Abstract
Milk is seen as a chief source of protein and other biologically available nutrients for human beings. Pakistan, the fourth largest milk-producing country, is badly affected by the contagious transboundary apthoviral disease of ungulate animals; the foot and mouth disease (FMD) virus. FMD is endemic in Pakistan and has caused significant economic loss to the dairy industry in the form of a profound decrease in milk production and increased morbidity and deaths of dairy animals. Inclusively, the case fatality ratio of FMD was 15.11%. Of the seven FMDV serotypes, (O, A, C, Asia 1, SAT 1, SAT 2, and SAT 3), three serotypes (O, A, and Asia-1) are endemic in Pakistan. Rapid and highly sensitive diagnostic tools are required for efficient control of this disease. Presently, FMD in the laboratory is diagnosed via ELISA and molecular approaches, i.e., RT-PCR. Serotype-specific RT-PCR analysis not only confirms ELISA serotyping results but can also be used for the screening of ELISA negative samples. Genotypically, FMDV serotype O has a topotype (Middle East–South Asia (ME–SA) and lineage PanAsia-2) that is reported frequently from different areas of Pakistan. Confirmed cases of serotype A and Asia-1 are also reported. The information gathered can be used for understanding the molecular epidemiology of FMD in Pakistan. Further studies on the molecular dynamics of FMD could be useful for ensuring the timely diagnosis of this deadly pathogen, which would ultimately be beneficial for the mass vaccination programs of FMD in Pakistan.
1. Introduction
Milk is seen as the chief source of protein and other biologically available nutrients (i.e., calcium, phosphorus, magnesium, zinc, and vitamins) for human beings. The dairy industry plays an important role in strengthening the overall economy of the country by providing income and job opportunities to individuals [1]. The worldwide increasing demand for milk and other dairy products is due to the growing population and the increase in per capita consumption of dairy products [2]. In 2017, 828 million tons of milk were produced globally, but the figure grew to 838 million tons in 2018. South Asian countries are also the key players in milk production [3]. About 20% of the global milk, that is, about 200 million tons of milk, is produced by South Asian countries annually [4]. The global increase in milk consumption could serve as an opportunity for dairy countries such as Pakistan to increase their exports and earn foreign exchange by supplying dairy products (milk and milk products) to milk-deficient countries [5].
Pakistan is the fourth-largest milk-producing country, with approximately 80% milk produced in rural areas, 5% in urban areas, and 15% in peri-urban areas. Dairy farming is an indispensable asset and white gold for Pakistan. In 2019, the country produced 48 million tons of milk, adding 11.2% of the gross-domestic product (GDP) to the country’s economy, showing that the dairy industry has a flourishing bright future in Pakistan (Economic Survey of Pakistan 2018–2019) [3]. The dairy industry, despite having primary importance in the country’s economy, has been hit by potentially contagious diseases especially foot and mouth disease [6].
Foot and mouth disease (FMD), caused by the foot and mouth disease virus (FMDV), is an Apthovirus belonging to family Picornaviridae and consisting of seven discrete serotypes (O, A, Asia-1, C, South African Territories (SAT 1, 2 and 3)). These serotypes are reported frequently from one part or another part of the world. The low infectious dose of the virus, rapid replication, prolonged environmental survival, the incubation period of 7–14 days, and high virus titer in nasal secretions contributes to the rapid transmission of the virus to the susceptible animal population [7]. The disease-causing ability of FMDV is different for different animals, which may depend on different factors such as the duration of virus present in the body, abrasion through which virus enters the body, and release of virus titer [8]. Besides animal–animal transmission via respiratory aerosols, the virus is disseminated easily via mechanical routes, i.e., fomites, shoes, clothes, vehicles, and veterinary surgical instruments. Furthermore, the uncontrolled transboundary movements of animals have also aggravated its spread [7].
FMD is a highly contagious, transboundary vesicular disease of ungulates and wild animals. The disease is rendered as a serious economic threat to the dairy industry and agriculture worldwide in the form of high morbidity in adult animals and mortality in juveniles [9]. Cattle, buffalo, goats, sheep, and pigs are the animal species that are susceptible to this communicable disease. The disease is characterized by fever, anorexia, excessive salivation, and the appearance of vesicular blisters on the tongue, nose, mouth, teats, snout, feet, and other parts of glabrous skin, which sometimes leads to food abstinence and lameness [9]. Those animals who have developed lesions over the teat become susceptible to mastitis, and they do not milk. The disease rate is 100% in the vulnerable population; however, in young suckling calves, the fatality rate is also 100%, and the death rate may increase to 50% due to myocarditis [10].
FMDV is not completely removed in some hosts from the pharynx, so these animals act as carriers and can be a threat for healthy animals such as pigs [11], sheep, and goats due to its property of persistency. The mechanism of persistence is also influenced by the characteristics of the host [12]. In 1922, Vallee and Carre discovered that cattle that once recovered from FMDV may get infected again when kept with infected animals [13]. The spread of the virus depends on several factors such as newly purchased animals, entry of infected or carrier animal in the flock, seasonal effects, nutritional deficiency, and rate of movement of wildlife, as well as ruminants within the flock [14].
The FMD has been placed on the list of notifiable animal diseases by the OIE (Office International des Epizooties) due to its high transmission power, cross-border dispersal nature, and high infectivity rate [15]. FMD thus is scrutinized as a hidden foe for industrialized countries due to the trafficking ban. In the dairy industry, both direct and indirect economic losses due to FMD are reported; directly in the form of reduced milk and meat production and indirectly in the form of diseases, abortion, infertility, weight loss, and loss of cart power in draught animals [16]. According to Ministry of Planning and Special Initiatives (February 2020), the annual loss due to FMD in Pakistan has exceeded USD 692 million, demonstrating that it has badly hit dairy animals [17]. Previously reported outbreaks of FMD in different countries, i.e., Korea (2010), South America (2011) Libya and Saudi Arabia (2013), and Algeria and Tunisia (2015) created awareness among the local public about the devastation and catastrophic loss caused by this virus on animal welfare and economy [18]. There is a chance that the persistent occurrence of FMDV serotypes in Pakistan can also paralyze our dairy sector by negatively impacting the agriculture and economy. Therefore, this review is undertaken to determine the molecular dynamics of different FMDV serotypes prevailing in Pakistan.
2. Etiology
Frosch and Loeffler in 1897 first described FMDV as the etiological agent of FMD, laying the foundation of the science of virology. Initial details about the characteristic symptoms of the disease in cattle were given by Hieronymus Fracastorius in Venice in 1514 [19]. These details bear a resemblance to the disease reported in Germany (1751) and the United Kingdom (1839). At that time, this disease emerged globally, beyond New Zealand, and still, this disease is endemic in different parts of Asia, Africa, and South America [19].
