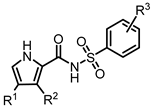
Abstract
We describe the design and synthesis of a series of N-[arylsulfonyl]-1H-pyrrole-2-carboxamides as hybrid analogs of the DCAF15 binders E7820 and tasisulam, two representative SPLAMs (sulfonamide-containing molecular glues). These hybrid molecules were designed to combine the key interactions of both parent ligands within the DCAF15 binding site, as supported by docking studies. Binding affinity was evaluated using fluorescence polarization assays, and structure–activity relationships were established, highlighting the importance of dichlorinated pyrrole moieties. Selected compounds were also tested in HCT116 cells to assess in vitro activity.
1. Introduction
DCAF15 (DDB1- and CUL4-associated factor 15) is a key regulatory protein in the ubiquitin–proteasome system (UPS) that plays an essential role in maintaining cellular homeostasis through the selective degradation of target proteins [1,2]. By recruiting specific compounds for ubiquitination, DCAF15 facilitates the degradation of proteins involved in various cellular processes, including cell cycle regulation, DNA repair, and stress responses. This makes DCAF15 an attractive target for therapeutic strategies focused on manipulating protein degradation, particularly in the context of cancer [1].
While traditional small molecules focus on inhibiting specific protein functions, discovering DCAF15 binders offers a different therapeutic approach, one that could facilitate the degradation of harmful proteins as molecular glues [3]. Potent DCAF15 binders can be used in the design of more sophisticated compounds such as PROTACs (proteolysis-targeting chimeras), which leverage the cell’s own machinery to degrade disease-associated proteins [4,5]. In addition, small molecule DCAF15 binders can be chemically modified, relying on X-ray co-crystal structural information to act as molecular glues for ubiquitination and protein degradation via the proteasome. However, without knowledge of their binding abilities to target proteins of interest, it is difficult to design potent binders [6].
The process of optimizing DCAF15 binding affinity requires a detailed understanding of ligand interactions with key regions of the protein. E7820 and tasisulam, both SPLAMs (SPLicing inhibitor SulfonAMides), are molecular glues known for their anticancer activities [1,7]. In combination with DCAF15 acting as a scaffold with other factors, the resulting complexes are known to degrade the RNA splicing neosubstrate molecule RBM39 via the ubiquitin pathway.
Indisulam has been shown to induce RBM39 degradation in HCT116 colon carcinoma cells, illustrating its mechanism of action via DCAF15-mediated ubiquitination [2].
Elegant studies by Lucas and coworkers [8] have commented on the DCAF15-mediated degradation of certain degrons and concluded that the process may require further scrutiny. Nevertheless, starting with a sulfonamide flanked by a 3,4-dichloro 4-amino indole and a furan carboxamide (1, IC50 = 0.221 µM), extensive optimization led to a potent DCAF15 binder (2, IC50 = 53 nM) (Figure 1). Incorporation into a PROTAC (IC50 = 74 nM) maintained activity that degraded BRD4 but not other proteins. Interestingly, the lead molecule could be co-crystallized with DCAF15 with relevant interactions in the binding site [8,9]. Despite the excellent binding to DCAF15, it was concluded that degradation by the corresponding PROTAC was independent of the presence of the ligase. To the best of our knowledge, this represents the first example of a co-crystal structure of a small molecule binder with DCAF15 alone. As such, this structural information may reveal other aspects of DCAF15 binding and augurs well for further exploration. Thus, the prospects of finding novel effective binders to DCAF15 as a ligase on its own merits further study.
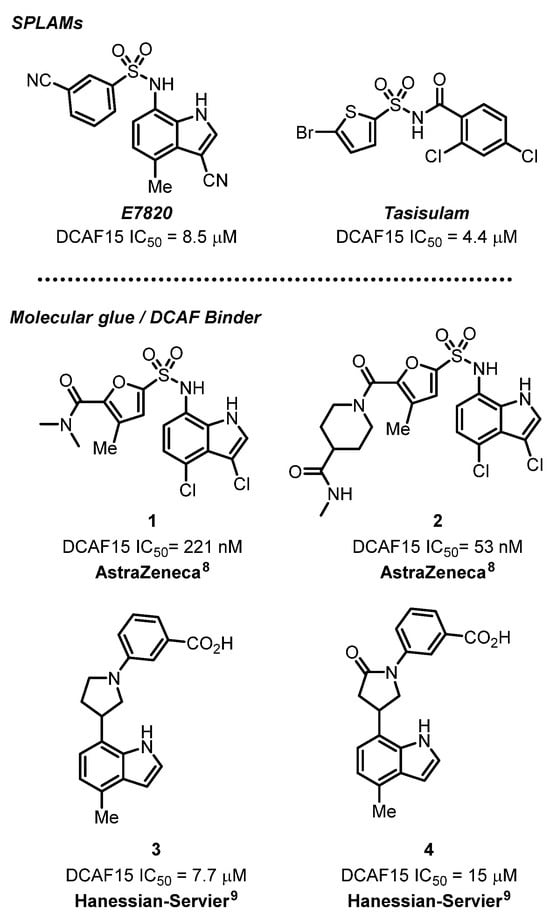
Figure 1.
Structures of SPLAMs and molecular glues as DCAF binders 1–4 [8,9].
In a previous publication [9], we explored the notion of designing molecules that would act as surrogates of the known DCAF15 binder E7820. We introduced the idea of azacyclic linkers to replace a sulfonamide group to join an indole moiety with an aryl group (3 and 4, Figure 1). A pyrrolidine group linker (3, AC50 = 7.7 µM) was found to be the closest analog of E7820 (AC50 = 8.5 µM), as revealed by a binding assay.
