Abstract
Photocatalytic oxidation technology harnesses solar energy for pollutant mineralization, presenting significant potential for environmental applications. A critical bottleneck remains the development of high-performance photocatalysts. This study centers on the non-metallic semiconductor material graphitic carbon nitride (g-C3N4). To overcome the inherent limitations of pristine g-C3N4, including limited surface area, rapid charge carrier recombination, and inadequate active sites, it implements surface engineering strategies employing acidic (H2SO4) or basic (K2CO3) agents to modulate microstructure, introduce defect sites (cyano/amino groups), and optimize bandgap engineering. These modifications synergistically enhanced photogenerated charge carrier separation efficiency and surface reactivity, leading to efficient dye degradation. Notably, the K2CO3-modified catalyst (g-C3N4-OH), synthesized with a mass ratio of m(g-C3N4):m(K2CO3) = 1:1, achieved 92.2% Rhodamine B degradation within 50 min under visible light, surpassing pristine g-C3N4 (20.6%), the optimized H2SO4-modified sample (g-C3N4-HS, 60.9%), and even template-synthesized g-C3N4-SBA (79.6%). The g-C3N4-OH catalyst demonstrated exceptional performance under both visible light and natural solar illumination. Combining facile synthesis, cost-effectiveness, superior activity, and robust stability, this work provides a novel approach for developing high-efficiency non-metallic photocatalysts applicable to dye wastewater.
1. Introduction
The rapid expansion of printing, dyeing, textile, and chemical industries in recent years has resulted in substantial discharge of persistent organic dyes into natural aquatic systems through industrial effluents, creating significant water contamination issues [1,2,3,4]. These hazardous substances exhibit multiple toxic characteristics including carcinogenic and teratogenic properties, while their ability to accumulate through food chain magnification presents substantial risks to both ecological systems and public health [5,6,7]. Conventional wastewater treatment approaches, including biological degradation [8,9], adsorptive separation [10,11], and chemical oxidation [12,13], are constrained by their limited effectiveness, elevated operational expenses, or potential for creating secondary environmental contamination. This technological impasse has necessitated the exploration of innovative, sustainable water purification strategies. Photocatalytic oxidation technology emerges as a promising alternative by harnessing solar energy to facilitate complete pollutant mineralization under relatively mild reaction conditions [14,15,16,17]. Nevertheless, the advancement of highly efficient photocatalytic materials continues to represent the fundamental challenge limiting broader implementation of this technology [18,19].
G-C3N4 has garnered significant attention as an emerging non-metallic semiconductor photocatalyst. Characterized by its distinctive electronic configuration, exceptional chemical durability, economic viability, and ecologically friendly nature, this material exhibits a band structure optimized for visible light absorption. Through efficient separation of photogenerated charge carriers, this material facilitates the breakdown of various pollutants, positioning it as a prominent focus area within photocatalytic research [20,21,22,23,24]. Nevertheless, the pristine form of g-C3N4 demonstrates inherent limitations, including restricted specific surface area, rapid recombination of photogenerated carriers, and inadequate surface-active sites [25,26,27]. These constraints collectively result in photocatalytic efficiency significantly below theoretical predictions, thereby severely restricting its practical deployment in environmental remediation applications such as dye degradation processes [28]. To address these fundamental challenges, surface engineering approaches have emerged as pivotal strategies for enhancing photocatalytic performance. By precisely modulating surface chemical composition, morphological architecture, or introducing synergistic active sites, these modifications enable substantial enhancements in light absorption capacity, charge separation dynamics, and surface reaction kinetics [21,29,30]. While these modification methods have demonstrated certain advancements, each approach retains intrinsic limitations. Current investigations suggest that intentional introduction of nitrogen defects into the g-C3N4 framework significantly enhances the photocatalytic activity under visible light [31]. To date, methods for synthesizing nitrogen-doped g-C3N4 include high-temperature pyrolysis [32], hydrothermal synthesis [33], and hydrogen reduction [34]. However, these methods exhibit limited precision in controlling the type and concentration of nitrogen defects generated. Consequently, the development of photocatalysts featuring simplified synthesis procedures, cost-effectiveness, and superior performance characteristics continues to represent a domain requiring further investigation and innovation.
Based on this, this study introduces a surface modification strategy based on an acid (H2SO4) or base (K2CO3). This methodology effectively enhances photogenerated charge separation efficiency and surface reactivity by regulating the microstructure of the materials, incorporating defect structures (cyano/amino functional groups), and optimizing band structural characteristics [21]. Under visible light illumination, the catalyst demonstrates optimal performance with a mass ratio of g-C3N4: K2CO3 = 1:1, achieving a remarkable RhB degradation rate of 92.2% within 50 min. The acid-modified sample exhibiting superior catalytic activity is designated as g-C3N4-HS, while the base-modified counterpart with optimal performance at a 1:1 ratio is labeled as g-C3N4-OH. A templated reference material (g-C3N4-SBA), synthesized using the SBA-15 hard template, serves as control for morphological analysis. All surface modification strategies significantly surpass the 20.6% degradation rate exhibited by unmodified g-C3N4 (g-C3N4-HS, 60.9% and g-C3N4-SBA, 79.6%). Notably, the material maintains high degradation efficiency under natural sunlight conditions, while maintaining catalytic activity stability across three consecutive reuse cycles, demonstrating that the material possesses a broad range of applicability and stable cyclic stability. Mechanistic studies indicate that superoxide radicals play a dominant role in the photocatalytic degradation of RhB, whilst photogenerated electrons also influence the degradation process.
2. Materials and Methods
2.1. Materials
Unless otherwise specified, all analytical reagents and solvents were commercially available. Melamine, ammonium hydrogen fluoride, and ethyl orthosilicate (TEOS) were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). P123 (EO20PO70EO20, MW = 5800) was purchased from SIGMA-ALDRICH. Rhodamine B was purchased from McLean Biochemical Technology Co., Ltd. (Shanghai, China). Potassium bromide and barium sulfate were purchased from Aladdin Biochemical Technology Co., Ltd. (Shanghai, China). Deionized water was prepared using a Sartorius 611DI water purification system.
2.2. Instrument Parameters
The instrument parameters, as well as the testing of properties, are described in detail in the Supplementary Information.
2.3. Preparation of Catalysts
2.3.1. Preparation of g-C3N4
Place the white melamine solid into an agate mortar and grind for 30 min. Weigh 16 g of the ground melamine, transfer it to a porcelain crucible, and cover it. Place the porcelain crucible in a muffle furnace. Set the temperature to 550 °C (heating rate: 5 °C/min), hold for 4 h, and then allow it to cool naturally. This yields 6.56 g of yellow powdered solid g-C3N4.
2.3.2. Acid Treatment of g-C3N4
Weigh 1 g of g-C3N4 and add 20 mL of concentrated sulfuric acid. Heat to 100 °C to ensure complete dissolution. Maintain heating at 100 °C for 2 h and then allow it to cool naturally to room temperature, yielding a yellow, clear liquid. Slowly add 60 mL of anhydrous ethanol dropwise to the yellow clear solution. A white solid gradually precipitates. Wash the white solid with water until neutral and then freeze-dry for approximately 18 h. Calcine the resulting white fluffy solid at 550 °C for 5 h under a nitrogen atmosphere (heating rate: 2.3 °C/min). After cooling to room temperature, 0.2 g of yellow fluffy solid is obtained, identified as g-C3N4-HS-20. Replace concentrated sulfuric acid with 15 mL and 25 mL, respectively, and repeat the above experiment to obtain g-C3N4-HS-15 and g-C3N4-HS-25.
