Abstract
Ceramic dental implants, particularly one-piece zirconia, offer a biocompatible and aesthetic alternative to titanium, with high strength and improved oral hygiene. By eliminating the implant–abutment micro-gap, they reduce bacterial accumulation because of their low plaque affinity and enhance stability. However, challenges remain, including alignment precision, limited retrievability, and sensitivity to mechanical stress. Misalignment can affect occlusal and functional outcomes, and zirconia’s rigidity complicates crown removal and modification. This case report explores the use of PEEK (polyether ether ketone) primary telescopic crowns to overcome these limitations, improving force distribution, enabling minor adjustments, and enhancing prosthetic retrievability in full-mouth zirconia restorations. A 62-year-old male patient seeking a fixed solution to replace removable dentures received 16 one-piece zirconia implants (eight per jaw). PEEK telescopic crowns were used over implant abutment copings, finalized with aesthetic zirconia bridges. The report details surgical and prosthetic procedures, along with a brief literature review on zirconia implants and PEEK applications. PEEK integration in telescopic prosthetic designs marks a notable advancement in prosthodontics. Its shock-absorbing, biocompatible, and stress-modulating properties make it valuable for implant-supported and hybrid restorations. As digital workflows advance, PEEK-based telescopic restorations may increasingly replace traditional metal-based solutions, improving long-term clinical outcomes. Further clinical research on a larger sample is needed.
1. Introduction
Ceramic dental implants have emerged as a strong alternative to traditional titanium implants, offering high mechanical strength, fracture toughness, and excellent aesthetics, particularly for patients with thin gingival biotypes or metal sensitivities [1,2]. Their hypoallergenic nature reduces the risk of adverse reactions, while their corrosion resistance, low plaque affinity, and thermal non-conductivity enhance durability, oral hygiene, and patient comfort [3]. Surface adjustments, such as polishing, sandblasting, acid etching, biofunctionalization, coating, laser treatment, and ultraviolet light treatment, further enhance zirconia implants by improving osseointegration, mechanical properties, and bacterial resistance, ultimately contributing to their long-term success [4].
Among ceramic implant designs, one-piece implants have gained popularity due to their simplified structure, which eliminates the micro-gap between the implant and abutment, thereby reducing plaque affinity and bacterial adhesion potential while enhancing mechanical strength. Their monolithic design lowers the risk of failure while improving biocompatibility and minimizing the likelihood of peri-implantitis [5,6,7]. Additionally, one-piece ceramic implants offer shorter treatment times with single-stage placement, prevent micro-movement, and provide superior aesthetics by eliminating the risk of metal show-through, making them a favorable choice for both clinicians and patients [8,9].
Restoring zirconia implants with zirconia crowns presents challenges due to the rigidity of both materials, which limits shock absorption and increases the risk of fractures under excessive occlusal forces [10]. Additionally, retrieving cemented zirconia crowns from zirconia implants is complex due to zirconia’s high strength, making conventional removal methods, such as diamond burs, time-consuming and potentially damaging to both the crown and the abutment [11]. The similar color of the restoration and implant further complicates the process by reducing visibility during removal. While pulsed erbium lasers (Er:YAG and Er,Cr:YSGG) have been effective in detaching ceramic restorations from titanium and zirconia abutments, their use in removing cemented zirconia crowns from one-piece zirconia implants remains limited. These lasers work by breaking down cement through the absorption of mid-infrared wavelengths, offering a potential non-invasive solution for crown retrieval without damaging the implant structure [12].
Precise placement of zirconia implants is crucial, especially for one-piece designs, as improper positioning can lead to significant complications. Unlike two-piece implants, which allow for abutment angulation adjustments, one-piece zirconia implants integrate the implant and abutment into a single structure, meaning any placement error directly affects the final prosthetic outcome. A significant consequence of misalignment is the difficulty in achieving a proper occlusal relationship, which can result in unfavorable load distribution and increase the risk of crown fractures or implant failure due to excessive occlusal forces. Additionally, improper positioning can make it challenging to restore the implant with a prosthesis that is both aesthetically and functionally acceptable [13].
Despite their advantages, one-piece ceramic implants pose challenges, particularly regarding alignment and retrievability. Unlike two-piece designs that allow for angulation adjustments and abutment replacement, one-piece implants require precise placement, making corrections difficult once integrated [14]. Modifying a one-piece ceramic implant to correct malalignment carries risks, as grinding can introduce microcracks, weakening the structure and increasing the likelihood of fractures under occlusal forces. Excessive grinding can also generate heat, potentially compromising the implant material, necessitating careful water cooling [15]. Additionally, adjustments may affect the prosthetic connection, leading to difficulties with crown retention and improper load distribution. Any roughened surface left after modification can promote bacterial adhesion, increasing the risk of peri-implantitis [16].
Since one-piece implants do not allow abutment replacement, any modifications are permanent, making precision during placement and adjustments crucial. Early clinical applications of these implants highlighted alignment difficulties, especially in single-unit restorations. Additionally, zirconia implants have limitations, including limited long-term data compared to titanium, raising concerns about their durability and performance over time [11]. Their higher cost and limited availability, due to specialized manufacturing and training requirements, further hinder their widespread use [17]. While zirconia is strong, its brittle nature makes it more prone to fracture under stress, unlike the more flexible titanium. Surface imperfections can also contribute to premature failure, increasing the risk of implant fractures. Given these challenges, precise placement is essential, as improper positioning can lead to significant restorative complications, particularly in one-piece implant designs [18].
Polyether ether ketone (PEEK) has emerged as a highly versatile material in implant and superstructure applications owing to its outstanding physical and mechanical properties. Its use spans several components of implant-retained prostheses, including implants, custom abutments, frameworks for fixed implant-supported prostheses, and overdenture frameworks. This is facilitated by the ease of machining and compatibility with CAD/CAM systems, enabling precise customization of PEEK-based components that conform precisely to patient-specific anatomical requirements, thereby enhancing the overall fit and function of the prosthesis [19]. When used as a framework material, PEEK offers a modulus of elasticity closer to that of bone compared to traditional metals, thereby promoting more favorable stress distribution and reducing the risk of stress shielding [20]. PEEK’s biocompatibility, chemical stability, and low plaque affinity make it further ideal for long-term intra-oral applications, ensuring minimal adverse tissue reactions. It contributes to patient comfort and better load distribution during the critical healing phase [21]. Additionally, its inherent tooth-like color contributes to superior aesthetics, especially in anterior restorations where metal components may be visually unappealing [22,23]. In provisional restorations, PEEK’s lightweight nature and shock-absorbing properties contribute to patient comfort and better load distribution during the critical healing phase [24]. With all these advantages, challenges remain regarding its bond strength with veneering materials, marginal fit, and fracture resistance, which necessitate further exploration to optimize its performance in clinical settings [25,26].