E7820 and tasisulam have each been co-crystallized as ternary complexes with RBM39 and DCAF15 [10,11]. Within the respective complexes, both compounds share a hydrogen bond between their sulfonamide moiety and the Ala234 and Phe235 residues of DCAF15. However, they differ in their additional interactions: E7820 forms a hydrogen bond between the NH of its indole group and Phe231, while tasisulam interacts with Gln232 via its carboxamide group (Figure 2A,B). A superimposition of the X-ray structures of E7820 and tasisulam within the DCAF15 binding pocket (Figure 2C) accentuates their complementary binding modes.
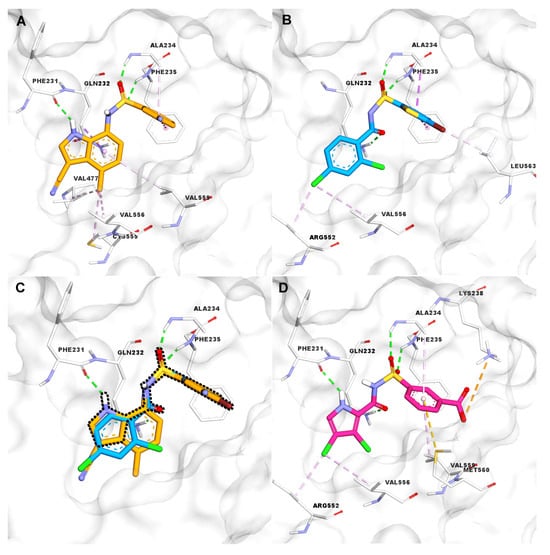
Figure 2.
(A) Key interactions of E7820 (orange) with DCAF15; (B) key interactions of tasisulam (blue) with DCAF15; (C) superposition of E7820 (orange) and tasisulam (blue) within DCAF15, showing a combined key H-bond and the shape of the hybrid analog (black dashed line); (D) docking pose of the 3,4-dichloro N-m-carboxyphenylsulphonamide hybrid analog (7) (pink) in DCAF15. Interaction color code: hydrogen bonds (green), π–π stacking (violet), halogen or hydrophobic contacts (light pink/yellow), charge interaction (orange).
We speculated whether combining key interactions of E7820 and tasisulam with DCAF15 would provide a hybrid molecule that would maintain, or possibly improve, the binding of either molecule individually. Toward this objective, we envisaged a hybrid molecule based on a N-[arylsulfonyl]-1H-pyrrole-2-carboxamide scaffold represented by structure (I) (Figure 3). We performed docking studies using AutoDock Vina 1.1.2 (La Jolla, CA, USA) [12,13], with the hybrid analogs retaining the critical interactions of E7820 and tasisulam with DCAF15. The docking pose of a representative hybrid analog 7 illustrates its optimal fit within the DCAF15 binding pocket (Figure 2D). Thus, the pyrrole NH of the hybrid engages with Phe231 via a hydrogen bond and the common sulfonamide linker group interacts with Phe235 and Ala234, while the amide carbonyl forms a hydrogen bond with Glu232. This design strategy would combine the critical interactions of both SPLAMs with DCAF15 in structure (I) and offer the option to generate hybrid analogs (I) (Figure 2D and Figure 3).
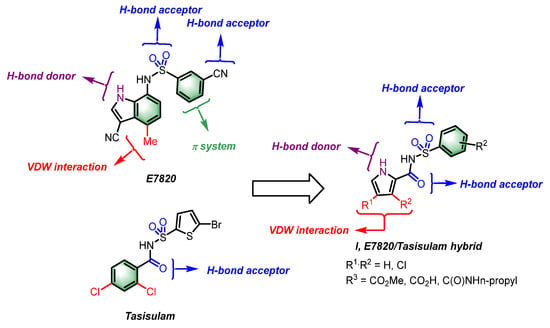
Figure 3.
Structural comparison of E7820, tasisulam, and proposed hybrid analogs (I), highlighting key interactions with DCAF15.
The N-[arylsulfonyl]-1H-pyrrole-2-carboxamide motif has been used in a variety of applications in medicinal chemistry and agrochemistry, as illustrated in Figure 4 [14,15,16,17,18,19]. It has been incorporated into compounds targeting a variety of biological pathways, including metabolic disorders such as uric acid-lowering agents [14] and GPR119 agonists [17], respiratory diseases through CFTR modulation [16], and bacterial or lipid biosynthesis inhibition via EVOLV6 [16] and UPPS inhibitors [19]. Its use in herbicidal agents [18] further underscores the versatility of this structural motif beyond pharmaceutical applications. The broad range of substitution patterns tolerated on both the pyrrole and arylsulfonyl moieties further supports its value as a modular platform for bioactive molecule design.
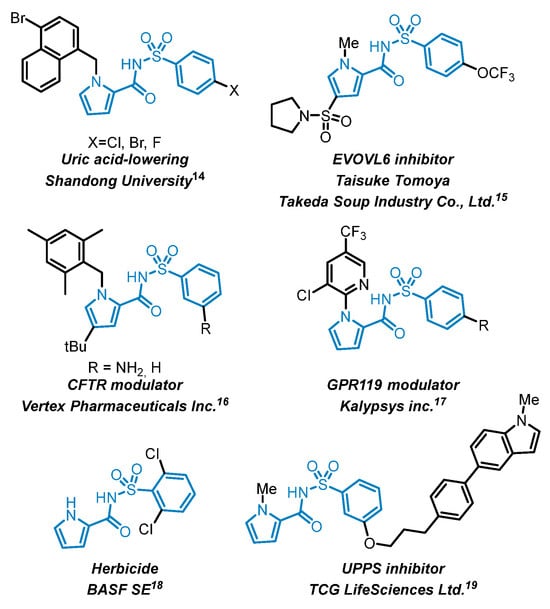
Figure 4.
