Physical Properties of PMMA Denture Base with Added Organoselenium as an Antifungal
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples Production and Experimental Grouping
2.2. Flexural Stress and Elastic Modulus
2.3. Microhardness Test
2.4. Scanning Electron Microscope Examination
2.5. Sample Size and Power Calculation
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- National Institutes of Health. World’s Older Population Grows Dramatically (2016). Available online: https://www.nia.nih.gov/news/worlds-older-population-grows-dramatically (accessed on 2 October 2025).
- Perić, M.; Miličić, B.; Pfićer, J.K.; Živković, R.; Arsenijević, V.A. A Systematic Review of Denture Stomatitis: Predisposing Factors, Clinical Features, Etiology, and Global Candida spp. Distribution. J. Fungi 2024, 10, 328. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gendreau, L.; Loewy, Z.G. Epidemiology and etiology of denture stomatitis. J. Prosthodont. 2011, 20, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Yarborough, A.; Cooper, L.; Duqum, I.; Mendonca, G.; McGraw, K.; Stoner, L. Evidence Regarding the Treatment of Denture Stomatitis. J. Prosthodont. 2016, 25, 288–301. [Google Scholar] [CrossRef] [PubMed]
- Mayer, F.L.; Wilson, D.; Hube, B. Candida albicanspathogenicity mechanisms. Virulence 2013, 4, 119–128. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Finkel, J.S.; Mitchell, A.P. Genetic control of Candida albicans biofilm development. Nat. Rev. Microbiol. 2011, 9, 109–118. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Susewind, S.; Lang, R.; Hahnel, S. Biofilm formation and Candida albicans morphology on the surface of denture base materials. Mycoses 2015, 58, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Gad, M.M.; Al-Thobity, A.M.; Fouda, S.M.; Näpänkangas, R.; Raustia, A. Flexural and Surface Properties of PMMA Denture Base Material Modified with Thymoquinone as an Antifungal Agent. J. Prosthodont. 2020, 29, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Fouda, S.M.; Gad, M.M.; Ellakany, P.; Al-Thobity, A.M.; Al-Harbi, F.A.; Virtanen, J.I.; Raustia, A. The effect of nanodiamonds on candida albicans adhesion and surface characteristics of PMMA denture base material—An in vitro study. J. Appl. Oral Sci. 2019, 27, e20180779. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dhir, G.; Berzins, D.W.; Dhuru, V.B.; Periathamby, A.R.; Dentino, A. Physical properties of denture base resins potentially resistant to Candida adhesion. J. Prosthodont. 