Synthesis and Structure-Chirality Relationship Analysis of Steroidal Quinoxalines to Design and Develop New Chiral Drugs
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Instruments
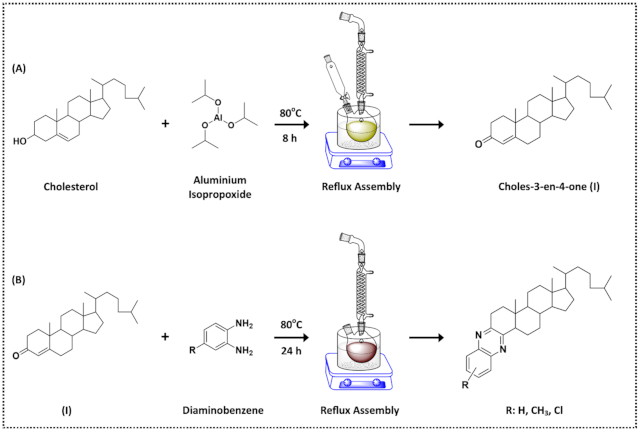
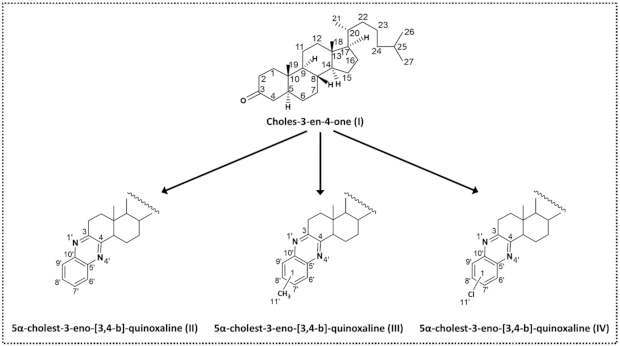
2.2. Synthesis and Characterisation
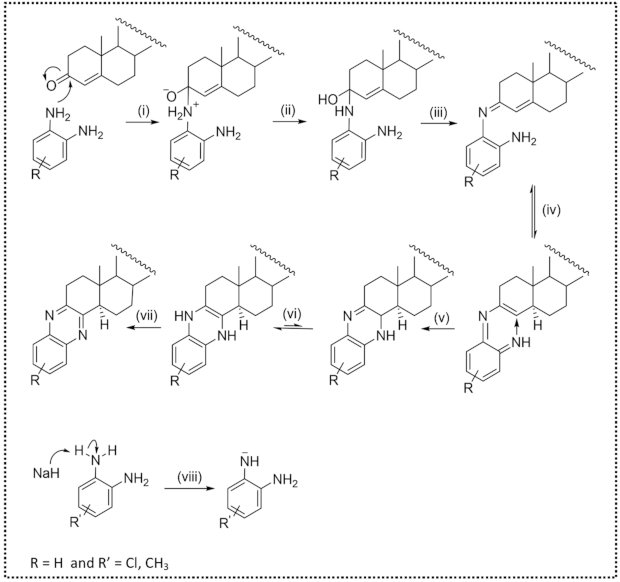
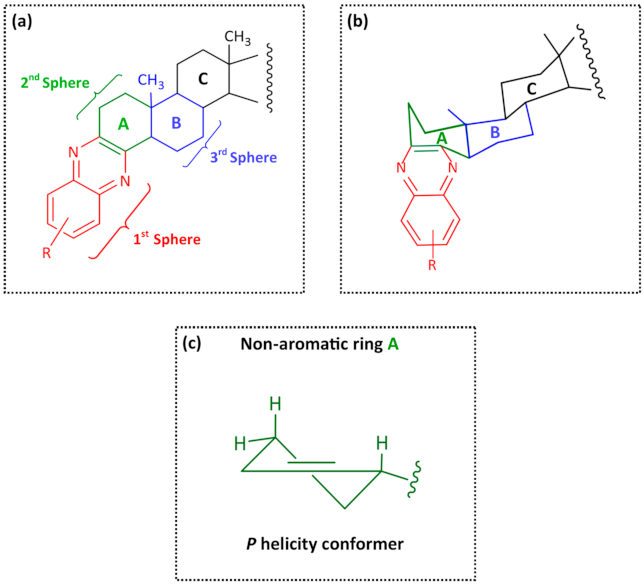
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Feringa, B.L. The Art of Building Small: From molecular switches to motors (Nobel Lecture). Angew. Chem. Int. Ed. 2017, 56, 11060–11078. [Google Scholar] [CrossRef]
- Brooks, W.H.; Guida, W.C.; Daniel, K.G. The significance of chirality in drug design and development. Curr. Top. Med. Chem. 2011, 11, 760–770. [Google Scholar] [CrossRef] [PubMed]
- Agranat, I.; Caner, H.; Caldwell, J. Putting chirality to work: The strategy of chiral switches. Nat. Rev. Drug Discov. 2002, 7, 753–768. [Google Scholar] [CrossRef] [PubMed]
- Fratta, I.D.; Sigg, E.B.; Maiorana, K. Teratogenic effects of thalidomide in rabbits, rats, hamsters, and mice. Toxicol. Appl. Pharmacol. 1965, 7, 268–286. [Google Scholar] [CrossRef]
- Kuhn, W.L.; Van Maanen, E.F. Central nervous system effects of thalidomide. J. Pharmacol. Exp. Ther. 1961, 134, 60–68. [Google Scholar] [PubMed]
- McConathy, J.; Owen, M.J. Stereochemistry in drug action. Prim. Care Companion J. Clin. Psychiatry 2003, 5, 70–73. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.A.; He, H.; Pham-Huy, C. Chiral drugs: An overview. Int. J. Biomed. Sci. 2006, 2, 85–100. [Google Scholar] [PubMed]
- Husain, A.; Rashid, M.; Mishra, R.; Parveen, S.; Shin, D.S.; Kumar, D. Benzimidazole bearing oxadiazole and triazolo-thiadiazoles nucleus: Design and synthesis as anticancer agents. Bioorganic Med. Chem. Lett. 2012, 22, 5438–5444. [Google Scholar] [CrossRef]
- Verma, A.; Joshi, S.; Singh, D. Imidazole: Having versatile biological activities. J. Chem. 2013, 2013, 1–12. [Google Scholar] [CrossRef]
- Sharma, G.V.M.; Ramesh, A.; Singh, A.; Srikanth, G.; Jayaram, V.; Duscharla, D.; Jun, J.H.; Ummanni, R.; Malhotra, S.V. Imidazole derivatives show anticancer potential by inducing apoptosis and cellular senescence. Med. Chem. Commun. 2014, 5, 1751–1760. [Google Scholar] [CrossRef]
- Khan, I.; Ibrar, A.; Abbas, N. Triazolothiadiazoles and triazolothiadiazines-biologically attractive scaffolds. Eur. J. Med. Chem. 2013, 63, 854–868. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, S.; Silakari, O.; Singh, P.K. Key updates on the chemistry and biological roles of thiazine scaffold: A review. Mini Rev. Med. Chem. 2018, 18, 1452–1478. [Google Scholar] [CrossRef] [PubMed]