Selected examples of N-[arylsulfonyl]-1H-pyrrole-2-carboxamides [14,15,16,17,18,19].
However, to the best of our knowledge, this combination of a pyrrole linked to an aryl moiety by a carboxamidyl N-sulfonyl spacer as a hybrid between E7820 and tasisulam has not been used in the context of binders to DCAF15 (Figure 1). Furthermore, to study the effect of chlorine substitutions, and to closely simulate the electronic properties of the 3,5-dichloroaryl moiety in tasisulam, we proceeded with analogs containing one, two, or no chlorine atoms in the pyrrole ring, while varying the substituents on the arylsulfonamide portion.
2. Chemical Synthesis
The synthesis of N-[arylsulfonyl]-1H-pyrrole-2-carboxamide derivatives was carried out in three steps as depicted in Scheme 1 for the 3,4-dichloro pyrrole analogs. In the first step, a coupling reaction between 3,4-dichloropyrrole-2-carboxylic acid derivatives 1 [20] and various arylsulfonamides [21,22] was performed using EDCI and DMAP in the presence of triethylamine in dichloromethane [23]. This reaction afforded the corresponding N-arylsulfonyl 3,4-dichloro pyrrole-2-carboxamides (2–5) in moderate yields (23–46%). It was observed that the electronic nature of the arylsulfonamide substituent had minimal impact on the efficiency of the reaction; however, chlorination of the pyrrole moiety appeared to reduce the yields (Scheme 1 and Scheme S1 in Supporting Information).
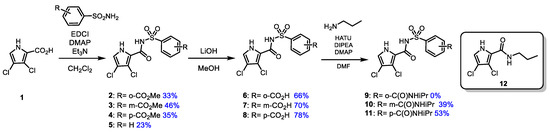
Scheme 1.
Synthesis of N-[arylsulfonyl]-1H-3,4-dichloropyrrole-2-carboxamides 2–5, 6–7, and 11.
In the second step, hydrolysis of the methyl esters (2–5) was carried out using LiOH in methanol to afford the corresponding 3,4-dichloro carboxylic acids (6–8) in moderate to good yields (66–78%). Amide formation of the carboxylic acids (6–8) with isopropylamine was achieved using HATU and DIPEA in DMF to yield the final N-arylsulfonylpyrrole-2-carboxamides 10–11 in moderate yields (39–53%). The same protocol was adopted for the synthesis of the corresponding N-[arylsulfonyl]-1H-pyrrole-2-carboxamide derivatives and the 3-monochloro pyrrole congeners (Scheme S1 in Supporting Information). However, this step was unsuccessful for the ortho-substituted analog 9, resulting in an attack of isopropylamine on the carboxamide moiety and the formation of 3,4-dichloro-N-propyl-1H-pyrrole-2-carboxamide (12).
3. Results and Discussion
With the hybrid analogs in hand, we performed preliminary binding assays using fluorescence polarization with a fluorescent derivative of E7820 as a tracer [7] (see Supporting Information). All compounds were also tested in vitro against HCT116 colon carcinoma cell lines. The binding activity of representative analogs is shown in Table 1 and plotted in Figure 4.

Table 1.
Binding affinity of hybrid analogs.
In general, a clear SAR was not observed for the nonchlorinated pyrroles (Table 1, entries 1–9). However, binding activity was improved when chlorine atoms were present in the pyrrole moiety. For example, in the simplest case where the phenyl group is not substituted, the nonchlorinated pyrrole analog (S7, IC50 = 93 µM) was less active than the monochlorinated pyrrole (S11, IC50 = 24 µM), which was itself less active than the 3,4-dichlorinated pyrrole (5, IC50 = 4 µM) (entries 1, 11, and 14, Table 1). Substitution on the phenyl group was tolerated regardless of the position or nature in the case of 3,4-dichloro pyrroles (Table 1, entries 14–21). Thus, the pyrrole part of the hybrid molecule appears to be more important in binding affinity toward DCAF15 than the phenyl part. This is also evident from the affinity plot, where three distinct clusters are observed corresponding to the type of functionalization on the pyrrole moiety (Figure 5).
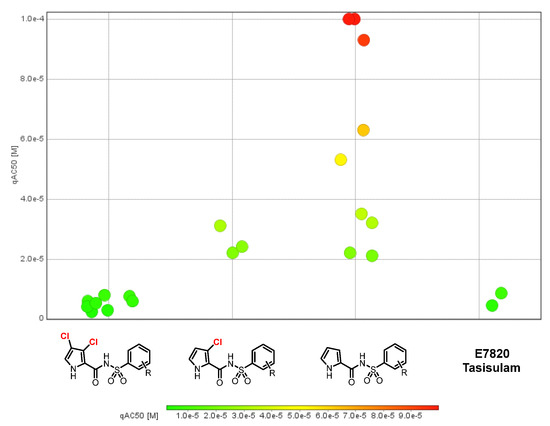
Figure 5.
Activity plot of compounds by category.
Considering this interesting trend, we performed molecular modeling studies comparing the docking poses with the DCAF15 of three N-[arylsulfonyl]-1H-pyrrole-2-carboxamide analogs: S7 (nonchlorinated pyrrole), S11 (4-chloro pyrrole), and 5 (3,4-dichloro pyrrole), shown in Figure 6A–C. The nonchlorinated analog, S7, does not fit well within the DCAF15 pocket. Although the sulfonyl part creates interactions with Ala234 and Phe235, the pyrrole NH does not form a hydrogen bond with Phe231, and its orientation is not optimal (Figure 6A). In contrast, the 4-chloro analog S11 provides better alignment, allowing the pyrrole NH to form a hydrogen bond with Phe231 while still interacting with other key residues like Ala234 and Phe235 and having a secondary interaction with the residue Gln232 (Figure 6B). Unlike the 3-chloro pyrrole and the unsubstituted pyrrole analogs, 3,4-dichloro pyrrole analog 5 is fully engaged within the binding site with interactions involving the 4-chlorine atom, the pyrrole core itself, and the pyrrole NH (Figure 6C). Compound 5 forms a well-organized hydrogen bond network involving the pyrrole NH (Phe231), the sulfonamide oxygens (Ala234, Phe235), and the amide carbonyl (Gln232). This pattern of interactions is similar to E7820 and tasisulam and aligns with the higher binding affinity (IC50 = 4 µM) observed, highlighting how chlorine substitution improves binding through better positioning and enhanced hydrophobic contacts.
