Theoretical Study on the Electronic Structure of Fe(0)–, Pd(0)–, and Pt(0)–Phosphine–Carbon Dioxide Complexes
Abstract
1. Introduction
2. Computational Details
3. Results and Discussion
3.1. QTAIM Analysis: Bonding Trends and Metal—CO2 Interactions
3.2. Geometric and IR Trends: CO2 Activation Efficiency
3.3. EDA-NOCV Analysis: Charge Transfer and Orbital Interactions
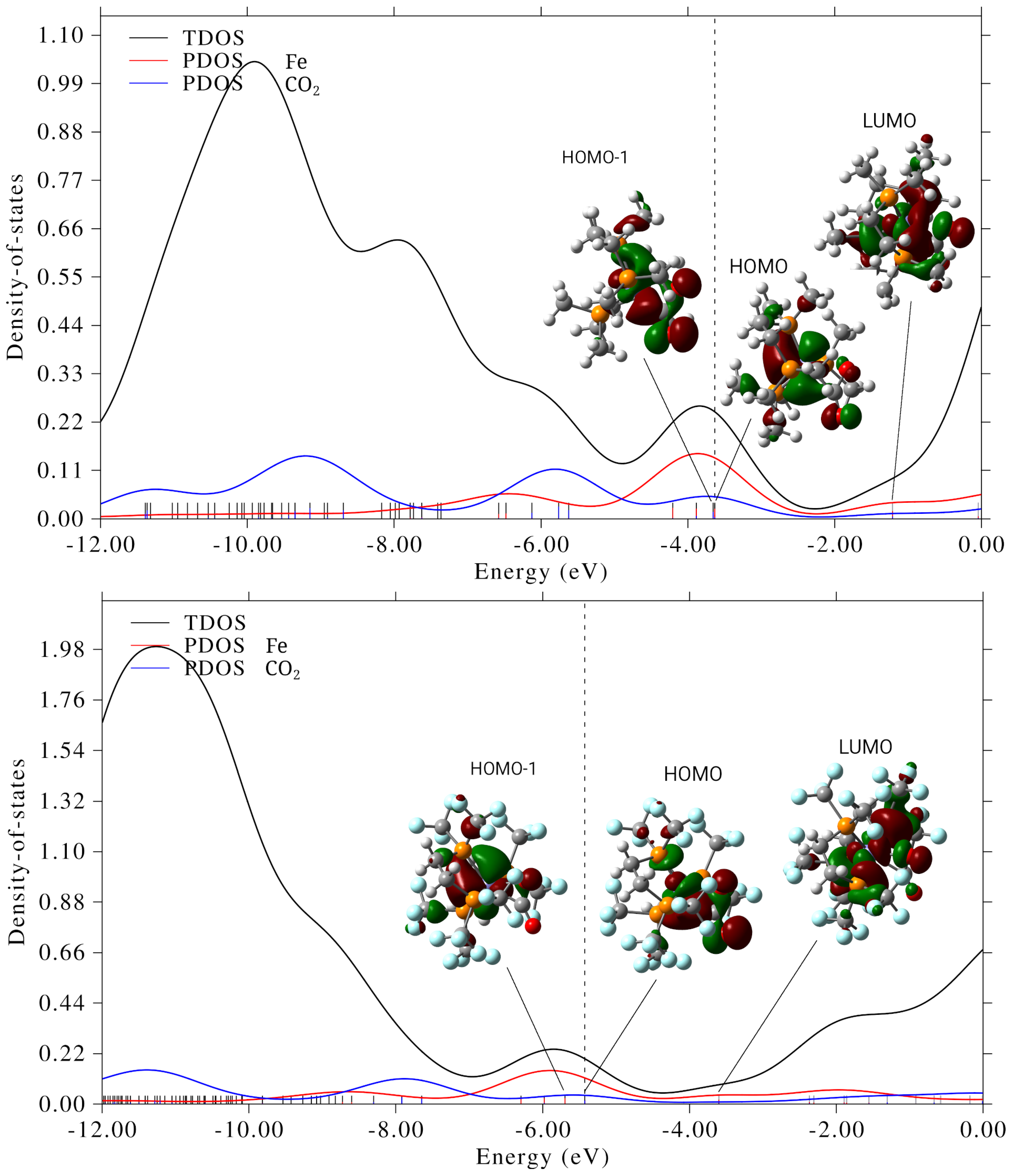
3.4. DOS/PDOS Analysis: Electronic Structure Depending on Phosphine Substituents
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yin, X.; Moss, J.R. Recent developments in the activation of carbon dioxide by metal complexes. Coord. Chem. Rev. 1999, 181, 27–59. [Google Scholar] [CrossRef]
- Braunstein, P.; Matt, D.; Nobel, D. Reactions of carbon dioxide with carbon-carbon bond formation catalyzed by transition-metal complexes. Chem. Rev. 1988, 88, 747–764. [Google Scholar] [CrossRef]
- Behr, A. Carbon dioxide as an alternative C1 synthetic unit: Activation by transition-metal complexes. Angew. Chem. Int. Ed. 1988, 27, 661–678. [Google Scholar] [CrossRef]
- Paparo, A.; Okuda, J. Carbon dioxide complexes: Bonding modes and synthetic methods. Coord. Chem. Rev. 2017, 334, 136–149. [Google Scholar] [CrossRef]
- Yang, Z.Z.; He, L.N.; Gao, J.; Liu, A.H.; Yu, B. Carbon dioxide utilization with C–N bond formation: Carbon dioxide capture and subsequent conversion. Energy Environ. Sci. 2012, 5, 6602–6639. [Google Scholar] [CrossRef]
- Yuan, Z.; Eden, M.R.; Gani, R. Toward the development and deployment of large-scale carbon dioxide capture and conversion processes. Ind. Eng. Chem. Res. 2016, 55, 3383–3419. [Google Scholar] [CrossRef]
- Lu, B.; Fan, Y.; Zhi, X.; Han, Z.; Wu, F.; Li, X.; Luo, C.; Zhang, L. Material design and prospect of dual-functional materials for integrated carbon dioxide capture and conversion. Carbon Capture Sci. Technol. 2024, 12, 100207. [Google Scholar] [CrossRef]
