Abstract
Selenium, an essential metalloid, plays a dual role in biological systems: while crucial for maintaining normal biological processes, excessive levels can be toxic. Organisms mitigate selenium toxicity through a biochemical process known as methylation, in which inorganic selenium species are enzymatically converted into less toxic, excretable organic metabolites. This review synthesizes recent biochemical and environmental findings (with an emphasis on the past decade) related to selenium methylation. It outlines the enzymatic mechanisms—particularly involving glutathione reductase, SAM-dependent methyltransferases, and selenocysteine lyase—through which selenite and selenate are reduced and methylated to intermediates such as hydrogen selenide (H2Se), ultimately yielding MMSe, DMSe, and TMSe+. The role of enzymes such as selenocysteine lyase in processing organic selenium and factors affecting the efficiency of these processes, including environmental conditions, are discussed. The role of enzymes such as selenocysteine lyase in metabolizing organic selenium species is also discussed, along with how environmental conditions (e.g., soil composition, redox potential) and genetic variability influence methylation efficiency and selenium speciation. In conclusion, this paper explores selenium methylation in plants, focusing on rice and corn, and how their selenium uptake and metabolism are affected by environmental factors. It examines the conversion of selenium into organic forms like selenomethionine and selenocysteine, and the role of methylation in managing excess selenium. The findings offer insights into selenium chemistry, with implications for food safety, nutrition, and environmental management, addressing key knowledge gaps and enhancing our understanding of selenium’s biological and chemical roles.
1. Introduction
Selenium, a metalloid with both essential and toxicological properties, has garnered significant scientific interest since its discovery in 1817 [1]. It plays a crucial role in various physiological processes, including antioxidant defense, immune function, and thyroid hormone metabolism, primarily through its incorporation into selenoproteins such as glutathione peroxidases and thioredoxin reductases [2,3,4,5,6]. However, selenium toxicity can arise when intake exceeds the narrow margin between essential and harmful levels, leading to selenosis, which is characterized by symptoms such as gastrointestinal distress, neurological disorders, and brittle hair and nails [7,8,9]. One of the primary mechanisms through which organisms regulate selenium homeostasis and mitigate toxicity is methylation—a biochemical process that transforms inorganic selenium species (e.g., selenate and selenite) into less harmful organic forms, such as methylselenol, dimethylselenide, and trimethylselenonium [10,11,12,13]. This detoxification pathway involves a series of enzymatic reactions catalyzed by methyltransferases, which facilitate the stepwise addition of methyl groups, thereby increasing selenium’s water solubility and excretion efficiency [11,12,13,14,15,16]. Despite its critical role in selenium metabolism, the specific enzymatic pathways, regulatory mechanisms, and environmental influences that govern selenium methylation remain poorly understood. While it is established that methylation modulates selenium bioavailability and toxicity, the precise molecular actors, their regulation, and the conditions under which these transformations occur are still unclear.
Although the general biochemical schemes of selenium methylation have been known for several decades, the last ten years have brought significant advancements in understanding the finer details of this process. These include the identification of novel intermediate compounds, the improved characterization of enzyme specificities (e.g., selenocysteine lyase and SAM-dependent methyltransferases), and the influence of genetic and environmental factors on methylation efficiency. Additionally, there has been growing interest in the role of gut microbiota in modulating selenium metabolism and in plant-based methylation mechanisms, particularly in staple crops like rice and corn.
This review seeks to synthesize these recent developments, emphasizing progress made in the past decade regarding the enzymatic mechanisms, regulatory networks, and environmental determinants of selenium methylation. Recent studies have suggested that genetic factors, dietary components, and gut microbiota may significantly influence methylation efficiency and selenium bioavailability [17,18,19,20], but a comprehensive understanding of how these factors interact remains elusive.
This limited knowledge impedes progress in several key areas. In human health, an incomplete understanding of selenium methylation hinders the development of targeted nutritional guidelines and therapeutic strategies, especially in regions with selenium-deficient or selenium-rich environments. In food safety and environmental management, uncertainty around methylation dynamics limits our ability to predict selenium mobility, bioavailability, and long-term ecological impact.
Despite the known importance of selenium methylation in detoxifying excess selenium and modulating its biological function [21,22,23], major knowledge gaps persist in the characterization of intermediate compounds, the identification of specific enzymes and cofactors, and the regulation of methylation in diverse environmental and physiological contexts [24,25]. Furthermore, it identifies persistent gaps in knowledge and emerging research directions, especially in relation to human health, food safety, and environmental management.
What distinguishes this manuscript from earlier reviews is its focus on the integration of recent biochemical, agricultural, and ecological insights—offering an updated and multidisciplinary perspective on selenium methylation. This manuscript aims to address these challenges by an in-depth examination of the primary chemical reactions and pathways involved in selenium methylation [22,26]. We explore the specific reaction intermediates, transition states, and conditions that facilitate these transformations, offering critical insights into the fundamental processes of selenium chemistry. Ultimately, advancing our understanding of selenium methylation holds significant promises for improving food safety, guiding environmental remediation strategies, and promoting human and ecosystem health. By doing so, it provides a contemporary foundation for advancing both theoretical understanding and practical applications in selenium research.
A structured literature search was conducted to identify relevant studies on the biochemical and enzymatic mechanisms of selenium methylation. Peer-reviewed articles published between 1980 and 2024 were retrieved from databases including PubMed, Scopus, Web of Science, and Google Scholar. The search employed controlled vocabulary and free-text keywords such as “selenium methylation,” “SAM-dependent methyltransferases,” “selenium speciation,” and “plant selenium metabolism,” refined with Boolean operators (AND, OR). After screening and quality appraisal, 176 articles were included for review, focusing on chemical pathways, enzymatic functions, and environmental relevance. Studies were thematically categorized to guide the synthesis of biochemical models and comparative tables.
2. Selenium Metabolism and Methylation
This review synthesizes recent scientific advancements from the past decade on the chemical and enzymatic processes underlying selenium methylation across biological systems. While foundational aspects of selenium biochemistry have long been established, this review specifically focuses on emerging insights into enzymatic mechanisms—particularly those involving glutathione reductase, SAM-dependent methyltransferases, and selenocysteine lyase—that have deepened our understanding of selenium detoxification. Recent studies on selenium behavior in staple crops like rice and corn are highlighted to illustrate species-specific methylation patterns and metabolic pathways. Furthermore, the review integrates new data on environmental and physiological factors influencing the rate, selectivity, and efficiency of methylation reactions. By consolidating updated mechanistic findings, metabolic routes, and interspecies comparisons, this work offers a current and comprehensive overview that advances the discourse on selenium methylation beyond classical biochemical reaction schemes.
2.1. Reaction Mechanisms and Pathways
2.1.1. Primary Chemical Reactions in Selenium Methylation
In the evolving domain of organoselenium chemistry, recent advances have significantly expanded our understanding of the mechanistic pathways involved in selenium methylation particularly within the last decade [22,26,27]. This section builds upon classical concepts by integrating current findings on the enzymatic and non-enzymatic reactions that drive the methylation of selenium atoms into various organoselenium species [28,29]. Special attention is given to newly characterized intermediates, revised mechanistic models, and updated insights into the catalytic roles of glutathione reductase, SAM-dependent methyltransferases, and selenocysteine lyase [28,30]. These updates provide a more critical understanding of Se transition states and reaction conditions than earlier models.
Selenium, Se, which was first recognized in 1817, is a metalloid belonging to the group 16 elements. Its most common oxidation numbers are −II, 0, +IV, and +VI [31,32]. Selenium occurs naturally in two different forms: organically, for example, in the form of selenocysteine; and inorganically, for example, in the form of selenite or selenate. Even though selenium is an essential dietary element, its toxicity in animals is established at relatively low levels [33,34,35,36,37].
Living organisms, however, have developed mechanisms to detoxify excess selenium primarily through methylation [26,38,39], a key process in organoselenium chemistry. Understanding the reaction mechanisms and pathways involved in selenium methylation is crucial for elucidating selenium’s chemical behavior and its biological significance [1].
This section focuses on the primary chemical reactions underpinning selenium methylation, detailing the mechanistic pathways through which selenium atoms are methylated to form various organoselenium species [2,3]. We analyze the specific reaction intermediates, transition states, and conditions that facilitate these transformations. A comprehensive examination of these primary reactions provides critical insights into the fundamental processes governing selenium chemistry.
2.1.2. Detoxification of Inorganic Forms of Selenium
The detoxification of inorganic selenium compounds, such as selenite (SeO32−), begins with a non-enzymatic reaction with glutathione (GSH) [40,41], forming selenodiglutathione (GS–Se–SG). This intermediate is then enzymatically reduced by glutathione reductase (GSH-R) [40,41] in the presence of NADPH, producing glutathione selenopersulfide (GSSeH). Under further reduction, GSSeH yields hydrogen selenide (H2Se), a key reactive intermediate [42,43]. This sequence represents a six-electron reduction process, converting selenium from a +IV oxidation state in selenite to −II in selenide [42,43,44,45,46,47,48].
Under anaerobic conditions, glutathione selenopersulfide (GSSeH) is further reduced by glutathione reductase, producing hydrogen selenide (H2Se) (Scheme 1), a key reactive intermediate [48,49]. Because methylated forms of selenide are significantly less toxic compared to inorganic forms, hydrogen selenide (H2Se) undergoes enzymatic methylation as the next step in the detoxification pathway [50,51]. This reaction is catalyzed by class I SAM-dependent methyltransferases (MTases) (Scheme 1), which transfer a methyl group from S-adenosylmethionine (SAM) to H2Se in an SN2 nucleophilic substitution substitution mechanism [50,52,53].
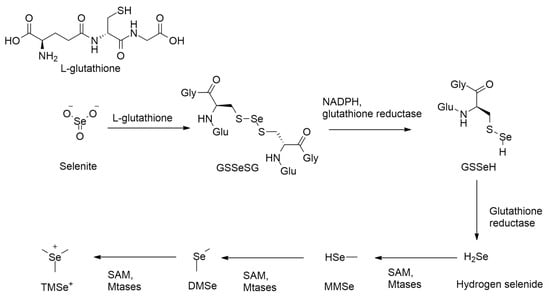
Scheme 1.
Methylation pathway of inorganic selenite.
The methylation of hydrogen selenide proceeds sequentially: first to monomethylselenide (MMSe), then to form dimethyl selenide (DMSe), and finally to trimethylselenonium ion (TMSe+) (Scheme 1) [52,54]. DMSe, being volatile, is expelled via exhalation, while TMSe+ is excreted in urine, representing a major detoxification route [16,54,55].