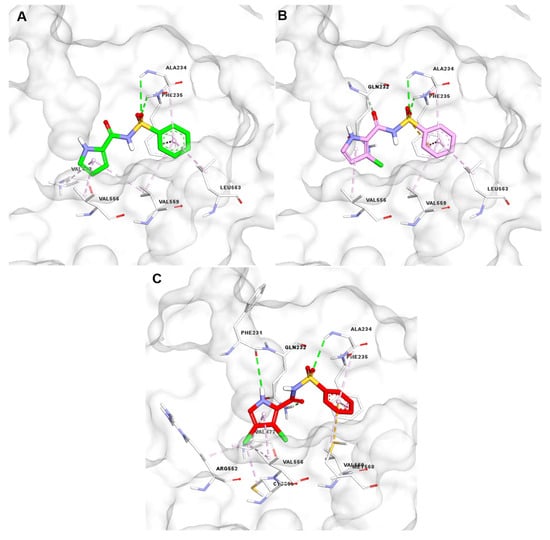
Figure 6.
(A) Docking pose of nonchlorinated analog S7 inside DCAF15. (B) Docking pose of 3-chlorinated analog S11 inside DCAF15. (C) Docking pose of 3,4-chlorinated analog 5 inside DCAF15. Interaction color code: hydrogen bonds (green), π–π stacking (violet), halogen or hydrophobic contacts (light pink/yellow), charge interaction (orange).
Unlike classical enzyme active sites, where inhibitors can be rationally designed to form stable tetrahedral adducts in the case of protease inhibitors [24], the development of DCAF15 binders relies primarily on optimizing non-covalent interactions within a relatively shallow binding pocket. This highlights the challenge of designing effective small molecules that can interfere with protein–protein interactions [25,26].
In previous reports, DCAF15 binders have also been tested for anticancer activity against colon carcinoma cell lines HCT116, although the activity was not necessarily associated with DCAF15 [9]. Only weak in vitro inhibition was observed against colon carcinoma cell lines HCT116 for analogs 7 and 13 (IC50 = 20 µM and 46 µM, respectively) and tasisulam (IC50 = 24 µM). On the other hand, a possible correlation between binding (AC50 = 10 µM) and HCT116 inhibition (IC50 = 0.7 µM) may exist in the case of E7820. However, the colon carcinoma cell line HCT116 activities cannot be attributed to DCAF15 binding activity without further experimentation.
4. Conclusions
In this study, the concept of synthesizing hybrid molecules based on E7820 and tasisulam, two known low-µM binders to DCAF15, was applied relying on molecular modeling. Our results indicate that, in general, regardless of the substitution on the N-aryl sulfonyl moiety in N-[arylsulfonyl]-1H-pyrrole-2-carboxamide analogs, the 3,4-dichloro pyrrole analogs are better binders to DCAF15 compared with the 3-monochloro or the nonchlorinated pyrrole counterparts. The SAR data appear to be more consistent with little variation depending on the position and the nature of the substituent on the N-[arylsulfonyl]-1H-pyrrole-2-carboxamide derivatives within the series of o-, m- or p-substituents. It is possible that the introduction of chlorine atoms in the pyrrole ring confers stability via hydrophobic interactions. Although a slight improvement in binding affinity was observed over E7820 and equaled that of tasisulam, the new series of DCAF15 binders reported herein further demonstrate the importance of chlorine substitution in the design of bioactive molecules [27,28].
5. Materials and Methods
5.1. DCAF15 Target Engagement—Polarized Fluorescence
A total of 100 nL of each tested compound was dispensed in an 11 dose-response in a 384-well plate (Perkin Elmer, Waltham, MA, USA), targeting a maximum final concentration of 100 µM in DMSO (semi-log dilution step). A solution with a 12.5 nM final concentration of the tracer was prepared, as well as a solution with a 5 nM final concentration of DCAF15 (provided by NOVALIX, Lyon, France) [7]. To start the experiment, we dispensed 4 µL of a freshly prepared buffer (50 mM HEPES, 200 mM NaCl, 4% DMSO, and 0.01% BSA, pH 7.5), 3 µL of the DCAF15 solution, and 3 µL of the tracer solution. The plate was centrifuged for 2 min at 900 rpm and then incubated at room temperature for 10 min. The fluorescence polarization (FP 635-20/680-20 module) was read in a PheraSTAR plate reader (BMG Labtech, Offenburg, Germany) as the total fluorescence intensity (FI 640/680 module).