2.3.3. Alkaline Treatment of g-C3N4
Weigh 4 g of g-C3N4 and 4 g of K2CO3, place them in a beaker, and mix thoroughly. Add 30 mL of distilled water. Sonicate for 30 min and then remove the distilled water by rotary evaporation to obtain a yellow solid. Place the solid in a muffle furnace and calcine at 400 °C for 4 h (heating rate: 2.3 °C/min). After reaction completion, allow to cool naturally to room temperature, yielding a yellow solid. Add 2 mol·L−1 dilute hydrochloric acid solution and sonicate to completely disperse any undissolved solid, ensuring all unreacted K2CO3 is removed. Filter under vacuum to obtain a yellow solid. Wash the filter cake with distilled water until neutral. Dry the resulting at 80 °C to yield a pale yellow solid, designated as g-C3N4-OH-1:1. Repeat the experiemt with K2CO3 quantities of 2 g and 6 g to obtain g-C3N4-OH-1:0.5 and g-C3N4-OH-1:1.5, respectively.
2.3.4. Preparation of Ordered Mesoporous g-C3N4
Synthesize according to reference [35]. Place 1 g of SBA-15 in a beaker, add 100 mL of water, sonicate for 5 min, and stir for 1 h until fully dispersed. Weigh 2 g of melamine, add it to the dispersion, and heat under stirring until dry (80 °C), yielding a white solid powder. Thoroughly grind the resulting solid powder in an agate mortar. Under air atmosphere, heat in a muffle furnace to 550 °C (heating rate 5 °C/min), and hold for 6 h, yielding a yellow solid powder. In a PTFE beaker, disperse the yellow solid powder into ammonium hydrogen fluoride solution. Stir for 48 h to etch away SBA-15. Filter under vacuum and dry to obtain a pale yellow powdery solid. Denote this as g-C3N4-SBA.
2.3.5. Photocatalytic Degradation of Dyes
Rhodamine B (RhB) is a common nitrogen-containing dye pollutant with a highly stable structure, making it a widely applicable target for photocatalytic degradation. All experiments were conducted under constant-temperature conditions, uniformly employing a 15 W LED light source to simulate sunlight. RhB dye was dissolved in deionized water to prepare a 1 × 10−5 mol·L−1 aqueous solution. Then, 10 mg of catalyst was added to 10 mL of the dye solution. Dark adsorption was first allowed for 1 h under dark conditions to achieve adsorption–desorption equilibrium. Subsequently, the light exposure experiment was conducted. At regular intervals, a fixed volume of liquid was removed, a clear solution was obtained by centrifugation, and its absorbance was measured at 554 nm using a UV–visible spectrophotometer to determine the concentration of RhB.
3. Results
3.1. Structural Analysis of g-C3N4 and Its Modified Materials
PXRD analysis indicates (Figure 1a) that g-C3N4 monomers exhibit two distinct characteristic peaks at 2θ = 12.9° and 27.8°, confirming that g-C3N4 belongs to the hexagonal crystal system [36]. The characteristic absorption peak at 12.9° corresponds to the (100) crystal plane of g-C3N4, attributed to the in-plane structural stacking peak of graphitic carbon nitride. The characteristic peak at 27.8° corresponds to the (002) crystal plane of g-C3N4, representing an absorption peak from the superimposed conjugated aromatic system within g-C3N4. This confirms that the prepared g-C3N4 sample is indeed graphitic carbon nitride material [37,38]. The strong peak at 27.8° for g-C3N4-HS, g-C3N4-OH, and g-C3N4-SBA remains unchanged, indicating that the interlayer stacking structure of the three materials has not altered. Interestingly, the peak at 12.9° for g-C3N4-OH has disappeared, indicating that the addition of the base etches the regular structure of g-C3N4, leading to the loss of ordered framework structures. The SBA-15 template restricts the growth of g-C3N4, causing a slight shift in the peak of g-C3N4-SBA at 27.8° [39,40].
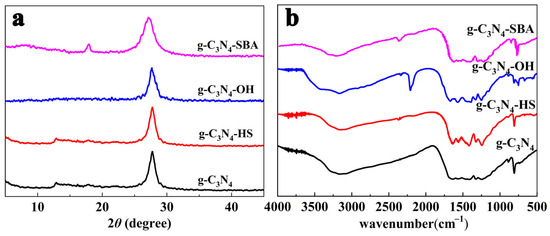
Figure 1.
(a) PXRD patterns of g-C3N4, g-C3N4-HS, g-C3N4-OH, and g-C3N4-SBA; (b) FT-IR spectra of g-C3N4, g-C3N4-HS, g-C3N4-OH, and g-C3N4-SBA.
The Fourier transform infrared spectra of all test samples (Figure 1b) reveal a characteristic peak at 810 cm−1, corresponding to the typical out-of-plane bending vibration of the triazine ring [41]. Additionally, multiple characteristic peaks observed in the 1000–1800 cm−1 range may be attributed to typical stretching modes of C-N and C=N heterocyclic bonds [42]. The presence of a triazine structure in the synthesized compound is confirmed by the characteristic stretching vibration modes of the aromatic CN heterocycle appearing at 810 cm−1 and 1200–1600 cm−1. Multiple broad peaks in the 3000–3700 cm−1 region correspond to N-H stretching vibrations and the physical adsorption of H2O. Compared to g-C3N4, the infrared spectra of g-C3N4-SBA and g-C3N4-HS show little change, while two distinct shifts are observed in the infrared spectrum of g-C3N4-OH. First, a new peak appeared at 2185 cm−1, corresponding to the asymmetric stretching vibration of the cyano group (-C≡N). This indicates that the addition of K2CO3 caused partial decomposition of the triazine skeleton, resulting in the presence of -C≡N groups in the synthesized g-C3N4-OH [43,44,45]. Second, the intensity of the N-H stretching peak between 3000 and 3300 cm−1 increases. This is attributed to the K2CO3 treatment reducing the regularity of the bulk g-C3N4 structure, etching its surface to expose more edge amino groups.
The SBET of g-C3N4 samples synthesized using melamine as a precursor was only 16.5 m2·g−1 (Figure 2a). This was attributed to its high degree of thermal polymerization, which resulted in a coarse-grained microstructure and, consequently, a low specific surface area. The pore size distribution is also relatively dispersed, which is presumed to result from interlayer stacking between g-C3N4 layers (Figure S2a). Figure 2b shows that the SBET of acid-treated g-C3N4-HS exhibits a certain degree of improvement, reaching 48.5 m2·g−1. However, pore sizes ranging from 20 nm to 80 nm indicate that defects etched during the acid treatment process are uncontrollable, leading to the non-uniformity in pore size distribution (Figure S2b). The nitrogen adsorption–desorption isotherm of g-C3N4-OH is shown in Figure 2c. A distinct loop appears within the relative pressure range of 0.5–0.9, likely due to alkali etching exposing additional edge amine groups, which impedes desorption and causes a desorption lag. The SBET of g-C3N4-OH showed a significant increase, reaching 78.5 m2·g−1. The pore size distribution ranged from ~20 nm to ~40 nm (Figure S2c). In comparison, the SBET of the mesoporous g-C3N4-SBA synthesized by us reached 83.7 m2·g−1, with its pore size primarily distributed around 21 nm (Figure 2d and Figure S2d). Compared to SBA-15 (Figure S1b), the pores are slightly smaller.