As for the one-piece ceramic implants, challenges such as angulation correction, retrievability, and load distribution remain unresolved, particularly in multiple-loaded implants. A significant gap exists in clinical practice regarding the management of minor implant angulation discrepancies without compromising prosthetic function or long-term stability. PEEK (polyether ether ketone) has emerged as a promising solution, offering a shock-absorbing interface in a telescopic crown system that enhances force distribution, facilitates minor adjustments, and improves retrievability. Its biocompatibility and adaptability near the marginal bone further expand its potential applications in implant dentistry [27]. By integrating PEEK components as a functional buffer, one-piece ceramic implants can overcome many of their inherent limitations, paving the way for improved clinical outcomes. Further research is needed to refine this approach, optimizing implant-prosthetic integration while ensuring long-term success.
The present study introduces, for the first time, the adaptation of PEEK telescopic crowns for fixed full-arch restorations on one-piece zirconia implants, representing a novel approach in implant prosthodontics that addresses the gap, thereby underscoring its originality and clinical relevance. This case report examines the potential of PEEK (polyether ether ketone) components to address common challenges associated with one-piece ceramic implants, including alignment issues, retrieval difficulties, and stress distribution. This report focuses on demonstrating full-mouth fixed zirconia bridges supported by one-piece zirconia dental implants (Bredent WhiteSKY ZIRCONIA tissue line, bredent GmbH & Co. KG, Senden, Germany) featuring PEEK primary telescopic crowns (bredent GmbH & Co. KG, Senden, Germany).
2. Materials and Methods
2.1. First Visit
This is a retrospective case report of a 62-year-old patient who presented to our clinic seeking fixed prosthetic rehabilitation to replace missing teeth in the maxillary arch and partially missing teeth in the mandibular arch. The patient is a heavy smoker, consuming two packs per day, and has a medical history that includes the placement of a coronary artery stent two years before the first visit.
The clinical dental assessment, at the time, indicated a completely edentulous maxilla and a partially edentulous mandible. The residual mandibular teeth exhibited periodontal compromise, classified as Grade 3 mobility according to the Miller Classification, with multiple cavities and a poor overall prognosis (Figure 1).
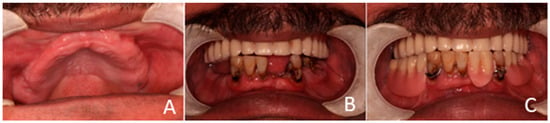
Figure 1.
Preoperative oral condition. (A) The edentulous maxilla. (B) Partially edentulous mandible. (C) Maxillary denture and mandibular partial denture.
The suggested treatment plan was to clear the compromised remaining mandibular teeth and to perform full-mouth rehabilitation using cement-retained fixed bridges over zirconia implants. According to the manufacturer’s guidelines, the number of abutments in a cement-retained bridge over zirconia implants should not exceed one pontic. To follow the recommendations, the plan was to do four bridges over eight implants in the maxilla and three bridges over eight implants in the mandible. The treatment plan was discussed with and approved by the patient, who signed a consent form. The treatment started with the extraction of the remaining lower teeth (Figure 2).
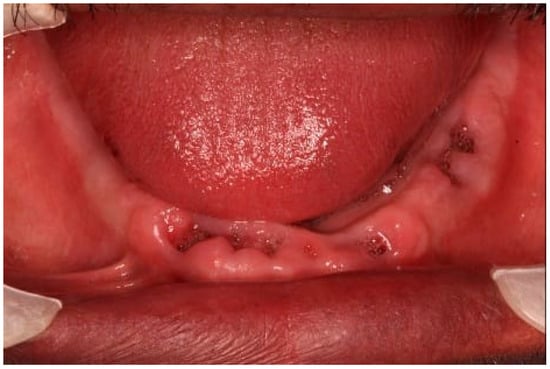
Figure 2.
After the extraction of the lower teeth.
2.2. Second Visit
The edentulous spaces in the lower partial denture were then restored to replace the extracted teeth. The existing upper denture was positioned with the lower denture to re-evaluate the vertical dimension, aesthetics, and phonetics (Figure 3).
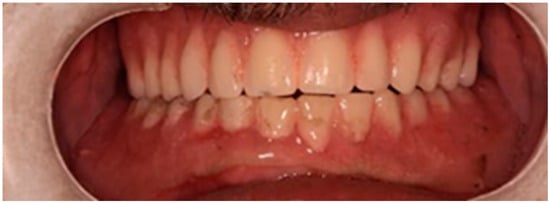
Figure 3.
Image showing the upper and lower dentures.
The dentures were utilized to develop a prosthetically guided implant placement protocol. Each tooth of the dentures was perforated from the occlusal aspect to facilitate the insertion of a gutta–percha cone at the center of each tooth (Figure 4).
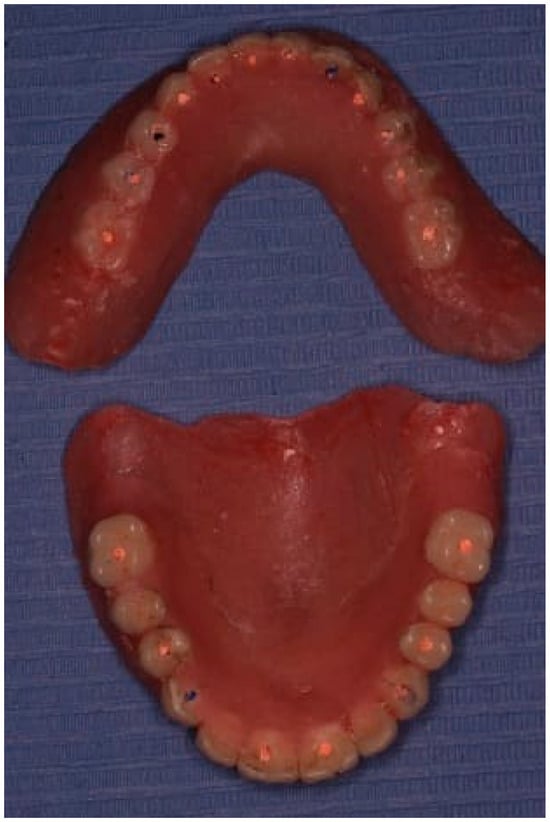
Figure 4.
Gutta–percha cones are inserted into the dentures.