5.2. General Information
Physical data and spectroscopic measurements NMR spectra were recorded on a Bruker 300 spectrometer (75 MHz for 13C), a Bruker AVANCETM 400 RG spectrometer (400 MHz for 1 H), and a Bruker AVANCETM 500 Ultrashield Plus spectrometer (500 MHz for 1 H, 126 MHz for 13C and 471 MHz for 19F) in chloroform-d, dmdo-d6, or methanol-d4. Spectra were taken in the indicated solvent at ambient temperature. Chemical shifts (δ) are given in parts per million (ppm) with tetramethylsilane as an internal standard. Multiplicities are recorded as follows: s = singlet, d = doublet, t = triplet, q = quartet, br = broad. Coupling constants (J values) are given in Hz. Structural assignments were performed with additional information from gCOSY, gHSQC, and gHMBC experiments. Accurate mass measurements were performed on an LC-TOF instrument from Agilent Technologies in positive electrospray mode. Protonated molecular ions (M + H)+ and/or sodium adducts (M + Na)+ were used for empirical formula confirmation. Melting points were determined on a Büchi B-540 melting point apparatus. Crystal measurements were performed on a Bruker Venture Metaljet diffractometer. Thin-layer chromatography (TLC) was performed on pre-coated Silicycle silica gel (250 µM, 60 Å) plates with F-254 indicator. Visualization was performed with a UV light (254 nm) or with stains (KMnO4, p-anisaldehyde or ninhydrin). ZEO prep 60 (0.040–0.063 mm) silica gel was used for all column chromatography analyses.
All reactions involving air- or moisture-sensitive compounds were performed under a nitrogen atmosphere in flame-dried glassware. Other solvents used for reactions were purified according to standard procedures. Starting reagents were purchased from commercial suppliers and used without further purification unless otherwise specified.
5.3. Experimental Procedure
- General procedure A: sulfamoylamide formation
To a solution of the pyrrole (1.41 mmol, 1.0 equiv.) in CH2Cl2 (14.5 mL), we added EDC·HCl (2.82 mmol, 2.0 equiv.), DMAP (4.23 mmol, 3.0 equiv.), triethylamine (4.23 mmol, 3.0 equiv.), and arylsulfonamide (1.41 mmol, 1.0 equiv.). The mixture was stirred for 48 h at room temperature. Progress was monitored by TLC (hexane/ethyl acetate, 7:3, with 5% AcOH; visualized with p-anisaldehyde). The reaction mixture was diluted with dichloromethane, and 1 M aqueous HCl was added to reach an acidic pH. The aqueous phase was extracted twice with dichloromethane (2 × 10 mL). The combined organic layers were dried over Na2SO4, filtered, and concentrated under reduced pressure. The crude product was purified by flash chromatography (hexane/ethyl acetate, 9:1, with 5% AcOH). Excess AcOH was removed by coevaporation with benzene. Final purification was achieved by reverse-phase C18 column chromatography (gradient: 0–30% ACN in water), and the compound was dissolved in DMSO for injection. The desired sulfamoylamide was obtained as a white powder (23–86% yield).
- General procedure B: saponification
To a solution of methyl ester (345 µmol, 1.0 equiv.) in MeOH (821 µL), we added 2 M aqueous LiOH (1.72 mL, 3.45 mmol, 10.0 equiv.) under stirring. A clear solution formed and was stirred for 16 h at room temperature. The reaction was monitored by TLC (hexane/ethyl acetate, 6:4, with 5% AcOH; visualized with p-anisaldehyde). Methanol was removed under reduced pressure. The residue was diluted with dichloromethane and acidified with 3 M aqueous HCl to an acidic pH. The aqueous layer was extracted twice with dichloromethane (2 × 5 mL). The combined organic layers were dried over Na2SO4, filtered, and concentrated. The product was purified by flash chromatography using a gradient of hexane/ethyl acetate (9:1 to 7:3) with 5% AcOH. Excess AcOH was removed by repeated coevaporation with benzene. The resulting carboxylic acid was isolated as a white powder (31–96% yield).
- General procedure C: Amide formation
To a solution of pyrrole benzoic acid (119 µmol, 1.0 equiv.) in DMF (4.15 mL), we added HATU (155 µmol, 1.3 equiv.), DMAP (24 µmol, 0.2 equiv.), and DIPEA (262 µmol, 2.2 equiv.) sequentially. Upon activation of the ester, the reaction mixture turned yellow, and propylamine (0.238 mmol, 2.0 equiv.) was added. The mixture was stirred for 24 h at room temperature, and progress was monitored by TLC (hexane/ethyl acetate, 5:5, with 0.3% AcOH; visualized with p-anisaldehyde). The mixture was diluted with ethyl acetate (15 mL) and transferred to a separatory funnel, where it was washed successively with 3 M aqueous HCl (4 × 20 mL) and saturated aqueous NaCl (2 × 10 mL). The organic layer was dried over MgSO4, filtered, and concentrated under reduced pressure. The crude product, a viscous oil, was purified by reverse-phase chromatography (0–30% acetonitrile in water). The desired propylamide was obtained as a white powder (33–53% yield).
Methyl 2-(N-(3,4-dichloro-1H-pyrrole-2-carbonyl)sulfamoyl)benzoate (2): Compound 2 was prepared following general procedure A from pyrrole 1 (254 mg, 1.41 mmol) and obtained as a white powder (175 mg, 33%). 1H NMR (700 MHz, acetone-d6) δ 11.55 (s, 1H), 8.35–8.30 (m, 1H), 7.90–7.81 (m, 3H), 7.35 (s, 1H), 3.96 (s, 3H); 13C NMR (176 MHz, acetone-d6) δ 167.28, 155.64, 137.29, 134.02, 131.84, 131.64, 131.25, 130.26, 124.75, 121.96, 114.08, 112.61, 52.84; HRMS (ESI) m/z: [M + H]+ calcd for C13H11Cl2N2O5S: 376.97602, found: 376.97666.
