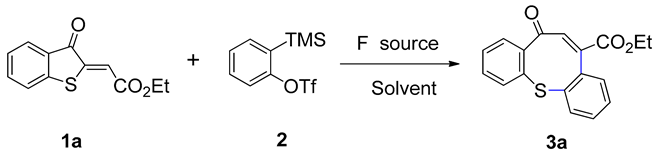
Abstract
In this study, we develop a concise and efficient synthetic strategy for the construction of eight-membered cyclic diaryl sulfides by undertaking [3+2] cycloaddition, 1,2-hydrogen shift, and C(sp2)-S bond cleavage steps on 2-methylenebenzothiophene-3-ones with aryne, using TBAT as the fluorine source. This transformation proceeds well under mild conditions and affords the target products in high to excellent yields (up to 93% yields). The process provides a practical route to achieving sulfur-containing medium-sized heterocycles.
1. Introduction
Sulfur-containing heterocycles are prominent structural motifs, which constitute the core structure of a significant number of natural products [1,2,3,4,5], pharmaceuticals [6,7,8,9,10,11], functional materials [12,13,14,15,16,17], and active biological molecules [18,19,20,21,22]. For instance, as shown in Scheme 1, diltiazem (calcium channel blocker) is used in the treatment of hypertension and angina [23,24]. Raloxifene is a widely recognized therapeutic agent for the treatment of osteoporosis [25]; Zaltoprofen is a non-steroidal anti-inflammatory drug [26,27,28]; and DNTT is a well-known organic thin-film transistor [29,30]. Additionally, thiolactomycin exhibits interesting antibacterial activity [31,32,33]. Compound A was found to be an effective inhibitor of stomach secretion [34], compound B showed anti-tumor activity [35], and compound C exhibited anti-ischemic activity [36]. As a result, many efforts have been devoted to the construction of sulfur-containing heterocycles [37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52]. Notably, there has been far less research focused on efficient methods for the synthesis of sulfur-containing benzo-fused eight-membered heterocycles. Likely due to unfavorable entropic and enthalpic constraints [53,54], the construction of these eight-membered heterocycles is a challenge.
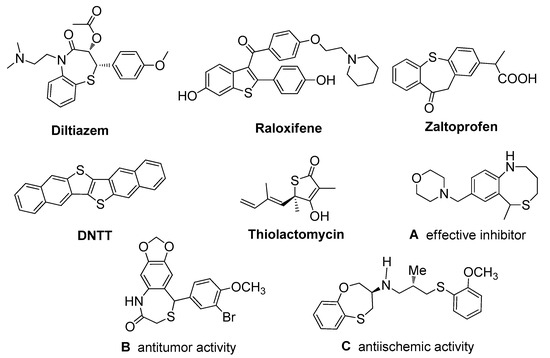
Scheme 1.
Representative examples of sulfur-containing heterocycles.
A survey of the relevant literature revealed that only several very limited examples have been reported. In 1987, Hellwinkel reported the connection of the head and tail of a sulfide using Dieckmann cyclization for the synthesis of benzo-fused eight-membered cyclic sulfides [55]. Biehl reported the synthesis of benzene-fused eight-membered rings containing nitrogen and sulfur via a benzyne ring closure reaction [56]. Foubelo reported the cyclisation of sulfanyl alcohol under acidic conditions to yield the eight-membered sulfur-containing heterocycle [57]. Jiang reported straightforward protocols for diarylannulated sulfide construction through S-I exchange [58,59]. Asai reported using the thio-alkylation of thiosalicylic acid and then intramolecular Friedel–Crafts acylation to synthesize diaryl sulfide [60]. In 2019, Chen’s and Meng’s groups reported the construction of benzo-fused eight-membered cyclic sulfides via the domino reaction of arynes and methylenebenzothiopheneones with CsF as the fluorine source [61,62]. When unsubstituted methylenebenzothiopheneone reacted with an unsubstituted aryne precursor, only a 31% yield was obtained. In other cases, there is no substrate scope of the reaction with 2-(trimethylsilyl)phenyl trifluoromethanesulfonate as the benzyne precursor. Reddy reported using copper (I)-catalyzed interrupted click-sulfenylation of O-/N-propargyl benzyl thiosulfonates with organic azides to construct triazole-fused eight-membered heterocycles [63]. Despite some efforts made in the past, the synthetic chemistry of sulfur-containing benzo-fused eight-membered heterocycles still suffers from considerable limitations, such as low yields and narrow substrate scope.
Considering the importance of eight-membered cyclic diaryl sulfides, we here report undertaking the [3+2] cycloaddition, 1,2-hydrogen shift and C(sp2)-S bond cleavage of 2-(trimethylsilyl)phenyl trifluoromethanesulfonate and 2-methylenebenzothiophene-3-ones with TBAT as fluorine source for the preparation of eight-membered cyclic diaryl sulfides.
2. Results and Discussion
In our initial attempts, we commenced with the reaction of 2-methylenebenzothiophene-3-one (1a) and 2-(trimethylsilyl)phenyl trifluoromethanesulfonate (unsubstituted aryne precursor) (2) for the exploration of the optimal reaction conditions. As shown in Table 1, first, the reaction was conducted with CsF as the fluorine source in ethyl acetate at 30 °C. Regrettably, only a trace amount of the anticipated product (3a) was obtained (Table 1, entry 1). Various solvents were screened (Table 1, entries 2–7). Similarly, only a trace amount of desired product (3a) was generated with THF, CH2Cl2, and EtOH (Table 1, entries 2–4). When the solvent was toluene, the desired product (3a) was not obtained (Table 1, entry 5). Fortunately, a significant improvement was noted when acetone was used as the solvent, resulting in 33% yield of the product (Table 1, entry 6). Further experimentation revealed that CH3CN was an optimal solvent, affording a 52% yield (Table 1, entry 7). Subsequently, the screening of different fluorine sources for the reaction was carried out. A yield of only 16% was generated when using KF as fluorine source (Table 1, entry 8). When using NaF or TBAF, trace amounts of the desired product (3a) were obtained (Table 1, entries 9 and 10). Gratifyingly, when TBAT was employed as the fluorine source, a remarkable 92% yield of product 3a was achieved (Table 1, entry 11). The effect of the additive 18-crown-6 was also evaluated, and the yield of 3a was substantially reduced to 73% (Table 1, entry 12). Then, further evaluations of the effect of temperature revealed that, when the reaction was carried out at 60 °C, no distinct change was detected (91% yield, Table 1, entry 15). At other temperatures, the reaction decreased yields (77% and 84% yields, Table 1, entries 13 and 14). Finally, the equivalent of TBAT was screened. Further increases in the equivalence of TBAT and 2 did not improve the yield of 3a (Table 1, entries 16 and 17).

