Abstract
Background/Objectives: The hepatitis C virus (HCV) is a significant threat to people with persistent infections. In recent years, the treatment of chronic hepatitis C (CHC) has been transformed significantly with the use of Direct-Acting Antivirals (DAAs). Hematological changes are seen in patients suffering from CHC due to both the disease and its therapy. This study aims to address the gaps in knowledge by thoroughly evaluating the hematological parameter alterations in CHC patients treated with Mavyret. Methods: This study reported that it took place over six months in 2024 on 101 patients observed at the gastroenterology department of Pitesti County Hospital in Romania, who had confirmed diagnoses of CHC and who were receiving the DAA Mavyret. Results: The values of leukocytes significantly decreased after treatment (8.69 ± 2.96 vs. 7.93 ± 1.78, p = 0.009). Similarly, neutrophils showed a statistically significant decrease after using Mavyret (5.45 ± 2.06 vs. 4.91 ± 1.29, p = 0.018). In the case of lymphocytes, the values slightly increased from 2.14 ± 0.69 before treatment to 2.20 ± 1.19 after it, but without a statistically significant difference (p = 0.320). The values of monocytes and eosinophils significantly decreased after antiviral therapy (0.81 ± 1.02 vs. 0.59 ± 0.15, p = 0.020; 0.21 ± 0.15 vs. 0.14 ± 0.09, p < 0.001). For basophils, the mean values remained almost unchanged after DAA therapy (0.09 ± 0.08 vs. 0.09 ± 0.15, p = 0.433). Conclusions: Mavyret has proven to be a safe medication for administering to patients with CHC, but its minor adverse effects on red blood cells, white blood cells, and platelets require constant monitoring, mainly when used for an extended period and in patients who are vulnerable in this regard.
1. Introduction
Chronic hepatitis C (CHC) is a major world health problem; this virus infects between 2 and 3 million people in the USA and 170 to 200 million globally [1]. In 2022, countries in the European region reported a hepatitis C virus (HCV) infection rate of 1333 per 100,000 inhabitants, with Romania registering 6.5 cases per 100,000 people [2,3].
The HCV, with primary risk factors being in blood, is a major threat to people with persistent infections [4]. If untreated, the HCV has the potential to develop severe liver diseases, including fibrosis, cirrhosis, and hepatocellular carcinoma (HCC), and, thus, it has significant morbidity and mortality [1]. The burden of CHC extends beyond health, as it strains healthcare systems and imposes economic challenges due to the long-term management of liver diseases and associated complications.
In recent years, the treatment of CHC has been transformed significantly with the use of Direct-Acting Antivirals (DAAs) [5]. Compared with conventional therapy, DAAs focus on viral proteins crucial to HCV replication and are more effective with shorter treatment courses [6]. Of the mentioned drugs, Mavyret has caught the most attention, as it is a fixed-dose combination of glecaprevir (protease inhibitor targeting the NS3/4A protein) and pibrentasvir (inhibitor of the NS5A protein), effective against all the key HCV genotypes [6]. Mavyret has cure rates above 95%, a short treatment duration of 8 to 12 weeks, and a favorable safety profile, making it a potential treatment regime for many patients, especially those with the more challenging HCV genotypes [6]. Since Mavyret is effective in clinical trials, much is still unknown regarding its physiological effects beyond the treatment of the HCV, particularly on hematological parameters [7].
Hematological changes are seen in patients suffering from CHC due to both the disease and its therapy. The liver is involved in the processes of extramedullary hematopoiesis [8]. Thrombocytopenia, which is a frequent occurrence, may stem from splenic pooling or thrombopoietin reduction, while anemia could be attributed to chronic inflammation or bone marrow suppression [8]. DAAs can also exacerbate these blood count abnormalities by inducing cytopenias, hence the need for the frequent monitoring of hematological parameters during treatment [9].
Monitoring these blood parameters is crucial for identifying drug-induced cytopenia, which may have implications for the patient’s response and the safety of antiviral therapies [9]. Hemoglobin counts, white blood cell counts (WBCs), and platelet counts (PLTs) can decline due to adverse drug reactions and may also predict the patient’s recovery from HCV-related disease [9]. However, further research exploring how DAAs, including Mavyret, affect hematological status in patients with hepatitis C virus infection has been lacking [7].