2007, 16, 465–472. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kanie, T.; Arikawa, H.; Fujii, K.; Inoue, K. Physical and mechanical properties of PMMA resins containing γ-methacryloxypropyltrimethoxysilane. J. Oral Rehabil. 2004, 31, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Cunha, T.R.; Regis, R.R.; Bonatti, M.R.; de Souza, R.F. Influence of incorporation of fluoroalkyl methacrylates on roughness and flexural strength of a denture base acrylic resin. J. Appl. Oral Sci. 2009, 17, 103–107. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Mirizadeh, A.; Atai, M.; Ebrahimi, S. Fabrication of denture base materials with antimicrobial properties. J. Prosthet. Dent. 2018, 119, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Gong, H.; Zhang, J.; Guo, X.; Yan, M.; Zhu, S. Effects of antibacterial coating on monomer exudation and the mechanical properties of denture base resins. J. Prosthet. Dent. 2017, 117, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Al-Harbi, F.A.; Abdel-Halim, M.S.; Gad, M.M.; Fouda, S.M.; Baba, N.Z.; AlRumaih, H.S.; Akhtar, S. Effect of Nanodiamond Addition on Flexural Strength, Impact Strength, and Surface Roughness of PMMA Denture Base. J. Prosthodont. 2019, 28, e417–e425. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Sun, J.; Lan, J.; Qi, Q. Effect of a denture base acrylic resin containing silver nanoparticles on Candida albicans adhesion and biofilm formation. Gerodontology 2016, 33, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Compagnoni, M.A.; Pero, A.C.; Ramos, S.M.M.; Marra, J.; Paleari, A.G.; Rodriguez, L.S. Antimicrobial activity and surface properties of an acrylic resin containing a biocide polymer. Gerodontology 2012, 31, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Darwish, G.; Huang, S.; Knoernschild, K.; Sukotjo, C.; Campbell, S.; Bishal, A.K.; Barão, V.A.; Wu, C.D.; Taukodis, C.G.; Yang, B. Improving Polymethyl Methacrylate Resin Using a Novel Titanium Dioxide Coating. J. Prosthodont. 2019, 28, 1011–1017. [Google Scholar] [CrossRef] [PubMed]
- Mu, M.; Liu, S.; DeFlorio, W.; Hao, L.; Wang, X.; Salazar, K.S.; Taylor, M.; Castillo, A.; Cisneros-Zevallos, L.; Oh, J.K.; et al. Influence of Surface Roughness, Nanostructure, and Wetting on Bacterial Adhesion. Langmuir 2023, 39, 5426–5439. [Google Scholar] [CrossRef]