Table 1.
Optimization of the reaction conditions a.
Thus, the optimal reaction conditions were found to be 1.0 equiv. of 2-methylenebenzothiophene-3-one 1a, 2.0 equiv. of 2-(trimethylsilyl)phenyl trifluoromethanesulfonate 2, and 2.0 equiv. of TBAT in 0.5 mL of CH3CN at 30 °C for 4 h.
With the optimal reaction conditions established, we further explored the substrate range of 2-methylenebenzothiophene-3-ones 1 (Scheme 2). First, we investigated the substrates with R2 = CO2Et. When the methyl group was substituted at the C5 or C6 positions, the corresponding products 3b and 3c were obtained in 85% and 87% yields, respectively. Product 3d was obtained in 93% yield when the methyl substitution was located at the C7 position. Next, when the R2 group of substrate 1 was changed from CO2Et to CO2Me, substrates that had a C5-, C6-, or C7-substituent phenyl ring with a methyl group were also subjected to general reaction conditions, producing the compounds 3e–3h in excellent yields (91–92% yields). When the ester moiety of substrate 1 changes to sterically hindered CO2tBu, the reaction of the substrate-bearing methyl group at the C5–C7 positions of the phenyl ring resulted in 80–84% yields of products 3i–3l. In addition, substrates with benzyl on the ester moiety were well tolerated and afforded products 3m–3p in high yields (70–78% yields). Unfortunately, when the substituents on the benzene ring were electron-withdrawing groups (Cl or Br), the desired products could not be generated.
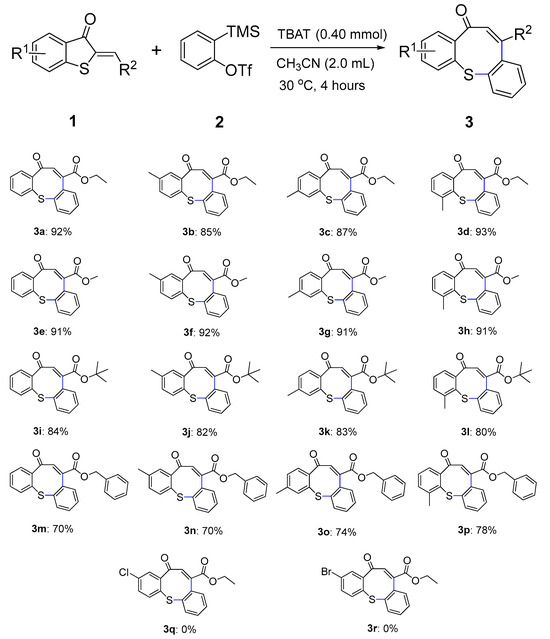
Scheme 2.
Substrate scope for the domino reaction between various 2-methylenebenzothiophene-3-ones 1 and 2-(trimethylsilyl)phenyl trifluoromethanesulfonate 2. Reaction conditions: 1 (0.20 mmol), 2 (0.40 mmol), and TBAT (0.40 mmol) in CH3CN (2.0 mL) at 30 °C; isolated yields.
Furthermore, to demonstrate the applicability of this reaction, we carried out a gram-scale experiment under optimized conditions. As shown in Scheme 3, when using 4.0 mmol (0.99 g) of methylenebenzothiophene-one 1d for cycloaddition, the reaction still proceeded well, producing 3d with a yield of 81% (1.05 g).
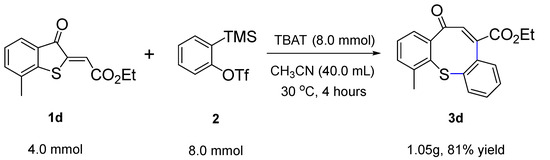
Scheme 3.
Scale-up reaction.
3. Conclusions
In summary, a variety of eight-membered cyclic diaryl sulfides were effectively synthesized. Utilizing TBAT as the fluorine source, the [3+2] cycloaddition of 2-methylenebenzothiophene-3-ones with aryne, following a 1,2-hydrogen shift and C-S bond cleavage, proceeded smoothly to afford the target products in high to excellent yields (70–93% yields). The reaction could be conducted on a gram scale. Our laboratory is undertaking further investigations of the potential of using arynes to construct sulfur-containing heterocycles.
4. Materials and Methods
The 1H and 13C NMR spectra of all compounds were recorded on a Bruker 400 advance III spectrometer (1H = 400 MHz and 13C = 101 MHz) (Bruker corporation) at room temperature (25 °C) using CDCl3 (Adamas-Beta, Shanghai, China) as the solvent. The processing of NMR spectra was performed using MestReNova Software (version 14.2.1-27684; Mestrelab Research, 2021). The chemical shifts were recorded in ppm relative to tetramethylsilane and with the solvent resonance as the internal standard. Data were reported as follows: chemical shift, multiplicity (s = singlet, d = doublet, t = triplet, m = multiplet, br = broad), coupling constants (Hz), and integration. The melting points were determined using Melting-Point Apparatus (HUAZHI HMX-1B, Fujian, China). HRMS data were acquired using a Thermo Scientific Q Exactive Plus Quadrupole-Orbitrap mass spectrometer (Thermo Fisher Scientific, Bremen, Germany) equipped with a Heated Electrospray Ionization (HESI) source. Flash chromatography was performed using silica gel (Qingdao Haiyang chemical corporation, Qingdao, China). Thin-layer chromatography (TLC): Huanghai HSGF254, Yantai, China. 1H and 13C-NMR spectra of compounds 3a–3p are in Supplementary Materials.