The FMD is caused by a small, positive sense, non-enveloped icosahedral RNA virus of the class Pisoniviricetes and order Picornavirales. The virus has a diameter of 26 nm, and its genome is 8.5 kb long, coding for four structural proteins (VP1, VP2, VP3, and VP4) and 10 non-structural proteins (NSPs; L, 2A, 2B, 2C, 3A; 3B1, 3B2, 3B3; 3C and 3D) [20]. The viral structural proteins (VP1, VP2, and VP3) encode the capsid and are highly immunogenic bearing antigenic determining sites while VP4 is found on the inner surface of the capsid. Some of the non-structural proteins (3ABC and 3D) participate in virus replication, whilst 3D is RNA-dependent RNA polymerase. Both structural and non-structural proteins are responsible for antibodies production in infected cattle [20].
3. FMDV Serotypes
The FMDV RNA lacks proof-reading activity, due to which it is prone to errors and hence genetic diversity [21]. FMDV consists of seven discrete serotypes (O, A, Asia-1, C, SAT 1, SAT 2, and SAT 3) and 61 subtypes. Serotype O has caused outbreaks worldwide. Except for Asia-1, all six serotypes are reported from Africa. Three serotypes (A, O, Asia-1) have caused outbreaks in Asia and three (A, O, C) in South America. The two serotypes, SAT 1 and SAT 2 have been reported previously from Africa and the Middle East. Notably, Serotype C was lastly reported from Brazil and Kenya in 2004 and now is extinct [22].
4. Insights into Genetic/Molecular Dynamics of FMD
The FMD eradication is difficult through vaccination due to the diverse genetic nature of FMDV RNA [23]. The occurrence and co-occurrence of different genetic variants of FMD in different or same geographical populations have drawn our attention to initiate FMD eradication and mass vaccination programs [23]. The discrete seven serotypes of FMDV (O, A, Asia-1, C, SAT-1, SAT-2, and SAT-3) are classified based on their capsid protein’s chemistry [24]. However, the worldwide prevalent serotype of FMDV, i.e., serotype O, is not genetically diverse; serotype A and SAT-1 are the highly diverse serotypes [25]. The nucleotide coding sequence of the VP1 region recovered from the viral sample can be used for the identification of FMDV virus serotypes [26]. The FMDV serotypes share approximately 86% similarity, but some of the capsid genes are genetically distinct with 30–50% variation in the VP1 region between the serotypes [27]. It is notable that, within each serotype, several geographically diverse genetic lineages (also called topotypes) are present, which are further subdivided into sub lineages and strains (genotypes), with 61 described to date. If two of the viruses have a 15% difference in their VP1 region, then they are grouped into another genotype [26]. In general, different topotypes of FMDV are distributed in the specified geographic area and do not jump often between boundaries. Based on this, seven viral pools have been recognized, but only specific serotypes are present within each pool (Table 1). The emergence of new virus strains in the certain locality can be challenging, because the already available vaccination strategy became ineffective rendering huge economic concerns [28]. The global proliferation of trade and commerce, people’s movement, and the lack of transboundary animal movement control is crucial for understanding the molecular epidemiology of FMDV [29].

Table 1.
FMDV pools with their endemic serotypes.
The FMDV genome encodes a polyprotein precursor that is firstly cleaved into three structural proteins, viz, VP1, VP3 and VP0, and later, during capsid assembly, it is divided into VP2 and VP4 along with 10 non-structural proteins (NSPs) [36]. The three structural proteins (VP1, VP2, and VP3) are surfaced exposed, while VP4 is highly conserved between serotypes [37]. The highly antigenic protein is VP1 and is used mostly for epidemiological investigations because it comprises B- cell epitope responsible for producing antibody-mediated immunity [38].
The FMDV enters the host cell via integrin receptors through clathrin-mediated endocytosis. The integrins are the intrinsic proteins responsible mainly for cell adhesion, proliferation, apoptosis, and signal transduction. The low pH of the endosome facilitates the uncoating of viral nucleic acid [39]. The G-H loop situated between 135 and 160 amino acids in the hypervariable region VP1 contain a motif called arginine-glycine-aspartic acid (RGD motif). This motif forms major epitopes on the virion and hence attaches the virus to the host integrin receptor [39]. The virus–integrin complex makes ungulate animals vulnerable to infection [39]. In the VP1 region, the RGD sequence at the amino acid position 145 to 147 is highly conserved among the seven serotypes, except for ALo61strain, which contains RSGD (arginine-serine-glycine-aspartic acid) sequence at the same position [40]. Besides the RGD sequence in the VP1 region, an additional sequence epitope is present at the C-terminus. Predictive models of VP1 structure have placed the RGD region and the C-terminal region of VP1 nearby on the virus surface, but whether amino acids from the C-terminal region contribute directly to attachment or indirectly by providing conformational support for the RGD site is not clear [41]. In either case, variations in this region may be responsible for the different receptor specificities amongst FMDV strains [42]. In some cases, however, activity has been shown in related peptides, for example, Gly-Arg-Gly-Glu-Ser with a conservative substitution of the aspartic acid residue, Arg-Glu-Asp-Val with a less conservative glutamic acid substitution for glycine, and even the reverse sequence, Ser-Asp-Gly-Arg [28]. Extraneous cofactors also contribute to RGD attachment, e.g., both FMDV attachment to cells and integrin receptor activity are dependent on divalent cations [40], and there is evidence that disialogangliosides contribute to the attachment of melanoma cells to RGD-containing substrates [28]. Amino acids of the VP2 region (1–33) are also capable of producing antibody response elicited by the VP1 region, but it is weakly immunogenic because the N-terminus of the VP2 protein is positioned at the bottom of the three-fold symmetry axis of the virus protein coat [43].
It has been anticipated that changes occurring in the amino acid of the VP1 region result in the formation of different serotypes. This aspect is used for vaccine production for both inter and intra-serotypes [39]. In the case of FMDV serotype O, the VP1 region contains the RGDLXXL motif used for virus attachment to the integrin receptor and is highly conserved among O serotypes strains; therefore, it can be used to trace the origin and transmission pathway of the disease [44]. Based on the variation in the amino acid sequence of the VP1 region, it is also possible to determine the variation between different O sub-lineages. For example, differences exist between PanAsia and PanAsia-2 serotype O viruses circulating in Pakistan and Afghanistan. Therefore, FMDV is thought to occur as a group of related but non-identical genomes known as quasispecies [14]. The high spontaneous mutation rates, antigenic drift and shift with virulence potential, and broad host range results in vast genetic diversity and immunization failures. Employing RNA interference targeting different viral genes can be used to control FMD infection [39].
5. Geographical Distribution of FMD
In the middle part of the twentieth century, the prevalence of FMD was at its peak worldwide but timely control measures and mitigation strategies have limited its prevalence [45]. The OIE officially recognized 70 countries as FMD free without vaccination [46], while Pakistan, India, and 100 other countries are still endemic for FMD. FMD outbreaks have been reported from regions wherever livestock is present, except New Zealand [20].
