Abstract
We hereby report a simple and efficient method for the preparation of (E)-3-aryl-2-styryl-2,3-dihydroquinazolin-4-(1H)-ones, from isatoic anhydride, anilines, and cinnamaldehydes in the presence of 20 mol% citric acid in methanol at 60 °C for 2 h. The styryl-dihydroquinazolin-4-(1H)-one products were obtained in moderate and good yields (30–80%) through the three-component condensation reaction, under an environment-friendly protocol. The latter were easily transformed into styrylquinazolin-4-(3H)-one derivatives with 57–91% yields using a mild oxidation with an I2/DMSO system for less than 60 min.
1. Introduction
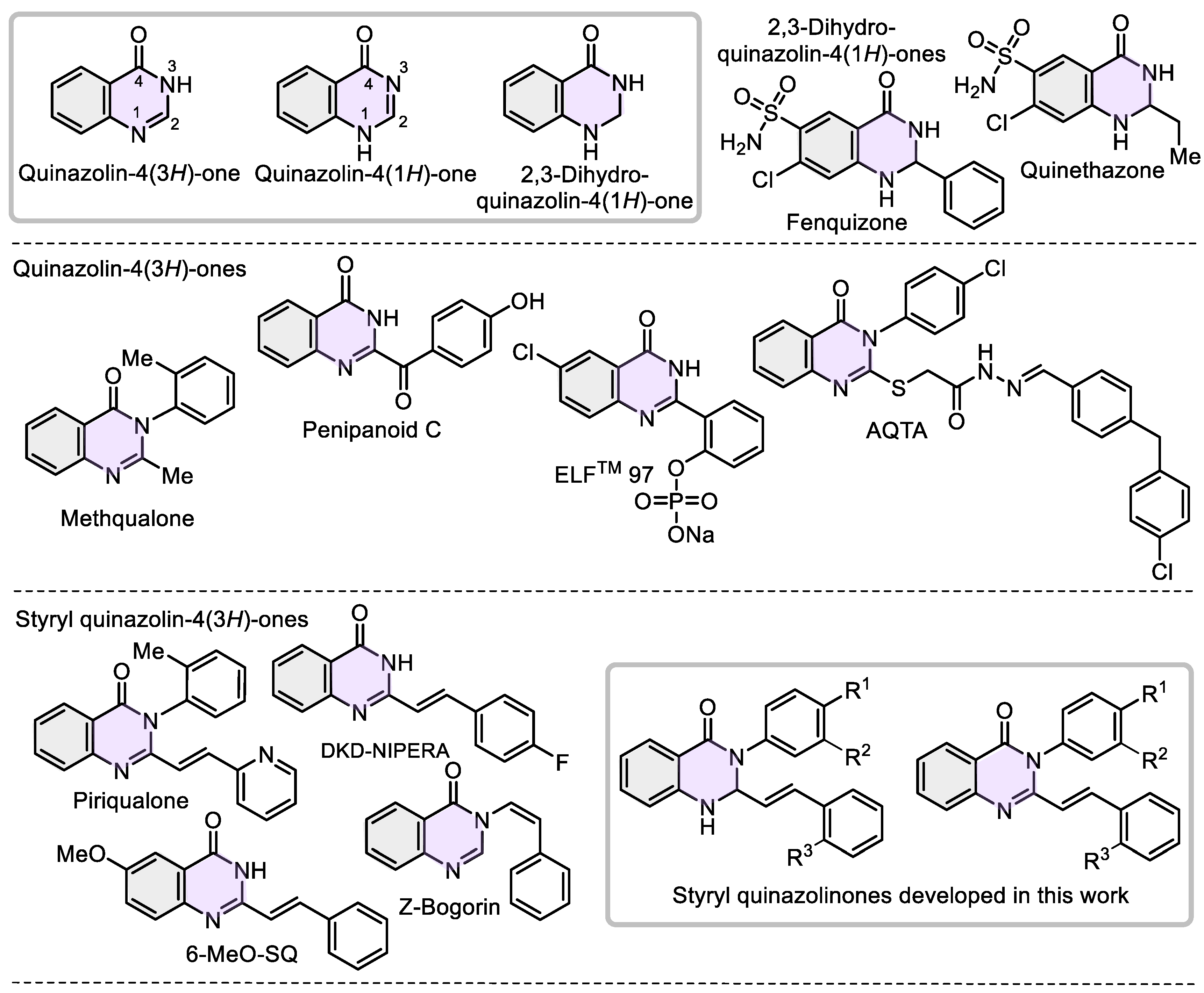
The quinazolinone skeletons (dihydroquinazolin-4-(1H)-ones and quinazolin-4-(3H)-ones) are the main structural parts for numerous secondary metabolites and privileged scaffolds in medicinal chemistry [1,2,3,4,5], especially in cancer drug research [6,7] and epilepsy treatment [8]. The chemical characteristics of the quinazolinone core (aromatic ring, two not equivalent nitrogen atoms in the pyrimidine ring, group C=O, π-conjugated lactam-aryl motif, and polarized endocyclic imine C=N function) make simple functionalized quinazolinones attractive, proper, and versatile models or/and precursors for use in agrochemical and luminescent settings [9,10,11,12].
Particularly, Quinethazone and Fenquizone are diuretics employed to treat hypertension [13], while Methaqualone (“Quaaludes”) is a sedative–hypnotic medication with effects similar to barbiturates. Currently, it has no accepted medical use due to its addictive nature [14] (Figure 1). The alkaloid Penipanoid C displayed high cytotoxic activity [15]. Fluorophore ELFTM 97 is insoluble in aqueous media but exhibits strong fluorescence in the solid state [16]. The 4-oxo-3-dihydroquinazolin-2-yl derivative AQTA exhibited superior sub-micromolar antiproliferative activity against the NSC lung cancer cell line NCI-H460, functioning as a potent EGFR inhibitor [17]. Piriqualone serves as an anticonvulsant agent for treating neurodegenerative and CNS-trauma-related conditions [18]. The compound 6-MeO-SQ inhibited tubulin polymerization and the growth of murine leukemia cells L12102 [19], while the fluorinated 2-styryl quinazolinone DKD-NIPERA derivative shows promise for oral cancer, demonstrating cytotoxicity in CAL-27 cancer cells (squamous cell carcinoma tumor) [20]. The alkaloid Z-Bogorin displayed good antifungal activity and moderate cytotoxic activity against Artemia salina [21].
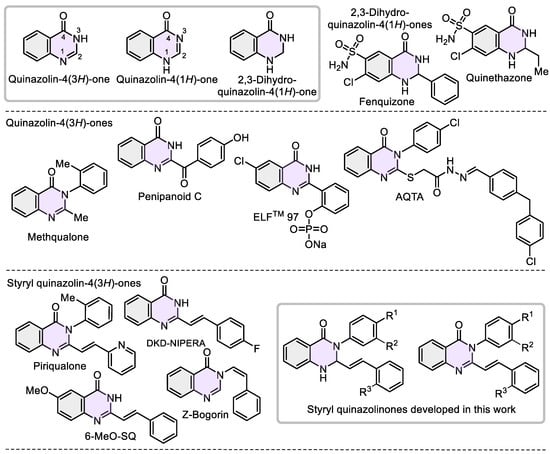
Figure 1.
Selected quinazolinone skeletons as pharmaceuticals and fluorophores according to structural classification, and styryl quinazolinones developed in this work.
Subsequently, there is a considerable review of the literature on their synthesis [22,23,24,25,26,27,28,29]. Among the synthesis methods, environmentally benign approaches, i.e., solid acid-catalyzed, nanocatalyzed, or organocatalyzed syntheses, stand out as promising green alternative methods for constructing quinazolinone skeletons [27,28,29].
Generally, the substituted 2,3-dihydroquinazolin-4(1H)-ones and quinazoline-4(3H)-ones are easily prepared using different principal starting materials, including 2-substituted aryl amines, such as anthranilic acid (2-aminobenzoic acid), its close derivatives, or 2-aminobenzaldehydes and 2-aminoaryl ketones. Direct synthesis of quinazolinone derivatives through cyclocondensation of 2-aminobenzamide derivatives and aldehydes in the presence of various metal catalysts remains the most popular method [25], although several new techniques have been developed for the synthesis of the substituted quinazolinone derivatives [30,31,32,33]. Another equally popular starting material in synthesizing 2,3-dihydroquinazolin-4(1H)-ones and quinazolin-4(3H)-ones, which are valuable intermediates in organic synthesis, are isatoic anhydrides. Several methods for the synthesis of 2-aryl(alkyl)- or 2,3-diaryl-(2,3-dihydro)-quinazolinones have been reported, which are usually based on the condensation of isatoic anhydride, aldehydes, and ammonium salts or primary amines in the presence of numerous different catalysts [34], including organocatalysts such as p-TsOH [35], ethylene diamine diacetate [36], dodecylbenzenesulfonic acid [37], L-proline [38], β-cyclodextrin [39], room-temperature ionic liquids [40], acetic [41], glutamic [42] or citric [43] acids, etc. The latter is the organocatalyst of choice in the present work. Notably, none of the organocatalysts mentioned above were used in the title compounds.
Although 2-styryl-dihydroquinazolin-4(1H)-ones and 2-styrylquinazolin-4(3H)-ones, compounds that merge the medicinally significant stilbene and quinazolinone frameworks, are of considerable interest to synthetic and medicinal chemists, their synthetic methodologies are less developed than those for 2-aryl- or 2,3-diaryl-quinazolinones and exhibit notable limitations. These methods utilizing starting materials such as 2-aminobenzamide, 2-methyl-3,1-benzoxazin-4-one, or 2-methylquinazolin-4(3H)-one derivatives typically require multi-step reactions, harsh conditions, prolonged reaction times, and the use of toxic or costly catalysts [44,45,46,47,48,49]. Notably, even the few reports describing one-pot procedures for synthesizing 2-styryl-2,3-dihydroquinazolin-4(1H)-ones and 2-styrylquinazolin-4(3H)-ones from readily available isatoic anhydride suffer similar drawbacks [50,51,52]. Specifically, Zhang and co-workers reported just two examples of synthesizing 3-phenyl (or 2-methoxyphenyl)-2-styryl-2,3-dihydroquinazolin-4(1H)-ones in the presence of a CuO-nanocatalyst in aqueous ethanol using an ultrasound irradiation technique [52]. Although the yields of these styryl derivatives are excellent (84–85%), copper oxide nanoparticles (CuO NPs) have limitations, including toxicity, high production costs, and the use of hazardous materials [53,54]. In contrast, citric acid is a compound naturally found in citrus and many other foods. It is generally recognized as safe (GRAS) by the U.S. Food and Drug Administration (FDA) [55].