General procedure for the synthesis of 3:
In a test tube, 2 (0.40 mmol) was added to a solution of 1 (0.20 mmol) and TBAT (0.40 mmol) in CH3CN (2.0 mL). The mixture was stirred at 30 °C for 4 h. After the reaction was complete (monitored by TLC), the solvent was removed under reduced pressure, and the residue was purified via column chromatography to afford the pure product 3 (ethyl acetate: petroleum ether = 1:20).
Ethyl (E)-5-oxo-5H-dibenzo[b,g]thiocine-7-carboxylate (3a):
Yellow solid (57.2 mg, 92% yield), mp 134–136 °C, Rf = 0.68 (Ethyl acetate: Petroleum ether = 1:5). 1H NMR (400 MHz, CDCl3) δ 7.82–7.73 (m, 1H), 7.71–7.61 (m, 2H), 7.56–7.36 (m, 4H), 7.34–7.25 (m, 2H), 4.37–4.26 (m, 2H), 1.33 (t, J = 7.1 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 195.93, 165.17, 140.96, 140.81, 139.57, 136.48, 136.41, 135.96, 132.76, 132.58, 131.99, 131.57, 130.08, 129.84, 129.58, 127.51, 61.87, 14.21. HRMS (ESI): m/z calcd for C18H14O3S [M + H]+: 311.0736, found: 311.0736.
Ethyl (E)-3-methyl-5-oxo-5H-dibenzo[b,g]thiocine-7-carboxylate (3b):
Yellow solid (55.2 mg, 85% yield), mp 134–135 °C, Rf = 0.64 (Ethyl acetate: Petroleum ether = 1:5). 1H NMR (400 MHz, CDCl3) δ 7.76–7.62 (m, 2H), 7.58–7.46 (m, 2H), 7.44–7.37 (m, 2H), 7.29–7.24 (m, 1H), 7.29–7.24 (m, 1H), 4.36–4.27 (m, 2H), 2.39 (s, 3H), 1.33 (t, J = 7.1 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 195.32, 165.30, 143.98, 140.96, 140.68, 139.72, 136.30, 135.45, 134.05, 132.89, 132.67, 131.74, 129.95, 129.87, 129.48, 128.51, 61.83, 21.37, 14.22. HRMS (ESI): m/z calcd for C19H16O3S [M + H]+: 325.0893, found: 325.0894.
Ethyl (E)-2-methyl-5-oxo-5H-dibenzo[b,g]thiocine-7-carboxylate (3c):
Yellow solid (56.5 mg, 87% yield), Rf = 0.64 (Ethyl acetate: Petroleum ether = 1:5). 1H NMR (400 MHz, CDCl3) δ 7.72–7.52 (m, 2H), 7.50–7.38 (m, 2H), 7.37–7.25 (m, 2H), 7.21–7.15 (m, 1H), 7.08–7.00 (m, 1H), 4.30–4.16 (m, 2H), 2.31 (s, 3H), 1.25 (t, J = 7.1 Hz, 3H). HRMS (ESI): m/z calcd for C19H16O3S [M + H]+: 325.0893, found: 325.0892.
Ethyl (E)-1-methyl-5-oxo-5H-dibenzo[b,g]thiocine-7-carboxylate (3d):
Yellow solid (60.3 mg, 93% yield), mp 143–144 °C, Rf = 0.64 (Ethyl acetate: Petroleum ether = 1:5). 1H NMR (400 MHz, Chloroform-d) δ 7.66–7.59 (m 1H), 7.58–7.48 (m, 2H), 7.46–7.33 (m, 3H), 7.28–7.23 (m, 1H), 7.18 (t, J = 7.6 Hz, 1H), 4.38–4.26 (m, 2H), 2.61 (s, 3H), 1.33 (t, J = 7.1 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 196.72, 165.06, 141.78, 140.77, 140.20, 138.46, 137.13, 136.85, 136.20, 134.14, 132.35, 130.17, 129.68, 129.57, 129.34, 126.49, 61.79, 21.47, 14.24. HRMS (ESI): m/z calcd for C19H16O3S [M + H]+: 325.0893, found: 325.0893.
Methyl (E)-5-oxo-5H-dibenzo[b,g]thiocine-7-carboxylate (3e):
Light yellow solid (53.7 mg, 91% yield), mp 146–147 °C, Rf = 0.66 (Ethyl acetate: Petroleum ether = 1:5)). 1H NMR (400 MHz, CDCl3) δ 7.79–7.72 (m, 1H), 7.71–7.65 (m, 2H), 7.50 (d, J = 7.7 Hz, 1H), 7.46–7.40 (m, 3H), 7.34–7.26 (m, 2H), 3.86 (s, 3H). HRMS (ESI): m/z calcd for C17H12O3S [M + H]+: 297.0580, found: 297.0584.
Methyl (E)-3-methyl-5-oxo-5H-dibenzo[b,g]thiocine-7-carboxylate (3f):
Light yellow solid (57.1 mg, 92% yield), mp 159–161 °C, Rf = 0.62 (Ethyl acetate: Petroleum ether = 1:5)). 1H NMR (400 MHz, CDCl3) δ 7.73–7.65 (m, 1H), 7.63–7.52 (m, 2H), 7.51–7.34 (m, 3H), 7.30–7.24 (m, 2H), 3.85 (s, 3H), 2.33 (s, 3H). HRMS (ESI): m/z calcd for C18H14O3S [M + H]+: 311.0736, found: 311.0736.
