An Alternative Method for Synthesizing N,2,3-Trimethyl-2H-indazol-6-amine as a Key Component in the Preparation of Pazopanib
Abstract
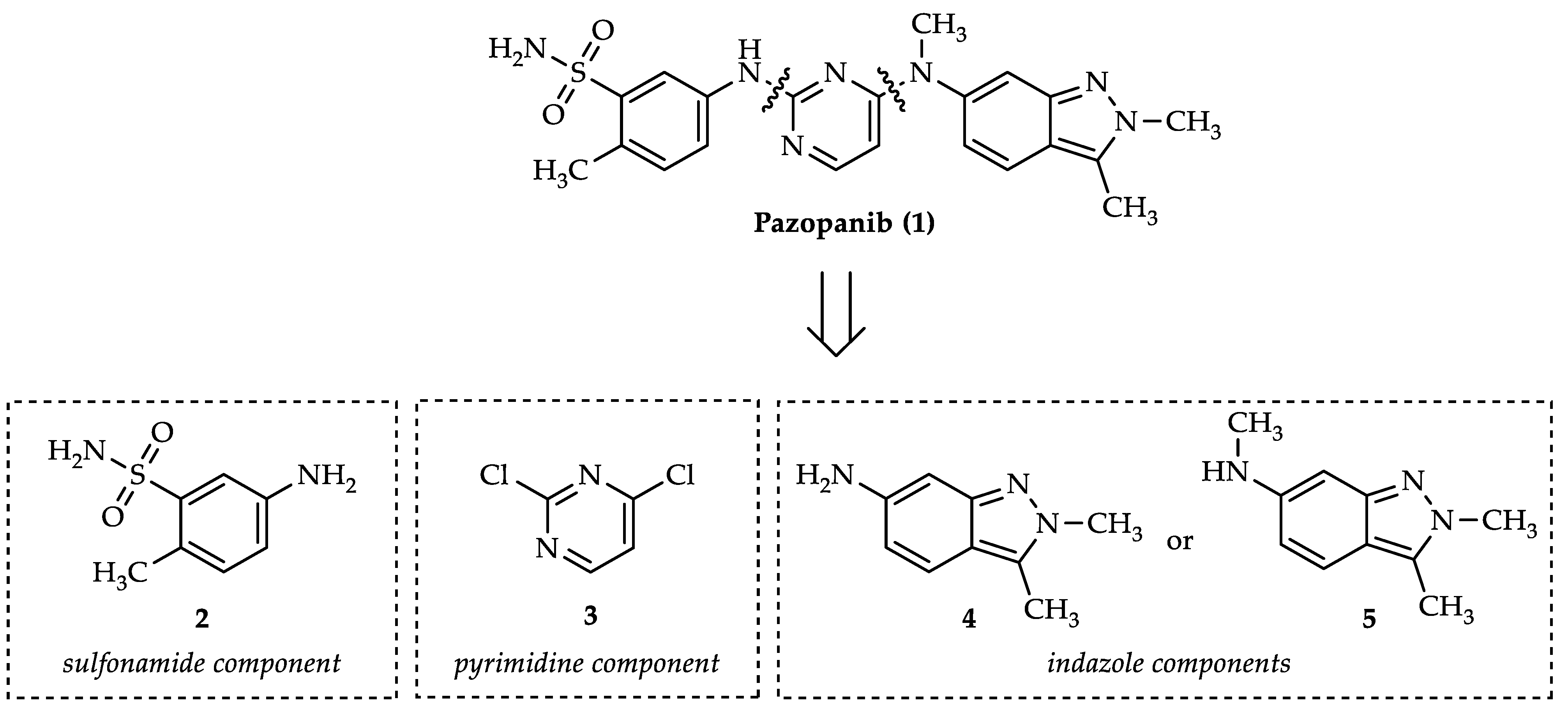
1. Introduction
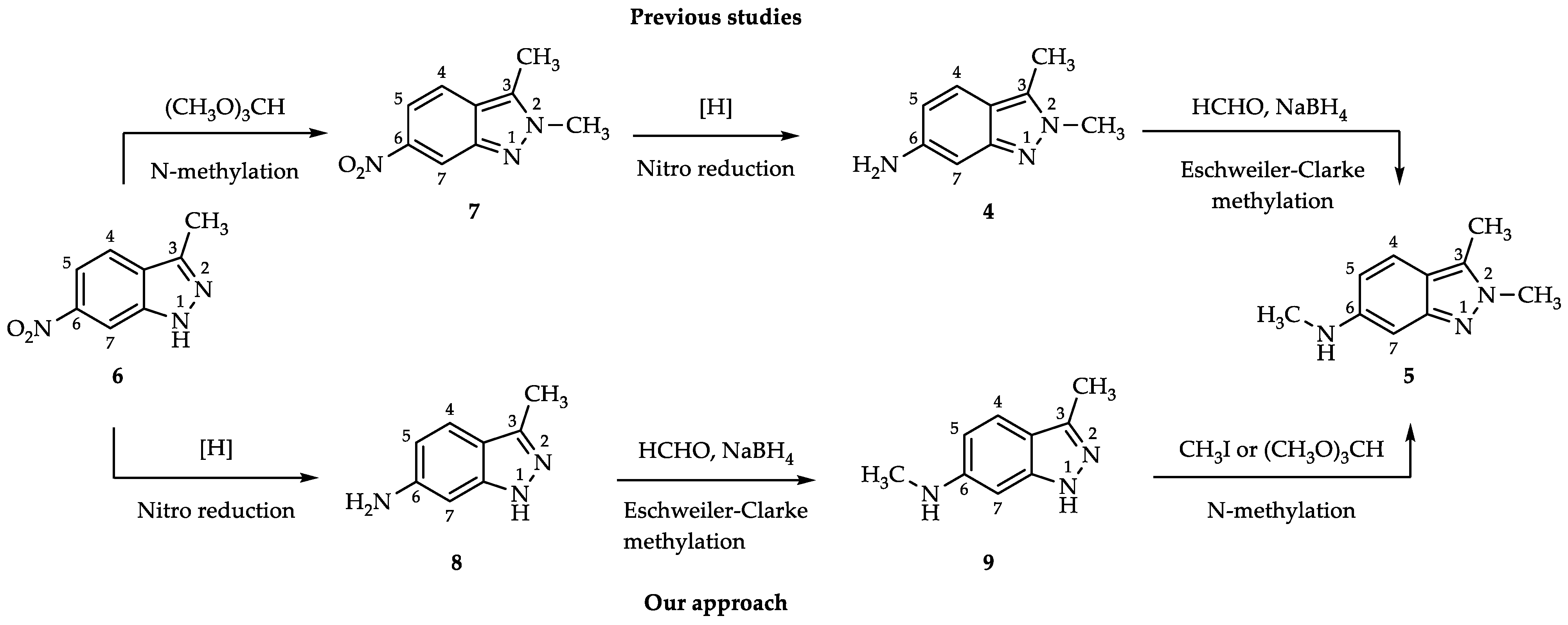
2. Results and Discussion
2.1. Process for 3-Methyl-1H-indazol-6-amine (8)
2.2. Process for N,3-Dimethyl-1H-indazol-6-amine (9) via Reductive Amination
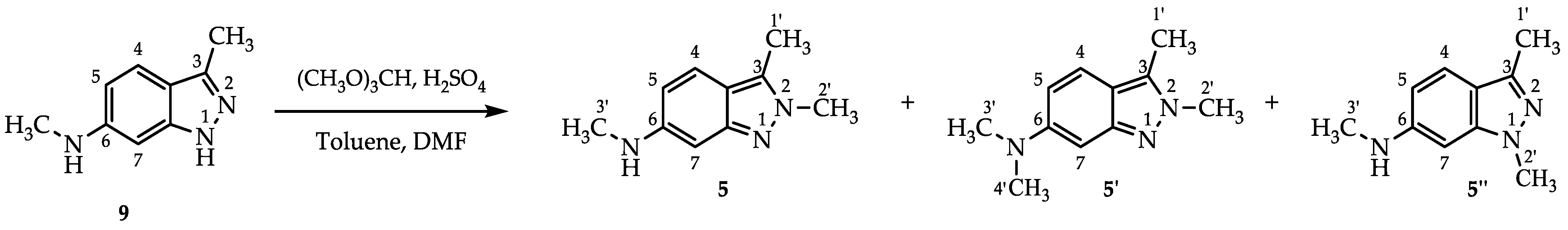
2.3. Process for N,2,3-Trimethyl-2H-indazol-6-amine (5)
3. Materials and Methods
3.1. General Information
3.2. Synthetic Procedure
3.2.1. 3-Methyl-1H-indazol-6-amine (8)
3.2.2. N,3-Dimethyl-1H-indazol-6-amine (9)
3.2.3. N,2,3-Trimethyl-2H-indazol-6-amine (5)
3.2.4. N,N,2,3-Tetratramethyl-2H-indazol-6-amine (5′)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Medicines Agency. EPAR Summary for the Public, Votrient, Procedure No. EMEA/H/C/001141; European Medicines Agency: London, UK, 2012. [Google Scholar]
