lack of comparative studies, insufficient research on structure–property correlations, limited practical applications, and high synthesis costs. In the future, it is necessary to explore new synthetic pathways and seek; inexpensive alternative raw materials.
1. Introduction
Metal–organic frameworks (MOFs) are a class of crystalline porous materials possessing a periodic network structure. They are formed by interconnecting inorganic metal centers (metal ions or metal clusters) with bridging organic ligands through self-assembly. The commonly used metal centers in MOFs include iron, zinc, cobalt, nickel, and copper. The widely used organic ligands typically employed are carboxylic acids or nitrogen-containing aromatic compounds. By selecting different metal centers and organic ligands or altering the synthesis method, MOFs with diverse compositions, crystal structures, and morphologies can be synthesized. In recent years, MOFs have been widely employed in gas adsorption and separation, storage, controlled drug release, and catalysis. This is because MOFs have a high specific surface area, many active sites, good thermal stability, and an adjustable pore size [1].
Electrochemical sensors are widely used in various fields, including environmental monitoring, food quality control, medical diagnostics, and chemical substance detection. Sensitivity and selectivity are the most important factors when evaluating the performance and utility of electrochemical detection. MOFs have high porosity and a large specific surface area, and they are capable of loading guest molecules, providing intrinsic sensitivity for electrochemical detection [2]. Their pore structure allows the diffusion of guest molecules into the native structure, and the shape and size of the pores can selectively accommodate incoming guests [2,3,4]. Moreover, chemical-specific interactions between the guest molecules and the MOFs—such as hydrogen bonding, π-π interactions, open metal sites, and van der Waals interactions—enable the selection of guest molecules. Although MOFs have relatively poor electrical conductivity and mechanical strength [5], combining them with conductive materials, such as carbon nanomaterials, noble metal nanoparticles, metals, metal oxides, and conductive polymers, can compensate for these shortcomings and expand the range of sensing applications. It is these multifaceted advantages that give MOF composites extremely promising potential for development in the field of electrochemical sensing.
This review primarily summarizes research outcomes regarding MOF composites that integrate several common conductive materials in electrochemical sensing. Additionally, we discuss the common methods of synthesizing MOF composites and the challenges in electrochemical detection applications and look ahead to the research and application prospects of these composites.
2. Synthesis Methods
The approach used to synthesize MOF composites is determined by the nature of the metal species, the structure of the organic linker, and/or the characteristics of the targeting agent [6]. The synthesis method and conditions also affect the composite’s morphology, crystal structure and porosity, which in turn affect its functionality. The common synthesis methods for MOF composites are shown in Table 1.

Table 1.
Advantages and disadvantages of synthesis methods for MOF composites.
3. MOF/Metal Nanoparticle Composites
Metal nanoparticles are tiny particles composed of metal atoms in the nanoscale range. Over the past two decades, metal nanoparticles have gradually become popular materials for sensing applications because of their excellent electrical conductivity and catalytic properties. Precious-metal nanoparticles, such as gold, silver, and platinum, are particularly well-suited for detecting and identifying various chemicals and biomolecules due to their high sensitivity and stability [7,8,9]. Unfortunately, due to their high surface energy, metal nanoparticles tend to aggregate during catalytic reactions, leading to a decrease in the number of active sites, a reduction in the specific surface-area-to-volume ratio, and, consequently, a decrease in catalytic activity [10,11]. To obtain well-dispersed metal nanoparticles, they must be confined to porous materials such as porous silica, zeolite, and porous carbon [12,13,14]. Among these porous materials, MOFs are the most popular choice because (1) they can be tailored to meet the specific needs of metal nanoparticles with different pore sizes and shapes for a wide range of applications; (2) they possess very high porosity and a very high specific surface area, which are ideal for hosting metal nanoparticles; and (3) MOFs have well-defined structures, and their pore structures can be easily customized, creating a well-defined surrounding environment for metal nanoparticles and being advantageous for understanding catalytic processes [15]. Recent studies have shown there is a synergistic effect between metal nanoparticles and MOFs. The metal nanoparticles not only offer electronic activity for signal enhancement but also create active sites that enhance catalytic performance [16,17].
3.1. MOF/Gold Nanoparticle Composites
Gold nanoparticles (AuNPs) have drawn significant attention in the electrochemical field due to their biocompatibility, outstanding electrical and thermal conductivity, high chemical stability, and large surface-to-volume ratio [18,19,20,21]. Therefore, researchers have begun exploring the combination of AuNPs with MOFs to create composites and utilizing them in electrochemical detection.
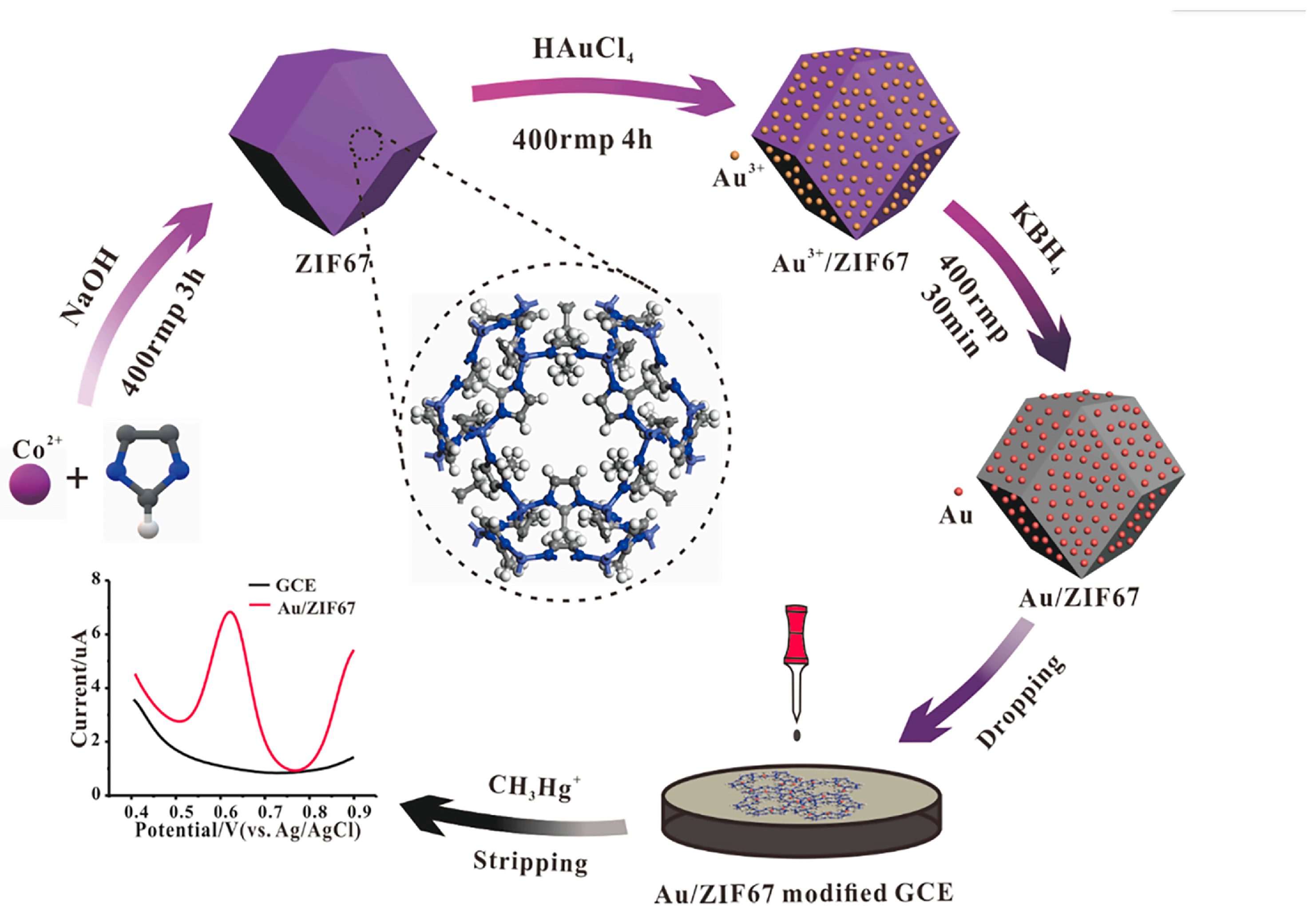
For example, Wang et al. [22] successfully synthesized AuNP/MMPF-6(Fe) composite materials by immobilizing AuNPs on metal–metal porphyrin frameworks (MMPF-6(Fe)) through electrostatic adsorption. The composite was modified on a glassy carbon electrode to detect hydroxylamine. MMPF-6(Fe) exhibits several advantageous properties, including large pores, an extensive surface area, outstanding chemical stability in both water and organic media, and excellent biomimetic catalytic activity. When combined with the favorable electrical conductivity of AuNPs, which can enhance electron transfer and amplify electrochemical signals, the synergy between these two materials empowers the sensor to reduce the anode overpotential of hydroxylamine. Consequently, the anode peak current is notably increased. In addition, the sensor has a low detection limit and a wide linear range at the nanomolar level. Under optimal experimental conditions, the electrode showed two linear ranges, namely, 0.01–1.0 and 1.0–20.0 μmol/L, and a low detection limit of 0.004 μmol/L (S/N = 3). Liu et al. [23] successfully developed an electrochemical sensor for detecting CH3Hg+ by modifying a glassy carbon electrode (AuNPs/ZIF67 GCE) using AuNPs and zeolitic imidazolate framework-67 (AuNPs/ZIF67), as shown in Figure 1. The AuNPs showed good performance for the detection of mercury species, but they were prone to agglomeration, reducing surface active sites and thus affecting catalytic performance. The unique hollow structure of ZIF67 inhibits the agglomeration of gold nanoparticles. Therefore, the AuNPs/ZIF67 GCE performed outstandingly in the detection of CH3Hg+ and had good stability in natural water bodies without interference from metal ions in the matrix. The linear range of this sensor is 1–25 µg/L [CH3Hg+], with a sensitivity of 0.571 μA/μgL−1 and a detection limit of 0.05 μg/L. This research result expands the application range of MOFs in the development of electrochemical sensors and provides a fast and reliable sensor scheme for the screening of CH3Hg+ in environmental samples. Wang et al. [24] synthesized AuNP/ZIF-L composite materials by decorating AuNPs on leafy zeolitic imidazolate frameworks (ZIF-L) for the detection of acetaminophen (AP). It is well known that AuNPs exhibit strong electrocatalytic performance for small molecules. Additionally, ZIF-L, serving as the loading platform for AuNPs, possesses a large specific surface area and high porosity. The AuNP/ZIF-L nano-hybrid provides an excellent microenvironment for the transfer of analytes in solution, which is conducive to accelerating electron transfer between the electrode and the analytes. When detecting AP, this composite material exhibits a linear range from 3.50 μmol/L to 0.056 mmol/L with a sensitivity of 37.28 μA/mmol L−1. When the linear range is from 0.056 to 0.56 mmol/L, the sensitivity is 25.10 μA/mmol L−1, and the lowest detection limit is 1.02 μmol/L. The prepared Au/ZIF-L electrode can be employed to accurately determine the content of AP in drug samples.