- Maekawa, T.; Sakai, N.; Tawada, H.; Murase, K.; Hazama, M.; Sugiyama, Y.; Momose, Y. Synthesis and biological activity of novel 5-(omega-aryloxyalkyl) oxazole derivatives as brain-derived neurotrophic factor inducers. Chem. Pharm. Bull. 2003, 51, 565–573. [Google Scholar] [CrossRef]
- Zhang, H.Z.; Zhao, Z.L.; Zhou, C.H. Recent advance in oxazole-based medicinal chemistry. Eur. J. Med. Chem. 2018, 144, 444–492. [Google Scholar] [CrossRef]
- Zhou, C.H.; Wang, Y. Recent research in triazole compounds as medicinal drugs. Curr. Med. Chem. 2012, 19, 239–280. [Google Scholar] [CrossRef] [PubMed]
- Pettit, G.R.; Xu, J.P.; Chapuis, J.C.; Melody, N. The cephalostatins. 24. Isolation, structure, and cancer cell growth inhibition of cephalostatin 20. J. Nat. Prod. 2015, 78, 1446–1450. [Google Scholar] [CrossRef]
- Dirsch, V.M.; Müller, I.M.; Eichhorst, S.T.; Pettit, G.R.; Kamano, Y.; Inoue, M.; Xu, J.P.; Ichihara, Y.; Wanner, G.; Vollmar, A.M. Cephalostatin 1 selectively triggers the release of Smac/DIABLO and subsequent apoptosis that is characterized by an increased density of the mitochondrial matrix. Cancer Res. 2003, 63, 8869–8876. [Google Scholar]
- Ingle, R.; Marathe, R.; Magar, D.; Patel, H.M.; Surana, S.J. Sulphonamido-quinoxalines: Search for anticancer agent. Eur. J. Med. Chem. 2013, 65, 168–186. [Google Scholar] [CrossRef] [PubMed]
- Refaat, H.M.; Moneer, A.A.; Khalil, O.M. Synthesis and antimicrobial activity of certain novel quinoxalines. Arch. Pharm. Res. 2004, 27, 1093–1098. [Google Scholar] [CrossRef] [PubMed]
- Mielcke, T.R.; Mascarello, A.; Fillipi-Chiela, E.; Zanin, R.F.; Lenz, G.; Leal, P.C.; Chiaradia, L.D.; Yunes, R.A.; Nunes, R.J.; Battastinie, A.M.O.; et al. Activity of novel quinoxaline-derived chalcones on in vitro glioma cell proliferation. Eur. J. Med. Chem. 2012, 48, 255–264. [Google Scholar] [CrossRef]
- Rigas, J.R.; Miller, V.A.; Tong, W.P.; Roistacher, N.; Kris, M.G.; Orazem, J.P.; Young, C.W.; Warrell, R.P., Jr. Clinical and pharmacology study of chloroquinoxaline sulfonamide given on a weekly schedule. Cancer Chemother. Pharmacol. 1995, 35, 483–488. [Google Scholar] [CrossRef]
- Hui, X.; Desrivot, J.; Bories, C.; Loiseau, P.M.; Franck, X.; Hocquemiller, R.; Figadere, B. Synthesis and antiprotozoal activity of some new synthetic substituted quinoxalines. Bioorganic Med. Chem. Lett. 2006, 16, 815–820. [Google Scholar] [CrossRef] [PubMed]