A cone–beam computed tomography (CBCT) scan was performed on the patient with the dentures in situ, providing visualizations of the gutta–percha markers and the alveolar bone’s anatomical dimensions (Figure 5).
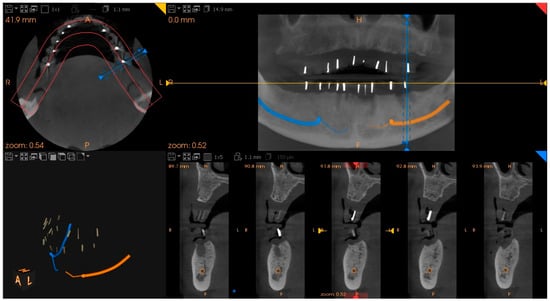
Figure 5.
CT scan showing the radiopaque gutta–percha cones inserted in the dentures, which mark the locations of the intended dental implants: voxel size 150, voltage 90 kV, current 4 mA, exposure time 15 s.
2.3. Third Visit
The positioning of the maxillary implants was strategically planned to coincide with the sites of the central incisors, canines, first premolars, and first molars. For the mandibular implants, the designated locations included the lateral incisors, canines, second premolars, and first molars. Consequently, the gutta–percha was excised from the dentures, and the resultant apertures served as a guide for the initial osteotomy, allowing for the accurate marking of the bone through the overlying soft tissue once the dentures were seated on the alveolar ridges.
The surgical procedure was performed under local anesthesia with 2% Lidocaine with 1:100,000 epinephrine and plain 2% Lidocaine, adhering to the guidelines of local anesthesia. A crestal incision was made along the ridge of the maxilla, extending from the area posterior to the most distal landmark (end of the denture) on the left side to the region behind the most distal landmark on the right side. A full-thickness mucoperiosteal flap was elevated conservatively, exposing only the occlusal surface of the alveolar ridge.
In the anterior mandibular region, flap reflection was unnecessary due to recent dental extractions. However, crestal flaps were elevated bilaterally in the molar areas.
Bone drilling was conducted to ensure as much parallelism as possible between the implants to be connected with a single bridge. The protocol for implant placement in this study followed a standardized methodology to ensure consistency across all cases. The implants used are zirconia one-piece implants with a rough surface at the bone level, followed by a distinct ledge marking the transition to the smooth coronal portion, which extends up to the abutment margin. Placement was performed using a drill specifically matched to the implant dimensions. A bone file with a stopper was employed to reach the precise depth corresponding to the link, ensuring that the implant was seated until the first deepest point of the implant neck, above the flutes and the roughened portion. Given the one-piece design, the abutment margin occupies the gingival area with the smooth surface, while the rough surface is fully embedded in the bone. This approach allowed for uniform positioning and optimal bone–implant interface in all cases.
In the present study, the peri-implant bone was predominantly compact in all regions surrounding the implant margins, indicating favorable bone quality for implant stability. The insertion torque ranged from 40 to 50 Ncm (Mean 46) in the maxilla and from 45 to 56 Ncm (Mean 50) in the mandible, according to the manufacturer’s guidelines.
Following implant placement, the incision wounds were left to heal by secondary intention, which resulted in augmentation of the buccal soft tissue (Figure 6).
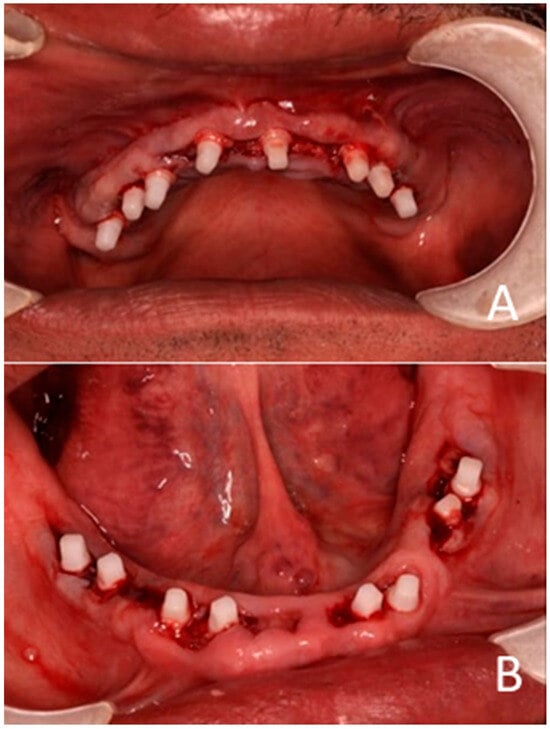
Figure 6.
One-piece zirconia implants and abutments are shown. (A) Maxilla. (B) Mandible.
The dentures were modified by enlarging the pre-existing guide holes to facilitate complete seating on the alveolar ridges. The flanges of the lower denture and the palatal portion of the upper implant-supported dentures were excised to expose the hard palate and the lingual aspect of the lower ridge, thereby providing a reference surface for the subsequent impression.
Plastic caps were placed on the abutments of the implants, and Teflon tape was packed into the open incision lines and extraction sockets to prevent the injectable crown bridge material from entering and solidifying within them, as this could compromise the surgical success of the implants (Figure 7).
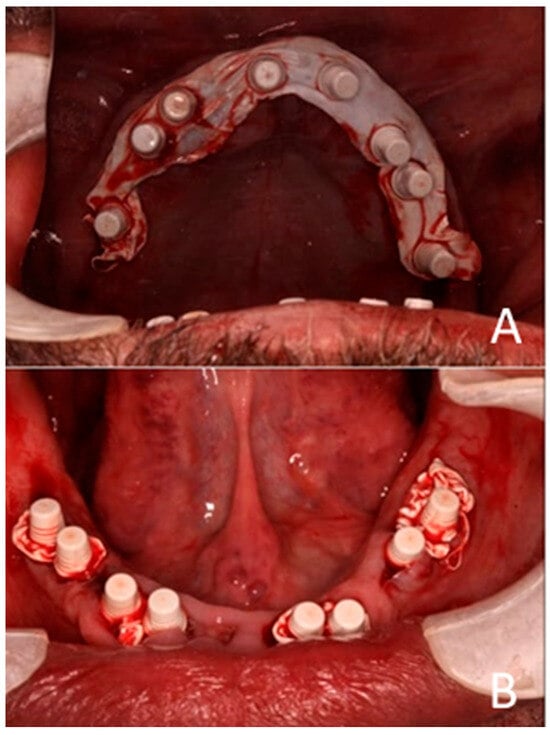
Figure 7.