- Cokoja, M.; Bruckmeier, C.; Rieger, B.; Herrmann, W.A.; Kühn, F.E. Transformation of carbon dioxide with homogeneous transition-metal catalysts: A molecular solution to a global challenge? Angew. Chem. Int. Ed. 2011, 50, 8510–8537. [Google Scholar] [CrossRef]
- Aresta, M.; Nobile, C.F.; Albano, V.G.; Forni, E.; Manassero, M. New nickel–carbon dioxide complex: Synthesis, properties, and crystallographic characterization of (carbon dioxide)-bis (tricyclohexylphosphine) nickel. J. Chem. Soc. Chem. Commun. 1975, 636–637. [Google Scholar] [CrossRef]
- Durfy, C.S.; Zurakowski, J.A.; Jobin, G.; Drover, M.W. An Investigation of Allyl-Substituted Bis (Diphosphine) Iron Complexes: Towards Precursors for Cooperative CO2 Activation. Chem. J. 2024, 30, e202302721. [Google Scholar] [CrossRef]
- Metters, O.J.; Forrest, S.J.; Sparkes, H.A.; Manners, I.; Wass, D.F. Small molecule activation by intermolecular Zr (IV)-phosphine frustrated Lewis pairs. J. Am. Chem. Soc. 2016, 138, 1994–2003. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Gong, J.K.; Collins, A.W.; Grove, L.J.; Seyler, J.W. Theoretical study of carbon dioxide coordination in palladium complexes. Appl. Organomet. Chem. 2001, 15, 95–98. [Google Scholar] [CrossRef]
- Kégl, T.; Ponec, R.; Kollár, L. Theoretical Insights into the Nature of Nickel Carbon Dioxide Interactions in Pt(PH3)2(η2-CO2). J. Phys. Chem. A 2011, 115, 12463–12473. [Google Scholar] [CrossRef] [PubMed]
- Kégl, T.R.; Carrilho, R.M.; Kégl, T. Theoretical insights into the electronic structure of nickel (0)-diphosphine-carbon dioxide complexes. J. Organomet. Chem. 2020, 924, 121462. [Google Scholar] [CrossRef]
- Kégl, T.R.; Kégl, T. Comparative Analysis of Nickel–Phosphine Complexes with Cumulated Double Bond Ligands: Structural Insights and Electronic Interactions via ETS-NOCV and QTAIM Approaches. Molecules 2024, 29, 324. [Google Scholar] [CrossRef]