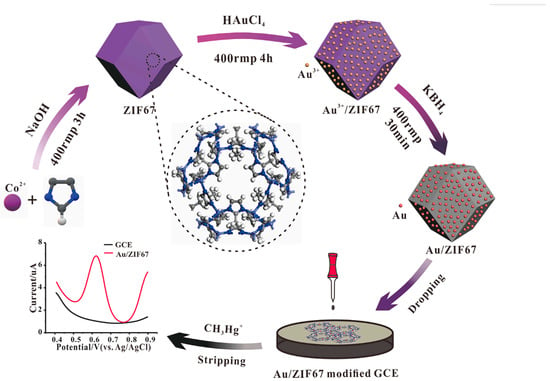
Figure 1.
Schematic illustration of the synthesis of the Au/ZIF67, which was used to construct electrochemical sensors for CH3Hg+. Image was reprinted with permission from [23].
3.2. MOF/Silver Nanoparticle Composites
Silver nanoparticles (AgNPs) stand out among various metal nanoparticles because of their high conductivity, good biocompatibility, and strong catalytic activity [25,26,27]. Furthermore, AgNPs can effectively suppress the electron–hole recombination process when acting as an electron reservoir. Consequently, in this process, a substantial number of holes can be utilized for electrochemical reactions, thereby enhancing electrochemical performance [28]. These outstanding characteristics have kindled people’s interest in devising silver-containing electrochemical sensors.
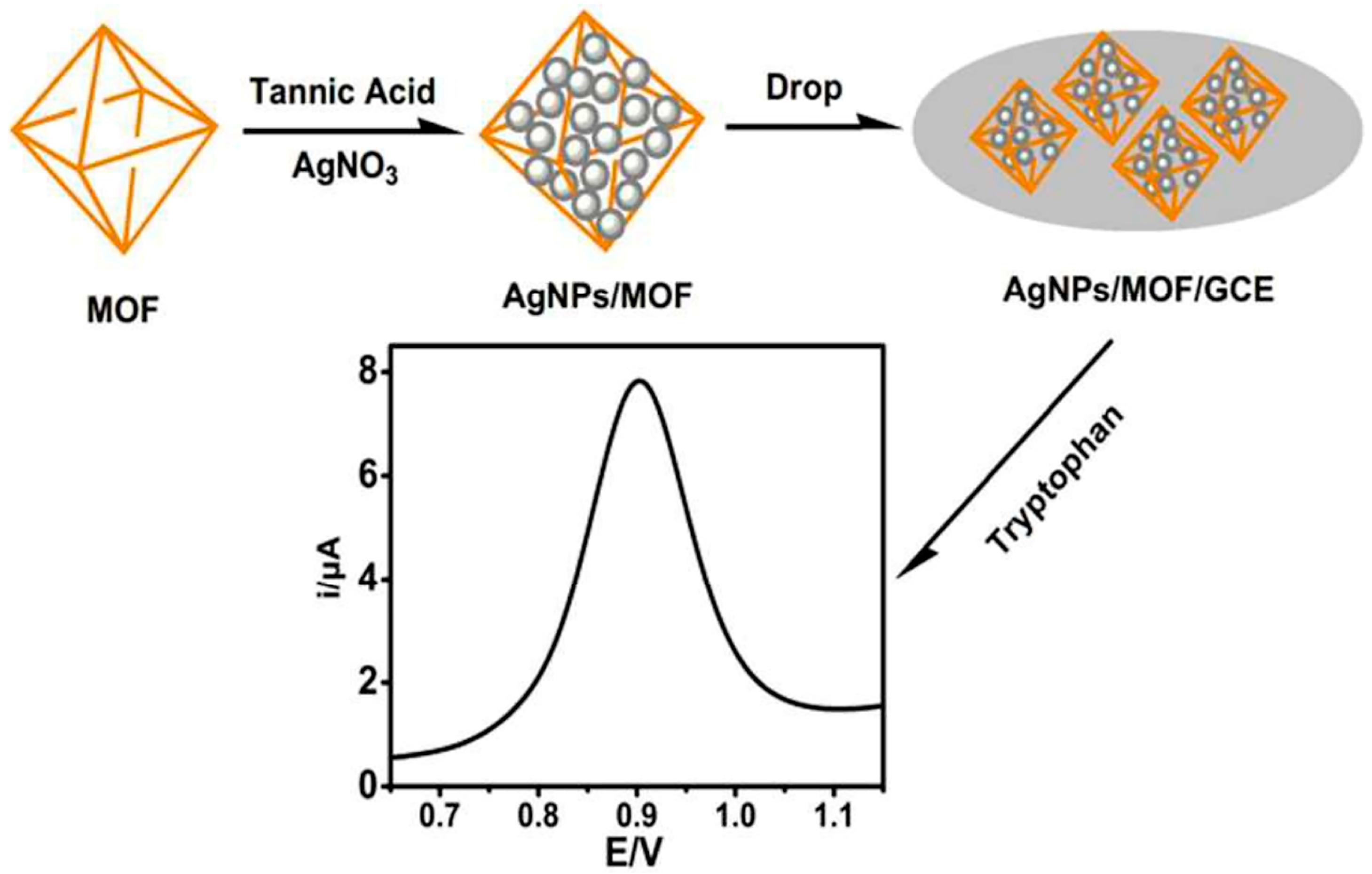
Chen et al. [29] successfully synthesized AgNP/2D Zn-MOFs materials by depositing AgNPs on two-dimensional zinc-based metal–organic frameworks (2D Zn-MOFs) via an electrodeposition method. These materials were employed for detecting H2O2 released by living cells (including normal cells and tumor cells). The utilization of two-dimensional MOFs can enhance the dispersion and stability of active metal components, offer a larger specific surface area, and improve conductivity. Regarding the reported non-enzymatic H2O2 electrochemical sensors, precious-metal-based materials possess strong catalytic activity towards H2O2 due to their small size and large specific surface area. Among common metal nanoparticles, AgNPs exhibit better reduction electrocatalytic activity for H2O2 than PtNPs and AuNPs. Under optimal conditions, the detection limit of the AgNP/2D Zn-MOF-modified electrode is relatively low, at 1.67 μmol/L (S/N = 3), and the linear range extends from 5.0 mmol/L to 70 mmol/L. This electrode, based on a metal nanoparticle/MOF enzyme, can function as an ideal platform for detecting biomarkers and evaluating drugs. Peng et al. [30] modified the surface of a self-made iron-based metal–organic framework (MIL-101(Fe)) with AgNPs via the reduction method to form the AgNP/MIL-101 complex for the detection of tryptophan, as shown in Figure 2. Owing to the large specific surface area of MIL-101 and the excellent conductivity and strong electromagnetic field of AgNPs, the accumulation of tryptophan molecules on the surface of MIL-101 was enhanced. Consequently, an electrode modified with this composite material could effectively oxidize tryptophan and exhibit a significant electrocatalytic effect. Under optimal experimental conditions, when the tryptophan concentration ranged from 1 to 50 μmol/L and from 50 to 150 μmol/L, the oxidation peak current was proportional to the tryptophan concentration. The detection limit of this method is 0.14 μmol/L (S/N = 3), and it was successfully applied to the determination of the tryptophan content of urine, demonstrating good recovery. Zhao et al. [31] designed and synthesized iron-based metal–organic frameworks (Fe-MOFs) adorned with silver nanoparticles (AgNPs) on the outer surface for the determination of an important tumor prognostic biomarker, α2,6-sialylated glycans (α2,6-sial-Gs). AgNP/Fe-MOFs display good endogenous redox mediator properties for generating electrons, constituting the basic mechanism for electronic signal amplification. A large specific surface area allows more nanoprobes to be carried. The detection range of this sensor extends from 1 fg/mL to 1 ng/mL, and the detection limit is 0.09 fg/mL. Additionally, this biosensor possesses good specificity and stability, indicating that this new nanobiotechnology platform can be utilized for the potential monitoring of biomarkers in serum.