Methyl (E)-2-methyl-5-oxo-5H-dibenzo[b,g]thiocine-7-carboxylate (3g):
Orange waxy solid (56.6 mg, 91% yield), Rf = 0.62 (Ethyl acetate: Petroleum ether = 1:5)). 1H NMR (400 MHz, CDCl3) δ 7.69 (t, J = 7.3 Hz, 2H), 7.53–7.37 (m, 4H), 7.30–7.24 (m, 1H), 7.13 (d, J = 7.8 Hz, 1H), 3.85 (s, 3H), 2.34 (s, 3H). HRMS (ESI): m/z calcd for C18H14O3S [M + H]+: 311.0736, found: 311.0736.
Methyl (E)-1-methyl-5-oxo-5H-dibenzo[b,g]thiocine-7-carboxylate (3h):
Light yellow solid (56.6 mg, 91% yield), mp 128–129 °C, Rf = 0.62 (Ethyl acetate: Petroleum ether = 1:5). 1H NMR (400 MHz, CDCl3) δ 7.65–7.61 (m, 1H), 7.58–7.49 (m, 2H), 7.47–7.31 (m, 3H), 7.30–7.24 (m, 1H), 7.19 (t, J = 7.6 Hz, 1H), 3.86 (s, 3H), 2.61 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 196.57, 165.56, 141.66, 140.72, 140.52, 138.49, 137.08, 136.87, 135.92, 134.17, 132.39, 130.22, 129.63, 129.36, 126.51, 52.75, 29.73, 21.48. HRMS (ESI): m/z calcd for C18H14O3S [M + H]+: 311.0736, found: 311.0740.
tert-Butyl (E)-5-oxo-5H-dibenzo[b,g]thiocine-7-carboxylate (3i):
Green oil (57.0 mg, 84% yield), Rf = 0.73 (Ethyl acetate: Petroleum ether = 1:5). 1H NMR (400 MHz, CDCl3) δ 7.73 -7.65 (m, 1H), 7.64–7.56 (m, 2H), 7.46–7.40 (m, 1H), 7.39–7.29 (m, 2H), 7.26–7.15 (m, 3H), 1.46 (s, 9H). HRMS (ESI): m/z calcd for C20H18O3S [M + H]+: 339.1049, found: 339.1052.
tert-Butyl (E)-3-methyl-5-oxo-5H-dibenzo[b,g]thiocine-7-carboxylate (3j):
Orange solid (58.0 mg, 82% yield), mp 113–115 °C, Rf = 0.75 (Ethyl acetate: Petroleum ether = 1:5)). 1H NMR (400 MHz, CDCl3) δ 7.70–7.63 (m, 1H), 7.62–7.53 (m, 2H), 7.52–7.45 (m, 1H), 7.42–7.31 (m, 2H), 7.27–7.24 (m, 2H), 2.33 (s, 3H), 1.53 (s, 9H). 13C NMR (101 MHz, CDCl3) δ 196.30, 164.32, 141.00, 138.68, 137.83, 137.64, 137.15, 136.39, 136.16, 133.63, 133.03, 132.11, 131.95, 129.95, 129.73, 129.32, 82.28, 28.05, 20.76. HRMS (ESI): m/z calcd for C21H20O3S [M + H]+: 353.1206, found: 353.1207.
tert-Butyl (E)-2-methyl-5-oxo-5H-dibenzo[b,g]thiocine-7-carboxylate (3k):
Bright yellow waxy solid (58.7 mg, 83% yield), Rf = 0.71 (Ethyl acetate: Petroleum ether = 1:5). 1H NMR (400 MHz, CDCl3) δ 7.72–7.64 (m, 2H), 7.54–7.47 (m, 2H), 7.42–7.36 (m, 1H), 7.34 (s, 1H), 7.28–7.24 (m, 1H), 7.14–7.11 (m, 1H), 2.39 (s, 3H), 1.53 (s, 9H). HRMS (ESI): m/z calcd for C21H20O3S [M + H]+: 353.1206, found: 353.1206.
tert-Butyl (E)-1-methyl-5-oxo-5H-dibenzo[b,g]thiocine-7-carboxylate (3l):
Orange waxy solid (56.5 mg, 80% yield), Rf = 0.72 (Ethyl acetate: Petroleum ether = 1:5). 1H NMR (400 MHz, CDCl3) δ 7.62 (d, J = 7.8 Hz, 1H), 7.53 (t, J = 8.4 Hz, 2H), 7.43–7.35 (m, 2H), 7.30 (s, 1H), 7.26 (s, 1H), 7.19 (t, J = 7.6 Hz, 1H), 2.62 (s, 3H), 1.54 (s, 9H). 13C NMR (101 MHz, CDCl3) δ 197.09, 164.13, 142.07, 140.86, 139.29, 138.39, 137.55, 137.20, 136.77, 134.03, 132.28, 129.99, 129.77, 129.36, 129.28, 126.39, 82.25, 28.07, 21.44. HRMS (ESI): m/z calcd for C21H20O3S [M + Na]+: 375.1025, found: 375.1025.
Benzyl (E)-5-oxo-5H-dibenzo[b,g]thiocine-7-carboxylate (3m):
Orange waxy solid (52.0 mg, 70% yield), Rf = 0.64 (Ethyl acetate: Petroleum ether = 1:5). 1H NMR (400 MHz, CDCl3) δ 7.75 (d, J = 7.8 Hz, 1H), 7.68 (t, J = 7.0 Hz, 2H), 7.51 (d, J = 7.7 Hz, 1H), 7.48–7.14 (m, 10H), 5.36–5.24 (m, 2H). HRMS (ESI): m/z calcd for C23H16O3S [M + H]+: 373.0893, found: 373.0894.