- Kégl, T.R.; Kollár, L.; Kégl, T. DFT Study on the Mechanism of Iron-Catalyzed Diazocarbonylation. Molecules 2020, 25, 5860. [Google Scholar] [CrossRef]
- Pálinkás, N.; Kollár, L.; Kégl, T. Nature of the Metal-Ligand Interactions in Complexes M(PH3)2(η2-L)(M = Ni, Pd, Pt; L = CO2, COS, CS2): A Theoretical Study. ChemistrySelect 2017, 2, 5740–5750. [Google Scholar] [CrossRef]
- Komiya, S.; Akita, M.; Kasuga, N.; Hirano, M.; Fukuoka, A. Synthesis, structure and reactions of a carbon dioxide complex of iron (0) containing 1, 2-bis (diethylphosphino) ethane ligands. J. Chem. Soc. Chem. Comun. 1994, 1115–1116. [Google Scholar] [CrossRef]
- Jegat, C.; Fouassier, M.; Tranquille, M.; Mascetti, J.; Tommasi, I.; Aresta, M.; Ingold, F.; Dedieu, A. Carbon dioxide coordination chemistry. 3. Vibrational, NMR, theoretical studies of (carbon dioxide)bis(tricyclohexylphosphine)nickel. Inorg. Chem. 1993, 32, 1279–1289. [Google Scholar] [CrossRef]
- Navarro-Jaén, S.; Virginie, M.; Bonin, J.; Robert, M.; Wojcieszak, R.; Khodakov, A.Y. Highlights and challenges in the selective reduction of carbon dioxide to methanol. Nat. Rev. Chem. 2021, 5, 564–579. [Google Scholar] [CrossRef]
- Boutin, E.; Wang, M.; Lin, J.C.; Mesnage, M.; Mendoza, D.; Lassalle-Kaiser, B.; Hahn, C.; Jaramillo, T.F.; Robert, M. Aqueous electrochemical reduction of carbon dioxide and carbon monoxide into methanol with cobalt phthalocyanine. Angew. Chem. Int. Ed. 2019, 58, 16172–16176. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Kortlever, R.; Kas, R.; Birdja, Y.Y.; Diaz-Morales, O.; Kwon, Y.; Ledezma-Yanez, I.; Schouten, K.J.P.; Mul, G.; Koper, M.T. Electrocatalytic reduction of carbon dioxide to carbon monoxide and methane at an immobilized cobalt protoporphyrin. Nat. Commun. 2015, 6, 8177. [Google Scholar] [CrossRef]