- AlMojel, N.; AbdulAzees, P.A.; Lamb, E.M.; Amaechi, B.T. Determining growth inhibition of Candida albicans biofilm on denture materials after application of an organoselenium-containing dental sealant. J. Prosthet. Dent. 2023, 129, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Tran, P.; Enos, T.; Luth, K.; Hamood, A.; Ray, C.; Mitchell, K.; Reid, T.W. Organo-Selenium-Containing Polyester Bandage Inhibits Bacterial Biofilm Growth on the Bandage and in the Wound. Biomedicines 2020, 8, 62. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tran, P.; Hamood, A.; Mosley, T.; Gray, T.; Jarvis, C.; Webster, D.; Amaechi, B.; Enos, T.; Reid, T. Organo-selenium-containing dental sealant inhibits bacterial biofilm. J. Dent. Res. 2013, 92, 461–466. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tran, P.; Arnett, A.; Jarvis, C.; Mosley, T.; Tran, K.; Hanes, R.; Webster, D.; Mitchell, K.; Dominguez, L.; Hamood, A.; et al. Organo-Selenium Coatings Inhibit Gram-Negative and Gram-Positive Bacterial Attachment to Ophthalmic Scleral Buckle Material. Transl. Vis. Sci. Technol. 2017, 6, 1. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Tran, P.L.; Hammond, A.A.; Mosley, T.; Cortez, J.; Gray, T.; Colmer-Hamood, J.A.; Shashtri, M.; Spallholz, J.E.; Hamood, A.N.; Reid, T.W. Organoselenium coating on cellulose inhibits the formation of biofilms by Pseudomonas aeruginosa and Staphylococcus aureus. Appl. Environ. Microbiol. 2009, 75, 3586–3592. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alawadi, A.; AbdulAzees, P.A.; Lin, C.; Haney, S.J.; Hanlon, J.P.; Angelara, K.; Taft, R.M.; Amaechi, B.T. Application of organoselenium in inhibiting Candida albicans biofilm adhesion on 3D printed denture base material. J. Prosthodont. 2024, 33, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, S.; Fantozzi, G.; Bernardi, S.; Antonouli, S.; Continenza, M.A.; Macchiarelli, G. Commercial oral hygiene products and implant collar surfaces: Scanning electron microscopy observations. Can. J. Dent. Hyg. 2020, 54, 26–31. [Google Scholar] [PubMed] [PubMed Central]
- Bernardi, S.; Stinziani, S.d.S.; Di Girolamo, M.; Ruparelia, K.C.; Continenza, M.A. Application of Natural Extracts After Dental Air-Polishing Procedures: What Should We Know? Altern. Complement. Ther. 2019, 25, 151–154. [Google Scholar] [CrossRef]