- Gerspacher, M.; Furet, P.; Vangrevelinghe, E.; Pissot, S.C.; Gaul, C.; Holzer, P. Preparation of quinoxalines, particularly heterocyclyl-substituted diarylquinoxalines, as inhibitors of the tyrosine kinase activity of Janus kinases for use in the treatment of immune and proliferative disorders. PCT Int. Appl. 2008, 177. Available online: https://www.researchgate.net/publication/233952648_Preparation_of_quinoxalines_particularly_heterocyclyl-substituted_diarylquinoxalines_as_inhibitors_of_the_tyrosine_kinase_activity_of_Janus_kinases_for_use_in_the_treatment_of_immune_and_proliferative (accessed on 22 December 2020).
- Aguirre, G.; Cerecetto, H.; Di Maio, R.; González, M.; Montoya Alfaro, M.E.; Jaso, A.; Zarranz, B.; Ortega, M.A.; Aldana, I.; Monge-Vega, A. Quinoxaline N,N′-dioxide derivatives and related compounds as growth inhibitors of Trypanosoma cruzi. Structure-activity relationships. Bioorganic Med. Chem. Lett. 2004, 14, 3835–3839. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Cho, S.; Namgoong, K.; Jung, J.K.; Cho, J.; Yang, S.I. Synthesis and in vitro evaluation of 7-dialkylaminomethylbenzo[g]quinoxaline-5,10-diones. Bioorganic Med. Chem. Lett. 2004, 14, 1235–1237. [Google Scholar] [CrossRef]
- Noolvi, M.N.; Patel, H.M.; Bhardwaj, V.; Chauhan, A. Synthesis and in vitro antitumor activity of substituted quinazoline and quinoxaline derivatives: Search for anticancer agent. Eur. J. Med. Chem. 2011, 46, 2327–2346. [Google Scholar] [CrossRef]
- Michael, J.W.; Ben-Hadda, T.; Kotchevan, A.T.; Ramdani, A.; Touzani, R.; Elkadiri, S.; Hakkou, A.; Boukka, M.; Elli, T. 2, 3-bifunctionalized quinoxalines: Synthesis, DNA interactions and evaluation of anticancer, anti-tuberculosis and antifungal Activity. Molecules 2002, 7, 641–656. [Google Scholar]
- Rodrigues, F.A.; Bomfim Ida, S.; Cavalcanti, B.C.; Pessoa Cdo, Ó.; Wardell, J.L.; Wardell, S.M.; Pinheiro, A.C.; Kaiser, C.R.; Nogueira, T.C.; Low, J.N.; et al. Design, synthesis, and biological evaluation of (E)-2-(2-arylhydrazinyl) quinoxalines, a promising and potent new class of anticancer agents. Bioorganic Med. Chem. Lett. 2014, 24, 934–939. [Google Scholar] [CrossRef] [PubMed]
- Ghanbarimasir, Z.; Bekhradnia, A.; Morteza-Semnani, K.; Rafiei, A.; Razzaghi-Asl, N.; Kardan, M. Design, synthesis, biological assessment, and molecular docking studies of new 2-aminoimidazole-quinoxaline hybrids as potential anticancer agents. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 194, 21–35. [Google Scholar] [CrossRef]
- Ahmed, H.E.A.; Ihmaid, S.K.; Omar, A.M.; Shehata, A.M.; Rateb, H.S.; Zayed, M.F.; Ahmed, S.; Elaasser, M.M. Design, synthesis, molecular docking of new lipophilic acetamide derivatives affording potential anticancer and antimicrobial agents. Bioorganic Chem. 2018, 76, 332–342. [Google Scholar] [CrossRef]