- Mantiero, M.; Bini, M.; Polignano, M.; Porcu, L.; Sanfilippo, R.; Fabbroni, C.; Parma, G.; Lapresa, M.; Calidona, C.; Silvestri, C.; et al. A ten-year real-life experience with pazopanib in uterine leyomiosarcoma in two high-specialized centers in Italy: Effectiveness and safety. Cancers 2024, 16, 192. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.J.; Yun, K.H.; Shin, S.J.; Lee, Y.H.; Kim, S.H.; Baek, W.; Han, Y.D.; Kim, S.K.; Ryu, H.J.; Lee, J.; et al. Durvalumab plus pazopanib combination in patients with advanced soft tissue sarcomas: A phase II trial. Nat. Commun. 2024, 15, 685. [Google Scholar] [CrossRef] [PubMed]
- Keisner, S.V.; Shah, S.R. Pazopanib: The newest tyrosine kinase inhibitor for the treatment of advanced or metastatic renal cell carcinoma. Drugs 2011, 71, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Sternberg, C.N.; Davis, I.D.; Mardiak, J.; Szczylik, C.; Lee, E.; Wagstaff, J.; Barrios, C.H.; Salman, P.; Gladkov, O.A.; Kavina, A.; et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: Results of a randomized phase III trial. J. Clin. Oncol. 