- Alqutaibi, A.Y.; Baik, A.; Almuzaini, S.A.; Farghal, A.E.; Alnazzawi, A.A.; Borzangy, S.; Aboalrejal, A.N.; AbdElaziz, M.H.; Mahmoud, I.I.; Zafar, M.S. Polymeric Denture Base Materials: A Review. Polymers 2023, 15, 3258. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Council on Dental Materials and Devices. Revised American Dental Association specification no. 12 for denture base polymers. J. Am. Dent. Assoc. 1975, 90, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Zeidan, A.A.E.-L.; Elrahim, R.A.A.; El Hakim, A.F.A.; Harby, N.M.; Helal, M.A. Evaluation of Surface Properties and Elastic Modulus of CAD-CAM Milled, 3D Printed, and Compression Moulded Denture Base Resins: An In Vitro Study. J. Int. Soc. Prev. Community Dent. 2022, 12, 630–637. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Consani, R.L.; Pucciarelli, M.G.; Mesquita, M.F.; Nogueira, M.C.; Barão, V.A. Polymerization cycles on hardness and surface gloss of denture bases. Int. J. Contemp. Dent. Med. Rev. 2014, 2014, 041114. [Google Scholar] [CrossRef]
- Choi, J.J.E.; Uy, C.E.; Ramani, R.S.; Waddell, J.N. Evaluation of surface roughness, hardness and elastic modulus of nanoparticle containing light-polymerized denture glaze materials. J. Mech. Behav. Biomed. Mater. 2020, 103, 103601. [Google Scholar] [CrossRef]
- Bortolozo, F.; Menezes, H.S.; Paschoal, Â.S.; Rinaldi, M.; Rêgo, B.; de Souza, P. Scanning electron microscope analysis of PMMA third generation: A comparative experimental study. MedNEXT J. Med. Health Sci. 2022, 3. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.-G. Statistical power analyses using G*power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef] [PubMed]
- Alzayyat, S.T.; Almutiri, G.A.; Aljandan, J.K.; Algarzai, R.M.; Khan, S.Q.; Akhtar, S.; Ateeq, I.S.; Gad, M.M. Effects of SiO2 Incorporation on the Flexural Properties of a Denture Base Resin: An In Vitro Study. Eur. J. Dent. 2022, 16, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Balos, S.; Puskar, T.; Potran, M.; Milekic, B.; Koprivica, D.D.; Terzija, J.L.; Gusic, I. Modulus, strength and cytotoxicity of PMMA-silica nanocomposites. Coatings 2020, 10, 583. [Google Scholar] [CrossRef]
- de Castro, D.T.; Valente, M.L.; da Silva, C.H.; Watanabe, E.; Siqueira, R.L.; Schiavon, M.A.; Alves, O.L.; dos Reis, A.C. Evaluation of antibiofilm and mechanical properties of new nanocomposites based on acrylic resins and silver vanadate nanoparticles. Arch. Oral Biol. 2016, 67, 46–53. [Google Scholar] [CrossRef]
- Regis, R.R.; Zanini, A.P.; Della Vecchia, M.P.; Silva-Lovato, C.H.; Paranhos, H.F.O.; de Souza, R.F. Physical Properties of an Acrylic Resin after Incorporation of an Antimicrobial Monomer. J. Prosthodont. 2011, 20, 372–379. [Google Scholar] [CrossRef]
