Merging Neuroscience and Engineering Through Regenerative Peripheral Nerve Interfaces
Abstract
1. Introduction
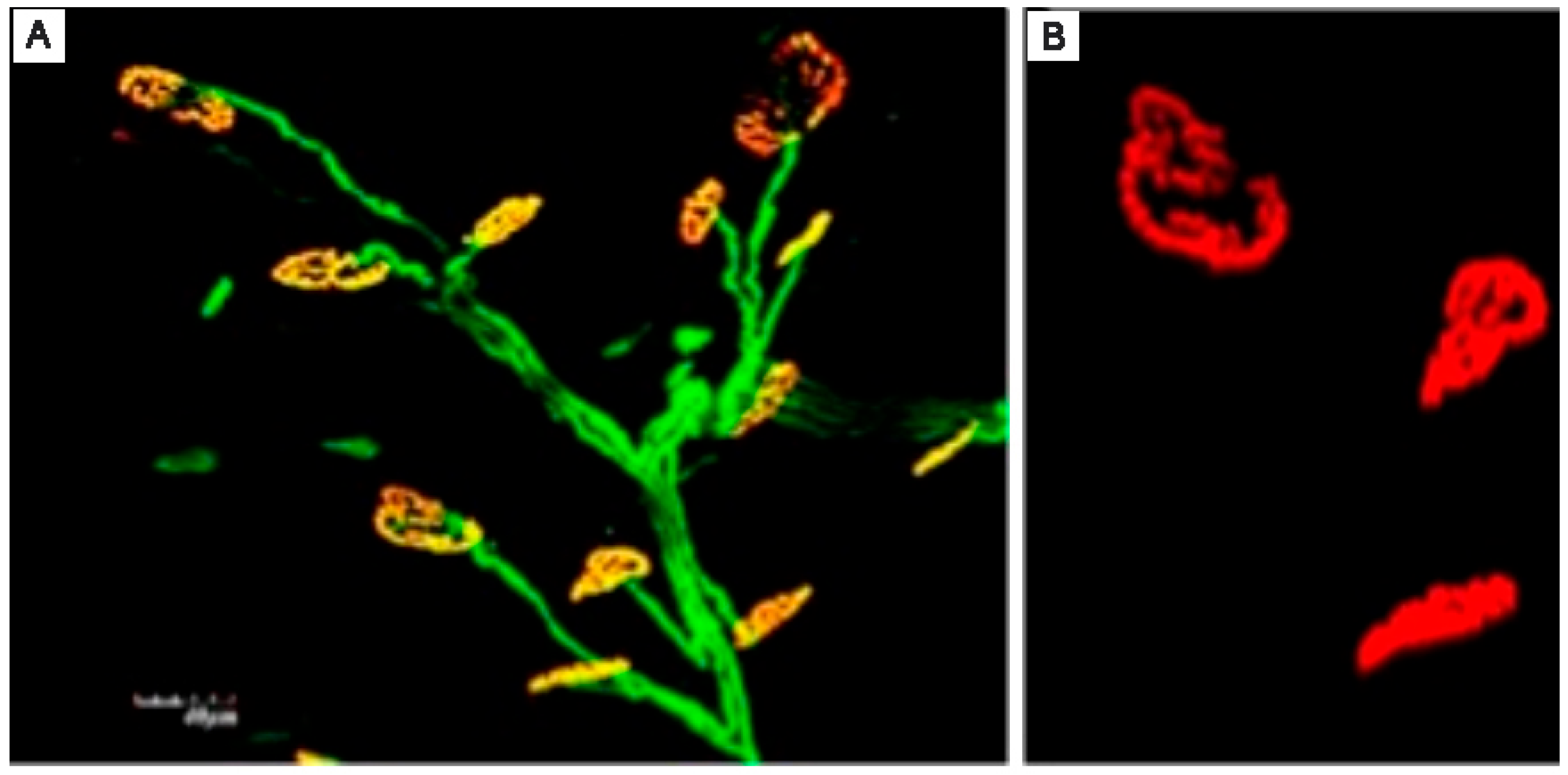
2. Basic Science: The Catalyst for RPNI Progress
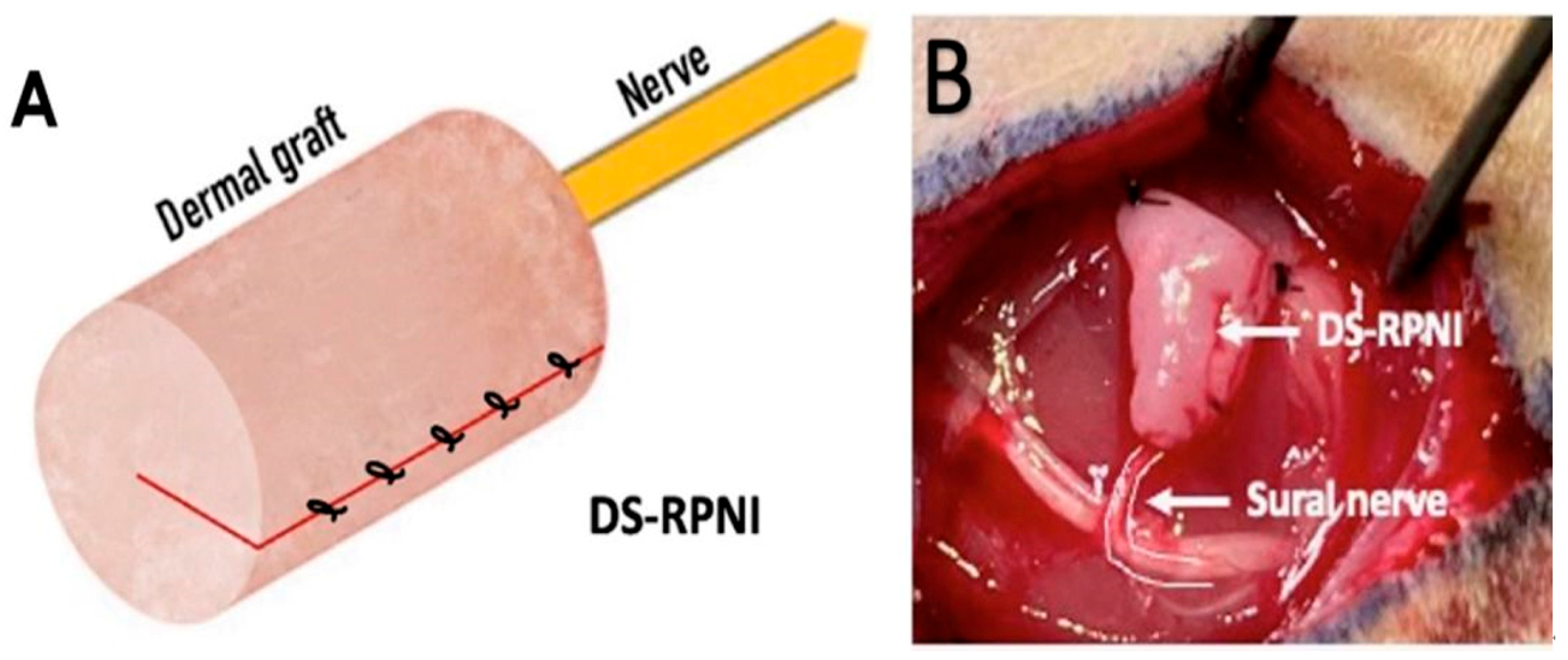
3. RPNI for the Prevention and Treatment of Neuroma Pain
4. Bridging Neuroscience and Engineering Through RPNI Surgery
5. Future Research Direction
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Kubiak, C.A.; Adidharma, W.; Kung, T.A.; Kemp, S.W.P.; Cederna, P.S.; Vemuri, C. Decreasing Postamputation Pain with the Regenerative Peripheral Nerve Interface (RPNI). Ann. Vasc. Surg. 2022, 79, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Leach, G.A.; Dean, R.A.; Kumar, N.G.; Tsai, C.; Chiarappa, F.E.; Cederna, P.S.; Kung, T.A.; Reid, C.M. Regenerative Peripheral Nerve Interface Surgery: Anatomic and Technical Guide. Plast. Reconstr. Surg. Glob. Open 2023, 11, e5127. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Adewole, D.O.; Serruya, M.D.; Harris, J.P.; Burrell, J.C.; Petrov, D.; Chen, H.I.; Wolf, J.A.; Cullen, D.K. The Evolution of Neuroprosthetic Interfaces. Crit. Rev. Biomed. Eng. 2016, 44, 123–152. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Burke, K.L.; Dumanian, G.A.; Cederna, P.S. Emergence of Nerve Interfaces with Robotic Applications. In Landmark Papers in Plastic Surgery; Khajuria, A., Hong, J.P., Neligan, P., Eds.; Springer: Cham, Switzerland, 2014. [Google Scholar] [CrossRef]
- Pettersen, E.; Sassu, P.; Pedrini, F.A.; Granberg, H.; Reinholdt, C.; Breyer, J.M.; Roche, A.; Hart, A.; Ladak, A.; Power, H.A.; et al. Regenerative Peripheral Nerve Interface: Surgical Protocol for a Randomized Controlled Trial in Postamputation Pain. J. Vis. Exp. 2024, 205, e66378. [Google Scholar] [CrossRef] [PubMed]
- Frost, C.M.; Ursu, D.C.; Flattery, S.M.; Nedic, A.; Hassett, C.A.; Moon, J.D.; Buchanan, P.J.; Brent Gillespie, R.; Kung, T.A.; Kemp, S.W.P.; et al. Regenerative peripheral nerve interfaces for real-time, proportional control of a Neuroprosthetic hand. J. Neuroeng. Rehabil. 2018, 15, 108. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ursu, D.; Nedic, A.; Urbanchek, M.; Cederna, P.; Gillespie, R.B. Adjacent regenerative peripheral nerve interfaces produce phase-antagonist signals during voluntary walking in rats. J. Neuroeng. Rehabil. 