Abstract
Background/Objectives: Silicone elastomers are widely used in maxillofacial prostheses, but their service life is typically limited to 6–24 months due to progressive degradation. Reinforcement with zirconium silicate (ZrSiO4) nanoparticles has been proposed to improve durability, yet evidence on their long-term performance under storage remains limited. This study evaluated the effect of two years of dark storage on the mechanical properties of room-temperature-vulcanized (RTV) silicone reinforced with 1.5 wt% ZrSiO4 nanoparticles. Materials and Methods: Zirconium silicate (ZrSiO4) nanoparticles at 1.5 wt% were incorporated into A-2186 RTV silicone specimens, which were randomly divided into two equal groups: baseline specimens stored for 24 h and aged specimens stored for 24 months under dark conditions. Mechanical properties were assessed by measuring tensile strength, elongation at break, tear resistance, and Shore A hardness in accordance with standardized protocols. Fourier transform infrared (FTIR) spectroscopy was performed to verify the structural characteristics of the ZrSiO4 nanopowder. Statistical analysis was conducted using independent-samples t-tests, with significance set at p < 0.05. Results: After 24 months of dark storage, tensile strength and elongation at break decreased significantly (p < 0.05), indicating reduced elasticity. Tear resistance and hardness showed slight but non-significant reductions. FTIR confirmed the preservation of ZrSiO4 structural features. Conclusions: Dark storage selectively reduced reinforced silicone’s tensile and elongation properties, while tear resistance and hardness remained relatively stable. ZrSiO4 nanoparticles provided partial reinforcement, enhancing stability but not entirely preventing RTV silicone aging.
1. Introduction
Patient satisfaction and quality of life (QOL) are essential indicators of healthcare outcomes, with treatment success often best reflected through patients’ self-evaluation. Studies have shown that maxillofacial prosthetic rehabilitation markedly improves QOL by restoring facial form and function, shielding surgically affected or defective areas, and providing a safe, non-invasive means of esthetic recovery. These benefits enhance self-esteem, support psychological adaptation, and promote social reintegration [,,].
Maxillofacial prosthetic materials vary widely in composition, resulting in distinct physical properties ranging from the rigidity of polymers and alloys to the flexibility of elastomers. Commonly used materials include latex, poly(methyl methacrylate) (PMMA), poly(vinyl chloride) (PVC), polyurethanes, and silicone rubbers. Clinically, the ideal material should combine high tear and tensile strength with sufficient elongation, optimal surface wettability, minimal hardness, and low water absorption, these features essential for comfort, durability, and long-term suitability in maxillofacial prosthetic applications [,].
Silicone remains the preferred material for maxillofacial prostheses because of its flexibility, skin-like texture, and thermal stability, which collectively enhance comfort, function, and esthetics [,,,]. Its performance primarily depends on the interaction between polydimethylsiloxane (PDMS) chains and silica fillers, a relationship that governs the strength and durability of the material [].
Room-temperature-vulcanized (RTV) silicone elastomers are widely recognized as the standard material for fabricating facial prostheses, with addition-cured systems offering superior mechanical properties, consistent pigmentation, color stability, and biocompatibility. Their performance depends on formulation parameters such as the cross-linking reaction, PDMS molecular weight, and filler content, as well as initiator type, additive concentration, and curing conditions [,,,]. Among these, Factor II A-2186, a platinum-catalyzed RTV silicone is particularly valued for its stable color incorporation, high tensile and tear strength, and soft, skin-like texture that yields thin, durable margins. These properties are attributed to its high filler concentration, greater polymer molecular weight, and ability to vulcanize at low temperatures through an addition-type cross-linking mechanism [,,,,].
Although silicone elastomers remain the preferred material for maxillofacial prostheses, their clinical durability is limited, as deterioration often begins within months of use, manifesting as surface roughness, marginal distortion, reduced tear strength, and color instability. These drawbacks shorten prosthesis longevity to an average of 1.5–2 years, with failure commonly attributed to microbial colonization, material rupture, and the cumulative effects of adhesives, skin secretions, and cleansing procedures [,,,,]. External factors such as air pollution, ultraviolet (UV) radiation, temperature fluctuations, and patient handling and hygiene further accelerate discoloration and degradation, necessitating periodic follow-up and timely replacement to preserve esthetics and function [,,,,].
The persistence of oxygen-induced reactions during dark storage can promote additional cross-linking, leading to a denser silicone framework. However, the simultaneous occurrence of chain scission diminishes overall structural integrity, ultimately yielding a material with reduced rigidity [].
Efforts to improve the color stability of silicone prosthetic materials have included incorporating nanoparticles such as zinc oxide (ZnO), barium sulfate (BaSO4), and titanium dioxide (TiO2). These opacifiers, known for their high refractive index, are also used in sunscreens to block ultraviolet radiation []. Their inclusion has been shown to enhance silicone’s resistance to discoloration and extend service life [,]. However, long-term durability depends not only on color stability but also on mechanical integrity, as nanoparticle reinforcement improves hardness, tensile and tear strength, elongation, and even imparts antifungal activity [].
Zirconia has since emerged as a valuable nano-additive, offering high flexural strength, fracture toughness, and hardness []. Zirconium rarely exists in a pure state and is usually found as silicate oxide (ZrO2·SiO2) or free oxide (ZrO2); however, these raw forms contain metallic impurities that affect color and therefore require purification to yield zirconia powders suitable for biomaterial use []. Incorporation of nano-ZrO2 has improved the mechanical performance of room-temperature vulcanizing silicone, while ZrO2/SiO2 sols enhance the ultraviolet resistance of coated textiles []. In addition to these mechanical benefits, zirconia nanoparticles (ZrO2 NPs) exhibit antibacterial, antifungal, and antioxidant activities due to their unique physicochemical characteristics [].
Incorporating nanoparticles into polymer matrices enables the development of materials that retain the inherent flexibility of organic polymers while gaining mechanical reinforcement. Owing to their rigidity and high shear modulus, nanoparticles strengthen the polymer network, while their large surface area and high interfacial reactivity promote strong bonding with the silicone matrix. This interaction facilitates the mobility of polymer chains around the nanoparticles, enhancing cross-linking between adjacent PDMS chains. As a result, nanoparticle incorporation improves the elastomer’s physical and optical properties and increases resistance to stress-induced fracture and long-term degradation [,]. Accordingly, the incorporation of ZrO2 nanoparticles into maxillofacial silicone elastomers has been investigated to improve mechanical performance. Their stress-induced phase-transformation toughening enhances reinforcement efficiency, contributing to greater durability and clinical longevity of the prosthesis [].
Weathering alters the physical and chemical characteristics of polymers, causing significant changes in their mechanical and optical behavior. In maxillofacial prostheses, factors such as sweat, sebum, and UV radiation accelerate silicone degradation, as UV exposure promotes both cross-linking and bond cleavage, leading to color instability and material breakdown []. To simulate clinical conditions, silicone elastomers have been tested under perspiration and sebum exposure, artificial daylight, outdoor weathering, and cleaning regimens. However, while accelerated tests provide useful insights, they do not fully replicate natural degradation [,,].
