Abstract
The success of platinum-based chemotherapeutic drugs for clinical cancer treatments has inspired tremendous research efforts on developing new metallic anticancer agents with improved cytotoxic activity and reduced side effects. 2,2′;6′,2″-Terpyridine and its 4′-substituted derivatives have showed great potential as ligand compartments for designing new anticancer drug candidates involving base metals. In this work, we synthesized a series of cobalt and iron coordination compounds based on 4′-pyridyl-2,2′;6′,2″-terpyridine, including homoleptic complexes, a dinuclear bridged complex and 1- and 2-dimensional coordination polymers/networks. The polymorphism of two homoleptic CoII and FeII complexes has been described along with the structural characterization of a CoII coordination polymer and dinuclear FeIII complex by X-ray crystallography. These compounds were tested preliminarily as precatalysts for the regioselective hydrosilylation of styrene. Their cytotoxic activities against two human breast cancer cell lines (MCF-7 and MDA-MB 468) and a normal breast epithelial cell line (MCF-10A) were investigated in order to observe the best-performing drug candidates.
1. Introduction
2,2′;6′,2″-terpyridine (tpy) is a classical tridentate metal-binding motif that has been well explored for decades and has found widespread applications in metallosupramolecular and materials chemistry, catalysis and biomedical research [1,2,3,4,5,6,7]. The facile modification on the 4′-position of tpy makes it highly versatile for the preparation of diverse metal coordination complexes/frameworks with tunable properties [8,9,10,11]. For instance, 4′-pyridyl-2,2′;6′,2″-terpyridine (pytpy) containing an additional N-coordination site in the 4′-position has been employed to form extended 1-D and 2-D coordination polymers/networks as well as supramolecular assemblies with various transition metals [12,13,14]. In particular, we have previously reported the superior catalytic performance of several CoII and FeII coordination polymers of pytpy for the highly efficient, chemo- and regioselective hydroboration of carbonyl compounds, alkenes and alkynes [15,16,17,18,19]. In addition, the controlled synthesis of homoleptic mononuclear CoII and FeII complexes has been investigated in order to compare their catalytic properties with polymeric networks [20].
On the other hand, the most intriguing property of tpy-based ligands in biological study is their strong intercalation with DNA, and indeed, because of this ability, their transition metal complexes have been numerously reported to be antiproliferative agents and hence promising chemotherapeutic drug candidates for cancer and infectious diseases [6,7,21,22,23]. The research in metal-based anticancer drugs has attracted enormous attention for several decades, largely owing to the success of platinum-based compounds such as cisplatin, oxaliplatin and carboplatin as clinical drugs for more than 50% chemotherapeutic treatments worldwide [24,25,26]. However, cancer treatments using platinum-based drugs were not ideal and suffered from not only drug resistance but also numerous side effects including nerve and kidney damage, nausea, vomiting and bone marrow suppression, etc. [27,28,29]. Therefore, there has been increasing interest in finding other metal-based anticancer drugs that outperform the platinum-based ones [30,31,32,33]. Among many earth-abundant transition metals, we are particularly interested in using cobalt and iron as alternative metals for the development of new metallic anticancer agents, as many of them proved to be cytotoxic compounds against various cancers while coordinating to suitable organic ligands [34,35,36,37,38]. Tpy-type ligands are a good choice for the preparation of such cobalt and iron complexes as potential anticancer agents, as the tpy core features a “non-innocent” ligand capable of stabilizing low valency metals. The notable binding interactions between metal–tpy complexes and DNA have been established from a number of experimental and theoretical reports [6,7]. However, over the past decade, the majority of research contributions to cytotoxic metal–tpy compounds was focused on Cu, Zn, Ru and Pt complexes of some 4′-substituted tpys, while cobalt and iron tpy complexes were comparatively underexplored [6].
In this work, we aim to report on a series of cobalt(II) and iron(II/III) coordination complexes/polymers assembled from pytpy and various metal salts (compounds 1–9, Scheme 1). In addition to their structural diversity, we compared their preliminary catalytic activities toward the hydrosilylation reaction of styrene. While the in-vitro cytotoxicity experiments reveal that these compounds display diverse activities in inhibiting the growth of both cancer and noncancerous cells, an ionic iron(II/III) coordination network (9) could be an excellent candidate for practical anticancer agents that may outperform cisplatin.
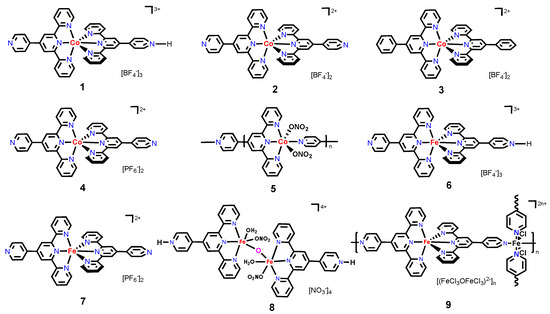
Scheme 1.
The structures of cobalt and iron compounds 1–9 studied in this work.
2. Materials and Methods
2.1. Materials
Unless specified otherwise, the synthesis was carried out under ambient conditions, while the catalytic reactions were conducted under a N2 atmosphere using a standard glovebox technique. All chemicals of analytical grade were used as received from Thermo Fisher Scientific (Waltham, MA, USA) without further purification. Three cell lines from ATCC (American Type Culture Collection, Manassas, VA, USA) were used for this study. Two of these cells are cancer cells: the MCF-7 cell line, which is a human breast cell line and MDA-MB-468, which is a human triple negative breast cancer cell line. The MCF-10A cell line is a human mammary non-tumorigenic epithelial cell line. Cell culture medium and reagents were obtained from Gibco (Grand Island, NY, USA), Thermo Fisher Scientific. FT-IR spectra were recorded on a Shimadzu 8400S instrument (Shimadzu, Kyoto, Japan) with solid samples using a Golden Gate ATR accessory (Specac Ltd., Orpington, UK). Microanalysis was conducted by the Midwest Microlab LLC in the US (Indianapolis, IN, USA). The Agilent 6550 QToF coupled to an Agilent 1290 Infinity LC system was used to acquire HR-MS data (Agilent Technologies, Inc., Santa Clara, CA, USA). GC-MS analysis was conducted on a Shimadzu GCMS-QP2010S gas chromatograph mass spectrometer (Shimadzu, Kyoto, Japan) (column: SHRX1-5MS; conditions: 30–200 °C, 10 °C/min, injection temperature: 100 °C; solvent cutoff: 3 min). UV-Vis absorption spectra were obtained on a Shimadzu UV-2700i spectrophotometer (Shimadzu, Kyoto, Japan). Compounds [CoII(pytpy)(H-pytpy)][BF4]3 (1), [CoII(4′-phenyltpy)2][BF4]2 (3), [CoII(pytpy)2][PF6]2 (4), [FeII(pytpy)2][PF6]2 (7) and [(FeII)3(pytpy)4Cl2][Cl3FeOFeCl3]2 (9) were prepared according to the procedure published previously [17,20].