In light of the aforementioned facts and our ongoing commitment to exploring the synthesis of novel bioactive small heterocyclic molecules under environmentally friendly reaction conditions [56,57,58], we established a simple green procedure for the synthesis of (E)-3-aryl-2-styryl-2,3-dihydroquinazolin-4-(1H)-ones and corresponding quinazolin-4(3H)-one derivatives. Therefore, this study presents new practical and direct syntheses of potentially pharmacologically active styryl-quinazolinone derivatives through a three-component condensation reaction involving isatoic anhydride, anilines, and cinnamaldehydes. The reaction, conducted in methanol with 20 mol% citric acid, yields (E)-3-aryl-2-styryl-2,3-dihydroquinazolin-4(1H)-ones. Notably, three-component reactions are excellent for multiple bond-forming events between several substrates. They are highly efficient one-pot processes in which three reactants combine to form target molecules, effectively incorporating most starting materials. These reactions occur in a single step and show excellent atom economy, reducing the number of synthetic operations [59,60].
The obtained dihydroquinazolinone intermediates are subsequently oxidized using an environmentally friendly iodine/DMSO catalytic system to produce smoothly the corresponding 3-aryl-2-styryl-quinazolin-4(3H)-ones. A key highlight of our research is the development of two simple, cost-effective procedures under sustainable reaction conditions, enabling the preparation of a diverse series of styryl-quinazolinones. These compounds represent privileged scaffolds and valuable medicinal chemistry and organic synthesis building blocks.
2. Materials and Methods
2.1. Materials and Instruments
The solvents and reagents used for synthesizing the intermediate and final compounds were of synthesis-grade purity. All chemicals were sourced from Merck and Aldrich Chemical Co (Sigma-Aldrich and Merck, St. Louis, MO, USA) and were utilized without additional purification. Reaction progress and product purity were monitored using thin-layer chromatography (TLC) on Silufol UV254 plates (0.25 mm thickness). Visualization was performed under UV light at 254 nm or using an ethanolic solution of phosphomolybdic–sulfuric acids. Melting points were determined with Fisher-Johns apparatus and are reported as uncorrected values.
Nuclear magnetic resonance (NMR) spectra for 1H and 13C were acquired using a Bruker Avance–400 spectrometer (400 MHz for 1H and 101 MHz for 13C). Chemical shifts (δ) are reported in parts per million (ppm), referenced to solvent signals: DMSO-d6: δ 2.50 ppm (comp. 4a, 4c–4e) or CDCl3: δ 7.28 ppm (comp. 4b, 5a–5d). Coupling constants (J) are provided in Hz, and signal multiplicity is denoted as follows: (s) singlet, (d) doublet, (dd) doublet of doublets, (ddd) doublet of doublet of doublets, and (m) multiplet. The coupling constants J are expressed in Hz. The aromatic protons of the N3-aryl fragment are designated as HPh, and those of 2-styryl moiety are shown as HAr.
Infrared spectra were recorded using a Bruker Tensor 27 FTIR spectrophotometer (Bruker Corporation, Billerica, MA, USA) equipped with a platinum ATR cell, operating at 31 scans with a resolution of 2 cm−1. Elemental analyses were performed on a Thermo Scientific CHNS-O analyzer (model: Flash 2000) (Thermo Fisher Scientific, Waltham, MA, USA) with results within ±0.4 of theoretical values.
2.2. General Procedure for the Synthesis of (E)-3-Aryl-2-styryl-2,3-dihydroquinazolin-4(1H)-one derivatives 4a–e
To a solution of isatoic anhydride 1 (1.4 mmol) in 1 mL of methanol, 20 mol% of citric acid monohydrate (CAM) was added, and the mixture was stirred for 20 min. Subsequently, the respective aniline derivatives 2a–e (1.6 mmol) and cinnamaldehydes 3a–b (1.3 mmol) were added sequentially. The resulting mixture was heated at 60 °C for 2 h, with progress monitored by TLC. After the reaction was complete, the mixture was cooled to room temperature. Methanol was distilled off, and the crude solids were washed with a cold solution of 85% ethanol and filtered to yield products 4a–e, which were purified by column chromatography, using alumina as the stationary phase and a petroleum ether/ethyl acetate (1:2) mixture as the eluent. Characterization data for the styryl-dihydroquinazolinone compounds 4a–e are provided below.
2.2.1. 3-(4-Methoxyphenyl)-2-styryl-2,3-dihydroquinazolin-4(1H)-one (4a)
3-(4-Methoxyphenyl)-2-styryl-2,3-dihydroquinazolin-4(1H)-one (4a) was synthesized following the general procedure using isatoic anhydride 1 (0.23 g, 1.32 mmol), 4-methoxyaniline 2a (0.20 g, 1.62 mmol), cinnamaldehyde 3a (0.17 mL, 1.27 mmol), and 20 mol% CAM (65 mg, 0.31 mmol). After reaction, isolation, and recrystallization, a white solid (0.40 g, 1.12 mmol, 80%) was obtained. Rf = 0.27 (1:2, petroleum ether/ethyl acetate); Mp = 234–236 °C (ethanol). IR (ATR, νmax): 3311 (N–H), 2810 (OCH3), 1630 (C=O), 1507 (ArC=C), 1389 and 1239 (C–N), 762 and 535 (ArC–H) cm−1. 1H NMR (400 MHz, DMSO-d6) δ (ppm): 7.72 (1H, d, J = 7.7 Hz, 5-H), 7.37–7.34 (2H, m, HAr), 7.34–7.31 (1H, m, 7-H), 7.27 (5H, m, HPh and HAr), 7.23 (1H, m, 6-H), 6.98–6.91 (2H, m, HPh), 6.82 (1H, d, J = 15.1 Hz, =HαCAr), 6.75 (1H, m, J = 15.0 Hz, =HβCQuin), 6.47 (2H, d, J = 3.1 Hz, 8-H and N–H), 5.61–5.57 (1H, m, 2-H), 3.74 (3H, s, OCH3). 13C NMR (101 MHz, DMSO-d6) δ (ppm): 161.8, 157.6, 146.8, 135.4, 133.5, 133.3, 131.9, 128.7 (2), 128.6 (2), 128.1, 127.9, 126.7, 126.6 (2), 117.4, 115.0, 114.7, 113.9 (2), 73.0, 55.2. Anal. calcd. for C23H20N2O2 (356.43): C, 77.51; H, 5.66; N, 7.86%. Found: C, 77.76; H, 5.53; N, 7.61%.
2.2.2. 3-Phenyl-2-styryl-2,3-dihydroquinazolin-4(1H)-one (4b)
3-Phenyl-2-styryl-2,3-dihydroquinazolin-4(1H)-one (4b) was synthesized following the general procedure using isatoic anhydride 1 (0.25 g, 1.53 mmol), aniline 2b (0.14 mL, 1.52 mmol), cinnamaldehyde 3a (0.19 mL, 1.52 mmol), and 20 mol% CAM (69 mg, 0.33 mmol). After reaction, isolation, and recrystallization, a pale yellow solid (0.27 g, 0.83 mmol, 54% yield) was obtained. Rf = 0.23 (1:2, petroleum ether/ethyl acetate); Mp = 192–194 °C (ethanol) (lit. 198–200 °C [52]). IR (ATR, νmax): 3310 (N–H), 3058 (ArC–H), 1631 (C=O), 1488 (ArC=C), 1395 (C-N), 748 and 629 (ArC–H) cm−1. 1H NMR (400 MHz, CDCl3) δ (ppm): 7.76 (1H, dd, J = 7.8, 1.6 Hz, 5-H), 7.43–7.38 (4H, m, HPh), 7.36–7.32 (5H, m, HAr), 7.28–7.21 (3H, m, 6-H, 7-H, and HPh), 6.85 (1H, d, J = 15.0 Hz, =HαCAr), 6.76 (1H, m, J = 15.0 Hz, =HβCQuin), 6.49 (2H, d, J = 4.7 Hz, 8-H and N-H), 5.69 (1H, m, 2-H). 13C NMR (101 MHz, CDCl3) δ (ppm): 161.7, 146.7, 140.6, 135.4, 133.6, 131.9, 128.7 (2), 128.6 (2), 128.1, 128.0, 127.0 (2), 126.7, 126.6 (2), 126.4, 117.5, 115.1, 114.8, 72.5. Anal. calcd. for C22H18N2O (326.40): C, 80.96; H, 5.56; N, 8.58%. Found: C, 80.84; H, 5.71; N, 8.40%.
