The Role of TiO2 during the Accelerated Aging of Recycled Rubber Tiles
Abstract
1. Introduction
2. Experimental Part
2.1. Materials
2.2. Preparation of Novel Rubber Tile Mixture
2.3. Characterization
2.3.1. The Experimental Investigation of the Accelerated Aging Test
2.3.2. Mechanical Properties Testing
Abrasion Testing
Tensile Strength Test
Hardness Test
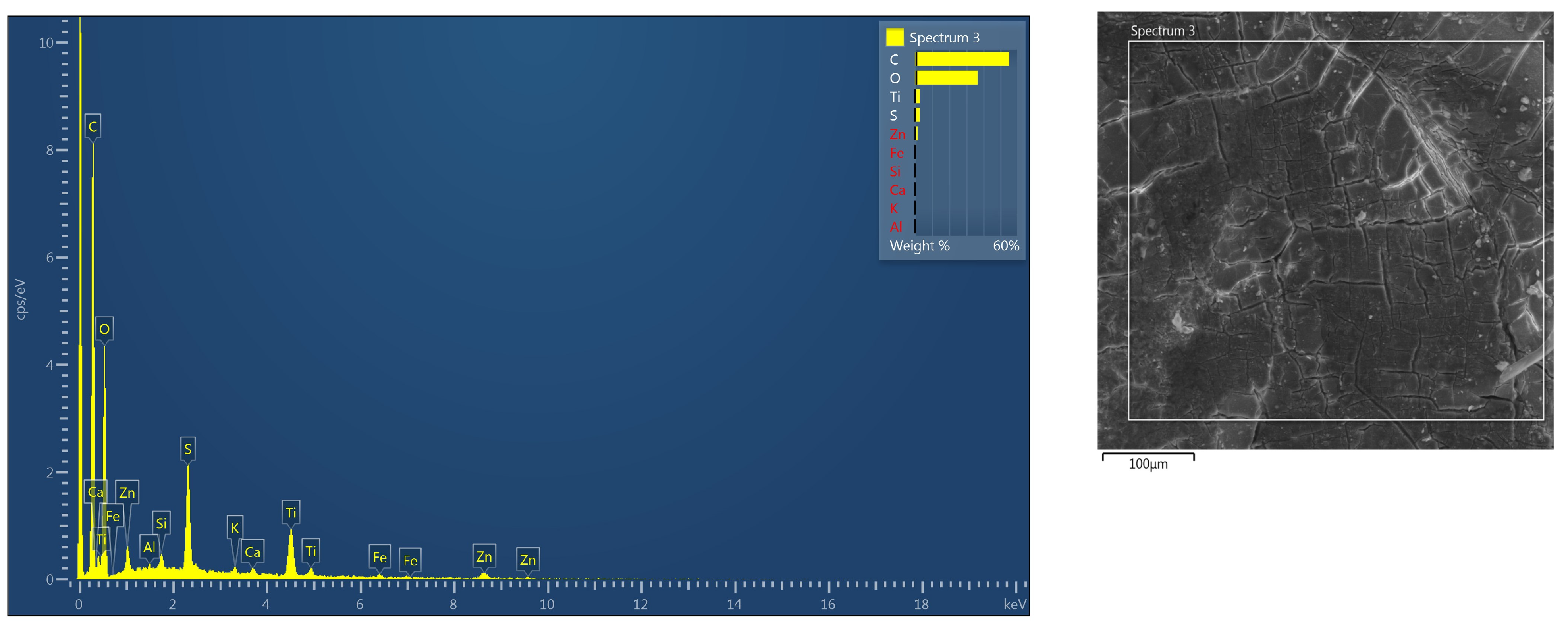
2.3.3. Scanning Electron Microscopy (SEM) and Energy Dispersion Spectroscopy (EDS)
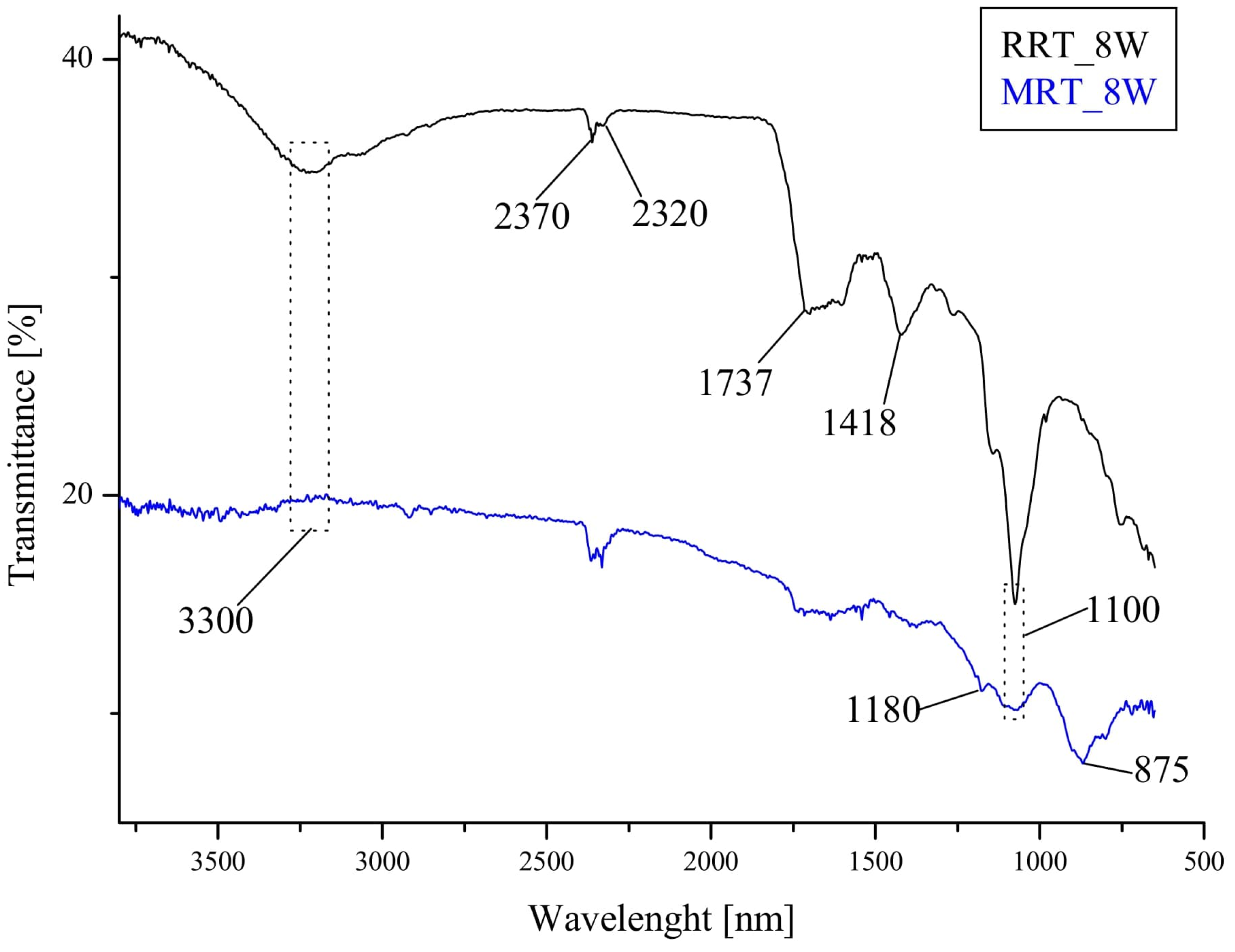
2.3.4. Fourier Transform Infrared Spectroscopy (FTIR)
2.3.5. Leaching Test
3. Results and Discussion
3.1. Results before Accelerated Aging Test
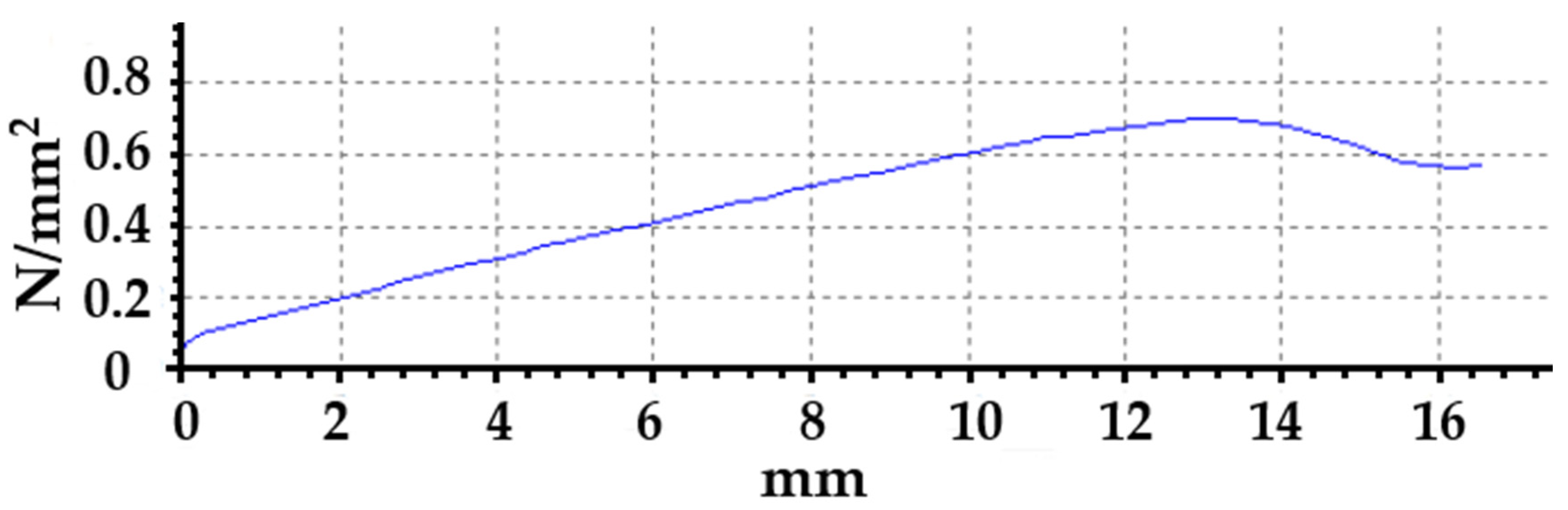
3.1.1. Mechanical Properties (Before Accelerated Aging Test)
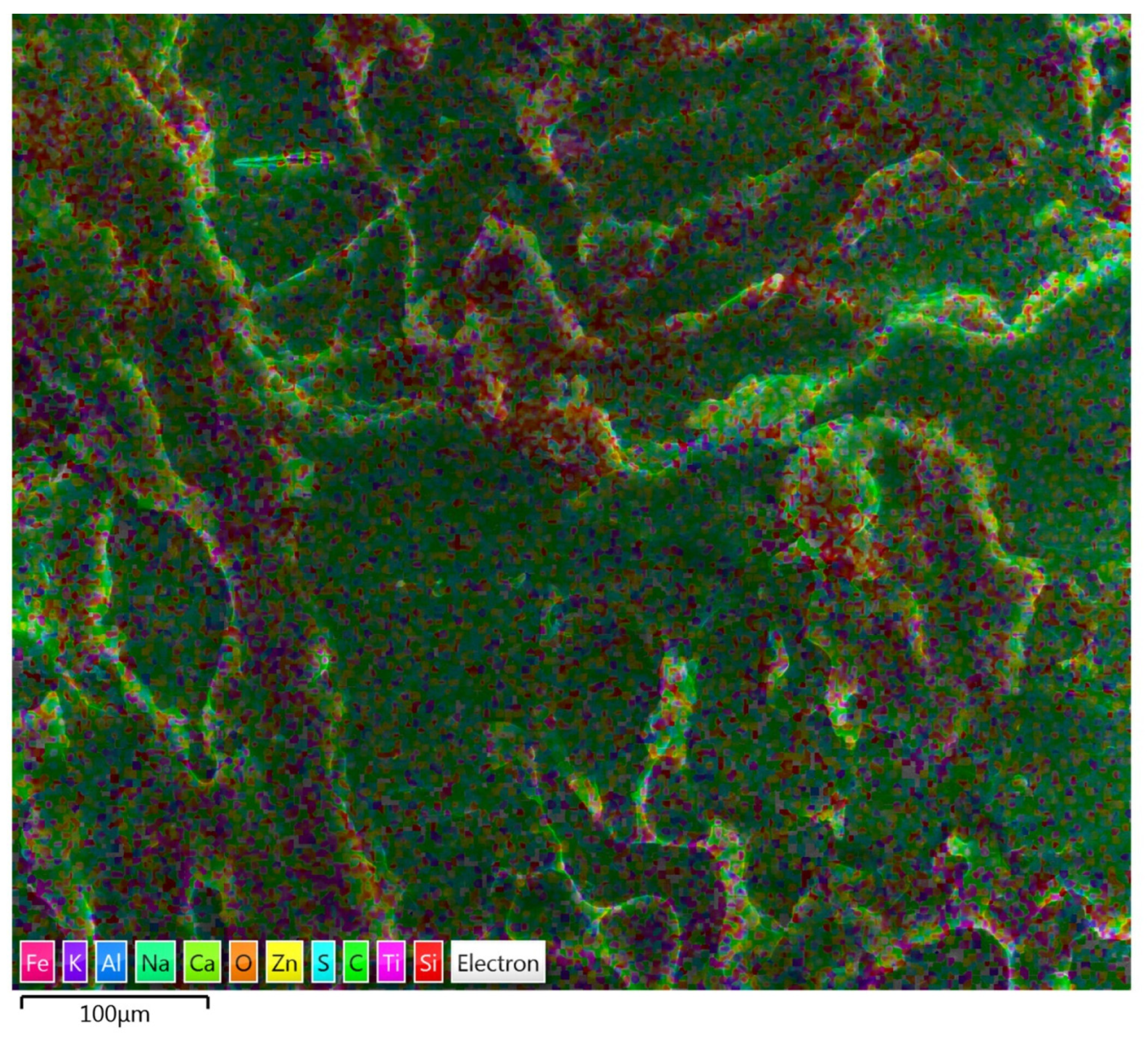
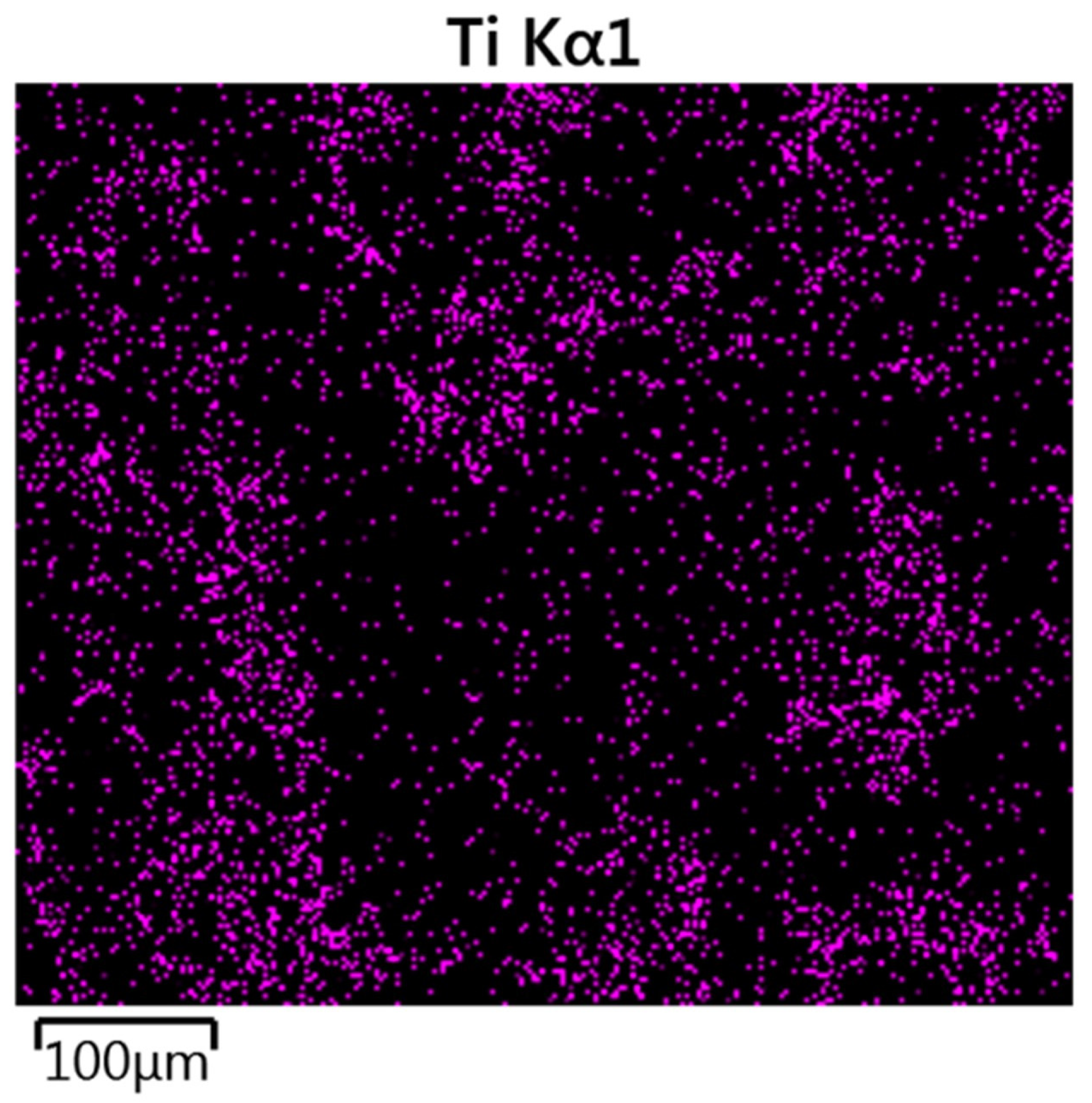
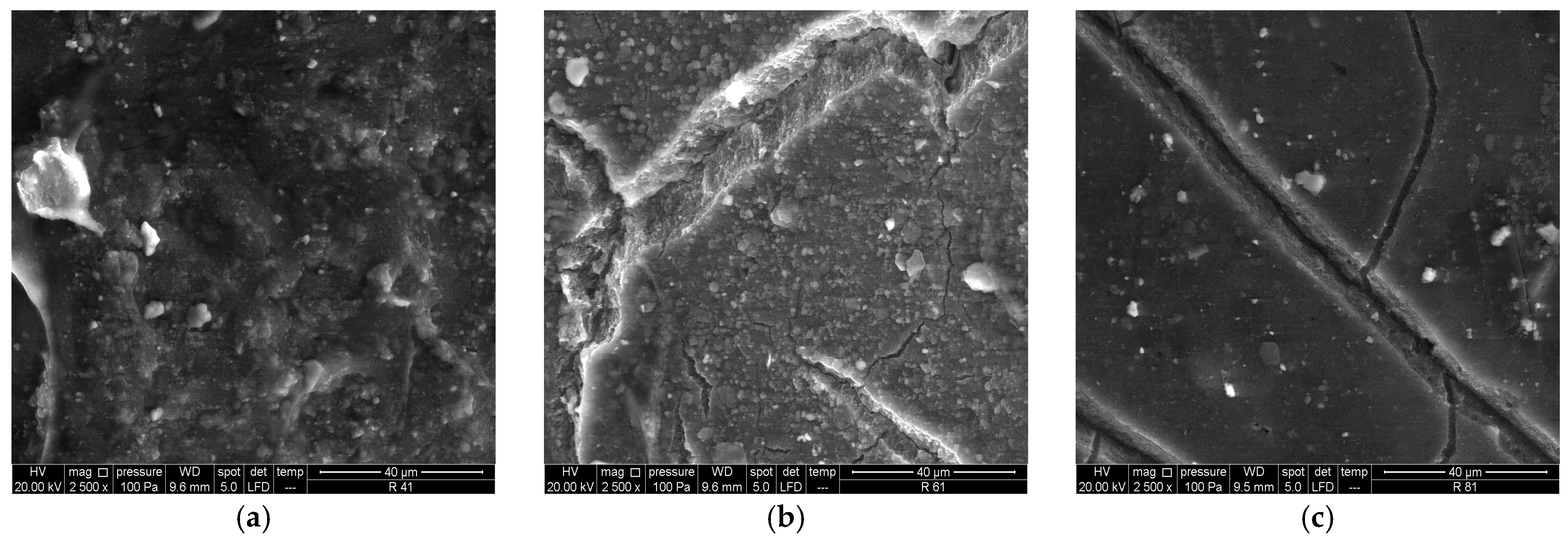
3.1.2. SEM/EDS Analysis (Before Accelerated Aging Test)
3.1.3. FTIR Analysis (Before Accelerated Aging Test)
3.1.4. Leaching Test Analysis (Before Accelerated Aging Test)
3.2. Results after Accelerated Aging Test
3.2.1. Mechanical Properties (After Accelerated Aging Test)
3.2.2. SEM/EDS Analysis (After Accelerated Aging Test)
3.2.3. FTIR Analysis (After Accelerated Aging Test)
3.2.4. Leaching Test (After Accelerated Aging Test)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kulkarni, P.; Jose, B.; Sambhudevan, S.; Shankar, B. Influence of SiC and TiO2 on the cure characteristics and mechanical properties of natural rubber composites. Mater. Today Proc. 2020, 46, 4451–4453. [Google Scholar] [CrossRef]