Despite the increasing use of Mavyret, prior studies designed to assess the drug’s impact have primarily centered on broader endpoints with a shortage of concentration on hematologic manifestations [7]. Clinical studies have reported the overall side effects to be mild, and few hematological severe adverse effects have been observed with Mavyret [7]. Nevertheless, empirical evidence about how this DAA affects the blood parameters in various groups of patients is limited [10]. This shortcoming is significant because there is no thorough, in-depth study to ensure that patients benefit from the best and safest care during their treatment [10].
This study aims to address the gaps in knowledge by thoroughly evaluating the hematological parameter alterations in CHC patients treated with Mavyret. Specifically, we will assess changes in blood components, including red blood cell counts (RBCs), WBCs, platelet counts, and other critical blood parameters before and after treatment. By systematically analyzing these alterations, we hope to provide deeper insights into the broader physiological effects of Mavyret, thereby contributing to improved management strategies for chronic hepatitis C patients.
2. Results
The current study initially selected 150 patients. Of these, 39 were excluded, 10 were lost during this study, and 101 patients completed this study. Table 1 presents the study group’s clinical and demographic characteristics.

Table 1.
The clinical and demographic characteristics of the study group.
The values of leukocytes significantly decreased after treatment (8.69 ± 2.96 vs. 7.93 ± 1.78, p = 0.009). Similarly, neutrophils showed a statistically significant decrease after using Mavyret (5.45 ± 2.06 vs. 4.91 ± 1.29, p = 0.018). In the case of lymphocytes, the values slightly increased from 2.14 ± 0.69 before treatment to 2.20 ± 1.19 after it, but without a statistically significant difference (p = 0.320). The values of monocytes and eosinophils significantly decreased after antiviral therapy (0.81 ± 1.02 vs. 0.59 ± 0.15, p = 0.020; 0.21 ± 0.15 vs. 0.14 ± 0.09, p < 0.001). For basophils, the mean values remained almost unchanged after DAA therapy (0.09 ± 0.08 vs. 0.09 ± 0.15, p = 0.433), as shown in Figure 1.
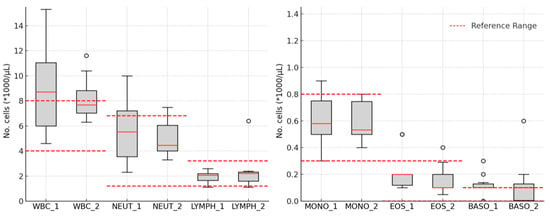
Figure 1.
The leukocytes’ variations: white blood cell count—WBC; neutrophil count—Neut; lymphocyte count—Lymph; monocyte count—Mono; eosinophil count—Eos; basophil count—Baso; 1—in the morning on the day of starting DAA therapy; 2—24 h after the completion of DAA therapy.
The erythrocyte parameters significantly decreased after the treatment period (RBC: 4.57 ± 0.62 vs. 4.32 ± 0.71, p < 0.001; HGB: 17.77 ± 18.30 vs. 16.16 ± 14.96, p < 0.001; HCT: 39.88 ± 6.16 vs. 37.46 ± 5.39, p < 0.001), as shown in Figure 2.
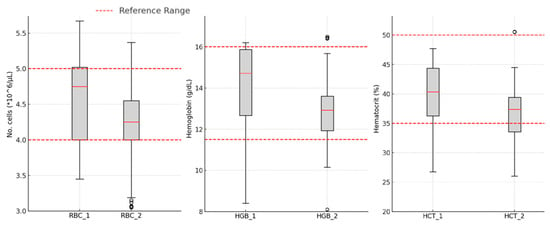
Figure 2.
Variations in erythrocyte line parameters: red blood cell count—RBC; hemoglobin concentration—HGB; hematocrit percentage—HCT; 1—in the morning on the day of starting DAA therapy; 2—24 h after the completion of DAA therapy.