- Agarwala, H.; Chen, X.; Lyonnet, J.R.; Johnson, B.A.; Ahlquist, M.; Ott, S. Alternating Metal-Ligand Coordination Improves Electrocatalytic CO2 Reduction by a Mononuclear Ru Catalyst. Angew. Chem. Int. Ed. 2023, 135, e202218728. [Google Scholar] [CrossRef]
- Yao, Y.; Wu, J.H.; Liu, G.; Zhang, R.; Yang, Z.S.; Gao, S.; Lau, T.C.; Zhang, J.L. A Bio-Inspired Bimetallic Fe-M Catalyst for Electro-and Photochemical CO2 Reduction. ChemCatChem 2024, 16, e202301705. [Google Scholar] [CrossRef]
- Guo, L.; Lamb, K.J.; North, M. Recent developments in organocatalysed transformations of epoxides and carbon dioxide into cyclic carbonates. Green Chem. 2021, 23, 77–118. [Google Scholar] [CrossRef]
- Cokoja, M.; Wilhelm, M.E.; Anthofer, M.H.; Herrmann, W.A.; Kühn, F.E. Synthesis of cyclic carbonates from epoxides and carbon dioxide by using organocatalysts. ChemSusChem 2015, 8, 2436–2454. [Google Scholar] [CrossRef]
- Lopes, E.J.; Ribeiro, A.P.; Martins, L.M. New trends in the conversion of CO2 to cyclic carbonates. Catalysts 2020, 10, 479. [Google Scholar] [CrossRef]
- Alves, M.; Grignard, B.; Méreau, R.; Jerome, C.; Tassaing, T.; Detrembleur, C. Organocatalyzed coupling of carbon dioxide with epoxides for the synthesis of cyclic carbonates: Catalyst design and mechanistic studies. Catal. Sci. Technol. 2017, 7, 2651–2684. [Google Scholar] [CrossRef]
- Richard, F.; Bader, R. Atoms in Molecules: A Quantum Theory; Oxford Academic: Oxford, UK, 1990. [Google Scholar]
- Bader, R.F. Bond paths are not chemical bonds. J. Phys. Chem. A 2009, 113, 10391–10396. [Google Scholar] [CrossRef]
- Mitoraj, M.; Michalak, A. Natural orbitals for chemical valence as descriptors of chemical bonding in transition metal complexes. J. Mol. Model. 2007, 13, 347–355. [Google Scholar] [CrossRef]