- Keri, R.S.; Pandule, S.S.; Budagumpi, S.; Nagaraja, B.M. Quinoxaline and quinoxaline-1,4-di-N-oxides: An emerging class of antimycobacterials. Arch. Pharm. 2018, 351, e1700325. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.C.; Tzeng, S.L.; Chang, C.K.; Kao, Y.F.; Waring, M.J.; Hou, M.H. Cooperative recognition of T:T mismatch by echinomycin causes structural distortions in DNA duplex. Nucleic Acids Res. 2018, 46, 7396–7404. [Google Scholar] [CrossRef]
- Fieser, L.F.; Stevenson, R. Cholesterol and companions. IX. Oxidation of Δ5-cholestene-3-one with lead tetraacetate. J. Am. Chem. Soc. 1954, 76, 1728–1733. [Google Scholar]
- Kirk, D.N.; Patel, D.K.; Petrow, V. Modified steroid hormones. Part I. some 4-brouno-3-oxo derivatives. J. Chem. Soc. 1956, 627–629. [Google Scholar]
- Hasan, M.; Rashid, N.; Voelter, W.; Snatzke, G.; Duddeck, H. Synthesis and CD spectral studies of some fused cholestenopyrimidines. Pure Appl. Chem. 1994, 66, 2057–2062. [Google Scholar] [CrossRef]
- Hasan, M.; Rashid, N.; Khan, K.M.; Snatzke, G.; Duddeck, H.; Voelter, W. Syntheses and CD Studies of 5α-Cholesten0[3,2-d]-[2,3-d]- and -[3,4-d] pyrimidines. Liebigs Ann. 1995, 1995, 889–896. [Google Scholar] [CrossRef]
- Hasan, M.; Rashid, N.; Khan, M.K.; Perveen, S.; Snatzke, G.; Duddeck, H.; Voelter, W. Syntheses and CD studies of new cholesteno[4,3-d]- and-[7,6-d] pyrimidines. Liebigs Ann. 1995, 1995, 1871–1876. [Google Scholar] [CrossRef]
- Kurtán, T.; Baitz-Gács, E.; Majerc, Z.; Antus, S. Synthesis and circular dichroism of steroids with 2,3-dihydro-1-benzofuran and 4H-benzopyran chromophores; revision of the absolute configuration of some norneolignans from Krameria cystisoides. J. Chem. Soc. Perkin Trans. 2000, 1, 453–461. [Google Scholar]
- Li, X.-C.; Ferreira, D.; Ding, Y. Determination of absolute configuration of natural products: Theoretical calculation of electronic circular dichroism as a tool. Curr. Org. Chem. 2010, 14, 1678–1697. [Google Scholar] [CrossRef] [PubMed]
- Rashid, N.; Alam, S.; Hasan, M.; Khan, N.; Khan, K.M.; Duddeck, H.; Pescitelli, G.; Kenéz, A.; Antus, S.; Kurtán, T. Cis-diastereoselectivity in pictet-spengler reactions of L-tryptophan and electronic circular dichroism studies. Chirality 2012, 24, 789–795. [Google Scholar] [CrossRef]
- Mehmood, R.; Ariotti, N.; Koshy, P.; Yang, J.-L.; Sorrell, C.C. pH-Responsive Morphology-controlled redox behaviour and cellular uptake of nanoceria in fibrosarcoma. ACS Biomater. Sci. Eng. 2018, 4, 1064–1072. [Google Scholar] [CrossRef]
- Xu, Y.; Mofarah, S.S.; Mehmood, R.; Cazorla, C.; Koshy, P.; Sorrell, C.C. Design strategies for ceria nanomaterials: Untangling key mechanistic concepts. Mater. Horiz. 2021, 8, 102–123. [Google Scholar] [CrossRef]