In addition to its detoxification via methylation, selenium homeostasis is further regulated through its incorporation into selenoproteins. These specialized proteins, which include glutathione peroxidase and thioredoxin reductase, are integral to the cellular antioxidant defense system [56,57]. They utilize selenium in the form of the amino acid selenocysteine at their active sites, enabling them to catalyze redox reactions that neutralize reactive oxygen species (ROS) [58]. Through this mechanism, selenoproteins help to maintain cellular redox balance and prevent oxidative damage to biomolecules such as DNA, lipids, and proteins. This incorporation into functional biomolecules represents a critical biological use of selenium, contributing to both its essentiality and its tightly regulated metabolism in living systems.
Selenium metabolism and detoxification efficiency vary widely among organisms due to a combination of environmental, dietary, and genetic factors [59,60,61]. One of the primary determinants of selenium bioavailability is soil composition, which directly influences the selenium content of crops consumed by humans and livestock [62,63,64]. On the other hand, agricultural practices—such as fertilizer application, irrigation methods, and crop selection—further modulate selenium uptake by plants, thereby affecting dietary selenium intake across different regions. In addition, genetic variations among species and individuals can alter the expression and activity of selenium-related enzymes and transporters, influencing how efficiently selenium is absorbed, metabolized, and detoxified. Environmental exposures, including pollutants and co-occurring trace elements, also interact with these biological pathways, collectively shaping selenium homeostasis and its associated health outcomes.
These sequential biochemical transformations alongside tightly regulated physiological mechanisms such as methylation, selenoprotein synthesis, cellular uptake, and excretion play a critical role in maintaining selenium homeostasis and preventing toxic accumulation in tissues and organs across various biological systems. Understanding these pathways is essential for developing strategies to manage selenium exposure in both environmental and clinical contexts, including the design of targeted nutritional supplementation, environmental remediation in high-selenium regions, and therapeutic interventions for selenium-related disorders such as selenosis or deficiency syndromes.
2.1.3. Intermediate and Excretory Metabolites in Selenium Methylation
Organic forms of selenium undergo metabolic transformation prior to excretion through a defined biochemical pathway [65,66,67]. These compounds are initially converted into hydrogen selenide (H2Se), a key reactive intermediate that undergoes methylation to form excretory metabolites [68,69]. In rat studies, the organic selenium compound selenodicystine, CySeSec, was shown to react with glutathione in a substitution-type reaction (Scheme 1), producing selenocysteine-glutathione selenyl sulfide (CySeSG) [70]. CySeSG can then be reduced to CySeH via two pathways: (1) non-enzymatically under anaerobic conditions with excess glutathione, or (2) enzymatically via glutathione reductase in the presence of NADPH (Scheme 2) [67]. CySeH is subsequently cleaved by selenocysteine β-lyase to yield H2Se, which follows a methylation pathway similar to that of selenite metabolism [69,70].
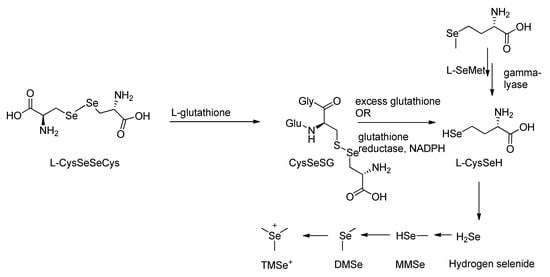
Scheme 2.
Methylation pathway of organic selenocysteine.
The metabolism of selenomethionine (SeMet) has also been well-characterized. Studies by Hasegawa et al. and others show that SeMet is directly reduced by γ-lyase to monomethylselenide, which then undergoes stepwise methylation to form dimethylselenide (DMSe) and trimethylselenonium (TMSe+) (Scheme 1 and Scheme 2), the primary excretory products (Scheme 2) [67,68,70].
2.2. Enzymes in Selenium Methylation
2.2.1. Role of Enzyme Glutathione Reductase
The first enzymatically catalyzed reaction in the methylation pathway of seleno-compounds utilizes the enzyme glutathione reductase [71]. This enzyme, which is highly conserved in nature, consists of two Rossmann folding domains, one of which binds NADPH and the other is an interface binding domain [72,73,74,75]. Glutathione, which is a cysteine-containing molecule, is readily oxidized. Hence, in the presence of inorganic selenite, or organic forms of selenium, glutathione is oxidized resulting in the formation of a GS-Se-GS bond [72,73,74,75,76] (refer to Scheme 1 and Scheme 2). Glutathione reductase thereafter catalyzes the recycling of oxidized glutathione back to its reduced form by catalyzing the breaking of the GS-Se bond in GS-Se-SG, in the presence of NADPH (Scheme 2). This catalysis is extremely vital, as it is key in the formation of hydrogen selenide.
Glutathione reductase is essential for maintaining cellular redox balance by continuously regenerating reduced glutathione, a key molecule involved in detoxification and metabolic pathways [77,78,79,80,81]. In addition to its role in selenium metabolism, this enzyme plays a significant part in shielding cells from oxidative stress by restoring reduced glutathione, which acts as a powerful antioxidant against reactive oxygen species (ROS) [80,82,83,84,85]. Glutathione reductase activity is tightly regulated, as fluctuations can disrupt redox balance and compromise cellular defenses against oxidative damage [71,81]. Furthermore, this enzyme supports biochemical processes that depend on reduced thiol groups, thereby contributing to overall cellular stability and function [71].
2.2.2. Role of SAM-Dependent Methyltransferases
The methylation of the selenide intermediate is catalyzed by SAM-dependent methyltransferases (Scheme 3). This crucial enzyme contributes to redox regulation, which in turn influences the structural integrity, post-translational modification, and proper functioning of proteins, as well as the synthesis and stability of nucleic acids (DNA/RNA) [86,87]. Initially, upon the binding of the substrate, flexible loops of the enzyme undergo reorientation. This is followed by a nucleophilic substitution with an SN2 mechanism, whereby a nucleophilic attack of the substrate is carried out on SAM, the electrophilic methyl donor, resulting in the cleavage of the C-S bond and subsequent formation of a C-Se bond [86,87,88]. The nucleophilic attack on SAM takes place due to the strong electrophilic character of its methyl group. Following methyl transfer, S-adenosyl-homocysteine (SAH) is formed, which is thereafter hydrolyzed to homocysteine by the enzyme SAH hydrolases (Scheme 3). Methionine is formed by the enzyme catalyzed methylation of homocysteine, and SAM is regenerated thereafter by the reaction of methionine with ATP, catalyzed by the enzyme SAM synthetase (Scheme 3).
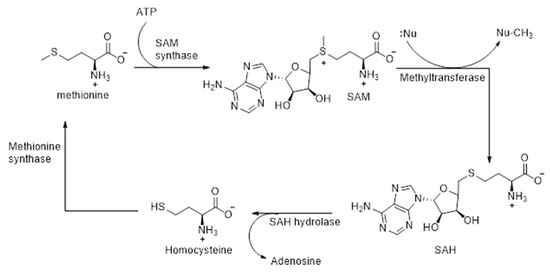
Scheme 3.
Recycling of SAM in the methylation of selenide intermediates.
2.2.3. Role of Selenocysteine Lyase Enzyme
Concerning organo-selenium compounds, a third type of enzyme is involved in the mechanistic pathway for methylation: selenocysteine lyase (SCL). This enzyme was first identified by Soda et al. in 1981 in mammalian tissues, specifically from rat liver extracts, and was the first proven enzyme to act specifically on selenium-containing substrates [89,90,91]. Selenocysteine lyases belong to a class of pyridoxal-5′-phosphate (PLP)-dependent aminotransferases that feature an evolutionarily conserved domain. In human SCL, for instance, a lysine residue (Lys-251) forms a Schiff base with PLP, enabling the formation of a homodimer that constitutes the enzyme’s active site pocket [90,91,92,93].
Each subunit of the human selenocysteine lyase enzyme comprises a small and large domain, with two active site cavities located at their interface. Upon the binding of L-selenocysteine, an extended lobe becomes ordered, positioning the substrate correctly in the active site [94]. In the mouse SCL, the selenium atom of L-selenocysteine specifically interacts with Cys375, a conserved cysteine residue in the active site, through a selenoate-thiol interaction. This interaction forms a Schiff base with PLP, resulting in a protonated aldimine intermediate. It is this unique interaction between the selenium atom and the thiol group of Cys375 that enables the enzyme to distinguish between selenium- and sulfur-containing substrates [91,95]. We note that, while these structural and mechanistic details are consistent across mammalian species such as humans and mice, variations may occur in non-mammalian organisms, potentially affecting substrate specificity and enzymatic activity [91,95]. The enzyme, when complexed with cysteine, forms a non-productive adduct and no chemical reaction takes place. ʟ-Selenocysteine is thus deprotonated to form a selenosulfide intermediate before being decomposed to ʟ-alanine. Selenium is released and is subsequently non-enzymatically reduced to hydrogen selenide. The role of selenocysteine lyases is therefore vital, as they ultimately recycle the selenium atom. The selenide that is generated re-enters the selenocysteine pathway by binding to a phosphate to generate selenophosphate (Figure 1), which is utilized to generate selenocysteine on its cognate tRNA and then incorporated into newly translated selenoproteins (Figure 2) [96,97]
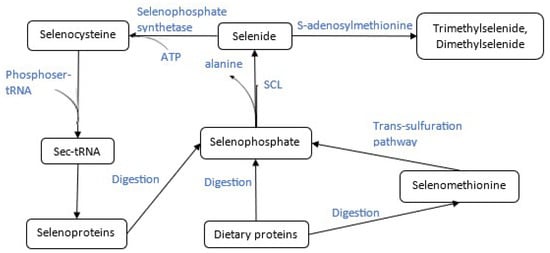
Figure 1.
Role of cystathionine γ-lyase enzyme in the recycling of selenium, outlining the metabolic conversion of selenium compounds in biological systems, highlighting key intermediates and potential toxic outcomes. Starting from inorganic selenium forms, such as selenate (SeO42−) and selenite (SeO32−), selenium is metabolized through pathways involving the formation of bis(alkylthio)selenide and subsequent oxidation products. These intermediates participate in S-adenosylmethionine (SAM)-dependent methylation reactions, producing methylated selenium species. Figure 1 also illustrates the incorporation of selenium into selenocysteine (Sec), which, at elevated concentrations, may contribute to Sec-related toxicity. Both Sec and excessive selenate exposure are linked to toxic effects, underlining the importance of tightly regulated selenium metabolism in plants and animals.
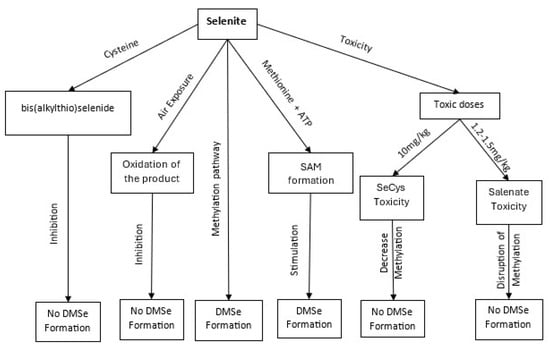
Figure 2.