Time-passage changes, as well as environmental and clinical factors, gradually reduce silicone prostheses’ tensile strength, tear resistance, hardness, and color stability. Accelerated aging is often used to estimate durability; however, the extreme conditions may alter degradation mechanisms and produce results that differ from those observed clinically. Most elastomers employed in facial prostheses are not ordinarily subjected to the moisture or thermal cycling levels imposed by artificial protocols. While accelerated weathering condenses deterioration into a shorter period without fully replicating natural exposure, outdoor weathering better reflects real conditions but is challenging to standardize due to seasonal and climatic variability [,,]. Given these limitations, silicone-based maxillofacial prostheses require replacement within 6–24 months of clinical service [,,].
Kareem et al. [] demonstrated that adding zirconium silicate (ZrSiO4) nanofiller to room-temperature-vulcanized silicone enhanced Shore A hardness, tear strength, tensile strength, and elongation, with the most significant improvement observed at a 1.5 wt.% concentration.
This study aimed to evaluate the long-term effect of dark aging on the mechanical properties of room-temperature-vulcanized (RTV) silicone reinforced with 1.5 wt.% zirconium silicate (ZrSiO4) nanoparticles. Unlike previous investigations using accelerated or UV aging, the present work employed two years of dark storage to isolate the reinforced silicone from environmental factors, allowing the exact effect of dark aging to be determined. This approach provides a realistic assessment of the material’s intrinsic stability and mechanical performance under conditions simulating clinical storage. The null hypothesis proposed that two years of dark aging would not significantly affect the mechanical properties of the reinforced RTV silicone.
2. Materials and Methods
2.1. Materials
Four experimental groups were prepared using a room-temperature-vulcanized (RTV) maxillofacial silicone elastomer (A-2186, Factor II Inc., Lakeside, AZ, USA), which served as the base material. The silicone was prepared following the manufacturer’s recommendations at a base-to-catalyst ratio of 10:1. Each group comprised ten specimens assigned to a specific mechanical test (tensile strength, elongation percentage, tear resistance, and hardness). The identical specimens used for tensile testing were also assessed for elongation percentage. To assess the influence of conditioning, every group was further divided into two equal subgroups, each exposed to a different conditioning protocol. In total, thirty specimens were prepared and evaluated across all groups. The sample size per group (n = 10) was selected in line with previous investigations on maxillofacial silicone elastomers, which demonstrated sufficient statistical power with comparable specimen numbers for mechanical evaluations [].
Zirconium silicate (ZrSiO4) nanopowder was selected as the reinforcing filler. It was procured from Sigma-Aldrich (Chemie GmbH, Eschenstrasse, Taufkirchen, Germany) under the label Zirconium (IV) silicate nanopowder (<100 nm particle size, 98.5% trace metals basis, CAS No. 10101-52-7, Molecular Weight: 183.31 g/mol, Product Number: 634395). According to Kareem et al., the nanopowder was incorporated into the silicone matrix at a concentration of 1.5 wt.% of the total weight []. Its structural characteristics were verified by Fourier-transform infrared spectroscopy (FTIR), with complementary morphological and crystalline features already established by Kareem et al. [] scanning electron microscopy (SEM) and X-ray diffraction (XRD).
Specimen geometries were designed using AutoCAD 2013 software and fabricated with a computer numerical control (CNC) milling machine. Transparent acrylic sheets were employed to construct the molds, which consisted of a base, frame, and cover. All components were verified by direct measurement to ensure dimensional accuracy.
2.2. Methods
2.2.1. Preparation of Zirconium-Silicate-Reinforced Silicone Nanocomposite
The preparation of the nanocomposite silicone followed a standardized multi-step process:
- Weighing and initial blending: The zirconium silicate nanofiller was accurately weighed (1.5 wt.% relative to the silicone base) using a precision analytical balance (Nimbus Analytical, Adam Equipment, Oxford, CT, USA; accuracy 0.0001 g) and transferred into the bowl of a vacuum mixer (Model AX-2000, Aixin Medical Equipment Co., Ltd., Tianjin, China). The silicone base (Part A) was weighed separately using a digital electronic scale and added over the nanofiller. The components were manually blended with a clean spatula for one minute.
- Mechanical mixing: The blend of silicone base and zirconium silicate nanofiller was homogenized in a vacuum mixer at a speed of 360 rpm for ten minutes. During the initial three minutes, mixing was carried out without vacuum, followed by seven minutes under a vacuum pressure of −0.09 bar. Subsequently, the catalyst (Part B) was incorporated into the mixture and mixed under vacuum for five minutes. To eliminate entrapped air bubbles, the mixture was placed in a vacuum chamber for fifteen minutes and maintained for three more minutes without vacuum to allow material settlement.
- Molding and curing: The prepared silicone mixture was poured into prefabricated acrylic molds, which were secured with G-clamps to permit the escape of excess material. Curing was performed at room temperature for 24 h. Following polymerization, specimens were carefully removed from the molds, cleaned, and stored under controlled laboratory conditions until testing conditions (room temperature 23 ± 2 °C, relative humidity 50 ± 5%).
2.2.2. Specimen Storage
Following fabrication and initial measurements, specimens were divided into two storage groups:
- Baseline group: Specimens were conditioned for a minimum of 16 h after curing and tested after 24 h in accordance with ISO 37:2017 and ISO 20795-1:2013 standards [,]. During this period, they were stored in hermetically sealed, light-resistant containers under controlled laboratory conditions (room temperature 23 ± 2 °C, relative humidity 50 ± 5%) [].
- Dark-storage group: Specimens were stored for two years (from 2021 to 2023) in sealed, pigment-free polyethylene bags maintained at room temperature (23 ± 2 °C) and relative humidity (50 ± 5%). The containers were placed inside a lightproof wooden box to prevent light exposure. At the end of the storage period, specimens were retrieved and subjected to testing [].
2.2.3. Mechanical Testing
Mechanical properties were evaluated at baseline (24 h after curing) and after two years of dark storage, with five specimens tested per group at each interval. All procedures followed the relevant ISO and ASTM standards for vulcanized rubber.
Tensile Strength
Tensile testing complied with ISO 37:2017 [], using type two dumbbell-shaped specimens, Figure 1. The thickness of each specimen was measured at three locations (both ends and the center) with a digital caliper (INGCO, China), and the average value was used for subsequent calculations. All measurements and tests were performed under controlled laboratory conditions (23 ± 1 °C and 50 ± 5% relative humidity). The tensile strength tests were performed using a computer-controlled universal testing machine (Laryee UE34100, Beijing, China). Each specimen was mounted in the machine grips with careful alignment to ensure uniform distribution of the applied load along the narrow section. The specimens were elongated at a crosshead speed of 500 mm/min until rupture, and the testing software automatically recorded the maximum load at fracture. Any specimens that fractured outside the central narrow region or showed irregular deformation were excluded from the analysis.
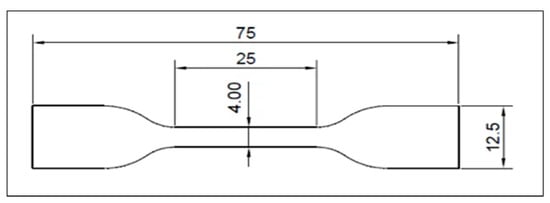
Figure 1.
The Dimensions of The Tensile Test Specimen.
The ultimate tensile strength (Ts) was calculated by dividing the maximum recorded load (F, in newtons) by the original cross-sectional area of the narrow section of the specimen, determined as the product of width (W) and thickness (T). The calculation followed Equation (1):
where the following abbreviations are used:
Ts (MPa) = F(N)/W × T (mm2)
F = maximum force at rupture (N);
W = width of narrow portion (mm);
T = thickness of narrow portion (mm).