2017, 14, 33. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gates, D.H.; Gonzalez, M.; Kung, T.A.; Chestek, C.A. Toward the use of muscle reinnveration for chronic bi-directional control of prostheses to improve functional outcomes of end users. Curr. Opin. Biomed. Eng. 2023, 28, 100497. [Google Scholar] [CrossRef]
- Burke, K.L.; Kung, T.A.; Hooper, R.C.; Kemp, S.W.P.; Cederna, P.S. Regenerative peripheral nerve interfaces (RPNIs): Current status and future direction. Plast. Aesthetic Res. 2022, 9, 48. [Google Scholar] [CrossRef]
- Ursu, D.C.; Urbanchek, M.G.; Nedic, A.; Cederna, P.S.; Gillespie, R.B. In vivo characterization of regenerative peripheral nerve interface function. J. Neural. Eng. 2016, 13, 026012. [Google Scholar] [CrossRef] [PubMed]
- Urbanchek, M.G.; Kung, T.A.; Frost, C.M.; Martin, D.C.; Larkin, L.M.; Wollstein, A.; Cederna, P.S. Development of a Regenerative Peripheral Nerve Interface for Control of a Neuroprosthetic Limb. Biomed. Res. Int. 2016, 2016, 5726730. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kubiak, C.A.; Svientek, S.R.; Dehdashtian, A.; Lawera, N.G.; Nadarajan, V.; Bratley, J.V.; Kung, T.A.; Cederna, P.S.; Kemp, S.W.P. Physiologic signaling and viability of the muscle cuff regenerative peripheral nerve interface (MC-RPNI) for intact peripheral nerves. J. Neural. Eng. 2021, 18, 0460d5. [Google Scholar] [CrossRef] [PubMed]
- Irwin, Z.T.; Schroeder, K.E.; Vu, P.P.; Tat, D.M.; Bullard, A.J.; Woo, S.L.; Sando, I.C.; Urbanchek, M.G.; Cederna, P.S.; Chestek, C.A. Chronic recording of hand prosthesis control signals via a regenerative peripheral nerve interface in a rhesus macaque. J. Neural. Eng. 2016, 13, 046007. [Google Scholar] [CrossRef] [PubMed]
- Vu, P.P.; Irwin, Z.T.; Bullard, A.J.; Ambani, S.W.; Sando, I.C.; Urbanchek, M.G.; Cederna, P.S.; Chestek, C.A. Closed-Loop Continuous Hand Control via Chronic Recording of Regenerative Peripheral Nerve Interfaces. IEEE Trans. Neural. Syst. Rehabil. Eng. 2018, 26, 515–526. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.; Kubiak, C.A.; Best, C.S.W.; Hamill, J.B.; Ki, J.; Kim, H.M.; Roth, R.S.; Kozlow, J.H.; Tinney, M.J.; Geisser, M.E.; et al. Regenerative Peripheral Nerve Interface Surgery to Treat Chronic Postamputation Pain: A Prospective Study in Major Lower Limb Amputation Patients. Ann. Surg. Open. 2025, 6, e535. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ganesh Kumar, N.; Cederna, P.S.; Kung, T.A. Regenerative Peripheral Nerve Interfaces for Treatment of Symptomatic Neuromas. In Contemporary Neuroma Management; Eberlin, K.R., Ducic, I., Moore, A., Cederna, P.S., Valerio, I.L., Dumanian, G.A., Eds.; Springer: Cham, Switzerland, 2024. [Google Scholar] [CrossRef]
- Hwang, C.D.; Hoftiezer, Y.A.J.; Raasveld, F.V.; Gomez-Eslava, B.; van der Heijden, E.P.A.; Jayakar, S.; Black, B.J.; Johnston, B.R.; Wainger, B.J.; Renthal, W.; et al. Biology and pathophysiology of symptomatic neuromas. Pain 2024, 165, 550–564. [Google Scholar] [CrossRef] [PubMed]
- Chou, J.; Liston, J.M.; DeGeorge, B.R. Traditional Neuroma Management Strategies: A Systematic Review. Ann. Plast. Surg. 2023, 90 (Suppl. 4), S350–S355. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, E.; Sassu, P.; Reinholdt, C.; Dahm, P.; Rolfson, O.; Björkman, A.; Innocenti, M.; Pedrini, F.A.; Breyer, J.M.; Roche, A.; et al. Surgical treatments for postamputation pain: Study protocol for an international, double-blind, randomised controlled trial. Trials 2023, 24, 304. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kubiak, C.A.; Kemp, S.W.P.; Cederna, P.S.; Kung, T.A. Prophylactic Regenerative Peripheral Nerve Interfaces to Prevent Postamputation Pain. Plast. Reconstr. Surg. 2019, 144, 421e–430e. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, A.; Cederna, P.; Kemp, S.; Kung, T. Prophylactic Regenerative Peripheral Nerve Interface (RPNI) Surgery in Pediatric Lower Limb Amputation Patients. Ann. Surg. 2025, 282, 346–351. [Google Scholar] [CrossRef]
- Gonzalez, M.A.; Nwokeabia, C.; Vaskov, A.K.; Vu, P.P.; Lu, C.W.; Patil, P.G.; Cederna, P.S.; Chestek, C.A.; Gates, D.H. Electrical Stimulation of Regenerative Peripheral Nerve Interfaces (RPNIs) Induces Referred Sensations in People With Upper Limb Loss. IEEE Trans. Neural Syst. Rehabil. Eng. 2024, 32, 339–349. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Irwin, Z.T. Restoring Fine Motor Skills Through Neural Interface Technology. Ph.D. Thesis, The University of Michigan, Ann Arbor, MI, USA, 2016. [Google Scholar]