Pathways of selenium methylation and associated toxicological outcomes, illustrating key biochemical pathways and intermediates involved in selenium (Se) methylation. The figure traces the progression from inorganic selenium compounds such as selenite and selenate to various organic and methylated selenium species. The transformation begins with the formation of bis (alkylthio)selenide, followed by its oxidation to more reactive intermediates. Subsequent steps involve the integration of selenium into S-adenosylmethionine (SAM)-dependent pathways.
2.3. Factors Influencing Rate and Selectivity
The rate limiting step in the detoxifying-methylation pathway of selenium containing compounds occurs in the methylation of selenide, which leads to the formation of DMSe [50,70,98,99,100]. In a set of studies conducted by the research group of Ganther et al. [101], it was found that the conversion of selenite to DMSe is inhibited by the presence of cysteine, presumably due to the reaction between the thiol functionality of cysteine and selenite to form bis(alkylthio)selenide. By way of their studies, it was also ascertained that the presence of S-adenosylmethionine stimulates the conversion to DMSe almost sixfold, compared to when only methionine and ATP are present as the methyl source, indicating that it is the formation of S-adenosylmethionine from methionine and ATP which is the specific rate-limiting step in the set of steps leading to the formation of DMSe (Scheme 1). In addition, it was found that the formation of DMSe requires the complete exclusion of air, presumably due to the resulting oxidation by air of the labile products produced as a result of selenium reduction. These findings were also supported by the research group of Hasegawa et al. [66], who investigated the effect of a toxic dose of selenocysteine (Sec) on the seleno-methylation pathway (Table 1; Figure 2).

Table 1.
Acute toxicity (LD50) of selenium compounds in various organisms.
The results concluded that a toxic dose of Sec causes a decrease in the rate of selenium methylation, and this is due to the inactivation by Sec of the enzyme methionine adenosyltransferase, which is involved in the synthesis of SAM from methionine and ATP. Similar results were obtained by Hasegawa et al. [66], whereby a toxic dose of CySeSeCy inhibited SAM formation and thus caused a decrease in selenium methylation (Table 1). In addition, it was reported by Hoffman [110] that an inactivation of the enzyme in the liver of mice occurs due to high concentrations of selenite. The minimum lethal dose of selenium in the form of selenite and selenate has been found to be on average 1.2–1.5 mg/kg body weight in rats, cats, and rabbits (Table 1) [102,103,111,112,113,114]. In mice, the toxic dose of selenocysteine was found to average 10 mg/kg body weight (Figure 2). To explain the inactivation of methionine adenosyltransferase, it has been suggested that the active site of the enzyme is sensitive to nucleophilic agents such as selenides and thus is inhibited when toxic doses of selenide are formed from the metabolism of organic and inorganic forms of selenium [66].
2.4. Pathways of Selenium Compounds Methylation in Rice and Corn
Animals obtain their share of selenium from their diet. For humans, the main sources of selenium are animal foods and edible plants, primarily in the form of organic selenium compounds. Cereals are the most common plant-based sources of Se in humans worldwide, being consumed on a daily basis as a staple food. Rice and millet are widely consumed in Asia as a staple food, whereas the staple foods in Africa constitute corn, oats and sorghum, and wheat in Europe and America [115,116,117,118]. Compared to animal foods, however, cereals have relatively low Se content [115]. Cereals such as corn and rice generally take up inorganic forms of selenium from the soil. The concentration of selenium in soil is dependent upon various factors such as oxidation-/reduction-favoring conditions, geographical location, soil drainage, and soil pH [119,120]. In alkaline and well-oxidized soils, the most prevalent source of selenium found is selenate, whereas, in well-drained soils with pH ranging from acidic to neutral, the predominant form of inorganic selenium is selenite. Selenite, however, is less bioavailable to plants in soil as compared to selenate due to the greater adsorption affinity of selenite with iron oxides and hydroxides [119]. Rice is grown in flooded conditions, and the resulting paddy soils favor reducing conditions; as such, rice plants mostly take up selenium in the form of selenite.
Group I sulfate transporters (SULTR 1;1 and SULTR 1;2) are most likely involved in the uptake of inorganic selenium in rice and corn plants, whereas inorganic phosphate transporters may be capable of taking up selenite at the root [121,122,123]. Selenate is transported into the chloroplasts of the plants, where it is metabolized into organic selenium. Selenate is initially reduced to selenite (Figure 2), which is further reduced non-enzymatically to the highly reactive species hydrogen selenide. Selenide subsequently enters the synthetic pathway to selenoamino acids, with selenocysteine being the first seleno-organic compound formed in the chloroplasts of the plants [115,120]. However, higher plants such as Arabidopsis thaliana and Oryza sativa (rice) lack both selenoproteins and the Sec (selenocysteine) insertion machinery, which is consistent with evidence that these components were lost during the evolutionary transition from aquatic to terrestrial environments [124,125]. In these plants, Sec is not incorporated into proteins but is instead metabolized into other non-protein selenium compounds. Alternatively, Sec is methylated to form selenomethionine (SeMet), methylselenocysteine (MeSec), or methylselenomethionine (MeSeMet) (Figure 2), with subsequent transformations leading to the formation of volatile dimethylselenide (DMSe) that is released via the stomata to remove excess selenium (Figure 2) [50,115,120]. Moreover, some SeMet and MeSec are translocated to the grains of rice and corn, serving as an organic dietary selenium source (Figure 3) [115].
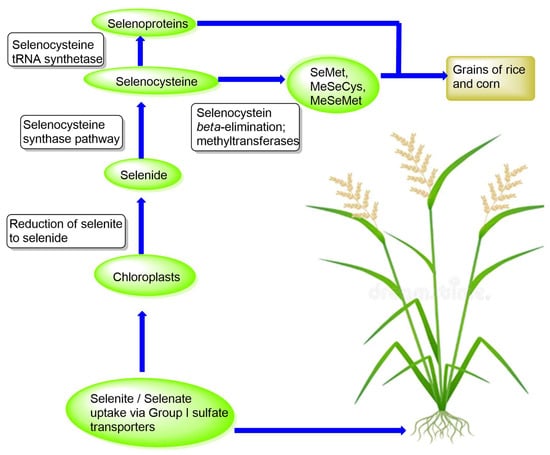
Figure 3.
Overview of uptake and metabolic fate of inorganic selenium in rice and corn plants.
2.5. Chemical Structures’ Impact on Stability and Reactivity
Elemental selenium is relatively nontoxic and can either be oxidized to the +IV oxidation state (selenite) or to the +VI oxidation state (selenate). Alternatively, it can be reduced to the −II oxidation state (selenide), which is the most common reduced form of selenium [126,127,128]. Selenium in living organisms, when metabolized, is always reduced to the selenide form rather than being oxidized. The selenide form of selenium is the one that is most commonly found in organo-selenium species containing C-Se bonds. Because the C-Se bond is weaker (234 kJ/mol bond strength) as compared to the C-S bond (272 kJ/mol bond strength), organo-selenium species are generally more nucleophilic and, hence, more reactive as compared to their organo-sulfur counterparts [129]. The inorganic forms of selenium, i.e., selenates and selenites, are the most mobile and biogeochemical forms of selenium, and are generally more toxic and less bioavailable to humans as compared to organo-selenium compounds [130,131]. Hydrogen selenide is too reactive to be isolated, and inorganic selenite is also considered to be too rapidly metabolized to be possible to quantify [132].
The main organo-selenium compounds are selenols (R-SeH), of which selenocysteine is the most common, selenides (R-Se-R), seleninic acids (R-Se-OOH), selenaheterocyclic compounds, and several more [129]. Selenenic acids (RSe-OH) are highly unstable and will readily and rapidly disproportionate into selenides and seleninic acids, which are usually more stable. As such, selenenic acids are difficult to observe spectroscopically [129]. Selenols are quite reactive and act as strong reducing agents [133]. Selenocysteine, a selenol-containing amino acid, is relatively unstable at physiological pH and can readily decompose or react with other molecules. Despite its instability, it has the ability to reduce hydrogen peroxide [134]. Selenocysteine is the key amino acid located in the active site of the enzyme glutathione peroxidase, which catalyzes the breakdown of hydrogen peroxide [135]. Selenoamino acids such as selenocysteine are converted into selenides, which are generally stable selenides, through the hydrolysis of the carbon–selenium (C–Se) bond at the β-position [134]. In contrast, methylselenoamino acids are first hydrolyzed to form methylselenol. For example, in selenomethionine, this occurs via the cleavage of the C–Se bond at the γ-position, whereas, in methylselenocysteine (MeSec), the bond is cleaved at the β-position [134]. MeSec is hydrolyzed to methylselonol (MeSe at physiological pH) by selenocysteine Se-conjugate β-lyases. Due to its high volatility and reactivity, MeSe has not been identified or isolated; rather, its formation has been monitored indirectly [132]. On the other hand, selenoglutathione (GSSeSG), as well as cysteine-glutathione selenotrisulfide, are both stable enough to be isolated, as they have been detected and quantified in rat liver cytosol and pig epithelial cells homogenates. Furthermore, selenomethionine (SeMet) is stable enough to be administered as a supplemental drug and it has a low toxicity [132]. Seleno-organic compounds have also been found to exhibit several important biological activities. MeSec, SeMet, and other Sec conjugates undergo transamination reactions that result in the production of the seleno-α-keto acids α-keto-γ-methylselenobutyrate and β-methylselenopyruvate [132]. This impacts the molecules’ biological activities in terms of redox activity, and antioxidant as well as prooxidant properties [136]. Selenocyanates (R-Se-CN) are also known to exhibit important biological properties such as antioxidant, anticarcinogenic, and antimutagenic properties [132,137].
2.6. Roles of Biomolecules in Selenium Methylation
The methylation of seleno-compounds would not be possible without the incredibly vital biomolecule S-adenosylmethionine (SAM). This molecule serves as the principal methyl donor in transmethylation reactions, whereby a methyl group is transferred to an acceptor substrate, which, in the case of selenium methylation, is a selenide species. During the transmethylation process, SAM is converted to S-adenosylhomocysteine (SAH), which acts as a competitive inhibitor of SAM-dependent methyltransferases enzyme, leading to its removal by SAH hydrolase enzyme [87,138,139]. The enzyme hydrolyzes SAM to adenosine and homocysteine, which is an important step in the methylation pathway to avoid the inhibition of methyltransferases [140]. SAM also functions as a precursor to the biomolecule glutathione through its conversion to cysteine via the trans-sulfuration pathway [72,138]. Glutathione (GSH) is involved in the reduction of selenite to selenide through the formation of the intermediate selenodiglutathione (GSSeSG), which is subsequently reduced to hydrogen selenide, before being subjected to methylation conditions.