- Sivaselvi, K.; Varma, V.S.; Harikumar, A.; Jayaprakash, A.; Sankar, S.; Krishna, C.Y.; Gopal, K. Improving the mechanical properties of natural rubber composite with carbon black (N220) as filler. Mater. Today Proc. 2020, 42, 921–925. [Google Scholar] [CrossRef]
- Datta, J.; Kosiorek, P.; Włoch, M. Effect of high loading of titanium dioxide particles on the morphology, mechanical and thermo-mechanical properties of the natural rubber-based composites. Iran. Polym. J. 2016, 25, 1021–1035. [Google Scholar] [CrossRef]
- Che, X.C.; Jin, Y.Z.; Lee, Y.S. Preparation of nano-TiO2/polyurethane emulsions via in situ RAFT polymerization. Prog. Org. Coat. 2010, 69, 534–538. [Google Scholar] [CrossRef]
- Król, P.; Szlachta, M.; Pielichowska, K. Hydrophilic and hydrophobic films based on polyurethane cationomers containing TiO2 nanofiller. Prog. Org. Coat. 2022, 162, 106524. [Google Scholar] [CrossRef]
- Mahesh, K.P.O.; Kuo, D.H.; Huang, B.R.; Ujihara, M.; Imae, T. Chemically modified polyurethane-SiO2/TiO2 hybrid composite film and its reusability for photocatalytic degradation of Acid Black 1 (AB 1) under UV light. Appl. Catal. A Gen. 2014, 475, 235–241. [Google Scholar] [CrossRef]
- Athir, N.; Shah, S.A.A.; Shehzad, F.K.; Cheng, J.; Zhang, J.; Shi, L. Rutile TiO2 integrated zwitterion polyurethane composite films as an efficient photostable food packaging material. React. Funct. Polym. 2020, 157, 104733. [Google Scholar] [CrossRef]
- Kasanen, J.; Suvanto, M.; Pakkanen, T.T. Self-cleaning, titanium dioxide based, multilayer coating fabricated on polymer and glass surfaces. J. Appl. Polym. Sci. 2009, 111, 2597–2606. [Google Scholar] [CrossRef]