2023, 41, 1957–1964. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Knick, V.B.; Rudolph, S.K.; Johnson, J.H.; Crosby, R.M.; Crouthamel, M.C.; Hopper, T.M.; Miller, C.G.; Harrington, L.E.; Onori, J.A.; et al. Pharmacokinetic-pharmacodynamic correlation from mouse to human with pazopanib, a multikinase angiogenesis inhibitor with potent antitumor and antiangiogenic activity. Mol. Cancer Ther. 2007, 6, 2012–2021. [Google Scholar] [CrossRef] [PubMed]
- McCormack, P.L. Pazopanib: A review of its use in the management of advanced renal cell carcinoma. Drugs 2014, 74, 1111–1125. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Zhang, J.; Feng, J.; Xu, F.; Pan, H.; Xu, W. Design, synthesis and biological evaluation of pazopanib derivatives as antitumor agents. Chem. Biol. Drug Des. 2014, 83, 306–316. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.A.; Boloor, A.; Cheung, M.; Kumar, R.; Crosby, R.M.; Davis-Ward, R.G.; Epperly, A.H.; Hinkle, K.W.; Hunter III, R.N.; Johnson, J.H.; et al. Discovery of 5-[[4-[(2,3-dimethyl-2H-indazol-6-yl)methylamino]-2-pyrimidinyl]amino]-2-methyl-benzenesulfonamide (Pazopanib), a novel and potent vascular endothelial growth factor receptor inhibitor. J. Med. Chem. 2008, 51, 4632–4640. [Google Scholar] [CrossRef] [PubMed]
- Mei, Y.C.; Yang, B.W.; Chen, W.; Huang, D.D.; Li, Y.; Deng, X.; Liu, B.M.; Wang, J.J.; Qian, H.; Huang, W.L. A novel practical synthesis of pazopanib: An anticancer drug. Lett. Org. Chem. 2012, 9, 276–279. [Google Scholar] [CrossRef]
- Boloor, A.; Cheung, M.; Davis, R.; Harris, P.A.; Hinkle, K.; Mook, R.A., Jr.; Stafford, J.A.; Veal, J.M. Pyrimidineamines as Angiogenesis Modulators. U.S. Patent US7105530B2, 12 September 2006. [Google Scholar]
- Mei, Y.C.; Yang, B.W. The regioselective alkylation of some indazoles using trialkyl orthoformate. Indian J. Heterocycl. Chem. 2017, 27, 275–280. [Google Scholar]
- Boloor, A.; Cheung, M. Chemical Process. WO2003106416A2, 24 December 2003. [Google Scholar]