In cereals such as rice and corn, and in microorganisms, the conversion of selenide to selenoamino acids highly necessitates the involvement of the biomolecule selenophosphate [57,66,141,142,143,144]. Bacterial tRNA is first enzymatically ligated with a serine residue to form seryl-tRNA by seryl-tRNA synthetase. Seryl-tRNA then reacts with selenophosphate, whereby the seryl residue of seryl-tRNA, which is bound to pyridoxal phosphate, is converted to a selenocystyl residue and is mediated by the enzyme selenocysteine synthetase [65]. The synthesis of selenophosphate takes place through the combination of ATP with the protein perselenide, which comes from GSSeSG, in the presence of water, resulting in the production of selenophosphate, AMP, and orthophosphate [65]. The selenocystyl-tRNA is then converted to selenocysteine, which is incorporated into proteins or methylated to eventually form DMSe (Scheme 1).
2.7. Environmental and Species-Specific Factors
2.7.1. Participation of Different Selenium Species in Methylation
The chemical form, or speciation, of selenium is important, as it affects the bioavailability, mobility, toxicity, and nutritional value of selenium [132,145,146]. Selenium broadly exists as either inorganic species or as organic species with direct Se-C bonds, depending on the environment/source. In soil and in natural waters, the predominant form of selenium present is the inorganic forms selenate and selenite, as well as elemental selenium, or the Se(-II) species in the form of biselenide (HSe-) [147]. Organic selenium species can also be found. For example, in plankton, selenium can be found in the form of selenoamino acids, and, in marine algae, high concentrations of selenomethionine (SeMet) have been found [130]. In plants, fungi, and bacteria, the predominant uptake of inorganic forms of selenium leads to methylation, forming less toxic methylated seleno-compounds such as the excretory metabolites DMSe and TMSe+ (Figure 2).
In foods commonly consumed by humans—particularly grains, legumes, and vegetables—selenium predominantly occurs in its organic forms. The most frequently encountered organic selenium species include selenomethionine (SeMet), selenocysteine (Sec), and methylselenocysteine (MeSec) [132,148]. The distribution and concentration of these compounds vary across different plant species. For instance, plants from the Allium and Brassicaceae (formerly Cruciferae) families tend to accumulate higher levels of MeSec than SeMet, while cereal crops generally contain significant quantities of SeMet [132,149,150,151,152]. Animal-derived foods are richer in Sec. It is important to note that selenium compounds exhibit a dual role in biological systems, especially in the context of cancer. Depending on the dose, chemical form, and cellular environment, selenium can act as either an antioxidant—enhancing cellular defense against oxidative stress—or a pro-oxidant—inducing oxidative damage and apoptosis in malignant cells [132,148]. This dichotomy underscores the need for caution when interpreting selenium’s role in cancer prevention or therapy, particularly as certain selenium species may have both protective and harmful effects, depending on the biochemical context. Thus, organic forms of selenium are more readily bioavailable to humans and animals as compared to the inorganic forms of selenium [153,154,155]. A significant amount of evidence relates to the participation of these methylated organic forms of selenium towards disease prevention. In particular, studies have found that MeSec and methylseleninic acid (MeSeA) exhibit significant roles in the control of cancerous tumors by inhibiting the progression and metastasis of prostate cancers and lung carcinoma in mice [132]. Even though humans mostly take up organic forms of selenium, both inorganic as well as organic forms of selenium can be utilized by the body [156]. They are reduced to the key intermediate hydrogen selenide before being transformed into selenophosphates and selenocystyl-tRNA for incorporation into proteins. Excess selenium is then subjected to the methylation pathway and excreted as volatile DMSe in breath or as TMSe+ in urine.
2.7.2. Environmental Conditions for Selenium Methylation in Rice and Corn
For cereals such as rice and corn, an adequate supply of water is necessary to support the growth of the plants and maintain productive yields. However, in arid and semi-arid regions—particularly in developing countries facing on-going climate challenges—irregular rainfall and drought conditions impose significant stress on maize. Drought stress inhibits key metabolic processes. leading to stunted growth and reduced yields. Maize plants respond to such conditions by altering their protein synthesis pattern, producing stress-responsive proteins that enhance tolerance [157,158,159,160,161,162]. Notably, selenium supplementation has been associated with increased levels of the amino acid proline, which contributes to drought tolerance by maintaining cell turgor and rigidity and reducing the increased levels of radical oxygen species brought about, reducing oxidative stress and supporting cellular integrity [157,163].
Selenium has also been found to encourage drought stress tolerance in maize by limiting the reduction in plant biomass due to water shortage and by limiting the oxidative damage by enhancing the plant’s antioxidant defense system, thus improving the yield and quality of maize produced under water-deficit conditions [157,164,165]. Similarly, selenium application attenuates the effects of water stress on rice plants, enabling rice to grow to its full or almost complete potential, even under conditions of drought [166,167,168]. Selenium improves the plants’ CO2 assimilation rate, their transpiration rate, water use efficiency, and, in general, their antioxidant-defense mechanism [166]. During these processes, selenium is metabolized as described previously; some is converted into Sec and some into SeMet. Although Sec plays a key role in selenium metabolism, its presence in cereals is typically negligible, as noted by Bocchini et al. [157]. When excess selenium is present, any Sec that is formed is rapidly methylated to non-toxic forms such as methyl-Sec and Se-methylselenocysteine (SeMeSec) [157], which are not incorporated into proteins and can be safely accumulated in plant tissues. These methylated compounds may serve as precursors to volatile excretory metabolites like dimethylselenide (DMSe) and trimethylselenonium ion (TMSe+) (Figure 2). Selenium may also play a significant role in the uptake of certain contaminants, as occurs, for instance, with the rice plants. Even though rice is consumed in most parts of the world and is one of the most common sources of selenium, it is also inadvertently a source of mercury, especially when grown in mercury-contaminated soils [169,170,171]. In a study conducted by Zhang et al. [172], it was found that an insoluble Hg-Se covalent complex was formed in the rhizospores/roots of rice plants treated with selenium, which subsequently affected the translocation of mercury up to the aerial parts of the plant. One possible explanation of this phenomenon is that, by forming an insoluble complex with mercury, either through the reaction of elemental Hg with elemental Se, or through the reaction of Hg2+ with Se2−, the selenium in the soil eventually leads to a decrease in the bioavailability of Hg2+ in the soil. A decrease in the bioavailability of Hg2+ consequently leads to a decrease or inhibition of mercury methylation in soil by microorganisms. The result is a decrease in the uptake of Hg2+ and MeHg through the roots of the plant [172].
3. Conclusions
This review offers a thorough examination of selenium methylation, elucidating the biochemical processes crucial for managing selenium toxicity and modulating its biological effects. It outlines the key chemical reactions, enzymatic pathways, and environmental impacts involved in converting excess selenium into less harmful forms. Key findings include a detailed analysis of the chemical reactions, such as the reduction of selenium species to hydrogen selenide and its subsequent methylation to monomethylselenide (MMSe) and dimethylselenide (DMSe). The roles of critical enzymes, including glutathione reductase and SAM-dependent methyltransferases, are highlighted, along with the function of selenocysteine lyase in processing organic selenium compounds. The manuscript also explores how plants like rice and corn manage selenium through methylation, detailing how soil conditions affect selenium uptake and metabolism. The review identifies current knowledge and gaps in the understanding of molecular mechanisms and environmental factors influencing selenium methylation efficiency. It also summarizes how agricultural practices may affect selenium uptake and detoxification, with implications for food safety and plant nutrition. The review also identifies areas requiring further investigation, such as the molecular mechanisms and environmental factors influencing methylation efficiency. Consideration is given to agricultural practices that may optimize selenium management in crops, emphasizing the importance of soil conditions and selenium availability. In summary, this review synthesizes current findings on selenium methylation and provides a foundation for future research relevant to health, agriculture, and environmental management.
Author Contributions
Conceptualization, A.T.M. and F.J.; methodology, A.T.M. and F.J.; software, A.T.M.; validation, A.T.M., F.J., L.K.B., P.N., S.N., N.J.K. and D.C.T.; formal analysis, A.T.M.; investigation, F.J.; resources, A.T.M.; data curation, S.N.; writing—original draft preparation, F.J. and F.J.; writing—review and editing, A.T.M., F.J., L.K.B., P.N., S.N., N.J.K. and D.C.T.; visualization, A.T.M., F.J., L.K.B., P.N., S.N., N.J.K. and D.C.T.; supervision, A.T.M.; project administration, F.J.; funding acquisition, A.T.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No new data were created.
Acknowledgments
The authors express profound gratitude to Natural Resources College (NRC) for the financial support of the study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Schwarz, K.; Foltz, C.M. Selenium as an Integral Part of Factor 3 Against Dietary Necrotic Liver Degeneration. J. Am. Chem. Soc. 1957, 79, 3292–3293. [Google Scholar] [CrossRef]
- Hariharan, S.; Dharmaraj, S. Selenium and selenoproteins: It’s role in regulation of inflammation. Inflammopharmacology 2020, 28, 667–695. [Google Scholar] [CrossRef]
- Shahidin; Wang, Y.; Wu, Y.; Chen, T.; Wu, X.; Yuan, W.; Zhu, Q.; Wang, X.; Zi, C. Selenium and Selenoproteins: Mechanisms, Health Functions, and Emerging Applications. Molecules 2025, 30, 437. [Google Scholar] [CrossRef] [PubMed]
- Batyrova, G.; Taskozhina, G.; Umarova, G.; Umarov, Y.; Morenko, M.; Iriskulov, B.; Kudabayeva, K.; Bazargaliyev, Y. Unveiling the Role of Selenium in Child Development: Impacts on Growth, Neurodevelopment and Immunity. J. Clin. Med. 2025, 14, 1274. [Google Scholar] [CrossRef] [PubMed]
- Cilla, A.; Barberá, R.; Lagarda, M.J.; Farré, R. Nutriential Hazards: Micronutrients: Vitamins and Minerals. Encycl. Food Saf. 2014, 3, 86–94. [Google Scholar] [CrossRef]
- Zhang, F.; Li, X.; Wei, Y. Selenium and Selenoproteins in Health. Biomolecules 2023, 13, 799. [Google Scholar] [CrossRef]
- Kosnett, M.; Chan, L.; Faustman, E.; Foldy, S.; Miller, G.; Takaro, T.; Ahmed, S.; Allen, P.; Boehme, J.; Gabriel, M.; et al. A Review of Human Health Impacts of Selenium in Aquatic Systems. A Report Submitted to the International Joint Commission by the Health Professionals Advisory Board. 2020. Available online: https://ijc.org/sites/default/files/2020-09/HPAB_SeleniumHealthReview_2020.pdf (accessed on 15 July 2025).