Teflon tape is used to cover the incision wounds. Plastic caps are shown. (A) Maxillary arch. (B) Mandibular arch.
An impression was made using additional silicone, combined with soft and hard putty, for a one-step pickup impression. After the impression material set, the dentures were removed and used as a key or index to fabricate chairside temporary bridges (Figure 8).
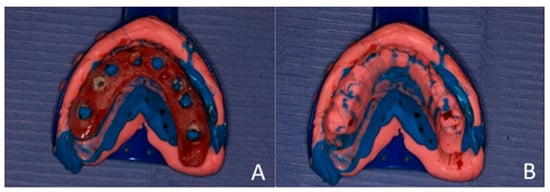
Figure 8.
(A) Fabrication of temporary bridges. The modified upper denture was picked with the additional silicone impression material. (B) Index for the temporary bridge.
The injectable acrylic material was then injected into the impressions, which were subsequently repositioned on their respective arches, one at a time, and allowed to cure. Any excess acrylic material was trimmed away, and the bridge was refined and smoothed to address any high spots in occlusal contact. The patient was advised to maintain a soft diet for three months (Figure 9).
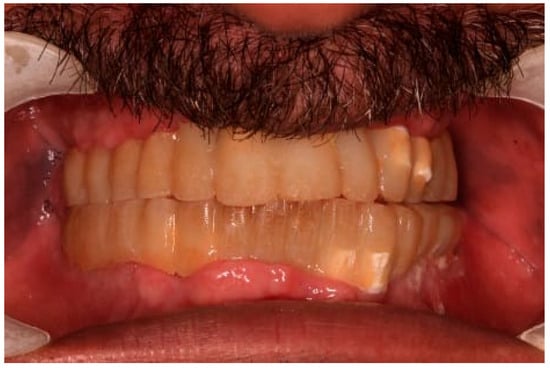
Figure 9.
Temporary bridges in place.
2.4. Fourth Visit
After 4 months, the provisional prostheses were removed, and the implants’ stability was evaluated using an AnyCheck machine (Kohdent/Kompass Co., Ltd., Seongnam, Republic of Korea)), which showed stable readings. This device provides an objective indication of implant stability. The recorded values ranged from 72 to 94, with the majority of implants demonstrating stability values in the 80 s, and one was 67. These results suggest that implants placed in compact bone achieved good primary stability, which is essential for predictable osseointegration and long-term success (Figure 10).

Figure 10.
Measurements of implant stability immediately before fixed prosthetic procedures.
A 3D scan of the maxillary and mandibular arches was performed using the Sirona Omnicam scanner (Dentsply Sirona, Bensheim, Germany)). The occlusal record was obtained by positioning half of each provisional bridge (upper and lower) on one side of the dental arch while the patient was in centric occlusion, then scanning the opposite side to capture the bite registration.
The single-piece dental implants exhibited non-parallel alignment, as confirmed on the final STL file. To manage this, a conventional, clinically approved approach was used. Polyether ether ketone (PEEK) telescopic crowns were fabricated to harmonize the prosthetic path of insertion, align the implants, and provide a cushioning effect beneath the planned rigid bridges. The STL files guided fabrication to achieve a parallel insertion path.
PEEK has been conventionally used for single crowns and for larger cross-arch removable partial dentures [27]. The novelty in this case lies in adapting PEEK as telescopic crowns to support a fixed bridge over single-piece implants, thereby aligning non-parallel fixtures and achieving a predictable path of insertion. As this report describes a single retrospective case treated within the scope of established clinical practice, institutional review board (IRB) approval was not required. However, IRB clearance was later obtained to support publication and ensure compliance with ethical reporting standards.
Each telescopic crown was sandblasted on the internal surfaces, then placed in an ultrasonic bath and treated with the manufacturer-specified “Bredent” solution. A resin cement was utilized to bond each telescopic crown to its respective zirconia abutment. The arches were scanned once again and sent to the lab for the fabrication of segmented zirconia bridges, which were subsequently provisionalized with PEEK crowns (Figure 11, Figure 12 and Figure 13).
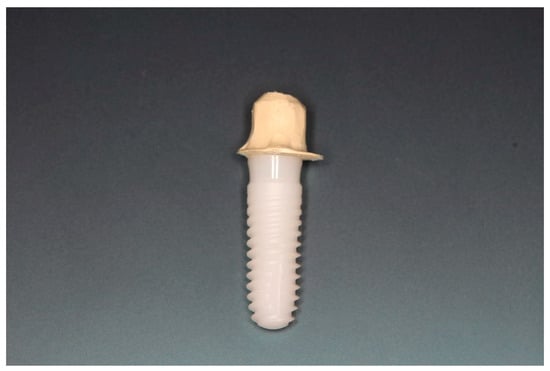
Figure 11.
Zirconia implant cemented to the PEEK telescopic crown.
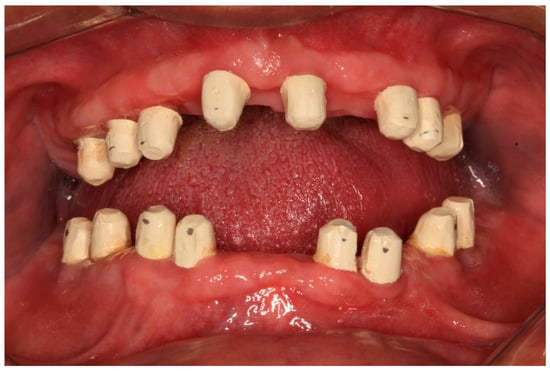
Figure 12.
PEEK telescopic crowns are shown covering the implants’ abutments.
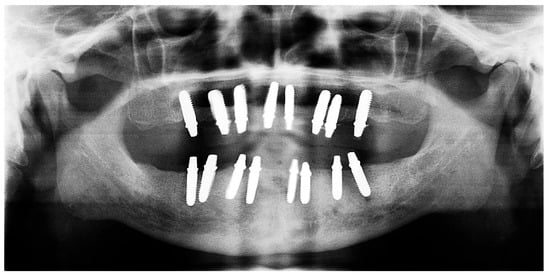
Figure 13.
Panoramic X-ray showing the inserted dental implants (taken 10 October 2022).
Radiographic evaluation in this study was limited to qualitative and linear assessment of crestal bone stability. While X-rays provide reliable detection of changes in marginal bone level, they cannot quantify bone mineral density (BMD). Hence, the radiographs presented here serve as indicators of implant stability rather than bone density evaluation. Quantitative assessment of bone density would require modalities such as CBCT voxel analysis or dual-energy X-ray absorptiometry (DEXA), which were not part of this retrospective case.