- Rodriguez, L.S.; Paleari, A.G.; Giro, G.; Junior, N.M.D.O.; Pero, A.C.; Compagnoni, M.A. Chemical Characterization and Flexural Strength of a Denture Base Acrylic Resin with Monomer 2-Tert-Butylaminoethyl Methacrylate. J. Prosthodont. 2012, 22, 292–297. [Google Scholar] [CrossRef]
- Al-Naggar, T.I.; El-Badry, B. Structural, optical and electrical properties of Poly(Methyl Methacrylate) polymer under alpha radiation. Nucl. Instrum. Methods Phys. Res. B 2021, 508, 24–28. [Google Scholar] [CrossRef]
- Cacciafesta, V.; Sfondrini, M.F.; Lena, A.; Scribante, A.; Vallittu, P.K.; Lassila, L.V. Flexural strengths of fiber-reinforced composites polymerized with conventional light-curing and additional postcuring. Am. J. Orthod. Dentofac. Orthop. 2007, 132, 524–527. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahim, R.A.; Ezzeldine, A.A.; Abdellah, M.; Elghazawi, S.S. Effect of Thermal Ageing on Flexural Strength and Microhardness of Novel High-Performance Polymer (Nanoksa G-Plus) in Comparison to a Widely Used Bio-HPP/PEEK. Dent. J. 2025, 13, 370. [Google Scholar] [CrossRef]
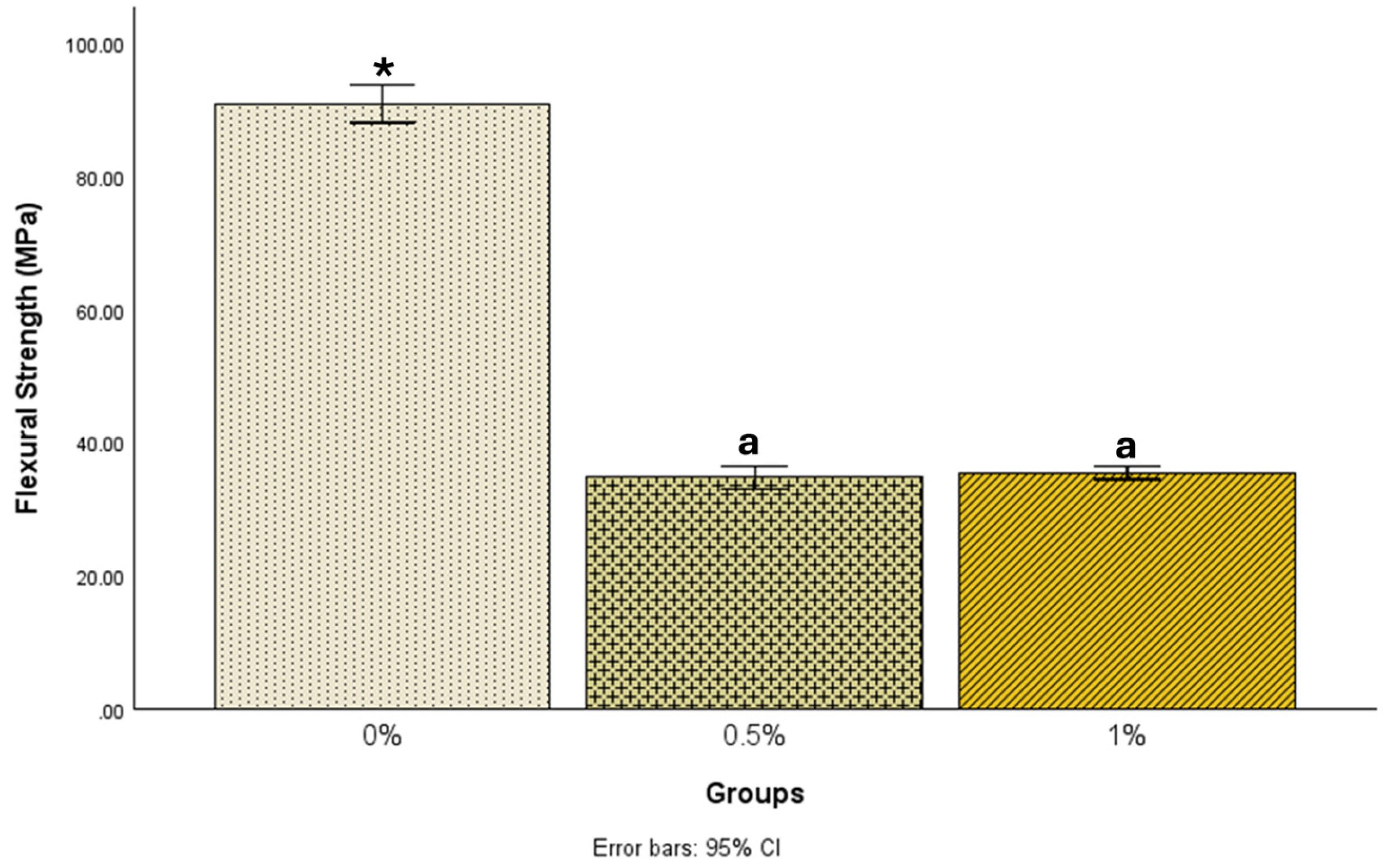

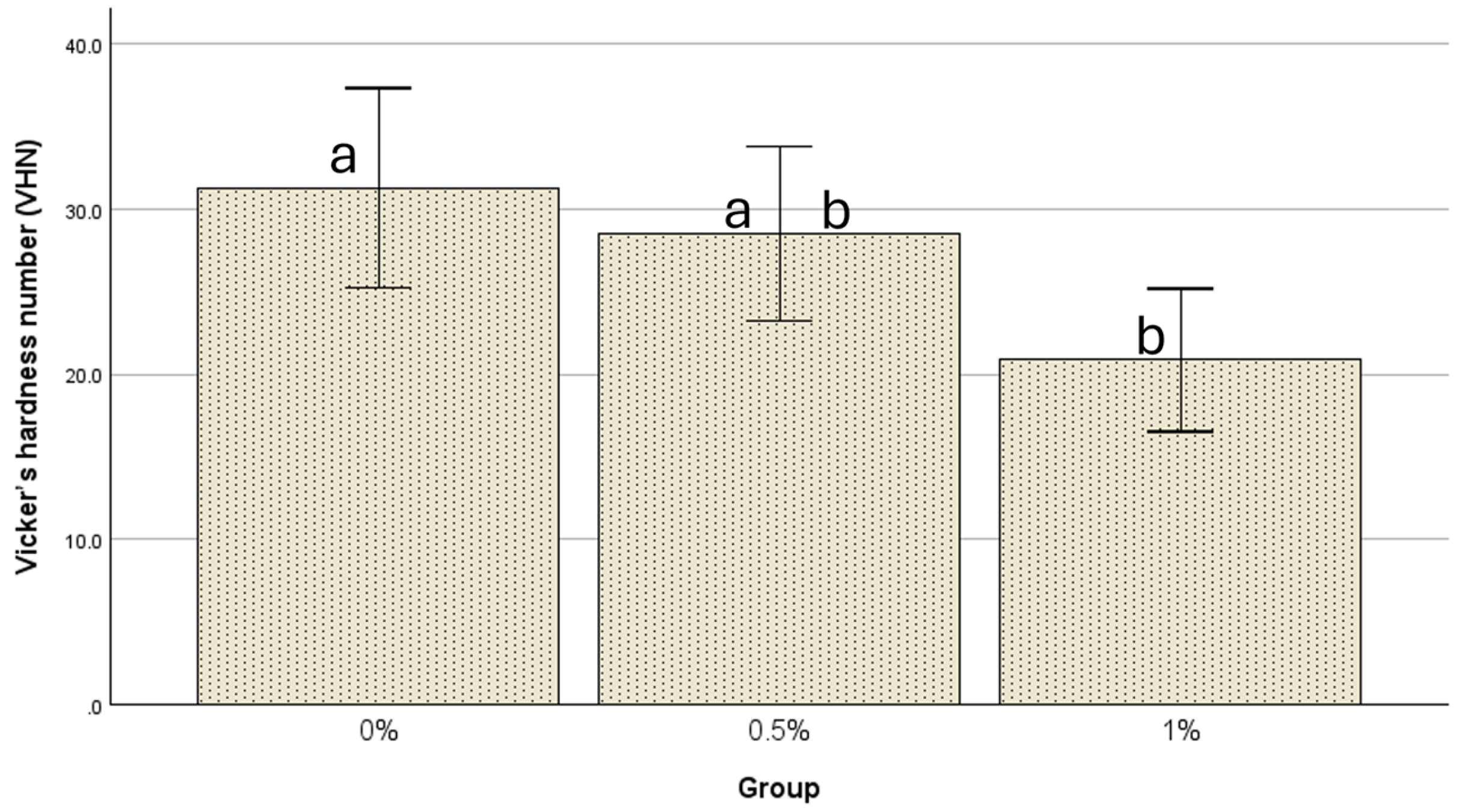


| N | Mean ± Std. Deviation | 95% Confidence Interval for Mean | Minimum | Maximum | ||
|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | |||||
| 0% | 25 | 90.9 ± 6.9 | 88.1 | 93.7 | 76.9 | 104.1 |
| 0.5% | 24 | 34.7 ± 3.9 | 33.0 | 36.4 | 25.5 | 41.1 |
| 1% | 22 | 35.4 ± 2.3 | 34.4 | 36.5 | 31.2 | 39.2 |
| Groups | Mean Difference (I–J) | Std. Error | Sig. | 95% Confidence Interval | ||
|---|---|---|---|---|---|---|
| (I) | (J) | Lower Bound | Upper Bound | |||
| 0% | 0.5% | 56.18872 * | 1.38549 | <0.001 | 52.8690 | 59.5085 |
| 1% | 55.46035 * | 1.41725 | <0.001 | 52.0645 | 58.8562 | |
| 0.5% | 0% | −56.18872 * | 1.38549 | <0.001 | −59.5085 | −52.8690 |
| 1% | −0.72837 | 1.43101 | 0.867 | −4.1572 | 2.7004 | |
| 1% | 0% | −55.46035 * | 1.41725 | <0.001 | −58.8562 | −52.0645 |
| 0.5% | 0.72837 | 1.43101 | 0.867 | −2.7004 | 4.1572 | |
| N | Mean ± Std. Deviation | 95% Confidence Interval for Mean | Minimum | Maximum | ||
|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | |||||
| 0% | 25 | 1801.8 ± 213.4 | 1713.7 | 1889.9 | 1356.2 | 2322.4 |
| 0.5% | 24 | 822.4 ± 151.5 | 758.4 | 886.4 | 415.8 | 1097.8 |
| 1% | 22 | 930.3 ± 130.4 | 872.5 | 988.1 | 696.8 | 1113.2 |
| Groups | Mean Difference (I–J) | Std. Error | Sig. | 95% Confidence Interval | ||
|---|---|---|---|---|---|---|
| (I) | (J) | Lower Bound | Upper Bound | |||
| 0% | 0.5% | 979.42788 * | 48.73897 | <0.001 | 862.6452 | 1096.2106 |