- Antonyak, H.; Malavolta, M.; Mocchegiani, E. Healthy Ageing and Longevity. In Trace Elements and Minerals in Health and Longevity; Malavolta, M., Mocchegiani, E., Eds.; Springer: Berlin/Heidelberg, Germany, 2018; Volume 8. [Google Scholar]
- Agarwal, P.; Mehta, R.; Panse, G.; Patil, S. Selenium toxicity: A rare diagnosis. Indian J. Dermatol. Venereol. Leprol. 2016, 82, 690–693. [Google Scholar] [CrossRef]
- Mehdi, Y.; Hornick, J.-L.; Istasse, L.; Dufrasne, I. Selenium in the environment, metabolism and involvement in body functions. Molecules 2013, 18, 3292–3311. [Google Scholar] [CrossRef]
- Misra, S.; Boylan, M.; Selvam, A.K.; Spallholz, J.E.; Björnstedt, M.; Fernandes, A.P. Redox-active selenium compounds—From toxicity and cell death to cancer treatment. Nutrients 2015, 7, 3536–3556. [Google Scholar] [CrossRef]
- Grosicka-Maciąg, E.; Kurhaluk, N.; Zielińska, A.; Skrajnowska, D. Biomedical effects of selenium in a human organism. J. Elem. 2017, 22, 1269–1284. [Google Scholar] [CrossRef]
- Qi, Z.; Duan, A.; Ng, K. Selenoproteins in Health. Molecules 2023, 29, 136. [Google Scholar] [CrossRef]
- Hodges, R.E.; Minich, D.M. Modulation of Metabolic Detoxification Pathways Using Foods and Food-Derived Components: A Scientific Review with Clinical Application. J. Nutr. Metab. 2015, 2015, 760689. [Google Scholar] [CrossRef]
- Hassoun, B.S.; Stohs, S.J.; Bagchi, D. Selenium detoxification by methylation. Res. Commun. Mol. Pathol. Pharmacol. 1995, 90, 133–142. [Google Scholar] [PubMed]
- Fukumoto, Y.; Rin Kyono, R.; Shibukawa, Y.; Tanaka, Y.; Suzuki, N.; Ogra, Y. Differential molecular mechanisms of substrate recognition by selenium methyltransferases, INMT and TPMT, in selenium detoxification and excretion. J. Biol. Chem. 2024, 300, 105599. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; He, F.; Li, L.; Guo, L.; Zhang, B.; Yu, S.; Zhao, W. Bioavailability Based on the Gut Microbiota: A New Perspective. Microbiol. Mol. Biol. Rev. 2020, 84, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.L.U.; Sena-Evangelista, K.C.M.; de Azevedo, E.P.; Pinheiro, F.I.; Ney Cobucci, R.; Pedrosa, L.F.C. Selenium in Human Health and Gut Microflora: Bioavailability of Selenocompounds and Relationship With Diseases. Front. Nutr. 2021, 8, 685317. [Google Scholar] [CrossRef]
- Ferenc, K.; Śliżewska, K.; Czech, A.; Grela, E.R. Modulation of the Gut Microbiota by Nutrition and Its Relationship to Epigenetics. Int. J. Mol. Sci. 2024, 25, 1228. [Google Scholar] [CrossRef]
- Paul, B.; Barnes, S.; Demark-Wahnefried, W.; Morrow, C.D.; Salvador, C.; Skibola, C.F.; Tollefsbol, T.O. Influences of Diet and the Gut Microbiome on Epigenetic Modulation in Cancer and Other Diseases. Clin. Epigenetics 2015, 7, 112. [Google Scholar] [CrossRef]
- Genchi, G.; Lauria, G.; Catalano, A.; Carocci, A.; Sinicropi, M.S. Biological Activity of Selenium and Its Impact on Human Health. Int. J. Mol. Sci. 2023, 24, 2663. [Google Scholar] [CrossRef]
- Xu, M.; Li, S.; Chen, X.; Wang, J.; Qian, W.; Zhang, Y. Pivotal Biological Processes and Proteins for Selenite Reduction and Methylation in Ganoderma lucidum. J. Hazard. Mater. 2023, 444, 130409. [Google Scholar] [CrossRef]
- Chen, M.; Nie, Y.; Xu, Y.; Li, X.; Xu, Y.; Zang, J.; Zhang, Y.; Zhang, H.; Wu, Z.; Yang, J. Identification and Functional Characterization of a Novel Selenocysteine Methyltransferase from Brassica juncea. J. Exp. Bot. 2019, 70, 6401–6416. [Google Scholar] [CrossRef]
- Ullah, H.; Ullah, W.; Hussain, A.; Khan, M.; Bilal, M.; Mehmood, S.; Mahmood, Q. A Comprehensive Review on Environmental Transformation of Selenium: Recent Advances and Research Perspectives. Environ. Geochem. Health 2019, 41, 1003–1035. [Google Scholar] [CrossRef]
- Fukumoto, Y.; Masuda, H.; Kamiyama, S.; Matsumoto, A.; Nakanishi, H.; Endo, Y.; Ohkubo, T.; Terao, T. Production of a Urinary Selenium Metabolite, Trimethylselenonium, by Thiopurine S-Methyltransferase and Indolethylamine N-Methyltransferase. Chem. Res. Toxicol. 2021, 33, 2467–2474. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Liu, C.; He, W.; Zhang, X.; Wang, H.; Jin, C.; Liu, J. Unveiling the Vital Role of Soil Microorganisms in Selenium Cycling: A Review. Front. Microbiol. 2024, 15, 1448539. [Google Scholar] [CrossRef] [PubMed]
- Mlangeni, A.T.; Jagot, F.; Namaumbo, S.; Kapito, N.J.; Tsukuluza, D.C.; Botha, L.; Ndovi, P.; Kumambala, P. Analytical Strategies for Quantifying Methylated Selenium Species in Staple Crops: Methods and Emerging Techniques. Chin. J. Anal. Chem. 2025, 53, 100511. [Google Scholar] [CrossRef]
- Reich, H.J.; Hondal, R.J. Why Nature Chose Selenium. ACS Chem. Biol. 2016, 11, 821–841. [Google Scholar] [CrossRef]
- Madabeni, A.; Bortoli, M.; Nogara, P.A.; Ribaudo, G.; Dalla Tiezza, M.; Flohé, L.; Rocha, J.B.T.; Orian, L. 50 years of organoselenium chemistry, biochemistry and reactivity: Mechanistic understanding, successful and controversial stories. Chem. A Eur. J. 2024, 30, e202403003. [Google Scholar] [CrossRef]
- Orian, L.; Flohé, L. Selenium-catalyzed reduction of hydroperoxides in chemistry and biology. Antioxidants 2021, 10, 1560. [Google Scholar] [CrossRef]
- Naga Jyothi, M.S.V.; Ramaiah, B.J.; Maliyekkal, S.M. Occurrence, contamination, speciation and analysis of selenium in the environment. In Measurement, Analysis and Remediation of Environmental Pollutants; Springer Nature: Berlin/Heidelberg, Germany, 2020; pp. 245–269. [Google Scholar] [CrossRef]
- Sigel, H.; Sigel, A. The bio-relevant metals of the periodic table of the elements. Z. Für Naturforschung B 2019, 74, 461–471. [Google Scholar] [CrossRef]
- Rayman, M.P. Selenium intake, status, and health: A complex relationship. Hormones 2020, 19, 9–14. [Google Scholar] [CrossRef]
- Sun, H.J.; Rathinasabapathi, B.; Wu, B.; Luo, J.; Pu, L.-P.; Ma, L.Q. Arsenic and selenium toxicity and their interactive effects in humans. Environ. Int. 2014, 69, 148–158. [Google Scholar] [CrossRef]
- Mangiapane, E.; Pessione, A.; Pessione, E. Selenium and Selenoproteins: An Overview on Different Biological Systems. Curr. Protein Pept. Sci. 2014, 15, 598–607. [Google Scholar] [CrossRef]
- Dolgova, N.V.; Hackett, M.J.; MacDonald, T.C.; Nehzati, S.; James, A.K.; Krone, P.H.; George, G.N.; Pickering, I.J. Distribution of selenium in zebrafish larvae after exposure to organic and inorganic selenium forms. Metallomics 2016, 8, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Surai, P.F.; Kochish, I.I.; Fisinin, V.I.; Velichko, O.A. Selenium in poultry nutrition: From sodium selenite to organic selenium sources. J. Poult. Sci. 2018, 55, 79–93. [Google Scholar] [CrossRef] [PubMed]
- Vriens, B.P. The Role of Methylation in the Biogeochemical Selenium Cycle. Ph.D. Thesis, ETH Zurich, Zurich, Switzerland, 2015. Available online: https://www.research-collection.ethz.ch/bitstream/handle/20.500.11850/155269/2/ETH22919.pdf (accessed on 25 February 2025).