2.2. Synthesis of [CoII(pytpy)2][BF4]2 (2)
The synthesis of 2 followed the same procedure reported in the literature [20]. The ligand pytpy (31.0 mg, 0.100 mmol) was dissolved in MeOH/CH2Cl2 (8 mL, 1:3, v/v), and a MeOH (3 mL) solution of CoCl2·6H2O (11.9 mg, 0.050 mmol) was then added. The mixture was stirred for 15 min, and a MeOH (2 mL) solution of NaBF4 (66.0 mg, 0.600 mmol) was added dropwise. Brownish precipitate appeared and was collected, washed with MeOH and then dried in air. The crude product was redissolved in MeOH/CH2Cl2 (1:4, v/v) and allowed to slowly evaporate over two days. Orange blocks (72%) were collected and washed with MeOH and dried in the air. HR-MS (ESI positive): 679.1766 (M-2(BF4−), cald. 679.1769). The polymorphism was confirmed by X-ray crystallography.
2.3. Synthesis of [CoII(pytpy)(NO3)2]n (5)
In a test tube, pytpy (31.0 mg, 0.100 mmol) was dissolved in MeOH/CH2Cl2 (10 mL, 1:3, v/v). A solvent mixture of MeOH/CH2Cl2 (4 mL, 1:1, v/v) was layered on the top, and then the solution of Co(NO3)2·6H2O (29.1 mg, 0.100 mmol) in MeOH (8 mL) was carefully added as the upper layer. The tube was sealed and after one week, X-ray quality crystals of 5 as brown plates have grown. The crystals were collected by filtration, washed with MeOH and then dried in air. Yield: 37 mg (75%). FT-IR (solid, cm−1): 3075 w, 1614 s, 1548 m, 1477 w, 1426 s, 1282 s, 1165 m, 1077 w, 1021 s, 896 w, 794 s. Anal. Calcd. for C20H14CoN6O6: C 48.70, H 2.86, N 17.04%. Found C 48.42, H 2.98, N 16.71%.
2.4. Synthesis of [FeII(pytpy)(H-pytpy)][BF4]3 (6)
In a test tube, a solution of pytpy (31.0 mg, 0.100 mmol) in methanol/chlorobenzene (10 mL, 1:3, v/v) was added, and then a blank solution of methanol/chlorobenzene (4 mL, 1:1, v/v) was layered on the top. A solution of Fe(BF4)2·6H2O (33.8 mg, 0.100 mmol) in MeOH (8 mL) was finally added to the upper layer. The tube was sealed at room temperature. After two weeks, X-ray quality brown blocks have grown at the bottom of the tube. The crystals were collected, washed with MeOH and then dried in air. Yield: 40.4 mg (77% based on pytpy). HR-MS (ESI positive): 676.1774 ([M-3(BF4−)-H+], cald. 676.1780). The polymorphism was confirmed as compared to that reported by X-ray crystallography [20].
2.5. Synthesis of {[FeIII(H-pytpy)(OH2)(ONO2)]2(O)}[NO3]4 (8)
The ligand pytpy (31.0 mg, 0.100 mmol) was dissolved in MeOH/CH2Cl2 (10 mL, 1:3, v/v) in a test tube. A solvent mixture of MeOH/CH2Cl2 (4 mL, 1: 1, v/v) was then layered on the top of the ligand, which was followed by a MeOH (8 mL) solution of Fe(NO3)3·9H2O (40.4 mg, 0.100 mmol). The test tube was sealed at room temperature. After one week, X-ray quality crystals of 8 as orange plates have grown. The crystals were collected, washed with MeOH and dried in air. Yield: 40.5 mg (70%). FT-IR (solid, cm−1): 3091 m, 1619 m, 1572 m, 1491 s, 1417 s, 1393 s, 1327 s, 1292 s, 1239 m, 1165 s, 1039 w, 1023 s, 871 s, 829 s, 795 s, 745 s. Anal. Calcd. for C40H34Fe2N14O21: C 41.47, H 2.96, N 16.93%. Found C 41.08, H 3.07, N 16.69%.
2.6. General Procedure for Catalytic Hydrosilylation of Styrene
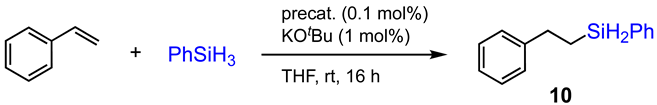
Compounds 1–9 (0.5 μmol, 0.1 mol%) and KOtBu (0.56 mg, 1 mol%) were dissolved in THF (1 mL) in a 3.8 mL glass vial equipped with a small stir bar under N2 atmosphere. The mixture was allowed to stir for 1 min before styrene (52 mg, 0.5 mmol) and phenylsilane (64.8 mg, 0.6 mmol) were added. The reaction was then allowed to proceed at room temperature for 16 h, after which time the reaction was quenched by exposing the solution to the air. The product was examined by GC-MS analysis using hexamethylbenzene as an internal standard.