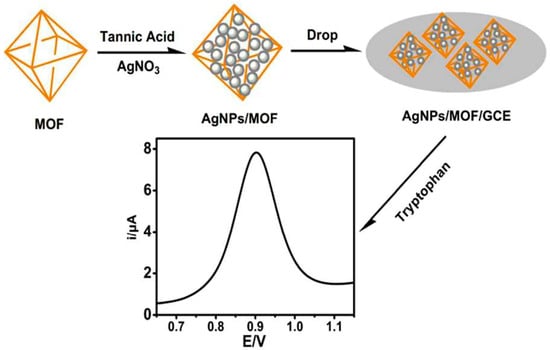
Figure 2.
Steps for preparation of AgNP/MIL-101(Fe)-modified electrode. Image was reprinted with permission from [30].
3.3. MOF/Platinum Nanoparticle Composites
Platinum nanoparticles (PtNPs) are extensively utilized in sensors on account of their outstanding catalytic activity, electrical conductivity, biocompatibility, and adsorption capacity. Additionally, PtNPs possess a sufficient working potential range and can take part in numerous electrochemical reactions on an electrode’s surface [32].
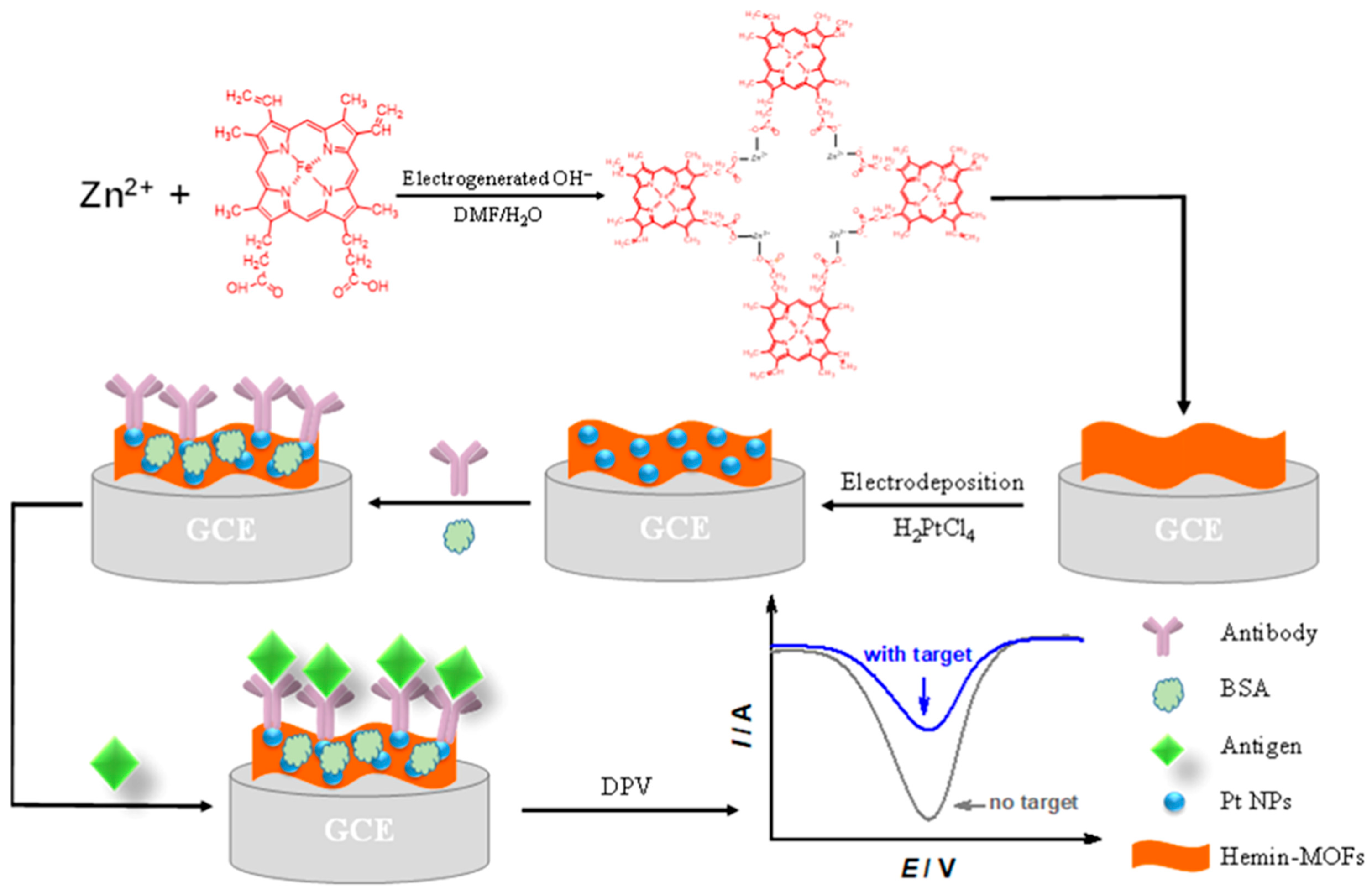
Ling et al. [33] discovered that the electrocatalysis of PtNPs upon the oxidation of sodium borohydride (NaBH4) is multi-electronic (involving up to eight electrons). Consequently, the electrochemical signal generated in this process is significantly higher than that generated by electrochemical reactions involving one-electron or two-electron oxidation. Considering the structural diversity and adjustability of MOFs, this research team synthesized a PtNP-and-MOF composite (PtNPs@UiO-66-NH2) and sequentially immobilized capture DNA (cDNA) so that it could serve as a signal probe. By utilizing the multi-electron electrocatalysis of PtNPs@UiO-66-NH2 upon the oxidation of NaBH4, an electrochemical sensor for detecting telomerase activity was successfully fabricated. The results indicated that telomerase activity demonstrated a wide dynamic correlation between 5 × 102 and 107 HeLa cells per milliliter, and the telomerase activity of a single HeLa cell was determined to be 2.0 × 10−11 IU via calculation. This significant development offers a powerful platform for the accurate detection of telomerase activity. Xu et al. [34] successfully fabricated a PtNPs@UiO-66-2 composite material by encapsulating PtNPs within the metal–organic framework UiO-66 matrix. As a zirconium-based MOF, UiO-66 possesses a compact structure and high chemical and thermal stability owing to strong Zr−O bonds. Additionally, the narrow penetration channel offered by its triangular window only permits small molecules to pass through, facilitating selective detection of H2O2. Electrochemical test results indicated that a glassy carbon electrode modified with the PtNPs@UiO-66-2 sample exhibited good electrocatalytic activity for the oxidation of H2O2. Under the condition that the interference concentration is identical to the H2O2 concentration, this electrode demonstrated excellent anti-interference performance, and its linear range extended from 5 μmol/L to 14.75 mmol/L. Additionally, this composite material possesses a low detection limit, good stability, and reproducibility. Tang et al. [35] fabricated 2D hemoglobin-bridged MOF flakes (Hemin-MOFs) using the in situ electrochemical synthesis method and grew PtNPs on 2D Hemin-MOF flakes, as shown in Figure 3. This composite material can serve as a peroxidase mimic, and PtNPs can act as an anchor point to capture antibodies. Consequently, the hybrid nanosheet-modified electrode can serve as an electrochemical sensing platform for Streptococcus erysipelatis surface protective antigen (Spa) protein. The results indicated that the linear range of this electrode is 50 fg/mL to 500 ng/mL, and the theoretical detection limit is 4.3 fg/mL (S/N = 3). Moreover, an immunosensor modified by hemin–MOF possessed good reproducibility, stability, and high repeatability, providing a new approach for the electrochemical synthesis of two-dimensional/three-dimensional MOF hybrid nanocomposites with a high specific surface area and biomimetic catalysts.
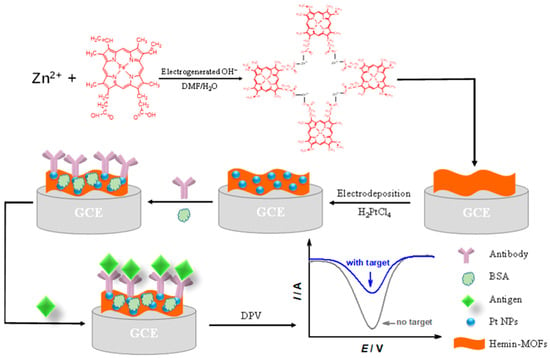
Figure 3.
Scheme for preparation of the hemin–MOF-modified electrode and immunosensor. Image was reprinted with permission from [35].