- Eswayah, A.S.; Smith, T.J.; Gardiner, P.H.E. Microbial Transformations of Selenium Species of Relevance to Bioremediation. Appl. Environ. Microbiol. 2016, 82, 4848–4859. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, U.D.; Rana, M.; Chakrapani, H. Phenacylselenoesters allow facile selenium transfer and hydrogen selenide generation. Chem. Sci. 2024, 15, 19315–19321. [Google Scholar] [CrossRef]
- Shalini, S.; Bansal, M.P. Co-operative effect of glutathione depletion and selenium induced oxidative stress on AP1 and NFκB expression in testicular cells in vitro: Insights to regulation of spermatogenesis. Biol. Res. 2007, 40, 307–317. [Google Scholar] [CrossRef]
- Paydary, P.; Schellenger, A.E.P.; Teli, M.; Jaisi, D.P.; Onnis-Hayden, A.; Larese-Casanova, P. Chemical oxidation of selenite to selenate: Evaluation of reactive oxygen species and O transfer pathways. Chem. Geol. 2021, 575, 120229. [Google Scholar] [CrossRef]
- Ting, K.K.Y.; Black, A.J.; Zheng, S.; Nguyen, T.; Lui, J.; Le, V.; Day, B.J.; Stocker, R.; Sobey, C.G.; Drummond, G.R.; et al. Measuring the rate of NADPH consumption by glutathione reductase in the cytosol and mitochondria. PLoS ONE 2024, 19, e0309886. [Google Scholar] [CrossRef]
- Koju, N.; Song, J.; You, J.; Lim, S.; Park, S.; Jo, J.; Kim, D.K.; Lee, J. Reduced nicotinamide adenine dinucleotide phosphate in redox balance and diseases: A friend or foe? Acta Pharmacol. Sin. 2022, 43, 1889–1904. [Google Scholar] [CrossRef]
- Gansemer, E.R.; Rutkowski, D.T. Pathways Linking Nicotinamide Adenine Dinucleotide Phosphate Production to Endoplasmic Reticulum Protein Oxidation and Stress. Front. Mol. Biosci. 2022, 9, 858142. [Google Scholar] [CrossRef]
- Spagnoletta, A.; Pastore, D.; Tozzi, M.G.; Pierri, C.L. Modulatory Effect of Nicotinamide Adenine Dinucleotide Phosphate (NADPH) on the 2-Oxoglutarate Mitochondrial Carrier. Molecules 2024, 29, 5154. [Google Scholar] [CrossRef] [PubMed]
- Kuganesan, M.; Haque, M.E.; Kageyama, Y.; Kar, S.; Saif, S.R.; Shimizu, T.; Tomita, K.; Fukuto, J.M.; Nagasawa, H.; Akaike, T. Selenium and hydrogen selenide: Essential micronutrient and the fourth gasotransmitter? Intensive Care Med. Exp. 2019, 7, 71. [Google Scholar] [CrossRef]
- Peyroche, G.; Saveanu, C.; Dauplais, M.; Lazard, M.; Beuneu, F.; Decourty, L.; Malabat, C.; Jacquier, A.; Blanquet, S.; Plateau, P. Sodium selenide toxicity is mediated by O2-dependent DNA breaks. PLoS ONE 2012, 7, e36343. [Google Scholar] [CrossRef] [PubMed]
- Zwolak, I.; Zaporowska, H. Selenium interactions and toxicity: A review. Cell Biol. Toxicol. 2012, 28, 31–46. [Google Scholar] [CrossRef]
- Wang, F.; Chen, Z.; Du, S.; Tan, Y.; Wang, L.; Zhang, Z.; Li, Y.; Li, L. Selenium volatilization in plants, microalgae, and microorganisms. Heliyon 2024, 10, e26023. [Google Scholar] [CrossRef]
- Nguyen, T.-Q.; Nicolet, Y. Structure and Catalytic Mechanism of Radical SAM Methylases. Life 2022, 12, 1732. [Google Scholar] [CrossRef]
- Zhang, C.; Weng, J.; Liu, X.; Liu, H.; Deng, Z.; Qu, X.; Zhang, Q. Biotechnological applications of S-adenosyl-methionine-dependent methyltransferases for natural products biosynthesis and diversification. Bioresour. Bioprocess. 2021, 8, 72. [Google Scholar] [CrossRef]
- Lin, H. S-Adenosylmethionine-dependent alkylation reactions: When are radical reactions used? Bioorganic Chem. 2021, 39, 161–170. [Google Scholar] [CrossRef]
- Taylor, D.; Reilly, C.; Burk, R. Recent developments in selenium research. Br. J. Biomed. Sci. 2009, 66, 107–116. [Google Scholar] [CrossRef]
- Rayman, M.P.; Infante, H.G.; Sargent, M. Food-chain selenium and human health: Spotlight on speciation. Br. J. Nutr. 2008, 100, 238–253. [Google Scholar] [CrossRef]
- Kang, D.; Lee, J.; Jung, Y.; Zhang, X.; Lee, Y.J.; Choi, Y.; Lee, S.; Kim, Y.; Lee, E.B.; Bae, S.C.; et al. The role of selenium metabolism and selenoproteins in cartilage homeostasis and arthropathies. Exp. Mol. Med. 2020, 52, 1198–1208. [Google Scholar] [CrossRef]
- Minich, W.B. Selenium Metabolism and Biosynthesis of Selenoproteins in the Human Body. Biochemistry 2022, 87, S168–S177. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, C.; Yao, Q.; Li, J. Role of selenoproteins in redox regulation of signaling and the antioxidant system: A review. Antioxidants 2020, 9, 383. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Wang, L.; Li, J.; Zhang, Y. Effects and Impact of Selenium on Human Health, A Review. Molecules 2024, 30, 50. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Wang, C. Factors affecting selenium-enrichment efficiency, metabolic mechanisms and physiological functions of selenium-enriched lactic acid bacteria. J. Future Foods 2022, 2, 285–293. [Google Scholar] [CrossRef]
- Schneider, H.A. Selenium in Nutrition. Science 1936, 83, 32–34. [Google Scholar] [CrossRef]
- Li, Z.; Liang, D.; Peng, Q.; Cui, Z.; Huang, Q. Interaction between selenium and soil organic matter and its impact on soil selenium bioavailability: A review. Geoderma 2017, 295, 69–79. [Google Scholar] [CrossRef]
- Shini, S.; Sultan, A.; Bryden, W.L.; Downing, J. Selenium Biochemistry and Bioavailability: Implications for Animal Agriculture. Agriculture 2015, 5, 1277–1288. [Google Scholar] [CrossRef]
- Mutonhodza, B.; Joy, E.J.M.; Broadley, M.R.; King, J.C.; Watts, M.J. Linkages between soil, crop, livestock, and human selenium status in Sub-Saharan Africa: A scoping review. Int. J. Food Sci. Technol. 2022, 57, 6336–6349. [Google Scholar] [CrossRef]
- Chasteen, T.G.; Bentley, R. Biomethylation of Selenium and Tellurium: Microorganisms and Plants. Chem. Rev. 2003, 103, 1–26. [Google Scholar] [CrossRef]
- Hasegawa, T.; Suzuki, K.T. Inorganic compounds Mechanisms of Selenium Methylation and Toxicity in Mice Treated with Selenocystine. Arch. Toxicol. 1996, 71, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Nakamuro, K.; Sayato, Y.; Yamanaka, K.; Yamane, Y. Metabolism of Selenoamino Acids and Their Contributionof Selenium Methylation to Their Toxicity. J. Health Sci. 2000, 46, 418–421. [Google Scholar] [CrossRef]
- Fernandes, A.P.; Wallenberg, M.; Gandin, V.; Misra, S.; Tisato, F.; Marzano, C.; Rigobello, M.P.; Kumar, S.; Björnstedt, M. Methylselenol Formed by Spontaneous Methylation of Selenide Is a Superior Selenium Substrate to the Thioredoxin and Glutaredoxin Systems. PLoS ONE 2012, 7, e50727. [Google Scholar] [CrossRef] [PubMed]
- Chau, Y.K.; Wong, P.T.S.; Silverberg, B.A.; Luxon, P.L.; Bengert, G.A. Methylation of selenium in the aquatic environment. Science 1976, 192, 1130–1131. [Google Scholar] [CrossRef]
- Ohta, Y.; Suzuki, K.T. Methylation and demethylation of intermediates selenide and methylselenol in the metabolism of selenium. Toxicol. Appl. Pharmacol. 2008, 226, 169–177. [Google Scholar] [CrossRef]
- Couto, N.; Wood, J.; Barber, J. The role of glutathione reductase and related enzymes on cellular redox homoeostasis network. Free Radic. Biol. Med. 2016, 95, 27–42. [Google Scholar] [CrossRef]
- Meister, A.; Anderson, M.E. Glutathione. Annu. Rev. Biochem. 1983, 52, 711–760. [Google Scholar] [CrossRef]
- Vaškov, J.; Trudičová, M.; Dobrota, D. Glutathione-Related Enzymes and Proteins: A Review. Molecules 2023, 28, 1447. [Google Scholar] [CrossRef]
- Smith, I.K.; Vierheller, T.L.; Thorne, C.A. Properties and functions of glutathione reductase in plants. Physiol. Plant. 1989, 77, 449–456. [Google Scholar] [CrossRef]
- Nuttall, K.L. Elemental selenium and glutathione reductase. Med. Hypotheses 1985, 16, 155–158. [Google Scholar] [CrossRef] [PubMed]
- Rubino, F.M. Toxicity of glutathione-binding metals: A review of targets and mechanisms. Toxics 2015, 3, 20–62. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, K.; Nakaki, T. Glutathione in Cellular Redox Homeostasis: Association with the Excitatory Amino Acid Carrier 1 (EAAC1). Molecules 2015, 20, 8742–8758. [Google Scholar] [CrossRef]
- Lushchak, V.I. Glutathione Homeostasis and Functions: Potential Targets for Medical Interventions. J. Amino Acids 2012, 2012, 736837. [Google Scholar] [CrossRef] [PubMed]
- Csiszár, J.; Horváth, E.; Bela, K.; Gallé, Á.; Erdei, L.; Györgyey, J.; Brunner, S.; Tari, I. Glutathione-Related Enzyme System: Glutathione Reductase (GR), Glutathione Transferases (GSTs) and Glutathione Peroxidases (GPXs). In Redox State as a Central Regulator of Plant-Cell Stress Responses; Springer: Berlin/Heidelberg, Germany, 2016; pp. 137–158. [Google Scholar] [CrossRef]
- Zhi, Y.; Qin, Y.; Wang, L.; Chen, Y.; Hu, X.; Yu, Z.; Li, Y. Glutathione reductase modulates endogenous oxidative stress and affects growth and virulence in Avibacterium paragallinarum. Vet. Res. 2025, 56, 1. [Google Scholar] [CrossRef]
- Aquilano, K.; Baldelli, S.; Ciriolo, M.R. Glutathione: New roles in redox signaling for an old antioxidant. Front. Pharmocology 2014, 5, 196. [Google Scholar] [CrossRef]
- Armutcu, F.; Akyol, S.; Ustunsoy, S.; Turan, F.F. The interaction of glutathione and thymoquinone and their antioxidant properties. Electron. J. Gen. Med. 2018, 15, 4–11. [Google Scholar] [CrossRef]
- Matuz-Mares, D.; Hernández-López, V.M.; Becerra, A.; Vázquez-Meza, H. Glutathione participation in the prevention of cardiovascular diseases. Antioxidants 2021, 10, 1220. [Google Scholar] [CrossRef]
- Lubos, E.; Loscalzo, J.; Handy, D.E. Glutathione Peroxidase-1 in Health and Disease: From molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal. 2011, 15, 1957–1997. [Google Scholar] [CrossRef]