- Vu, P. Restoring Fine Motor Prosthetic Hand Control via Peripheral Neural Technology. Ph.D. Thesis, The University of Michigan, Ann Arbor, MI, USA, 2019. [Google Scholar]
- Vu, P.P.; Lu, C.W.; Vaskov, A.K.; Gates, D.H.; Gillespie, R.B.; Kemp, S.W.P.; Patil, P.G.; Chestek, C.A.; Cederna, P.S.; Kung, T.A. Restoration of Proprioceptive and Cutaneous Sensation Using Regenerative Peripheral Nerve Interfaces in Humans with Upper Limb Amputations. Plast Reconstr Surg. 2022, 149, 1149e–1154e. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sando, I.C.; Adidharma, W.; Nedic, A.; Ursu, D.C.; Mays, E.A.; Hu, Y.; Kubiak, C.A.; Sugg, K.B.; Kung, T.A.; Cederna, P.S.; et al. Dermal Sensory Regenerative Peripheral Nerve Interface for Reestablishing Sensory Nerve Feedback in Peripheral Afferents in the Rat. Plast. Reconstr. Surg. 2023, 151, 804e–813e. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, C.; Nehrdich, S.; Seifert, S.; Blume, K.R.; Miltner, W.H.R.; Hofmann, G.O.; Weiss, T. Leg Prosthesis With Somatosensory Feedback Reduces Phantom Limb Pain and Increases Functionality. Front. Neurol. 2018, 9, 270. [Google Scholar] [CrossRef]
- Nanivadekar, A.C.; Bose, R.; Petersen, B.A.; Okorokova, E.V.; Sarma, D.; Madonna, T.J.; Barra, B.; Farooqui, J.; Dalrymple, A.D.; Levy, I.; et al. Restoration of sensory feedback from the foot and reduction of phantom limb pain via closed-loop spinal cord stimulation. Nat. Biomed. Eng. 2023, 8, 992–1003. [Google Scholar] [CrossRef]
- Culp, C.J.; Abdi, S. Current Understanding of Phantom Pain and its Treatment. Pain Physician 2022, 25, E941–E957. [Google Scholar] [PubMed]
- Berberoglu, I.; Burke, K.L.; Cederna, P.S.; Kemp, S.W.P. Regenerative peripheral nerve interfaces (RPNIs): An overview of innovative surgical approaches. Plast. Aesthet. Res. 2024, 11, 14. [Google Scholar] [CrossRef]
- Svientek, S.R.; Ursu, D.C.; Cederna, P.S.; Kemp, S.W.P. Fabrication of the Composite Regenerative Peripheral Nerve Interface (C-RPNI) in the Adult Rat. J. Vis. Exp. 2020, 156, e60841. [Google Scholar] [CrossRef] [PubMed]
- Kubiak, C.A.; Ursu, D.C.; Moon, J.D.; Patil, P.G.; Kung, T.A.; Cederna, P.S.; Kemp, S.W.P. Abstract 36: Viability and Signal Transduction with the Composite Regenerative Peripheral Nerve Interface (C-RPNI). Plast. Reconstr. Surg. Glob. Open 2019, 7 (Suppl. 4), 26–27. [Google Scholar] [CrossRef] [PubMed Central]