- Naphon, P.; Wiriyasart, S.; Naphon, N. Thermal, mechanical, and electrical properties of rubber latex with TiO2 nanoparticles. Compos. Commun. 2020, 22, 100449. [Google Scholar] [CrossRef]
- Hayeemasae, N.; Rathnayake, W.G.I.U.; Ismail, H. Nano-sized TiO2 -Reinforced Natural Rubber Composites Prepared by Latex Compounding Method. J. Vinyl Addit. Technol. 2015, 23, 200–209. [Google Scholar] [CrossRef]
- Loddo, V.; Bellardita, M.; Camera-Roda, G.; Parrino, F.; Palmisano, L. Heterogeneous Photocatalysis: A Promising Advanced Oxidation Process. In Current Trends and Future Developments on (Bio-) Membranes; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Sriwong, C.; Wongnawa, S.; Patarapaiboolchai, O. Photocatalytic activity of rubber sheet impregnated with TiO2 particles and its recyclability. Catal. Commun. 2008, 9, 213–218. [Google Scholar] [CrossRef]
- Leng, Z.; Yu, H. Novel Method of Coating Titanium Dioxide on to Asphalt Mixture Based on the Breath Figure Process for Air-Purifying Purpose. J. Mater. Civ. Eng. 2016, 28, 04015188. [Google Scholar] [CrossRef]
- Boonen, E.; Beeldens, A. Recent photocatalytic applications for air purification in Belgium. Coatings 2014, 4, 553–573. [Google Scholar] [CrossRef]
- La Russa, M.F.; Rovella, N.; Alvarez De Buergo, M.; Belfiore, C.M.; Pezzino, A.; Crisci, G.M.; Ruffolo, S.A. Nano-TiO2 coatings for cultural heritage protection: The role of the binder on hydrophobic and self-cleaning efficacy. Prog. Org. Coat. 2016, 91, 1–8. [Google Scholar] [CrossRef]