Although the death rate of FMD is low, it is regarded as a global threat due to its direct impact on production losses and change in herd structure and consequential losses caused due to the cost of controlling FMD, limited use of advanced technology and poor access to market [15]. The disease is reported frequently in Africa, Asia, Middle East, and South America [7]. Shmeiger reported an FMD outbreak due to serotype O in the vaccinated dairy farms of Israel in the year 2021 [47]. In 2020, an investigation was carried out by Giza, in which he demonstrated that the major outbreaks reported in Ethiopia were due to serotype O, A, and SAT-2 [48]. Kenya is the world-leading consumer of dairy products. Kenya is an endemic country for FMDV serotypes (A, O, SAT-1, and SAT-2). A survey was conducted in 2010, in which it was shown that the seroprevalence of FMD in Kenya is 52.5%, and the estimated milk loss due to FMD is 42% [49]. Lyons and their colleagues reported an outbreak due to FMDV serotype SAT-2 that has occurred in Nakuru in 2015. The outbreak has resulted in decreased milk yield in the farm due to this catastrophic disease [50]. The FMD epidemic was also recorded in Japan in the year 2010. Despite effective control measures, disinfection, movement restriction, and stamping out, the country was failed to prevent the virus from entering the vulnerable FMD population. This outbreak was reported in an area with a large population of cattle making its spread easy and control difficult. Delay in the diagnosis of the disease has also increased the number of outbreaks over the period. This outbreak has severely damaged the dairy sector of Japan [51]. Still, the disease is absent in many countries including Morocco, Zimbabwe, Tunisia, and South Africa, but they lack official OIE FMD free status, and South America, except Venezuela, has eradicated FMD with vaccination and is now disease free [46]. Great Britain also claims to be FMD free after the contentious FMDV PanAsia O serotype outbreak that was reported in 2001 and was controlled after culling 6.5 million infected animals because no ring vaccination policy was implemented [52].
FMD caused a huge economical loss during the 19th century due to more trade of animals in many countries [53]. After successful identification and genetic studies, the first experimental level FMDV vaccine was published by Vallee et al., (1922) [13], and later, production of FMDV vaccine at the commercial level started in France [54] and Argentina [55]. The vaccine proved itself as a safeguard in the prevention of disease occurrence at a significant level in European countries. Mass vaccination programs helped in the eradication of FMD in Siberia [56]. In 1960, using calf kidney cells, vaccine production was started at an industrial scale [57], but difficulty in kidney processing led to employing baby hamster kidney cells (BHK-21) for vaccine production [57]. For inactivation of FMDV, formaldehyde was used, and later, this was replaced with ethyleneimine [58].
5.1. FMD in South Asia
South Asian countries are considered a hub for dairy farming, as they have a large population of cattle and buffaloes. These countries comprise India, Bangladesh, Bhutan, Nepal, and Sri Lanka. In these countries, animals are used not only for milk and meat production but also for draught power. The cross-border movement of dairy animals results in the dissemination and maintenance of FMD [31]. South Asian countries are endemic for the FMDV. It has been documented that serotype A, O, and Asia-1 are the frequently circulating serotypes in these countries. In India, 80% of outbreaks were due to FMDV serotype O. The predominant topotype was Middle East–South Asia (ME–SA), and the resulting lineages were Ind-2001, Pan-Asia, and Pan-Asia 2 (O/ME-SA/Ind-2001, O/ME-SA/Pan-Asia, O/ME-SA/Pan-Asia 2) [31]. The FMDV O lineage O/ME-SA/Ind-2001 is also reported from Bangladesh, Bhutan, and Nepal. Sri Lanka reported O/ME-SA/Pan-Asia 2 in 2012 and O/ME-SA/Ind-2001 in 2013, respectively [59]. Besides the prevalent O serotype, FMDV A serotype that has topotype ASIA and lineage G-VII has been frequently reported since 2001 from India [60]. The same lineage G-VII was also reported from Bangladesh in 2013. In the FMDV viral pool II, Asia-1 genotype II has caused outbreaks in India and Bangladesh [60].
The entry of FMD from other localities or the active animal flow from southern Asia into eastern countries (Afghanistan, Pakistan, Iraq, and Iran) has resulted in an increased incidence of FMD in these countries. FMDV O topotype ME-SA and lineage PanAsia-2 is the extensively distributed lineage in Iraq, Iran, Afghanistan, and Pakistan [59].
5.2. The Situation of FMD in Pakistan
Pakistan is the largest livestock population country in South Asia. Agriculture accounts for approximately 18.5% of gross domestic product (GDP), with nearly 60.5% of the total contribution to livestock [61]. The livestock division has previously played an important role in the country’s economy with the potential to lessen poverty, thereby contributing to food security and improved production [62].
Cattle diseases are the principal source of economic losses in Pakistan, and FMD has been recognized as having a significant impact on dairy production, fertility, and animal selling prices, besides retaining a profitable export market to other territories [63]. The country is currently placed at stage 2 of progressive control pathway (PCP) by OIE, with its aim to limit virus circulation in the dairy unit [64].
Pakistan is endemic for FMD for decades with three prevalent serotypes (A, O, and Asia-1). Serotype O was first detected in 1952 in Pakistan soon after its sovereignty in 1947. Serotype Asia-1 and A were detected in preceding years, i.e., 1954 and 1955 by Pirbright institute [63]. Mixed types of infections of FMD have also been reported [63]. Serotype C was also reported in 1954 with its major outbreak in 1963 in the northern region of the country, but this serotype was completely eradicated from Pakistan in 1995 [63]. The three frequently reported serotypes (Asia-1, O, and A) have rendered major epidemics in the region sharing a similar genealogical history with other FMD viruses circulating in pool 3. The FMD pool 3 viruses commonly reported in the Middle East and West Eurasia belong to O lineage: O/ME-SA/PanAsia-2 [65]. The dominant sub-lineage of serotype O that is commonly detected in Pakistan is O/ME-SA/PanAsia-2/ANT-10 [44]. Serotype A lineage: A/Asia/Iran 05 and Asia-1 lineage Sindh-08 (Group VII) [65] has also been described from Pakistan. The prevalence of serotypes O, Asia 1, and A reported in the previous years are 70%, 25%, and 4.7%, respectively [66], with high existence of serotype O and A in Sindh and A in Punjab province [16]. Seasonal changes with animal movement are a significant risk factor in virus transmission, because outbreaks have been seen in January to March at the holy festival Eid ul Azha due to the massive movement of animals among specific countries [31].
6. Phylogenetic Analysis of FMDV Serotypes
The coding sequence of the VP1 gene is an ideal marker for the characterization of FMDV into lineages and sub-lineages [67]. Additionally, the VP1 protein coat is used significantly for determining the phylogenetic and genetic relatedness among serotypes, genotypes, and topotypes [68]. Several molecular assays are commonly used for early and effective diagnosis and control of deadly diseases. RT-PCR-based detection with universal primers P1/P2 and IF/IR along with phylogenetic sequence analysis can detect FMD rapidly in infected animals [69]. Phylogenetic analysis also helps in tracing the FMD outbreak and, most importantly, gives information about the strain involved [40]. The phylogenetic analysis of FMD serotypes O, A, and Asia-1 circulating in Pakistan is given in detail below.