- Mitoraj, M.; Michalak, A. Applications of natural orbitals for chemical valence in a description of bonding in conjugated molecules. J. Mol. Model. 2008, 14, 681–687. [Google Scholar] [CrossRef]
- Mitoraj, M.P.; Michalak, A.; Ziegler, T. A combined charge and energy decomposition scheme for bond analysis. J. Chem. Theory Comput. 2009, 5, 962–975. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision C.01; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Kégl, T.; Pálinkás, N.; Kollár, L.; Kégl, T. Computational Characterization of Bidentate P-Donor Ligands: Direct Comparison to Tolman’s Electronic Parameters. Molecules 2018, 23, 3176. [Google Scholar] [CrossRef]
- Papp, T.; Nagy, P.R.; Kegl, T. Advanced computation of enthalpies for a range of hydroformylation reactions with a predictive power to match experiments. Chem. Phys. Lett. 2025, 861, 141833. [Google Scholar] [CrossRef]
- Felgueiras, A.P.; Rodrigues, F.M.; Carrilho, R.M.; Cruz, P.F.; Rodrigues, V.H.; Kégl, T.; Kollár, L.; Pereira, M.M. Stereoisomeric Tris-BINOL-Menthol Bulky Monophosphites: Synthesis, Characterisation and Application in Rhodium-Catalysed Hydroformylation. Molecules 2022, 27, 1989. [Google Scholar] [CrossRef]
- Keith, T.A. AIMAll, Version 15.05.18; TK Gristmill Software: Overland Park, KS, USA, 2015; Available online: https://aim.tkgristmill.com (accessed on 3 May 2024).
- AMS2025; SCM, Theoretical Chemistry; Vrije Universiteit: Amsterdam, The Netherlands, 2025; Available online: http://www.scm.com (accessed on 8 August 2025).
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Lu, T. A comprehensive electron wavefunction analysis toolbox for chemists, Multiwfn. J. Chem. Phys. 2024, 161, 082503. [Google Scholar] [CrossRef]
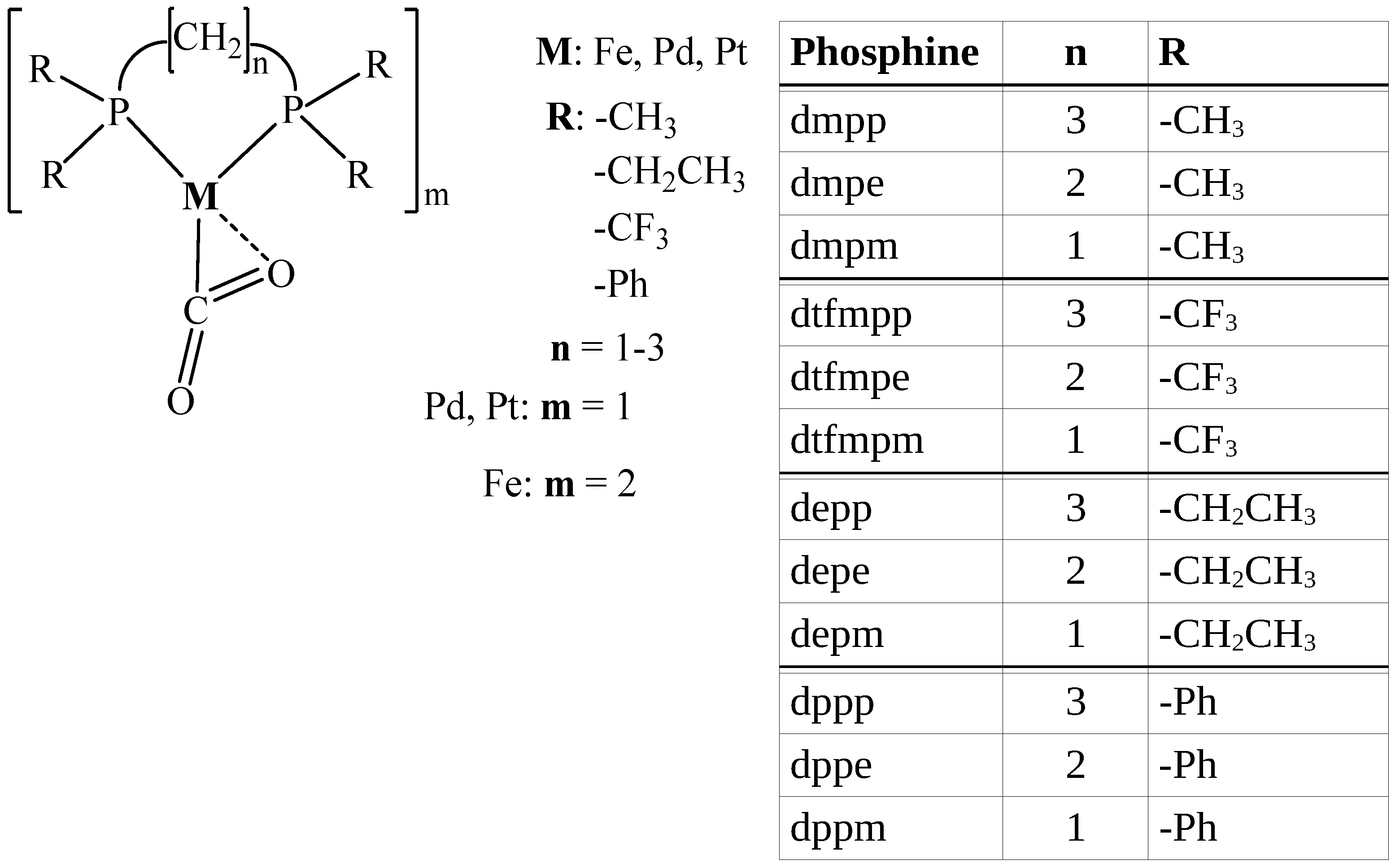
| M | Ligand | C= | C= | DI C= | DI C= | M–C | DI M–C | V/G |
|---|---|---|---|---|---|---|---|---|
| Fe | dmpp | 0.3521 | 0.4059 | 1.0571 | 1.2453 | 0.1224 | 0.7303 | 1.538 |