2.2.3. 3-(3,4-Dimethoxyphenyl)-2-styryl-2,3-dihydroquinazolin-4(1H)-one (4c)
3-(3,4-Dimethoxyphenyl)-2-styryl-2,3-dihydroquinazolin-4(1H)-one (4c) was synthesized following the general procedure using isatoic anhydride 1 (0.21 g, 1.30 mmol), 3,4-dimethoxyaniline 2c (0.22 g, 1.44 mmol), cinnamaldehyde 3a (0.16 mL, 1.30 mmol), and 20 mol% CAM (58 mg, 0.28 mmol). After reaction, isolation, and recrystallization, a white solid (0.35 g, 0.90 mmol, 70 %) was obtained. Rf = 0.10 (1:2, petroleum ether/ethyl acetate); Mp = 227–228 °C (ethanol). IR (ATR, νmax): 3306 (N–H), 2919 (OCH3), 1630 (C=O), 1507 (ArC=C), 1389 and 1230 (C–N), 752 (ArC–H) cm−1. 1H NMR (400 MHz, DMSO-d6) δ (ppm): 7.73 (1H, d, J = 7.8 Hz, 5-H), 7.39–7.35 (2H, m, HAr), 7.34–7.31 (1H, m, 7-H), 7.29 (3H, d, J = 3.7 Hz, HAr), 7.27–7.22 (1H, m, HPh), 6.98–6.95 (1H, m, 6-H), 6.94 (1H, s, HPh), 6.87 (1H, dd, J = 8.5, 2.3 Hz, HPh), 6.83 (1H, d, J = 15.0 Hz, =HαCAr), 6.75 (1H, m, J = 15.0 Hz, =HβCQuin), 6.50 (2H, d, J = 3.2 Hz, 8-H and N–H), 5.64–5.57 (1H, m, 2-H), 3.74 (3H, s, OCH3), 3.70 (3H, s, OCH3). 13C NMR (101 MHz, DMSO-d6) δ (ppm): 161.8, 148.5, 147.4, 146.8, 135.5, 133.5 (2), 132.0, 128.6 (2), 128.1, 128.0, 126.8, 126.6 (2), 119.6, 117.4, 115.0, 114.7, 111.8, 111.4, 73.0, 55.5 (2). Anal. calcd. for C24H22N2O3 (386.45): C, 74.59; H, 5.74; N, 7.25 %. Found: C, 74.40; H, 5.89; N, 7.13 %.
2.2.4. 3-(4-Methoxyphenyl)-2-(2-methoxystyryl)-2,3-dihydroquinazolin-4(1H)-one (4d)
3-(4-Methoxyphenyl)-2-(2-methoxystyryl)-2,3-dihydroquinazolin-4(1H)-one (4d) was synthesized following the general procedure using isatoic anhydride 1 (0.21g, 1.30 mmol), 4-methoxyaniline 2a (0.17 g, 1.43 mmol), 3-(2-methoxyphenyl)acrylaldehyde 3b (0.21 g, 1.29 mmol), and 20 mol% CAM (59 mg, 0.28 mmol). After reaction, isolation, and recrystallization, a brown solid (0.31 g, 0.80 mmol, 61%) was obtained. Rf = 0.16 (1:2, petroleum ether/ethyl acetate); Mp = 162–164 °C (ethanol). IR (ATR, νmax): 3306 (N–H), 2832 (OCH3), 1628 (C=O), 1604 and 1508 (ArC=C), 1391 and 1242 (C–N), 751 (ArC–H) cm−1. 1H NMR (400 MHz, DMSO-d6) δ (ppm): 7.72 (1H, dd, J = 7.8, 1.4 Hz, 5-H), 7.37 (1H, dd, J = 7.7, 1.6 Hz, HAr), 7.33–7.30 (1H, m, 7-H), 7.29–7.26 (3H, m, HAr), 7.25–7.20 (1H, m, 6-H), 6.97–6.92 (3H, m, 8-H and HPh), 6.88–6.80 (3H, m, HPh and N–H), 6.76–6.69 (1H, m, J = 15.0, =HαCAr), 6.43 (1H, m, J = 15.9, =HβCQuin), 5.60 (1H, m, 2-H), 3.74 (3H, s, OCH3), 3.72 (3H, s, OCH3). 13C NMR (101 MHz, DMSO-d6) δ (ppm): 161.9, 157.5, 156.4, 146.9, 133.4, 133.3, 129.4, 128.7 (2), 127.9, 127.0, 126.7, 126.5, 123.9, 120.5, 117.3, 115.0, 114.7, 113.9 (2), 111.4, 73.3, 55.4, 55. Anal. calcd. for C24H22N2O (386.45): C, 74.59; H, 5.74; N, 7.25%. Found: C, 74.68; H, 5.61; N, 7.33%.
2.2.5. 3-(4-Bromophenyl)-2-styryl-2,3-dihydroquinazolin-4(1H)-one (4e)
3-(4-Bromophenyl)-2-styryl-2,3-dihydroquinazolin-4(1H)-one (4e) was synthesized following the general procedure using isatoic anhydride 1 (0.20 g, 1.30 mmol), 4-bromoaniline 2d (0.23 g, 1.35 mmol), cinnamaldehyde 3a (0.15 mL, 1.23 mmol), and 20 mol% CAM (63 mg, 0.30 mmol). After reaction, isolation, and recrystallization, a white solid (0.14 g, 1.12 mmol, 30%) was obtained. Rf = 0.46 (1:2, petroleum ether/ethyl acetate); Mp = 183–185 °C (ethanol). IR (ATR, νmax): 3306 (N–H), 3067 (ArC–H), 1726 (C=O), 1613 and 1485 (ArC=C), 1391 (C–N), 1010 (C–Br), 751 (ArC–H) cm−1. 1H NMR (400 MHz, DMSO-d6) δ (ppm): 7.92 (1H, dd, J = 7.9, 1.1 Hz, 5-H), 7.60–7.57 (2H, m, HPh), 7.38–7.34 (5H, m, HAr), 7.27–7.24 (2H, m, HPh), 7.16 (2H, d, J = 8.2 Hz, 6-H and 7-H), 6.84 (1H, d, J = 15.2 Hz, =HαCAr), 6.74–6.69 (1H, m, J = 15.0 Hz, =HβCQuin), 6.51–6.46 (2H, m, 8-H and N–H), 5.71 (1H, m, 2-H). 13C NMR (101 MHz, DMSO-d6) δ (ppm): 161.8, 159.8, 147.0, 146.8, 141.3, 132.5, 132.1, 131.6 (2), 128.6 (2), 128.4, 128.2, 126.6 (2), 126.5, 123.4, 117.5, 115.32 (2), 114.9, 72.3. Anal. calcd. for C22H17BrN2O (405.30): C, 65.20; H, 4.23; N, 6.91%. Found: C, 65.37; H, 4.11; N, 6.82%.
2.3. General Procedure for the Synthesis of (E)-3-Aryl-2-styrylquinazolin-4(3H)-one derivatives 5a–d
The oxidation of 2,3-dihydroquinazolin-4(3H)-ones (4a–d) was conducted as follows: A total of 0.20 g (0.56 mmol) of the corresponding substrate, dissolved in 2 mL of DMSO was introduced into a vial under constant stirring. Subsequently, 20 mol% of I2 was added, and the reaction was maintained at 100 °C, with progress monitored via TLC for 1 h. The reaction mixture was then extracted with ethyl acetate and washed with brine (3 × 30 mL). The organic phase was dried over anhydrous Na2SO4, filtered, and concentrated under reduced pressure. Final purification was achieved through column chromatography (silica gel) using petroleum ether/ethyl acetate mixtures (10:1). Characterization data for the synthesized compounds 5a–d are provided below.
2.3.1. 3-(4-Methoxyphenyl)-2-styrylquinazolin-4(3H)-one (5a)
3-(4-Methoxyphenyl)-2-styrylquinazolin-4(3H)-one (5a) was synthesized following the general procedure using 2,3-dihydroquinazolinone 4a (0.20 g, 56 mmol) and 20 mol% of I2 (28 mg, 0.11 mmol), dissolved in 2 mL of DMSO. The reaction mixture was heated at 100 °C. After reaction, isolation, and recrystallization, a pale yellow solid (0.18 g, 0.52 mmol, 91%) was obtained. Rf = 0.53 (1:2, petroleum ether/ethyl acetate); Mp = 166–168 °C (ethanol). IR (ATR, νmax): 2922 (ArC–H), 2838 (OCH3), 1671 (C=O), 1548 (ArC=C, ArC=N), 1245 (C–N), 689 (ArC–H) cm−1. 1H NMR (400 MHz, CDCl3) δ (ppm): 8.30 (1H, d, J = 7.9 Hz, 5-H), 7.97 (1H, d, J = 15.5 Hz, =HαCAr), 7.80–7.76 (2H, m, 6-H and 7-H), 7.46 (1H, d, J = 8.2 8-H), 7.38–7.29 (5H, m, HAr), 7.25–7.19 (2H, m, HPh), 7.11–7.05 (2H, m, HPh), 6.47 (1H, d, J = 15.5 Hz, =HβCQuin), 3.91 (3H, s, 4′-OCH3). 13C NMR (101 MHz, DMSO-d6) δ (ppm): 162.7, 160.0, 152.2, 147.9, 139.9, 135.4, 134.6, 129.8 (2), 129.7, 129.5, 128.9 (2), 127.9 (2), 127.4, 127.3, 126.6, 121.0, 120.1, 115.2 (2), 55.7. Anal. calcd. for C23H18N2O2 (354.41): C, 77.95; H, 5.12; N, 7.90%. Found: C, 77.82; H, 5.37; N, 7.76%.
2.3.2. 3-Phenyl-2-styrylquinazolin-4(3H)-one (5b)
3-Phenyl-2-styrylquinazolin-4(3H)-one (5b) was synthesized following the general procedure using 2,3-dihydroquinazolinone 4b (0.20 g, 0.61 mmol) and 20 mol% of I2 (31 mg, 0.12 mmol), dissolved in 2 mL of DMSO. The reaction mixture was heated at 100 °C. After reaction, isolation, and recrystallization, a pale yellow solid (0.11 g, 0.35 mmol, 57%) was obtained. Rf = 0.50 (1:2, petroleum ether/ethyl acetate); Mp = 195–197 °C (ethanol). (lit. 150–152 °C [49], 196–197 °C [50]). IR (ATR, νmax): 3055 (ArC–H), 1665 (C=O), 1550 (ArC=N), 1467 (ArC=C), 1352 (C–N), 689 (ArC–H) cm−1. 1H NMR (400 MHz, CDCl3) δ (ppm): 8.31 (1H, d, J = 7.7 Hz, 5-H), 7.98 (1H, d, J = 15.5 Hz, =HαCAr), 7.81–7.78 (2H, m, 6-H and 7-H), 7.63–7.55 (3H, m, HPh), 7.47 (1H, d, J = 8.2 Hz, 8-H), 7.35–7.32 (2H, m, HPh), 7.32–7.29 (5H, m, HAr), 6.40 (1H, d, J = 15.5 Hz, =HβCQuin). 13C NMR (101 MHz, CDCl3) δ (ppm): 162.4, 151.8, 147.9, 140.0, 137.1, 135.4, 134.7, 130.0 (2), 129.7, 129.4, 128.9 (2), 128.8 (2), 127.8 (2), 127.4, 127.2, 126.7, 121.0, 120.0. Anal. calcd. for C22H16N2O (324.38): C, 81.46; H, 4.97; N, 8.64%. Found: C, 81.21; H, 4.82; N, 8.53%.