The erythrocyte parameters MCH and MCHC significantly decreased after antiviral treatment, as shown in Table 2.

Table 2.
The erythrocyte parameters.
The platelet count values slightly decreased without statistical significance after completing antiviral therapy (260,901.3 ± 99,072.11 vs. 257,206.2 ± 62,772.92, p = 0.343), as shown in Figure 3.
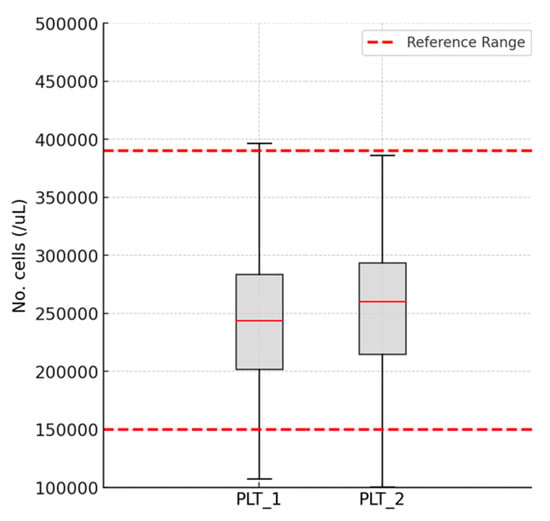
Figure 3.
Variations in the platelet count: platelet count—PLT; 1—in the morning on the day of starting DAA therapy; 2—24 h after the completion of DAA therapy.
3. Materials and Methods
3.1. Study Design
This study took place over six months, from 1 January to 30 June 2024, focusing on patients observed at the gastroenterology department of Pitesti County Hospital in Romania. The population consisted of treatment-naive individuals with CHC.
Informed consent to participate in this study was obtained from all participants. The research was approved by the Ethics Committee of Pitesti County Hospital, Romania, under Opinion Number 2600/21.04.2023. We can confirm that this study was conducted according to the ethical standards described in the 2000 Declaration of Helsinki and the applicable law concerning bioethics.
All study participants received the Direct-Acting Antiviral (DAA) with Mavyret (100 mg of glecaprevir plus 40 mg of pibrentasvir). According to the standard protocol for naive patients with CHC, the recommended dose was three tablets administered orally with food once daily for eight weeks. The inclusion and exclusion criteria were applied to the initial patients, as shown in Figure 4.
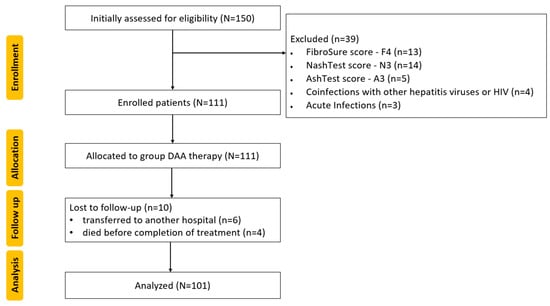
Figure 4.
CONSORT flow diagram of this study: N, n—Number; Human Immunodeficiency Virus—HIV; Direct-Acting Antiviral—DAA.
Inclusion Criteria:
- The inclusion criteria used were as follows:
- Aged over 18 years old;
- Confirmed diagnosis of chronic hepatitis C;
- Signed informed consent to participate in this study.
Exclusion Criteria:
- The exclusion criteria were as follows:
- FibroSure score—F4;
- NashTest score—N3;
- AshTest score—A3;
- Coinfections with other hepatitis viruses or Human Immunodeficiency Virus (HIV);
- Previous antiviral treatment for chronic hepatitis C;
- Liver/kidney transplant;
- Change in the treatment of associated diseases during this study;
- Acute infections;
- Pre-existing hematological disorders;
- Severe anemia;
- Treatment with drugs that influence RBC, WBC, or PLT (erythropoietin, corticosteroids, heparin, or thrombopoietin receptor agonists);
- Cancer.
3.2. Data Collection
Table 3 shows the hematological parameters evaluated before Mavyret therapy and 24 h after the end-point of DAA.

Table 3.