3.4. MOF/Bimetal Nanoparticle Composites
Bimetallic nanoparticles have been reported to display higher electrochemical catalytic activity and stability compared to single-component nanoparticles because of the strong metal synergistic effect. In comparison with single-metal nanoparticle/MOF composites, MOF composites incorporating bimetallic nanoparticles possess better catalytic effects. However, currently, there are a limited number of reports on the application of bimetallic nanoparticle/MOF composites in electrochemical detection [36,37].
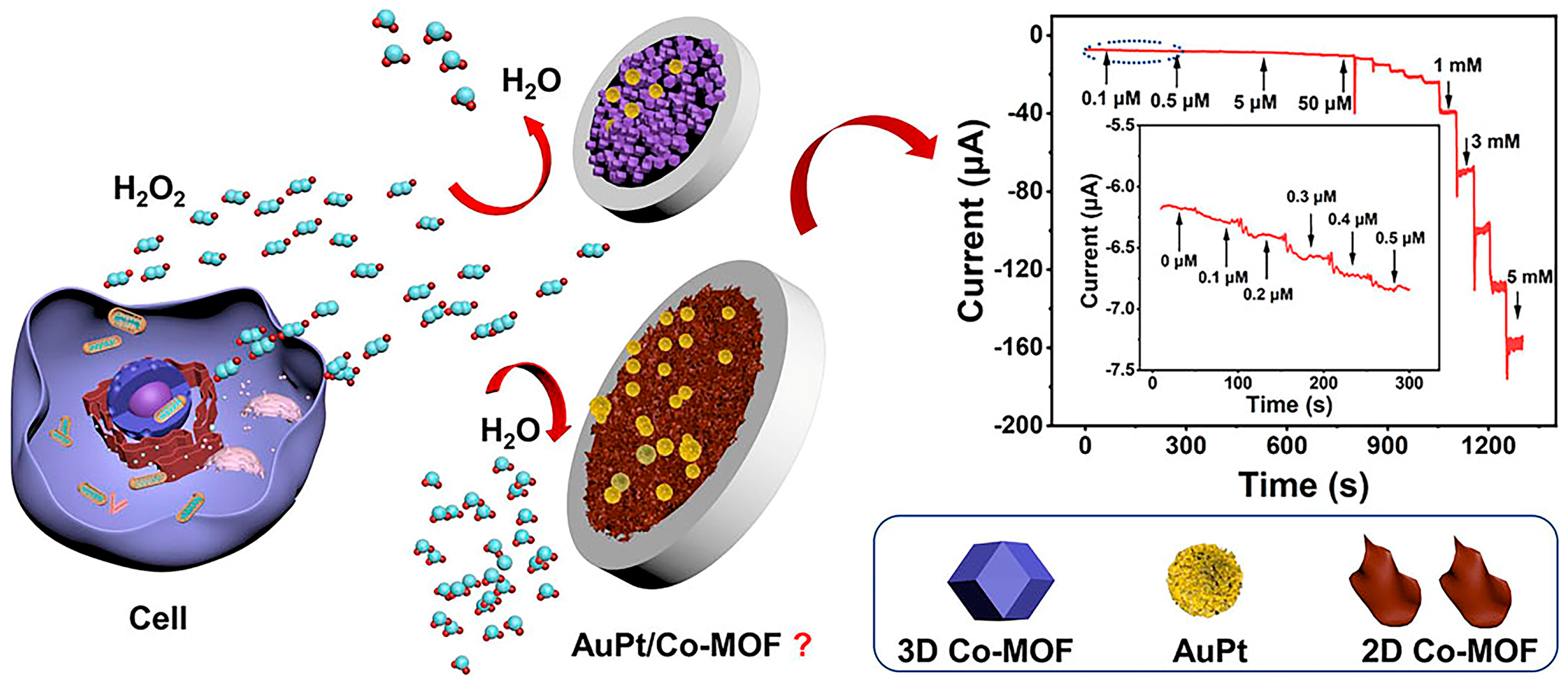
In an ethanol–aqueous solution, Yang et al. [38] employed a novel adsorption/reduction approach to fabricate highly dispersed bimetallic nanoparticles, AuPdNPs (3–6 nm in size), on an amino-functionalized Zr(IV)-based MOF, UiO-66-NH2. UiO-66-NH2 plays a dual role, acting as both a supporting platform and a protective agent. The pores within UiO-66-NH2 prevent the aggregation and migration of the AuPd bimetallic nanoparticles. The resulting UiO-66-NH2 catalyst features a large specific surface area and demonstrates excellent stability and dispersion in aqueous environments. When this composite material is employed to construct a nitrite sensor, owing to its strong metal synergistic effect, its catalytic activity is superior to that of single-metal nanoparticle/MOF composites (Au/UiO-66-NH2 and Pd/UiO-66-NH2). Electrochemical research indicated that the linear response concentration range for nitrite detection of the AuPd/UiO-66-NH2 sensor is 0.05–5666 μmol/L, and the detection limit is 0.01 μmol/L. This method was successfully utilized for the determination of nitrite in sausage and pickled vegetable samples. Xu et al. [39] reported a sandwich immunosensor supported by AuPtNP/MOF for the detection of lymphocyte activation gene-3 (LAG-3) protein. In comparison with pure platinum and pure gold nanoparticles, AuPt bimetallic nanoparticles possess greater catalytic activity. This sandwich immunosensor exhibits a high degree of sensitivity, particularly in the presence of a low concentration of LAG-3 protein. Under optimal conditions, the detection sensitivity range of this sensor for LAG-3 protein is 0.01 ng/mL–1 μg/mL, and the lowest detection limit is 1.1 pg/mL (based on 3σ). This ultrasensitive biosensor can be utilized for the detection of LAG-3 protein in early clinical tumor diagnosis. Xie et al. [40] constructed an electrochemical sensor for the detection of H2O2 based on bimetallic gold-platinum nanoparticles (Au3Pt7 NPs) supported by Co-based metal organic framework (Co-MOF). By one-step electrodeposition, Au3Pt7 NPs with optimal electrocatalytic activity and accessible active surface can be deposited on the surface of the Co-MOF–modified glassy carbon electrodes (AuPt/Co-MOF). After evaluating the electrocatalytic activities of 3D Co-MOF and 2D Co-MOF modified electrodes, it was found that the 2D Co-MOF nanosheets, as the supporting material, exhibited better electrocatalytic performance than 3D Co-MOF crystals in reducing H2O2. The results show that the catalytic current of the prepared Au3Pt7/2D Co-MOF sensor has a linear relationship with the H2O2 concentration in the range of 0.1–5 mmol/L and 5–60 mmol/L. The low detection limit is 0.02 μmol/L (S/N = 3). The excellent electroanalytical performance of the Au3Pt7/2D Co-MOF sensor was ascribed to the synergistic effect between the high dispersion of Au3Pt7 NPs and the high specific surface area of 2D Co-MOFs. Additionally, this sensor was successfully utilized to detect the concentration of H2O2 released by Hela cells (Figure 4).
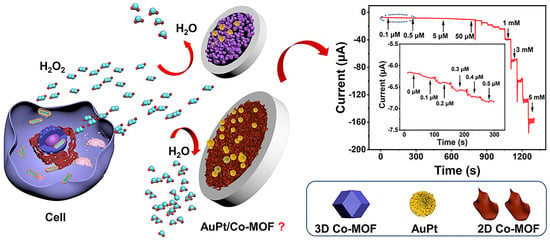
Figure 4.
The aforementioned electrochemical sensor for detecting H2O2 secreted by HeLa cells in response to drug stimulation. Image was reprinted with permission from [40].
4. MOF/Carbon-Based Material Composites
Carbon-based materials primarily encompass carbon nanotubes, carbon nanofibers, graphene, diamond, and graphdiyne, among others. They are extensively utilized due to their advantageous characteristics, such as a rich pore structure, a high specific surface area, adjustable surface functionality, excellent electrical conductivity, facile chemical functionalization, good stability, good biocompatibility, and a relatively low cost [41,42,43].