Benzyl (E)-3-methyl-5-oxo-5H-dibenzo[b,g]thiocine-7-carboxylate (3n):
Orange waxy solid (54.4 mg, 70% yield), Rf = 0.62 (Ethyl acetate: Petroleum ether = 1:5). 1H NMR (400 MHz, CDCl3) δ 7.68 (d, J = 7.8 Hz, 1H), 7.63–7.54 (m, 2H), 7.52–7.44 (m, 2H), 7.42–7.30 (m, 6H), 7.28–7.18 (m, 2H), 5.35–5.24 (m, 2H), 2.32 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 195.78, 165.12, 140.56, 140.03, 137.76, 137.71, 136.28, 135.52, 133.76, 133.19, 132.17, 132.00, 129.94, 129.85, 129.61, 128.63, 128.38, 128.15, 67.43, 20.78. HRMS (ESI): m/z calcd for C24H18O3S [M + H]+: 387.1049, found: 387.1052.
Benzyl (E)-2-methyl-5-oxo-5H-dibenzo[b,g]thiocine-7-carboxylate (3o):
Light yellow waxy solid (57.4 mg, 74% yield), Rf = 0.62 (Ethyl acetate: Petroleum ether = 1:5). 1H NMR (400 MHz, CDCl3) δ 7.73–7.53 (m, 2H), 7.53–7.45 (m, 2H), 7.43–7.22 (m, 8H), 7.12 (d, J = 7.9 Hz, 1H), 5.34–5.24 (m, 2H), 2.33 (d, J = 41.6 Hz, 3H). HRMS (ESI): m/z calcd for C24H18O3S [M + H]+: 387.1049, found: 387.1049.
Benzyl (E)-1-methyl-5-oxo-5H-dibenzo[b,g]thiocine-7-carboxylate (3p):
Orange waxy solid (60.6 mg, 78% yield), Rf = 0.62 (Ethyl acetate: Petroleum ether = 1:5). 1H NMR (400 MHz, CDCl3) δ 7.62 (d, J = 7.8 Hz, 1H), 7.53 (d, J = 7.9 Hz, 2H), 7.46–7.30 (m, 8H), 7.29–7.23 (m, 1H), 7.17 (t, J = 7.6 Hz, 1H), 5.30 (d, J = 7.2 Hz, 2H), 2.61 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 196.59, 164.90, 141.62, 140.76, 140.66, 138.48, 137.08, 136.90, 135.91, 135.52, 134.18, 132.41, 130.21, 129.69, 129.66, 129.35, 128.65, 128.41, 128.21, 126.50, 67.42, 21.48. HRMS (ESI): m/z calcd for C24H18O3S [M + H]+: 387.1049, found: 387.1051.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/reactions6020035/s1, 1H and 13C-NMR spectra of compounds 3a–3p.
Author Contributions
J.F.: experimental design, writing—original draft preparation, and writing—review and editing. W.Z. and H.Z.: experiments and data collection. Q.H., A.H., K.L. and G.Y.: data analysis. All authors revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in this study are included in the article and Supplementary Materials. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Suchý, M.; Kutschy, P.; Monde, K.; Goto, H.; Harada, N.; Takasugi, M.; Dzurilla, M.; Balentová, E. Synthesis, Absolute Configuration, and Enantiomeric Enrichment of a Cruciferous Oxindole Phytoalexin, (S)-(−)-Spirobrassinin, and Its Oxazoline Analog. J. Org. Chem. 2001, 66, 3940–3947. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chen, X.; Bois-Choussy, M.; Zhu, J. Total Synthesis of Ecteinascidin 743. J. Am. Chem. Soc. 2006, 128, 87–89. [Google Scholar] [CrossRef] [PubMed]
- Nicolaou, K.C.; Hale, C.R.H.; Nilewski, C.; Ioannidou, H.A. Constructing Molecular Complexity and Diversity: Total Synthesis of Natural Products of Biological and Medicinal Importance. Chem. Soc. Rev. 2012, 41, 5185–5238. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Y.; Jiang, X. Sulfur-Center-Involved Photocatalyzed Reactions. Chem. Asian J. 2018, 13, 2208–2242. [Google Scholar] [CrossRef]
- He, W.; Zhang, Z.; Ma, D. A Scalable Total Synthesis of the Antitumor Agents Et-743 and Lurbinectedin. Angew. Chem. Int. Ed. 2019, 58, 3972–3975. [Google Scholar] [CrossRef]
- Yoneya, T.; Taniguchi, K.; Nakamura, R.; Tsunenari, T.; Ohizumi, I.; Kanbe, Y.; Morikawa, K.; Kaiho, S.-I.; Yamada-Okabe, H. Thiochroman Derivative CH4986399, A New Nonsteroidal Estrogen Receptor Down-regulator, Is Effective in Breast Cancer Models. Anticancer Res. 2010, 30, 873–878. [Google Scholar]
- Berrade, L.; Aisa, B.; Ramirez, M.J.; Galiano, S.; Guccione, S.; Moltzau, L.R.; Levy, F.O.; Nicoletti, F.; Battaglia, G.; Molinaro, G.; et al. Novel Benzo[b]thiophene Derivatives as New Potential Antidepressants with Rapid Onset of Action. J. Med. Chem. 2011, 54, 3086–3090. [Google Scholar] [CrossRef]
- Zaher, A.F.; Abuel-Maaty, S.M.; El-Nassan, H.B.; Amer, S.A.S.; Abdelghany, T.M. Synthesis, Antitumor Screening and Cell Cycle Analysis of Novel Benzothieno[3,2-b]pyran Derivatives. J. Enzyme Inhib. Med. Chem. 2016, 31, 145–153. [Google Scholar] [CrossRef]
- Boyd-Kimball, D.; Gonczy, K.; Lewis, B.; Mason, T.; Siliko, N.; Wolfe, J. Classics in Chemical Neuroscience: Chlorpromazine. ACS Chem. Neurosci. 2019, 10, 79–88. [Google Scholar] [CrossRef]