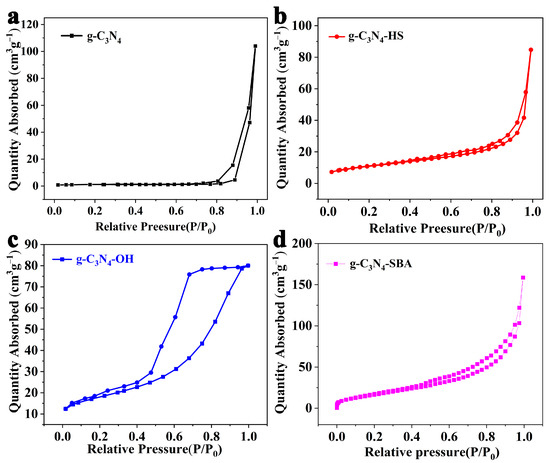
Figure 2.
N2 adsorption–desorption isotherms of (a) g-C3N4 at 77 K, (b) g-C3N4-HS, (c) g-C3N4-OH, and (d) g-C3N4-SBA.
3.2. Morphological Structure
The morphologies of g-C3N4, g-C3N4-HS, g-C3N4-OH, and g-C3N4-SBA were observed using scanning electron microscopy (SEM) and transmission electron microscopy (TEM). As shown in Figure 3a–c, g-C3N4, g-C3N4-HS, and g-C3N4-OH all exhibit blocky structures composed of plate-like nanocrystals. These plate-like nanocrystals possess irregular geometric shapes, with dimensions at the micrometer level and thicknesses at the nanometer level. Figure 3d indicates that the size of g-C3N4 synthesized on the SBA-15 template was successfully restricted, with a structure differing from other non-templated materials in that it consists of a layered structure formed by the stacking of nanosheets. As shown in Figure 4a, g-C3N4 exhibits a characteristic layered stacking structure with closely packed layers. In contrast, g-C3N4-HS, g-C3N4-OH, and g-C3N4-SBA all display multilayer overlapping nanosheets at the edges (Figure 4b–d). The sheets maintain a certain distance between each other, forming an open-pore structure. This unique nanosheet architecture significantly increases the material’s specific surface area, providing more active sites for catalytic reactions.
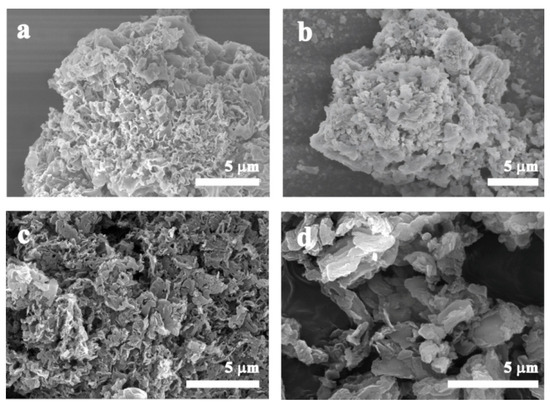
Figure 3.
SEM images of (a) g-C3N4, (b) g-C3N4-HS, (c) g-C3N4-OH, and (d) g-C3N4-SBA.
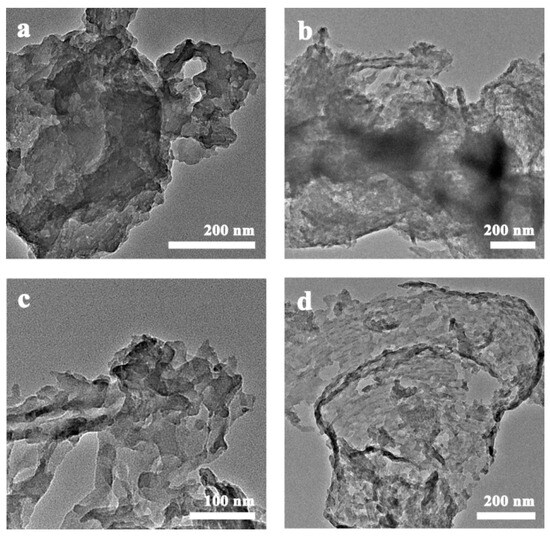
Figure 4.
TEM images of (a) g-C3N4, (b) g-C3N4-HS, (c) g-C3N4-OH, and (d) g-C3N4-SBA.
3.3. Thermal Stability
As shown in Figure S3, the thermal stability of four materials was evaluated via thermogravimetric analysis (TGA) under an air atmosphere. The g-C3N4 sample (Figure S3a) remained stable without significant weight loss until 550 °C. In contrast, the g-C3N4-HS (Figure S3b) and g-C3N4-OH (Figure S3c) samples exhibited gradual weight loss starting at 528 °C and 500 °C, respectively. This reduced stability is strongly correlated with the presence of structural defects within these materials. The etching by acids or alkalis disrupts the regularity and polymerization degree of g-C3N4 itself, leading to structural collapse and the formation of voids. Simultaneously, it results in the generation of partially oligomeric g-C3N4 fragments, thereby reducing its stability. Due to template-induced size constraints reducing polymerization degree, g-C3N4-SBA (Figure S3d) exhibits slightly diminished stability compared to pristine g-C3N4. All four materials show no significant weight loss below 500 °C, indicating stability under the following photocatalytic dye degradation test conditions.
3.4. X-Ray Photoelectron Spectroscopy
X-ray photoelectron spectroscopy (XPS) was further employed to determine the atomic valence states and bonding environment. The peak at 284.6 eV is attributed to C-C bonds arising from carbon adhering to the XPS instrument itself, and this peak was utilized to calibrate all XPS peak positions. The C 1s XPS spectrum exhibits a distinct and sharp peak accompanied by a pronounced shoulder peak (Figure S4a–d). After fitting, three peaks were observed in all four samples, with binding energies of 288.2, 286.1, and 284.6 eV. The peaks located at 288.2 and 286.1 eV are attributed to hybridized carbon atoms containing the C-(N)3 and N-C=N group [46]. Among the four samples, only the g-C3N4-OH sample exhibited a double peak for potassium in its C spectrum, which is attributable to residual K from K2CO3 introduced during the preparation process. As shown in Figure S5, N 1s could be fitted into four peaks, located at 404.6, 400.8, and 398.9 eV. The peak at 398.9 eV is assigned to sp2-hybridized aromatic N bound to C atoms (C=N-C), while the signal at the binding energy of 400.8 eV indicates tertiary nitrogen, N-(C)3. And the peak at 404.6 eV is assigned to C-N-H groups and charging effects [21,47].
3.5. Photocatalytic Studies
Experimental results indicate that when treating g-C3N4 with H2SO4, the amount of acid used has little effect on catalytic performance (Figure 5a), whereas the amount of base used significantly impacts catalytic performance (Figure 5b). Photocatalytic degradation of RhB within 50 min showed a degradation rate of 20.6% for the g-C3N4 catalyst, while the g-C3N4: K2CO3 = 1:0.5~1.5 catalysts exhibited degradation rates of 64.8%, 92.2%, and 83.2%, respectively. Within 50 min, the degradation rate of the g-C3N4: K2CO3 = 1:1 (g-C3N4-OH) catalyst was 4.5 times that of g-C3N4. All surface modification approaches demonstrated substantially higher degradation efficiencies compared to pristine g-C3N4. Specifically, the acid-modified g-C3N4-HS sample reached 60.9% degradation efficiency, while the template-synthesized g-C3N4-SBA exhibited 79.6% degradation efficiency under identical conditions. Compared with the mesoporous g-C3N4-SBA synthesized via the template method, which is recognized for its excellent catalytic activity, g-C3N4-OH exhibits superior catalytic performance (Figure 5c).