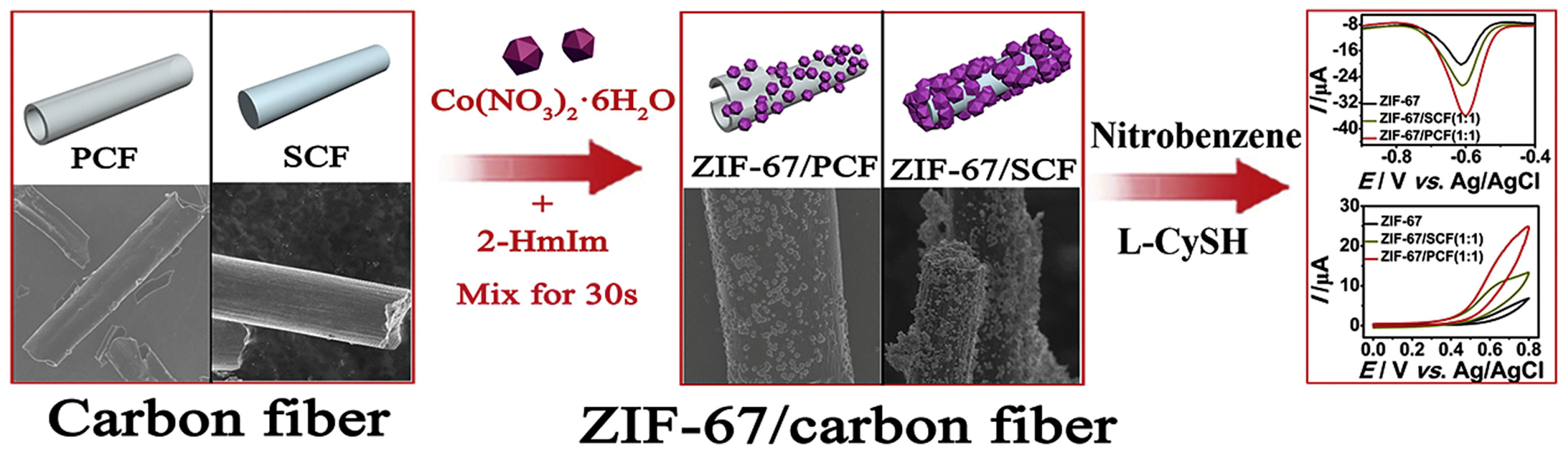
Li et al. [44] combined an MOF (UiO-66-NH2), which is composed of zirconium chloride (ZrCl4) and terephthalic acid, with carbon nanotubes (CNTs) to form UiO-66-NH2@CNT. Subsequently, they constructed an electrochemical sensor for detecting dopamine (DA) and acetaminophen (AC). In comparison with UiO-66-NH2/GCE and CNT/GCE, the sensor constructed using UiO-66-NH2@CNT/GCE exhibits a significant enhancement effect on the oxidation of DA and AC. Its linear range is from 0.03–2.0 mmol/L, and the LOD is 15 nmol/L and 9 nmol/L, respectively. Zhang et al. [45] developed a novel method for fabricating a portable sensor for synephrine detection. They first dispersed graphene (GN) and UiO-66 within a Nafion solution. The resulting homogeneous mixture was then utilized to modify a screen-printed carbon electrode (SPCE), leading to the creation of a unique sensor, Nafion/UiO-66/GN/SPCE. It displayed an extremely low detection limit of 0.04 μmol/L, and its linear response range extended from 0.5 to 60 μmol/L, with a high correlation coefficient (r2 = 0.995). Furthermore, this sensor demonstrated excellent reproducibility and stability, suggesting its reliability and potential for practical application in the quantitative analysis of synephrine. Feng et al. [46] prepared ZIF-67@CF composite material from ZIF-67 and carbon fiber (CF) and used it for electrode modification to build an electrochemical sensor for nitrobenzene detection, as shown in Figure 5. This sensor exhibits excellent linearity in two concentration ranges, namely, from 0.3 to 50 μmol/L and from 50 to 390 μmol/L. The detection limit is as low as 0.16 μmol/L, indicating its high sensitivity. Additionally, the sensor also possesses versatility and can effectively detect L-cysteine with linear ranges from 5 to 160 μmol/L and from 160 to 1580 μmol/L. It has a fast response (<1.0 s), good stability, and an anti-interference ability.
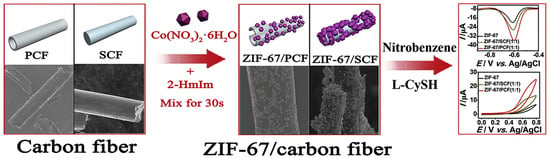
Figure 5.
Schematic diagram of synthesis of ZIF-67@CF composite and detection of nitrobenzene and L-cysteine. Image was reprinted with permission from [46].
5. MOF/Conductive Polymer Composites
Conductive polymers, also referred to as conductive macromolecules or conductive plastics, are a category of polymer compounds possessing special conductive properties. Through doping and other approaches, their conductivity can be enhanced to the range of semiconductors or conductors, while they retain the inherent characteristics of high strength and good toughness of polymer materials [47,48,49]. In the field of electrochemical sensors, conductive polymers such as polyaniline (PAN) and polypyrrole are especially prevalent, and they have been widely applied due to their excellent performance.
Arushi et al. [50] investigated a composite material consisting of a copper–MOF (Cu3(BTC)2) and PAN plated on SPCE. By integrating this conductive film with the antibody against the cardiac marker troponin (cTnI), a sensor for cTnI detection was developed. This immunosensor enables impedance detection of cTnI antigen within a concentration range of 1–400 ng/mL. The entire antigen analysis process can be accomplished within 5 min. This detection method is specific to cTnI, even in the presence of other possible interfering proteins. Rezaei et al. [51] synthesized zinc- and titanium-based metal–organic frameworks and employed them in conjunction with a polypyrrole conductive polymer for the determination of perfluorooctane sulfonate (PSOF) content. The response of the Zn/Ti/C-MOF–magnetic molecularly imprinted polymer/carbon paste electrode (MOFMMIP/CPE) to PSOF increases logarithmically with the concentration ranging from 0.002 to 165 μmol/L, and the detection limit is 0.7 nmol/L. The recovery rate of PFOS in tap water, river water, and well water samples was 96.00–104.14%, the deviation was –4.00–4.14%, and the relative standard deviation (RSD) was 1.89–3.74%. Comparing the data obtained using this sensor with those obtained using high-performance liquid chromatography (HPLC) indicates that the two are highly consistent, highlighting the high accuracy of this method in PSOF detection.
6. MOF/Multiple Conductive Material Composites
As the research and development of conductive materials continues to expand and deepen, researchers are no longer content with merely combining MOFs with a single type of conductive material. They have commenced attempting to innovatively combine MOFs with multiple diverse types of conductive materials to further enhance the conductivity, mechanical strength, and chemical stability of composite materials.
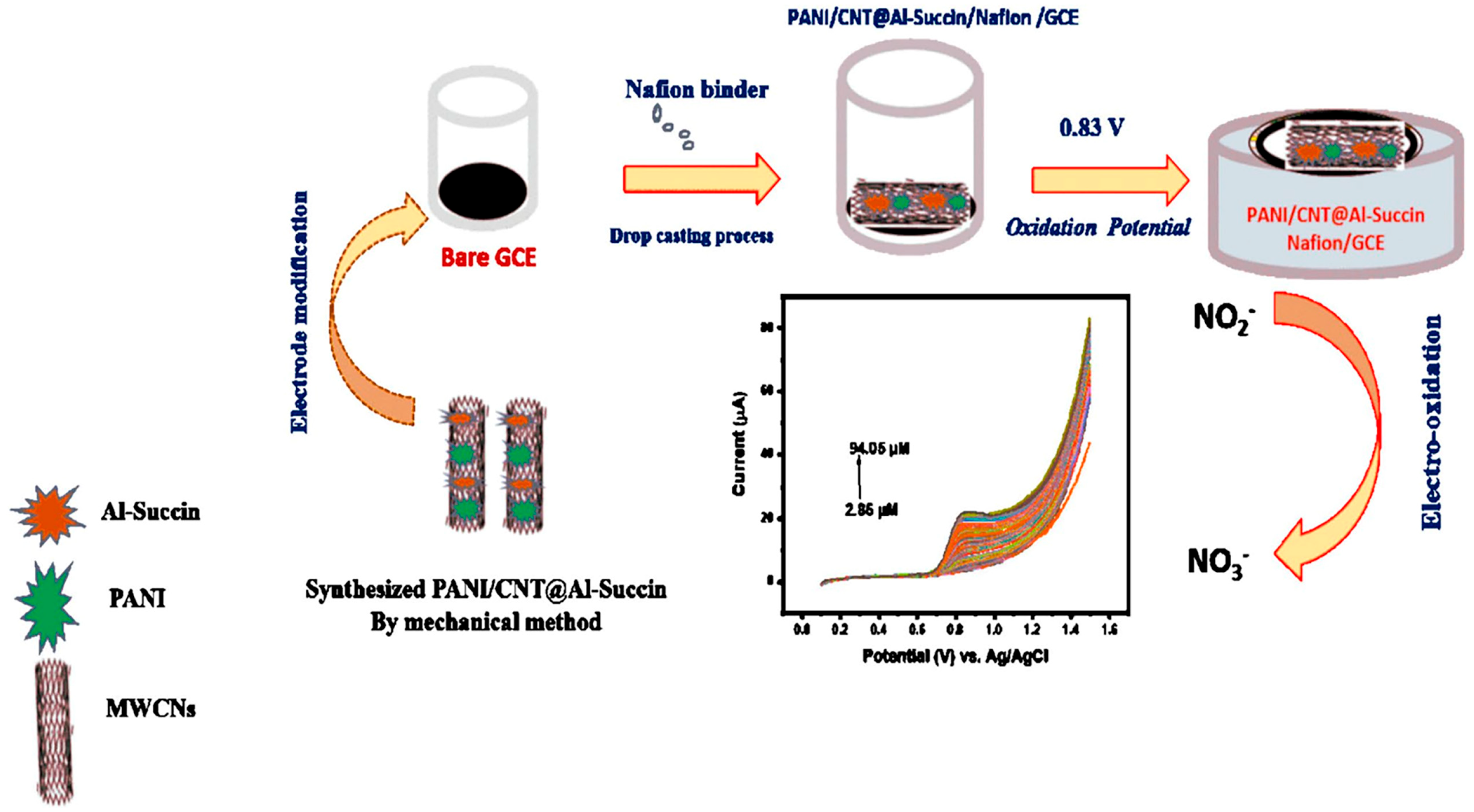
Chen et al. [52] successfully prepared multi-layer Ti3C2–carbon nanotube–gold nanoparticle (Ti3C2-CNTs-AuNP) and cyclodextrin metal–organic framework–carbon nanotube (CD-MOF-CNT) composites using the in situ growth method. Subsequently, they combined the two materials for the efficient detection of rutin. Ti3C2 contains numerous metal active sites, carbon nanotubes (CNTs) possess high electron transfer efficiency, and gold nanoparticles (AuNPs) exhibit excellent catalytic properties. These characteristics synergistically contribute to significantly enhancing the electrochemical properties of the composite nanomaterials. On the other hand, CD-MOF endows the composite material with outstanding enrichment performance and high selectivity for rutin due to its unique host–guest recognition ability and abundant cavities, molecular gaps, and surface reaction groups. Under optimal conditions, the linear range of this new sensor extends from 2 × 10−9 to 8 × 10−7 mol/L, with a detection limit of 6.5 × 10−10 mol/L. Additionally, this sensor can still maintain excellent anti-interference with rutin in a complex flavonoid compound system, providing a reliable means of detecting rutin in natural plant samples such as buckwheat, lemongrass, and sophora flower. Shu et al. [53] fabricated a stretchable electrode for detecting DA released by C6 living cells by coating Ni-MOF composite/AuNPs on CNTs and then depositing the resulting product on a polydimethylsiloxane (PDMS) film. The final product’s linear detection range is as wide as 50 nmol/L to 15 μmol/L, and its sensitivity is as high as 1250 mA/(cm2 mol L−1). At the same time, the electrode exhibits good cytocompatibility. C6 living cells can be cultured and proliferated on it and strongly adhere to it. Consequently, this stretchable sensor can monitor the release of DA by C6 living cells in stretched and unstretched states in real time. This finding reveals that the flexible Ni-MOF