- Jansen, T.P.J.; Konst, R.E.; de Vos, A.; Paradies, V.; Teerenstra, S.; van den Oord, S.C.H.; Dimitriu-Leen, A.; Maas, A.H.E.M.; Smits, P.C.; Damman, P.; et al. Efficacy of Diltiazem to Improve Coronary Vasomotor Dysfunction in ANOCA: The EDIT-CMD Randomized Clinical Trial. JACC Cardiovasc. Imaging 2022, 15, 1473–1484. [Google Scholar] [CrossRef]
- Viscardi, S.; Topola, E.; Sobieraj, J.; Duda-Madej, A. Novel Siderophore Cephalosporin and Combinations of Cephalosporins with β-Lactamase Inhibitors as an Advancement in Treatment of Ventilator-Associated Pneumonia. Antibiotics 2024, 13, 445. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Ma, C.-Q.; Bäuerle, P. Functional Oligothiophenes: Molecular Design for Multidimensional Nanoarchitectures and Their Applications. Chem. Rev. 2009, 109, 1141–1276. [Google Scholar] [CrossRef] [PubMed]
- Takimiya, K.; Shinamura, S.; Osaka, I.; Miyazaki, E. Thienoacene-Based Organic Semiconductors. Adv. Mater. 2011, 23, 4347–4370. [Google Scholar] [CrossRef] [PubMed]
- Lehnherr, D.; Hallani, R.; McDonald, R.; Anthony, J.E.; Tykwinski, R.R. Synthesis and Properties of Isomerically Pure Anthrabisbenzothiophenes. Org. Lett. 2012, 14, 62–65. [Google Scholar] [CrossRef]
- Takimiya, K.; Osaka, I.; Mori, T.; Nakano, M. Organic Semiconductors Based on [1]Benzothieno[3,2-b][1]benzothiophene Substructure. Acc. Chem. Res. 2014, 47, 1493–1502. [Google Scholar] [CrossRef]
- Cinar, M.E.; Ozturk, T. Thienothiophenes, Dithienothiophenes, and Thienoacenes: Syntheses, Oligomers, Polymers, and Properties. Chem. Rev. 2015, 115, 3036–3140. [Google Scholar] [CrossRef]
- Zhan, X.; Wu, Z.; Lin, Y.; Tang, S.; Yang, J.; Hu, J.; Peng, Q.; Ma, D.; Li, Q.; Li, Z. New AIEgens Containing Dibenzothiophene-S,S-dioxide and Tetraphenylethene Moieties: Similar Structures but Much Different Hole/Electron Transport Properties. J. Mater. Chem. C 2015, 3, 5903–5909. [Google Scholar] [CrossRef]
- Banerjee, T.; Sharma, S.K.; Kapoor, N.; Dwivedi, V.; Surolia, N.; Surolia, A. Benzothiophene Carboxamide Derivatives as Inhibitors of Plasmodium Falciparum Enoyl-ACP Reductase. IUBMB Life 2011, 63, 1101–1110. [Google Scholar] [CrossRef]
- Jain, A.K.; Vaidya, A.; Ravichandran, V.; Kashaw, S.K.; Agrawal, R.K. Recent Developments and Biological Activities of Thiazolidinone Derivatives: A Review. Bioorg. Med. Chem. 2012, 20, 3378–3395. [Google Scholar] [CrossRef]
- Romagnoli, R.; Baraldi, P.G.; Lopez-Cara, C.; Preti, D.; Tabrizi, M.A.; Balzarini, J.; Bassetto, M.; Brancale, A.; Fu, X.-H.; Gao, Y.; et al. Concise Synthesis and Biological Evaluation of 2-Aroyl-5-Amino Benzo[b]thiophene Derivatives As a Novel Class of Potent Antimitotic Agents. J. Med. Chem. 2013, 56, 9296–9309. [Google Scholar] [CrossRef]
- Scott, S.A.; Spencer, C.T.; O’Reilly, M.C.; Brown, K.A.; Lavieri, R.R.; Cho, C.-H.; Jung, D.-I.; Larock, R.C.; Alex Brown, H.; Lindsley, C.W. Discovery of Desketoraloxifene Analogues as Inhibitors of Mammalian, Pseudomonas aeruginosa, and NAPE Phospholipase D Enzymes. ACS Chem. Biol. 2015, 10, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Dao, P.; Ye, F.; Liu, Y.; Du, Z.Y.; Zhang, K.; Dong, C.Z.; Meunier, B.; Chen, H. Development of Phenothiazine-Based Theranostic Compounds That Act Both as Inhibitors of β-Amyloid Aggregation and as Imaging Probes for Amyloid Plaques in Alzheimer’s Disease. ACS Chem. Neurosci. 2017, 8, 798–806. [Google Scholar] [CrossRef] [PubMed]
- Kugita, H.; Inoue, H.; Ikezaki, M.; Konda, M.; Takeo, S. Synthesis of 1,5-Benzothiazepine Derivatives. II. Chem. Pharm. Bull. 1970, 18, 2284–2299. [Google Scholar] [CrossRef]
- Asano, K.; Matsubara, S. Catalytic Approaches to Optically Active 1,5-Benzothiazepines. ACS Catal. 2018, 8, 6273–6282. [Google Scholar] [CrossRef]
- Jones, C.D.; Jevnikar, M.G.; Pike, A.J.; Peters, M.K.; Black, L.J.; Thompson, A.R.; Falcone, J.F.; Clemens, J.A. Antiestrogens. 2. Structure-Activity Studies in a Series of 3-Aroyl-2-arylbenzo[b]thiophene Derivatives Leading to [6-Hydroxy-2-(4-hydroxyphenyl) benzo[b]thien-3-yl] [4-[2-(1-piperidinyl) ethoxy]-phenyl] methanone Hydrochloride (LY156758), a Remarkably Effective Estrogen Antagonist with Only Minimal Intrinsic Estrogenicity. J. Med. Chem. 1984, 27, 1057–1066. [Google Scholar]
- Yasuo, F.; Shigeru, Y. DE2941869. 1980. Available online: https://patents.google.com/patent/DE2941869C2/d (accessed on 26 May 2025).