- Terentjeva, S.; Muceniece, D.; Lūsis, V. Process-related impurities of pazopanib. Org. Process Res. Dev. 2019, 23, 2057–2068. [Google Scholar] [CrossRef]
- Frizzo, C.P. Alkyl orthoformate: A versatile reagent in organic synthesis. Synlett 2009, 2009, 1019–1020. [Google Scholar] [CrossRef][Green Version]
- Kim, D.J.; Oh, K.H.; Park, J.K. A general and direct synthesis of imidazolium ionic liquids using orthoesters. Green Chem. 2014, 16, 4098–4101. [Google Scholar] [CrossRef]
- Escudero-Ortiz, V.; Pérez-Ruixo, J.J.; Valenzuela, B. Development and validation of an HPLC-UV method for pazopanib quantification in human plasma and application to patients with cancer in routine clinical practice. Ther. Drug Monit. 2015, 37, 172–179. [Google Scholar] [CrossRef] [PubMed]

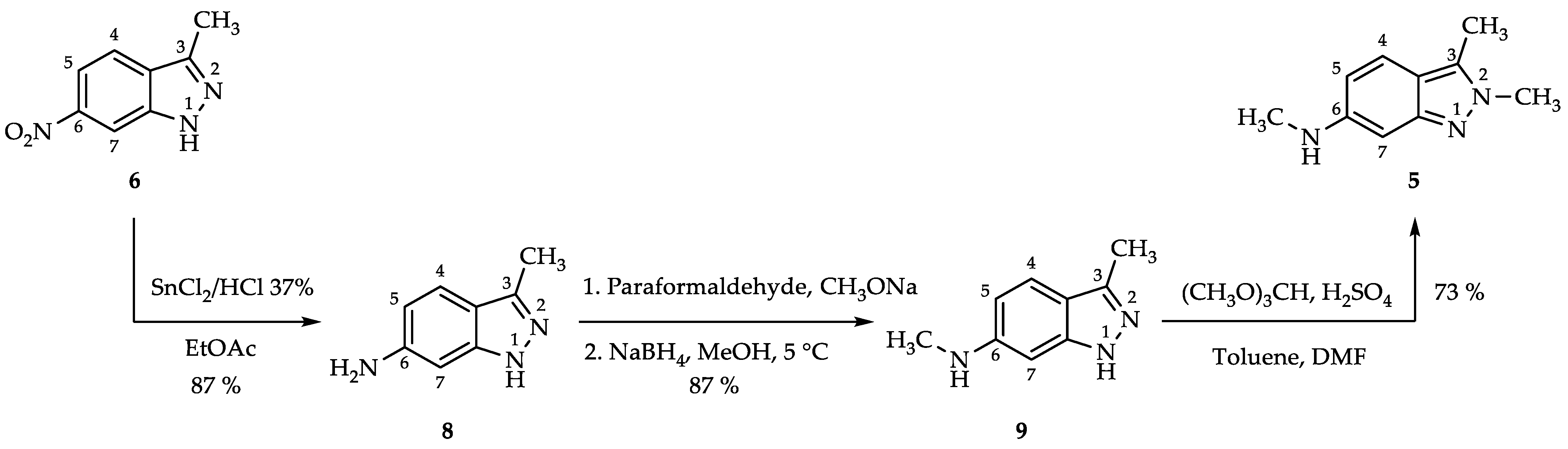
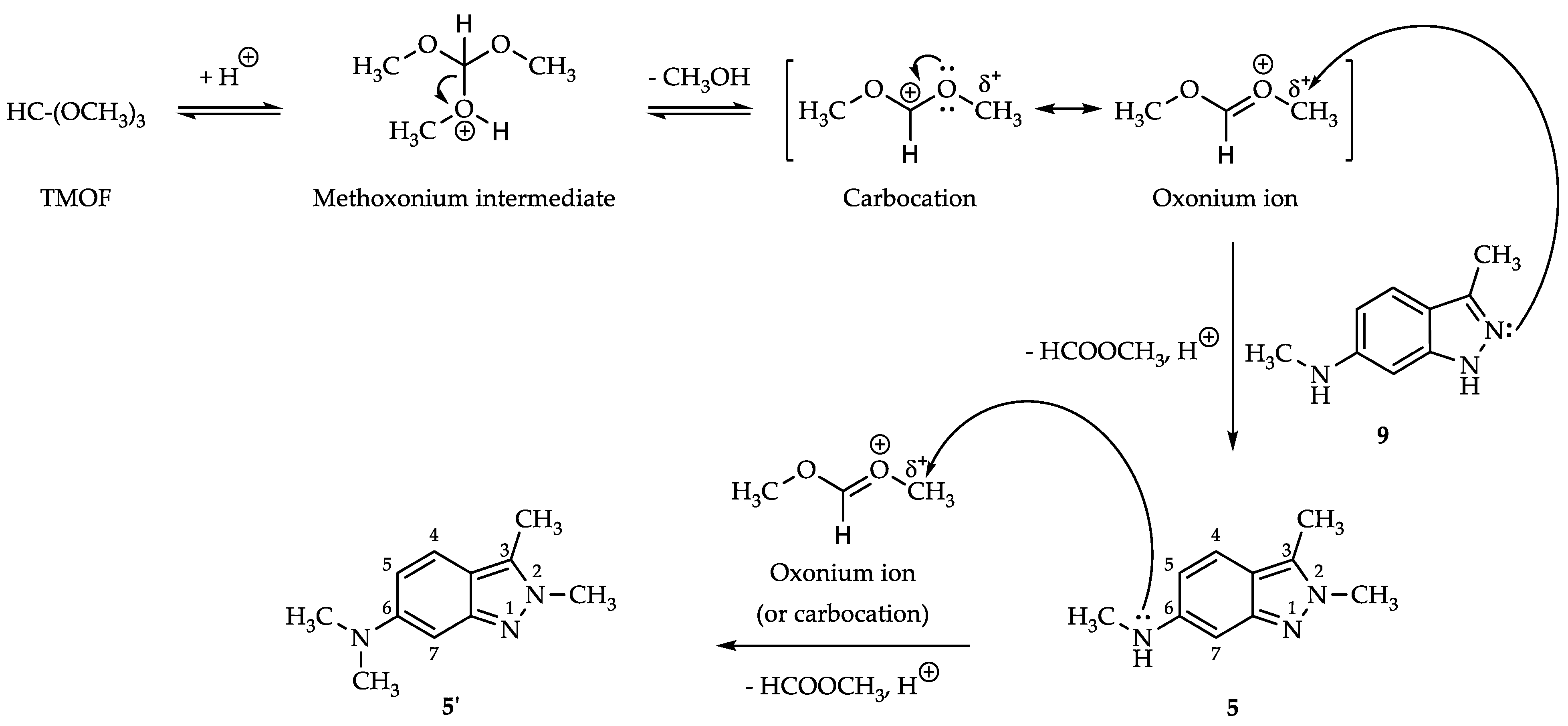
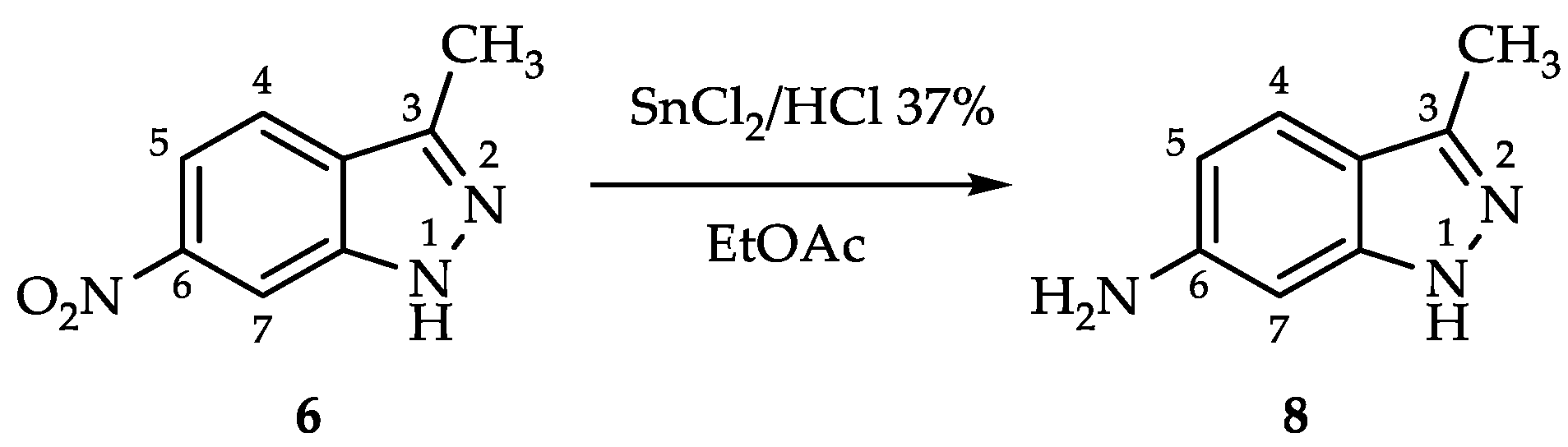 | ||||
|---|---|---|---|---|
| Entry | Ratio (SnCl2:Compound 6) | Time (h) | Product Mass (g) | Yield (%) |