2.3.3. 3-(3,4-Dimethoxyphenyl)-2-styrylquinazolin-4(3H)-one (5c)
3-(3,4-Dimethoxyphenyl)-2-styrylquinazolin-4(3H)-one (5c) was synthesized following the general procedure using 2,3-dihydroquinazolinone 4c (0.20 g, 52 mmol) and 20 mol% of I2 (26 mg, 0.10 mmol), dissolved in 2 mL of DMSO. The reaction mixture was heated at 100 °C. After reaction, isolation, and recrystallization, a pale yellow solid (0.17 g, 0.47 mmol, 87 %) was obtained. Rf = 0.30 (1:2, petroleum ether/ethyl acetate); Mp = 232–233 °C (ethanol). IR (ATR, νmax): 3005 (ArC–H), 2915 (OCH3), 1673 (C=O), 1551 (ArC=C, ArC=N), 1228 (C–N), 697 (ArC–H) cm−1. 1H NMR (400 MHz, CDCl3) δ (ppm): 8.30 (1H, d, J = 7.9 Hz, 5-H), 7.98 (1H, d, J = 15.5 Hz, =HαCAr), 7.82–7.75 (2H, m, 6-H and 7-H), 7.50–7.43 (1H, m, 8-H), 7.37–7.30 (5H, m, HAr), 7.03 (1H, d, J = 8.4 Hz, HPh), 6.87 (1H, dd, J = 8.4, 2.3 Hz, HPh), 6.82 (1H, d, J = 2.4 Hz, HPh), 6.48 (1H, d, J = 15.5 Hz, =HβCQuin), 3.98 (3H, s, OCH3), 3.88 (3H, s, OCH3). 13C NMR (101 MHz, CDCl3) δ (ppm): 162.7, 152.1, 150.0, 149.6, 147.9, 140.0, 135.4, 134.7, 129.8, 129.7, 128.9 (2), 127.9 (2), 127.4, 127.2, 126.7, 121.0 (2), 120.0, 111.7, 111.6, 56.2 (2). Anal. calcd. for C24H20N2O3 (384.44): C, 74.98; H, 5.24; N, 7.29%. Found: C, 74.71; H, 5.37; N, 7.40%.
2.3.4. 3-(4-Methoxyphenyl)-2-(2-methoxystyryl)-quinazolin-4(3H)-one (5d)
3-(4-Methoxyphenyl)-2-(2-methoxystyryl)-quinazolin-4(3H)-one (5d) was synthesized following the general procedure using 2,3-dihydroquinazolinone 4d (0.20 g, 52 mmol) and 20 mol% of I2 (26 mg, 0.10 mmol), dissolved in 2 mL of DMSO. The reaction mixture was heated at 100 °C. After reaction, isolation, and recrystallization, a pale yellow solid (0.17 g, 0.45 mmol, 86%) was obtained. Rf = 0.43 (1:2, petroleum ether/ethyl acetate); Mp = 189–190 °C (ethanol). IR (ATR, νmax): 3057 (ArC–H), 2935 (OCH3), 1671 (C=O), 1550 (ArC=C, ArC=N), 1245 (C–N), 772 (ArC–H) cm−1. 1H NMR (400 MHz, CDCl3) δ (ppm): 8.29 (1H, ddd, J = 8.0, 1.4, 0.6 Hz, 5-H), 8.19 (1H, d, J = 15.6 Hz, =HαCAr), 7.82–7.74 (2H, m, 6-H and 7-H), 7.44 (1H, d, J = 8.2, 6.6, 1.7 Hz, 8-H), 7.29–7.25 (2H, m, HAr), 7.23 (2H, d, J = 9.0 Hz, HPh), 7.08 (2H, d, J = 9.0 Hz, HPh), 6.92–6.84 (2H, m, HAr), 6.67 (1H, d, J = 15.6 Hz, =HβCQuin), 3.90 (3H, s, OCH3), 3.76 (3H, s, 4OCH3). 13C NMR (101 MHz, CDCl3) δ (ppm): 162.8, 159.9, 158.3, 152.9, 148.0, 135.6, 134.5, 130.8, 129.9, 129.8 (2), 129.5, 127.5, 127.2, 126.4, 124.5, 121.3, 120.9, 120.7, 115.1 (2), 111.1, 55.7, 55.3. Anal. calcd. for C24H20N2O3 (384.44): C, 74.98; H, 5.24; N, 7.29%. Found: C, 74.83; H, 5.41; N, 7.11%.
3. Results and Discussion
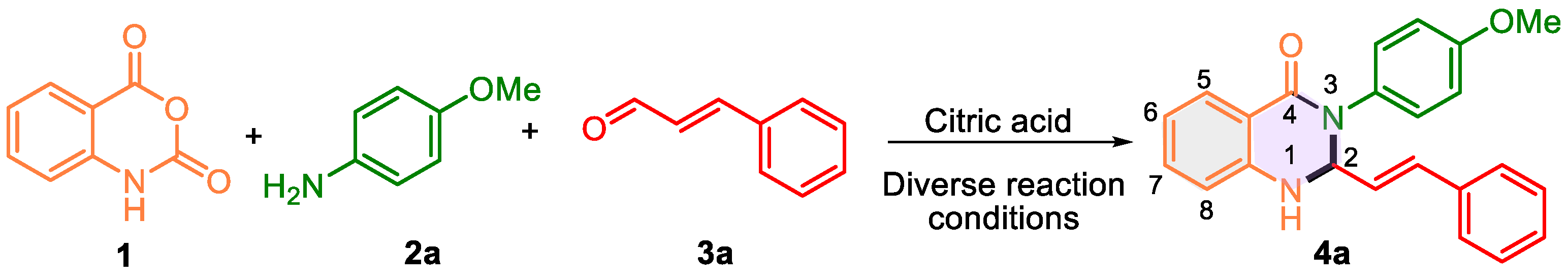
Drawing on previous reports of 3-aryl-2,3-dihydroquinazolione synthesis catalyzed by organocatalysts [34,35,36,37,38,39,40,41,42,43], we selected citric acid as the catalyst. This naturally occurring Brønsted acid is inexpensive, readily available, and has performed well in preparing such heterocycles [43]. To explore its efficacy, we investigated the reaction of isatoic anhydride 1, 4-methoxyaniline 2a, and trans-cinnamaldehyde 3a to synthesize 3-(4-methoxyphenyl)-2-styryl-2,3-dihydroquinazolin-4(1H)-one 4a using citric acid under varying reaction conditions (Scheme 1, Table 1).
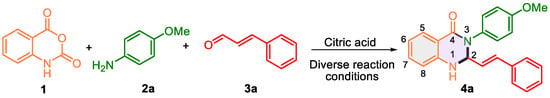
Scheme 1.
The model reaction of isatoic anhydride 1, 4-methoxyaniline 2a, and trans-cinnamaldehyde 3a to afford 3-(4-methoxyphenyl)-2-styryl-2,3-dihydroquinazolin-4(1H)-one 4a evaluating reaction parameters.

Table 1.
Study of the optimal reaction conditions for the preparation of 3-(4-methoxyphenyl)-2-styryl-2,3-dihydroquinazolin-4(1H)-one (4a) a.
Based on the prior literature on the construction of similar systems, the formation of the target compound 4a was initially investigated using 40 mol% citric acid as a catalyst, yielding 76% (Entry 1, Table 1). Despite this promising result, our goal was to develop a more user-friendly and efficient protocol to accelerate the reaction rate and improve the selective synthesis of dihydroquinazolinones. To this end, microwave radiation (MW) was employed as a heating source under various time and temperature conditions. While MW-assisted synthesis of similar 2,3-dihydroquinazolinones has been documented [61,62,63], no reports exist for synthesizing 2-styryl derivatives. Experiments using MW revealed a significant decrease in yield (40% and 20%, respectively) as the temperature and reaction time increased (Entries 2–3, Table 1), indicating that these conditions adversely affected the reaction’s progress toward the desired 2-styryl-2,3-dihydroquinazoline systems.
Subsequently, citric acid catalyst loading was reduced to 20 mol% under conventional heating. The reaction, monitored via TLC and completed in two hours, successfully yielded 4a with an improved 80% yield (Entry 4, Table 1). Finally, a urea/zinc chloride eutectic solvent system (3.5:1 molar ratio) was tested, inspired by recent reports on synthesizing 2,3-diaryl-dihydroquinazolinones in deep eutectic solvents [64]. However, under these conditions, the efficient formation of 4a could not be achieved (Entry 5, Table 1).
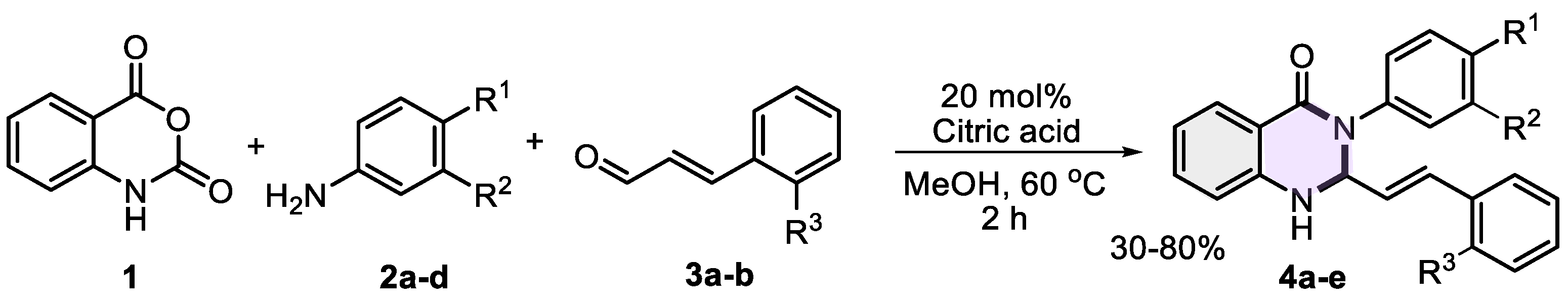
Following the optimization of reaction conditions, a small series of 2-styryl-dihydroquinazolinones 4a–e was successfully synthesized (Scheme 2). The reaction employed isatoic anhydride 1, selected anilines 2a–d, and cinnamaldehydes 3a–b in the presence of 20 mol% citric acid as a catalyst using methanol as the solvent at 60 °C for 2 h. The resulting 2,3-dihydroquinazolin-4(1H)-ones 4a–e were obtained in yields ranging from 30% to 80%.