Methyl 3-(N-(3,4-dichloro-1H-pyrrole-2-carbonyl)sulfamoyl)benzoate (3): Compound 3 was prepared following general procedure A from pyrrole 1 (254 mg, 1.41 mmol) and obtained as a white powder (245 mg, 46%). 1H NMR (400 MHz, acetone-d6) δ 11.15 (s, 1H), 8.62 (t, J = 1.8 Hz, 1H), 8.27 (d, J = 7.9, 1.5 Hz, 1H), 8.13 (d, J = 7.8 Hz, 1H), 7.62 (t, J = 7.8 Hz, 1H), 7.04 (s, 1H), 3.89 (s, 3H); 13C NMR (101 MHz, acetone-d6) δ 165.40, 143.97, 132.36, 131.47, 130.54, 128.88, 127.97, 122.93, 118.59, 113.85, 111.94, 51.83; HRMS (ESI) m/z: [M + H]+ calcd for C13H11Cl2N2O5S: 376.97602, found: 376.97652.
Methyl 4-(N-(3,4-dichloro-1H-pyrrole-2-carbonyl)sulfamoyl)benzoate (4): Compound 4 was prepared following general procedure A from pyrrole 1 (254 mg, 1.41 mmol) and obtained as a white powder (186 mg, 35%). 1H NMR (400 MHz, acetone-d6) δ 11.44 (s, 1H), 8.23–8.14 (m, 4H), 7.27 (s, 1H), 3.90 (s, 3H); 13C NMR (101 MHz, acetone-d6) δ 165.12, 155.93, 143.45, 134.76, 129.74, 128.67, 121.69, 119.44, 114.75, 112.63; HRMS (ESI) m/z: [M + H]+ calcd for C13H11Cl2N2O5S: 376.97602, found: 376.97625.
3,4-Dichloro-N-(phenylsulfonyl)-1H-pyrrole-2-carboxamide (5): Compound 5 was prepared following general procedure A from pyrrole 1 (42 mg, 233 μmol) and obtained as a white powder (15 mg, 20%). 1H NMR (700 MHz, acetone-d6) δ 11.27 (s, 1H), 8.07 (d, J = 7.7 Hz, 2H), 7.58 (d, J = 54.8 Hz, 3H), 7.15 (s, 1H); 13C NMR (176 MHz, acetone-d6) δ 158.97, 141.74, 132.64, 128.56, 127.60, 121.55, 119.72, 113.94, 112.11; HRMS (ESI) m/z: [M + H]+ calcd for C11H9Cl2N2O3S: 340.95249, found: 340.95240.
2-(N-(3,4-Dichloro-1H-pyrrole-2-carbonyl)sulfamoyl)benzoic acid (6): Compound 6 was prepared following general procedure B from methylester 2 (65 mg, 172 μmol) and obtained as a white powder (41 mg, 66%). 1H NMR (700 MHz, acetone-d6) δ 11.54 (s, 1H), 8.32 (d, J = 7.4, 1.8 Hz, 1H), 7.96 (dd, J = 7.1, 1.8 Hz, 1H), 7.89–7.82 (m, 2H), 7.34 (s, 1H);13C NMR (176 MHz, acetone-d6) δ 167.83, 155.82, 137.50, 133.96, 132.37, 131.57, 131.22, 130.56, 128.26, 121.89, 113.97, 112.56; HRMS (ESI) m/z: [M + H]+ calcd for C12H9Cl2N2O5S: 362.96037, found: 362.96073.
3-(N-(3,4-Dichloro-1H-pyrrole-2-carbonyl)sulfamoyl)benzoic acid (7): Compound 7 was prepared following general procedure B from methylester 3 (50 mg, 133 μmol) and obtained as a white powder (34 mg, 70%). 1H NMR (700 MHz, acetone-d6) δ 11.48 (s, 1H), 8.73 (s, 1H), 8.35 (dd, J = 10.9, 7.9, 1.5 Hz, 4H), 7.81 (t, J = 7.8 Hz, 2H), 7.32 (s, 2H); 13C NMR (176 MHz, acetone-d6) δ 165.24, 156.00, 140.29, 134.51, 132.62, 131.46, 129.53, 129.44, 121.66, 119.50, 114.66, 112.58; HRMS (ESI) m/z: [M + H]+ calcd for C12H9Cl2N2O5S: 362.96037, found: 362.96029.
4-(N-(3,4-Dichloro-1H-pyrrole-2-carbonyl)sulfamoyl)benzoic acid (8): Compound 8 was prepared following general procedure B from methylester 4 (65 mg, 172 μmol) and obtained as a white powder (49 mg, 78%). 1H NMR (400 MHz, acetone-d6) δ 11.48 (s, 1H), 8.26–8.22 (m, 4H), 7.31 (s, 1H), 13C NMR (101 MHz, acetone-d6) δ 165.42, 155.98, 143.43, 135.18, 129.97, 128.61, 121.62, 119.52, 114.69, 112.59, HRMS (ESI) m/z: [M + H]+ calcd for C12H9Cl2N2O5S: 362.96037, found: 362.96092.
3,4-Dichloro-N-((3-(propylcarbamoyl)phenyl)sulfonyl)-1H-pyrrole-2-carboxamide (10): Compound 10 was prepared following general procedure C from carboxylic acid 7 (82 mg, 226 μmol) and obtained as a white powder (36 mg, 39%). 1H NMR (700 MHz, Acetone) δ 11.43 (s, 1H), 8.54 (s, 1H), 8.21 (d, J = 7.9, 1.4 Hz, 1H), 8.17 (d, J = 7.7 Hz, 1H), 8.09 (s, 1H), 7.70 (t, J = 7.8 Hz, 1H), 3.38 (q, 2H), 1.63 (h, J = 7.3 Hz, 2H), 0.95 (t, J = 7.4 Hz, 3H); 13C NMR (176 MHz, Acetone) δ 165.87, 157.90, 141.80, 136.87, 136.84, 132.67, 131.40, 129.86, 127.90, 121.76, 121.20, 115.26, 113.32, 42.44, 23.53, 23.51, 11.83; HRMS (ESI) m/z: [M + H]+ calcd for C15H16Cl2N3O4S: 404.02331, found: 404.02363.