2.7. Cytotoxicity Measurement
Cells were cultured in an incubation at 37 °C with 5% CO2. The complete media for human mammary epithelial MCF-10A cells contained Dulbecco’s modified Eagle’s medium (DMEM) with 5% fetal bovine serum (FBS), 50 µg/mL gentamicin and a mixture of supplements: 10 µg/mL human insulin, 20 ng/mL hEGF, 100 ng/mL Cholera toxin, and 0.5 µg/mL hydrocortisone. The complete media for MCF-7 contained DMEM with 10% FBS and 50 µg/mL gentamicin. The complete media for MDA-MB-468 contained DMEM with 10% FBS, 2 mM L-glutamine and 50 µg/mL gentamicin. On the day prior to chemical treatment, cells were seeded into 96-well plates with a seeding density of 1 × 104 cells per well to ensure that there is about 80% confluence when exposed to chemicals. Dimethyl sulfoxide (DMSO) was used as a solvent to prepare a 100 mM stock solution for each compound. Compounds were then diluted in the culture media to 0 to 100 µM prior to chemical treatments. The cytotoxicity of samples was determined using CCK-8 cytotoxicity assay following the manufacturer’s protocol (Sigma-Aldrich, St. Louis, MO, USA). The absorbance signals were detected by a BioTek Cytation 7 cell imaging multimode reader (Agilent Technologies, Inc., Santa Clara, CA, USA) at 450 nm. Three separate experiments were conducted for statistical analysis. IC50 was calculated using AAT Bioquest analysis (https://www.aatbio.com/tools/ic50-calculator (accessed on 9 August 2023).
2.8. UV-Vis Absorption Measurement
Solution samples of compounds 1–9 for the UV-Vis absorption measurements were prepared by the sample procedure: a stock solution in DMSO (5 mL) was first prepared at a concentration of 1 × 10−3 M. A 50 µL solution was then taken and added to 5 mL of distilled water to give the final solution of 1 × 10−5 M for measurement. After the UV-Vis spectrum was recorded, the solution was kept at room temperature for 24 h and after which time the UV-Vis spectrum was recorded again. The solution was then warmed up to 37 °C and kept at this temperature for an additional 2 h before the third UV-Vis spectrum was recorded.
2.9. X-ray Crystallography
A Bruker X8 Kappa Apex II diffractometer using Mo Kα radiation was used to collect single crystal X-ray data for 5 and 6, while a Bruker D8 VENTURE diffractometer using Cu Kα radiation was used for 2 and 8. TWINABS was used to account for non-merohedral twinning in 5 [39]. The single crystal structures were initially solved using a dual-space method and standard difference map techniques, and these solutions were further refined by full-matrix least-squares procedures on F2 with SHELXTL [40,41]. Most hydrogen atoms were placed in calculated positions and refined with a riding model [Uiso(H) = 1.2–1.5Ueq(C)], but the hydrogen atoms bound to nitrogen were located on the difference map and freely refined to elucidate the relevant hydrogen bonding. The crystallographic refinement data are listed in Table S1 (see Supplementary Material). The original data have been submitted to the CCDC (deposition nos. 2375496-2375499). These data can be freely obtained via https://www.ccdc.cam.ac.uk/structures/ (accessed on 17 September 2024) or by e-mail at deposit@ccdc.cam.ac.uk.
3. Results
3.1. Synthesis and Crystal Structures
Homoleptic cobalt(II) complexes (1–4) and iron(II) complexes (6 and 7) based on 4′-pytpy and 4′-Phtpy ligands have been previously reported [20]. The 2-dimensional coordination framework (9) has been also synthesized and characterized as an active catalyst for alkyne hydroboration reaction [17]. While reproducing the synthesis of compounds 2 and 6, we observed that changing the reaction and/or crystallization conditions led to the formation of these two compounds as new polymorphs, respectively. Thus, complex [CoII(pytpy)2][BF4]2 (2) was first synthesized by reacting pytpy with CoCl2·6H2O followed by an anion exchange with an excess amount of NaBF4. The crude product was then recrystallized from a CH2Cl2-MeOH solution at room temperature by slow evaporation over two days. Orange block-like crystals of 2 have been harvested in 77% yield. The bulk sample was then characterized by spectroscopic techniques. Although the solution 1H NMR spectrum of 2 shows some broadened signals that could not be unambiguously assigned due to its paramagnetic nature, the ESI-MS spectrum of 2 reveals the peak envelope centered at 679.1766 that can be assigned to the cation of the complex, and the isotope pattern matches with that simulated. X-ray crystallographic analysis reveals that 2 crystallizes in the monoclinic space group P21/c. One independent molecule of 2 was found in the asymmetric unit, and no solvent molecules were included, unlike its other polymorph that contains one acetonitrile molecule in each unit cell, as reported previously [20]. The molecular structure of the cation of 2 is shown in Figure 1A. The 4′-pyridyl group in one of the ligands was found to be disordered, as well as one of BF4− counterions, which was modeled with three-fold disorder. The Co-N bond lengths around the cobalt center in 2 range from 1.8819(15) to 2.1345(16) Å (see the caption of Figure 1). These are unexceptional when compared to previous complexes containing the Co(pytpy)2 cation [20,42]. The non-coordinated 4′-pyridyl ring is distorted from the plane of the tpy backbone in both ligands. The corresponding torsion angle for the ligand molecule containing atoms N2 and N4 is 26.1(3)°. Due to the two-fold disorder, the other ligand molecule containing N6 has two torsion angles of 32(2)° (primary ring containing N8) and 20(2)° (secondary ring containing N8A). In addition, π⋯π stacking interactions between the ‘side-arm’ pyridyl and 4′-pyridyl rings were found to dominate the intermolecular packing mode in the cation of 2. The shortest C⋯C distances for the π-stacking are 3.477(2) and 3.320(5) Å, respectively.
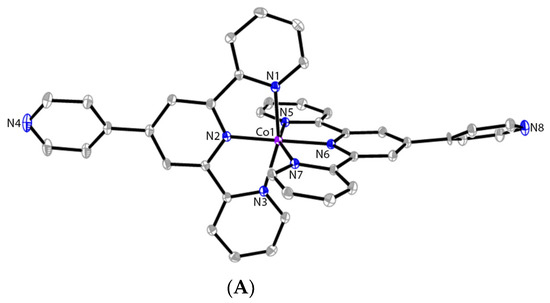
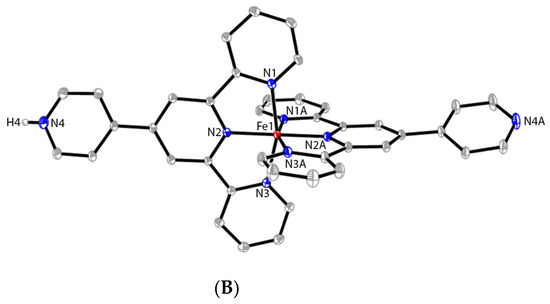
Figure 1.