- Klinkhammer, C.; Matthes, R.; Stufler, S.; Barton, A.; von Woedtke, T.; Weltmann, K.-D.; Partecke, L.I.; Masur, K.; Harms, M. Elucidation of Plasma-induced Chemical Modifications on Glutathione and Glutathione Disulphide. Sci. Rep. 2017, 7, 13828. [Google Scholar] [CrossRef]
- Abdelraheem, E.M.M.; Busch, F.; Hanefeld, U.; Hollmann, F. Methyltransferases: Functions and applications. ChemBioChem 2022, 23, e202200212. [Google Scholar] [CrossRef] [PubMed]
- Liscombe, D.K.; Louie, G.V.; Noel, J.P. Architectures, mechanisms and molecular evolution of natural product methyltransferases. Nat. Prod. Rep. 2012, 29, 1238–1250. [Google Scholar] [CrossRef] [PubMed]
- Grillo, M.A.; Colombatto, S. S-adenosylmethionine and its products. Amino Acids 2008, 34, 187–193. [Google Scholar] [CrossRef]
- Esaki, N.; Soda, K.; Tanaka, H.; Kojima, Y.; Yamauchi, K.; Tani, Y.; Suda, H. Selenocysteine lyase, a novel enzyme that specifically acts on selenocysteine. Mammalian distribution and purification and properties of pig liver enzyme. J. Biol. Chem. 1982, 257, 4386–4391. [Google Scholar] [CrossRef]
- Esaki, N.; Soda, K.; Suda, H. Mechanism of Reactions Catalyzed by Selenocysteine β-Lyase. Arch. Biochem. Biophys. 1985, 238, 418–423. [Google Scholar] [CrossRef]
- Mihara, H.; Esaki, N. Selenocysteine lyase: Mechanism, structure, and biological role. In Selenium: Its Molecular Biology and Role in Human Health; Springer: New York, NY, USA, 2012; pp. 95–105. [Google Scholar]
- Mihara, H.; Esaki, N. Mechanism, structure, and biological role of selenocysteine lyase. In Selenium: Its Molecular Biology and Role in Human Health, 4th ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 113–123. [Google Scholar]
- Suzuki, K.T.; Ogra, Y.; Imura, N. Selenocysteine β-lyase and methylselenol demethylase in the metabolism of Se-methylated selenocompounds into selenide. Biochim. Et Biophys. Acta Gen. Subj. 2007, 1770, 1053–1061. [Google Scholar] [CrossRef]
- Seale, L.A. Selenocysteine β-lyase: Biochemistry, regulation and physiological role of the selenocysteine decomposition enzyme. Antioxidants 2019, 8, 357. [Google Scholar] [CrossRef]
- Omi, R.; Sakakibara, Y.; Yamaguchi, Y.; Hatakeyama, T.; Fujisawa, K.; Esaki, N.; Suzuki, T. Reaction mechanism and molecular basis for selenium/sulfur discrimination of selenocysteine lyase. J. Biol. Chem. 2010, 285, 12133–12139. [Google Scholar] [CrossRef]
- Seale, L.A.; Torres, S.; Berry, M.J.; Burk, R.F.; Van Den Veyver, I.B.; Ogra, Y.; Hoffmann, V.; Schweizer, U.; Sunde, R.A. Relationship between selenoprotein P and selenocysteine lyase: Insights into selenium metabolism. Free Radic. Biol. Med. 2018, 127, 182–189. [Google Scholar] [CrossRef]
- Seale, L.A.; Torres, S.; Schweizer, U.; Berry, M.J.; Burk, R.F.; Sunde, R.A. Disruption of the Selenocysteine Lyase-Mediated Selenium Recycling Pathway Leads to Metabolic Syndrome in Mice. Mol. Cell. Biol. 2012, 32, 4141–4154. [Google Scholar] [CrossRef]
- De Souza, M.P.; Pilon-Smits, E.A.; Terry, N. Rate-Limiting Steps in Selenium Assimilation and Volatilization by Indian Mustard 1. Plant Physiol. 1988, 117, 1487–1494. [Google Scholar] [CrossRef] [PubMed]
- Pilon-Smits, E.A.; Quinn, C.F.; Tappero, R.; Cotter-Howells, J.; Hwang, S.; Lytle, C.M.; Pilon, M. Physiological functions of beneficial elements. Curr. Opin. Plant Biol. 2009, 12, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Skrypnik, L.; Skrypnik, K.; Medvedeva, I.; Ederli, L.; Inzaghi, C.; Pardossi, A. The Integral Boosting Effect of Selenium on the Secondary Metabolism of Higher Plants. Plants 2022, 11, 3432. [Google Scholar] [CrossRef] [PubMed]
- Ganther, H.E. Enzymic Synthesis of Dimethyl Selenide from Sodium Selenite in Mouse Liver Extracts. Biochemistry 1966, 5, 1089–1098. [Google Scholar] [CrossRef]
- Cummins, L.M.; Kimura, E.T. Safety evaluation of selenium sulfide antidandruff shampoos. Toxicol. Appl. Pharmacol. 1971, 20, 89–96. [Google Scholar] [CrossRef]
- Pletnikova, I.P. Biological effect and tolerance level of Se in drinking water. Gig. Sanit. 1970, 2, 14–19. [Google Scholar]
- Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for Selenium; Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 2003. Available online: https://www.ncbi.nlm.nih.gov/books/NBK600364/ (accessed on 12 February 2025).
- Sayato, Y.; Fukuda, M.; Fujii, M.; Kurokawa, Y.; Saito, H. Acute and subacute oral toxicity of selenocystine in mice. Eisei Kagaku 1993, 39, 289–296. [Google Scholar] [CrossRef]
- Power, R.; Lyons, T.P.; Jacques, K.A. (Eds.) English, Book chapter Conference paper, UK, Stamford, Nutritional biotechnology in the feed and food industries. In Proceedings of the Alltech’s 21st Annual Symposium, Lexington, KY, USA, 22–25 May 2005; pp. 135–145. [Google Scholar]
- McConnell, K.P.; Portman, O.W. Toxicity of dimethyl selenide in the rat and mouse. Proc. Soc. Exp. Biol. Med. 1952, 79, 230–231. [Google Scholar] [CrossRef]
- Obermeyer, B.D.; Sultatos, L.G.; Ganther, H.E.l. Toxicity of trimethylselenonium chloride in the rat with and without arsenite. Toxicol. Appl. Pharmacol. 1971, 20, 135–146. [Google Scholar] [CrossRef]
- Wilber, C.G. Toxicology of selenium: A review. Clin. Toxicol. 1980, 17, 171–230. [Google Scholar] [CrossRef]
- Hoffman, J.L. Selenite Toxicity, Depletion of Liver S-Adenosylmethionine, and Inactivation of Methionine Adenosyltransferase. Arch. Biochem. Biophys. 1977, 179, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Hadrup, N.; Ravn-Haren, G. Acute human toxicity and mortality after selenium ingestion: A review. J. Trace Elem. Med. Biol. 2020, 58, 126435. [Google Scholar] [CrossRef] [PubMed]
- Hadrup, N.; Ravn-Haren, G. Toxicity of repeated oral intake of organic selenium, inorganic selenium, and selenium nanoparticles: A review. J. Trace Elem. Med. Biol. 2023, 79, 127235. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Jia, X. Safety evaluation of Se-methylselenocysteine as nutritional selenium supplement: Acute toxicity, genotoxicity and subchronic toxicity. Regul. Toxicol. Pharmacol. 2014, 70, 720–727. [Google Scholar] [CrossRef]
- Jacevic, V.; Djordjevic, A.; Petrovic, D.; Vucinic, S. Acute toxicity of sodium selenite in rodents: Pathomorphological study. Mil. Med. Sci. Lett. 2011, 80, 90–96. [Google Scholar] [CrossRef]
- Xie, M.; Wang, Y.; Zhang, F.; Mao, J.; Zhang, Y.; Li, Y. Selenium in cereals: Insight into species of the element from total amount. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2914–2940. [Google Scholar] [CrossRef]
- Rayman, M.P. Selenium and human health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef]
- Rayman, M.P. The importance of selenium to human health. Lancet 2000, 356, 233–241. [Google Scholar] [CrossRef]
- Soetan, K.O.; Olaiya, C.O.; Oyewole, O.E. The importance of mineral elements for humans, domestic animals and plants: A review. Afr. J. Food Sci. 2010, 4, 200–222. [Google Scholar]
- Zhu, Y.G.; Pilon-Smits, E.A.H.; Zhao, F.J.; Williams, P.N.; Meharg, A.A. Selenium in higher plants: Understanding mechanisms for biofortification and phytoremediation. Trends Plant Sci. 2009, 14, 436–442. [Google Scholar] [CrossRef]
- Zhang, L.; Chu, C. Selenium Uptake, Transport, Metabolism, Reutilization, and Biofortification in Rice. Rice 2022, 15, 30. [Google Scholar] [CrossRef]
- Trippe, R.C.; Pilon-Smits, E.A.H. Selenium transport and metabolism in plants: Phytoremediation and biofortification implications. J. Hazard. Mater. 2021, 404, 124178. [Google Scholar] [CrossRef]
- El Mehdawi, A.F.; Jiang, Y.; Guignardi, Z.S.; Esmat, A.; Pilon, M.; Pilon-Smits, E.A.H. Influence of sulfate supply on selenium uptake dynamics and expression of sulfate/selenate transporters in selenium hyperaccumulator and nonhyperaccumulator Brassicaceae. New Phytol. 2018, 217, 194–205. [Google Scholar] [CrossRef]
- Cabannes, E.; Buchner, P.; Broadley, M.R.; Hawkesford, M.J. A comparison of sulfate and selenium accumulation in relation to the expression of sulfate transporter genes in Astragalus species. Plant Physiol. 2011, 157, 2227–2239. [Google Scholar] [CrossRef]
- Lu, J.; Holmgren, A. Selenoproteins. J. Biol. Chem. 2009, 284, 723–727. [Google Scholar] [CrossRef]
- Johansson, L.; Gafvelin, G.; Arnér, E.S.J. Selenocysteine in proteins—Properties and biotechnological use. Biochim. Biophys. Acta Gen. Subj. 2005, 1726, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Blum, J.S.; Bindi, A.B.; Buzzelli, J.; Stolz, J.F.; Oremland, R.S. Reduction of elemental selenium to selenide: Experiments with anoxic sediments and bacteria that respire se-oxyanions. Geomicrobiol. J. 2003, 20, 587–602. [Google Scholar] [CrossRef]
- Guo, J.; Lin, Z.; Wang, L.; Zhang, X.; Ma, Y.; Wang, D.; Zhang, L.; Ma, J. Contributions of selenium-oxidizing bacteria to selenium biofortification and cadmium bioremediation in a native seleniferous Cd-polluted sandy loam soil. Ecotoxicol. Environ. Saf. 2024, 272, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Laliberte, M.; De Ladurantaye-Noel, M. Removal of Selenium by Reduction to Selenite and Surface Complexation. In Proceedings of the Mine Water Solutions, Vancouver, BC, Canada, 14–16 June 2022; pp. 295–301. [Google Scholar]
- Perrone, D.; Lavorgna, M.; Preedy, V.R. Selenium: Chemistry, Analysis, Function and Effects; Preedy, V.R., Ed.; The Royal Society of Chemistry: London, UK, 2015; Available online: www.rsc.org (accessed on 12 June 2025).