The evaluated parameters.
The pathological morphological changes in the liver were assessed in all subjects included in this study using noninvasive tests [fibrosis (FibroTest), steatosis (SteatoTest), necroinflammation (ActiTest), non-alcoholic steatohepatitis (NashTest), and alcoholic steatohepatitis (AshTest)], Figure 5.
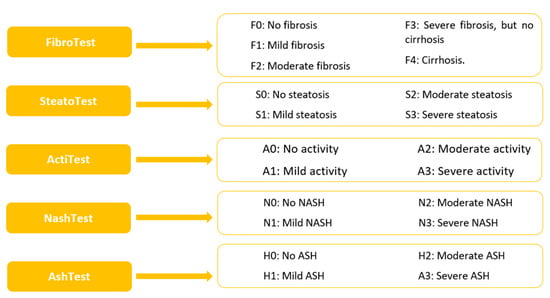
Figure 5.
Noninvasive hepatic tests: Non-alcoholic steatohepatitis—NASH; alcoholic steatohepatitis—ASH.
Blood samples were obtained for each subject at two time points:
1—In the morning on the day of the start of DAA therapy;
2—A total of 24 h after the completion of DAA therapy.
Laboratory parameters were assessed using standardized laboratory techniques. All patients underwent an initial evaluation regarding the following biochemical parameters: ALT [normal values (NV) 0–55 U/L], AST (NV 0–34 U/L), GGT (NV 0–64 U/L), TB (NV < 1.2 mg/dL), TC (NV 0–200 mg/dL), TG (NV 0–1.7 mmol/L), HbA1c (NV 4.8–5.6%), and creatinine (NV 0.5–1.5 mg/dL g/L). The samples were collected after fasting, using standard collection, transport, and storage procedures, and subsequently processed in the hospital laboratory.
3.3. Statistical Analysis
The data assigned to each subject (demographic, laboratory parameters) were processed using IBM SPSS Statistics software [version 29.0.2.0 (20)]. The evaluation of the blood count parameters at the two time points was performed by calculating the means, standard deviations, and paired t-tests (a p-value < 0.05 was marked as statistically significant). The graphs were edited using Microsoft Excel version 2021 (Redmond, WA, USA), Matplotlib version 3.7.1, Python version 3.11.2, and Adobe Photoshop version 24.5.
4. Discussion
The HCV, responsible for the most common form of chronic viral hepatitis, has six genotypes that manifest clinically in the same way. However, regarding antiviral treatment, these genotypes can cause issues with sustained viral responses. Identifying them is not easy for clinicians, so a medication that is effective regardless of genotype is very useful due to the additive and synergistic actions of the two components [11,12]. Mavyret is an effective medication in patients with CHC, regardless of the viral genotype. This is a major advantage for this molecule, but as is well known, any treatment has both positive and adverse effects. The latter often pose challenges with regard to continuing therapy or complicating the clinical picture. What may seem like a trivial relationship between certain molecules and erythrocytes, leukocytes, or platelets can lead to decreases or increases that are nearly imperceptible, except for patients who are already sensitive to any minor fluctuations.
Patients with CHC suffer from a deficiency in clotting factors and a vitamin K deficiency and, thus, have an increased tendency to bleed. Additionally, the presence of the HCV may lead to a decrease in platelet count due to the reduced synthesis of thrombopoietin (a protein involved in stimulating platelet production in the bone marrow) through the production of antiplatelet antibodies or their destruction through hypersplenism. A drop in platelets, which are involved in blood clotting, even if still within the normal range, can exacerbate this already existing deficiency, with devastating effects on the blood clotting of CHC patients. This study observed a mild but statistically insignificant decrease in platelet count, with values remaining within the reference range. The absence of thrombocytopenia is essential when selecting an antiviral product for this group of patients. Forns X. and colleagues reported the presence of thrombocytopenia in 4.7% (175/3754) of patients enrolled in a cohort of clinical studies in 2021 [13]. The data reported by Jordan J. Feld et al. (2022) show that G/P treatment was well tolerated, including in patients with thrombocytopenia [14].