Elongation at Break
The elongation percentage was determined in accordance with ISO 37:2017 [], simultaneously considering the tensile strength evaluation and up to the point of specimen failure. Each specimen’s initial gauge length (Lo) was established before testing using a digital caliper (INGCO, China).
Two reference marks were placed on the narrow section of each dumbbell-shaped specimen with a fine-tipped permanent marker. These were positioned 20 ± 0.5 mm apart, equidistant from the specimen’s center, and perpendicular to the longitudinal axis.
During tensile loading, the specimen was elongated until rupture. The final length between the two reference marks (Lb) was recorded at fracture, and the percentage of elongation at break was calculated using Equation (2):
where the following abbreviations are used:
Elongation percentage at break = 100 × (Lb − Lo)/Lo
Lo = initial gauge length (20 mm);
Lb = gauge length at rupture (mm).
Tear Strength
Tear resistance was measured in compliance with ISO 34-1:2015 [] using trouser-shaped specimens, as shown in Figure 2. Each specimen was clamped to a depth of 30 mm and aligned with the pull direction. Testing was performed on the Laryee UE34100 universal testing machine at a 500 mm/min crosshead speed. Each specimen’s thickness (d) and width were measured three times at different points using a digital caliper (INGCO, China), and the mean values were used in the calculations. Tear strength in newtons per millimeter (T, N/mm) was determined using Equation (3):
where the following abbreviations are used:
Tear strength (T) = F/d
F = maximum force at rupture (N);
D = specimen thickness (mm).
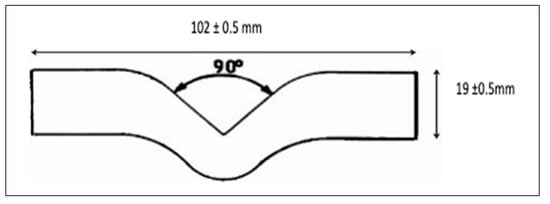
Figure 2.
Dimensions of The Tear Test Specimen.
Hardness
Shore A hardness was determined per ASTM D2240-15 (2021) []. Test specimens were fabricated as square plates measuring 40 × 40 × 6 mm [], Figure 3. Six test locations were identified on each specimen, positioned at least 12 mm from the edges and spaced 6 mm apart.
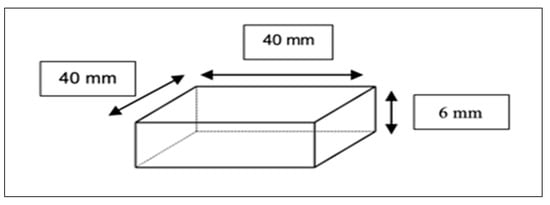
Figure 3.
Dimensions of Hardness Test Specimen.
Measurements were carried out using a Shore A durometer (Model: LX-A; total measure force: 10 N; needle stroke: 2.5 mm; needlepoint size: Φ 0.79 mm; resolution: 0.5 HA; dial value: 0~100 HA; recommended range of measurements: 10~90 HA, B089YDB9LL Co., China Ltd.). The durometer was applied vertically with the presser foot resting parallel to the specimen surface. Indentation values were recorded after a contact period of five seconds, and the average of the six readings was reported as the final hardness value for each specimen.
The specimen configurations used for mechanical testing, including tensile strength (a), tear strength (b), and hardness (c), are elucidated in Figure 4. The flowchart of the study design is presented in Figure 5.
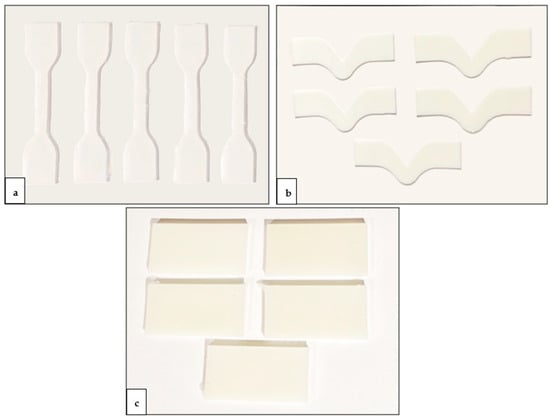
Figure 4.
Specimen configurations for mechanical testing: (a) tensile strength, (b) tear strength, and (c) hardness.
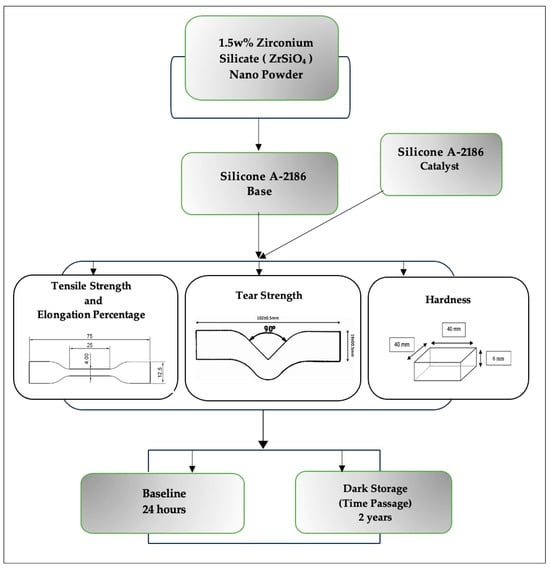
Figure 5.
Flow chart of study design.
2.3. Statistical Analysis
Statistical analyses were performed using SPSS software (version 16.0; SPSS Inc., Chicago, IL, USA). Descriptive statistics were expressed as mean ± standard deviation (SD). The normality of the data distribution was assessed using the Shapiro–Wilk test (p > 0.05). Comparisons between baseline (24 h) and two years of dark storage were conducted using independent-samples t-tests. A 95% confidence interval (CI) of the mean difference was calculated, and effect sizes were determined using Cohen’s d. Statistical significance was set at p < 0.05.
3. Results
3.1. FTIR Characterization
The FTIR spectrum of zirconium silicate (ZrSiO4) nanopowder shows O-Si-O bending vibration at 478 cm−1 and 585 cm−1. The Zr-O bond also appears at 540 cm−1. The peaks at 810, 894, and 1103 cm−1 were attributed to Si-O-Si stretching vibration. O-H stretching vibration bond also appeared at 3444 cm−1. These findings are presented in Figure 6. The detailed FTIR vibration bands corresponding to Figure 6 are provided in Table S1 of the Supplementary Materials for reference. These spectral features are consistent with previously reported findings [,], confirming the structural integrity of the nanopowder.
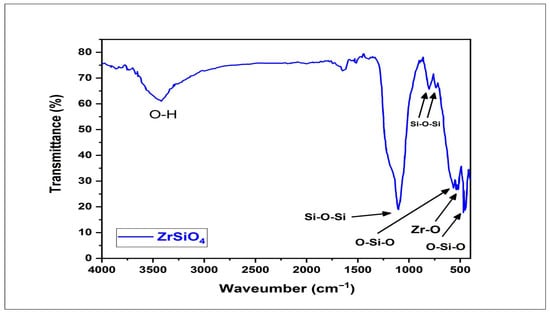
Figure 6.
The FTIR spectrum of zirconium silicate (ZrSiO4) nanopowder confirming the characteristic vibrational bands associated with ZrSiO4 functional groups.