composite/AuNP/CNTs/PDMS (NACP) film electrode presents additional possibilities for the detection of chemical signals emitted by cells and soft organisms. Notably, it can perform this function even when it is in a mechanically deformed condition. Alsafrani et al. [54] added aluminum succinate MOF (Al-succin) to PAN/carbon nanotube (CNT) on a glassy carbon electrode (GCE), thereby creating an efficient electrochemical sensor for the detection of nitrite, as shown in Figure 6. The current response and electrocatalytic performance of Pani/CNT@Al-succin-modified GCE for nitrite are superior to those of bare GCE. Its linear ranges are from 5.7 to 74.1 μmol/L (CV) and from 8.55 to 92.62 (LSV) μmol/L, and the LOD values are 1.16 μmol/L (CV) and 0.08 μmol/L (LSV). These LOD values are significantly lower than the acceptable nitrite limit in drinking water as defined by the World Health Organization (WHO). Moreover, when this sensor, PANI/CNT@Al-Succin, was utilized for the analysis of real samples, including apple juice, orange juice, and bottled water, the achieved recovery rates reached 98.92%, 99.38%, and 99.90%, respectively. To sum up, the advantages and disadvantages of different MOF composite are shown in Table 2.
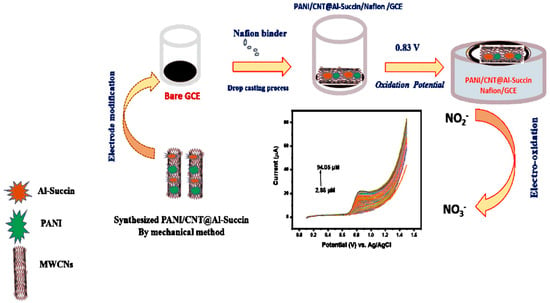
Figure 6.
Schematic clarification of the synthesis of the modified electrode and the electro-oxidation detection of nitrite. This image was reprinted with permission from [54].

Table 2.
Advantages and disadvantages of MOF composites.
7. The Selection of MOF Composite Combinations
When choosing MOF composites for a specific application, one must take into account both the requirements of the application and the properties of the MOFs and other conductive materials. For the design of fluorescent sensors, MOF-76, which possesses fluorescent properties, is an ideal choice [55]. To enhance specific recognition and enrichment, MOFs with specific pore structures and functional groups can be utilized. For instance, ZIF-67, which incorporates the organic ligand imidazole, has a large number of gas-diffusion pores and exhibits excellent sensing properties for hydrogen sulfide gas [56,57,58]. If the goal is to boost the catalytic activity for signal amplification, MOFs with unsaturated metal sites, such as MIL-101(Fe) and UIO-66, can be selected [59,60]. Additionally, synergism is a crucial factor to consider when choosing composites. Improving electrical conductivity and electron-transfer efficiency can contribute to enhancing the response speed and sensitivity of detection. Metal nanoparticles, carbon-based composites, and the conductive polymers mentioned previously can accelerate electron transfer between the electrode and the detector. Simultaneously, targeted selection can also be carried out based on the substances to be detected. For example, when detecting certain biomolecules, biocompatible metal nanoparticles can synergize with MOFs through the surface modification of specific antibodies or aptamers. For instance, Zheng et al. [61] constructed an electrochemical aptasensor based on an aptamer-enzyme-catalyzed signal-amplification approach. Polyethyleneimine-functionalized molybdenum disulfide Au@PtPd nanobipyramids (MoS2/Au@PtPd NBPs) were utilized as the modifying material in this study for the detection of ATP. Melamine, melamine-cyanuric acid (MCA), and cobalt-based metal–organic frameworks (Co-MOFs) were employed as the signaling tags for detecting ATP, and π-π stacking interactions could increase the loading efficiency and surface concentration of the signaling tags for ATP. In the presence of Mg2+, the signal tag was ring-cut to amplify the signal. MoS2/Au@PtPd NBPs feature a large specific surface area and excellent electrical conductivity. These properties endow them with the capacity to adsorb a substantial quantity of DNA strands. Moreover, they can amplify the redox signal of methylene blue (MB). Owing to these advantages, MoS2/Au@PtPd NBPs hold great promise for diverse applications in areas such as quality supervision and food safety.
In summary, when selecting MOF combinations, it is essential to have a comprehensive understanding of the properties of various types of MOFs, commonly used composites, and the objects to be detected. This understanding is beneficial for the preparation of more sensitive electrochemical sensors.
8. Conclusions
Benefiting from the adjustability and porous nature of MOFs as well as the diversity and excellent catalytic activity of conductive materials, MOF composites have been enhanced in terms of stability and conductivity, thereby providing an ideal platform for electrocatalytic reactions. These composites not only possess high activity and selectivity but also can effectively catalyze various reactions, thus significantly reducing the amount of catalyst used.
However, currently, there are still several issues related to MOF composites that need to be addressed: (1) Current research on MOF composites is primarily focused on summarizing the discoveries and impacts of a single MOF or its combination, lacking appropriate comparative studies. (2) MOFs and their conductive materials are often randomly selected or created based on hypothesized effects, and there is a lack of systematic research on structure–property correlations. (3) Most electrochemical detection demonstrations in current research are still in the laboratory stage, and although it is possible to test the actual samples, there is still a long way to go to achieve truly practical applications. (4) The synthesis of MOF composites typically requires the use of relatively expensive raw materials and complex synthesis processes. For instance, some metal ions and organic ligands are costly, and the synthesis process may demand strict reaction conditions and a long reaction time. Due to cost considerations, this restricts the large-scale production and application of MOF composites.
Future Research Directions: (1) Establish a database to integrate experimental and simulation data to provide a scientific basis for the rational selection and design of MOFs and their conductive materials, and abolish the status quo of random selection and creation. (2) Explore new synthetic pathways, such as the use of green solvent thermal synthesis, microwave-assisted synthesis, and other new methods, to simplify the process and shorten the reaction time. (3) Tap into inexpensive alternative raw materials, search for affordable metal sources and organic ligands with comparable performance, and reduce the cost of raw materials so as to promote the large-scale production and application of MOF composites.