2.5. Fifth Visit
The restoration plan involved placing a bridge that connects the central incisors to the canines, along with an additional bridge spanning the first premolar to the first molar on each side of the maxillary arch. In the mandibular arch, the plan included three bridges: one connecting the lateral incisors bilaterally and another bridging the canines to the second premolars and first molars on each side (Figure 14 and Figure 15). Placing was confirmed using Panoramic radiographs obtained using the PantOs DG XP panoramic dental X-ray system (Fona S.r.l., Assago, Italy; CE 0051). The unit operates on a 230 V, 50/60 Hz input line with an 8 A fuse. Standard panoramic exposure parameters were applied (90 kVp, 10 mA, exposure time 14 s) following the manufacturer’s safety recommendations. A trained radiology technician performed all scans, and patient positioning was standardized using the built-in cephalostat support and light-beam alignment to ensure reproducibility of serial images.
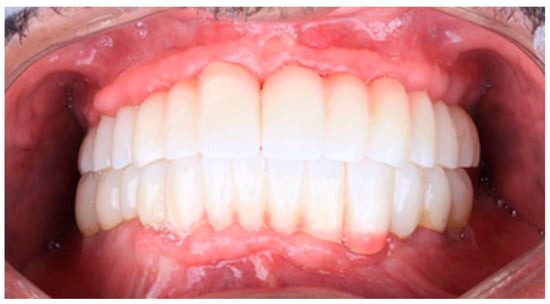
Figure 14.
The final prostheses.
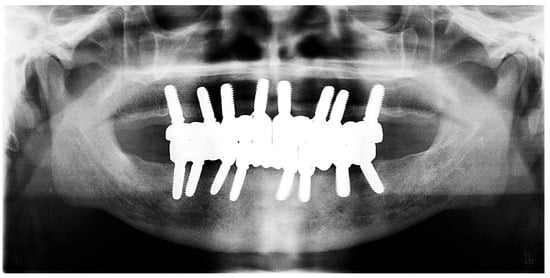
Figure 15.
Panoramic X-ray showing the final prostheses (taken 21 November 2023).
2.6. Sixth Visit
During the three-year follow-up period from October 2022 through September 2025, the patient was regularly recalled for evaluation. The peri-implant soft tissues remained healthy throughout the observation period. The gingiva surrounding the implants was consistently pink, firm, and showed no bleeding or suppuration upon probing of the peri-implant sulcus. At the last visit, probing depths ranged from 2–4 mm (mean 3 mm) in the maxillary arch and 1–3 mm (mean 2 mm) in the mandibular arch. No clinical signs of inflammation, edema, or granulation tissue were observed, indicating stable peri-implant mucosa and sustained biocompatibility of the materials used. Radiographic assessment revealed no crestal bone loss, and the prosthetic components remained functionally and esthetically intact, with no evidence of wear, fracture, or loosening. The patient reported high satisfaction regarding comfort, function, and appearance, further supporting the clinical success and reliability of this treatment approach (Figure 16 and Figure 17).
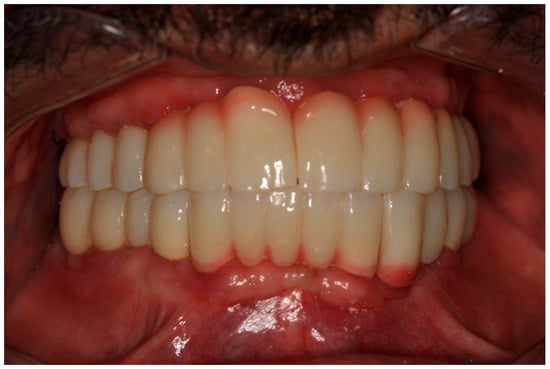
Figure 16.
Follow-up photo.
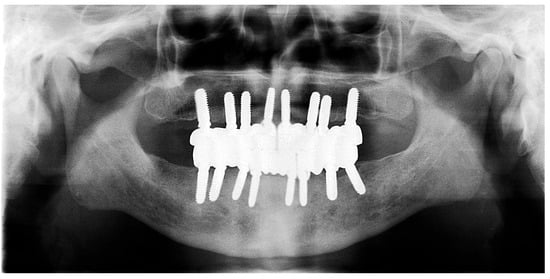
Figure 17.
Panoramic X-ray at follow-up visit (taken 9 September 2025).
3. Discussion
When a multi-implant-loaded prosthesis is fabricated, alignment is crucial to ensure complete seating and favorable stress distributions. If single implants are not aligned, several correction methods have been explored, including multi-unit abutments, angled screw channels, and telescopic crowns. Among these, telescopic crowns have gained attention for their ability to provide a stable, passive fit for implant-supported restorations, similar to tooth-supported restorations. These restorations employ a two-layered system comprising a primary crown (inner coping) and a secondary crown (outer prosthetic component). This design enables a more adaptable connection, reducing the impact of minor misalignments while improving retention and load distribution [28,29].
In a telescopic system, the secondary crowns are typically fabricated from high-performance polymers, such as polyether ether ketone (PEEK), which offers excellent wear resistance, biocompatibility, and stress-modulating properties. PEEK’s resilience and ability to create a friction-retained interface with the primary crown make it an attractive choice for telescopic prostheses [30]. Additionally, its CAD/CAM compatibility ensures precise fabrication, optimizing both retention and prosthetic stability [31]. The present study introduces, for the first time, the adaptation of PEEK telescopic crowns for fixed full-arch restorations on one-piece zirconia implants, representing a novel approach in implant prosthodontics. While previous studies have explored PEEK for removable or single-unit restorations, its application in full-arch fixed rehabilitations on zirconia implants has not been reported.
The primary crown, which serves as the foundation for the secondary crown, plays a critical role in ensuring the overall success of the telescopic system. Traditionally, primary crowns have been fabricated from metal alloys, such as gold or cobalt-chromium, which provide excellent strength and wear resistance. The introduction of PEEK in multiple in vitro studies has provided an alternative with distinct advantages in terms of weight, flexibility, and biocompatibility [32,33]. Numerous studies have used these PEEK copings, characterized by their low modulus of elasticity (4 GPa), as part of removable overdentures and have found that repeated removals contribute to variations in the material’s performance. PEEK absorbs occlusal forces and exhibits gradual wear over time [24,34,35,36]. A final study showed that the combination of PEEK primary and secondary crowns demonstrated performance comparable to conventional material combinations (zirconia, PEEK, and chrome cobalt alloys), successfully withstanding 10,000 pull-off cycles without a significant loss of retention force. This durability corresponds to an estimated average service life of approximately 13 years. Consequently, PEEK appears to be a suitable material for fabricating primary crowns when paired with PEEK secondary crowns.