3.2. Mechanical Test Results
The data distribution fulfilled normality assumptions according to the Shapiro–Wilk test (p > 0.05); therefore, parametric tests were applied. Descriptive statistics (mean and standard deviation) for all mechanical properties of the silicone samples reinforced with ZrSiO4 nanopowder at baseline (24 h) and after two years of dark storage are presented in Table 1 and Figure 7.

Table 1.
Mean values ± standard deviation of the mechanical tests of silicone reinforced with ZrSiO4 samples, measured at baseline (24 h) and after dark storage for two years.
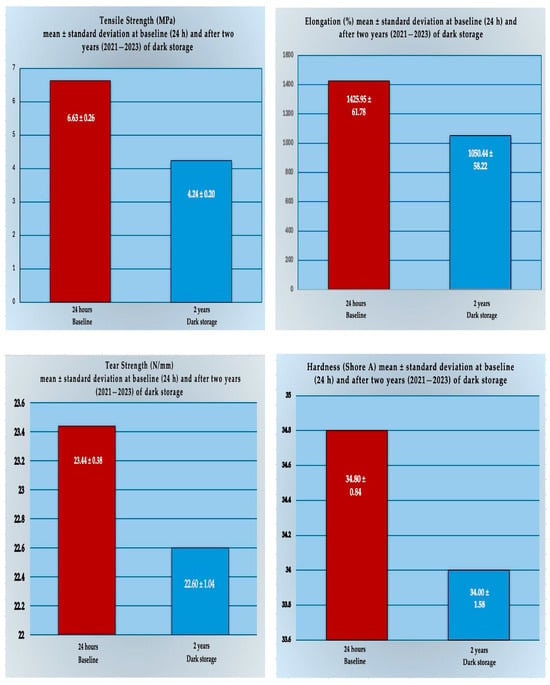
Figure 7.
Comparative multiple bar charts illustrating the mean values of all measured mechanical properties (tensile strength, elongation, tear strength, and hardness) of the silicone specimens reinforced with ZrSiO4 nanopowder across the two evaluation time periods at baseline (24 h) and after dark storage for two years.
Furthermore, the independent-samples t-test in Table 2 revealed that tensile strength and elongation were significantly reduced after aging. Tensile strength decreased from 6.63 ± 0.26 MPa to 4.24 ± 0.20 MPa, t (8) = 12.73, p < 0.001, with a considerable effect size (Cohen’s d = 5.69). Similarly, elongation declined from 1425.95 ± 61.78% to 1050.44 ± 58.22%, t (8) = 8.81, p = 0.001, also with a considerable effect size (Cohen’s d = 3.94).

Table 2.
The results of independent-samples t-tests comparing the mechanical properties (tensile strength, elongation %, tear strength, and hardness) of the silicone specimens reinforced with ZrSiO4, measured at baseline (24 h) and after dark storage for two years.
In contrast, differences in tear strength (23.44 ± 0.38 N/mm versus 22.60 ± 1.04 N/mm) and hardness (34.80 ± 0.84 versus 34.00 ± 1.58 Shore A) were not statistically significant, t(8) = 1.42, p = 0.228, and t (8) = 0.78, p = 0.477, respectively. Both outcomes showed only small-to-medium effect sizes, indicating minimal influence of long-term dark storage on these properties.
4. Discussion
Maxillofacial silicones are limited by inadequate mechanical performance, compromising their ability to replicate natural tissues. Since environmental aging accelerates these shortcomings and prosthesis replacement is costly, enhancing material durability is essential [].
Reinforcing silicone with suitable nanofillers has been proposed to mitigate this aging-related decline as an effective strategy to improve durability. Enhancing the mechanical strength of polymeric materials often requires the incorporation of reinforcing fillers, with the resulting properties influenced by factors such as filler morphology, surface area, particle size, surface chemistry, reactivity, and loading level, as well as the nature of the polymer matrix and processing conditions [,].
This study selected zirconia nanoparticles as the reinforcing agent because of their favorable characteristics, including biocompatibility, high strength, toughness, chemical stability, and resistance to abrasion and corrosion. Their large surface area also enables them to resist crack propagation. Clinically, incorporating zirconia nanofillers into maxillofacial silicone optimizes essential mechanical properties critical for daily use and extends the prosthesis service life [,].
In the present study, FTIR analysis confirmed the characteristic structural features of zirconium silicate (ZrSiO4) nanopowder, validating its composition prior to further evaluation. The spectrum displayed a broad absorption band at ~3444 cm−1, assigned to O–H stretching vibrations of surface hydroxyl groups and adsorbed water, while a medium band at ~1630 cm−1 corresponded to H–O–H bending. The strong signals at 1103, 894, and 810 cm−1 were attributed to Si–O–Si stretching vibrations, confirming the silicate framework. Additional absorptions observed at 585, 540, and 478 cm−1 were assigned to O–Si–O bending and Zr–O stretching modes, characteristic of the zircon lattice. Similar findings were reported by Dahham et al. [], who observed distinct Zr–O peaks in pure zirconia (ZrO2), and by Sun et al. [], who noted overlapping Si–O–Si and Zr–O bands in zirconium-silicate systems due to shared oxygen coordination within the silicate lattice. The present spectrum followed the latter pattern, showing partially merged bands rather than distinct peaks, which is typical for ZrSiO4 structures and confirms the presence of Zr–O bonding within the silicate matrix.
The findings of this study partially rejected the null hypothesis. After two years of dark storage, the maxillofacial silicone reinforced with 1.5 wt.% zirconium silicate (ZrSiO4) nanoparticles exhibited significant reductions in tensile strength and elongation at break, indicating that prolonged dark aging negatively affected these properties. In contrast, no statistically significant changes were detected in the tear strength or hardness of the reinforced silicone, suggesting that these characteristics remained relatively stable under two years of dark aging.
Among the evaluated properties, tensile strength, elongation, tear resistance, and hardness are particularly critical for assessing the performance of maxillofacial silicones. Tensile strength reflects the capacity of silicone elastomers to endure stress. At the same time, elongation indicates flexibility and resistance to rupture during routine handling of maxillofacial prostheses; the high tensile strength and elongation together are necessary to prevent deformation or damage during insertion and removal. High tear strength is essential since thin margins, particularly in orbital and nasal regions, are prone to irreversible damage once torn. Hardness determines silicone’s ability to replicate facial tissue texture, with optimal values ranging between 25 and 35 Shore A, primarily governed by cross-link density and the structural integrity of PDMS chains [,,,].
Incorporating zirconium silicate (ZrSiO4) nanopowder into Factor II maxillofacial silicone at varying concentrations has been shown to enhance tensile strength, tear resistance, elongation, and hardness, with 1.5 wt.% producing the most favorable improvements over the unreinforced control []. However, that investigation did not evaluate the effect of aging. To address this gap, the present study assessed RTV silicone reinforced with 1.5 wt% ZrSiO4 after two years of dark storage.
Polymeric biomaterials are susceptible to degradation owing to their limited resistance to thermal fluctuations and ultraviolet radiation, with photo-oxidation recognized as the primary mechanism of deterioration. Weathering exacerbates these changes through UV exposure, oxidation, and hydrolysis combined. As clinical conditions differ from those simulated in laboratory chambers, accelerated aging may not reliably represent in-service performance and can overestimate the extent of degradation compared with dark storage or routine prosthesis use [,].