- Mehmood, R.; Mofarah, S.S.; Chen, W.-F.; Koshy, P.; Sorrell, C.C. Surface, subsurface, and bulk oxygen vacancies quantified by decoupling and deconvolution of the defect structure of redox-active nanoceria. Inorg. Chem. 2019, 58, 6016–6027. [Google Scholar]
- Mehmood, R.; Mofarah, S.S.; Rawal, A.; Tomasetig, F.; Wang, X.; Yang, J.-L.; Koshy, P.; Sorrell, C.C. Green synthesis of zwitterion-functionalized nano-octahedral ceria for enhanced intracellular delivery and cancer therapy. ACS Sustain. Chem. Eng. 2019, 7, 9189–9201. [Google Scholar] [CrossRef]
- Chen, W.-F.; Malacco, C.M.D.S.; Mehmood, R.; Johnson, K.K.; Yang, J.-L.; Sorrell, C.C.; Koshy, P. Impact of morphology and collagen-functionalization on the redox equilibria of nanoceria for cancer therapies. Mater. Sci. Eng. C 2021, 120, 111663. [Google Scholar]
- Reeta, R.; Mehmood, R.; Amaldoss, M.J.N. Fabrication and characterisation of lavender oil—Plant phospholipid based Sumatriptan succinate hybrid solid lipid nanoparticles. Pharm. Biomed. Res. 2020, 6, 91–104. [Google Scholar]
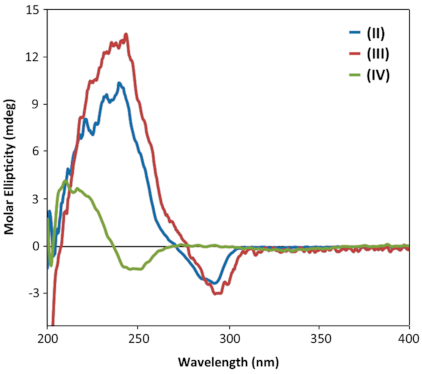
| Compound | Solvent | Conc. [mol/L] | Cell [mm] | CD Cotton Effects [mdeg] | ||
|---|---|---|---|---|---|---|
| 1st Band (Wavelength) | 2nd Band (Wavelength) | 3rd Band (Wavelength) | ||||
| (II) | Acetonitrile | 2.05 × 10−4 | 1 | 9.7 (238 nm) | −1.3 (282 nm) | - |
| (III) | Acetonitrile | 1.91 × 10−4 | 1 | 13.0 (240 nm) | 2.4 (287 nm) | - |
| (IV) | Acetonitrile | 3.28 × 10−4 | 1 | 3.9 (212 nm) | −1.9 (244 nm) | −0.016 (359 nm) |
| (IV) | Acetonitrile | 1.75 × 10−4 | 0.5 | 3.6 (212 nm) | −1.4 (244 nm) | −0.013 (359 nm) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mehmood, R.; Rashid, N.; Ullah, S.; Amaldoss, M.J.N.; Sorrell, C.C. Synthesis and Structure-Chirality Relationship Analysis of Steroidal Quinoxalines to Design and Develop New Chiral Drugs. Chemistry 2021, 3, 402-410. https://doi.org/10.3390/chemistry3010030
Mehmood R, Rashid N, Ullah S, Amaldoss MJN, Sorrell CC. Synthesis and Structure-Chirality Relationship Analysis of Steroidal Quinoxalines to Design and Develop New Chiral Drugs. Chemistry. 2021; 3(1):402-410. https://doi.org/10.3390/chemistry3010030
Chicago/Turabian StyleMehmood, Rashid, Naghmana Rashid, Shakir Ullah, Maria John Newton Amaldoss, and Charles Christopher Sorrell. 2021. "Synthesis and Structure-Chirality Relationship Analysis of Steroidal Quinoxalines to Design and Develop New Chiral Drugs" Chemistry 3, no. 1: 402-410. https://doi.org/10.3390/chemistry3010030
APA StyleMehmood, R., Rashid, N., Ullah, S., Amaldoss, M. J. N., & Sorrell, C. C. (2021). Synthesis and Structure-Chirality Relationship Analysis of Steroidal Quinoxalines to Design and Develop New Chiral Drugs. Chemistry, 3(1), 402-410. https://doi.org/10.3390/chemistry3010030