Author Contributions
Conceptualization, S.W.; data curation, P.L., M.W. and J.Y.; writing—original draft preparation, P.L. and S.W.; writing—review and editing, Z.C. and Q.C.; visualization, M.H.; project administration, Q.C. and S.W.; funding acquisition, Q.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Medical Fund of the Hubei University of Science and Technology (No.: 2023YKY04). The authors gratefully acknowledge the financial support provided by the National Natural Science Foundation of Hubei Province of China (No.: 2024AFB502).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Li, S.; Huo, F. Metal–organic framework composites: From fundamentals to applications. Nanoscale 2015, 7, 7482–7501. [Google Scholar] [CrossRef]
- Liu, W.; Yin, X.B. Metal–organic frameworks for electrochemical applications. TrAC Trends Anal. Chem. 2016, 75, 86–96. [Google Scholar] [CrossRef]
- Kumar, P.; Deep, A.; Kim, K.H. Metal organic frameworks for sensing applications. TrAC Trends Anal. Chem. 2015, 73, 39–53. [Google Scholar] [CrossRef]
- Lei, J.P.; Qian, R.C.; Ling, P.H.; Cui, L.; Ju, H.X. Design and sensing applications of metal–organic framework composites. TrAC Trends Anal. Chem. 2014, 58, 71–78. [Google Scholar] [CrossRef]
- Liu, C.S.; Li, J.; Pang, H. Metal-organic framework-based materials as an emerging platform for advanced electrochemical sensing. Coord. Chem. Rev. 2020, 410, 213222. [Google Scholar] [CrossRef]
- Cui, J.D.; Ren, S.Z.; Sun, B.T.; Jia, S.R. Optimization protocols and improved strategies for metal-organic frameworks for immobilizing enzymes: Current development and future challenges. Coord. Chem. Rev. 2018, 370, 22–41. [Google Scholar] [CrossRef]
- Hasan, M.R.; Islam, T.; Hasan, M.M.; Chowdhury, A.-N.; Ahammad, A.J.S.; Reaz, A.H.; Roy, C.K.; Shah, S.S.; Al-Imran; Aziz, M.A. Evaluating the electrochemical detection of nitrite using a platinum nanoparticle coated jute carbon modified glassy carbon electrode and voltametric analysis. J. Phys. Chem. Solids 2022, 165, 110659. [Google Scholar] [CrossRef]
- Timakwe, S.; Silwana, B.; Matoetoe, M.C. Electrochemistry as a complementary technique for revealing the influence of reducing agent concentration on AgNPs. ACS Omega 2022, 7, 4921–4931. [Google Scholar] [CrossRef]
- Thi Thanh, C.; Nguyen Duc Duoc, P.; Van Trinh, P.; Thi Huyen, N.; Van Tu, N.; Tuan Anh, C.; Van Hai, P.; Yoshida, K.; Abe, H.; Van Chuc, N. 3D porous graphene/double-walled carbon nanotubes/gold nanoparticles hybrid film for modifying electrochemical electrode. Mater. Lett. 2023, 330, 133308. [Google Scholar] [CrossRef]
- Mahugo, R.; Mayoral, A.; Sánchez-Sánchez, M.; Diaz, I. Observation of Ag nanoparticles in/on Ag@MIL-100 (Fe) prepared through different procedures. Front. Chem. 2019, 7, 686. [Google Scholar] [CrossRef]
- Wang, Q.J.; Tsumori, N.; Kitta, M.; Xu, Q. Fast dehydrogenation of formic acid over palladium nanoparticles immobilized in nitrogen-doped hierarchically porous carbon. ACS Catal. 2018, 8, 12041–12045. [Google Scholar] [CrossRef]
- White, R.J.; Luque, R.; Budarin, V.L.; Clark, J.H.; Macquarrie, D.J. Supported metal nanoparticles on porous materials. Methods and applications. J. Chem. Soc. Rev. 2009, 38, 481–494. [Google Scholar] [CrossRef]
- Goel, S.; Wu, Z.J.; Zones, S.I.; Iglesia, E. Synthesis and catalytic properties of metal clusters encapsulated within small-pore (SOD, GIS, ANA) zeolites. J. Am. Chem. Soc. 2012, 134, 17688–17695. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.L.; Xu, Q. Immobilization of ultrafine metal nanoparticles to high-surface-area materials and their catalytic applications. Chem. Soc. Rev. 2016, 1, 220–245. [Google Scholar] [CrossRef]
- Yang, Q.H.; Xu, Q.; Jiang, H.L. Metal–organic frameworks meet metal nanoparticles: Synergistic effect for enhanced catalysis. Chem. Soc. Rev. 2017, 46, 4774–4808. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Zhu, M. Tailoring the photoluminescence of atomically precise nanoclusters. Chem. Soc. Rev. 2019, 48, 2422–2457. [Google Scholar] [CrossRef]
- Yu, H.Z.; Rao, B.; Jiang, W.; Yang, S.; Zhu, M.Z. The photoluminescent metal nanoclusters with atomic precision. Coord. Chem. Rev. 2019, 378, 595–617. [Google Scholar] [CrossRef]
- Su, S.; Sun, H.F.; Cao, W.F.; Chao, J.; Peng, H.Z.; Zuo, X.L.; Yuwen, L.H.; Fan, C.H.; Wang, L.H. Dual-target electrochemical biosensing based on DNA structural switching on gold nanoparticle-decorated MoS2 nanosheets. ACS Appl. Mater. Interfaces 2016, 8, 6826–6833. [Google Scholar] [CrossRef]
- Wang, N.; Lin, M.; Dai, H.X.; Ma, H.Y. Functionalized gold nanoparticles/reduced graphene oxide nanocomposites for ultrasensitive electrochemical sensing of mercury ions based on thymine–mercury–thymine structure. Biosens. Bioelectron. 2016, 79, 320–326. [Google Scholar] [CrossRef]
- Kim, D.; Resasco, J.; Yu, Y.; Asiri, A.M.; Yang, P. Synergistic geometric and electronic effects for electrochemical reduction of carbon dioxide using gold–copper bimetallic nanoparticles. Nat. Commun. 2014, 5, 4948. [Google Scholar] [CrossRef]
- Rezaei, B.; Boroujeni, M.K.; Ensafi, A.A. Fabrication of DNA, o-phenylenediamine, and gold nanoparticle bioimprinted polymer electrochemical sensor for the determination of dopamine. Biosens. Bioelectron. 2015, 66, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, L.; Chen, H.H.; Hu, X.Y.; Ma, S.Q. Fabrication of highly sensitive and stable hydroxylamine electrochemical sensor based on gold nanoparticles and metal–metalloporphyrin framework modified electrode. ACS Appl. Mater. Interfaces 2016, 8, 18173–18181. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Weerasooriya, R.; Chen, X. The metal-organic framework supported gold nanoparticles as a highly sensitive platform for electrochemical detection of methyl mercury species in the aqueous environment. J. Hazard. Mater. 2022, 431, 128608. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Meng, T.J.; Fan, Y.W.; Chen, C.X.; Guo, Z.W.; Wang, H.; Zhang, Y.F. Electrochemical study of acetaminophen oxidation by gold nanoparticles supported on a leaf-like zeolitic imidazolate framework. J. Colloid Interface Sci. 2018, 524, 1–7. [Google Scholar] [CrossRef]
- Meng, W.; Wen, Y.Y.; Dai, L.; He, Z.X.; Wang, L. A novel electrochemical sensor for glucose detection based on Ag@ZIF-67 nanocomposite. Sens. Actuators B Chem. 2018, 260, 852–860. [Google Scholar] [CrossRef]
- Ham, Y.S.; Choe, S.; Kim, M.J.; Lim, T.; Kim, S.-K.; Kim, J.J. Electrodeposited Ag catalysts for the electrochemical reduction of CO2 to CO. Appl. Catal. B Environ. 2017, 208, 35–43. [Google Scholar] [CrossRef]
- Li, X.C.; He, C.J.; Zheng, J.; Wu, D.N.; Duan, Y.T.; Li, Y.F.; Rao, P.H.; Tang, B.; Rui, Y.C. Flocculent Cu caused by the Jahn–Teller effect improved the performance of Mg-MOF-74 as an anode material for lithium-ion batteries. ACS Appl. Mater. Interfaces 2020, 12, 52864–52872. [Google Scholar] [CrossRef]
- He, X.L.; Cai, Y.Y.; Zhang, H.; Liang, C.H. Photocatalytic degradation of organic pollutants with Ag decorated free-standing TiO2 nanotube arrays and interface electrochemical response. J. Mater. Chem. 2011, 21, 475–480. [Google Scholar] [CrossRef]
- Chen, S.Y.; Xie, Y.X.; Guo, X.J.; Sun, D.P. Self-supporting electrochemical sensors for monitoring of cell-released H2O2 based on metal nanoparticle/MOF nanozymes. Microchem. J. 2022, 181, 107715. [Google Scholar] [CrossRef]
- Peng, Z.W.; Jiang, Z.W.; Huang, X.; Li, Y.F. A novel electrochemical sensor of tryptophan based on silver nanoparticles/metal–organic framework composite modified glassy carbon electrode. RSC Adv. 2016, 6, 13742–13748. [Google Scholar] [CrossRef]
- Zhao, Y.F.; Chen, J.; Zhong, H.T.; Zhang, C.L.; Zhou, Y.; Mao, W.R.; Yu, C. Functionalized Ag/Fe-MOFs nanocomposite as a novel endogenous redox mediator for determination of α2,6-sialylated glycans in serum. Microchim. Acta 2020, 187, 649. [Google Scholar] [CrossRef]