Weathering has been shown to cause marked changes in polymers’ mechanical and physical properties. Photo-oxidative degradation generally progresses through three stages. In the initiation phase, free radicals are generated, either by photon-induced cleavage of polymer chains or by the presence of impurities such as residual catalyst metals. The propagation phase involves the reaction of these radicals with oxygen, forming oxy- and peroxy-radicals along with secondary radicals, ultimately leading to chain scission. In the termination phase, free radicals combine, which may increase cross-link density by forming new inter-chain or inter-monomer bonds, making the polymer more rigid. Conversely, when chain scission dominates, the network density decreases, resulting in greater material flexibility [,]. Chain fragmentation disrupts bonds within or between polymer chains, weakening the overall structure and reducing tensile strength and elongation capacity. In silicone rubbers, aging is primarily attributed to chemical degradation of the linkages between polysiloxane chains and alterations in the methyl groups attached to the silicon atoms [,,].
During dark storage, degradation processes initiated by oxygen uptake before sealing may persist, promoting additional cross-linking and increasing the density of the silicone network. At the same time, chain scission may occur, reducing structural integrity and yielding a softer material []. Polymers incorporating aromatic rings and C=C bonds within their structure are particularly susceptible to molecular rearrangements during aging. These transformations, which may involve chain scission, radical formation, and other molecular instabilities, progressively compromise structural integrity. Such changes are commonly reflected in the mechanical behavior of the material, where reduced flexibility and increased stiffness occur as disrupted amorphous regions reorganize into more ordered crystalline domains, a phenomenon often described as chemical crystallization [].
Silicone-based maxillofacial prostheses generally demonstrate a limited clinical service life of approximately 6–24 months, primarily due to progressive declines in their mechanical, physical, and optical properties [,,]. To simulate this time frame, evaluation under twenty-four months of dark storage offers a consistent and clinically relevant method for assessing time-dependent degradation. Unlike accelerated weathering, which may overstate deterioration, dark storage offers a more realistic long-term performance measure under controlled conditions.
The present findings demonstrated that the 1.5% ZrSiO4-reinforced RTV maxillofacial silicone exhibited a significant decline in both tensile strength and elongation percentage after 24 months (two years) of dark storage compared to specimens tested after 24 h (baseline). Tear resistance and hardness did not differ significantly; nevertheless, both parameters slightly decreased their mean values following dark storage.
The observed behavior can be explained by the combined effects of cross-linking and chain scission within the polymer network, where densely cross-linked regions are gradually disrupted by scission reactions []. These processes align with the present findings, as increased cross-link density would be expected to raise the modulus and alter the refractive index, whereas chain cleavage lowers tensile strength and elongation at break. Even without ultraviolet radiation or thermal cycling, molecular rearrangements progress over time, reducing chain flexibility and weakening intermolecular cohesion. Consequently, the elastomer becomes less able to withstand tensile forces and sustain elongation before rupture. In contrast, tear strength and hardness remained largely unaffected, indicating that these properties are less sensitive to dark aging and depend more strongly on stable filler–matrix interactions.
Kareem et al. [] reported that incorporating 1.5% zirconium silicate nanoparticles reduced deterioration, as reinforced specimens demonstrated superior mechanical performance compared with the non-reinforced material. In contrast, the present study found that although 1.5% zirconium silicate contributed to maintaining structural integrity, it did not entirely prevent the progressive decline of mechanical properties after two years of dark storage. At the molecular level, this outcome can be attributed to the interplay between cross-linking and chain-scission reactions: cross-linking increases network density and rigidity through the formation of additional interchain bonds, whereas chain scission disrupts main or interchain bonds, leading to reduced mechanical performance [,].
The gradual reduction in tensile strength and elongation observed after two years of dark storage may also be explained by the incorporation of nanoparticles. These promote the formation of multiple cross-links and entanglements that restrict polymer chain mobility and limit extensibility by aging. As polymer chains become more tightly anchored around the nanoparticles, their interaction with the surrounding silicone matrix is weakened; at the same time, the nanoparticles form bonds with silicone chains, and these multifunctional cross-links increase the overall cross-linking density of the cured elastomer. This nano-size effect generates numerous junction points within the composite, resulting in a broader particle distribution, a stiffer network, and reduced extensibility [,,,]. In addition, nanoparticles may interfere with chain entanglement during curing, slowing the initial polymerization rate. Although the degree of polymerization was reduced, the nanoparticles could not prevent the continuous polymerization of silicone, which is known to progress during aging [].
Azeez et al. observed that incorporating 1.5% zeolite into maxillofacial silicone led to reduced tensile strength and elongation, which they attributed to the formation of agglomerates. These clusters, held together by weak Van der Waals forces, act as stress concentration points within the polymer matrix and may fracture under load, initiating crack propagation and weakening the material []. In the present study, a comparable mechanism may also apply, as adding 1.5% zirconium silicate nanoparticles could generate localized stress sites that contribute to the deterioration of mechanical properties during prolonged dark aging.
During aging, the absorption of energy by polymer molecules can destabilize their structure. The excess energy may be released through several pathways, such as transfer to neighboring molecules or relaxation of functional groups back to their ground state. If this energy is not dissipated, it can induce bond cleavage through photochemical degradation, contributing to molecular deterioration, resulting in dimensional alterations, changes in color and brightness, loss of opacity, surface cracking, and increased hardness [].
Tear strength largely depends on the polymer matrix’s ability to dissipate energy at the crack tip during propagation. Well-dispersed nanoparticles can support this mechanism by scattering strain energy and functioning as multifunctional cross-links, anchoring polymer chains, and enabling redistribution of fracture energy throughout the network. This reinforcement enhances cross-link density and energy transfer, resulting in a stiffer, stronger material that resists tearing until higher deformation [,]. In the present study, this balance may explain the slight but non-significant decrease in tear strength: while aging-induced molecular degradation could weaken the silicone, the presence of zirconium silicate nanoparticles helped to dissipate energy and stabilize the matrix, preventing a statistically significant decline during aging.
In contrast with the findings of Sonnahalli and Chowdhary and Azeez et al., who emphasized that the reduction in tear strength may result from nanoparticle agglomeration, the present study demonstrated only a slight, non-significant decrease after aging. However, at higher filler concentrations, poor dispersion and the formation of agglomerates can create voids and stress concentrators, which may explain the reductions in elongation, increased modulus, and the decline in tear resistance reported in their studies. [,,].
Hardness in silicone elastomers has been shown to vary depending on the type of nanofiller used. Çevik et al. [] reported that titanium dioxide nanoparticles increased hardness, whereas the present study demonstrated a minor decrease without statistical significance after two years of dark storage with 1.5% zirconium silicate reinforcement. This difference may be attributed to variations in filler chemistry and the nature of filler–matrix interactions. It has also been noted that aging increases the degree of polymerization in facial silicones, which can influence hardness outcomes [].
Azeez et al. reported that Shore A hardness increased with higher filler concentrations, noting that at 1.5% silver–zinc zeolite, stronger adsorption of polymer chains onto the filler surface enhanced intermolecular forces and produced a stiffer matrix with greater resistance to permanent deformation under penetration. In the present study, although the same 1.5% concentration was used, zirconium silicate reinforcement after two years of dark storage showed only a minor, non-significant decrease in hardness, underscoring the role of filler type and aging conditions in determining hardness behavior [].
Haug et al. and Hatamleh et al. reported that maxillofacial silicones can exhibit measurable changes in mechanical properties even when stored in the dark, with variations attributed to silicone type, cross-link density, and intrinsic material stability. These observations are consistent with the present findings, where alterations were detected after two years of dark storage [,].