- Gherardi, F.; Maravelaki, P.N. Advances in the application of nanomaterials for natural stone conservation. RILEM Tech. Lett. 2022, 7, 20–29. [Google Scholar] [CrossRef]
- Ruffolo, S.A.; Francesco, M.; Russa, L. Nanostructured Coatings for Stone Protection: An Overview. Front. Mater. 2019, 6, 147. [Google Scholar] [CrossRef]
- Esposito, C.; Ingrosso, C.; Petronella, F.; Comparelli, R.; Striccoli, M.; Agostiano, A.; Frigione, M.; Curri, M.L. Progress in Organic Coatings A designed UV—Vis light curable coating nanocomposite based on colloidal TiO2 NRs in a hybrid resin for stone protection. Prog. Org. Coat. 2018, 122, 290–301. [Google Scholar] [CrossRef]
- Nazir, M.; Irfan, M.; Ali, I.; Abdul, M. Photonics and Nanostructures—Fundamentals and Applications Revealing antimicrobial and contrasting photocatalytic behavior of metal chalcogenide deposited P25-TiO2 nanoparticles. Photonics Nanostruct.—Fundam. Appl. 2019, 36, 100721. [Google Scholar] [CrossRef]
- Dds, A.S.; Bahador, A.; Khalil, S.; Saffar, A.; Dds, S.; Zaman, M. The effect of TiO2 and SiO2 nanoparticles on flexural strength of poly (methyl methacrylate) acrylic resins. J. Prosthodont. Res. 2013, 57, 15–19. [Google Scholar] [CrossRef]
- Salama, A.; Kamel, B.M.; Osman, T.A.; Rashad, R.M. Investigation of mechanical properties of UHMWPE composites reinforced with HAP þ TiO2 fabricated by solvent dispersing technique. J. Mater. Res. Technol. 2022, 21, 4330–4343. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Q.; Jing, D.; Zhou, S.; Shao, L. ScienceDirect Biomechanical properties of nano-TiO2 addition to a medical silicone elastomer: The effect of artificial ageing. J. Dent. 2014, 42, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Elsaka, S.E.; Hamouda, I.M.; Swain, M.V. Titanium dioxide nanoparticles addition to a conventional glass-ionomer restorative: Influence on physical and antibacterial properties. J. Dent. 2011, 39, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Zhang, X.; Emeline, A.V.; Numata, T.; Murakami, T.; Fujishima, A. Surface modification of natural rubber by TiO2 film. Surf. Coat. Technol. 2008, 202, 1364–1370. [Google Scholar] [CrossRef]
- Grčić, I.; Papić, S.; Brnardić, I. Photocatalytic Activity of TiO2 Thin Films: Kinetic and Efficiency Study. Int. J. Chem. React. Eng. 2018, 16, 20160153. [Google Scholar] [CrossRef]
- Kockott, D. New method for accelerated testing of the aging behavior of polymeric materials as a function of radiation and temperature. Polym. Test. 2022, 110, 107550. [Google Scholar] [CrossRef]
- Krug, N.; Zarges, J.-C.; Heim, H.-P. Influence of ethylene oxide and gamma irradiation sterilization processes on the degradation behaviour of poly(lactic acid) (PLA) in the course of artificially accelerated aging. Polym. Test. 2024, 132, 108362. [Google Scholar] [CrossRef]
- Lamberti, M.; Maurel-Pantel, A.; Lebon, F. Experimental and numerical evaluation of hydro-thermal ageing’s effects on adhesive connections in offshore structures. Ocean Eng. 2023, 290, 116303. [Google Scholar] [CrossRef]
- Determination of Abrasion Resistance, Dtsch. Norm. (1987). Available online: https://www.scribd.com/document/491624773/DIN-53516-1987 (accessed on 24 September 2024).