| 1 | 3:1 | 8 | 1.35 | 81 |
| 2 | 4:1 | 3 | 1.45 | 87 |
| 3 | 5:1 | 3 | 1.37 | 83 |
| 4 | 6:1 | 3 | 1.39 | 84 |
 | ||||
|---|---|---|---|---|
| Entry | Base Catalyst | Time (h) | Product Mass (g) | Yield (%) |
| 1 | CH3ONa | 6 | 1.90 | 87 |
| 2 | t-BuOK | 6 | 1.74 | 79 |
| 3 | K2CO3 | 10 | 1.43 | 65 |
| 4 | Na2CO3 | 10 | 1.46 | 67 |
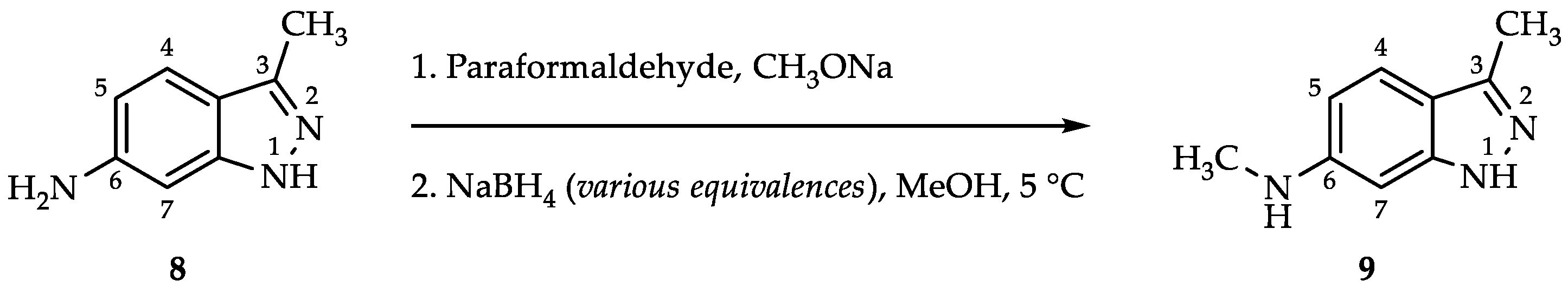 | |||
|---|---|---|---|
| Entry | Molar Ratio of Compound 8:NaBH4 | Time (h) | Yield (%) |
| 1 | 1:2 | 14 | 76 |
| 2 | 1:3 | 9 | 79 |
| 3 | 1:4 | 6 | 87 |
| 4 | 1:5 | 6 | 87 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bui, T.T.C.; Luu, H.L.; Luong, T.T.; Nguyen, T.N.; Dao, N.S.H.; Nguyen, V.G.; Nguyen, D.L.; Trinh, N.T.; Nguyen, V.H. An Alternative Method for Synthesizing N,2,3-Trimethyl-2H-indazol-6-amine as a Key Component in the Preparation of Pazopanib. Chemistry 2024, 6, 1089-1098. https://doi.org/10.3390/chemistry6050063