The results are inconsistent, but few studies in the medical literature address this aspect.
The cellular anti-infectious defense system, specifically WBC, is crucial in patients suffering from hypoproteinemia and, consequently, a deficient humoral immune response. The HCV is responsible for manipulating (by producing autoantibodies that attack various host structures) and exhausting the host’s immune response in an ineffective attempt to eliminate the virus-infected cells. It is well known that neutrophils are involved in the body’s antibacterial and antifungal defense. In this study, a statistically significant decrease in WBC and neutrophil counts was observed, although they remained within the reference range. However, this decrease can weaken the defense against these two types of microorganisms. The medical literature lacks sufficient studies on these cell types, but isolated cases of neutropenia following Mavyret treatment have been reported [11]. Monocytes, which can differentiate into macrophages and dendritic cells, play an important antimicrobial role against viruses, fungi, and bacteria through phagocytosis and antigen presentation. The statistically significant decrease in monocyte count noted in this study, though still within the reference range, raises concerns about Mavyret’s potential ability to compromise the anti-infectious defense systems of patients undergoing this treatment. To the best of our knowledge, the effect of this medication on monocytes has not been sufficiently studied. Eosinophils, cells capable of fighting parasites, are another important element in the body’s cellular defense mechanisms. This study recorded a statistically significant decrease in eosinophil count under Mavyret treatment, though it was still within the reference range. This effect has not yet been documented in the medical literature.
Hepcidin is a protein synthesized by the liver, playing a role in iron metabolism. In inflammation, such as in hepatitis, the liver increases hepcidin levels, reducing the intestinal absorption of iron and preventing its release from hepatic stores. The result is iron deficiency, leading to iron-deficiency anemia. Mavyret administration resulted in a statistically significant decrease in all erythrocyte indices (RBC, hemoglobin, hematocrit, MCHC, MCH), but they remained within the reference range. MCV decreases but not to a statistically significant degree, and when correlated with the other indices, a mild case of iron-deficiency anemia can be inferred. If we consider the possible interference of hepcidin in the inflammatory context of CHC, the situation could evolve in the long term towards clinically significant anemia. Antiviral medication interferes with the red cell line, but during the period monitored in this study, the changes were mild. The medical literature is sparse regarding the study of Mavyret’s effects on the erythrocyte line [11].
Previously used specific treatments cause adverse reactions such as anemia and neutropenia in patients with liver cirrhosis or chronic hepatitis [14].
Treatment with interferon–ribavirin administered to patients with chronic hepatitis C significantly reduces hemoglobin levels (hemolytic phenomena) and platelet counts [15,16]. The trials conducted that evaluate the efficacy and safety of G/P administration report data on genotypes, antiviral response, clinical adverse reactions, and median platelet values, but they provide less data on the complete blood count. The SURVEYOR-II Part 3 study supports the idea that these reactions are rarely encountered or even absent [17].
The EXPEDITION-8 trial reports the presence of leukopenia and neutropenia in 1 patient out of 334 enrolled patients after 8 weeks of G/P administration [11].
All these data support the safety of administering this type of pangenotypic treatment for treating bleeding disorders [15].
4.1. Strengths and Limitations
The strength of this analysis is represented by the period evaluated, namely after the approval of the marketing of the combination, when the number of published studies increased dramatically. This study’s limitations consist of it using a single database with a smaller number of articles, but their value, the quantification of the number of citations, can turn this limitation into a strength.
4.2. Future Direction
For treating relapsed forms, guidelines recommend administering G/P combined with sofosbuvir and ribavirin or sofosbuvir/velpatasvir/voxilaprevir. The administration of these combinations in patients after liver transplantation has also been studied [18,19].
5. Conclusions
Mavyret has proven to be a safe medication for administering to patients with CHC, but its minor adverse effects on red blood cells, white blood cells, and platelets require constant monitoring, especially when used for an extended period and in patients who are vulnerable in this regard. Future studies are needed to closely monitor the potential adverse reactions of this molecule, especially when opting for administration periods longer than 8 weeks.
