Factors Associated with Days Alive and at Home within 30 Days (DAH30) Scores Following Surgery: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Risk of Bias (ROB) Assessment
2.4. Data Extraction and Synthesis
3. Results
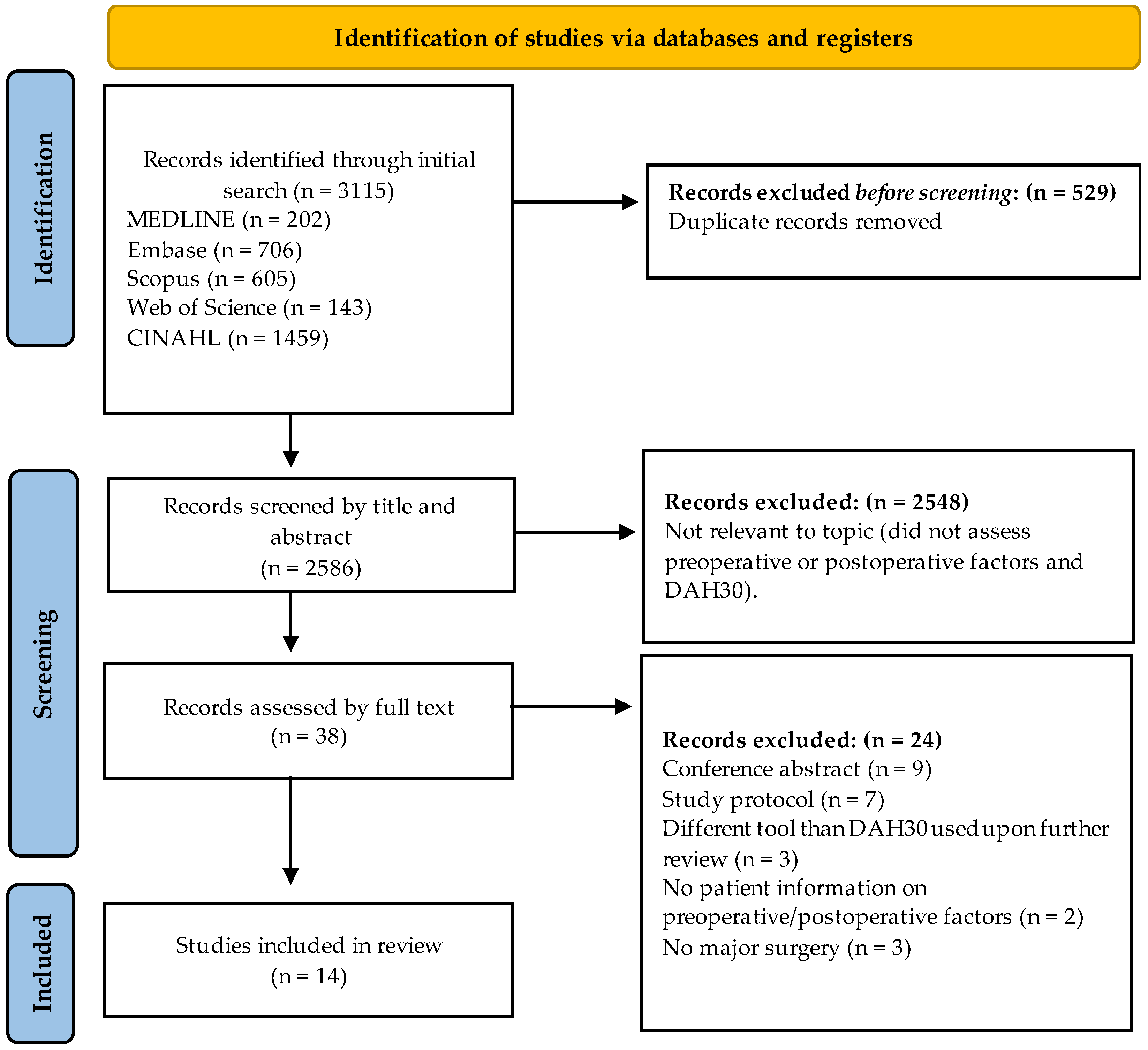
3.1. Study Selection and Characteristics
3.2. Risk of Bias
3.3. Predictors of DAH30
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| MEDLINE via Ovid | |
| #1 | (Surger* OR operat* OR surgical procedure*).mp |
| #2 | (((DAH30 OR “days at home up to 30 days after surgery” OR days alive and at home OR days at home* OR postoperative 30 days) OR ((postoperative* OR preoperative* OR after surgery* OR after procedure*) adj3 (days alive and at home) OR days at home OR DAH30))).mp |
| #3 | (#1 AND #2) |
| #4 | Limit #3 to humans |
| Embase via Ovid | |
| #1 | (Surger* OR operat* OR surgical procedure).mp |
| #2 | (((DAH30 OR “days at home up to 30 days after surgery” OR days alive and at home OR days at home* OR postoperative 30 days) OR ((postoperative* OR preoperative* OR after surgery* OR after procedure*) adj3 (days alive and at home) OR days at home OR DAH30))).mp |
| #3 | (#1 AND #2) |
| #4 | Limit #3 to humans |
| AMED via Ovid | |
| #1 | (Surger* OR operat* OR surgical procedure).mp |
| #2 | (((DAH30 OR “days at home up to 30 days after surgery” OR days alive and at home OR days at home* OR postoperative 30 days) OR ((postoperative* OR preoperative* OR after surgery* OR after procedure*) adj3 (days alive and at home) OR days at home OR DAH30))).mp |
| #3 | (#1 AND #2) |
| #4 | Limit #3 to humans |
| Scopus | |
| #1 | (Surger* OR operat* OR surgical procedure).mp |
| #2 | (DAH30 OR “days at home up to 30 days after surgery” OR “days alive and at home” OR “days at home” OR “postoperative 30 days”).mp |
| #3 | (#1 AND #2) |
| #4 | Limit #3 to humans |
| Web of Science | |
| #1 | (Surger* OR operat* OR surgical procedure).mp |
| #2 | (((DAH30 OR “days at home up to 30 days after surgery” OR “days alive and at home” OR “days at home*” OR postoperative 30 days) OR ((postoperative* OR preoperative* OR after surgery* OR after procedure*) “NEAR/3” (“days alive and at home” OR “days at home” OR DAH30))).mp |
| #3 | (#1 AND #2) |
| #4 | Limit #3 to humans |
| CINAHL | |
| #1 | (Surger* OR operat* OR surgical procedure).mp |
| #2 | (DAH30 OR “days at home up to 30 days after surgery” OR days alive and at home OR days at home* OR postoperative 30 days).mp |
| #3 | (#1 AND #2) |
| #4 | Limit #3 to humans |
References
- Quene, T.; Bust, L.; Louw, J.; Mwandri, M.; Chu, K.M. Global surgery is an essential component of global health. Surgeon 2022, 20, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Meara, J.G.; Leather, A.J.M.; Hagander, L.; Alkire, B.C.; Alonso, N.; Ameh, E.A.; Bickler, S.W.; Conteh, L.; Dare, A.J.; Davies, J.; et al. Global Surgery 2030: Evidence and solutions for achieving health, welfare, and economic development. Lancet 2015, 386, 569–624. [Google Scholar] [CrossRef] [PubMed]