The ORTEP structure of cations of [CoII(pytpy)2][BF4]2 (2) (A) and [FeII(pytpy)(H-pytpy)][BF4]3 (6) (B) with atomic displacement parameters drawn at the 30% probability level. BF4− counterions, H atoms bound to C, and disorder. Selected bond parameters for 2: Co1–N1 = 2.0393(16), Co1–N2 = 1.8819(15), Co1–N3 = 2.0279(15), Co1–N5 = 2.1286(16), Co1–N6 = 1.9225(15), Co1–N7 = 2.1345(16) Å; N1–Co1–N2 = 80.16(6), N1–Co1–N6 = 99.16(6), N2–Co1–N3 = 80.76(6), N5–Co1–N6 = 78.70(6), N6–Co1–N7 = 79.27(6)°; for 6: Fe1–N1 = 1.9766(10), Fe1–N2 = 1.8781(9), Fe1–N3 = 1.9718(10), N4–H4 = 0.80(4) Å; N1–Fe1–N2 = 80.82(4), N2–Fe1–N3 = 81.06(4), N1–Fe1–N2i = 98.98(4), N1–Fe1–N1i = 92.04(5), N2–Fe1–N2i = 179.71(6)°; i = −x, y, −z + 1/2.
Brown blocks of complex 6 were obtained by layering a solution of Fe(BF4)2·6H2O in MeOH onto a C6H5Cl-MeOH solution (10 mL, 3:1, v/v) of pytpy over two weeks, and X-ray crystallographic analysis reveals a chlorobenzene-solvated polymorph of the monoprotonated complex [FeII(pytpy)(H-pytpy)][BF4]3, where the cation (Figure 1B) has been reported previously.14 Due to the protonation of one of the 4′-pyridyl groups in the cationic complex, an intermolelcular hydrogen bond N4–H4⋯N4(i) (symmetry code i = −x + 1/2, −y + 1/2, −z; N4–H4 = 0.80(4) Å, H4⋯N8 = 1.91(4) Å, N4–H4⋯N4(i) = 178(5)°) has formed and led to a one-dimensional hydrogen-bonded chain similar to those reported [20].
A new coordination polymer, [CoII(pytpy)(NO3)2]n (5), has been synthesized by layering a solution of Co(NO3)2·6H2O in MeOH onto a CH2Cl2-MeOH solution (10 mL, 3:1, v/v) of pytpy for one week. Brown plates of 5 were characterized by X-ray crystallography in a triclinic P-1 space group, which reveals a one-dimensional (1-D) polymeric chain composed of one independent ligand and Co(NO3)2 salt in the repeating structural unit, as shown in Figure 2A. The square bipyramidal coordination mode of the CoII center is similar to that found in the similar 1-D coordination polymer, [CoII(pytpy)Cl2]n reported previously [15], except for the different axial co-ligands (NO3− vs. Cl−) in both structures. The cobalt atom is bound with one tpy-N3 cavity and one 4′-pyridyl-N atom from two adjacent ligand molecules to form a head-to-tail linking pattern. In addition, two NO3− counterions are bonded to each cobalt atom, leading to hexacoordinate CoII centers. However, the bond lengths found in compound 5 are notably different from those in [CoII(pytpy)Cl2]n [15]. As shown in the caption of Figure 2A, the Co-N lengths in 5 range from 2.0511(15) to 2.1890(17) Å, which are approximately 0.2 Å longer than the 1.876(5)–1.986(4) Å Co-N bond lengths in [CoII(pytpy)Cl2]n. While the tpy domain is almost planar, the 4′-pyridyl ring is severely twisted with respect to the tpy plane with a dihedral angle of 33.2(3)°. This is also in sharp contrast to that found in [CoII(pytpy)Cl2]n, where all aromatic rings are perfectly coplanar (merely 1.5° for the distortion angle between the 4′-pyridyl ring and tpy domain). This proves the importance of the coordinating counterions in determining the ligand conformations and consequently the molecular packing mode. The one-dimensional chains in 5 are packed along the crystallographic b axis with significant π⋯π interactions between the side pyridyl rings of tpy (the closest C⋯C distance is 3.317(3) Å), as shown in Figure 2B. However, in the 3-D molecular packing of [CoII(pytpy)Cl2]n, multiple π⋯stacking interactions have been observed between all coplanar pyridyl rings.
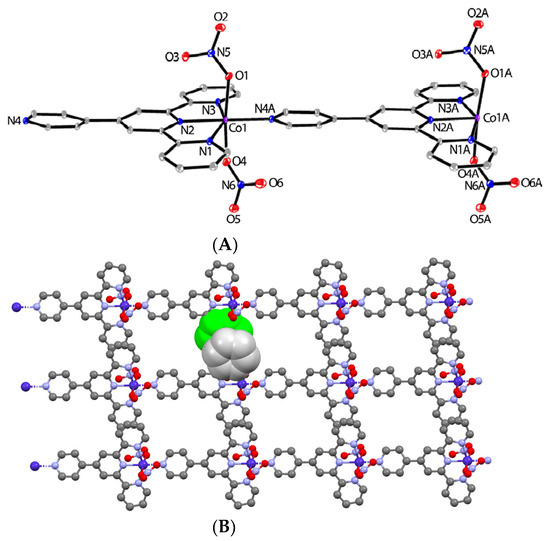
Figure 2.
(A) The ORTEP structure of cations of [CoII(pytpy)(NO3)2]n (5) with atomic displacement parameters drawn at the 30% probability level. Selected bond parameters: Co1–N1 = 2.1890(17), Co1–N2 = 2.0511(15), Co1–N3 = 2.1648(17), Co1–N4i = 2.0832(16), Co1–O1 = 2.1146(15), Co1–O4 = 2.1170(15) Å; N2–Co1–N4i = 178.82(7), N1–Co1–N2 = 76.35(6), N2–Co1–N3 = 76.42(6), N2–Co1–O1 = 98.63(6), N2–Co1–O4 = 87.15(6), O1–Co1–O4 = 174.02(6)°; i = x, y + 1, z. (B) The packing mode of chains in 5 dominated by interchain π⋯π stacking. The space-filling representation highlights one of the π-stacking domains.