6.1. Serotype O
Serotype O was given its name at the Department of Oise in France [41]. Serotype O is the extensively distributed serotype with worldwide occurrence [70]. Based on antigenic diversity, the serotype is divided into 11 topotypes, namely Europe–South America (Euro–SA), Middle East–South Asia (ME–SA), Southeast Asia (SEA), Cathay (CHY), West Africa (WA), East Africa 1 (EA-1), East Africa 2 (EA-2), East Africa 3 (EA-3), East Africa 4 (EA-4), Indonesia-1 (ISA-1), and Indonesia-2 (ISA-2) [70]. Phylogenetic analysis of the VP1 nucleotide coding sequence of isolates by RT-PCR showed that ME-SA topotype is the most reported topotype from Pakistan from 1997 to date [71]. The Pakistan ME-SA topotype has common genetic lineages (Pan-Asia, Iran 2001, and Pak 98). Pan-Asia is further classified into Pan-Asia 1, Pan-Asia II, and Pan-Asia III. Pan-Asia I was detected in the Eastern and Western parts of Pakistan in 2003. Pan-Asia II and III are mostly similar but have only 5% variation in their nucleotides. Pan-Asia II was reported from the East, west, and Southern parts of Pakistan from 2005–2008 [32] and is still being reported [71]. The lineage PanAsia-III was detected from 2008 to 2009 in Pakistan and was found to be responsible for epidemics during this period [32]. Pak 98 lineage was detected in 1998 in Southern parts of Pakistan, showing 94.8% similarity to the VP1 coding region [32]. The Iran 2001 lineages were endemic in Iran; however, phylogenetic analysis revealed that it shares more than 80% nucleotide homology with serotype O strain circulating in Pakistan [72]. Studies on FMDV in Pakistan have been conducted, especially in the Landhi Dairy Colony Karachi, to show the presence of Serotype O [73]. During 2005–2008, in most of the outbreaks reported from Punjab, serotype O was found high in numbers compared to other serotypes [69]. The constant diversification and variation of FMDV strains make the prevention and control of FMDV even more challenging.
6.2. Serotype A
Serotype A is known to be the highly diverse antigenic serotype among the seven serotypes. Serotype A was first confirmed in Europe in the early 1980s, and it was subsequently demonstrated that the serotype originated from South America [24]. Outbreaks caused by serotype A in India have followed a more complicated pattern. A sequencing study undertaken in the early 21st century indicated that different serotype A viruses were responsible for outbreaks in different areas of India [26]. In Africa, type A strains were divided into six genotypes. Others were indigenous to Africa [50]. Although there are many genetic subtypes of serotype A, there are three main geographically distinct topotypes: Euro–SA, ASIA, and AFRICA [26].
Based on phylogenetic studies of the VP1 coding region, it has been known that serotype A exhibits >15 differences in their VP1 genes [74]. With FMDV A, the topotypes are called genotypes; a study conducted in 2002 identified nine clades of serotype A (I-IX), and a total of 26 genotypes have been acknowledged to date [75]. These genotypes are further classified into strain or lineage (i.e., A/Iran-2005, A/Pak-09). Of the total 26 reported genotypes, 11 are placed in topotype ASIA, with two dominant lineages (A/ASIA/Iran-05 and G-VII, also known as G-18) reported from Pakistan and other South Asian countries [76]. The involved genotype in most of the reported outbreaks during 2004–2012 in Pakistan belonged to Genotype II, with prevailing lineages A/Iran-05 and A/Pak-09. The A/Pak-09 lineage depicts 8% variation within the VP1 region from the prototype A/Iran-05 [77].
A study was carried out to investigate the recently discovered genetic variant of serotype A in Iran in 2005 by Klein et al. (2007) [73]. This serotype has caused outbreaks during the period 2005–2007. The newly emerged sub-lineage A/IRN/2005 underwent genetic modification in the A22 region (most used vaccine sequence) of structural capsid, leading to its emergence. The extreme genetically diverse nature of FMDV A serotype hampers vaccine development for outbreak control [78].
6.3. Serotype Asia-1
Asia-1 FMD viruses are mostly confined to Asian countries, with periodic epidemics reported as far west as Greece (2000) and Turkey (2006), plus as far south as in Bahrain [30]. FMDV serotype Asia-1 was first identified by Pirbright in 1954 [63] in Pakistan from the water buffalo at Okara Pakistan [79]. Serotype Asia-1 has been present in Pakistan since 1940, and retrospective studies showed that outbreaks from 1943 to 1947 occurred due to Asia-1 [80]. During the period 1959–1960, an outbreak due to serotype Asia-1 was reported at Qadirabad cattle breeding farm, with high mortality rates in young animals [81]. After these outbreaks, serotype Asia-1 was not detected during the periods of 1978–1983, 1990–1995, and 1999–2001, but it was found to be associated with severe outbreaks from 1984–1987 [82]. Again, in the year 2003, a severe outbreak of serotype Asia-1 was reported in Pakistan [26]. Since then, frequent outbreaks of FMDV Asia-1 are reported from different areas of Pakistan. Most importantly, significant epidemics occurred during the period 2007–2012 in Pakistan [83].
The Asia-1 serotype is not antigenically diverse, as with the other serotypes, and their VP1 sequences show almost >85% similarity with one another; therefore, only one topotype is present for this serotype. In the case of Asia-1, the topotype is named “Groups” [84]. Phylogenetic evolutionary analysis revealed that three genetic groups, namely Group II, Group VI, and Group VII, were found circulating in Pakistan from 1998–2009 [32]. These groups have also been detected circulating in the neighboring country Afghanistan. An inter-serotypes recombinant virus with VP2-VP3-VP1-2A coding sequences resulting from Asia-1 Group-VII virus and the rest of the genome from a serotype A virus of the A-Iran05 AFG-07 sub-lineage was identified using whole-genome analysis [32].
7. Sequence Analysis of FMDV Strains from Pakistan
The VP1 gene sequences and complete genome of foot and mouth disease virus serotypes (O, A, and Asia-1) circulating in Pakistan were retrieved from GenBank and used for FMD evolutionary analysis. The phylogenetic tree was constructed using the maximum likelihood method and Tamura–Nei model [85]. The bootstrap values were inferred from 100 replicates of original data, and the values are shown next to each branch of the tree [86]. Branches matching to partitions reproduced in less than 50% bootstrap replicates are collapsed. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (100 replicates) are shown next to the branches [86]. MEGA X software was used for tree construction [87]. The phylogenetic tree of serotype O, A, and Asia-1 are depicted in the figures given below (Figure 1, Figure 2 and Figure 3).
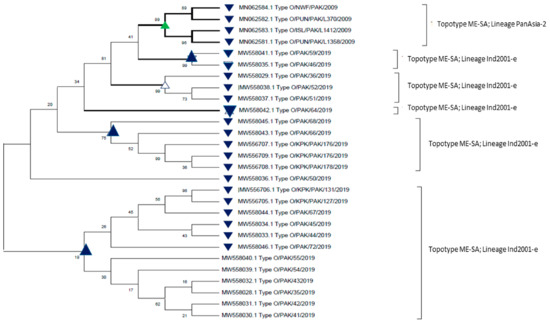
Figure 1.
A phylogenetic evolutionary (rooted) tree depicting the relationship between FMDV serotype O isolates of Pakistan that have been retrieved from GeneBank. The VP1 region sequences are used for the comparison of sequences. The phylogenetic tree was constructed with maximum-likelihood method based on Tamura–Nei model. The tree topology robustness was measured with bootstrap values (100 replicates). The values on the nodes show bootstrap values.