While the present work provides valuable insight into the mechanical behavior of silicone prostheses subjected to prolonged dark aging, it does not encompass the full spectrum of environmental and clinical factors that may influence the long-term stability of such materials. In clinical practice, maxillofacial prostheses are exposed to a combination of conditions including ultraviolet (UV) radiation, fluctuating temperature and humidity, repetitive heating and cooling cycles, oxidation, sebum and perspiration, as well as mechanical stress during patient use and cleaning procedures. These factors collectively contribute to progressive material degradation over time. Therefore, future investigations should extend beyond controlled dark aging and include comprehensive in vivo and simulated in vitro studies that replicate these environmental and biological challenges. Such studies will provide a more realistic evaluation of the overall durability, color stability, and mechanical reliability of silicone prostheses under actual service conditions. In addition to mechanical testing, optical properties, particularly color stability, should be assessed, since esthetic outcomes remain essential to clinical success. Moreover, although the earlier study by Kareem et al. [] characterized reinforced silicone prior to aging, further characterization after prolonged storage and aging is warranted to confirm structural stability and filler–matrix interactions over time. The development of hybrid nanofiller systems, combining zirconium silicate with biocompatible organic nanoparticles such as chitosan, represents a promising strategy to improve both durability and biological compatibility. Such approaches are likely to extend the service life of maxillofacial silicone materials and strengthen their clinical reliability.
5. Conclusions
This study reached the following conclusions:
- Although zirconium silicate nanoparticles enhanced the baseline properties of maxillofacial silicone, they did not entirely prevent aging-related deterioration. After two years of dark storage, significant reductions occurred in tensile strength and elongation at break, indicating susceptibility of these properties to long-term degradation.
- Tear strength and hardness showed no statistically significant changes after aging, suggesting that these characteristics are less sensitive to dark storage conditions.
- The observed mechanical changes can be attributed to the combined effects of cross-linking and chain scission within the polymer network, along with possible nanoparticle agglomeration and restricted chain mobility.
- The limited long-term durability observed highlights the need to optimize filler concentration, improve nanoparticle dispersion, and evaluate performance under clinically relevant conditions to achieve more reliable prostheses.
- Dark aging provides a realistic model of storage conditions, capturing the gradual changes that occur in silicone prostheses and offering a practical method to evaluate long-term material stability during typical service life.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/prosthesis7060149/s1, Table S1: FTIR peak assignments of zirconium silicate (ZrSiO4) nanopowder showing the main vibrational modes identified in the spectrum.
Author Contributions
Conceptualization, S.K.E. and F.K.A.-K.; methodology, S.K.E. and F.K.A.-K.; software, S.K.E., F.K.A.-K. and M.A.M.; validation, S.K.E., F.K.A.-K., J.F.A. and M.A.M.; formal analysis, F.K.A.-K.; investigation, F.K.A.-K.; resources, F.K.A.-K.; data curation, F.K.A.-K., J.F.A., M.A.M. and S.K.E.; writing—original draft preparation, F.K.A.-K.; writing—review and editing, F.K.A.-K.; visualization, S.K.E. and F.K.A.-K.; supervision, S.K.E., J.F.A. and M.A.M.; project administration, S.K.E., F.K.A.-K., J.F.A. and M.A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data supporting this study’s findings are available upon request from the corresponding author [Al-Kadi, F.K.] to preserve data integrity.
Acknowledgments
The authors would like to express their gratitude to Aqeel F. Hasan (University of Technology and Science, Baghdad, Iraq) for his assistance with the characterization tests. Special thanks are extended to the Assistant. Khalid K. Abbas (Department of Materials Engineering, University of Technology, Baghdad, Iraq) for conducting the mechanical testing.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ASTM | American Society for Testing and Materials (an international standards organization) |
| bar | Pressure unit |
| BaSO4 | barium sulfate |
| CI | confidence interval |
| cm−1 | Reciprocal centimeter (number of wave cycles per centimeter) |
| CNC | computer numerical control |
| d | specimen thickness |
| F | maximum force at rupture |
| FTIR | Fourier-transform infrared spectroscopy |
| g | Gram |
| g/mol | Gram per mole (Molarity unit) |
| h | Hour(s) |
| ISO | International Organization for Standardization |
| KN | Kilonewton |
| Lb | gauge length at rupture |
| Lo | initial gauge length |
| Mac. | Machine |
| min | Minute(s) |
| mm | Millimeter |
| MPa | Megapascal |
| N | Newton |
| n | Number |
| p | p-value (the calculated probability) |
| PDMS | polydimethylsiloxane |
| PMMA | Polymethyl Methacrylate |
| PVC | poly(vinyl chloride) |
| QOL | quality of life |
| rpm | Revolutions per minute |
| RTV | Room-Temperature-Vulcanizing Silicone |
| SD | Standard Deviation |
| SE | Standard Error |
| SPSS | Statistical Package for Social Sciences |
| t | t-statistic |
| T | Thickness |
| TiO2 | titanium dioxide |
| Ts | tensile strength |
| UTM | Universal Testing Machine |
| UV | Ultraviolet radiation |
| W | Width |
| wt% | Weight Percentage |
| ZnO | zinc oxide |
| ZrO2 NPs | zirconia nanoparticles |
| ZrSiO4 | zirconium silicate |
| % | Percent |
| °C | Degree Celsius |
| < | Less than |
| = | Equals |
| ± | The range around a value |
References
- Goiato, M.C.; Pesqueira, A.A.; Ramos da Silva, C.; Gennari Filho, H.; Micheline Dos Santos, D. Patient Satisfaction with Maxillofacial Prosthesis. Literature Review. J. Plast. Reconstr. Aesthetic Surg. 2009, 62, 175–180. [Google Scholar] [CrossRef]
- Kumar, S.; Rajtilak, G.; Rajasekar, V.; Kumar, M. Nasal Prosthesis for a Patient with Xeroderma Pigmentosum. J. Pharm. Bioallied Sci. 2013, 5, S176–S178. [Google Scholar] [CrossRef]