Following the procedure for polymer 5, we prepared a discrete dinuclear complex {[FeIII(H-pytpy)(OH2)(ONO2)]2(O)}[NO3]4 (8) by layering an equimolar solution of Fe(NO3)3·9H2O in MeOH onto a CH2Cl2-MeOH solution (10 mL, 3:1, v/v) of pytpy over three weeks. Orange plates of 8 were obtained in 70% yield as good-quality single crystals. X-ray crystallographic analysis confirmed its molecular structure as a dimeric dinuclear FeIII complex containing two monoprotonated ligands. Complex 8 (Figure 3) crystallizes in the triclinic space group P-1; one half of the dimer was contained in the asymmetric unit, and the other half was generated across the inversion center. The FeIII center is bonded to the tpy domain of the ligand, one NO3− and one water molecule. Two symmetric FeIII units are further bridged on the axial direction with an O atom to form a dimeric structure. Interestingly, the 4′-pyridyl groups of pytpy ligands are protonated and thus unavailable for further coordination unlike those in the polymeric chain found in 5. The protonated 4′-pyridyl ring deviates from the plane of the tpy domain by 19.3(4)°. In addition, the Fe–N bond lengths in 8 are found to be in the range of 2.158(2)–2.242(2) Å, which is significantly longer than those present in the homoleptic complex 6 where they are between 1.8781(9) and 1.9766(10) Å (Figure 1). The oxidation state of the iron centers is determined to be 3+, as there are four non-coordinating NO3− ions found for each of the dimeric complexes in the cell. These NO3− ions contribute to the formation of hydrogen bonds with the N-H of the protonated 4′-pyridyl rings and coordinated H2O molecules, which leads to the 3-D molecular packing, along with intermolecular π⋯π stacking interactions (the closest C⋯C contact between 4′-pyridyl rings is 3.533(5) Å).
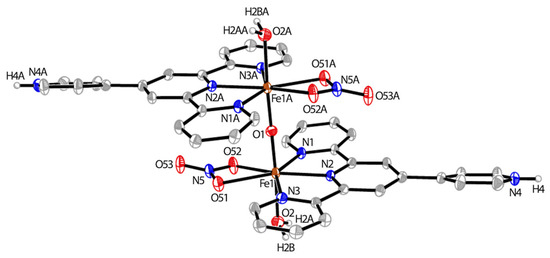
Figure 3.
The ORTEP structure of {[FeIII(H-pytpy)(OH2)(ONO2)]2(O)}[NO3]4 (8) with atomic displacement parameters drawn at the 30% probability level. Non-coordinating NO3− ions and H atoms bound to carbon are omitted for clarity. Selected bond parameters: Fe1–N1 = 2.242(2), Fe1–N2 = 2.158(2), Fe1–N3 = 2.219(2), Fe1–O1 = 1.7663(4), Fe1–O2 = 2.082(2), Fe1–O51 = 2.316(2), Fe1–O52 = 2.217(2) Å, N4–H4 = 0.87(4); O1–Fe1–O2 = 176.70(7), O1–Fe1–N2 = 93.54(6), O2–Fe1–N2 = 88.87(9), O1–Fe1–O51 = 89.08(6)°.
3.2. Catalytic Properties
In light of the excellent performance of cobalt(II) complexes/coordination polymers based on tpy derivatives as catalysts for the hydroboration reactions of polar and nonpolar unsaturated bonds that have been reported [15,16,17,18,19,20], we were interested in examining the catalytic properties of compounds 1–9 for the hydrosilylation of alkenes, which is relatively underexplored by using tpy-based metal catalysts [10]. Thus, in order to evaluate whether these CoII and FeII/III complexes/coordination polymers could be used as effective precatalysts for alkene hydrosilylation, we adopted the similar catalytic conditions used for the known hydroboration of styrene catalyzed by [CoII(pytpy)Cl2]n. The preliminary results of catalytic tests are summarized in Table 1. Interestingly, when precatalyst 1 (0.1 mol%) and a base additive KOtBu (1 mol%) were combined in THF, the hydrosilylation of styrene was conducted in 16 h to give the linear hydrosilylated product (10) in 75% yield with complete anti-Markovnikov selectivity (entry 1). In contrast, the hydroboration of styrene by using the same catalyst led to a mixture of linear and branched alkylboronate products, with the latter being the major product [20]. Improved yields were detected while using 2 and 3 as precatalysts, and the same anti-Markovnikov product was obtained. However, it turned out that complex 4 containing PF6− as counter anions performed poorly for the hydrosilylation reaction (entry 4). This indicates that the BF4− anion might have played an important role in initiating the catalytic cycle, which is reminiscent of the observation of the hidden role of organoboranes in the catalytic hydroboration reaction by Thomas [43]. The best-performing precatalyst was revealed to be the 1-D coordination polymer 5 which furnished the reaction with a quantitative yield and complete regioselectivity. The same result was obtained when using [CoII(pytpy)Cl2]n as a precatalyst (entries 5 and 10). It was surprising that all iron-based compounds (6–9) were inactive precatalysts under the same conditions regardless of the coordination chemistry (entries 6–9).

Table 1.
Preliminary catalytic tests for hydrosilylation of styrene a.