The above figure (Figure 1) shows that sequences of FMDV serotype O that have been collected during the period 2009–2019 belong to topotype Middle East–South Asia (ME–SA), and the circulating predominant lineages were PanAsia-2 and Ind-2001 (Table 2). The above figure also gives us an insight that the FMDV O strains isolated in 2009, clustered with FMDV O strains collected in 2019 (topotype ME–SA).

Table 2.
FMDV serotypes, topotypes, and their lineages circulating in Pakistan.
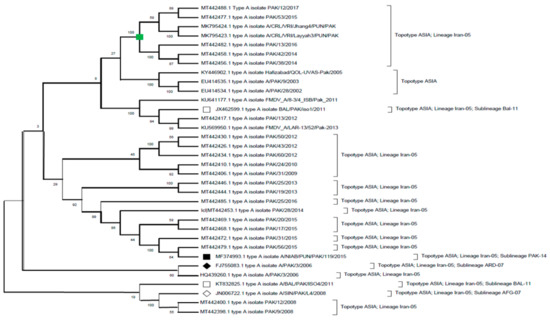
Figure 2.
A phylogenetic evolutionary (rooted) tree depicting the relationship between FMDV serotype A isolates of Pakistan that have been retrieved from GeneBank. The VP1 region sequences are used for the comparison of sequences. The phylogenetic tree was constructed with maximum-likelihood method based on Tamura–Nei model. The tree topology robustness was measured with bootstrap values (100 replicates). The values on the nodes show bootstrap values.
The above figure (Figure 2) shows that sequences of FMDV serotype A that have been collected during the period 2002–2017 belong to topotype ASIA (Table 2). (♦) (■) (□) (◊) indicates the predominant sub-lineages ARD-07, PAK-14, BAL-11, and AFG-07 that have been reported from Pakistan during this period.
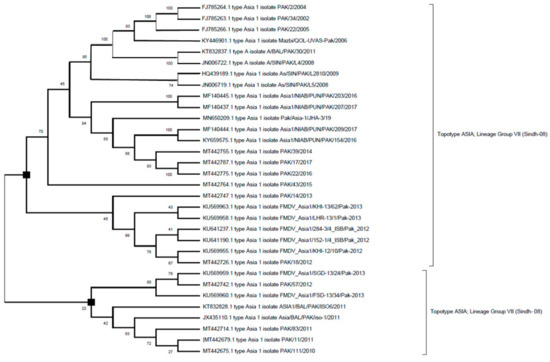
Figure 3.
Phylogenetic evolutionary (rooted) tree depicting relationship between FMDV serotype Asia-1 isolates of Pakistan that have been retrieved from GeneeBank. The VP1 region sequences are used for comparison of sequences. The phylogenetic tree was constructed with maximum likelihood method based on Tamura–Nei model. The tree topology robustness was measured with bootstrap values (100 replicates). The values on the nodes show bootstrap values.
The above figure (Figure 3) shows that sequences of FMDV serotype Asia-I that have been collected during the time period 2002–2017 belongs to topotype ASIA, and the circulating predominant lineage was Group VII (Sindh-08) (Table 2).
The above phylogenetic-based analysis will help us understand the genetics of FMDV strains circulating in Pakistan. Such analysis is extremely crucial for prevention strategies, i.e., diagnosis, rapid point of care detection, and development of recombinant vaccines.
8. Conclusions
This review delivers valuable information on the molecular dynamics of FMDV serotype A, O, and Asia-1 circulating in Pakistan. Molecular epidemiology and phylogenetic provide new insights to trace the origin, combat, and eradicate FMD. At present, FMDV O topotype ME-SA and lineage PanAsia-2 are reported frequently from different regions of Pakistan. The diverse nature of the FMDV serotype hinders vaccine development. The FMDV VP1 nucleotide sequence data produced from the structural capsid might play an important role in the selection of appropriate subtypes for novel vaccine development. The use of effective mass vaccination strategies accompanied by the control movement of dairy animals might lessen the disease burden. Presently, both FAO and OIE are embracing the Progressive Control Pathway for Foot and Mouth Disease (PCP-FMD) for the worldwide control of FMD. Continuous monitoring and timely surveillance programs are necessary to estimate the efficacy of vaccines, thereby preventing transmission and spread of the FMDV among dairy animals.
Author Contributions
M.A. and S.M. conceive the idea, M.A. and Z.S. prepared the paper structure. M.A., S.M. and M.J.A. reviewed and wrote paper. S.M. and Z.S. performed phylogenetic analysis. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References and Notes
- Alhaji, N.; Amin, J.; Aliyu, M.; Mohammad, B.; Babalobi, O.; Wungak, Y.; Odetokun, I. Economic impact assessment of foot-and-mouth disease burden and control in pastoral local dairy cattle production systems in Northern Nigeria: A cross-sectional survey. Prev. Vet. Med. 2020, 177, 104974. [Google Scholar] [CrossRef]
- Bulletins Archives—IDF—IDF Is the Leading Source of Scientific and Technical Expertise for All Stakeholders of the Dairy Chain. Available online: https://fil-idf.org/product-category/bulletin/ (accessed on 23 December 2021).
- Pakistan Finance Division. Pakistan Economic Survey 2018–2019; 2019. Available online: http://www.finance.gov.pk/survey/chapters_19/Economic_Survey_2018_19.pdf (accessed on 23 December 2021).
- Siddiky, M. Dairying in South Asian region: Opportunities, challenges and way forward. SAARC J. Agric. 2017, 15, 173–187. [Google Scholar] [CrossRef]
- Shaw, J. Milk: The Mammary Gland and Its Secretion; Food and Agriculture Organization of the United Nations: Rome, Italy, 2016; p. 89. [Google Scholar]
- Rushton, J. The Economics of Animal Health and Production; CABI: Wallingford, UK, 2009. [Google Scholar]
- Awel, S.; Dilba, G.; Abraha, B.; Zewde, D.; Wakjira, B.; Aliy, A. Seroprevalence and molecular detection of foot and mouth disease virus in dairy cattle around Addis Ababa, Central Ethiopia. Vet. Med. Res. Rep. 2021, 12, 187–197. [Google Scholar] [CrossRef]
- Lee, M.; Jo, H.; Park, S.; Ko, M.; Kim, S.; Kim, B.; Park, J. Advanced Foot-and-mouth disease vaccine platform for stimulation of simultaneous cellular and humoral immune responses. Vaccines 2020, 8, 254. [Google Scholar] [CrossRef]
- Azeem, A. A review on foot and mouth disease in dairy animals, etiology, pathogenesis and clinical findings. Pure Appl. Biol. 2020, 9, 821–832. [Google Scholar] [CrossRef]
- Upadhayay, U.; Ewam, P. Epidemiology and diagnosis of foot-and-mouth disease: A review. Indian J. Anim. Sci. 2012, 6, 543–551. [Google Scholar]
- Hietela, S.; Ardans, A. Molecular weapons against agricultural vulnerability and the war on terror. J. Vet. Med. Educ. 2003, 30, 155–156. [Google Scholar] [CrossRef] [PubMed]
- Samina, I. Host factors affecting the homologous and heterologous immune response of cattle to FMDV: Genetic background, age, virus strains and route of administration. Vaccines 1998, 16, 335–339. [Google Scholar] [CrossRef]
- Vallée, H.; Carré, H. Sur la pluralité du virus aphteux. CR Acad. Sci. Paris 1922, 174, 1498–1500. [Google Scholar]
- Abubakar, M. An evaluation of foot-and-mouth disease outbreak in relation to vaccination in a herd of cattle and buffaloes. Res. J. Vet. Pract. 2014, 2, 28–29. [Google Scholar] [CrossRef]
- Knight-Jones, T.J.; Rushton, J. The economic impacts of foot and mouth disease–What are they, how big are they and where do they occur? Prev. Vet. Med. 2013, 112, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Abubakar, M.; Khan, E.; Arshed, M.; Gonzales, J.; Ferrari, G.; Hussain, M.; Ali, Q. An appraisal on the occurrence of foot-and-mouth disease virus serotypes in cattle and buffaloes, Pakistan. Arch. Virol. 2015, 160, 1561–1564. [Google Scholar] [CrossRef]
- Feasibility Analysis for Cluster Development Based Agriculture Transformation. Cluster Development Based Agriculture Transformation Plan Vision-2025; Ministry of Planning Development and Special Initiatives: Islamabad, Pakistan, 2020.