Clinically, PEEK telescopic copings demonstrated performance similar to metallic telescopic systems (Co-Cr crowns) in terms of implant stability and crestal bone height changes when supporting a removable partial denture. However, PEEK exhibits lower periodontal pocket depths, suggesting better biocompatibility [37]. One drawback is that PEEK telescopic copings exhibit greater bacterial accumulation than metallic copings [38]. Despite this, it has been concluded that PEEK’s mechanical properties and biocompatibility make it a viable alternative to traditional materials in implant dentistry. However, further research is needed to confirm its long-term performance [39].
Currently, no studies have explored one-piece implant-supported telescopic prostheses, but PEEK is expected to serve as a stable primary crown material when cemented onto the abutment. Its shock-absorbing and force modulation properties could help distribute occlusal forces more evenly, reducing stress on implants, particularly in full-arch restorations. Beyond its mechanical advantages, PEEK enhances patient comfort due to its lightweight nature, while its biocompatibility and non-metallic composition make it ideal for patients with metal allergies. Additionally, its radiolucency allows for better radiographic assessment of underlying structures, unlike metal-based primary crowns. The clinical case presented illustrates the integration of PEEK-based telescopic crowns within an implant-supported prosthetic framework, emphasizing the advantages of digital workflows and material selection for optimized patient outcomes. Each procedural step was carefully designed to ensure a precise fit, stability, and long-term success of the restoration.
During the initial phase, modifications to the pre-existing dentures facilitated the creation of accurate impressions, ensuring a passive fit for subsequent restorations. The strategic removal of specific denture flanges provided a stable reference for the impression, enhancing the reproducibility of the final prosthesis. The use of additional silicone, combined with soft and hard putty, for a one-step pick-up impression contributed to the accurate capture of implant positions, which was critical for the fabrication of the temporary bridges. The application of plastic caps on the implant abutments, combined with Teflon tape to protect incision lines and extraction sockets, ensured that the injectable acrylic material did not interfere with healing, preventing any compromise to implant integration.
Upon fabrication and insertion of the provisional prostheses, occlusal refinement was performed to achieve a balanced occlusal relationship and ensure even load distribution across the implants. The patient’s adherence to a soft diet during the healing phase was an essential factor in minimizing excessive forces on the newly integrated implants.
After 4 months, the implants’ stability was assessed with a quantitative device, confirming their readiness for definitive restorations. The use of an intraoral scanner to capture 3D digital impressions allowed for precise prosthetic fabrication, minimizing errors associated with conventional impressions. The digital workflow also facilitated accurate occlusal registration, ensuring that the telescopic PEEK crowns were optimally positioned.
In vitro studies on PEEK cemented by resin to additively manufactured crown specimens showed the highest bond strength compared to bonded titanium and subtractively manufactured specimens [40], Another study showed that all bonding protocols provided a stable bond between PEEK and titanium bases, with no specimen failures after thermomechanical aging. The heat-pressing technique generally produced higher retention forces, particularly in non-aged groups, compared to the Monobond–Multilink hybrid combination. Adhesive failures occurred at the PEEK-cement interface rather than at the titanium–cement interface, indicating that the cement type primarily influenced bond strength [41]. In another study on PEEK, its mechanical properties when bonded to lithium disilicate were compared to those of zirconia, highlighting its flexibility and resilience under loading. While PEEK exhibited significantly lower bond strength to lithium disilicate than zirconia, its high flexural capacity prevented fracture under stress, unlike zirconia, which fractured at a defined load. These findings suggest that while PEEK may not provide the same rigid support as zirconia, its flexibility, and shock-absorbing properties make it a valuable material for applications where stress distribution and resilience are critical [42].
While the outcomes of this single-patient case are encouraging, several limitations should be considered when designing a complete clinical study. Future protocols should include standardized peri-implant soft tissue evaluation, using calibrated probing at multiple sites per implant to measure probing depth, bleeding on probing, suppuration, gingival color, and consistency. Radiographic assessments should be performed at baseline and regular intervals to quantify crestal bone levels and bone density using standardized reference points. Implant placement should follow a uniform methodology, employing depth stoppers, drills, and surgical guides matched to the implant dimensions to ensure reproducible positioning. Prosthetic evaluation should involve systematic monitoring of PEEK telescopic crowns for wear, fracture, loosening, and overall functional and esthetic performance. Patient-reported outcome measures should be integrated to assess comfort, function, and satisfaction in a validated manner. Studies should recruit a larger, diverse cohort with well-defined inclusion and exclusion criteria to improve generalizability. Additionally, comparative groups: while this study highlights the clinical performance of PEEK telescopic crowns, the discussion does not thoroughly compare them with conventional metallic telescopic systems. Future studies should consider well-designed, two-arm randomized controlled trials to directly compare PEEK and metallic telescopic crowns with respect to implant stability, peri-implant health, and patient-reported outcomes. Such studies would provide higher-quality evidence to support the clinical advantages and potential novelty of PEEK telescopic crowns beneath fixed prostheses. Collectively, these protocols will provide a rigorous framework for assessing the long-term success, biocompatibility, and clinical reliability of one-piece zirconia implants with PEEK telescopic crowns.
4. Conclusions
In conclusion, the integration of PEEK into telescopic prosthetic designs represents a significant advancement in the field of prosthodontics. As a primary coping material, it offers superior shock absorption, biocompatibility, and stress modulation, making it particularly valuable for implant-supported and hybrid restorations. As digital workflows continue to evolve, the refinement of PEEK-based telescopic restorations will likely enhance clinical outcomes, making them a viable alternative to traditional metal-based solutions. Future research should further investigate the long-term performance of PEEK telescopic prostheses, particularly regarding retention stability, material wear, and biofilm accumulation, to establish comprehensive clinical guidelines for their use in implant dentistry. However, this study is limited by the lack of a comparative arm with conventional metallic telescopic systems; future two-arm randomized controlled trials are recommended to directly compare outcomes and validate the clinical advantages of PEEK prostheses.
Author Contributions
Conceptualization, H.H.A.D.; methodology, H.H.A.D.; software, H.H.A.D.; validation, M.M.H. and H.H.H.; investigation, H.H.A.D.; resources, H.H.A.D.; data curation H.H.A.D.; writing—original draft preparation, M.M.H. and H.H.H.; writing—review and editing, L.A.A.-N.; supervision, L.A.A.-N.; project administration, H.H.A.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This case was treated in accordance with established clinical practice. Although IRB approval was not required, clearance was later obtained from the Institutional Review Board of An-Najah University, Nablus, Palestine (Dent 2025/31), to support publication. The novelty lies in using PEEK telescopic crowns to align and support a fixed bridge over non-parallel single-piece implants.