| Fe | dmpe | 0.3551 | 0.4068 | 1.0699 | 1.2542 | 0.1061 | 0.6014 | 1.555 |
| Fe | dmpm | 0.3574 | 0.4061 | 1.0808 | 1.2562 | 0.1252 | 0.7247 | 1.561 |
| Fe | dtfmpp | 0.3628 | 0.4228 | 1.0708 | 1.2941 | 0.1145 | 0.6682 | 1.550 |
| Fe | dtfmpe | 0.3755 | 0.4314 | 1.1077 | 1.3367 | 0.0971 | 0.5710 | 1.478 |
| Fe | dtfmpm | 0.3737 | 0.4300 | 1.1070 | 1.3341 | 0.1044 | 0.6038 | 1.516 |
| Fe | depp | 0.3498 | 0.4044 | 1.0451 | 1.2340 | 0.1234 | 0.7384 | 1.536 |
| Fe | depe | 0.3522 | 0.4041 | 1.0631 | 1.2407 | 0.1260 | 0.7313 | 1.554 |
| Fe | depm | 0.3544 | 0.4048 | 1.0733 | 1.2525 | 0.1274 | 0.7356 | 1.560 |
| Fe | dppp | 0.3519 | 0.4057 | 1.0498 | 1.2365 | 0.1329 | 0.7847 | 1.586 |
| Fe | dppe | 0.3562 | 0.4140 | 1.0524 | 1.2531 | 0.1238 | 0.7295 | 1.563 |
| Fe | dppm | 0.3527 | 0.4100 | 1.0534 | 1.2574 | 0.1266 | 0.7394 | 1.566 |
| Pd | dmpp | 0.3911 | 0.4195 | 1.1933 | 1.3006 | 0.1039 | 0.5968 | 1.481 |
| Pd | dmpe | 0.3898 | 0.4208 | 1.1897 | 1.3077 | 0.1061 | 0.6014 | 1.492 |
| Pd | dmpm | 0.3918 | 0.4256 | 1.1898 | 1.3186 | 0.1098 | 0.6135 | 1.515 |
| Pd | depp | 0.3895 | 0.4177 | 1.1896 | 1.2946 | 0.1058 | 0.6068 | 1.488 |
| Pd | depe | 0.3884 | 0.4193 | 1.1861 | 1.3000 | 0.1079 | 0.6102 | 1.500 |
| Pd | depm | 0.3895 | 0.4235 | 1.1845 | 1.3108 | 0.1103 | 0.6211 | 1.510 |
| Pd | dppp | 0.3896 | 0.4225 | 1.1884 | 1.3100 | 0.1028 | 0.5862 | 1.463 |
| Pd | dppe | 0.3889 | 0.4228 | 1.1849 | 1.3140 | 0.1028 | 0.5882 | 1.455 |
| Pd | dppm | 0.3922 | 0.4251 | 1.1900 | 1.1341 | 0.1096 | 0.6116 | 1.499 |
| Pt | dmpp | 0.3664 | 0.4178 | 1.1132 | 1.2979 | 0.1312 | 0.7320 | 1.709 |
| Pt | dmpe | 0.3632 | 0.4201 | 1.1050 | 1.3089 | 0.1324 | 0.7346 | 1.712 |
| Pt | dmpm | 0.3649 | 0.4242 | 1.1045 | 1.3157 | 0.1349 | 0.7428 | 1.731 |
| Pt | dtfmpp | 0.3840 | 0.4351 | 1.1519 | 1.3583 | 0.1035 | 0.5684 | 1.550 |