- Er, E.; Çelikkan, H.; Erka, N. An ultra-sensitive 2D electrochemical sensor based on a PtNPs@graphene/Nafion nanocomposite for determination of α1-AR antagonist silodosin in human plasma. Anal. Methods 2017, 9, 3782–3789. [Google Scholar] [CrossRef]
- Ling, P.H.; Lei, J.P.; Jia, L.; Ju, H.X. Platinum nanoparticles encapsulated metal–organic frameworks for the electrochemical detection of telomerase activity. Chem. Commun. 2016, 52, 1226–1229. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.D.; Yang, L.Z.; Xu, C.L. Pt@UiO-66 heterostructures for highly selective detection of hydrogen peroxide with an extended linear range. Anal. Chem. 2015, 87, 3438–3444. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.L.; Yang, X.L.; Wang, B.R.; Ding, Y.B.; Xu, S.Y.; Liu, J.J.; Peng, Y.; Yu, X.L.; Su, Z.H.; Qin, X.L. One-step electrochemical growth of 2D/3D Zn (II)-MOF hybrid nanocomposites on an electrode and utilization of a PtNPs@2D MOF nanocatalyst for electrochemical immunoassay. ACS Appl. Mater. Interfaces 2021, 13, 46225–46232. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.M.; Jin, H.L.; Suo, Z.G.; Shen, H.L.; Chen, X.H.; Liu, Y.; Zhu, J.M.; Wei, M.; He, B.S.; Zhao, R.Y. Highly conductive MXene-Au NPs and high current AuPd NPs/UiO-66 electrochemical sensor combining multiple signal amplification strategies for tetracycline detection. Chem. Eng. J. 2024, 504, 158980. [Google Scholar] [CrossRef]
- Hira, S.A.; Nallal, M.; Park, K.H. Fabrication of PdAg nanoparticle infused metal-organic framework for electrochemical and solution-chemical reduction and detection of toxic 4-nitrophenol. Sens. Actuators B Chem. 2019, 298, 126861. [Google Scholar] [CrossRef]
- Yang, J.; Yang, L.T.; Ye, H.L.; Zhao, F.Q.; Zeng, B.Z. Highly dispersed AuPd alloy nanoparticles immobilized on UiO-66-NH2 metal-organic framework for the detection of nitrite. Electrochim. Acta 2016, 219, 647–654. [Google Scholar] [CrossRef]
- Xu, W.; Qin, Z.; Hao, Y.; He, Q.; Chen, S.; Zhang, Z.; Peng, D.; Wen, H.; Chen, J.; Qiu, J.; et al. A signal-decreased electrochemical immunosensor for the sensitive detection of LAG-3 protein based on a hollow nanobox-MOFs/AuPt alloy. Biosens. Bioelectron. 2018, 113, 148–156. [Google Scholar] [CrossRef]
- Xie, Y.X.; Shi, X.H.; Chen, L.X.; Lu, J.; Lu, X.G.; Sun, D.P.; Zhang, L.Y. Direct electrodeposition of bimetallic nanostructures on Co-based MOFs for electrochemical sensing of hydrogen peroxide. Front. Chem. 2022, 10, 856003. [Google Scholar] [CrossRef]
- Maduraiveeran, G.; Jin, W. Carbon nanomaterials: Synthesis, properties and applications in electrochemical sensors and energy conversion systems. Mater. Sci. Eng. B 2021, 272, 115341. [Google Scholar] [CrossRef]
- Kannan, P.; Maduraiveeran, G. Carbon nanocomposites-based electrochemical sensors and biosensors for biomedical diagnostics. Curr. Med. Chem. 2024, 31, 3870–3881. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, B.R.; Govindhan, M.; Chen, A. Carbon nanomaterials based electrochemical sensors/biosensors for the sensitive detection of pharmaceutical and biological compounds. Sensors 2015, 15, 22490–22508. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Shen, Y.L.; Zhang, Y.Y.; Zeng, T.; Wan, Q.J.; Lai, G.S.; Yang, N.J. A UiO-66-NH2/carbon nanotube nanocomposite for simultaneous sensing of dopamine and acetaminophen. Anal. Chim. Acta 2021, 1158, 338419. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; You, Z.Y.; Liu, L.L.; Duan, S.W.; Xiao, A.P. Electrochemical determination of synephrine by using nafion/UiO-66/graphene-modified screen-printed carbon electrode. Curr. Res. Food Sci. 2022, 5, 1158–1166. [Google Scholar] [CrossRef]
- Feng, X.G.; Lin, S.R.; Li, M.; Bo, X.J.; Guo, L.P. Comparative study of carbon fiber structure on the electrocatalytic performance of ZIF-67. Anal. Chim. Acta 2017, 984, 96–106. [Google Scholar] [CrossRef]
- Kumar, D.; Sharma, R.C. Advances in conductive polymers. Eur. Polym. J. 1998, 34, 1053–1060. [Google Scholar] [CrossRef]
- Naveen, M.H.; Gurudatt, N.G.; Shim, Y.B. Applications of conducting polymer composites to electrochemical sensors: A review. Appl. Mater. Today 2017, 9, 419–433. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, A.; Han, Y.; Li, T. Sensors based on conductive polymers and their composites: A review. Polym. Int. 2020, 69, 7–17. [Google Scholar] [CrossRef]
- Gupta, A.; Sharma, S.K.; Pachauri, V.; Ingebrandt, S.; Singh, S.; Sharma, A.L.; Deep, A. Sensitive impedimetric detection of troponin I with metal–organic framework composite electrode. RSC Adv. 2021, 11, 2167–2174. [Google Scholar] [CrossRef]
- Rezaei, M.; Ghanavati, M.; Mohammadi, N.; Khani, S.; Nasirimoghadam, S.; Smiley, E.; Basiryanmahabadi, A. A new sensitive layer based on calcined Zn/Ti-MOF/magnetic molecularly imprinted polypyrrole: Application to preconcentration and electrochemical determination of perfluorooctane sulfonic acid by magnetic carbon paste electrode. Talanta 2024, 276, 126229. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Fei, M.H.; Ni, M.J.; Wang, Y.L.; Liu, Z.F.; Xie, Y.X.; Zhao, P.C.; Zhang, Z.Y.; Fei, J.J. Multilayer Ti3C2-CNTs-Au Loaded with Cyclodextrin-MOF for Enhanced Selective Detection of Rutin. Small 2024, 20, 2310217. [Google Scholar] [CrossRef] [PubMed]
- Shu, Y.; Lu, Q.; Yuan, F.; Tao, Q.; Jin, D.Q.; Yao, H.; Xu, Q.; Hu, X.Y. Stretchable electrochemical biosensing platform based on Ni-MOF composite/Au nanoparticle-coated carbon nanotubes for real-time monitoring of dopamine released from living cells. ACS Appl. Mater. Interfaces 2020, 12, 49480–49488. [Google Scholar] [CrossRef] [PubMed]
- Alsafrani, A.E.; Adeosun, W.A.; Alruwais, R.S.; Marwani, H.M.; Asiri, A.M.; Khan, A. Metal–organic frameworks (MOFs) composite of polyaniline-CNT@ aluminum succinate for non-enzymatic nitrite sensor. Environ. Sci. Pollut. Res. 2023, 30, 71322–71339. [Google Scholar] [CrossRef]
- Duan, N.; Chen, X.W.; Lin, X.F.; Ying, D.C.; Wang, Z.P.; Yuan, W.B.; Wu, S.J. Paper-based fluorometric sensing of malachite green using synergistic recognition of aptamer-molecularly imprinted polymers and luminescent metal–organic frameworks. Sens. Actuators B Chem. 2023, 384, 133665. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhao, D.L.; Nai, M.H.; Chen, S.B.; Chung, T.S. Nanovoid-enhanced thin-film composite reverse osmosis membranes using ZIF-67 nanoparticles as a sacrificial template. ACS Appl. Mater. Interfaces 2021, 13, 33024–33033. [Google Scholar] [CrossRef]
- Shang, H.Y.; Xu, H.; Jin, L.J.; Chen, C.Y.; Song, T.X.; Wang, C.; Du, Y.K. Electrochemical-photoelectrochemical dual-mode sensing platform based on advanced Cu9S8/polypyrrole/ZIF-67 heterojunction nanohybrid for the robust and selective detection of hydrogen sulfide. Sens. Actuators B Chem. 2019, 301, 127060. [Google Scholar] [CrossRef]
- Tan, J.; Hussain, S.; Ge, C.X.; Wang, M.S.; Shah, S.; Liu, G.W.; Qiao, G.J. ZIF-67 MOF-derived unique double-shelled Co3O4/NiCo2O4 nanocages for superior Gas-sensing performances. Sens. Actuators B Chem. 2020, 303, 127251. [Google Scholar] [CrossRef]
- Salman, F.; Zengin, A.; Çelik Kazici, H. Synthesis and characterization of Fe3O4-supported metal–organic framework MIL-101 (Fe) for a highly selective and sensitive hydrogen peroxide electrochemical sensor. Ionics 2020, 26, 5221–5232. [Google Scholar] [CrossRef]
- Zhang, T.; Wei, J.Z.; Sun, X.J.; Zhao, X.J.; Tang, H.L.; Yan, H.; Zhang, F.M. Rapid synthesis of UiO-66 by means of electrochemical cathode method with electrochemical detection of 2,4,6-TCP. Inorg. Chem. Commun. 2020, 111, 107671. [Google Scholar] [CrossRef]
- Zheng, R.N.; He, B.S.; Xie, L.L.; Yan, H.Y.; Jiang, L.Y.; Ren, W.J.; Suo, Z.G.; Xu, Y.W.; Wei, M.; Jin, H.L. Molecular Recognition-Triggered Aptazyme Sensor Using a Co-MOF@ MCA Hybrid Nanostructure as Signal Labels for Adenosine Triphosphate Detection in Food Samples. Anal. Chem. 2022, 94, 12866–12874. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).