3.3. In Vitro Anticancer Activities
Next, we were interested in determining whether these cobalt(II) and iron(II/III) compounds are promising drug candidates against cancer cells in comparison with the clinically approved drug cisplatin. Both cobalt and iron complexes 1–8 showed moderate solubility in water yet were highly soluble in DMSO. Surprisingly, compound 9 showed high solubility in water as a rare water-soluble 2-D ionic coordination framework. To be consistent, DMSO was used to dissolve the compounds (including cisplatin for comparison), and then cell culture media was added to make a homogenous aqueous solution (see experimental details). Thus, their in vitro cytotoxicity was investigated toward the human breast cancer cell (MCF-7), the triple-negative breast cancer cell (MDA-MB 468) and the non-tumorigenic epithelial cell line from the mammary gland (MCF-10A) using cell proliferation CCK-8 array. All the compounds were incubated for 72 h, after which time the cell viability was assessed. The results are summarized in Table 2. It was found that these compounds show diverse activities in inhibiting the growth of both the cancer cells and noncancerous cells. For example, the CoII complexes 1–5 all showed promisingly low IC50 values against MCF-7 and MDA-MB 468 cells; however, they are equally strong inhibitors toward the noncancerous cell MCF-10A, thus limiting their potential as drug candidates. Similarly, homoleptic FeII complexes 6 and 7 suffered from high toxicity toward noncancerous cells, even though compound 7 showed the highest cytotoxicity against the triple-negative breast cancer cell. Iron complex 8 was a poor inhibitor against cancer cell MCF-7 while displaying high toxicity in noncancerous cells. Pleasingly, the ionic coordination framework 9 performed the best among nine compounds employed here, making it promising as an anticancer drug candidate. Compound 9 showed relatively low toxicity toward the noncancerous cell MCF-10A, but it strongly inhibited the growth of tumorigenic cell MCF-7 with IC50 values as low as approximately 0.7 µM. Although its IC50 value against the MDA-MB 468 cells was higher than that of cisplatin, it remains at the low level as a potentially highly active drug. Previously, a mononuclear iron(II)-pytpy dichloride complex and its analogues have been thoroughly studied on their interaction modes with DNA and their subsequent cellular effects by Gattuso and coworkers [44]. In contrast, compound 9 is water soluble and adopts a unique 2-D supramolecular framework architecture, and its superior anticancer activity certainly deserves further investigation.

Table 2.
Half-maximal inhibitory concentration (IC50) values (µM) of compounds 1–9 against the breast cancer cell (MCF-7), the triple-negative breast cancer cell (MDA-MB 468) and the non-tumorigenic epithelial cell line from the mammary gland (MCF-10A).
The stability of all nine compounds in aqueous media has been examined by UV-Vis absorption spectroscopy in order to understand the robustness of these drug candidates under physiological environments. The water solutions of nine compounds with a concentration of 1 × 10−5 M were obtained by diluting the stock solutions for each of the samples in DMSO, which is similar to the conditions used for cytotoxicity measurement. The UV-Vis absorption of all samples has been measured as freshly prepared and after 24 h in the aqueous media (see Supplementary Material). The results reveal that compounds 1–4, 7 and 9 show good stability after 24 h. The main peaks of their UV-Vis absorption remained with a slight decrease in their intensity due to possible molecular aggregation and precipitation in water. Furthermore, their spectra showed no changes after the solutions were kept at 37 °C for 2 h. However, the UV-Vis absorption of compounds 5, 6 and 8 showed significant decrease in intensity, indicating their poorer stability in water.
4. Conclusions
In summary, we have synthesized and characterized diverse cobalt(II) and iron(II/III) complexes and coordination polymers using the ditopic pytpy ligand. The polymorphism of two known homoleptic complexes (2 and 6) has been explored by X-ray crystallography. A new 1-D coordination polymer of cobalt(II) nitrate with pytpy (5) has been prepared and structurally elucidated to be similar to [CoII(pytpy)Cl2]n in terms of the 1-D polymeric chain yet notably different in aspects of intermolecular interactions and 3-D molecular packing. Using the same reaction conditions with iron(III) nitrate, an oxo-bridged FeIII-pytpyH dimer has been isolated, where the 4′-pyridyl N atom of the pytpy ligand was protonated instead of participating in coordination with metal ions. These new compounds along with other analogues previously reported (1–9) have been preliminarily explored for the catalytic ability in the hydrosilylation of styrene. The results revealed that both 1-D coordination polymers 5 and [CoII (pytpy)Cl2]n were equally highly effective precatalysts for the regioselective hydrosilylation of styrene, affording a linear alkylphenylsilane product in quantitative yield. In vitro cytotoxicity studies revealed that these compounds all showed considerable anticancer effects on human breast cancer cells (MCF-7) and triple-negative breast cancer cells (MDA-MB 468). However, all of them but compound 9 suffered from high toxicity toward the noncancerous cells MCF-10A. Therefore, the water-soluble and stable compound 9 represents a potentially active anticancer drug candidate comparable to the clinically approved cisplatin and deserves further investigation.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/chemistry6050064/s1, Figure S1–S9: UV-Vis spectra for compounds 1–9; Table S1: X-ray crystallographic data for compounds 2, 5, 6 and 8.
Author Contributions
Conceptualization, S.-Y.C., Q.Z. and S.Z.; Synthesis, characterization, catalysis and cytotoxicity, Q.Z. and Q.T.; crystallographic analysis, M.C.N.; writing—original draft preparation, S.-Y.C. and Q.Z.; writing—review and editing, Q.Z., S.-Y.C., M.C.N. and S.Z.; supervision, S.-Y.C. and S.Z.; project administration, S.-Y.C. and S.Z.; funding acquisition, S.-Y.C. and M.C.N. All authors have read and agreed to the published version of the manuscript.
Funding
We are grateful to the support from the PRISM program at John Jay College of Criminal Justice, the City University of New York. United States Department of Education Title V Institutional Development of Hispanic Serving Institutions Grant (Award: P031S200200). United States Department of Education Title III—Part F Hispanic Serving Institutions—Science, Technology, Engineering, and Mathematics (HSI-STEM) and Articulation Programs Grant (Award: P031C210098). The Bruker D8 VENTURE X-ray diffractometer used for portions of this work was proved by the Air Force Office of Scientific Research under award number FA9550-20-1-0158.
Data Availability Statement
Data are contained within the article or Supplementary Material.