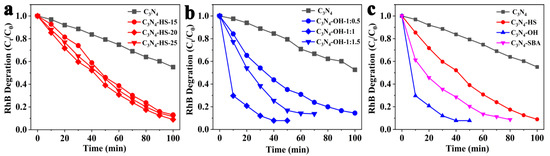
Figure 5.
(a) Effect of catalyst preparation with different acid dosages on photocatalytic degradation of RhB; (b) effect of catalyst preparation with different base dosages on photocatalytic degradation of RhB. (c) Catalytic activity of four catalysts in the photocatalytic degradation of RhB.
4. Discussion
4.1. Photophysical and Electrochemical Properties
To determine the optical properties of the four materials, ultraviolet–visible (UV) absorption and photoluminescence (PL) measurements were conducted. As shown in Figure 6a, g-C3N4 and g-C3N4-OH exhibit absorption edges at 430 nm, while the absorption edges of g-C3N4-HS and g-C3N4-SBA are red-shifted to approximately 510 nm. The three modified materials effectively retained the optical properties of g-C3N4. The charge transfer behavior of the four materials was investigated via fluorescence spectroscopy (PL) (Figure 6b). The strongest peak of g-C3N4 appeared at approximately 470 nm, indicating the lowest electron mobility and highest electron–hole recombination rate [48]. The three modified materials exhibit significantly reduced fluorescence intensity, particularly g-C3N4-OH and g-C3N4-SBA, which show the weakest peaks around 470 nm. This indicates that the modified materials effectively suppress electron–hole recombination.
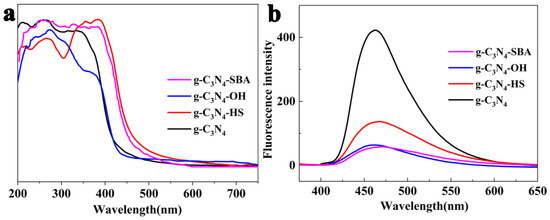
Figure 6.
(a) Solid-state UV-vis spectra of g-C3N4, g-C3N4-HS, g-C3N4-OH, and g-C3N4-SBA; (b) photoluminescence spectra of g-C3N4, g-C3N4-HS, g-C3N4-OH, and g-C3N4-SBA.
According to the Kubelka–Monk (K-M) function, the band gaps of these four materials (g-C3N4, g-C3N4 HS, g-C3N4-OH, and g-C3N4-SBA) are 3.02 eV, 2.84 eV, 2.78 eV, and 2.99 eV, respectively (Figure S3) [49]. The flat-band positions of the four materials (g-C3N4, g-C3N4 HS, g-C3N4-OH, and g-C3N4-SBA) obtained from the Mott–Schottky (M-S) plots relative to Ag/AgCl are −1.16 V, −1.27 V, −1.07 V, and −1.18 V, respectively (Figure S4). Since the bottom of the conduction band (CB) is approximately equal to the flat-band position, the conduction band edges of g-C3N4, g-C3N4 HS, g-C3N4-OH, and g-C3N4-SBA were determined to be −0.96 V, −1.07 V, −0.87 V, and −0.98 V relative to the standard hydrogen electrode (NHE). Therefore, based on the equation Eg = EVB − ECB, the valence-band (VB) positions of these materials are estimated to be 2.06 V, 1.71 V, 2.12 V, and 1.86 V, respectively (Figure 7a) [50,51]. Given their matching band structures, these four materials are thermodynamically suitable for photodegradation of dyes.
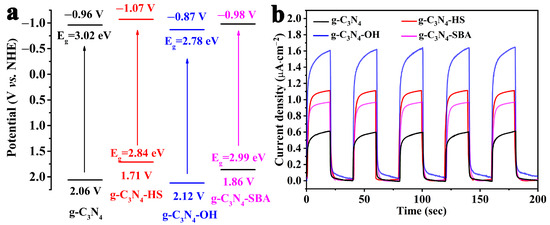
Figure 7.
(a) Band structure schematic diagram; (b) transient photocurrent responses.
Transient photocurrent response measurements were employed to investigate the photosensitivity of the materials [52]. As shown in Figure 7b, when exposed to light, the photocurrent generated in all four samples rapidly increased and gradually reached a steady state. However, upon light removal, the photocurrent rapidly decreased. This cycle could be repeated multiple times without a significant reduction in the maximum current value, indicating that these four materials are stable under illumination. Among them, g-C3N4-OH exhibited the strongest photocurrent response intensity, approximately three times that of pristine g-C3N4, indicating its superior photogenerated electron transport capability, which is conducive to photogenerated charge separation [53].
4.2. Study on Photocatalytic Degradation of Dyes
Based on the experimental results in Figure 5, we selected g-C3N4-HS and g-C3N4-OH as catalysts for further investigation into the photocatalytic degradation of RhB dye, with results shown in Figure 8. Under light irradiation without catalysts, the RhB solution exhibited negligible degradation. In the absence of light, the presence of g-C3N4-HS or g-C3N4-OH catalysts resulted in negligible degradation of the RhB solution. However, under light irradiation in the presence of these catalysts, the dye exhibited significant and substantial degradation. Furthermore, g-C3N4-OH demonstrated superior catalytic activity compared to g-C3N4-HS.
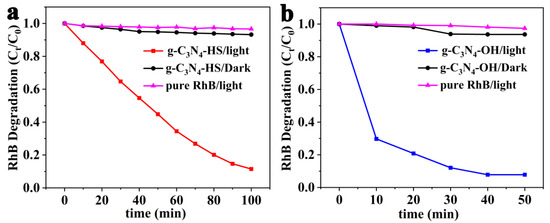
Figure 8.
Photocatalytic degradation of RhB under different conditions of (a) g-C3N4-HS and (b) g-C3N4-OH.
To further investigate the kinetic characteristics of g-C3N4-HS and g-C3N4-OH in degrading RhB, we conducted experiments on the photocatalytic degradation of RhB by g-C3N4-HS and g-C3N4-OH at different temperatures of 30 °C, 35 °C, 40 °C, 45 °C, and 50 °C. The experimental results are shown in Figure 9a,b. Plotting the logarithmic value of dye degradation rate (ln(Ct/C0)) against reaction time (t) yields excellent linear relationships with correlation coefficients exceeding 0.9, consistent with first-order reaction kinetics (Figure 9c,d). The slope of each line allows determination of the reaction rate constant (k) at different temperatures. Plotting ln k versus 1/T yields the results shown in Figure 9e,f. The activation energy (Ea) for catalyst-catalyzed RhB degradation can be determined from the slope of the line using the Arrhenius equation. The reaction rate constants, activation energies, and correlation coefficients for RhB degradation at different temperatures are summarized in Table 1.