- Brito, B.; Rodriguez, L.; Hammond, J.; Pinto, J.; Perez, A. Review of the global distribution of foot-and-mouth disease virus from 2007 to 2014. Transbound. Emerg. Dis. 2015, 64, 316–332. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, M. The causative agent of FMD. In Some RNA Viruses; IntechOpen Limited: London, UK, 2021; p. 79. [Google Scholar]
- Chakraborty, S. Foot-and-mouth disease, an economically important disease of animals. Adv. Anim. Vet. Sci. 2014, 2, 1–18. [Google Scholar] [CrossRef]
- Morelli, M.; Wright, C.; Knowles, N.; Juleff, N.; Paton, D.; King, D.; Haydon, D. Evolution of foot-and-mouth disease virus intra-sample sequence diversity during serial transmission in bovine hosts. Vet. Res. 2013, 44, 12. [Google Scholar] [CrossRef]
- Paton, D.J.; Di Nardo, A.; Knowles, N.J.; Wadsworth, J.; Pituco, E.M.; Cosivi, O.; Rivera, A.M.; Kassimi, L.B.; Brocchi, E.; de Clercq, K.; et al. The history of foot-and-mouth disease virus serotype C: The first known extinct serotype? Virus Evol. 2021, 7, veab009. [Google Scholar] [CrossRef]
- Rudreshappa, A.; Sanyal, A.; Mohapatra, J.; Subramaniam, S.; De, A.; Das, B.; Singanallur, N.; Jangam, A.; Muthukrishnan, M.; Villuppanoor, S.; et al. Emergence of antigenic variants with in serotype A foot and mouth disease virus in India and evaluation of a new vaccine candidate panel. Vet. Microbiol. 2012, 158, 405–409. [Google Scholar] [CrossRef]
- Ding, Y.; Chen, H.; Zhang, J.; Zhou, J.; Ma, L.; Zhang, L.; Gu, Y.; Liu, Y. An overview of control strategy and diagnostic technology for foot-and-mouth disease in China. Virol. J. 2013, 10, 78. [Google Scholar] [CrossRef]
- Doel, T. FMD Vaccines. Virus Res. 2003, 91, 81–99. [Google Scholar] [CrossRef]
- Knowles, N.; Samuel, A. Molecular epidemiology of foot-and-mouth disease virus. Virus Res. 2003, 91, 65–80. [Google Scholar] [CrossRef]
- Yang, M.; Holland, H.; Clavijo, A. Production of monoclonal antibodies against whole virus particles of foot-and-mouth disease virus serotype O and A and their potential use in quantification of intact virus for vaccine manufacture. Vaccines 2008, 26, 3377–3382. [Google Scholar] [CrossRef] [PubMed]
- Knight-Jones, T.; McLaws, M.; Rushton, J. Foot-and-mouth disease impact on smallholders—What do we know, what don’t we know and how can we find out more? Transbound. Emerg. Dis. 2016, 64, 1079–1094. [Google Scholar] [CrossRef] [PubMed]
- Mahapatra, M.; Parida, S. Foot and mouth disease vaccine strain selection: Current approaches and future perspectives. Expert Rev. Vaccines 2018, 17, 577–591. [Google Scholar] [CrossRef] [PubMed]
- WRLFMD. World Reference Laboratory for Foot and Mouth; OIE/FAO Reference Laboratory Contract Report; Annual Report; The Pirbright Institute: Pirbright, UK, 2016. [Google Scholar]
- Subramaniam, S.; Mohapatra, J.; Das, B.; Sanyal, A.; Pattnaik, B. Genetic and antigenic analysis of foot-and-mouth disease virus serotype O responsible for outbreaks in India during 2013. Infect. Genet. Evol. 2015, 30, 59–64. [Google Scholar] [CrossRef]
- Jamal, S.; Ferrari, G.; Ahmed, S.; Normann, P.; Belsham, G. Genetic diversity of foot-and-mouth disease virus serotype O in Pakistan and Afghanistan, 1997–2009. Infect. Genet. Evol. 2011, 11, 1229–1238. [Google Scholar] [CrossRef] [PubMed]
- Namatovu, A.; Belsham, G.; Ayebazibwe, C.; Dhikusooka, M.; Wekesa, S.; Siegismund, H.; Muwanika, V.; Tjørnehøj, K. Challenges for serology-based characterization of foot-and-mouth disease outbreaks in endemic areas; identification of two separate lineages of serotype O FMDV in Uganda In 2011. Transbound. Emerg. Dis. 2013, 62, 522–534. [Google Scholar] [CrossRef][Green Version]
- World Animal Health Information Database (WAHID)—Web Resources. Available online: https://webresources.articles411.com/world-animal-health-information-database-wahid (accessed on 23 December 2021).