3,4-Dichloro-N-((4-(propylcarbamoyl)phenyl)sulfonyl)-1H-pyrrole-2-carboxamide (11): Compound 11 was prepared following general procedure C from carboxylic acid 8 (52 mg, 143 μmol) and obtained as a white powder (31 mg, 53%). 1H NMR (700 MHz, acetone-d6) δ 11.19 (s, 1H), 8.02 (d, J = 8.1 Hz, 2H), 7.94–7.88 (m, 3H), 7.07 (s, 1H), 3.35–3.29 (m, 2H), 1.58 (h, J = 7.3 Hz, 2H), 0.90 (t, J = 7.4 Hz, 3H); 13C NMR (176 MHz, acetone-d6) δ 165.63, 127.33, 114.13, 112.11, 41.48, 22.55, 22.54, 10.87; HRMS (ESI) m/z: [M + H]+ calcd for C15H16Cl2N3O4S: 404.02331, found: 404.02363.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/reactions6020034/s1, Scheme S1. Synthesis of N-[arylsulfonyl]-1H-pyrrole-2-carboxamide derivatives and the 3-monochloro congeners S4–S20. Scheme S2. Synthesis of DCAF15 tracer S28. Table S1. Binding affinity of all analogs. Table S2. Cellular activity of analogs on colon carcinoma cell lines HCT116. Figure S1. Superposition of crystal structure of E7820 and docked pose obtained for E7820 (yellow) in DCAF15 using AutodockVina. Figure S2. A. X-ray visualization of E7820 inside DCAF15. B. E7820 interactions with proximal residues inside DCAF15. Figure S3. A. Docking pose of analog 7 inside DCAF15. B. Interactions of analog 7 with proximal residues inside DCAF15. Figure S4. A. Docking pose of nonchlorinated analog S7 inside DCAF15. B. Interactions of S7 with proximal residues inside DCAF15. Figure S5. A. Docking pose of monochlorinated analog S11 inside DCAF15. B. Interactions of S11 with proximal residues inside DCAF15. Figure S6. A. Docking pose of bichlorinated analog 4 inside DCAF15. B. Interactions of analog 4 with proximal residues inside DCAF15. Figure S7. Superposition of the docking pose for nonchlorinated analog S7 (green), monochlorinated analog S11 (pink), and bichlorinated analog 4 (red) inside DCAF15.
Author Contributions
Chemical synthesis of analogs, V.C. and R.C.; chemical synthesis of tracer, T.B.; docking study, S.H. (Sofiane Hocine); binding and cellular assays, J.A.B., S.T., L.C., L.K., and S.C.; conceptualization, S.H. (Sofiane Hocine); writing—original draft preparation, S.H. (Sofiane Hocine) and S.H. (Stephen Hanessian); writing—review and editing, S.H. (Sofiane Hocine) and S.H. (Stephen Hanessian); supervision, S.H. (Sofiane Hocine) and S.H. (Stephen Hanessian); project administration, A.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by NSERC/Servier Senior Industrial Research Chair program under grant number “IRCPJ 531309-17”.
Data Availability Statement
No data were used for the research described in this article.
Acknowledgments
We thank NSERC for financial assistance. The UdeM group acknowledges generous financial support from Servier in the context of the NSERC/Servier Senior Industrial Research Chair program. We also thank Isabelle Theret for her valuable advice and feedback on the docking studies.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Han, T.; Goralski, M.; Gaskill, N.; Capota, E.; Kim, J.; Ting, T.C.; Xie, Y.; Williams, N.S.; Nijhawan, D. Anticancer Sulfonamides Target Splicing by Inducing RBM39 Degradation via Recruitment to DCAF15. Science 2017, 356, eaal3755. [Google Scholar] [CrossRef] [PubMed]
- Bussiere, D.E.; Xie, L.; Srinivas, H.; Shu, W.; Burke, A.; Be, C.; Zhao, J.; Godbole, A.; King, D.; Karki, R.G.; et al. Structural Basis of Indisulam-Mediated RBM39 Recruitment to DCAF15 E3 Ligase Complex. Nat. Chem. Biol. 2020, 16, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Konstantinidou, M.; Arkin, M.R. Molecular Glues for Protein-Protein Interactions: Progressing toward a New Dream. Cell Chem. Biol. 2024, 31, 1064–1088. [Google Scholar] [CrossRef]
- Ishida, T.; Ciulli, A. E3 Ligase Ligands for PROTACs: How They Were Found and How to Discover New Ones. SLAS Discov. 2021, 26, 484–502. [Google Scholar] [CrossRef] [PubMed]
- Békés, M.; Langley, D.R.; Crews, C.M. PROTAC Targeted Protein Degraders: The Past Is Prologue. Nat. Rev. Drug Discov. 2022, 21, 181–200. [Google Scholar] [CrossRef]
- Holdgate, G.A.; Bardelle, C.; Berry, S.K.; Lanne, A.; Cuomo, M.E. Screening for Molecular Glues—Challenges and Opportunities. SLAS Discov. 2024, 29, 100136. [Google Scholar] [CrossRef]