- Vaskov, A.K.; Vu, P.P.; North, N.; Davis, A.J.; Kung, T.A.; Gates, D.H.; Cederna, P.S.; Chestek, C.A. Surgically Implanted Electrodes Enable Real-Time Finger and Grasp Pattern Recognition for Prosthetic Hands. IEEE Trans. Robot. 2022, 38, 2841–2857. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Graczyk, E.L.; Schiefer, M.A.; Saal, H.P.; Delhaye, B.P.; Bensmaia, S.J.; Tyler, D.J. The neural basis of perceived intensity in natural and artificial touch. Sci. Transl. Med. 2016, 8, 362ra142. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- George, J.A.; Kluger, D.T.; Davis, T.S.; Wendelken, S.M.; Okorokova, E.V.; He, Q.; Duncan, C.C.; Hutchinson, D.T.; Thumser, Z.C.; Beckler, D.T.; et al. Biomimetic sensory feedback through peripheral nerve stimulation improves dexterous use of a bionic hand. Sci. Robot. 2019, 4, eaax2352. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Catalan, M.; Guðmundsdóttir, R.A.; Kristoffersen, M.B.; Zepeda-Echavarria, A.; Caine-Winterberger, K.; Kulbacka-Ortiz, K.; Widehammar, C.; Eriksson, K.; Stockselius, A.; Ragnö, C.; et al. Phantom motor execution facilitated by machine learning and augmented reality as treatment for phantom limb pain: A single group, clinical trial in patients with chronic intractable phantom limb pain. Lancet 2016, 388, 2885–2894. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.J.; Kung, T.A.; Snyder-Warwick, A.K.; Cederna, P.S. Merging Neuroscience and Engineering Through Regenerative Peripheral Nerve Interfaces. Prosthesis 2025, 7, 97. https://doi.org/10.3390/prosthesis7040097
Wang MJ, Kung TA, Snyder-Warwick AK, Cederna PS. Merging Neuroscience and Engineering Through Regenerative Peripheral Nerve Interfaces. Prosthesis. 2025; 7(4):97. https://doi.org/10.3390/prosthesis7040097
Chicago/Turabian StyleWang, Melanie J., Theodore A. Kung, Alison K. Snyder-Warwick, and Paul S. Cederna. 2025. "Merging Neuroscience and Engineering Through Regenerative Peripheral Nerve Interfaces" Prosthesis 7, no. 4: 97. https://doi.org/10.3390/prosthesis7040097
APA StyleWang, M. J., Kung, T. A., Snyder-Warwick, A. K., & Cederna, P. S. (2025). Merging Neuroscience and Engineering Through Regenerative Peripheral Nerve Interfaces. Prosthesis, 7(4), 97. https://doi.org/10.3390/prosthesis7040097