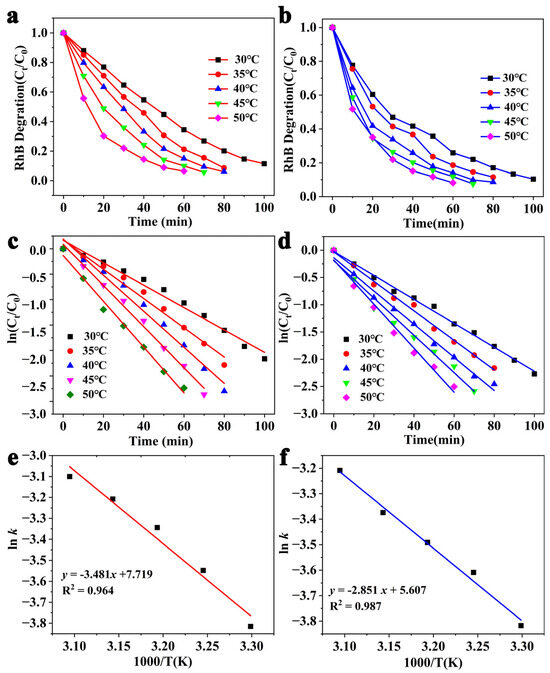
Figure 9.
Effect of temperature on dye degradation under visible light of (a,c,e) g-C3N4-HS and (b,d,f) g-C3N4-OH.

Table 1.
Reaction rate constants, activation energy, and correlation coefficients for RhB degradation at different temperatures.
To evaluate the stability of g-C3N4-HS and g-C3N4-OH, we recovered the g-C3N4-HS and g-C3N4-OH catalysts used for the catalytic degradation of RhB. These were sequentially washed with distilled water and ethanol, followed by vacuum activation. After five cycles of experiments, the test results are shown in Figure 10a,b. Compared to the degradation rate during the first cycle, no significant decrease was observed, indicating that both catalysts are stable during the photocatalytic degradation of RhB dye. The slight decrease in activity observed in each reaction cycle may be attributable to minor losses incurred during sample recovery.
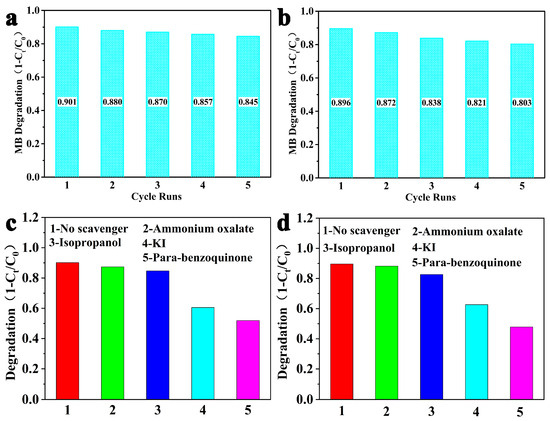
Figure 10.
Cycling runs for the photocatalytic degradation of RhB: (a) g-C3N4-HS and (b) g-C3N4-OH. The effects of different sacrificial agents on the degradation of RhB: (c) g-C3N4-HS and (d) g-C3N4-OH.
4.3. Mechanism Study
We conducted a preliminary investigation into the degradation mechanisms of RhB dye catalyzed by g-C3N4-HS and g-C3N4-OH. Photogenerated electrons, holes, hydroxyl radicals, and superoxide radicals are common reactive species [54,55,56]. Using potassium iodide as a photoelectron scavenger, ammonium oxalate as a hole scavenger, isopropanol as a hydroxyl radical scavenger, and p-benzoquinone as a superoxide radical scavenger, we conducted photocatalytic degradation experiments on RhB. The photodegradation rates after 300 min of irradiation are shown in Figure 10c,d. Compared to the control without scavengers, the photodegradation rate of RhB showed no significant change upon addition of ammonium oxalate and isopropanol solution, indicating that neither holes nor hydroxyl radicals participated in the photodegradation reaction of RhB. However, when p-benzoquinone was added, the degradation rate of RhB decreased significantly, suggesting that superoxide radicals play a dominant role in the photocatalytic degradation of RhB. The degradation rate of RhB also decreased upon addition of potassium iodide, indicating that photogenerated electrons also influence its degradation.
4.4. Solar-Powered Applications
To further validate the practicality of the g-C3N4-OH catalyst, we conducted experiments to degrade RhB under sunlight. We weighed 20 mg of g-C3N4-OH catalyst into a 100 mL beaker, added 50 mL of a 2 × 10−5 mol·dm−3 RhB solution, and exposed it to sunlight. Centrifugation was performed every 30 min, and the supernatant was tested for UV absorption spectra, with results shown in Figure 11. After 330 min of sunlight exposure, the RhB solution degraded from its initial pink color to near-colorless, indicating that the g-C3N4-OH catalyst retains satisfactory catalytic efficacy under sunlight and possesses practical applicability.
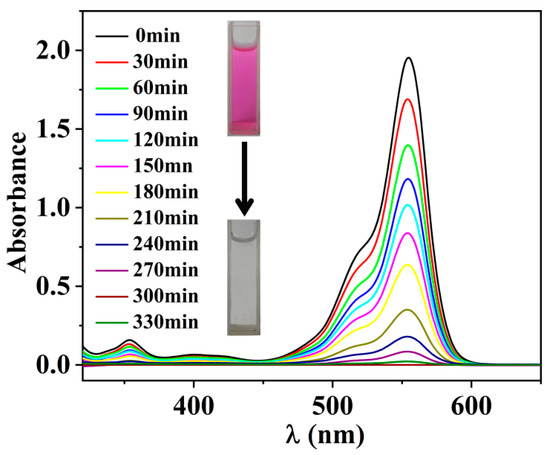
Figure 11.
Photocatalytic degradation of RhB by g-C3N4-OH under sunlight.
5. Conclusions
This study systematically examines the effects of acid and base surface modification strategies on the photocatalytic dye degradation performance of g-C3N4, establishing and refining their structure–function correlations. The modification strategy effectively manipulates the microstructural characteristics (increasing specific surface area), chemical composition (introducing cyano functionalities and revealing amino groups), and electronic properties (optimizing band configuration and facilitating charge separation) through etching mechanisms. Consequently, it significantly enhances the photocatalytic degradation activity and stability of the material toward the dye RhB. Notably, basic treatment demonstrates superior performance enhancement compared to acid or hard template modification. Under visible light irradiation, the g-C3N4-OH catalyst manifests a remarkable degradation rate of up to 92.2% for RhB, achieving significantly higher performance than pristine g-C3N4, optimal acid-modified g-C3N4-HS, and template-synthesized mesoporous g-C3N4-SBA. The g-C3N4-OH catalyst maintains exceptional performance under natural sunlight while exhibiting reduced activation energy requirements and outstanding cyclic stability. Mechanistic studies reveal that superoxide radicals constitute the primary reactive species driving RhB photodegradation, with photoinduced electrons playing a significant auxiliary role in the redox processes. These findings offer valuable perspectives and promising material candidates for the advancement of high-efficiency, durable, and economically viable non-metallic photocatalytic systems, particularly targeting environmental remediation applications in dye wastewater treatment.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/chemistry7050168/s1. Figure S1: (a) Fourier transform infrared spectrum of SBA-15. (b) Low-temperature N2 adsorp-tion-desorption isotherm of SBA-15. (c) Scanning electron micrograph of SBA-15. (d) Trans-mission electron micrograph of SBA-15; Figure S2: The pore widths of (a) g-C3N4 at 77 K, (b) g-C3N4-HS, (c) g-C3N4-OH, (d) g-C3N4-SBA; Figure S3: TGA curves of (a) g-C3N4, (b) g-C3N4-HS, (c) g-C3N4-OH, (d) g-C3N4-SBA; Figure S4: X-ray photoelectron spectroscopy (XPS) of C 1s for (a) g-C3N4, (b) g-C3N4-HS, (c) g-C3N4-OH and (d) g-C3N4-SBA; Figure S5: X-ray photoelectron spectroscopy (XPS) of N 1s for (a) g-C3N4, (b) g-C3N4-HS, (c) g-C3N4-OH and (d) g-C3N4-SBA; Figure S6: Kubelka-Munk-transformed reflectance spectra of (a) g-C3N4, (b) g-C3N4-HS, (c) g-C3N4-OH, (d) g-C3N4-SBA; Figure S7: Mott-Schottky plots of (a) g-C3N4, (b) g-C3N4-HS, (c) g-C3N4-OH, (d) g-C3N4-SBA; Table S1: The results of BET, band gap, CB and VB values of all the catalysts; Table S2: Some results of photocatalytic activity for Rhb degradation partially based on g-C3N4 as a catalyst; References [57,58,59,60,61,62,63,64] are cited in Supplementary Materials.