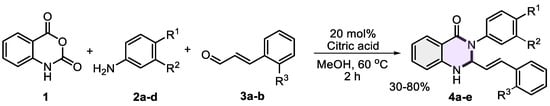
Scheme 2.
Synthesis of 2,3-dihydroquinazolin-4(1H)-ones 4a–e using citric acid as catalyst.
The lowest yield (30%) was observed for molecule 4e, likely due to the diminished nucleophilicity of 4-bromoaniline 2d, which impeded its interaction with isatoic anhydride during the initial condensation step. Moreover, no product was formed with 4-nitroaniline (not shown in the scheme), underscoring the essential role of aniline nucleophilicity in successfully creating the target compounds. In contrast, 2,3-dihydroquinazolin-4(1H)-one 4a and those bearing electron-donating substituents 4b–c were obtained in moderate to excellent yields. Purified through column chromatography (alumina) using petroleum ether/ethyl acetate mixtures (1:2), dihydroquinazolinones resulted in stable powders of different colors (from white to pale yellow and brown) with well-defined melting points (Table 2), allowing for determination of their characterization by spectroscopic techniques to elucidate the structure of these heterocyclic systems.

Table 2.
2-Styryl-dihydroquinazolinone derivatives 4 obtained through a citric acid-catalyzed three-component condensation reaction.
Their structures were elucidated using spectroscopic and analytical techniques, including IR, 1H NMR, 13C NMR, and elemental analysis. The 1H NMR spectra provided clear evidence for the molecular structure, with all proton signals and their couplings consistent with the spatial arrangement of the molecule. Key features confirming the skeleton construction included the C-2 proton and the olefinic protons of the styryl fragment, =HαCAr and =HβCQuin, which were observed in the regions of 5.57–5.73 ppm, 6.67–6.85 ppm, and 6.43–6.76 ppm, respectively. The olefinic protons were confirmed to have a trans-configuration, as indicated by the Hα signals appearing as doublets with coupling constants J = 15.0–15.9 Hz. Meanwhile, the Hβ protons were deshielded and appeared as multiplets due to interactions with the two quinazolinone nitrogen atoms and the adjacent C-2 proton (see Figure S17 for example).
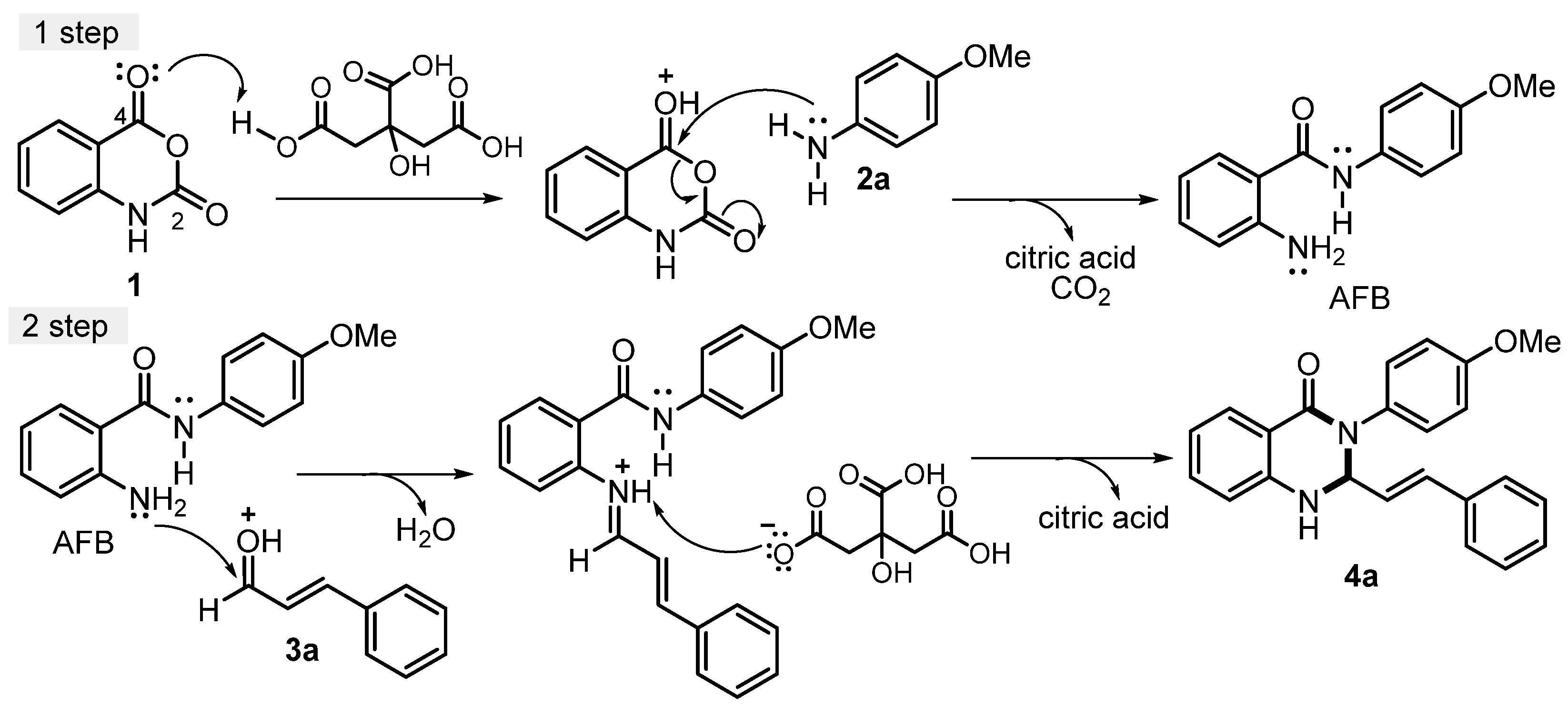
As noted in previous studies [34,62,65], the synthesis of the 2,3-dihydroquinazolin-4(1H)-one series depends mainly on the balance between the acidity of the reaction medium and the nucleophilicity of the starting anilines. In the initial step, the carbonyl group at the C-4 position of isatoic anhydride 1 undergoes protonation by citric acid, which enhances its electrophilicity and promotes a nucleophilic attack by the amino group of 4-methoxyaniline 2a. This reaction produces the intermediate 2-amino-N-phenylbenzamide (AFB), which then forms the desired product. Under the same conditions, the second step proceeds as the carbonyl group of cinnamaldehyde 3a, activated by citric acid, reacts with the amino group at the C-2 position of the intermediate AFB. This nucleophilic attack is followed by dehydration and subsequent protonation facilitated by citric acid in the reaction medium. These steps culminate in cyclization, yielding 2,3-dihydroquinazolin-4(1H)-one 4a as an example (Scheme 3).
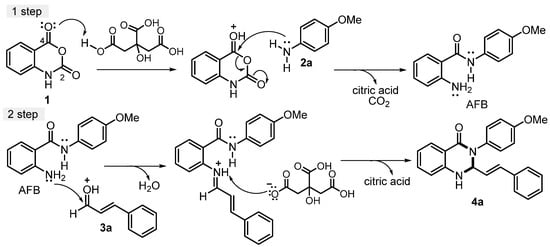
Scheme 3.
Proposed mechanism of 2,3-dihydroquinazolin-4(1H)-ones via multicomponent reaction.