- DIN 53504 Testing of Rubber—Determination of Tensile Strength at Break, Tensile Stress at Yield, Elongation at Break and Stress Values in a Tensile Test, Dtsch. Norm. (2009). Available online: https://www.scribd.com/document/604097259/DIN-53504-2009 (accessed on 24 September 2024).
- DIN 53505, Ind. Phys. (n.d.). Available online: https://industrialphysics.com/ (accessed on 24 September 2024).
- Narodne Novine, Službeni List Republike Hrvatske. Available online: https://narodne-novine.nn.hr/clanci/sluzbeni/2015_10_114_2184.html (accessed on 25 March 2024).
- USEPA-Method:1311; Toxicity Characteristic Leaching Procedure (TCLP). Hong Kong Accredit. LOR: Hong Kong, China, 2011.
- van der Sloot, H.A.; van Zomeren, A.; Meeussen, J.; Hoede, D.; Rietra, R.; Stenger, R.; Lang, T.; Schneider, M.; Spanka, G.; Stoltenberg-Hansson, E.; et al. Environmental Criteria for Cement Based Products Phase I: Ordinary Portland Cement Phase II: Blended Cements and Methodology for Impact Assessment; Energy Research Centre of the Netherlands ECN: Petten, The Netherlands, 2008; Volume 2011, p. 224. [Google Scholar]
- Fasahat, F.; Dastjerdi, R.; Mojtahedi, M.R.M.; Hoseini, P. Wear properties of high speed spun multi-component PA6 nanocomposite fabrics; abrasion resistance mechanism of nanocomposites. Wear 2015, 322–323, 117–125. [Google Scholar] [CrossRef]
- Elastomer Institut Richter Tensile Strength, Tear Resistance and Elongation at Break. Available online: https://elastomer-institut.de/en/ (accessed on 18 April 2024).
- Xometry Elongation at Break: Definition, Calculation, Benefits, Limitations, and Examples. Available online: https://www.xometry.com/ (accessed on 18 April 2024).
- Track, I.; Surface, S.; Specifications, T. IAAF Track Synthetic Surface Testing Specifications IAAF Certification System Track Synthetic Surface Testing Specifications. 2010. Available online: https://worldathletics.org/ (accessed on 14 April 2024).
- Edeskär, T. Technical and Environmental Properties of Tyre Shreds Focusing on Ground Engineering Applications; Luleå Tekniska Universitet: Luleå, Sweden, 2004; Available online: https://www.diva-portal.org/smash/record.jsf?pid=diva2%3A996312&dswid=-1211 (accessed on 15 May 2024).
- Baranowski, P.; Janiszewski, J.; Małachowski, J. Tire rubber testing procedure over a wide range of strain rates. J. Theor. Appl. Mech. 2017, 55, 727–739. [Google Scholar] [CrossRef]
- Janiszewski, J.; Bużantowicz, W.; Baranowski, P. Correction Procedure of Wave Signals for a Viscoelastic Split Hopkinson Pressure Bar. Probl. Mechatronics. Armament Aviat. Saf. Eng. 2016, 7, 17–30. [Google Scholar] [CrossRef]
- Ptiček Siročić, A.; Rešček, A.; Katančić, Z.; Hrnjak-Murgić, Z. Effect of modifiers; casein, zeolite and magnetite on the properties of bilayer polyethylene/polycaprolactone films. J. Adhes. Sci. Technol. 2020, 34, 2537–2550. [Google Scholar] [CrossRef]
- Ptiček Siročić, A.; Rešček, A.; Katančić, Z.; Hrnjak-Murgić, Z. Development of PE/PCL Bilayer Films Modified with Casein and Aluminum Oxide. Molecules 2021, 26, 3090. [Google Scholar] [CrossRef] [PubMed]
- Rešček, A.; Krehula, L.K.; Katančić, Z.; Hrnjak-Murgić, Z. Active bilayer PE/PCL films for food packaging modified with zinc oxide and casein. Croat. Chem. Acta 2015, 88, 461–473. [Google Scholar] [CrossRef]
- Jarnthong, M.; Liao, L.; Zhang, F.; Wang, Y.; Li, P.; Peng, Z.; Malawet, C.; Intharapat, P. Characterization of interaction between natural rubber and silica by FTIR. AIP Conf. Proc. 2017, 1846, 020014. [Google Scholar] [CrossRef]
- Manap, S.M.; Ahmad, A.; Anuar, F.H. Characterization of poly(L-lactide/Propylene glycol) based polyurethane films using ATR-FTIR spectroscopy. AIP Conf. Proc. 2016, 1784, 03002. [Google Scholar] [CrossRef]
- Badri, K.B.H.; Sien, W.C.; Shahrom, M.S.B.R.; Hao, L.C.; Baderuliksan, N.Y.; Norzali, N.R. Adawiyah Ftir Spectroscopy Analysis of the Prepolymerization of Palm-Base Polyurethane. Solid State Sci. Technol. 2010, 18, 1–8. [Google Scholar]
- Vijayalakshmi, U.; Chellappa, M.; Anjaneyulu, U.; Manivasagam, G.; Sethu, S. Influence of Coating Parameter and Sintering Atmosphere on the Corrosion Resistance Behavior of Electrophoretically Deposited Composite Coatings. Mater. Manuf. Process. 2016, 31, 95–106. [Google Scholar] [CrossRef]
- Sriwong, C.; Choojun, K.; Sriwong, S. High photocatalytic performance of 3D porous-structured TiO2@natural rubber hybrid sheet on the removal of indigo carmine dye in water. SN Appl. Sci. 2019, 1, 864. [Google Scholar] [CrossRef]
- Ingole, S.; Charanpahari, A.; Kakade, A.; Umare, S.S.; Bhatt, D.V.; Menghani, J. Tribological behavior of nano TiO2 as an additive in base oil. Wear 2013, 301, 776–785. [Google Scholar] [CrossRef]
- Panwar, K.; Jassal, M.; Agrawal, A.K. TiO2-SiO2 Janus particles with highly enhanced photocatalytic activity. RSC Adv. 2016, 6, 92754–92764. [Google Scholar] [CrossRef]
- Selbes, M. Leaching of Dissolved Organic Carbon and Carbon Dioxide Emission. 2009. Available online: https://open.clemson.edu/all_theses/664/ (accessed on 8 May 2024).