- Dings, J.P.J.; Merkx, M.A.W.; de Clonie Maclennan-Naphausen, M.T.P.; van de Pol, P.; Maal, T.J.J.; Meijer, G.J. Maxillofacial Prosthetic Rehabilitation: A Survey on the Quality of Life. J. Prosthet. Dent. 2018, 120, 780–786. [Google Scholar] [CrossRef]
- Aziz, T.; Waters, M.; Jagger, R. Analysis of the Properties of Silicone Rubber Maxillofacial Prosthetic Materials. J. Dent. 2003, 31, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Hatamleh, M.M.; Polyzois, G.L.; Nuseir, A.; Hatamleh, K.; Alnazzawi, A. Mechanical Properties and Simulated Aging of Silicone Maxillofacial Elastomers: Advancements in the Past 45 Years: Advancements in Maxillofacial Silicone Mechanical Properties. J. Prosthodont. 2016, 25, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Huber, H.; Studer, S.P. Materials and Techniques in Maxillofacial Prosthodontic Rehabilitation. Oral Maxillofac. Surg. Clin. N. Am. 2002, 14, 73–93. [Google Scholar] [CrossRef] [PubMed]
- Haddad, M.F.; Goiato, M.C.; Dos Santos, D.M.; Pesqueira, A.A.; Moreno, A.; Pellizzer, E.P. Influence of Pigment and Opacifier on Dimensional Stability and Detail Reproduction of Maxillofacial Silicone Elastomer. J. Craniofac. Surg. 2011, 22, 1612–1616. [Google Scholar] [CrossRef]
- Bangera, B.S.; Guttal, S.S. Evaluation of Varying Concentrations of Nano-Oxides as Ultraviolet Protective Agents When Incorporated in Maxillofacial Silicones: An in Vitro Study. J. Prosthet. Dent. 2014, 112, 1567–1572. [Google Scholar] [CrossRef]
- Mancuso, D.N.; Goiato, M.C.; de Carvalho Dekon, S.F.; Gennari-Filho, H. Visual Evaluation of Color Stability after Accelerated Aging of Pigmented and Nonpigmented Silicones to Be Used in Facial Prostheses. Indian J. Dent. Res. 2009, 20, 77–80. [Google Scholar] [CrossRef]
- Gandhi, D.S.; Sethuraman, R. Comparative Evaluation of Tensile Strength, Tear Strength, Color Stability, and Hardness of Conventional and 1% Trisnorbornenylisobutyl Polyhedralsilsesquioxane Modified Room Temperature Vulcanizing Maxillofacial Silicone after a Six-Month Artificial Aging Period. J. Indian Prosthodont. Soc. 2022, 22, 328–337. [Google Scholar] [CrossRef]
- Gunay, Y.; Kurtoglu, C.; Atay, A.; Karayazgan, B.; Gurbuz, C.C. Effect of Tulle on the Mechanical Properties of a Maxillofacial Silicone Elastomer. Dent. Mater. J. 2008, 27, 775–779. [Google Scholar] [CrossRef]
- Montgomery, P.C.; Kiat-Amnuay, S. Survey of Currently Used Materials for Fabrication of Extraoral Maxillofacial Prostheses in North America, Europe, Asia, and Australia: Survey of Maxillofacial Prostheses Materials Used. J. Prosthodont. 2009, 19, 482–490. [Google Scholar] [CrossRef]
- Nobrega, A.S.; Andreotti, A.M.; Moreno, A.; Sinhoreti, M.A.C.; Dos Santos, D.M.; Goiato, M.C. Influence of Adding Nanoparticles on the Hardness, Tear Strength, and Permanent Deformation of Facial Silicone Subjected to Accelerated Aging. J. Prosthet. Dent. 2016, 116, 623–629.e1. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, R.A.; Moore, D.J.; Cruz, D.L.; Chappell, R. Comparison of the Physical Properties of Two Types of Polydimethyl Siloxane for Fabrication of Facial Prostheses. J. Prosthet. Dent. 1992, 67, 679–682. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.H.; Wang, L.L.; Ko, C.C.; DeLong, R.L.; Hodges, J.S. New Organosilicon Maxillofacial Prosthetic Materials. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2002, 18, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Goiato, M.C.; Nobrega, A.S.; Freitas da Silva, E.V.; Micheline Dos Santos, D.; Pinheiro de Magalhães Bertoz, A.; Sonego, M.V.; Lamartine de Moraes Melo Neto, C. Tear Strength Analysis of MDX4-4210 and A-2186 Silicones with Different Intrinsic Pigments Incorporated by Mechanical and Industrial Methods. Int. J. Dent. 2019, 2019, 2573095. [Google Scholar] [CrossRef]
- Polyzois, G.L.; Eleni, P.N.; Krokida, M.K. Effect of Time Passage on Some Physical Properties of Silicone Maxillofacial Elastomers. J. Craniofac. Surg. 2011, 22, 1617–1621. [Google Scholar] [CrossRef]
- Ariani, N.; Visser, A.; Teulings, M.R.I.M.; Dijk, M.; Rahardjo, T.B.W.; Vissink, A.; Van Der Mei, H.C. Efficacy of Cleansing Agents in Killing Microorganisms in Mixed Species Biofilms Present on Silicone Facial Prostheses—An In Vitro Study. Clin. Oral Investig. 2015, 19, 2285–2293. [Google Scholar] [CrossRef]
- Goiato, M.C.; Pesqueira, A.A.; dos Santos, D.M.; Antenucci, R.M.F.; Ribeiro, P.d.P. Evaluation of Dimensional Change and Detail Reproduction in Silicones for Facial Prostheses. Acta Odontol. Latinoam. 2008, 21, 85–88. [Google Scholar]
- Eleni, P.N.; Katsavou, I.; Krokida, M.K.; Polyzois, G.L. Color Stability of Facial Silicon Prosthetic Elastomer after Artificial Weathering. Dent. Res. J. 2008, 5, 71–79. [Google Scholar]
- Nóbrega, A.S.; Neto, C.L.M.M.; Dos Santos, D.M.; Bertoz, A.P.M.; de MeloMoreno, A.L.; Goiato, M.C. Effect of Accelerated Aging on the Sorption and Solubility Percentages of Silicone Facial Prostheses. Eur. J. Dent. 2022, 116, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Chotprasert, N.; Shrestha, B.; Sipiyaruk, K. Effects of Disinfection Methods on the Color Stability of Precolored and Hand-Colored Maxillofacial Silicone: An In Vitro Study. Int. J. Biomater. 2022, 2022, 7744744. [Google Scholar] [CrossRef] [PubMed]
- Al-Kadi, F.K.; Abdulkareem, J.F.; Azhdar, B.A. Hybrid Chitosan-TiO2 Nanocomposite Impregnated in Type A-2186 Maxillofacial Silicone Subjected to Different Accelerated Aging Conditions: An Evaluation of Color Stability. Nanomaterials 2023, 13, 2379. [Google Scholar] [CrossRef]
- Alkahtany, M.; Beatty, M.W.; Alsalleeh, F.; Petro, T.M.; Simetich, B.; Zhou, Y.; Feely, D.; Polyzois, G. Color Stability, Physical Properties, and Antifungal Effects of ZrO2 Additions to Experimental Maxillofacial Silicones: Comparisons with TiO2. Prosthesis 2023, 5, 916–938. [Google Scholar] [CrossRef]
- Akay, C.; Cevik, P.; Karakis, D.; Sevim, H. In Vitro Cytotoxicity of Maxillofacial Silicone Elastomers: Effect of Nanoparticles. J. Prosthodont. 2018, 27, 584–587. [Google Scholar] [CrossRef]
- Saridag, S.; Tak, O.; Alniacik, G. Basic Properties and Types of Zirconia: An Overview. World J. Stomatol. 2013, 2, 40–47. [Google Scholar] [CrossRef]
- Balaji, S.; Mandal, B.K.; Ranjan, S.; Dasgupta, N.; Chidambaram, R. Nano-Zirconia—Evaluation of Its Antioxidant and Anticancer Activity. J. Photochem. Photobiol. B Biol. 2017, 170, 125–133. [Google Scholar] [CrossRef]