- Shoji, M.; Mikio, W. Production of 2-(10,11-dihydro-10-oxodibenzo(b,f)thiepin-2-yl) Propionic Acid. JP61251682A, 8 November 1986. [Google Scholar]
- Mitsuo, M.; Hiromitsu, T.; Naoya, M. Verfahren zur Herstellung von Dibenzothiepin-Derivaten. EP0309626 A1, 5 April 1989. [Google Scholar]
- Yamamoto, T.; Takimiya, K. Facile Synthesis of Highly π-Extended Heteroarenes, Dinaphtho [2,3-b: 2′, 3′-f] chalcogenopheno [3,2-b] chalcogenophenes, and Their Application to Field-Effect Transistors. J. Am. Chem. Soc. 2007, 129, 2224–2225. [Google Scholar] [CrossRef]
- Kang, M.J.; Doi, I.; Mori, H.; Miyazaki, E.; Takimiya, K.; Ikeda, M.; Kuwabara, H. Alkylated Dinaphtho [2,3-b:2′,3′-f] Thieno [3,2-b] Thiophenes (Cn-DNTTs): Organic Semiconductors for High-Performance Thin-Film Transistors. Adv. Mater. 2011, 23, 1222–1225. [Google Scholar] [CrossRef] [PubMed]
- Nishida, I.; Kawaguchi, A.; Yamada, M. Effect of Thiolactomycin on the Individual Enzymes of the Fatty Acid Synthase System in Escherichia coli1. J. Biochem. 1986, 99, 1447–1454. [Google Scholar] [CrossRef]
- Slayden, R.A.; Lee, R.E.; Armour, J.W.; Cooper, A.M.; Orme, I.M.; Brennan, P.J.; Besra, G.S. Antimycobacterial Action of Thiolactomycin: An Inhibitor of Fatty Acid and Mycolic Acid Synthesis. Antimicrob. Agents Chemother. 1996, 40, 2813–2819. [Google Scholar] [CrossRef]
- Heath, R.J.; White, S.W.; Rock, C.O. Lipid Biosynthesis as a Target for Antibacterial Agents. Prog. Lipid Res. 2001, 40, 467–497. [Google Scholar] [CrossRef]
- Tomita, K.; Sato, S.; Kobayashi, S. Condensed Heterocyclic Compound. JP 60132972 A, 12 December 1983. [Google Scholar]
- Wu, L.-Q.; Yang, X.-J.; Peng, Q.-J.; Sun, G.-F. Synthesis and Anti-proliferative Activity Evaluation of Novel Benzo[d][1,3] dioxoles-fused 1,4-Thiazepines. Eur. J. Med. Chem. 2017, 127, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Grand, B.L.; Pignier, C.; Létienne, R.; Cuisiat, F.; Rolland, F.; Mas, A.; Vacher, B. Sodium Late Current Blockers in Ischemia Reperfusion: Is the Bullet Magic? J. Med. Chem. 2008, 51, 3856–3866. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-J.; Chen, D.-L. Eight-Membered Thiocycloether via Indium-Mediated Ring Enlargement. Synlett 1999, 1999, 735–736. [Google Scholar] [CrossRef]
- Qiao, Z.; Liu, H.; Xiao, X.; Fu, Y.; Wei, J.; Li, Y.; Jiang, X. Efficient Access to 1,4-Benzothiazine: Palladium-Catalyzed Double C–S Bond Formation Using Na2S2O3 as Sulfurating Reagent. Org. Lett. 2013, 15, 2594–2597. [Google Scholar] [CrossRef] [PubMed]
- Muthusamy, S.; Sivaguru, M.; Suresh, E. Indium(III) Chloride Catalyzed Highly Diastereoselective Domino Synthesis of Indenodithiepines and Indenodithiocines. Chem. Commun. 2015, 51, 707–710. [Google Scholar] [CrossRef]
- Acharya, A.; Vijay Kumar, S.; Ila, H. Diversity-Oriented Synthesis of Substituted Benzo[b]thiophenes and Their Hetero-Fused Analogues through Palladium-Catalyzed Oxidative C-H Functionalization/Intramolecular Arylthiolation. Chem. Eur. J. 2015, 21, 17116–17125. [Google Scholar] [CrossRef]
- Masuya, Y.; Tobisu, M.; Chatani, N. Palladium-Catalyzed Synthesis of 2,3-Disubstituted Benzothiophenes via the Annulation of Aryl Sulfides with Alkynes. Org. Lett. 2016, 18, 4312–4315. [Google Scholar] [CrossRef]
- Meng, L.; Fujikawa, T.; Kuwayama, M.; Segawa, Y.; Itami, K. Thiophene-Fused π-Systems from Diarylacetylenes and Elemental Sulfur. J. Am. Chem. Soc. 2016, 138, 10351–10355. [Google Scholar] [CrossRef]
- Simlandy, A.K.; Mukherjee, S. Catalytic Enantioselective Synthesis of 3,4-Unsubstituted Thiochromenes through Sulfa-Michael/Julia–Kocienski Olefination Cascade Reaction. J. Org. Chem. 2017, 82, 4851–4858. [Google Scholar] [CrossRef]
- Yugandar, S.; Konda, S.; Ila, H. Synthesis of Substituted Benzo[b]thiophenes via Sequential One-Pot, Copper-Catalyzed Intermolecular C–S Bond Formation and Palladium-Catalyzed Intramolecular Arene–Alkene Coupling of Bis(het)aryl/alkyl-1,3-monothiodiketones and o-Bromoiodoarenes. Org. Lett. 2017, 19, 1512–1515. [Google Scholar] [CrossRef]