Author Contributions
Z.Z., J.Z. and H.P. conceived and designed the idea; H.P. designed the experiments collected and analyzed the data; G.L., X.W., S.L. and J.W. assisted with the experiments and characterizations; H.P. wrote the manuscript; J.C., J.Z. and Z.Z. helped with manuscript revising and data analysis. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the National Natural Science Foundation of China (No. 22401066), Hainan Provincial Natural Science Foundation (No. 225QN295), Hainan Provincial Education Department Foundation (No. Hnky2025-11), Weifang Institute of Science and Technology High-Level Talents Research Start-Up Fund Project (No. KJRC2023010), and the Natural Science Foundation of Weifang University of Science and Technology (No. 2023KJ08).
Data Availability Statement
The data that support the findings of this study are available from the cor-responding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| g-C3N4: Graphitic carbon nitride |
| RhB: Rhodamine B |
| PXRD: Powder X-ray diffraction |
| FT-IR: Fourier transform infrared spectrometer |
| BET: Brunauer–Emmett–Teller specific surface area analysis |
| SEM: Scanning electron microscope |
| TEM: Transmission electron microscope |
References
- Downing, A.L. Research on Water Pollution. Nature 1964, 204, 817–818. [Google Scholar] [CrossRef]
- Ran, W.; Zhao, H.; Zhang, X.; Li, S.; Sun, J.-F.; Liu, J.; Liu, R.; Jiang, G. Critical Review of Pd-Catalyzed Reduction Process for Treatment of Waterborne Pollutants. Environ. Sci. Technol. 2024, 58, 3079–3097. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-W.; Yu, H.-Q.; Rittmann, B.E. Chemistry: Reuse water pollutants. Nature 2015, 528, 29–31. [Google Scholar] [CrossRef] [PubMed]
- Yap, P.L.; Nine, M.J.; Hassan, K.; Tung, T.T.; Tran, D.N.H.; Losic, D. Graphene-Based Sorbents for Multipollutants Removal in Water: A Review of Recent Progress. Adv. Funct. Mater. 2021, 31, 2007356. [Google Scholar] [CrossRef]
- Khanam, S.; Rout, S.K. A Photocatalytic Hydrolysis and Degradation of Toxic Dyes by Using Plasmonic Metal–Semiconductor Heterostructures: A Review. Chemistry 2022, 4, 454–479. [Google Scholar] [CrossRef]
- Fu, Y.; Yin, Z.; Qin, L.; Huang, D.; Yi, H.; Liu, X.; Liu, S.; Zhang, M.; Li, B.; Li, L.; et al. Recent progress of noble metals with tailored features in catalytic oxidation for organic pollutants degradation. J. Hazard. Mater. 2022, 422, 126950. [Google Scholar] [CrossRef]
- Lu, F.; Astruc, D. Nanomaterials for removal of toxic elements from water. Coord. Chem. Rev. 2018, 356, 147–164. [Google Scholar] [CrossRef]
- Laishram, D.; Kim, S.-B.; Lee, S.-Y.; Park, S.-J. Advancements in Biochar as a Sustainable Adsorbent for Water Pollution Mitigation. Adv. Sci. 2025, 12, 2410383. [Google Scholar] [CrossRef]
- Guo, C.; Zhang, L.-Q.; Jiang, W. Biodegrading plastics with a synthetic non-biodegradable enzyme. Chem 2023, 9, 363–376. [Google Scholar] [CrossRef]
- Araujo, M.S.; Costa-Silva, D.; Izidoro, J.C.; Fungaro, D.A.; Castanho, S.M. Zeolite Synthesized from Solid Waste for Eco-System Remediation: Selective Adsorption in Wastewater. Chemistry 2024, 7, 3. [Google Scholar] [CrossRef]
- Saafie, N.; Zulfiqar, M.; Samsudin, M.F.R.; Sufian, S. Current Scenario of MXene-Based Nanomaterials for Wastewater Remediation: A Review. Chemistry 2022, 4, 1576–1608. [Google Scholar] [CrossRef]
- Li, Z.; Chen, Y.; Guo, Y.; Wang, Y.; Guan, T.; Bai, J.; Hu, D.; Li, H.; Du, J. Preparation of ultra-thin porous carbon nitride and its photocatalytic H2O2 production and photodegradation of RhB. Appl. Surf. Sci. 2022, 598, 153866. [Google Scholar] [CrossRef]
- Khanam, S.; Rout, S.K. Enhanced Photocatalytic Oxidation of RhB and MB Using Plasmonic Performance of Ag Deposited on Bi2WO6. Chemistry 2022, 4, 272–296. [Google Scholar] [CrossRef]
- Ashu Abey, S.; Reis, N.M.; Emanuelsson, E.A.C.; Expósito, A.J. Harnessing visible light: Advanced photocatalytic strategies for sustainable environmental reactions. Chem. Eng. J. 2025, 519, 164951. [Google Scholar] [CrossRef]
- Zheng, Z.; Tian, S.; Feng, Y.; Zhao, S.; Li, X.; Wang, S.; He, Z. Recent advances of photocatalytic coupling technologies for wastewater treatment. Chin. J. Catal. 2023, 54, 88–136. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X.; Liu, B.; Zhang, Y.; Zhao, Y.; Wang, S. Directional Regulation of Reactive Oxygen Species in Titanium Dioxide Boosting the Photocatalytic Degradation Performance of Azo Dyes. J. Colloid Interface Sci. 2024, 673, 275–283. [Google Scholar] [CrossRef]
- Dong, F.; Pang, Z.; Yang, S.; Lin, Q.; Song, S.; Li, C.; Ma, X.; Nie, S. Improving Wastewater Treatment by Triboelectric-Photo/Electric Coupling Effect. ACS Nano 2022, 16, 3449–3475. [Google Scholar] [CrossRef]
- Zhang, P.; He, M.; Teng, W.; Li, F.; Qiu, X.; Li, K.; Wang, H. Ordered mesoporous materials for water pollution treatment: Adsorption and catalysis. Green Energy Environ. 2024, 9, 1239–1256. [Google Scholar] [CrossRef]
- Song, Y.; Phipps, J.; Zhu, C.; Ma, S. Porous Materials for Water Purification. Angew. Chem. Int. Ed. 2023, 62, e202216724. [Google Scholar] [CrossRef]
- Cao, S.; Low, J.; Yu, J.; Jaroniec, M. Polymeric photocatalysts based on graphitic carbon nitride. Adv. Mater. 2015, 27, 2150–2176. [Google Scholar] [CrossRef]
- Ong, W.J.; Tan, L.L.; Ng, Y.H.; Yong, S.T.; Chai, S.P. Graphitic Carbon Nitride (g-C3N4)-Based Photocatalysts for Artificial Photosynthesis and Environmental Remediation: Are We a Step Closer to Achieving Sustainability? Chem. Rev. 2016, 116, 7159–7329. [Google Scholar] [CrossRef]