- Benjak, P.; Radetić, L.; Tomaš, M.; Brnardić, I.; Radetić, B.; Špada, V.; Grčić, I. Rubber Tiles Made from Secondary Raw Materials with Immobilized Titanium Dioxide as Passive Air Protection. Processes 2023, 11, 125. [Google Scholar] [CrossRef]
- Jimenez-Relinque, E.; Grande, M.; Duran, T.; Castillo, Á.; Castellote, M. Environmental impact of nano-functionalized construction materials: Leaching of titanium and nitrates from photocatalytic pavements under outdoor conditions. Sci. Total Environ. 2020, 744, 140817. [Google Scholar] [CrossRef] [PubMed]
- The European Chemicals Agency. Available online: https://echa.europa.eu/registration-dossier/-/registered-dossier/15560/6/2/1 (accessed on 13 March 2024).
- Kashi, S.; Varley, R.; De Souza, M.; Al-Assafi, S.; Di Pietro, A.; de Lavigne, C.; Fox, B. Mechanical, Thermal, and Morphological Behavior of Silicone Rubber during Accelerated Aging. Polym.—Plast. Technol. Eng. 2018, 57, 1687–1696. [Google Scholar] [CrossRef]
- Baldwin, O.M.; Bauer, D.R. Rubber oxidation and tire aging—A review. Rubber Chem. Technol. 2008, 81, 338–358. [Google Scholar] [CrossRef]
- Boulaouche, T.; Kherroub, D.E.; Khimeche, K.; Belbachir, M. Green strategy for the synthesis of polyurethane by a heterogeneous catalyst based on activated clay. Res. Chem. Intermed. 2019, 45, 3585–3600. [Google Scholar] [CrossRef]
- Asefnejad, A.; Khorasani, M.T.; Behnamghader, A.; Farsadzadeh, B.; Bonakdar, S. Manufacturing of biodegradable polyurethane scaffolds based on polycaprolactone using a phase separation method: Physical properties and in vitro assay. Int. J. Nanomed. 2011, 6, 2375–2384. [Google Scholar] [CrossRef] [PubMed]
- Riazian, M.; Montazeri, N.; Biazar, E. Nano structural properties of TiO2-SiO2. Orient. J. Chem. 2011, 27, 903–910. [Google Scholar]
- Luna, C.B.B.; Araújo, E.M.; Siqueira, D.D.; Morais, D.D.d.S.; Filho, E.A.d.S.; Fook, M.V.L. Incorporation of a recycled rubber compound from the shoe industry in polystyrene: Effect of SBS compatibilizer content. J. Elastomers Plast. 2020, 52, 3–28. [Google Scholar] [CrossRef]







| Mass of Rubber Granulates [kg] | Mass of Polyurethane [g] | Mass of Catalyst [g] | Mass of TiO2 [g] | |
|---|---|---|---|---|
| RRT | 9 | 380 | 5 | - |
| T/TiO2 (2% beginning) | 9 | 380 | 5 | 7.7 |
| T/TiO2 (2% end) | 9 | 380 | 5 | 7.7 |
| T/TiO2 (4% beginning) | 9 | 380 | 5 | 15.4 |
| T/TiO2 (4% end) | 9 | 380 | 5 | 15.4 |
| T/TiO2 (10% beginning)—failed | 9 | 380 | 5 | 38.5 |
| MRT | 9 | 700 | 7 | 38.5 |
| A [mm3] | SD | TS [N/mm2] | SD | TSb [N/mm2] | Eb [%] | SD | H [ShA] | SD | E [MPa] | |
|---|---|---|---|---|---|---|---|---|---|---|
| RRT | 144 | 21.0713 | 1.27 | 0.1572 | 1.27 | 64.48 | 5.3171 | 53.48 | 1.7108 | 1.97 |
| T/TiO2 (2% beginning) | 152 | 24.4336 | 0.44 | 0.1552 | 0.44 | 55.56 | 6.7396 | 34.82 | 5.9964 | 0.79 |
| T/TiO2 (2% end) | 168 | 21.7025 | 0.48 | 0.1744 | 0.48 | 49.48 | 6.7109 | 40.14 | 7.5550 | 0.97 |
| T/TiO2 (4% beginning) | 184 | 26.8887 | 0.32 | 0.1513 | 0.32 | 43.10 | 5.2608 | 37.78 | 4.8618 | 0.74 |
| T/TiO2 (4% end) | 206 | 23.3024 | 0.67 | 0.1873 | 0.64 | 63.22 | 5.9757 | 36.74 | 0.4506 | 1.06 |
| MRT | 112.21 | 24.3392 | 0.75 | 0.1375 | 0.75 | 72.01 | 5.9727 | 44.04 | 5.6123 | 1.04 |
| Parameter | RRT | MRT |
|---|---|---|