Conflicts of Interest
There are no conflicts to declare. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Momeni, B.Z.; Davarzani, N.; Janczak, J.; Ma, N.; Abd-El-Aziz, A.S. Progress in design and applications of supramolecular assembly of 2,2′:6′,2″-terpyridine-based first row d-block elements. Coord. Chem. Rev. 2024, 506, 215619. [Google Scholar] [CrossRef]
- Winter, A.; Newkome, G.R.; Schubert, U.S. The chemistry of the s-and p-block elements with 2,2′:6′,2″-terpyridine ligands. Inorg. Chem. Front. 2024, 11, 342–399. [Google Scholar] [CrossRef]
- Panicker, R.R.; Sivaramakrishna, A. Remarkably flexible 2,2′:6′,2″-terpyridines and their group 8–10 transition metal complexes–Chemistry and applications. Coord. Chem. Rev. 2022, 459, 214426. [Google Scholar] [CrossRef]
- Shi, J.; Wang, M. Self-Assembly Methods for Recently Reported Discrete Supramolecular Structures Based on Terpyridine. Chem.–Asian J. 2021, 16, 4037–4048. [Google Scholar] [CrossRef]
- Winter, A.; Schubert, U.S. Metal-Terpyridine Complexes in Catalytic Application—A Spotlight on the Last Decade. ChemCatChem 2020, 12, 2890–2941. [Google Scholar] [CrossRef]
- Abhijnakrishna, R.; Magesh, K.; Ayushi, A.; Velmathi, S. Advances in the biological studies of metal-terpyridine complexes: An overview from 2012 to 2022. Coord. Chem. Rev. 2023, 496, 215380. [Google Scholar] [CrossRef]
- Musiol, R.; Malecki, P.; Pacholczyk, M.; Mularski, J. Terpyridines as promising antitumor agents: An overview of their discovery and development. Exp. Opin. Drug Disc. 2022, 17, 259–271. [Google Scholar] [CrossRef]
- Elahi, S.M.; Raizada, M.; Sahu, P.K.; Konar, S. Terpyridine-Based 3D Metal–Organic-Frameworks: A Structure–Property Correlation. Chem. Eur. J. 2021, 27, 5858–5870. [Google Scholar] [CrossRef]
- Kainat, S.F.; Hawsawi, M.B.; Mughal, E.U.; Naeem, N.; Almohyawi, A.M.; Altass, H.M.; Hussein, E.M.; Sadiq, A.; Moussa, Z.; Abd-El-Aziz, A.S.; et al. Recent developments in the synthesis and applications of terpyridine-based metal complexes: A systematic review. RSC adv. 2024, 14, 21464–21537. [Google Scholar] [CrossRef]
- Attwood, M.; Turner, S.S. Back to back 2, 6-bis (pyrazol-1-yl) pyridine and 2,2′:6′,2″-terpyridine ligands: Untapped potential for spin crossover research and beyond. Coord. Chem. Rev. 2017, 353, 247–277. [Google Scholar] [CrossRef]
- Dickenson, J.C.; Haley, M.E.; Hyde, J.T.; Reid, Z.M.; Tarring, T.J.; Iovan, D.A.; Harrison, D.P. Fine-tuning metal and ligand-centered redox potentials of homoleptic bis-terpyridine complexes with 4′-aryl substituents. Inorg. Chem. 2021, 60, 9956–9969. [Google Scholar] [CrossRef] [PubMed]
- Beves, J.E.; Bray, D.J.; Clegg, J.K.; Constable, E.C.; Housecroft, C.E.; Jolliffe, K.A.; Kepert, C.J.; Lindoy, L.F.; Neuburger, M.; Price, D.J.; et al. Expanding the 4, 4′-bipyridine ligand: Structural variation in {M(pytpy)2}2+ complexes (pytpy= 4′-(4-pyridyl)-2,2′:6′,2″-terpyridine, M = Fe, Ni, Ru) and assembly of the hydrogen-bonded, one-dimensional polymer {[Ru(pytpy)(Hpytpy)]}n3n+. Inorg. Chim. Acta 2008, 361, 2582–2590. [Google Scholar] [CrossRef]
- Beves, J.E.; Constable, E.C.; Housecroft, C.E.; Kepert, C.J.; Neuburger, M.; Price, D.J.; Schaffner, S. The conjugate acid of bis{4′-(4-pyridyl)-2,2′:6′,2″-terpyridine} iron(II) as a self-complementary hydrogen-bonded building block. CrystEngComm 2007, 9, 1073–1077. [Google Scholar] [CrossRef]
- Beves, J.E.; Dunphy, E.L.; Constable, E.C.; Housecroft, C.E.; Kepert, C.J.; Neuburger, M.; Price, D.J.; Schaffner, S. Vectorial property dependence in bis {4′-(n-pyridyl)-2,2′:6′,2″-terpyridine} iron(II) and ruthenium(II) complexes with n = 2, 3 and 4. Dalton Trans. 2008, 3, 386–396. [Google Scholar] [CrossRef]
- Wu, J.; Zeng, H.S.; Cheng, J.; Zheng, S.; Golen, J.A.; Manke, D.R.; Zhang, G. Cobalt (II) coordination polymer as a precatalyst for selective hydroboration of aldehydes, ketones, and imines. J. Org. Chem. 2018, 83, 9442–9448. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Li, S.; Wu, J.; Zeng, H.; Mo, Z.; Davis, K.; Zheng, S. Highly efficient and selective hydroboration of terminal and internal alkynes catalysed by a cobalt (II) coordination polymer. Org. Chem. Front. 2019, 6, 3228–3233. [Google Scholar] [CrossRef]
- Zhang, G.; Zheng, S.; Neary, M.C. An ionic Fe-based metal–organic-framework with 4′-pyridyl-2,2′:6′,2″-terpyridine for catalytic hydroboration of alkynes. RSC Adv. 2023, 13, 2225–2232. [Google Scholar] [CrossRef]
- Zhang, G.; Wu, J.; Li, S.; Cass, S.; Zheng, S. Markovnikov-Selective Hydroboration of Vinylarenes Catalyzed by a Cobalt(II) Coordination Polymer. Org. Lett. 2018, 20, 7893–7897. [Google Scholar] [CrossRef]
- Zhang, G.; Zeng, H.; Li, S.; Johnson, J.; Mo, Z.; Neary, M.C.; Zheng, S. 1-D manganese (II)-terpyridine coordination polymers as precatalysts for hydrofunctionalisation of carbonyl compounds. Dalton Trans. 2020, 49, 2610–2615. [Google Scholar] [CrossRef]
- Zhang, G.; Zeng, H.; Zadori, N.; Marino, C.; Zheng, S.; Neary, M.C. Homoleptic octahedral CoII complexes as precatalysts for regioselective hydroboration of alkenes with high turnover frequencies. RSC Adv. 2023, 13, 28089–28096. [Google Scholar] [CrossRef]