- Khan, M.A.; Mutahir, S.; Shaheen, I.; Qunhui, Y.; Bououdina, M.; Humayun, M. Recent advances over the doped g-C3N4 in photocatalysis: A review. Coord. Chem. Rev. 2025, 522, 216227. [Google Scholar] [CrossRef]
- Yu, Y.; Huang, H. Coupled adsorption and photocatalysis of g-C3N4 based composites: Material synthesis, mechanism, and environmental applications. Chem. Eng. J. 2023, 453, 139755. [Google Scholar] [CrossRef]
- Wang, J.; Hao, D.; Ye, J.; Umezawa, N. Determination of Crystal Structure of Graphitic Carbon Nitride: Ab Initio Evolutionary Search and Experimental Validation. Chem. Mater. 2017, 29, 2694–2707. [Google Scholar] [CrossRef]
- Cai, W.; Cui, J.; Li, K.; Zhang, Z.; Xie, H.; Zhong, Q.; Qu, H. Insight into the surface property modification-enhanced C3N4 performance of photocatalytic nitrogen fixation. Chem. Commun. 2022, 58, 6502–6505. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Chen, W.; Sun, C.; Han, L.; Qin, C.; Chen, M.; Wang, X.; Wang, E.; Su, Z. Oxidative Polyoxometalates Modified Graphitic Carbon Nitride for Visible-Light CO2 Reduction. ACS Appl. Mater. Interfaces 2017, 9, 11689–11695. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, Z.; Liu, Y.; Peng, W.; Fu, X.; Zhou, J.; Han, L.; Hua, Y.; Zhou, Z.-Y. A multi-centre metal-free COF@g-C3N4 catalyst assembled with covalent bonds for photocatalytic CO2 reduction. Dalton Trans. 2025, 54, 1625–1632. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Wang, W.; Liu, G.; Chen, Z.; Lv, K.; Zhu, J. Photocatalytic performances of g-C3N4 based catalysts for RhB degradation: Effect of preparation conditions. Appl. Surf. Sci. 2015, 358, 313–318. [Google Scholar] [CrossRef]
- Yang, Q.; Chen, C.; Zhang, Q.; Zhang, Z.; Fang, X. Molecular engineering of supramolecular precursor to modulate g-C3N4 for boosting photocatalytic hydrogen evolution. Carbon 2020, 164, 337–348. [Google Scholar] [CrossRef]
- Ma, S.; Wang, S.; Wang, X.; Wang, S. Recent Progress on the Surface and Bulk Modification of Carbon Nitrides for Photocatalytic Carbon Dioxide Reduction. Energy Technol. 2024, 13, 2400136. [Google Scholar] [CrossRef]
- Yu, H.; Shi, R.; Zhao, Y.; Bian, T.; Zhao, Y.; Zhou, C.; Waterhouse, G.I.N.; Wu, L.-Z.; Tung, C.-H.; Zhang, T. Alkali-Assisted Synthesis of Nitrogen Deficient Graphitic Carbon Nitride with Tunable Band Structures for Efficient Visible-Light-Driven Hydrogen Evolution. Adv. Mater. 2017, 29, 1605148. [Google Scholar] [CrossRef]
- Wang, S.; Li, Y.; Wang, X.; Zi, G.; Zhou, C.; Liu, B.; Liu, G.; Wang, L.; Huang, W. One-step Supramolecular Preorganization Constructed Crinkly Graphitic Carbon Nitride Nanosheets with Enhanced Photocatalytic Activity. J. Mater. Sci. Technol. 2022, 104, 155–162. [Google Scholar] [CrossRef]
- Hong, Z.; Shen, B.; Chen, Y.; Lin, B.; Gao, B. Enhancement of photocatalytic H2 evolution over nitrogen-deficient graphitic carbon nitride. J. Mater. Chem. A 2013, 1, 11754–11761. [Google Scholar] [CrossRef]
- Niu, P.; Yin, L.-C.; Yang, Y.-Q.; Liu, G.; Cheng, H.-M. Increasing the Visible Light Absorption of Graphitic Carbon Nitride (Melon) Photocatalysts by Homogeneous Self-Modification with Nitrogen Vacancies. Adv. Mater. 2014, 26, 8046–8052. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, K.; Zu, S.-Z.; Han, B.-H.; Wei, Z. Hierarchical Nanocomposites of Polyaniline Nanowire Arrays on Graphene Oxide Sheets with Synergistic Effect for Energy Storage. ACS Nano 2010, 4, 5019–5026. [Google Scholar] [CrossRef]
- Sun, B.-w.; Yu, H.-y.; Yang, Y.-j.; Li, H.-j.; Zhai, C.-y.; Qian, D.-J.; Chen, M. New complete assignment of X-ray powder diffraction patterns in graphitic carbon nitride using discrete Fourier transform and direct experimental evidence. Phys. Chem. Chem. Phys. 2017, 19, 26072–26084. [Google Scholar] [CrossRef]
- Zuo, S.; Chen, J.; Liu, W.; Yao, C.; Mao, H.; Li, Y.; Fu, Y.; Liu, X. Photocatalytic activity of flower-like carbon/Ag3PO4 composite microspheres for pollutant degradation. Mater. Lett. 2017, 190, 134–137. [Google Scholar] [CrossRef]
- You, Y.; Wang, S.; Xiao, K.; Ma, T.; Zhang, Y.; Huang, H. Z-Scheme g-C3N4/Bi4NbO8Cl Heterojunction for Enhanced Photocatalytic Hydrogen Production. ACS Sustain. Chem. Eng. 2018, 6, 16219–16227. [Google Scholar] [CrossRef]
- Yuan, C.; Zhang, Y.; Zong, Z.; Zhou, S.; Cui, H.; Tan, H. In situ growth of an ultrathin Cu/g-C3N4 coating over SBA-15 for catalytic wet air oxidation of pollutants under extremely mild conditions. Green Chem. 2024, 26, 6601–6615. [Google Scholar] [CrossRef]
- Huang, Z.; Li, F.; Chen, B.; Lu, T.; Yuan, Y.; Yuan, G. Well-dispersed g-C3N4 nanophases in mesoporous silica channels and their catalytic activity for carbon dioxide activation and conversion. Appl. Catal. B Environ. 2013, 136–137, 269–277. [Google Scholar] [CrossRef]
- Ge, L.; Zuo, F.; Liu, J.; Ma, Q.; Wang, C.; Sun, D.; Bartels, L.; Feng, P. Synthesis and Efficient Visible Light Photocatalytic Hydrogen Evolution of Polymeric g-C3N4 Coupled with CdS Quantum Dots. J. Phys. Chem. C 2012, 116, 13708–13714. [Google Scholar] [CrossRef]
- Miao, X.; Yue, X.; Ji, Z.; Shen, X.; Zhou, H.; Liu, M.; Xu, K.; Zhu, J.; Zhu, G.; Kong, L.; et al. Nitrogen-doped carbon dots decorated on g-C3N4/Ag3PO4 photocatalyst with improved visible light photocatalytic activity and mechanism insight. Appl. Catal. B Environ. 2018, 227, 459–469. [Google Scholar] [CrossRef]
- Irran, E.; Jürgens, B.; Schnick, W. Synthesis, crystal structure determination from X-ray powder diffractometry and vibrational spectroscopy of the tricyanomelaminate monohydrates M3[C6N9]·H2O (M = K, Rb). Solid State Sci. 2002, 4, 1305–1311. [Google Scholar] [CrossRef]