| 1% | 871.48989 * | 49.85646 | <0.001 | 752.0296 | 990.9502 | |
| 0.5% | 0% | −979.42788 * | 48.73897 | <0.001 | −1096.2106 | −862.6452 |
| 1% | −107.93799 | 50.34031 | 0.089 | −228.5576 | 12.6817 | |
| 1% | 0% | −871.48989 * | 49.85646 | <0.001 | −990.9502 | −752.0296 |
| 0.5% | 107.93799 | 50.34031 | 0.089 | −12.6817 | 228.5576 | |
| N | Mean ± Std. Deviation | 95% Confidence Interval for Mean | Minimum | Maximum | ||
|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | |||||
| 0% | 21 | 31.3 ± 13.3 | 25.2254 | 37.3460 | 17.30 | 66.30 |
| 0.5% | 21 | 28.5 ± 11.7 | 23.2145 | 33.8236 | 12.70 | 49.30 |
| 1% | 21 | 20.9 ± 9.5 | 16.5305 | 25.1743 | 10.20 | 46.60 |
| Groups | Mean Difference (I–J) | Std. Error | Sig. | 95% Confidence Interval | ||
|---|---|---|---|---|---|---|
| (I) | (J) | Lower Bound | Upper Bound | |||
| 0% | 0.5% | 2.76667 | 3.57772 | 0.721 | −5.8314 | 11.3647 |
| 1% | 10.43333 * | 3.57772 | 0.014 | 1.8353 | 19.0314 | |
| 0.5% | 0% | −2.76667 | 3.57772 | 0.721 | −11.3647 | 5.8314 |
| 1% | 7.66667 | 3.57772 | 0.090 | −0.9314 | 16.2647 | |
| 1% | 0% | −10.43333 * | 3.57772 | 0.014 | −19.0314 | −1.8353 |
| 0.5% | −7.66667 | 3.57772 | 0.090 | −16.2647 | 0.9314 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
DuDash, A.; Amaechi, B.T.; Obiefuna, A.C.; Abdollahi, S.; Gohil, T.; Girnary, M.; Haney, S.J.; Vickers, V.A.; Omosebi, T.O.; Vijayaraghavan, M. Physical Properties of PMMA Denture Base with Added Organoselenium as an Antifungal. Prosthesis 2025, 7, 148. https://doi.org/10.3390/prosthesis7060148
DuDash A, Amaechi BT, Obiefuna AC, Abdollahi S, Gohil T, Girnary M, Haney SJ, Vickers VA, Omosebi TO, Vijayaraghavan M. Physical Properties of PMMA Denture Base with Added Organoselenium as an Antifungal. Prosthesis. 2025; 7(6):148. https://doi.org/10.3390/prosthesis7060148
Chicago/Turabian StyleDuDash, Alexis, Bennett T. Amaechi, Amos C. Obiefuna, Sima Abdollahi, Tejal Gohil, Mustafa Girnary, Stephan J. Haney, Victoria A. Vickers, Temitope O. Omosebi, and Mahalakshmi Vijayaraghavan. 2025. "Physical Properties of PMMA Denture Base with Added Organoselenium as an Antifungal" Prosthesis 7, no. 6: 148. https://doi.org/10.3390/prosthesis7060148
APA StyleDuDash, A., Amaechi, B. T., Obiefuna, A. C., Abdollahi, S., Gohil, T., Girnary, M., Haney, S. J., Vickers, V. A., Omosebi, T. O., & Vijayaraghavan, M. (2025). Physical Properties of PMMA Denture Base with Added Organoselenium as an Antifungal. Prosthesis, 7(6), 148. https://doi.org/10.3390/prosthesis7060148