- Forman, S.; Le Gall, F.; Belton, D.; Evans, B.; François, J.L.; Murray, G.; Sheesley, D.; Vandersmissen, A.; Yoshimura, S. Moving towards the global control of foot and mouth disease: An opportunity for donors. Rev. Sci. Tech. 2009, 28, 883. [Google Scholar] [CrossRef]
- Kumar, R.; Sreenivasa, B.; Tamilselvan, R. Construction and characterization of recombinant human adenovirus type 5 expressing foot-and-mouth disease virus capsid proteins of indian vaccine strain, O/IND/R2/75. Vet. World 2015, 8, 147–155. [Google Scholar] [CrossRef][Green Version]
- Ashkani, J.; Rees, D. The critical role of vp1 in forming the necessary cavities for receptor-mediated entry of FMDV to the host cell. Sci. Rep. 2016, 6, 27140. [Google Scholar] [CrossRef]
- Garcıía-Briones, M.; Blanco, E.; Chiva, C.; Andreu, D.; Ley, V.; Sobrino, F. immunogenicity and T cell recognition in swine of foot-and-mouth disease virus polymerase 3D. Virology 2004, 322, 264–275. [Google Scholar] [CrossRef]
- Singh, I.; Deb, R.; Kumar, S.; Singh, R.; Andonissamy, J.; Smita, S.; Sengar, G.; Kumar, R.; Ojha, K.; Sahoo, N.; et al. Deciphering foot-and-mouth disease (FMD) virus–host tropism. J. Biomol. Struct. Dyn. 2019, 37, 4779–4789. [Google Scholar] [CrossRef]
- Domingo, E.; Baranowski, E.; Escarmís, C.; Sobrino, F. Foot-and-mouth disease virus. Comp. Immunol. Microbiol. Infect. Dis. 2002, 25, 297–308. [Google Scholar] [CrossRef]
- Diaz-San Segundo, F.; Medina, G.; Stenfeldt, C.; Arzt, J.; de los Santos, T. Foot-and-mouth disease vaccines. Vet. Microbiol. 2017, 206, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Cox, S.; Barnett, P. Experimental evaluation of Foot-and-mouth disease vaccines for emergency use in ruminants and pigs: A review. Vet. Res. 2008, 40, 13. [Google Scholar] [CrossRef]
- Jiang, S.; Bai, X.; Li, P.; Zhang, M.; Bao, H.; Sun, P.; Lu, Z.; Cao, Y.; Chen, Y.; Li, D.; et al. Influence of foot-and-mouth disease virus O/CHN/Mya98/33-P strain leader protein on viral replication and host innate immunity. Viral Immunol. 2015, 28, 360–366. [Google Scholar] [CrossRef]
- Jackson, T.; Sharma, A.; Ghazaleh, R.; Blakemore, W.; Ellard, F.; Simmons, D.; Newman, J.; Stuart, D.; King, A. Arginine-Glycine-Aspartic Acid-specific binding by foot-and-mouth disease viruses to the purified integrin Alpha(V)Beta3 in vitro. J. Virol. 1997, 71, 8357–8361. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, I.; Malirat, V.; Falczuk, A. Evolving Perception On The Benefits Of Vaccination As A Foot And Mouth Disease Control Policy: Contributions Of South America. Expert Rev. Vaccines 2005, 4, 903–913. [Google Scholar] [CrossRef] [PubMed]
- Pinto, J. Foot-and-Mouth Disease Situation Food and Agriculture Organization of the United Nations Monthly Report; FAO: Roma, Italy, 2017; pp. 21–25. [Google Scholar]
- Shmeiger, Z.; Miculitzki, M.; Gelman, B.; Vaxman, I.; Goshen, T. The effect of foot and mouth disease morbidity influencing periparturient diseases and culling on Nir Yitzhak dairy cattle farm. Isr. J. Vet. Med. 2021, 76, 27. [Google Scholar]
- Gizaw, D.; Tesfaye, Y.; Wood, B.; Di Nardo, A.; Shegu, D.; Muluneh, A.; Bilata, T.; Belayneh, R.; Fentie, A.; Asgdome, H.; et al. Molecular characterization of foot-and-mouth disease viruses circulating in Ethiopia between 2008 and 2019. Transbound. Emerg. Dis. 2020, 67, 2983–2992. [Google Scholar] [CrossRef]
- Lyons, N.; Alexander, N.; Stärk, K.; Dulu, T.; Sumption, K.; James, A.; Rushton, J.; Fine, P. Impact of foot-and-mouth disease on milk production on a large-scale dairy farm in Kenya. Prev. Vet. Med. 2015, 120, 177–186. [Google Scholar] [CrossRef]
- Lyons, N.; Stärk, K.; van Maanen, C.; Thomas, S.; Chepkwony, E.; Sangula, A.; Dulu, T.; Fine, P. Epidemiological analysis of an outbreak of foot-and-mouth disease (serotype SAT2) on a large dairy farm in Kenya using regular vaccination. Acta Tropica 2015, 143, 103–111. [Google Scholar] [CrossRef]
- Muroga, N.; Hayama, Y.; Yamamoto, T.; Kurogi, A.; Tsuda, T.; Tsutsui, T. The 2010 foot-and-mouth disease epidemic in Japan. J. Vet. Med. Sci. 2012, 74, 399–404. [Google Scholar] [CrossRef]
- Haydon, D.; Kao, R.; Kitching, R. The UK foot-and-mouth disease outbreak—The aftermath. Nat. Rev. Microbiol. 2004, 2, 675–681. [Google Scholar] [CrossRef]
- O’Rourke, K.; Williamson, J. After columbus: Explaining europe’s overseas trade boom, 1500–1800. J. Econ. Hist. 2002, 62, 417–456. [Google Scholar] [CrossRef]
- Lombard, M.; Fussel, A. Antigen and vaccine banks: Technical requirements and the role of the european antigen bank in emergency foot and mouth disease vaccination. Revue Sci. et Tech. de l’OIE 2007, 26, 117–134. [Google Scholar] [CrossRef]
- Jamal, S.; Belsham, G. Foot-And-Mouth Disease: Past, present and future. Vet. Res. 2013, 44, 116. [Google Scholar] [CrossRef]
- Fodgeby, E. Review of Epizootology and Control of Foot-and-Mouth Disease in Europe from 1937 to 1961; European Commission for the Control of Foot-and-Mouth Disease, Food and Agriculture Organization of the United Nations: Rome, Italy, 1962; p. 108. [Google Scholar]
- Capstick, P.; Telling, R.; Chapman, W.; Stewart, D. Growth of a cloned strain of hamster kidney cells in suspended cultures and their susceptibility to the virus of foot-and-mouth disease. Nature 1962, 195, 1163–1164. [Google Scholar] [CrossRef] [PubMed]
- Bahnemann, H. Binary ethylenimine as an inactivant for foot-and-mouth disease virus and its application for vaccine production. Arch. Virol. 1975, 47, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Foot-and-Mouth Disease Situation Food and Agriculture Organization of the United Nations Monthly Report; FAO: Roma, Italy, 2019; p. 32.
- Bachanek-Bankowska, K.; Di Nardo, A.; Wadsworth, J.; Henry, E.K.; Parlak, Ü.; Timina, A.; Mischenko, A.; Qasim, I.A.; Abdollahi, D.; Sultana, M.; et al. Foot-and-Mouth Disease in the Middle East Caused by an A/ASIA/G-VII Virus Lineage, 2015–2016. Emerg. Infect. Dis. 2018, 24, 1073. [Google Scholar] [CrossRef] [PubMed]
- Agriculture-Ministry of Finance—Government of Pakistan-pdf—Chapter 2 Agriculture Pakistan’s Agriculture Sector plays a Central Role in the Course Hero. Available online: https://www.coursehero.com/file/45332132/Agriculture-Ministry-of-Finance-Government-of-Pakistan-pdf/ (accessed on 23 December 2021).