Informed Consent Statement
Informed consent was obtained from the patient involved in the study at the time when the treatment plan was executed. Written informed consent was obtained from the patient(s) to publish this paper.
Data Availability Statement
All data will be present upon request.
Acknowledgments
All the authors thank An-Najah National University for providing the institutional support and IRB approval that enabled the publication of this case report. We also acknowledge the assistance of the university staff who facilitated administrative and technical processes to prepare this work for publication. L.A. used OpenAI. 2025. ChatGPT (GPT-5) for the writing process to improve readability and language during the preparation of this manuscript. The outcomes after using ChatGPT were reviewed and edited by the authors (H.A., H.H., M.H.), who declare their full responsibility for the content and integrity of this work.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gautam, C.; Joyner, J.; Gautam, A.; Rao, J.; Vajtai, R. Zirconia-based dental ceramics: Structure, mechanical properties, biocompatibility, and applications. Dalton Trans. 2016, 45, 19194–19215. [Google Scholar] [CrossRef]
- Gökçen-Röhlig, B.; Saruhanoglu, A.; Cifter, E.D.; Evlioglu, G. Applicability of zirconia dental prostheses for metal allergy patients. Int. J. Prosthodont. 2010, 23, 562–565. [Google Scholar]
- Mohammed, M.K.; Alahmari, A.; Alkhalefah, H.; Abidi, M.H. Evaluation of zirconia ceramics fabricated through DLP 3D printing process for dental applications. Heliyon 2024, 10, e36725. [Google Scholar] [CrossRef]
- Karthigeyan, S.; Ravindran, A.J.; Bhat, R.T.R.; Nageshwarao, M.N.; Murugesan, S.V.; Angamuthu, V. Surface Modification Techniques for Zirconia-Based Bioceramics: A Review. J. Pharm. Bioallied Sci. 2019, 11, S131–S134. [Google Scholar] [CrossRef]
- Durrani, F.; Nahid, R.; Pandey, S.; Singh, P.; Pandey, A. One-piece implants: Careful approach for complex rehabilitation. Natl. J. Maxillofac. Surg. 2021, 12, 266–270. [Google Scholar] [CrossRef]
- Curiel-Aguilera, F.P.; Griffiths, G.R.; Rossmann, J.A.; Gonzalez, J.A. Titanium versus zirconia complete arch implant-supported fixed prostheses: A comparison of plaque accumulation. J. Prosthet. Dent. 2023, 130, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Prithviraj, D.R.; Deeksha, S.; Regish, K.M.; Anoop, N. A systematic review of zirconia as an implant material. Indian J. Dent. Res. 2012, 23, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Bollen, C.; Hakobayan, G.; Jörgens, M. One-piece versus two-piece ceramic dental implants. Br. Dent. J. 2024, 236, 383–387. [Google Scholar] [CrossRef]
- Prithviraj, D.R.; Gupta, V.; Muley, N.; Sandhu, P. One-piece implants: Placement timing, surgical technique, loading protocol, and marginal bone loss. J. Prosthodont. 2013, 22, 237–244. [Google Scholar] [CrossRef]
- Sadowsky, S.J. An overview of treatment considerations for esthetic restorations: A review of the literature. J. Prosthet. Dent. 2016, 116, 324–335. [Google Scholar] [CrossRef] [PubMed]
- Roehling, S.; Woelfler, H.; Hicklin, S.; Kniha, H. A retrospective clinical study of zirconia dental implants: 10-year follow-up. Clin. Oral Implants Res. 2019, 30, 1006–1015. [Google Scholar]
- Elkharashi, A.; Grzech-Leśniak, K.; Deeb, J.G.; Abdulmajeed, A.A.; Bencharit, S. Exploring the use of pulsed erbium lasers to retrieve a zirconia crown from a zirconia implant abutment. PLoS ONE 2020, 15, e0233536. [Google Scholar] [CrossRef] [PubMed]
- Sailer, I.; Karasan, D.; Todorovic, A.; Ligoutsikou, M.; Pjetursson, B.E. Prosthetic failures in dental implant therapy. Periodontology 2000 2022, 88, 130–144. [Google Scholar] [CrossRef]
- Schnutenhaus, S.; Edelmann, C.; Knipper, A.; Rudolph, H.; Luthardt, R. One-piece ceramic implants in the maxillary anterior region—Limitations and possibilities. Clin. Oral Implants Res. 2020, 31, 253. [Google Scholar] [CrossRef]
- Canneto, J.J.; Cattani-Lorente, M.; Durual, S.; Wiskott, A.H.; Scherrer, S.S. Grinding damage assessment on four high-strength ceramics. Dent. Mater. 2016, 32, 171–182. [Google Scholar] [CrossRef]
- Rashid, H. The effect of surface roughness on ceramics used in dentistry: A review of literature. Eur. J. Dent. 2014, 8, 571–579. [Google Scholar] [CrossRef]
- Cionca, N.; Hashim, D.; Mombelli, A. Zirconia dental implants: Where are we now, and where are we heading? Periodontology 2000 2017, 73, 241–258. [Google Scholar] [CrossRef]
- Nasti, S.; Anjum, S.; Kalekhan, S.M.; Ashok, A.; Bumb, P.P.; Puthenkandathil, R. Surgical guides: Precision redefined in implant placement. IP Int. J. Periodontol. Implantol. 2023, 8, 177–180. [Google Scholar] [CrossRef]
- Rahmitasari, F.; Ishida, Y.; Kurahashi, K.; Matsuda, T.; Watanabe, M.; Ichikawa, T. PEEK with Reinforced Materials and Modifications for Dental Implant Applications. Dent. J. 2017, 5, 35. [Google Scholar] [CrossRef] [PubMed]
- Khaohoen, A.; Sornsuwan, T.; Chaijareenont, P.; Poovarodom, P.; Rungsiyakull, C.; Rungsiyakull, P. Bio-materials and clinical application of dental implants in relation to bone density—A narrative review. J. Clin. Med. 2023, 12, 6924. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Tang, T. Current strategies to improve the bioactivity of PEEK. Int. J. Mol. Sci. 2014, 15, 5426–5445. [Google Scholar] [CrossRef] [PubMed]