- Gil-Moles, M.; Concepción Gimeno, M. The Therapeutic Potential in Cancer of Terpyridine-Based Metal Complexes Featuring Group 11 Elements. ChemMedChem 2024, 19, e202300645. [Google Scholar] [CrossRef] [PubMed]
- Winter, A.; Gottschaldt, M.R.; Newkome, G.; Schubert, U.S. Terpyridines and their complexes with first row transition metal ions: Cytotoxicity, nuclease activity and self-assembly of biomacromolecules. Curr. Top. Med. Chem. 2012, 12, 158–175. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Wu, Z.; Ge, Q.; Zheng, X.; Yang, Z. Antitumor activity of tridentate pincer and related metal complexes. Org. Biomol. Chem. 2021, 19, 5254–5273. [Google Scholar] [CrossRef] [PubMed]
- Karges, J. Combining inorganic chemistry and biology: The underestimated potential of metal complexes in medicine. ChemBioChem 2020, 21, 3044–3046. [Google Scholar] [CrossRef]
- Gasser, G. Metal complexes and medicine: A successful combination. Chimia 2015, 69, 442. [Google Scholar] [CrossRef]
- Johnstone, T.C.; Suntharalingam, K.; Lippard, S.J. The next generation of platinum drugs: Targeted Pt (II) agents, nanoparticle delivery, and Pt (IV) prodrugs. Chem. Rev. 2016, 116, 3436–3486. [Google Scholar] [CrossRef]
- Florea, A.M.; Büsselberg, D. Cisplatin as an anti-tumor drug: Cellular mechanisms of activity, drug resistance and induced side effects. Cancers 2011, 3, 1351–1371. [Google Scholar] [CrossRef]
- Karges, J.; Yempala, T.; Tharaud, M.; Gibson, D.; Gasser, G. A multi-action and multi-target RuII–PtIV conjugate combining cancer-activated chemotherapy and photodynamic therapy to overcome drug resistant cancers. Angew. Chem. Intern. Ed. 2020, 59, 7069–7075. [Google Scholar] [CrossRef]
- Alassadi, S.; Pisani, M.J.; Wheate, N.J. A chemical perspective on the clinical use of platinum-based anticancer drugs. Dalton Trans. 2022, 51, 10835–10846. [Google Scholar] [CrossRef]
- Paprocka, R.; Wiese-Szadkowska, M.; Janciauskiene, S.; Kosmalski, T.; Kulik, M.; Helmin-Basa, A. Latest developments in metal complexes as anticancer agents. Coord. Chem. Rev. 2022, 452, 214307. [Google Scholar] [CrossRef]
- Gourdon, L.; Cariou, K.; Gasser, G. Phototherapeutic anticancer strategies with first-row transition metal complexes: A critical review. Chem. Soc. Rev. 2022, 51, 1167–1195. [Google Scholar] [CrossRef]
- Sen, S.; Won, M.; Levine, M.S.; Noh, Y.; Sedgwick, A.C.; Kim, J.S.; Sessler, J.L.; Arambula, J.F. Metal-based anticancer agents as immunogenic cell death inducers: The past, present, and future. Chem. Soc. Rev. 2022, 51, 1212–1233. [Google Scholar] [CrossRef] [PubMed]
- Abdolmaleki, S.; Aliabadi, A.; Khaksar, S. Riding the metal wave: A review of the latest developments in metal-based anticancer agents. Coord. Chem. Rev. 2024, 501, 215579. [Google Scholar] [CrossRef]
- Kar, K.; Ghosh, D.; Kabi, B.; Chandra, A. A concise review on cobalt Schiff base complexes as anticancer agents. Polyhedron 2022, 222, 115890. [Google Scholar] [CrossRef]
- Munteanu, C.R.; Suntharalingam, K. Advances in cobalt complexes as anticancer agents. Dalton Trans. 2015, 44, 13796–13808. [Google Scholar] [CrossRef]
- Szlasa, W.; Gachowska, M.; Kiszka, K.; Rakoczy, K.; Kiełbik, A.; Wala, K.; Puchała, J.; Chorążykiewicz, K.; Saczko, J.; Kulbacka, J. Iron chelates in the anticancer therapy. Chem. Papers 2022, 76, 1285–1294. [Google Scholar] [CrossRef]
- Bouché, M.; Hognon, C.; Grandemange, S.; Monari, A.; Gros, P.C. Recent advances in iron-complexes as drug candidates for cancer therapy: Reactivity, mechanism of action and metabolites. Dalton Trans. 2020, 49, 11451–11466. [Google Scholar] [CrossRef]
- Basu, U.; Roy, M.; Chakravarty, A.R. Recent advances in the chemistry of iron-based chemotherapeutic agents. Coord. Chem. Rev. 2020, 417, 213339. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Twinabs; University of Göttingen: Göttingen, Germany, 2012. [Google Scholar]
- Sheldrick, G.M. SHELXTL, an Integrated System for Solving, Refining, and Displaying Crystal Structures from Diffraction Data; University of Göttingen: Göttingen, Germany, 1981. [Google Scholar]
- Sheldrick, G.M. SHELXT-Integrated Space-Group and Crystal-Structure Determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef]
- Guo, Y.; Yang, X.L.; Wei, R.J.; Zheng, L.S.; Tao, J. Spin transition and structural transformation in a mononuclear cobalt(II) complex. Inorg. Chem. 2015, 54, 7670–7672. [Google Scholar] [CrossRef]
- Bage, A.D.; Nicholson, K.; Hunt, T.A.; Langer, T.; Thomas, S.P. The hidden role of boranes and borohydrides in hydroboration catalysis. ACS Catal. 2020, 10, 13479–13486. [Google Scholar] [CrossRef]
- Gattuso, H.; Duchanois, T.; Besancenot, V.; Barbieux, C.; Assfeld, X.; Becuwe, P.; Gros, P.C.; Grandemange, S.; Monari, A. Interaction of Iron II Complexes with B-DNA. Insights from Molecular Modeling, Spectroscopy, and Cellular Biology. Front. Chem. 2015, 3, 67. [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).