Author Contributions
Conceptualization, A.M.C. and F.M.; Data curation, A.I.A., H.T.J., A.F., N.N. and F.M.; Formal analysis, R.N.S., H.T.J. and F.M.; Investigation, A.M.C.; Methodology, A.M.C., R.N.S., N.N. and A.F.; Resources, A.M.C. and A.F.; Software, H.T.J. and N.N.; Supervision, R.N.S.; Validation, R.N.S., A.I.A., A.F., N.N. and F.M.; Visualization, A.M.C., A.I.A., H.T.J., N.N. and A.F.; Writing—original draft, A.M.C., R.N.S., A.I.A., H.T.J., A.F., N.N. and F.M.; Writing—review and editing, R.N.S., A.I.A., H.T.J., N.N. and F.M. All authors have read and agreed to the published version of the manuscript.
Funding
The APC was funded by the University of Oradea, Romania.
Institutional Review Board Statement
This study was approved by the Ethics Commission of Pitesti County Hospital (Approval No. 2600/21.04.2023) and complied with the Declaration of Helsinki of the World Medical Association.
Informed Consent Statement
Informed consent was obtained from all subjects involved in this study.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding authors.
Acknowledgments
The all authors thank the University of Oradea for providing the logistic facilities that they used.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Petruzziello, A.; Marigliano, S.; Loquercio, G.; Cozzolino, A.; Cacciapuoti, C. Global epidemiology of hepatitis C virus infection: An up-date of the distribution and circulation of hepatitis C virus genotypes. World J. Gastroenterol. 2016, 22, 7824. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Hepatitis C in the WHO European Region—Fact Sheet. July 2022. Available online: https://www.who.int/europe/health-topics/hepatitis (accessed on 27 September 2024).
- European Centre for Disease Prevention and Control. Hepatitis C. In ECDC Annual Epidemiological Report for 2022; ECDC: Stockholm, Sweden, 2024. [Google Scholar]
- Polaris Observatory HCV Collaborators. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: A modelling study. Lancet Gastroenterol. Hepatol. 2017, 2, 161–176. [Google Scholar] [CrossRef]
- Razavi, H.; Waked, I.; Sarrazin, C.; Myers, R.P.; Idilman, R.; Calinas, F.; Vogel, W.; Mendes Correa, M.C.; Hézode, C.; Lázaro, P.; et al. The present and future disease burden of hepatitis C virus (HCV) infection with today’s treatment paradigm. J. Viral Hepat. 2014, 21, 34–59. [Google Scholar] [CrossRef]
- Feld, J.J.; Jacobson, I.M.; Hézode, C.; Asselah, T.; Ruane, P.J.; Gruener, N.; Abergel, A.; Mangia, A.; Lai, C.-L.; Chan, H.L.Y.; et al. Sofosbuvir and velpatasvir for HCV genotype 1, 2, 4, 5, and 6 infection. N. Engl. J. Med. 2015, 373, 2599–2607. [Google Scholar] [CrossRef]
- Puoti, M.; Foster, G.R.; Wang, S.; Mutimer, D.; Gane, E.; Moreno, C.; Chang, T.T.; Lee, S.S.; Marinho, R.; Dufour, J.F.; et al. High SVR12 with 8-week and 12-week glecaprevir/pibrentasvir therapy: An integrated analysis of HCV genotype 1-6 patients without cirrhosis. J. Hepatol. 2018, 69, 293–300. [Google Scholar] [CrossRef]
- Dahal, S.; Upadhyay, S.; Banjade, R.; Dhakal, P.; Khanal, N.; Bhatt, V.R. Thrombocytopenia in patients with chronic hepatitis C virus infection. Mediterr. J. Hematol. Infect. Dis. 2017, 9, e2017019. [Google Scholar] [CrossRef]