- Blench, R.; Chapman, R.; Slaymaker, T. A Study of the Role of Livestock in Poverty Reduction Strategy Papers; Food and Agriculture Organization of the United Nation: Rome, Italy, 2003; pp. 1–57. [Google Scholar]
- Jamal, S.; Ahmed, S.; Hussain, M.; Ali, Q. Status of foot-and-mouth disease in Pakistan. Arch. Virol. 2010, 155, 1487–1491. [Google Scholar] [CrossRef]
- Final Evaluation of the Project, Development of a Framework for the Progressive Control of Foot and Mouth Disease in Pakistan; FAO: Rome, Italy, 2016. Available online: http://www.fao.org/evaluation/digest/evaluation-detail/es/c/416084/ (accessed on 23 December 2021).
- Bachanek-Bankowska, K.; Di Nardo, A.; Wadsworth, J.; King, D.; Knowles, N. A47 reconstructing the evolutionary history of pandemic foot-and-mouth disease viruses: The impact of recombination within the emerging O/ME-SA/Ind-2001 lineage. Virus Evol. 2019, 5, vez002-046. [Google Scholar] [CrossRef]
- Zulfiqar, M. Draft Report for Development of National Disease Control Policy for Foot and Mouth Disease in Pakistan under the FAO Project Support for Emergency Prevention and Control of Main Trans-Boundary Animal Diseases in Pakistan Rinderpest; FMD PPR: Draft report Pakistan. 2003; p. 90.
- Abdul-Hamid, N.; Hussein, N.; Wadsworth, J.; Radford, A.; Knowles, N.; King, D. Phylogeography of foot-and-mouth disease virus types O and A In Malaysia and surrounding countries. Infect. Genet. Evol. 2011, 11, 320–328. [Google Scholar] [CrossRef]
- Burman, A.; Clark, S.; Abrescia, N.; Fry, E.; Stuart, D.; Jackson, T. Specificity of the VP1 GH loop of foot-and-mouth disease virus for Alphav integrins. J. Virol. 2006, 80, 9798–9810. [Google Scholar] [CrossRef] [PubMed]
- Saeed, A.; Khan, Q.; Waheed, U.; Arshad, M.; Asif, M.; Farooq, M. RT-pcr evaluation for identification and sequence analysis of foot-and-mouth disease serotype O from 2006 To 2007 In Punjab, Pakistan. Comp. Immunol. Microbiol. Infect. Dis. 2011, 34, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Knowles, N.; Samuel, A.; Davies, P.; Midgley, R.; Valarcher, J. Pandemic strain of foot-and-mouth disease virus serotype O. Emerg. Infect. Dis. 2005, 11, 1887–1893. [Google Scholar] [CrossRef] [PubMed]
- Hicks, H.; Wadsworth, J.; Azhar, M.; Afzal, M.; Manzoor, S.; Abubakar, M.; Khan, E.; King, D.; Knowles, N. Genome sequences of foot-and-mouth disease virus O/ME-SA/Ind-2001E strains isolated in Pakistan. Microbiol. Resour. Announc. 2020, 9, e00242-14. [Google Scholar] [CrossRef] [PubMed]
- Rashtibaf, M.; Sharifi, K.; Zibaee, S.; Dehghani, H. A survey on the frequency of foot-and-mouth disease virus carriers in cattle in North-East of Iran by RT-PCR: Implications for revising disease control strategy. Transbound. Emerg. Dis. 2012, 59, 482–489. [Google Scholar] [CrossRef]
- Klein, J.; Hussain, M.; Ahmad, M.; Normann, P.; Afzal, M.; Alexandersen, S. Genetic characterisation of the recent foot-and-mouth disease virus subtype A/IRN/2005. Virol. J. 2007, 4, 122. [Google Scholar] [CrossRef]
- Samuel, A.; Knowles, N. Foot-and-mouth disease type O viruses exhibit genetically and geographically distinct evolutionary lineages (topotypes). J. Gen. Virol. 2001, 82, 609–621. [Google Scholar] [CrossRef]
- Mohapatra, J.; Subramaniam, S.; Pandey, L.; Pawar, S.; De, A.; Das, B.; Sanyal, A.; Pattnaik, B. Phylogenetic structure of serotype A foot-and-mouth disease virus: Global diversity and the Indian perspective. J. Gen. Virol. 2011, 92, 873–879. [Google Scholar] [CrossRef]
- Jamal, S.; Belsham, G. Molecular epidemiology, evolution and phylogeny of foot-and-mouth disease virus. Infect. Genet. Evol. 2018, 59, 84–98. [Google Scholar] [CrossRef] [PubMed]
- Ali, W.; Habib, M. Molecular Evaluation of Vp1 Coding Region of Foot and Mouth Disease Virus Circulating in Pakistan. Sarhad J. Agric. 2018, 34, 979–985. [Google Scholar] [CrossRef]
- Kitching, R. The role of the world reference laboratories for foot-and-mouth disease and for rinderpest. Ann. N. Y. Acad. Sci. 2006, 916, 139–146. [Google Scholar] [CrossRef]
- Brooksby, J.; Rogers, J. Methods Used in Typing the Virus of Foot-and-Mouth Disease at Pirbright, 1950–1955: Methods of Typing and Cultivation of Foot-and-Mouth Disease Virus; European Productivity Agency of the Organization of European Cooperation (OEEC), The Pirbright Institute: Pirbright, UK, 1957; pp. 31–34. [Google Scholar]
- Yasin, S.; Huq, M. Foot-and-Mouth Disease in Pakistan. Off. Int. Epizoot. 1960, 54, 378–388. [Google Scholar]
- Sheikh, S. An outbreak of malignant Foot-and-mouth disease at government Dajjal cattle breeding farm, Qadirabad, District Montgomery. Healer 1960, 5, 3–7. [Google Scholar]
- Ullah, A.; Jamal, S.; Romey, A.; Gorna, K.; Kakar, M.; Abbas, F.; Ahmad, J.; Zientara, S.; Bakkali Kassimi, L. Genetic characterization of serotypes A and Asia-1 Foot-and-mouth disease viruses in Balochistan, Pakistan, in 2011. Transbound. Emerg. Dis. 2016, 64, 1569–1578. [Google Scholar] [CrossRef]
- Jamal, S.; Ferrari, G.; Ahmed, S.; Normann, P.; Belsham, G. Molecular characterization of serotype Asia-1 foot-and-mouth disease viruses in Pakistan and Afghanistan; emergence of a new genetic group and evidence for a novel recombinant virus. Infect. Genet. Evol. 2011, 11, 2049–2062. [Google Scholar] [CrossRef]
- Mahapatra, M.; Yuvaraj, S.; Madhanmohan, M.; Subramaniam, S.; Pattnaik, B.; Paton, D.; Srinivasan, V.; Parida, S. Antigenic and genetic comparison of foot-and-mouth disease virus serotype O Indian vaccine strain, O/IND/R2/75 against currently circulating viruses. Vaccines 2015, 33, 693–700. [Google Scholar] [CrossRef]
- Tamura, K.; Nei, M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol. Biol. Evol. 1993, 10, 512–526. [Google Scholar]
- Felsenstein, J. Phylogenies from restriction sites: A maximum-likelihood approach. Evolution 1992, 46, 159–173. [Google Scholar]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).