- Lei, W.; Portehault, D.; Dimova, R.; Antonietti, M. Boron Carbon Nitride Nanostructures from Salt Melts: Tunable Water-Soluble Phosphors. J. Am. Chem. Soc. 2011, 133, 7121–7127. [Google Scholar] [CrossRef]
- Cui, Y.; Ding, Z.; Fu, X.; Wang, X. Construction of Conjugated Carbon Nitride Nanoarchitectures in Solution at Low Temperatures for Photoredox Catalysis. Angew. Chem. Int. Ed. 2012, 51, 11814–11818. [Google Scholar] [CrossRef]
- Song, X.; Hu, Y.; Zheng, M.; Wei, C. Solvent-free in situ synthesis of g-C3N4 /{0 0 1}TiO2 composite with enhanced UV-and visible-light photocatalytic activity for NO oxidation. Appl. Catal. B 2016, 182, 587–597. [Google Scholar] [CrossRef]
- Han, H.; Wang, B.; Tang, Q.; Jia, S.; Liu, J.; Li, H.; Wang, C.; Xu, H.; Hua, Y. Non-metallic Nitrogen-Doped Graphene Quantum Dots Coupled with G-C3N4 Achieve Efficient Photocatalytic Performance. Appl. Surf. Sci. 2023, 649, 159171. [Google Scholar] [CrossRef]
- Zhou, J.; Dong, M.; Sun, Y.; Shan, G.G.; Sun, C.Y.; You, S.Q.; Wang, X.L.; Kang, Z.H.; Su, Z.M. Dynamic Interface with Enhanced Visible-Light Absorption and Electron Transfer for Direct Photoreduction of Flue Gas to Syngas. ACS Appl. Mater. Interfaces 2022, 14, 6476–6483. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Li, J.; Kan, L.; Zhang, L.; Huang, Q.; Yan, Y.; Chen, Y.; Liu, J.; Li, S.-L.; Lan, Y.-Q. Linking oxidative and reductive clusters to prepare crystalline porous catalysts for photocatalytic CO2 reduction with H2O. Nat. Commun. 2022, 13, 4681. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Li, C.-X.; Zhou, J.; Hua, Y.; Zhou, Z.-Y.; Su, Z.-M. A covalent organic framework integrating photocatalytic center and electron reservoir for photocatalytic CO2 reduction to HCOOH. Sep. Purif. Technol. 2024, 346, 127466. [Google Scholar] [CrossRef]
- Li, J.; Zhou, J.; Wang, X.-H.; Guo, C.; Li, R.-H.; Zhuang, H.; Feng, W.; Hua, Y.; Lan, Y.-Q. In situ Construction of Single-Atom Electronic Bridge on COF to Enhance Photocatalytic H2 Production. Angew. Chem. Int. Ed. 2024, 63, e202411721. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, N.; Khan, I.; Yamani, Z.H.; Qurashi, A. Sonochemical Assisted Solvothermal Synthesis of Gallium Oxynitride Nanosheets and their Solar-Driven Photoelectrochemical Water-Splitting Applications. Sci. Rep. 2016, 6, 32319. [Google Scholar] [CrossRef]
- Kumar, D.P.; Hong, S.; Reddy, D.A.; Kim, T.K. Ultrathin MoS2 layers anchored exfoliated reduced graphene oxide nanosheet hybrid as a highly efficient cocatalyst for CdS nanorods towards enhanced photocatalytic hydrogen production. Appl. Catal. B Environ. 2017, 212, 7–14. [Google Scholar] [CrossRef]
- Ye, Y.; Yang, H.; Wang, X.; Feng, W. Photocatalytic, Fenton and Photo-Fenton Degradation of RhB over Z-scheme g-C3N4/LaFeO3 Heterojunction Photocatalysts. Mater. Sci. Semicon. Proc. 2018, 82, 14–24. [Google Scholar] [CrossRef]
- Mousavi, M.; Habibi-Yangjeh, A.; Seifzadeh, D. Novel Ternary g-C3N4 /Fe3O4/MnWO4 Nanocomposites: Synthesis, Characterization, and Visible-Light Photocatalytic Performance for Environmental Purposes. J. Mater. Sci. Technol. 2018, 34, 1638–1651. [Google Scholar] [CrossRef]
- Lops, C.; Ancona, A.; Cesare, K.D.; Dumontel, B.; Garino, N.; Canavese, G.; Hernandez, S.; Cauda, V. Sonophotocatalytic Degradation Mechanisms of Rhodamine B Dye Via Radicals Generation by Micro- and Nano-Particles of ZnO. Appl. Catal. B Environ. 2019, 243, 629–640. [Google Scholar] [CrossRef]
- Jun, Y.-S.; Hong, W.H.; Antonietti, M.; Thomas, A. Mesoporous, 2D Hexagonal Carbon Nitride and Titanium Nitride/Carbon Composites. Adv. Mater. 2009, 21, 4270–4274. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, L.; Wang, D.; Zhang, Q.; Zeng, J.; Zhang, R. Facile synthesis of nitrogen-defective g-C3N4 for superior photocatalytic degradation of rhodamine B. RSC Adv. 2021, 11, 30503–30509. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhu, C.; Wan, W.; Qian, K.; Xie, J. Constructing graphite-like carbon nitride modified hierarchical yolk–shell TiO2 spheres for water pollution treatment and hydrogen production. J. Mater. Chem. A 2016, 4, 1806–1818. [Google Scholar] [CrossRef]
- Qiu, L.; Zhou, Z.; Ma, M.; Li, P.; Lu, J.; Hou, Y.; Chen, X.; Duo, S. Enhanced Visible-Light Photocatalytic Performance of SAPO-5-Based g-C3N4 Composite for Rhodamine B (RhB) Degradation. Materials 2019, 12, 3948. [Google Scholar] [CrossRef]
- Kolesnyk, I.; Kujawa, J.; Bubela, H.; Konovalova, V.; Burban, A.; Cyganiuk, A.; Kujawski, W. Photocatalytic properties of PVDF membranes modified with g-C3N4 in the process of Rhodamines decomposition. Sep. Purif. Technol. 2020, 250, 117231. [Google Scholar] [CrossRef]
- Janardhana, D.; Jayaramu, S.N.; Coetsee, E.; Motaung, D.E.; Swart, H.C. Nanosheet g-C3N4 decorated α-Bi2O3:Eu3+ needles for efficient photocatalytic degradation of rhodamine B dye. Mat. Sci. Semicon. Proc. 2024, 184, 108789. [Google Scholar] [CrossRef]
- Zhao, F.; Khaing, K.K.; Yin, D.; Liu, B.; Chen, T.; Wu, C.; Huang, K.; Deng, L.; Li, L. Large enhanced photocatalytic activity of g-C3N4 by fabrication of a nanocomposite with introducing upconversion nanocrystal and Ag nanoparticles. RSC Adv. 2018, 8, 42308–42321. [Google Scholar] [CrossRef]
- Nisha, V.; Sasi, S.C.; Krishnan, R.; Paravannoor, A.; Palantavida, S.; Hinder, S.J.; Pillai, S.C.; Kizhakkekilikoodayil Vijayan, B. S-doped g-C3N4 for fluorescence sensing of Fe3+ and photocatalytic dye degradation under solar light. Next Mater. 2024, 2, 100082. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).