Bui TTC, Luu HL, Luong TT, Nguyen TN, Dao NSH, Nguyen VG, Nguyen DL, Trinh NT, Nguyen VH. An Alternative Method for Synthesizing N,2,3-Trimethyl-2H-indazol-6-amine as a Key Component in the Preparation of Pazopanib. Chemistry. 2024; 6(5):1089-1098. https://doi.org/10.3390/chemistry6050063
Chicago/Turabian StyleBui, Thi Thanh Cham, Hue Linh Luu, Thi Thanh Luong, Thi Ngoc Nguyen, Nguyet Suong Huyen Dao, Van Giang Nguyen, Dinh Luyen Nguyen, Nguyen Trieu Trinh, and Van Hai Nguyen. 2024. "An Alternative Method for Synthesizing N,2,3-Trimethyl-2H-indazol-6-amine as a Key Component in the Preparation of Pazopanib" Chemistry 6, no. 5: 1089-1098. https://doi.org/10.3390/chemistry6050063
APA StyleBui, T. T. C., Luu, H. L., Luong, T. T., Nguyen, T. N., Dao, N. S. H., Nguyen, V. G., Nguyen, D. L., Trinh, N. T., & Nguyen, V. H. (2024). An Alternative Method for Synthesizing N,2,3-Trimethyl-2H-indazol-6-amine as a Key Component in the Preparation of Pazopanib. Chemistry, 6(5), 1089-1098. https://doi.org/10.3390/chemistry6050063