| Pt | dtfmpe | 0.3793 | 0.4352 | 1.1402 | 1.3605 | 0.1089 | 0.5906 | 1.582 |
| Pt | dtfmpm | 0.3952 | 0.4465 | 1.1722 | 1.3792 | 0.1014 | 0.5435 | 1.504 |
| Pt | depp | 0.3586 | 0.4187 | 1.0891 | 1.3022 | 0.1314 | 0.7504 | 1.68 |
| Pt | depe | 0.3616 | 0.4191 | 1.1015 | 1.3061 | 0.1333 | 0.7401 | 1.713 |
| Pt | depm | 0.3636 | 0.4213 | 1.1030 | 1.3045 | 0.1364 | 0.7499 | 1.737 |
| Pt | dppp | 0.3616 | 0.4207 | 1.0990 | 1.3070 | 0.1295 | 0.7263 | 1.680 |
| Pt | dppe | 0.3626 | 0.4198 | 1.1015 | 1.3035 | 0.1302 | 0.7281 | 1.684 |
| Pt | dppm | 0.3668 | 0.4227 | 1.1080 | 1.3046 | 0.1363 | 0.7445 | 1.738 |
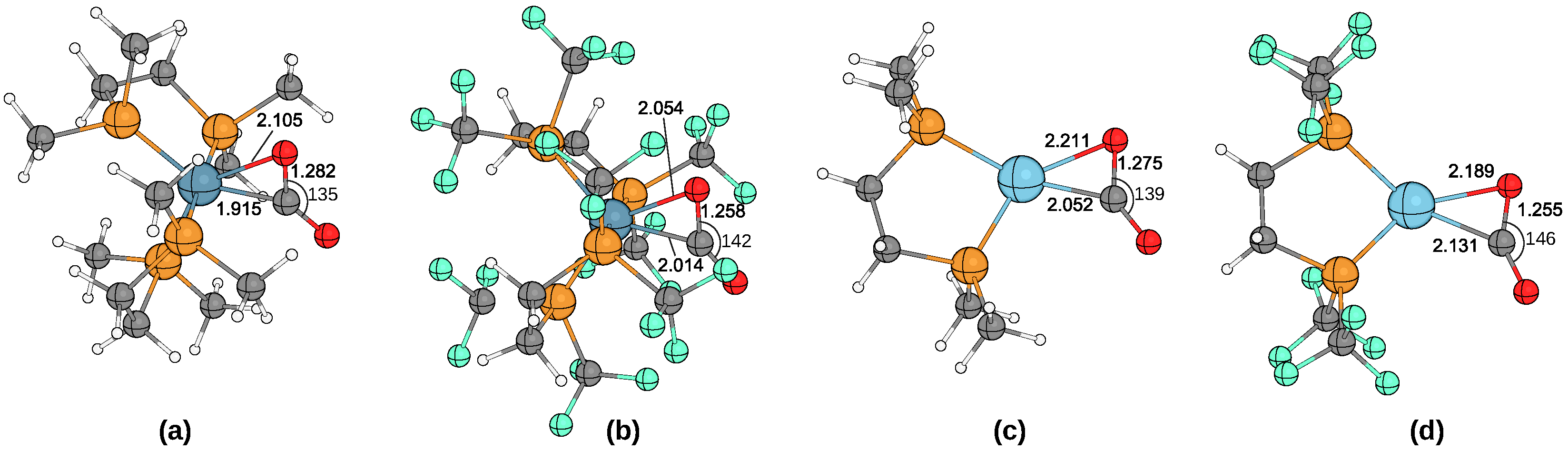
| M | Ligand | Bond Angle CO2 | Bond Length C= | Bond Length C= | Bond Length M–C |
|---|---|---|---|---|---|
| Fe | dmpp | 133.40 | 1.286 | 1.223 | 1.916 |
| Fe | dmpe | 134.76 | 1.282 | 1.222 | 1.915 |
| Fe | dmpm | 135.27 | 1.279 | 1.223 | 1.907 |
| Fe | dtfmpp | 138.82 | 1.274 | 1.204 | 1.948 |
| Fe | dtfmpe | 142.43 | 1.258 | 1.195 | 2.014 |
| Fe | dtfmpm | 142.25 | 1.260 | 1.196 | 1.985 |
| Fe | depp | 132.34 | 1.289 | 1.225 | 1.914 |
| Fe | depe | 133.92 | 1.286 | 1.225 | 1.906 |
| Fe | depm | 134.70 | 1.283 | 1.224 | 1.900 |
| Fe | dppp | 132.95 | 1.285 | 1.223 | 1.892 |
| Fe | dppe | 134.76 | 1.279 | 1.218 | 1.915 |
| Fe | dppm | 134.25 | 1.284 | 1.218 | 1.903 |
| Pd | dmpp | 143.55 | 1.240 | 1.208 | 2.095 |
| Pd | dmpe | 143.95 | 1.242 | 1.206 | 2.086 |
| Pd | dmpm | 145.81 | 1.239 | 1.201 | 2.067 |
| Pd | depp | 142.85 | 1.242 | 1.210 | 2.089 |
| Pd | depe | 143.33 | 1.243 | 1.208 | 2.079 |
| Pd | depm | 144.69 | 1.242 | 1.203 | 2.066 |
| Pd | dppp | 144.09 | 1.241 | 1.204 | 2.097 |
| Pd | dppe | 144.01 | 1.242 | 1.203 | 2.095 |
| Pd | dppm | 145.71 | 1.239 | 1.201 | 2.066 |
| Pt | dmpp | 139.19 | 1.271 | 1.210 | 2.057 |
| Pt | dmpe | 139.33 | 1.275 | 1.208 | 2.052 |
| Pt | dmpm | 140.59 | 1.273 | 1.204 | 2.041 |