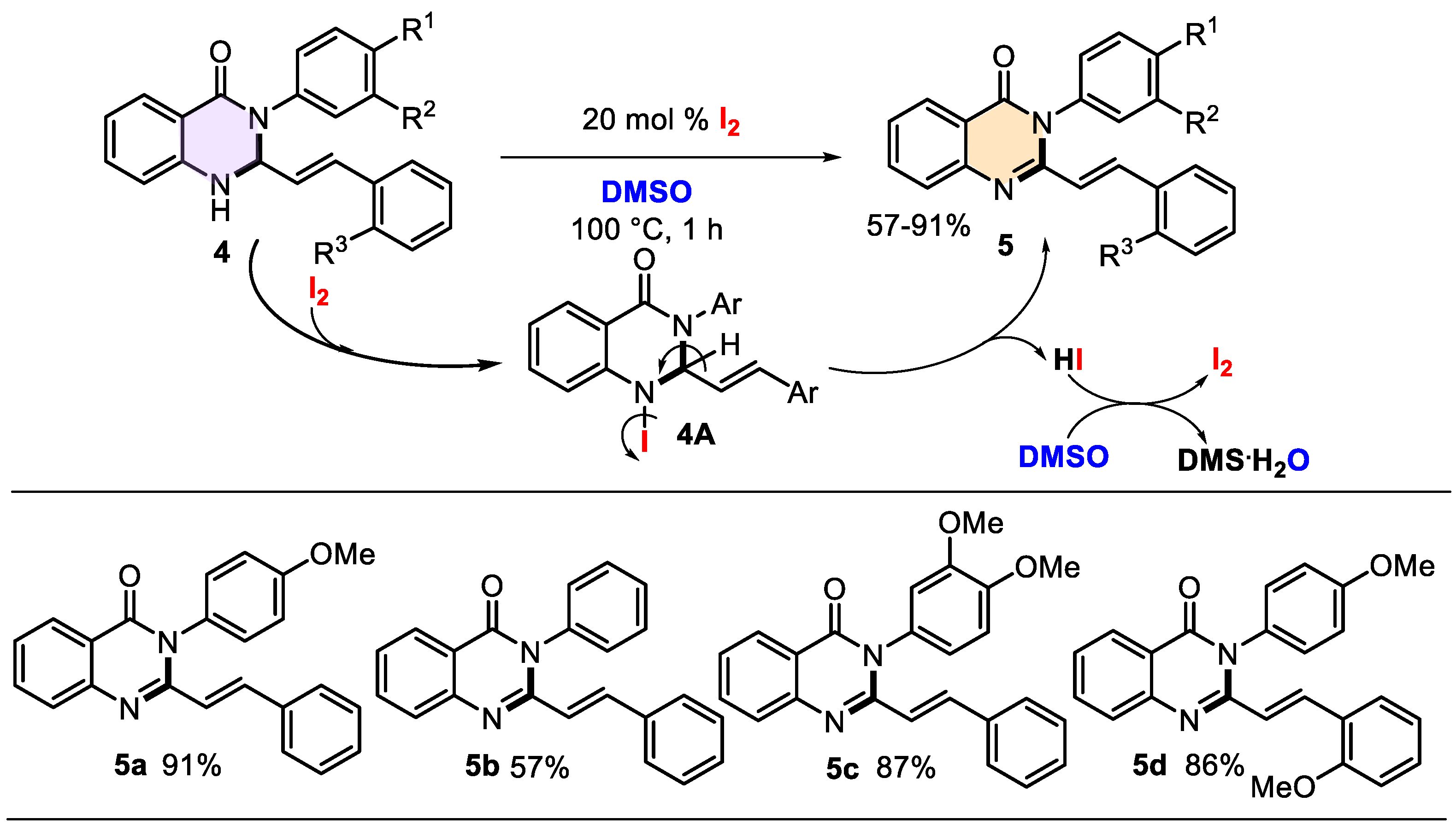
The products obtained hold significant value not only for medicinal applications but also for their synthetic potential. Typically, 2,3-diaryl-dihydroquinazolinones can be converted into their corresponding quinazolin-4(3H)-one derivatives through oxidation. Established methods include potassium tert-butoxide and tetrabutylammonium bromide in dry THF [66] for 4–6 h or 5% KMnO4 in acetone for 8 h [38]. Additionally, a catalyzed cyclization–oxidation coupling of isatoic anhydride with benzaldehydes and amines in the presence of iodine (1 equiv.) and acetic acid (10 mol%) in a MeCN-H2O mixture was reported in 2010, yielding 2,3-diarylquinazolin-4(3H)-ones [67]. Seeking an efficient method for the oxidation of 3-aryl-2-styryl-2,3-dihydroquinazolin-4(1H)-ones 4 to their respective 2-styrylquinazolinones 5, we explored the I2/DMSO catalytic system. This system has gained considerable attention due to its green chemistry attributes, high efficiency, atom economy, low cost, and mild reaction conditions [68,69,70]. Drawing on these advantages and our prior experience with the system [71], we subjected compounds 4 to oxidation using 20 mol% iodine in DMSO at 100 °C for 1 h. This approach successfully produced the corresponding quinazolin-4(3H)-one derivatives 5 in good to excellent yields, demonstrating that this oxidative catalytic system is also reproducible for this type of quinazoline system (Scheme 4).
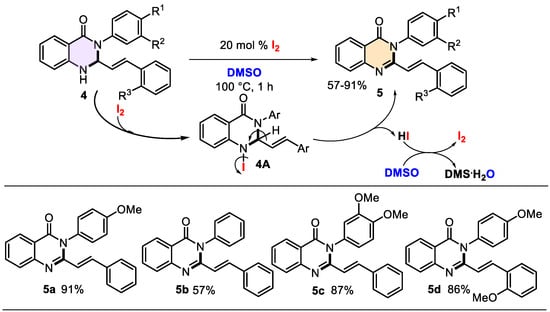
Scheme 4.
Synthesis of 3-aryl-2-styrylquinazolin-4(3H)-ones 5 promoted by the environmentally friendly iodine/DMSO catalytic system and its mechanistic insights.
Under the used reaction conditions, iodine acts as a mild Lewis acid catalyst, and DMSO acts as an oxidant, solvent, and oxygen source, as a general law [68,72]. Thus, the nitrogen (N1) lone pair of dihydroquinazolinones 4 first reacts with molecular iodine (I2) (i.e., N-iodination reaction) to form iodine intermediate 4A. The dehydroiodination process (intramolecular HI elimination) can expand the π-conjugation system to form cyclic imine bonds, producing the desired quinazolin-4(3H)-ones 5. The oxidation of I2 in the presence of DMSO produces (CH3)2S (DMS), water, and iodine, which can act as a catalyst that may be regenerated in the subsequent catalytic cycle [68] (Scheme 4). The reaction system’s remarkable characteristics are that it is metal-free, peroxide-free, and acid–base-free.
The obtained quinazolin-4(3H)-ones 5a–d were isolated as stable, pale yellow solids, facilitating the determination of their physical properties and characterization through infrared spectroscopy and nuclear magnetic resonance analysis. A clear comparison of the IR spectra of the dehydro-product 4d and its oxidized counterpart 5d reveals the absence of the characteristic peak at 3306 cm−1, corresponding to the NH group. This confirms the successful and complete oxidation of dihydroquinazolinone 4d (Figure S10). Additionally, the NMR spectra of the oxidized products display well-defined signals for the olefinic protons. The olefinic Hβ protons (=HβCQuin) appear as doublets at 6.40–6.67 ppm (J = 15.5–15.6 Hz), while the Hα protons (=HαCAr) are observed as doublets at 7.97–8.19 ppm, exhibiting identical coupling constants (see Figure S27 as an example).
4. Conclusions
In summary, we have developed an organocatalytic approach for synthesizing 3-aryl-quinazolinones incorporating a trans-stilbene unit. This synthetic method, executed in a straightforward and eco-friendly one-pot process, is particularly interesting in heterocyclic chemistry. Citric acid, the organocatalyst, exhibited remarkable efficiency in facilitating these dehydro-products. These intermediates were then efficiently transformed into 2-styryl-quinazolinones through a metal-free catalytic system using iodine/DMSO under mild oxidative conditions.
The synthetic strategies introduced here enable the construction of two diverse series of saturated and aromatic 3-aryl-2-styryl-quinazolinone frameworks. The workup procedures are also simple, cost-effective, and use readily available commercial reagents. Both structural series could have significant potential in medicinal chemistry, such as cancer drug discovery. This study presents an accessible and practical method for creating novel libraries of styryl-quinazolinones from various amines and cinnamaldehydes.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/chemistry7020042/s1, synthetic procedures, FT-IR and NMR spectra of the obtained compounds.
Author Contributions
Conceptualization, V.V.K.; methodology, A.P.G.; formal analysis, A.P.G., C.E.P.G. and V.V.K.; writing—original draft preparation, V.V.K.; writing—review and editing, C.E.P.G. and V.V.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Synthetic procedures and FT-IR and NMR are reported in Supplementary Materials.
Acknowledgments
We thank Escuela de Química of the Universidad Industrial de Santander for financial support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- He, D.; Wang, M.; Zhao, S.; Shu, Y.; Zeng, H.; Xiao, C.; Lu, C.; Liu, Y. Pharmaceutical prospects of naturally occurring quinazolinone and its derivatives. Fitoterapia 2017, 119, 136–149. [Google Scholar] [CrossRef]
- Hameed, A.; Al-Rashida, M.; Uroos, M.; Ali, S.A.; Arshia; Ishtiaq, M.; Khan, K.M. Quinazoline and quinazolinone as important medicinal scaffolds: A comparative patent review (2011–2016). Expert Opin. Ther. Pat. 2018, 28, 281–297. [Google Scholar] [CrossRef]
- Alsibaee, A.M.; Al-Yousef, H.M.; Al-Salem, H.S. Quinazolinones, the winning horse in drug discovery. Molecules 2023, 28, 978. [Google Scholar] [CrossRef]
- Tiwary, B.K.; Pradhan, K.; Nanda, A.K.; Chakraborty, R. Implication of Quinazoline-4(3H)-Ones in Medicinal Chemistry: A Brief Review. J. Chem. Biol. Ther. 2015, 1, 1000104. [Google Scholar]
- Mahato, A.; Srivastava, B.; Nithya, S. Chemistry Structure Activity Relationship and Biological Activity of Quinazoline-4 (3H)-One Derivatives. Inventi Rapid Med. Chem. 2011, 2, 13–19. [Google Scholar]
- Kaur, J.; Kaur, S.; Muskan; Kaur, N.; Kumar, V.; Anand, A. Unveiling the Therapeutic Potential of Quinazolinone Derivatives in Cancer Treatment: A Comprehensive Exploration. ChemistrySelect 2024, 9, e202401366. [Google Scholar] [CrossRef]
- Upadhyay, R.; Tandel, P.; Patel, A.B. Halogen-based quinazolin-4 (3H)-one derivatives as MCF-7 breast cancer inhibitors: Current developments and structure–activity relationship. Arch. Pharm. 2024, 358, e2400740. [Google Scholar] [CrossRef]
- Ugale, V.G.; Bari, S.B. Quinazolines: New horizons in anticonvulsant therapy. Eur. J. Med. Chem. 2014, 80, 447–501. [Google Scholar] [CrossRef]
- Utreja, D.; Salotra, R.; Kaur, G.; Sharma, S.; Kaushal, S. Chemistry of quinolines and their agrochemical potential. Curr. Org. Chem. 2022, 26, 1895–1913. [Google Scholar] [CrossRef]
- An, L.; Yang, L.; Yan, T.; Yi, M.; Liu, S.; Li, H.; Bao, X. Synthesis and agricultural antimicrobial evaluation of new quinazoline derivatives containing both a piperazine linker and the N-acetyl moiety. Pest Manag. Sci. 2024, 80, 5307–5321. [Google Scholar] [CrossRef]
- Ma, J.; Li, P.; Li, X.; Shi, Q.; Wan, Z.; Hu, D.; Jin, L.; Song, B. Synthesis and antiviral bioactivity of novel 3-((2-((1E,4E)-3-oxo-5-arylpenta-1,4-dien-1-yl) phenoxy) methyl)-4(3H)-quinazolinone derivatives. J. Agric. Food Chem. 2014, 62, 8928–8934. [Google Scholar] [CrossRef]
- Xing, Z.; Wu, W.; Miao, Y.; Tang, Y.; Zhou, Y.; Zheng, L.; Fu, Y.; Song, Z.; Peng, Y. Recent advances in quinazolinones as an emerging molecular platform for luminescent materials and bioimaging. Org. Chem. Front. 2021, 8, 1867–1889. [Google Scholar] [CrossRef]
- Selvam, T.P.; Kumar, P.V. Quinazoline marketed drugs. Res. Pharm. 2015, 1. Available online: https://updatepublishing.com/journal/index.php/rip/article/view/204 (accessed on 30 January 2025).