- Pyrzynska, K. Speciation Analysis of Some Organic Selenium Compounds: A Review. Analyst 1996, 121, 77R–83R. [Google Scholar] [CrossRef]
- Pyrzynska, K. Speciation of Selenium Compounds. Anal. Sci. 1998, 14, 479–483. [Google Scholar] [CrossRef][Green Version]
- Weekley, C.M.; Harris, H.H. Which form is that? the importance of selenium speciation and metabolism in the prevention and treatment of disease. Chem. Soc. Rev. 2013, 42, 8870–8894. [Google Scholar] [CrossRef]
- Tanini, D.; Capperucci, A. Synthesis and Applications of Organic Selenols. Adv. Synth. Catal. 2021, 363, 5360–5385. [Google Scholar] [CrossRef]
- Kieliszek, M.; Błazejak, S. Selenium: Significance, and outlook for supplementation. Nutrition 2013, 29, 713–718. [Google Scholar] [CrossRef] [PubMed]
- Forstrom, J.W.; Zakowski, J.J.; Tappel, A.L. Identification of the Catalytic Site of Rat Liver Glutathione Peroxidase as Selenocysteine. Biochemistry 1978, 17, 2639–2644. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.G.; Kunwar, A. Redox reactions of organoselenium compounds: Implication in their biological activity. Free Radic. Res. 2021, 55, 641–654. [Google Scholar] [CrossRef] [PubMed]
- Nie, Y.; Liu, Q.; Zhang, S.; Guo, H.; Song, X.; Chen, W. Synthesis and Potential Anticancer Activity of Some Novel Selenocyanates and Diselenides. Chem. Biodivers. 2020, 17, e1900603. [Google Scholar] [CrossRef]
- Lu, S.C. S-adenosylmethionine. Int. J. Biochem. Cell Biol. 2000, 32, 391–395. [Google Scholar] [CrossRef]
- Fontecave, M.; Atta, M.; Mulliez, E. S-adenosylmethionine: Nothing goes to waste. Trends Biochem. Sci. 2004, 9, 243–249. [Google Scholar] [CrossRef]
- Ueland, P.M. Pharmacological and Biochemical Aspects of S-Adenosylhomocysteine and S-Adenosylhomocysteine Hydrolase. Pharmacol. Rev. 1982, 34, 223–253. [Google Scholar] [CrossRef]
- Brown, K.; Arthur, J. Selenium, selenoproteins and human health: A review. Public Health Nutr. 2001, 4, 593–599. [Google Scholar] [CrossRef]
- Lacourciere, G.M.; Stadtman, T.C. Utilization of Selenocysteine as a Source of Selenium for Selenophosphate Biosynthesis. BioFactors 2001, 14, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Dodig, S.; Cepelak, I. The facts and controverses about selenium. Acta Pharm. 2004, 54, 261–276. [Google Scholar] [PubMed]
- Driscoll, D.M.; Copeland, P.R. Mechanism and regulation of selenoprotein synthesis. Annu. Rev. Nutr. 2003, 23, 17–40. [Google Scholar] [CrossRef] [PubMed]
- Winkel, L.H.E.; Vriens, B.; Jones, G.D.; Schneider, L.S.; Pilon-Smits, E.; Bañuelos, G.S. Selenium cycling across soil-plant-atmosphere interfaces: A critical review. Nutrients 2015, 7, 4199–4239. [Google Scholar] [CrossRef]
- Muleya, M.; Watts, M.J.; Nyirenda, G.K.C.; Chilimba, A.D.C.; Phiri, F.P.; Broadley, M.R.; Siyeni, D.; Chilima, B.; Chilima, B.Z.; Joy, E.J.M. Selenium speciation and bioaccessibility in Se-fertilised crops of dietary importance in Malawi. J. Food Compos. Anal. 2021, 98, 103841. [Google Scholar] [CrossRef]
- Cooke, T.D.; Bruland, K.W. Aquatic Chemistry of Selenium: Evidence of Biomethylationt. Environ. Sci. Technol. 1987, 21, 1214–1219. [Google Scholar] [CrossRef]
- Khanam, A.; Platel, K. Bioaccessibility of selenium, selenomethionine and selenocysteine from foods and influence of heat processing on the same. Food Chem. 2016, 194, 1293–1299. [Google Scholar] [CrossRef]
- Williams, P.N.; Lombi, E.; Sun, G.X.; Scheckel, K.; Zhu, Y.G.; Feng, X.; Zhu, J.; Carey, A.M.; Adomako, E.; Lawgali, Y.Y.; et al. Selenium characterization in the global rice supply chain. Environ. Sci. Technol. 2009, 43, 6024–6030. [Google Scholar] [CrossRef]
- Tani, L.S.K.; Dhanarajan, A.; Nair, R.; Gaman, M.A.; Efficace, E.; Sarac, I.; Aon, M.A. Selenium deficiency-from soil to thyroid cancer. Appl. Sci. 2020, 10, 5386. [Google Scholar]
- Abdalla, M.A.; Alotaibi, S.H.; Khattab, A.R.; El Sayed, A.M.; Elnakady, Y.A.; Shalaby, A.S.G.; Badr, G.; Abdel-Hady, H.; Alharthy, B.; Abdelmohsen, U.R. Regulation of Selenium/Sulfur Interactions to Enhance Chemopreventive Effects: Lessons to Learn from Brassicaceae. Molecules 2020, 25, 5846. [Google Scholar] [CrossRef]
- Sun, G.X.; Williams, P.N.; Carey, A.M.; Zhu, Y.G.; Deacon, C.; Raab, A.; Feldmann, J.; Islam, R.M.; Meharg, A.A. Distribution and translocation of selenium from soil to grain and its speciation in Paddy Rice (Oryza sativa L.). Environ. Sci. Technol. 2010, 44, 6706–6711. [Google Scholar] [CrossRef]
- Lyons, M.P.; Papazyan, T.T.; Surai, P.F. Selenium in food chain and animal nutrition: Lessons from nature-review. Asian Australas. J. Anim. Sci. 2007, 20, 1135–1155. [Google Scholar] [CrossRef]
- Bodnar, M.; Konieczka, P.; Namiesnik, J. The properties, functions, and use of selenium compounds in living organisms. J. Environ. Sci. Health Part C Environ. Carcinog. Ecotoxicol. Rev. 2012, 30, 225–252. [Google Scholar] [CrossRef] [PubMed]
- Mayland, H.F. Selenium in Plant and Animal Nutrition. In Selenium in the Environment, 1st ed.; CRC Press: Boca Raton, FL, USA, 1994; pp. 29–45. [Google Scholar]
- Pyrzyńska, K. Determination of selenium species in environmental samples. Mikrochim. Acta 2002, 140, 55–62. [Google Scholar] [CrossRef]
- Bocchini, M.; D’Amato, R.; Ciancaleoni, S.; Fontanella, M.C.; Palmerini, C.A.; Beone, G.M.; Onofri, A.; Negri, V.; Pilon-Smits, E.A.H.; Iannelli, M.A.; et al. Soil selenium (Se) biofortification changes the physiological, biochemical and epigenetic responses to water stress in Zea mays L. by inducing a higher drought tolerance. Front. Plant Sci. 2018, 9, 389. [Google Scholar] [CrossRef] [PubMed]
- Proietti, P.; Nasini, L.; Del Buono, D.; D’Amato, R.; Tedeschini, E.; Businelli, D. Selenium protects olive (Olea europaea L.) from drought stress. Sci. Hortic. 2013, 164, 165–171. [Google Scholar] [CrossRef]
- Liu, J.X.; Howell, S.H. Endoplasmic reticulum protein quality control and its relationship to environmental stress responses in plants. Plant Cell 2010, 22, 2930–2942. [Google Scholar] [CrossRef]
- Bohnert, H.J.; Nelson, D.E.; Jensen, R.G. Adaptations to Environmental Stresses. Plant Cell 1995, 7, 1099–1111. [Google Scholar] [CrossRef]
- Mareri, L.; Romi, M.; Cai, G. Environmental Stress and Plants. Int. J. Mol. Sci. 2022, 23, 5416. [Google Scholar] [CrossRef]
- Kosová, K.; Vítámvás, P.; Prášil, I.T.; Renaut, J. Plant proteoforms under environmental stress: Functional proteins arising from a single gene. Front. Plant Sci. 2021, 12, 793113. [Google Scholar] [CrossRef]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments: A review. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef]
- Nawaz, F.; Ashraf, M.Y.; Ahmad, R.; Waraich, E.A.; Khan, S.Z.; Ehsan, A. Selenium supplementation affects physiological and biochemical processes to improve fodder yield and quality of maize (Zea mays L.) under water deficit conditions. Front. Plant Sci. 2016, 7, 1438. [Google Scholar] [CrossRef] [PubMed]
- Naseem, M.; Ahmad, F.; Wang, X.; Ahmad, M.; Wu, D.; Hassan, M.J.; Chen, X. Influence of selenium on growth, physiology, and antioxidant responses in maize varies in a dose-dependent manner. J. Food Qual. 2021, 2021, 6642018. [Google Scholar] [CrossRef]
- Andrade, F.R.; Carvalho, R.F.; Campos, M.L.; Azevedo, R.A. Selenium protects rice plants from water deficit stress. Ecotoxicol. Environ. Saf. 2018, 164, 562–570. [Google Scholar] [CrossRef]
- Patnaik, G.P.; Dash, S.; Sahoo, A.; Mohapatra, S.; Tripathy, S.K.; Bastia, D.N. Selenium Application Improves Drought Tolerance during Reproductive Phase of Rice. Sustainability 2023, 15, 2730. [Google Scholar] [CrossRef]
- Ghouri, F.; Nawaz, M.A.; Farooq, N.; Javed, M.T.; Hussain, H.A.; Waraich, E.A.; Rasheed, R.; Irshad, M.K. Effects of Silicon and Selenium in Alleviation of Drought Stress in Rice. Silicon 2022, 14, 5453–5461. [Google Scholar] [CrossRef]
- Liu, M.; Li, Y.; Yin, R.; Zhang, H.; Zhang, L.; Wang, M.; Sommar, J.; Feng, X. Rice Life Cycle-Based Global Mercury Biotransport and Human Methylmercury Exposure. Nat. Commun. 2019, 10, 5164. [Google Scholar] [CrossRef]
- Palmieri, J.R.; Edelstein, M.R.; Angeloni, S.V. Implications and Significance of Mercury in Rice. J. Food Nutr. Metab. 2020, 3, 1–5. [Google Scholar] [CrossRef]
- Zhao, H.; Li, H.; Wang, Q.; Wang, Y.; Wang, M.; Zhang, M.; Liang, Y.; Zhu, Y. Mercury contents in rice and potential health risks across China. Environ. Int. 2019, 126, 406–412. [Google Scholar] [CrossRef]
- Zhang, H.; Feng, X.; Wang, X.; Larssen, T.; Shang, L.; Li, P. Selenium in soil inhibits mercury uptake and translocation in rice (Oryza sativa L.). Environ. Sci. Technol. 2012, 46, 10040–10046. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).