- Song, J.; Wu, H.; Sun, W.; Wang, S.; Sun, H.; Xiao, K.; Qian, Y.; Liu, C. A Pd-catalyzed Optional Approach for the Synthesis of Dibenzothiophenes. Org. Biomol. Chem. 2018, 16, 2083–2087. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Yang, Y.; Hou, X.; Zeng, W.; Yu, A.; Zhao, X.; Meng, X. Darzens Reaction of Thioisatins and Sulfonium Salts: Approach to the Synthesis of Thiochromenone Derivatives with Anticancer Potency. Org. Biomol. Chem. 2018, 16, 3487–3494. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Chen, L.; Qian, C.; Zhu, X.; Yang, Y.; Liu, J.; Yang, Y.; Liang, Y. Copper-Catalyzed Synthesis of 2-Acylbenzo[b]thiophenes from 3-(2-Iodophenyl)-1-arylpropan-1-ones and Potassium Sulfide under Aerobic Conditions. Org. Biomol. Chem. 2018, 16, 8020–8024. [Google Scholar] [CrossRef]
- Garg, P.; Singh, A. Unmasking Dipole Character of Acyl Ketene Dithioacetals via a Cascade Reaction with Arynes: Synthesis of Benzo[b]thiophenes. Org. Lett. 2018, 20, 1320–1323. [Google Scholar] [CrossRef] [PubMed]
- Uchida, S.; Kinoshita, H.; Miura, K. Diisobutylaluminum Hydride Promoted Selectivity-Switchable Synthesis of Benzothiophene Oxides and Benzothiophenes via an Al–Li-Dimetalated Intermediate. Org. Lett. 2020, 22, 3123–3127. [Google Scholar] [CrossRef]
- Tang, L.; Yang, Q.; Zhang, J.; Deng, G. Regioselective Reversal Cyclization to Access either Eight-Membered Sulfur-Containing Heterocycle-Fused γ-Pyrones or 2-(1,4-Dithianyl)-4-pyrones from the Same Precursors. J. Org. Chem. 2020, 85, 2575–2584. [Google Scholar] [CrossRef]
- An, Y.; Zhang, F.; Du, G.; Cai, Z.; He, L. Construction of 6H-benzo[c]thiochromenes via a Tandem Reaction of Arynes with Thionoesters. Org. Chem. Front. 2021, 8, 6979–6984. [Google Scholar] [CrossRef]
- Sundaravelu, N.; Nandy, A.; Sekar, G. Visible Light Mediated Photocatalyst Free C–S Cross Coupling: Domino Synthesis of Thiochromane Derivatives via Photoinduced Electron Transfer. Org. Lett. 2021, 23, 3115–3119. [Google Scholar] [CrossRef]
- Molander, G.A. Diverse Methods for Medium Ring Synthesis. Acc. Chem. Res. 1998, 31, 603–609. [Google Scholar] [CrossRef]
- Maier, M.E. Synthesis of Medium-Sized Rings by the Ring-Closing Metathesis Reaction. Angew. Chem. Int. Ed. 2000, 39, 2073–2077. [Google Scholar] [CrossRef]
- Mukherjee, C.; Biehl, E. An Efficient Synthesis of Benzene Fused Six-, Seven- and Eight-membered Rings Containing Nitrogen and Sulfur by Benzyne Ring Closure Reaction. Heterocycles 2004, 63, 2309–2318. [Google Scholar] [CrossRef]
- Lu, S.-M.; Alper, H. Intramolecular Carbonylation Reactions with Recyclable Palladium-Complexed Dendrimers on Silica: Synthesis of Oxygen, Nitrogen, or Sulfur-Containing Medium Ring Fused Heterocycles. J. Am. Chem. Soc. 2005, 127, 14776–14784. [Google Scholar] [CrossRef] [PubMed]
- Foubelo, F.; Moreno, B.; Soler, T.; Yus, M. Reductive Ring Opening of Dihydrodibenzothiepine and Dihydrodinaphtho-Oxepine and -Thiepine. Tetrahedron 2005, 61, 9082–9096. [Google Scholar] [CrossRef]
- Wang, M.; Fan, Q.; Jiang, X. Transition-Metal-Free Diarylannulated Sulfide and Selenide Construction via Radical/Anion-Mediated Sulfur−Iodine and Selenium−Iodine Exchange. Org. Lett. 2016, 18, 5756–5759. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wei, J.; Fan, Q.; Jiang, X. Cu(II)-catalyzed Sulfide Construction: Both Aryl Groups Utilization of Intermolecular and Intramolecular Diaryliodonium Salt. Chem. Commun. 2017, 53, 2918–2921. [Google Scholar] [CrossRef]
- Ogo, N.; Ishikawa, Y.; Sawada, J.; Matsuno, K.; Hashimoto, A.; Asai, A. Structure-Guided Design of Novel L-Cysteine Derivatives as Potent KSP Inhibitors. ACS Med. Chem. Lett. 2015, 6, 1004–1009. [Google Scholar] [CrossRef]
- Xiao, P.; Su, S.; Wang, W.; Cao, W.; Chen, J.; Li, J.; Chen, Y. Sequential Cycloaddition and Ring Expansion Reaction of Arynes and Methylenebenzothiopheneones: Synthesis of a Benzo-Fused Eight-Membered Ring via Sulfonium Ylides. RSC Adv. 2019, 9, 39119–39123. [Google Scholar] [CrossRef]
- Ding, W.; Yu, A.; Zhang, L.; Meng, X. Construction of Eight-Membered Cyclic Diaryl Sulfides via Domino Reaction of Arynes with Thioaurone Analogues and DFT Study on the Reaction Mechanism. Org. Lett. 2019, 21, 9014–9018. [Google Scholar] [CrossRef]
- Reddy, R.J.; Waheed, M.; Kumari, A.H.; Krishna, G.R. Interrupted CuAAC-Thiolation for the Construction of 1,2,3-Triazole-Fused Eight-Membered Heterocycles from O-/N-Propargyl derived Benzyl Thiosulfonates with Organic Azides. Adv. Synth. Catal. 2022, 364, 319–325. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).