- Inger, J.A.; Mihan, E.R.; Kolli, J.U.; Lindsley, C.W.; Bender, A.M. DARK classics in chemical neuroscience: Methaqualone. ACS Chem. Neurosci. 2023, 14, 340–350. [Google Scholar] [CrossRef]
- Ma, C.; Li, Y.; Niu, S.; Zhang, H.; Liu, X.; Che, Y. N-hydroxypyridones, phenylhydrazones, and a quinazolinone from Isaria farinosa. J. Nat. Prod. 2011, 74, 32–37. [Google Scholar] [CrossRef]
- Zi-Jun, C.A.I.; Kuang, Y.Q.; Dan, P.A.N.; Wei, L.I.U.; Jiang, J.H. Synthesis and characterization of a novel ELF-97-based fluorescent probe for hydrogen peroxide detection. Chin. J. Anal. Chem. 2015, 43, 1671–1675. [Google Scholar]
- Sonousi, A.; Hassan, R.A.; Osman, E.O.; Abdou, A.M.; Emam, S.H. Design and synthesis of novel quinazolinone-based derivatives as EGFR inhibitors with antitumor activity. J. Enzyme Inhib. Med. Chem. 2022, 37, 2644–2659. [Google Scholar] [CrossRef]
- Welch, W.M.; Ewing, F.E.; Huang, J.; Menniti, F.S.; Pagnozzi, M.J.; Kelly, K.; Seymour, P.A.; Guanowsky, V.; Guhan, S.; Guinn, M.R.; et al. Atropisomeric quinazolin-4-one derivatives are potent noncompetitive α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor antagonists. Bioorg. Med. Chem. Lett. 2001, 11, 177–181. [Google Scholar] [CrossRef]
- Jiang, J.B.; Hesson, D.P.; Dusak, B.A.; Dexter, D.L.; Kang, G.J.; Hamel, E. Synthesis and biological evaluation of 2-styrylquinazolin-4 (3H)-ones, a new class of antimitotic anticancer agents which inhibit tubulin polymerization. J. Med. Chem. 1990, 33, 1721–1728. [Google Scholar] [CrossRef]
- Satpute, D.P.; Shirwadkar, U.; Tharalla, A.K.; Shinde, S.D.; Vaidya, G.N.; Joshi, S.; Vatsa, P.P.; Jain, A.; Singh, A.A.; Garg, R.; et al. Discovery of fluorinated 2-Styryl-4(3H)-quinazolinone as potential therapeutic hit for oral cancer. Bioorg. Med. Chem. 2023, 81, 117193. [Google Scholar]
- Seger, C.; Vajrodaya, S.; Greger, H.; Hofer, O. Structure elucidation and synthesis of a new bioactive quinazolone derivative obtained from Glycosmis Cf. Chlorosperma. Chem. Pharm. Bull. 1998, 46, 1926–1928. [Google Scholar] [CrossRef]
- Connolly, D.J.; Cusack, D.; O’Sullivan, T.P.; Guiry, P.J. Synthesis of quinazolinones and quinazolines. Tetrahedron 2005, 61, 10153–10202. [Google Scholar] [CrossRef]
- Khan, I.; Ibrar, A.; Abbas, N.; Saeed, A. Recent advances in the structural library of functionalized quinazoline and quinazolinone scaffolds: Synthetic approaches and multifarious applications. Eur. J. Med. Chem. 2014, 76, 193–244. [Google Scholar] [CrossRef]
- Khan, I.; Ibrar, A.; Ahmed, W.; Saeed, A. Synthetic approaches, functionalization and therapeutic potential of quinazoline and quinazolinone skeletons: The advances continue. Eur. J. Med. Chem. 2015, 90, 124–169. [Google Scholar] [CrossRef]
- Maiden, T.M.M.; Harrity, J.P.A. Recent developments in transition metal catalysis for quinazolinone synthesis. Org. Biomol. Chem. 2016, 14, 8014–8025. [Google Scholar] [CrossRef]
- Reddy, M.M.; Sivaramakrishna, A. Remarkably flexible quinazolinones—Synthesis and biological applications. J. Heterocycl. Chem. 2020, 57, 942–954. [Google Scholar] [CrossRef]
- Kumar, P.; Tomar, V.; Joshi, R.K.; Nemiwal, M. Nanocatalyzed synthetic approach for quinazoline and quinazolinone derivatives: A review (2015–present). Synth. Commun. 2022, 52, 795–826. [Google Scholar] [CrossRef]
- Lodhi, A.; Maheria, K.C. Solid acid catalysed synthesis of biologically potent quinazolinones: Environmentally benign approaches. Sustain. Chem. Pharm. 2023, 36, 101265. [Google Scholar] [CrossRef]
- Borah, B.; Swain, S.; Patat, M.; Chowhan, L.R. Recent advances and prospects in the organocatalytic synthesis of quinazolinones. Front. Chem. 2022, 10, 991026. [Google Scholar] [CrossRef]
- Peng, J.-B.; Geng, H.-Q.; Wang, W.; Qi, X.; Ying, J.; Wu, X.-F. Palladium-catalyzed four-component carbonylative synthesis of 2,3-disubstituted quinazolin-4(3H)-ones: Convenient methaqualone preparation. J. Catal. 2018, 365, 10–13. [Google Scholar] [CrossRef]
- Wang, L.C.; Du, S.; Chen, Z.; Wu, X.F. FeCl3-Mediated Synthesis of 2-(Trifluoromethyl) quinazolin-4(3H)-ones from Isatins and Trifluoroacetimidoyl Chlorides. Org. Lett. 2020, 22, 5567–5571. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, L.C.; Zhang, J.; Wu, X.F. Palladium-catalyzed three-component carbonylative synthesis of 2-(trifluoromethyl) quinazolin-4(3H)-ones from trifluoroacetimidoyl chlorides and amines. Org. Chem. Front. 2020, 7, 2499–2504. [Google Scholar] [CrossRef]
- Wang, L.C.; Zhang, Y.; Chen, Z.; Wu, X.F. Palladium-Catalyzed Carbonylative Synthesis of 2-(Trifluoromethyl) quinazolin-4(3H)-ones from Trifluoroacetimidoyl Chlorides and Nitro Compounds. Adv. Synth. Catal. 2021, 363, 1417–1426. [Google Scholar] [CrossRef]
- Abbas, S.Y.; El-Bayouki, K.A.; Basyouni, W.M. Utilization of isatoic anhydride in the syntheses of various types of quinazoline and quinazolinone derivatives. Synth. Commun. 2016, 46, 993–1035. [Google Scholar] [CrossRef]
- Baghbanzadeh, M.; Salehi, P.; Dabiri, M.; Kozehgary, G. Water-accelerated synthesis of novel bis-2,3-dihydroquinazolin-4(1H)-one derivatives. Synthesis 2006, 2006, 344–348. [Google Scholar] [CrossRef]
- Narasimhulu, M.; Lee, Y.R. Ethylenediamine diacetate-catalyzed three-component reaction for the synthesis of 2, 3-dihydroquinazolin-4(1H)-ones and their spirooxindole derivatives. Tetrahedron 2011, 67, 9627–9634. [Google Scholar] [CrossRef]
- Chen, B.H.; Li, J.T.; Chen, G.F. Efficient synthesis of 2,3-disubstituted-2,3-dihydroquinazolin-4(1H)-ones catalyzed by dodecylbenzenesulfonic acid in aqueous media under ultrasound irradiation. Ultrason. Sonochem. 2015, 23, 59–65. [Google Scholar] [CrossRef]
- Mehta, H.B.; Dixit, B.C.; Dixit, R.B. L-Proline catalyzed one-pot multi-component synthesis of 2-(1,3-diphenyl-1H-pyrazol-4-yl) quinazolin-4(3H)-one derivatives and their biological studies. Chin. Chem. Lett. 2014, 25, 741–744. [Google Scholar] [CrossRef]
- Ramesh, K.; Karnakar, K.G.K.H.V.; Satish, G.; Reddy, K.H.V.; Nageswar, Y.V.D. Tandem supramolecular synthesis of substituted 2-aryl-2,3-dihydroquinazolin-4(1H)-ones in the presence of β-cyclodextrin in water. Tetrahedron Lett. 2012, 53, 6095–6099. [Google Scholar] [CrossRef]
- Darvatkar, N.B.; Bhilare, S.V.; Deorukhkar, A.R.; Raut, D.G.; Salunkhe, M.M. [bmim] HSO4: An efficient and reusable catalyst for one-pot three-component synthesis of 2,3-dihydro-4(1H)-quinazolinones. Green Chem. Lett. Rev. 2010, 3, 301–306. [Google Scholar] [CrossRef]
- Karimi-Jaberi, Z.; Arjmandi, R. Acetic acid-promoted, efficient, one-pot synthesis of 2,3-dihydroquinazolin-4(1H)-ones. Monatsh. Chem. 2011, 142, 631–635. [Google Scholar] [CrossRef]
- Mane, R.; Yaraguppi, D.A.; Ashok, A.K.; Gangadharappa, B.; Chandrakala, K.B.; Kamanna, K. Glutamic acid-catalyzed synthesis of dihydroquinazolinone: Anticancer activity, electrochemical behavior, molecular docking, dynamics, simulations and drug-likeness studies. Res. Chem. Intermed. 2024, 50, 3271–3303. [Google Scholar] [CrossRef]
- Fahimi, N.; Sardarian, A.R. Citric acid: A green bioorganic catalyst for one-pot three-component synthesis of 2,3-dihydroquinazoline-4 (1H)-ones. Curr. Organocatal. 2016, 3, 39–44. [Google Scholar] [CrossRef]