- Sonnahalli, N.K.; Chowdhary, R. Effect of Nanoparticles on Color Stability and Mechanical and Biological Properties of Maxillofacial Silicone Elastomer: A Systematic Review. J. Indian Prosthodont. Soc. 2020, 20, 244–254. [Google Scholar] [CrossRef]
- Goiato, M.C.; Haddad, M.F.; Sinhoreti, M.A.C.; dos Santos, D.M.; Pesqueira, A.A.; Moreno, A. Influence of Opacifiers on Dimensional Stability and Detail Reproduction of Maxillofacial Silicone Elastomer. Biomed. Eng. Online 2010, 9, 85. [Google Scholar] [CrossRef]
- Hussein, I.E.; Hasan, R.H. Effects of Nano Zirconium Oxide Addition on the Strength, Hardness, and Microstructure of Maxillofacial Silicone Material. Int. Med. J. 2021, 28 (Suppl. S1), 54–57. [Google Scholar]
- Hatamleh, M.M.; Polyzois, G.L.; Silikas, N.; Watts, D.C. Effect of Extraoral Aging Conditions on Mechanical Properties of Maxillofacial Silicone Elastomer. J. Prosthodont. 2011, 20, 439–446. [Google Scholar] [CrossRef]
- Nair, A.; Saratchandran, S. Comparative Evaluation of Colour Stability of Maxillofacial Silicones Following Accelerated Ageing Conditions. Face 2022, 3, 362–368. [Google Scholar] [CrossRef]
- Kiat-amnuay, S.; Mekayarajjananonth, T.; Powers, J.M.; Chambers, M.S.; Lemon, J.C. Interactions of Pigments and Opacifiers on Colour Stability of MDX4-4210/Type A Maxillofacial Elastomers Subjected to Artificial Ageing. J. Prosthet. Dent. 2006, 95, 249–257. [Google Scholar] [CrossRef]
- Radey, N.; Al Shimy, A.; Ahmed, D. Effect of Extraoral Aging Conditions on Mechanical Properties of Facial Silicone Elastomer Reinforced with Titanium Oxide Nanoparticles (In Vitro Study). Alex. Dent. J. 2020, 45, 29–36. [Google Scholar] [CrossRef]
- Gupta, P.; Deshpande, S.; Radke, U.; Ughade, S.; Sethuraman, R. The Color Stability of Maxillofacial Silicones: A Systematic Review and Meta-Analysis. J. Indian Prosthodont. Soc. 2021, 21, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Kareem, S.; Fatalla, A.; Moudhaffer, M.; Ali, M. Effects of Zirconium Silicate Nanofillers on Some Properties of Room-Vulcanized Maxillofacial Silicone Elastomers. Res. J. Pharm. Biol. Chem. Sci. 2018, 9, 1023–1031. [Google Scholar]
- Al-Kadi, F.K.; Abdulkareem, J.F.; Azhdar, B.A. Evaluation of the Mechanical and Physical Properties of Maxillofacial Silicone Type A-2186 Impregnated with a Hybrid Chitosan–TiO2 Nanocomposite Subjected to Different Accelerated Aging Conditions. Biomimetics 2023, 8, 539. [Google Scholar] [CrossRef]
- ISO 37:2017; Rubber, Vulcanized or Thermoplastic—Determination of Tensile Stress–Strain Properties. International Organization for Standardization: Geneva, Switzerland, 2017.
- ISO 20795-1:2013; Dentistry—Base Polymers—Part 1: Denture Base Polymers. International Organization for Standardization: Geneva, Switzerland, 2013.
- Çevik, P. Evaluation of Shore A hardness of maxillofacial silicones: The effect of dark storage and nanoparticles. Eur. Oral Res. 2018, 52, 99–104. [Google Scholar] [CrossRef]
- ISO 34-1; Rubber, Vulcanized or Thermoplastic—Determination of Tear Strength—Part 1. International Organization for Standardization: Geneva, Switzerland, 2010.
- ASTM D2240-15; Standard Test Method for Rubber Property—Durometer Hardness. ASTM International: West Conshohocken, PA, USA, 2012. [CrossRef]
- Dahham, O.S.; Hamzah, R.; Noriman, N.Z.; Alakrach, A.M.; Idrus, S.Z.S.; Shayfull, Z.; Adam, T. The Influences of Zirconium Dioxide on ENR-25/ZrO2 Composites: FTIR and TGA Analysis. J. Phys. Conf. Ser. 2018, 1019, 012055. [Google Scholar] [CrossRef]
- Sun, H.; Song, J.; Qi, T. Separation of Zr and Si in Zirconium Silicate by Sodium Hydroxide Sub-Molten Salt. Metals 2024, 14, 630. [Google Scholar] [CrossRef]
- Tukmachi, M.S.; Safi, I.N.; Ali, M.M.M. Evaluation of Mechanical Properties and Cytotoxicity of Maxillofacial Silicone Material after Incorporation of Zirconia Nanopowder. Mater. Today Proc. 2021, 42, 2209–2217. [Google Scholar] [CrossRef]
- Nguyen, C.T.; Chambers, M.S.; Powers, J.M.; Kiat-Amnuay, S. Effect of Opacifiers and UV Absorbers on Pigmented Maxillofacial Silicone Elastomer, Part 2: Mechanical Properties after Artificial Aging. J. Prosthet. Dent. 2013, 109, 402–410. [Google Scholar] [CrossRef]
- Eleni, P.N.; Krokida, M.K.; Polyzois, G.L. The Effect of Artificial Accelerated Weathering on the Mechanical Properties of Maxillofacial Polymers PDMS and CPE. Biomed. Mater. 2009, 4, 035001. [Google Scholar] [CrossRef]
- Rahman, A.M.; Jamayet, N.B.; Nizami, M.M.U.I.; Johari, Y.; Husein, A.; Alam, M.K. Effect of Tropical Outdoor Weathering on the Surface Roughness and Mechanical Properties of Maxillofacial Silicones. J. Prosthet. Dent. 2022, 127, 937–942. [Google Scholar] [CrossRef]
- Chang, H.; Wan, Z.; Chen, X.; Wan, J.; Luo, L.; Zhang, H.; Shu, S.; Tu, Z. Temperature and Humidity Effect on Aging of Silicone Rubbers as Sealing Materials for Proton Exchange Membrane Fuel Cell Applications. Appl. Therm. Eng. 2016, 104, 472–478. [Google Scholar] [CrossRef]
- Mouzakis, D.E.; Kandilioti, G.; Elenis, A.; Gregoriou, V.G. Ultraviolet Radiation Induced Cold Chemi-Crystallization in Syndio-tactic Polypropylene Clay-Nanocomposites. J. Macromol. Sci. Part A 2006, 43, 259–267. [Google Scholar] [CrossRef]
- Willett, E.S.; Beatty, M.W. Outdoor Weathering of Facial Prosthetic Elastomers Differing in Durometer Hardness. J. Prosthet. Dent. 2015, 113, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Al-Harbi, F.A.; Ayad, N.M.; Saber, M.A.; ArRejaie, A.S.; Morgano, S.M. Mechanical Behavior and Color Change of Facial Prosthetic Elastomers after Outdoor Weathering in a Hot and Humid Climate. J. Prosthet. Dent. 2015, 113, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, Q.; Jing, D.; Zhou, S.; Shao, L. Biomechanical Properties of Nano-TiO2 Addition to a Medical Silicone Elastomer: The Effect of Artificial Aging. J. Dent. 2014, 42, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Azeez, Z.; Tukmachi, M.; Mohammed, D.H. Effect of Silver-Zinc Zeolite Addition on Mechanical Properties of Maxillofacial Silicone. Int. J. Med. Res. Health Sci. 2018, 7, 19–29. [Google Scholar]
- Andreopoulos, A.G.; Evangelatou, M. Evaluation of Various Reinforcements for Maxillofacial Silicone Elastomers. J. Biomater. Appl. 1994, 8, 344–360. [Google Scholar] [CrossRef] [PubMed]
- Haug, S.P.; Moore, B.K.; Andres, C.J. Colour Stability and Colourant Effect on Maxillofacial Elastomers. Part II: Weathering Effect on Physical Properties. J. Prosthet. Dent. 1999, 81, 423–430. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).