| As | <0.05 µg/L | 0.054 µg/L |
| Ba | <0.35 µg/L | <0.35 µg/L |
| Cd | <0.002 µg/L | <0.002 µg/L |
| Cr | <0.003 mg/L | <0.003 mg/L |
| Cu | <0.0015 mg/L | <0.0015 mg/L |
| Hg | <0.009 µg/L | <0.009 µg/L |
| Mo | 1.22 µg/L | <0.03 µg/L |
| Ni | <0.07 µg/L | <0.07 µg/L |
| Pb | <0.05 µg/L | 0.14 µg/L |
| Se | <0.05 µg/L | <0.05 µg/L |
| Si | 1472.6 µg/L | 347.5 µg/L |
| Ti | 26.09 µg/L | 97.86 µg/L |
| Zn | 0.73 mg/L | 0.474 mg/L |
| Cl− | 2.1 mg/L | 2.4 mg/L |
| F− | 0.12 mg/L | 0.13 mg/L |
| SO42− | 1 mg/L | 1 mg/L |
| DOC | 39.5 mg/L | 24.07 mg/L |
| TDS | 13.4 mg/L | 15.8 mg/L |
| A [mm3] | SD | TS [N/mm2] | SD | TSb [N/mm2] | Eb [%] | SD | H [ShA] | SD | E [MPa] | |
|---|---|---|---|---|---|---|---|---|---|---|
| RRT 4W | 337 | 31.5753 | 1.55 | 0.1835 | 1.55 | 3.39 | 1.1001 | 59.6 | 6.7168 | |
| RRT 6W | 517 | 25.2389 | 0.44 | 0.1249 | 0.41 | 19.61 | 6.1592 | 80.7 | 6.7279 | |
| RRT 8W | 673 | 26.5141 | 0.10 | 0.0306 | 0.00 | 16.21 | 3.2618 | 79.5 | 6.5046 | 0.62 |
| MRT 4W | 662 | 27.4044 | 0.71 | 0.1699 | 0.70 | 38.88 | 7.0777 | 50.6 | 7.2536 | |
| MRT 6W | 917 | 25.5343 | 0.52 | 0.1833 | 0.50 | 28.65 | 7.3741 | 76.3 | 6.7724 | |
| MRT 8W | 767 | 31.1929 | 0.70 | 0.0236 | 0.57 | 66.24 | 5.6473 | 72.0 | 7.3144 | 1.06 |
| Element | Wt% |
|---|---|
| C | 70.56 |
| O | 22.95 |
| Na | 0.49 |
| Al | 0.11 |
| Si | 0.18 |
| S | 1.59 |
| Cl | 0.12 |
| K | 0.23 |
| Ca | 0.25 |
| Fe | 0.45 |
| Zn | 3.08 |
| Total: | 100.00 |
| Element | Wt% |
|---|---|
| C | 55.09 |
| O | 36.71 |
| Al | 0.10 |
| Si | 0.29 |
| S | 2.67 |
| K | 0.19 |
| Ca | 0.25 |
| Ti | 2.89 |
| Fe | 0.29 |
| Zn | 1.52 |
| Total: | 100.00 |
| Parameter | RRT 8W | MRT 8W |
|---|---|---|
| As | <0.05 µg/L | <0.05 µg/L |
| Ba | 76.09 µg/L | 35.31 µg/L |
| Cd | <0.002 µg/L | <0.002 µg/L |
| Cr | <0.003 mg/L | <0.003 mg/L |
| Cu | 0.010 mg/L | <0.0015 mg/L |
| Hg | 0.427 µg/L | 0.240 µg/L |
| Mo | 7.62 µg/L | 2.32 µg/L |
| Ni | 0.787 µg/L | 0.709 µg/L |
| Pb | <0.05 µg/L | 0.09 µg/L |
| Se | <0.05 µg/L | <0.05 µg/L |
| Si | 2104 µg/L | 244.4 µg/L |
| Ti | 18.82 µg/L | 116.1 µg/L |
| Zn | 8.52 mg/L | 4.22 mg/L |
| Cl− | 3.6 mg/L | 3.6 mg/L |
| F− | 0.05 mg/L | 0.09 mg/L |
| SO42- | 9 mg/L | 4 mg/L |
| DOC | 18.16 mg/L | 97.58 mg/L |
| TDS | 21.7 mg/L | 34.5 mg/L |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benjak, P.; Tomaš, M.; Ptiček Siročić, A.; Brnardić, I.; Florijanić, F.; Grčić, I. The Role of TiO2 during the Accelerated Aging of Recycled Rubber Tiles. Chemistry 2024, 6, 1111-1132. https://doi.org/10.3390/chemistry6050065
Benjak P, Tomaš M, Ptiček Siročić A, Brnardić I, Florijanić F, Grčić I. The Role of TiO2 during the Accelerated Aging of Recycled Rubber Tiles. Chemistry. 2024; 6(5):1111-1132. https://doi.org/10.3390/chemistry6050065
Chicago/Turabian StyleBenjak, Paula, Marija Tomaš, Anita Ptiček Siročić, Ivan Brnardić, Franjo Florijanić, and Ivana Grčić. 2024. "The Role of TiO2 during the Accelerated Aging of Recycled Rubber Tiles" Chemistry 6, no. 5: 1111-1132. https://doi.org/10.3390/chemistry6050065
APA StyleBenjak, P., Tomaš, M., Ptiček Siročić, A., Brnardić, I., Florijanić, F., & Grčić, I. (2024). The Role of TiO2 during the Accelerated Aging of Recycled Rubber Tiles. Chemistry, 6(5), 1111-1132. https://doi.org/10.3390/chemistry6050065