- Taymour, N.; Abd El-Fattah, A.; Kandil, S.; Fahmy, A.E.; Al-Qahtani, N.H.; Khaled, A.; Al-Dulaijan, Y.A.; Gepreel, M.A.-H. Revolutionizing dental polymers: The versatility and future potential of polyetheretherketone in restorative dentistry. Polymers 2024, 17, 80. [Google Scholar] [CrossRef] [PubMed]
- Jangade, M.; Sharma, R.; Sudharson, N.A.; Jain, R.; Sahu, S.K.; Dani, A. Evaluation of marginal accuracy of polyetheretherketone and zirconia copings fabricated using CAD/CAM technique: An in vitro study. J. Indian Prosthodont. Soc. 2025, 25, 95–101. [Google Scholar] [CrossRef]
- Shash, Y.H.; El-Wakad, M.T.; Eldosoky, M.A.A.; Dohiem, M.M. Evaluation of stress and strain on mandible caused using “All-on-Four” system from PEEK in hybrid prosthesis: Finite-element analysis. Odontology 2023, 111, 618–629. [Google Scholar] [CrossRef]
- Soares Machado, P.; Cadore Rodrigues, A.C.; Chaves, E.T.; Susin, A.H.; Valandro, L.F.; Pereira, G.K.R.; Rippe, M.P. Surface treatments and adhesives used to increase the bond strength between PEEK and resin-based dental materials: A scoping review. J. Adhes. Dent. 2022, 24, 233–245. [Google Scholar] [CrossRef]
- Gama, L.T.; Bezerra, A.P.; Schimmel, M.; Rodrigues Garcia, R.C.M.; de Luca Canto, G.; Gonçalves, T.M.S.V. Clinical performance of polymer frameworks in dental prostheses: A systematic review. J. Prosthet. Dent. 2024, 131, 579–590. [Google Scholar] [CrossRef]
- Papathanasiou, I.; Kamposiora, P.; Papavasiliou, G.; Ferrari, M. The use of PEEK in digital prosthodontics: A narrative review. BMC Oral Health 2020, 20, 217. [Google Scholar] [CrossRef]
- Cavallaro, J., Jr.; Greenstein, G. Angled implant abutments: A practical application of available knowledge. J. Am. Dent. Assoc. 2011, 142, 150–158. [Google Scholar] [CrossRef]
- Burkhardt, F.; Strietzel, F.P.; Bitter, K.; Spies, B.C. Guided implant surgery for one-piece ceramic implants: A digital workflow. Int. J. Comput. Dent. 2020, 23, 73–82. [Google Scholar] [PubMed]
- Jena, S.P.; Kumari, N.; Agarwal, S.; Afzal, V.A.; Magar, S.M.; Gulati, P. An in vitro assessment of retention force of ZrO2, PEEK, and ZrO2-PEEK telescopic attachments for mandibular overdentures. J. Pharm. Bioallied Sci. 2022, 14, S968–S970. [Google Scholar] [CrossRef] [PubMed]
- Schimmel, M.; Walther, M.; Al-Haj Husain, N.; Igarashi, K.; Wittneben, J.; Abou-Ayash, S. Retention forces between primary and secondary CAD/CAM manufactured telescopic crowns: An in vitro comparison of common material combinations. Clin. Oral Investig. 2021, 25, 6297–6307. [Google Scholar] [CrossRef]
- Abdel Hamid, S.M.; Selima, R.A.; Basiony, M.Z. Surface topography changes and wear resistance of different non-metallic telescopic crown attachment materials in implant retained overdentures: Prospective comparative in vitro study. BMC Oral Health 2024, 24, 1123. [Google Scholar] [CrossRef] [PubMed]
- Abdelhady, M.; Quassem, M.; Badr, W. Retention of zirconia-PEEK versus cobalt-chromium-PEEK telescopic attachments for implants retained mandibular overdenture. Al-Azhar J. Dent. Sci. 2024, 27, 479–486. [Google Scholar] [CrossRef]
- Gujjalapudi, M.; Verma, A.; Magar, S.; Balaji, D.; Dang, G.; Bhardwaj, A. An in vitro assessment of retention force for implant-retained overdentures using all-PEEK, zirconia, and titanium attachments. Int. J. Curr. Res. Rev. 2021, 13, 75–78. [Google Scholar] [CrossRef]
- Mahalakshmi, G.; Sruthi, T.N.; Sharma, P.K.; Thandava, M.; Mitra, N.; Patel, N.G.; Manas, A. An in vitro evaluation of retention force of all PEEK, all zirconia and zirconia-PEEK telescopic attachments for mandibular overdentures. J. Pharm. Bioallied Sci. 2023, 15, S910–S912. [Google Scholar] [CrossRef]
- Mohamed, S.S.; Emarah, A. Metal versus PEEK secondary copings for rigid telescopic retained implant-supported mandibular overdentures: Evaluation of clinical retention forces. Egypt. Dent. J. 2020, 66, 1769–1778. [Google Scholar] [CrossRef]
- Amin, A.; Fikry, G.; Rizk, F.; Badr, A. Comparative study of two different telescopic crown materials retaining lower partial overdenture. Indian J. Public Health Res. Dev. 2019, 10, 1214. [Google Scholar] [CrossRef]
- Abd El Azeem, A.; Hassan, A.; Agamy, E. Bacterial accumulation of different telescopic crowns: PEEK versus cobalt-chromium telescopes in implant-supported mandibular overdenture. Minia J. Med. Res. 2021, 32, 91–98. [Google Scholar] [CrossRef]
- Bojovic, M.; Todic, J.; Blagojevic, M. Comparative analysis of stress and deformation distribution in implant-supported telescopic systems made of different materials. Vojnosanit. Pregl. 2022, 80, 49–55. [Google Scholar] [CrossRef]
- Küçükekenci, A.S.; Dönmez, M.B.; Dede, D.Ö.; Çakmak, G.; Yilmaz, B. Bond strength of recently introduced CAD/CAM resin-based crown materials to PEEK and titanium. J. Prosthet. Dent. 2024, 132, 1066.e1–1066.e8. [Google Scholar] [CrossRef]
- Yilmaz, B.; Gouveia, D.; Schimmel, M.; Lu, W.E.; Özcan, M.; Abou-Ayash, S. Effect of adhesive system, resin cement, heat-pressing technique, and thermomechanical aging on the adhesion between titanium base and a high-performance polymer. J. Prosthet. Dent. 2024, 131, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Sloan, R.; Hollis, W.; Selecman, A.; Jain, V.; Versluis, A. Bond strength of lithium disilicate to PEEK. J. Prosthet. Dent. 2022, 128, 1351–1357. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).