- Du, X.; Volkov, O.A.; Czerwinski, R.M.; Tan, H.L.; Huerta, C.; Morton, E.R.; Rizzi, J.P.; Wehn, P.M.; Xu, R.; Nijhawan, D.; et al. Structural Basis and Kinetic Pathway of RBM39 Recruitment to DCAF15 by a Sulfonamide Molecular Glue E7820. Structure 2019, 27, 1625–1633.e3. [Google Scholar] [CrossRef]
- Lucas, S.C.C.; Ahmed, A.; Ashraf, S.N.; Argyrou, A.; Bauer, M.R.; De Donatis, G.M.; Demanze, S.; Eisele, F.; Fusani, L.; Hock, A.; et al. Optimization of Potent Ligands for the E3 Ligase DCAF15 and Evaluation of Their Use in Heterobifunctional Degraders. J. Med. Chem. 2024, 67, 5538–5566. [Google Scholar] [CrossRef]
- Devarajappa, R.; Kiyeleko, S.; Hocine, S.; Cosson, V.; Calandrino, R.; Baló, T.; Bordelo, J.A.; Triboulet, S.; Caruana, L.; Klipfel, L.; et al. Design and Synthesis of 7-(N-Aryl Pyrrolidinyl) Indoles as Potential DCAF15 Binders. Reactions 2025, 6, 20. [Google Scholar] [CrossRef]
- Faust, T.B.; Yoon, H.; Nowak, R.P.; Donovan, K.A.; Li, Z.; Cai, Q.; Eleuteri, N.A.; Zhang, T.; Gray, N.S.; Fischer, E.S. Structural Complementarity Facilitates E7820-Mediated Degradation of RBM39 by DCAF15. Nat. Chem. Biol. 2019, 16, 7–14. [Google Scholar] [CrossRef]
- Coomar, S.; Gillingham, D.G. Exploring DCAF15 for Reprogrammable Targeted Protein Degradation. bioRxiv 2019, 542506. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Eberhardt, J.; Santos-Martins, D.; Tillack, A.F.; Forli, S. AutoDock Vina 1.2.0: New Docking Methods, Expanded Force Field, and Python Bindings. J. Chem. Inf. Model. 2021, 61, 3891–3898. [Google Scholar] [CrossRef] [PubMed]
- Zhan, P.; Wang, Z.; Liu, X.; Yi, F.; Zhang, Z.; Shi, X.; Qi, D.; Yang, M.; Yang, Q. A Pyrrole Derivative and Its Preparation Method and Application. CN118344277A, 16 July 2024. [Google Scholar]
- Tomoya, T.; Yukawa, T. Aromatic Ring Compound. WO2011059042A1, 19 May 2011. [Google Scholar]
- Miller, M.T.; Anderson, C.; Arumugam, V.; Bear, B.R.; Binch, H.M.; Clemens, J.J.; Cleveland, T.; Conroy, E.; Coon, T.R.; Frieman, B.A.; et al. Modulators of Cystic Fibrosis Transmembrane Conductance Regulator. US20160095858A1, 7 April 2016. [Google Scholar]
- Heistracher, E.; Fischer, K.; Mayer, H.; Saupe, T.; Hamprecht, G.; Ditrich, K.; Kuekenhoehner, T.; Gerber, M.; Walter, H.; Westphalen, K.-O. Sulfonamide. DE4029753A1, 20 September 1990. [Google Scholar]
- Smith, N.; Bonnefous, C.; Govek, S.P.; Wu, D.; Pinkerton, A.B.; Kahraman, M.; Cook, T.; Noble, S.A.; Borchardt, A.J.; Prins, T. Heterocyclic Modulators of Gpr119 for Treatment of Disease. WO2009117421A2, 17 March 2009. [Google Scholar]
- Murthi, K.K.; Joshi, B.P. Preparation of N-Acylsulfonamide Derivatives as UPPS Inhibitors. IN2015KO00769, 8 December 2015. [Google Scholar]
- Senge, M.O.; Flögel, O.; Ruhlandt-Senge, K. 2,3,7,8,12,13,17,18-Octachloroporphyrin. J. Porphyr. Phthalocyanines 2001, 5, 503–506. [Google Scholar] [CrossRef]
- Wang, M.; Fan, Q.; Jiang, X. Metal-Free Construction of Primary Sulfonamides through Three Diverse Salts. Green Chem. 2018, 20, 5469–5473. [Google Scholar] [CrossRef]
- Mishra, C.B.; Kumari, S.; Angeli, A.; Bua, S.; Buonanno, M.; Monti, S.M.; Tiwari, M.; Supuran, C.T. Discovery of Potent Anti-Convulsant Carbonic Anhydrase Inhibitors: Design, Synthesis. Eur. J. Med. Chem. 2018, 156, 430–443. [Google Scholar] [CrossRef] [PubMed]
- Pelz, N.F.; Bian, Z.; Zhao, B.; Shaw, S.; Tarr, J.C.; Belmar, J.; Gregg, C.; Camper, D.M.V.; Goodwin, C.M.; Arnold, A.L.; et al. Discovery of 2-Indole-Acylsulfonamide Myeloid Cell Leukemia 1 (Mcl-1) Inhibitors Using Fragment-Based Methods. J. Med. Chem. 2016, 59, 2054–2066. [Google Scholar] [CrossRef]
- Hedstrom, L. Serine Protease Mechanism and Specificity. Chem. Rev. 2002, 102, 4501–4523. [Google Scholar] [CrossRef]
- Teng, M.; Gray, N.S. The Rise of Degrader Drugs. Cell Chem. Biol. 2023, 30, 864–878. [Google Scholar] [CrossRef]
- Schreiber, S.L. The Rise of Molecular Glues. Cell 2021, 184, 3–9. [Google Scholar] [CrossRef]
- Fang, W.Y.; Ravindar, L.; Rakesh, K.P.; Manukumar, H.M.; Shantharam, C.S.; Alharbi, N.S.; Qin, H.L. Synthetic Approaches and Pharmaceutical Applications of Chloro-Containing Molecules for Drug Discovery: A Critical Review. Eur. J. Med. Chem. 2019, 173, 117–153. [Google Scholar] [CrossRef] [PubMed]
- Chiodi, D.; Ishihara, Y. “Magic Chloro”: Profound Effects of the Chlorine Atom in Drug Discovery. J. Med. Chem. 2023, 66, 5305–5331. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).