- Jatav, V.; Mishra, P.; Kashaw, S.; Stables, J.P. Synthesis and CNS depressant activity of some novel 3-[5-substituted 1,3,4-thiadiazole-2-yl]-2-styryl quinazoline-4(3H)-ones. Eur. J. Med. Chem. 2008, 43, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Raffa, D.; Edler, M.C.; Daidone, G.; Maggio, B.; Merickech, M.; Plescia, S.; Schillaci, D.; Bai, R.; Hamel, E. Synthesis, cytotoxicity, and inhibitory effects on tubulin polymerization of a new 3-heterocyclo substituted 2-styrylquinazolinones. Eur. J. Med. Chem. 2004, 39, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Ovchinnikova, I.G.; Kim, G.A.; Matochkina, E.G.; Kodess, M.I.; Barykin, N.V.; El´tsov, O.S.; Nosova, E.V.; Rusinov, G.L.; Charushin, V.N. Synthesis, photochemical and luminescent properties of (E)-2-(2-hydroxyarylethylene)-3-phenylquinazolin-4(3H)-ones. Russ. Chem. Bull. 2014, 63, 2467–2477. [Google Scholar] [CrossRef]
- Baghbanzadeh, M.; Molnar, M.; Damm, M.; Reidlinger, C.; Dabiri, M.; Kappe, C.O. Parallel microwave synthesis of 2-styrylquinazolin-4(3H)-ones in a high-throughput platform using HPLC/GC vials as reaction vessels. J. Comb. Chem. 2009, 11, 676–684. [Google Scholar] [CrossRef]
- Srinivasa Reddy, B.; Naidu, A.; Dubey, P.K. PEG-600-mediated, green and efficient, tandem syntheses of N-subtituted-2-styrylquinazolin-4-ones. Green Chem. Lett. Rev. 2013, 6, 254–261. [Google Scholar] [CrossRef]
- Trashakhova, T.V.; Nosova, E.V.; Valova, M.S.; Slepukhin, P.A.; Lipunova, G.N.; Charushin, V.N. Synthesis and photophysical properties of 2-styrylquinazolin-4-ones. Russ. J. Org. Chem. 2011, 47, 753–761. [Google Scholar] [CrossRef]
- Kumar, D.; Jadhavar, P.S.; Nautiyal, M.; Sharma, H.; Meena, P.K.; Adane, L.; Pancholia, S.; Chakraborti, A.K. Convenient synthesis of 2,3-disubstituted quinazolin-4(3H)-ones and 2-styryl-3-substituted quinazolin-4 (3H)-ones: Applications towards the synthesis of drugs. RSC Adv. 2015, 5, 30819–30825. [Google Scholar] [CrossRef]
- Dabiri, M.; Baghbanzadeh, M.; Delbari, A.S. Novel and efficient one-Pot tandem synthesis of 2-Styryl-Substituted 4 (3H)-Quinazolinones. J. Comb. Chem. 2008, 10, 700–703. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ren, D.; Ma, Y.; Wang, W.; Wu, H. CuO nanoparticles catalyzed simple and efficient synthesis of 2,3-dihydroquinazolin-4(1H)-ones and quinazolin-4(3H)-ones under ultrasound irradiation in aqueous ethanol under ultrasound irradiation in aqueous ethanol. Tetrahedron 2014, 70, 5274–5282. [Google Scholar] [CrossRef]
- Naz, S.; Gul, A.; Zia, M. Toxicity of copper oxide nanoparticles: A review study. IET Nanobiotechn. 2020, 14, 1–13. [Google Scholar] [CrossRef]
- Naz, S.; Gul, A.; Zia, M.; Javed, R. Synthesis, biomedical applications, and toxicity of CuO nanoparticles. Appl. Microbiol. Biotechnol. 2023, 107, 1039–1061. [Google Scholar] [CrossRef] [PubMed]
- Fiume, M.M.; Heldreth, B.A.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G., Jr.; Shank, R.C.; Slaga, T.J.; et al. Safety assessment of citric acid, inorganic citrate salts, and alkyl citrate esters as used in cosmetics. Int. J. Toxicol. 2014, 33 (Suppl. 2), 16S–46S. [Google Scholar] [CrossRef]
- Rosado-Solano, D.N.; Barón-Rodríguez, M.A.; Sanabria-Florez, P.L.; Luna-Parada, L.K.; Puerto-Galvis, C.E.; Zorro-González, A.F.; Kouznetsov, V.V.; Vargas-Méndez, L.Y. Synthesis, biological evaluation and in silico computational studies of 7-chloro-4-(1H-1,2,3-triazol-1-yl)quinoline derivatives. Search for new controlling agents against Spodoptera frugiperda (Lepidoptera: Noctuidae) larvae. J. Agric. Food Chem. 2019, 67, 9210–9219. [Google Scholar] [CrossRef]
- Villamizar-Mogotocoro, A.F.; Bonilla-Castañeda, S.M.; Kouznetsov, V.V. Green conditions for the efficient two-step synthesis of new 6-arylphenanthridines from 2-bromoacetoanilides based on microwave-assisted Suzuki-Miyaura cross-coupling and modified Pictet-Spengler dehydrogenative cyclization in a zinc chloride/[Bmim]BF4 mixture. Green Chem. 2022, 24, 7996–8004. [Google Scholar]
- Becerra-Anaya, S.J.; Merchán Arenas, D.R.; Kouznetsov, V.V. A simple and effective protocol for the Pechmann reaction to obtain 4-methylcoumarin derivatives using a high-speed mixer ball mill process. Chemistry 2023, 5, 1077–1088. [Google Scholar] [CrossRef]
- Cioc, R.C.; Ruijter, E.; Orru, R.V. Multicomponent reactions: Advanced tools for sustainable organic synthesis. Green Chem. 2016, 16, 2958–2975. [Google Scholar] [CrossRef]
- Shen, X.; Hong, G.; Wang, L. Recent Advances in Green Multi-Component Reactions for Heterocyclic Compound Construction. Org. Biomol. Chem. 2025, 23, 2059–2078. [Google Scholar] [CrossRef]
- Gupta, A.D.; Sepay, N.; Mallik, A.K. An efficient microwave-assisted synthesis of 2,3-dihydroquinazolin-4(1H)-ones by a three component reaction under catalyst-and solvent-free conditions. Eur. Chem. Bull. 2016, 5, 185–188. [Google Scholar]
- Rupnar, B.D.; Kachave, T.R.; Jawale, P.D.; Shisodia, S.U.; Pawar, R.P. Green and efficient synthesis of 2,3-dihydroquinazolin-4(1H)-ones in aqueous medium using ZnFe2O4 catalyst under microwave irradiation. J. Iran. Chem. Soc. 2017, 14, 1853–1858. [Google Scholar] [CrossRef]
- Dutta, A.; Sarma, D. Base promoted metal-free approach towards synthesis of quinazolin-4(3H)-ones and 2,3-dihydroquinazolin-4(1H)-ones under microwave irradiation. Sustain. Chem. Pharm. 2021, 20, 100402. [Google Scholar] [CrossRef]
- Peña-Solórzano, D.; Guilombo, C.E.G.; Ochoa-Puentes, C. Rapid and eco-friendly high yield synthesis of dihydroquinazolinones mediated by urea/zinc chloride eutectic mixture. Sustain. Chem. Pharm. 2019, 14, 100167. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Lu, H.Y.; Yang, S.H.; Gao, J.W. Synthesis of 2,3-dihydroquinazolin-4(1H)-ones by three-component coupling of isatoic anhydride, amines, and aldehydes catalyzed by magnetic Fe3O4 nanoparticles in water. J. Comb. Chem. 2010, 12, 643–646. [Google Scholar] [CrossRef]
- Mahdavi, M.; Pedrood, K.; Safavi, M.; Saeedi, M.; Pordeli, M.; Ardestani, S.K.; Emami, S.; Adib, M.; Foroumadi, A.; Shafiee, A. Synthesis and anticancer activity of N-substituted 2-arylquinazolinones bearing trans-stilbene scaffold. Eur. J. Med. Chem. 2015, 95, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Dabiri, M.; Salehi, P.; Bahramnejad, M.; Alizadeh, M. A practical and versatile approach toward a one-pot synthesis of 2,3-disubstituted 4(3H)-quinazolinones. Monatsh. Chem. 2010, 141, 877–881. [Google Scholar] [CrossRef]
- Monga, A.; Bagchi, S.; Sharma, A. Iodine/DMSO oxidations: A contemporary paradigm in C–N bond chemistry. New J. Chem. 2018, 42, 1551–1576. [Google Scholar] [CrossRef]
- Wang, J.Q.; Zuo, Z.Y.; He, W. Recent advances of green catalytic system I2/DMSO in C–C and C–Heteroatom bonds formation. Catalysts 2022, 12, 821. [Google Scholar] [CrossRef]
- Singhal, R.; Choudhary, S.P.; Malik, B.; Pilania, M. I2/DMSO-mediated oxidative C–C and C–heteroatom bond formation: A sustainable approach to chemical synthesis. RSC Adv. 2024, 14, 5817–5845. [Google Scholar] [CrossRef]
- Peñaranda Gómez, A.; Puerto Galvis, C.E.; Macías, M.A.; Ochoa-Puentes, C.; Kouznetsov, V.V. I2/DMSO-Promoted the synthesis of chromeno[4,3-b]quinolines through an imine formation/aza-Diels-Alder/aromatization tandem reaction under metal-catalyst and photosensitizer-free conditions. Synthesis 2022, 54, 1857–1869. [Google Scholar]
- Wen, S.; Du, Y.; Liu, Y.; Cui, X.; Liu, Q.; Zhou, H. Access to 2-Arylquinazolin-4(3H)-ones through Intramolecular Oxidative C (sp3)− H/N− H Cross-Coupling Mediated by I2/DMSO. Eur. J. Org. Chem. 2022, 2022, e202101187. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).