| Pt | dtfmpp | 146.00 | 1.250 | 1.191 | 2.153 |
| Pt | dtfmpe | 145.55 | 1.255 | 1.191 | 2.131 |
| Pt | dtfmpm | 152.43 | 1.238 | 1.180 | 2.144 |
| Pt | depp | 137.55 | 1.280 | 1.210 | 2.053 |
| Pt | depe | 138.82 | 1.277 | 1.209 | 2.050 |
| Pt | depm | 139.70 | 1.275 | 1.207 | 2.038 |
| Pt | dppp | 138.80 | 1.276 | 1.207 | 2.059 |
| Pt | dppe | 138.83 | 1.276 | 1.208 | 2.057 |
| Pt | dppm | 140.65 | 1.271 | 1.205 | 2.036 |
| M | Ligand | ||||
|---|---|---|---|---|---|
| Fe | dmpe | −81.8 | 154.6 | −76.7 | −155.4 |
| Fe | dtfmpe | −35.8 | 130.1 | −53.5 | −107.9 |
| Pt | dmpe | −65.3 | 166.8 | −98.6 | −130.9 |
| Pt | dtfmpe | −42.4 | 129.2 | −75.8 | −95.8 |
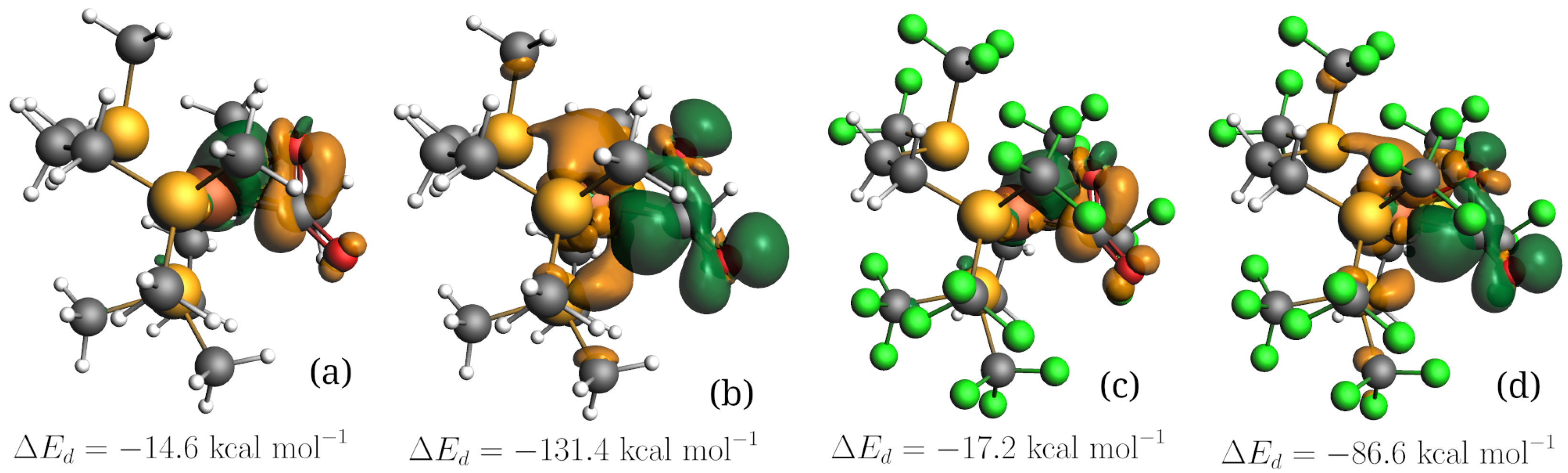
| M | Ligand | QH | ||
|---|---|---|---|---|
| Fe | dmpe | −131.4 (1.27) | −14.6 (0.43) | −0.532 |
| Fe | dtfmpe | −86.6 (1.12) | −17.2 (0.46) | −0.338 |
| Pt | dmpe | −105.3 (1.06) | −19.7 (0.42) | −0.374 |
| Pt | dtfmpe | −72.0 (0.92) | −20.3 (0.40) | −0.252 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kégl, T.R.; Kégl, T. Theoretical Study on the Electronic Structure of Fe(0)–, Pd(0)–, and Pt(0)–Phosphine–Carbon Dioxide Complexes. Chemistry 2025, 7, 152. https://doi.org/10.3390/chemistry7050152
Kégl TR, Kégl T. Theoretical Study on the Electronic Structure of Fe(0)–, Pd(0)–, and Pt(0)–Phosphine–Carbon Dioxide Complexes. Chemistry. 2025; 7(5):152. https://doi.org/10.3390/chemistry7050152
Chicago/Turabian StyleKégl, Tímea R., and Tamás Kégl. 2025. "Theoretical Study on the Electronic Structure of Fe(0)–, Pd(0)–, and Pt(0)–Phosphine–Carbon Dioxide Complexes" Chemistry 7, no. 5: 152. https://doi.org/10.3390/chemistry7050152
APA StyleKégl, T. R., & Kégl, T. (2025). Theoretical Study on the Electronic Structure of Fe(0)–, Pd(0)–, and Pt(0)–Phosphine–Carbon Dioxide Complexes. Chemistry, 7(5), 152. https://doi.org/10.3390/chemistry7050152