- Dybowska, D.; Zarębska-Michaluk, D.; Rzymski, P.; Berak, H.; Lorenc, B.; Sitko, M.; Dybowski, M.; Mazur, W.; Tudrujek-Zdunek, M.; Janocha-Litwin, J.; et al. Real-world effectiveness and safety of direct-acting antivirals in hepatitis C virus patients with mental disorders. World J. Gastroenterol. 2023, 29, 4085–4098. [Google Scholar] [CrossRef]
- Liu, X.; Hu, P. Efficacy and safety of Glecaprevir/Pibrentasvir in patients with chronic HCV infection. J. Clin. Transl. Hepatol. 2021, 9, 125–132. [Google Scholar] [CrossRef]
- Brown, R.S., Jr.; Buti, M.; Rodrigues, L.; Chulanov, V.; Chuang, W.-L.; Aguilar, H.; Horváth, G.; Zuckerman, E.; Carrion, B.R.; Rodriguez-Perez, F.; et al. Glecaprevir/pibrentasvir for 8 weeks in treatment-naive patients with chronic HCV genotypes 1–6 and compensated cirrhosis: The EXPEDITION-8 trial. J. Hepatol. 2020, 72, 441–449. [Google Scholar] [CrossRef]
- Ng, T.I.; Tripathi, R.; Reisch, T.; Lu, L.; Middleton, T.; Hopkins, T.A.; Pithawalla, R.; Irvin, M.; Dekhtyar, T.; Krishnan, P.; et al. In vitro antiviral activity and resistance profile of the next-generation hepatitis C virus NS3/4A protease inhibitor Glecaprevir. Antimicrob. Agents Chemother. 2017, 62. [Google Scholar] [CrossRef]
- Forns, X.; Feld, J.J.; Dylla, D.E.; Pol, S.; Chayama, K.; Hou, J.; Heo, J.; Lampertico, P.; Brown, A.; Bondin, M.; et al. Safety of Patients with Hepatitis C Virus Treated with Glecaprevir/Pibrentasvir from Clinical Trials and Real-World Cohorts. Adv. Ther. 2021, 38, 3409–3426. [Google Scholar] [CrossRef]
- Feld, J.J.; Forns, X.; Dylla, D.E.; Kumada, H.; de Ledinghen, V.; Wei, L.; Brown, R.S., Jr.; Flisiak, R.; Lampertico, P.; Thabut, D.; et al. Safety analysis of glecaprevir/pibrentasvir in patients with markers of advanced liver disease in clinical and real-world cohorts. J. Viral Hepat. 2022, 29, 1050–1061. [Google Scholar] [CrossRef]
- Smolders, E.J.; Jansen, A.M.E.; ter Horst, P.G.J.; Rockstroh, J.; Back, D.J.; Burger, D.M. Viral Hepatitis C Therapy: Pharmacokinetic and Pharmacodynamic Considerations: A 2019 Update. Clin. Pharmacokinet. 2019, 58, 1237–1263. [Google Scholar] [CrossRef]
- van Soest, H.; Renooij, W.; van Erpecum, K.J. Clinical and basal aspects of anemia during antiviral therapy for hepatitis C. Ann. Hepatol. 2009, 8, 316–324. [Google Scholar] [CrossRef]
- Wyles, D.; Poordad, F.; Wang, S.; Alric, L.; Felizarta, F.; Kwo, P.Y.; Maliakkal, B.; Agarwal, K.; Hassanein, T.; We-ilert, F.; et al. Glecaprevir/pibrentasvir for hepatitis C virus genotype 3 patients with cirrhosis and/or prior treatment experience: A partially randomized phase 3 clinical trial. Hepatology 2018, 67, 514–523. [Google Scholar] [CrossRef]
- Arora, R.; Martin, M.T.; Boike, J.; Patel, S. Glecaprevir/pibrentasvir + sofosbuvir for post-liver transplant recurrent hepatitis C virus treatment. World J. Hepatol. 2023, 15, 318–320. [Google Scholar] [CrossRef]
- García-Juárez, I.; Alonzo-García, C.; Contreras, A.G.; Romero-Hernández, F.; Servín-Rojas, M.; Fernández-Ramírez, A.; Ruiz, I. Sofosbuvir plus glecaprevir/pibrentasvir as salvage therapy after liver transplantation in NS5A inhibitor-experienced patients. A case series. Gac. Médica México 2023, 159, 338–344. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).