- Weiser, T.G.; Haynes, A.B.; Molina, G.; Lipsitz, S.R.; Esquivel, M.M.; Uribe-Leitz, T.; Fu, R.; Azad, T.; Chao, T.E.; Berry, W.R.; et al. Estimate of the global volume of surgery in 2012: An assessment supporting improved health outcomes. Lancet 2015, 385, S11. [Google Scholar] [CrossRef] [PubMed]
- Australian Institute of Health and Welfare. Hospitals at a Glance 2017–2018. 2019. Available online: https://www.aihw.gov.au/reports/hospitals/hospitals-at-a-glance-2017-18/contents/surgery-in-australia-s-hospitals (accessed on 15 December 2023).
- Etzioni, D.A.; Liu, J.H.; Maggard, M.A.; Ko, C.Y. The aging population and its impact on the surgery workforce. Ann. Surg. 2003, 238, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Birkmeyer, J.D.; Gust, C.; Dimick, J.B.; Birkmeyer, N.J.O.; Skinner, J.S. Hospital quality and the cost of inpatient surgery in the United States. Ann. Surg. 2012, 255, 1–5. [Google Scholar] [CrossRef]
- Billig, J.I.; Sears, E.D.; Travis, B.N.; Waljee, J.F. Patient-Reported Outcomes: Understanding Surgical Efficacy and Quality from the Patient’s Perspective. Ann. Surg. Oncol. 2020, 27, 56–64. [Google Scholar] [CrossRef]
- Tevis, S.E.; Kennedy, G.D. Postoperative complications and implications on patient-centered outcomes. J. Surg. Res. 2013, 181, 106–113. [Google Scholar] [CrossRef]
- Ferguson, M.; Kusre, S.; Myles, P. Minimal clinically important difference in days at home up to 30 days after surgery. Anaesthesia 2022, 77, 196–200. [Google Scholar] [CrossRef]
- Reilly, J.R.; Myles, P.S.; Wong, D.; Heritier, S.R.; Brown, W.A.; Richards, T.; Bell, M. Hospital costs and factors associated with days alive and at home after surgery (DAH(30)). Med. J. Aust. 2022, 217, 311–317. [Google Scholar] [CrossRef]
- Dellen, M.; Flanagan, M.; Pfafman, R.; Drouin, M.; Pater, J.; Pei, K.Y. Factors Potential Patients Deem Important for Decision-Making in High-Risk Surgical Scenarios. J. Am. Coll. Surg. 2023, 236, 93–98. [Google Scholar] [CrossRef]
- Gunaratnam, C.; Bernstein, M. Factors Affecting Surgical Decision-making-A Qualitative Study. Rambam Maimonides Med. J. 2018, 9, e0003. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- COVIDENCE. The World’s #1 Systematic Review Tool. 2024. Available online: https://www.covidence.org/ (accessed on 24 April 2024).
- Hayden, J.A.; van der Windt, D.A.; Cartwright, J.L.; Côté, P.; Bombardier, C. Assessing Bias in Studies of Prognostic Factors. Ann. Intern. Med. 2013, 158, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Fung, P.L.P.; Lau, V.N.M.; Ng, F.F.; Leung, W.W.; Mak, T.W.C.; Lee, A. Perioperative changes in haemoglobin and ferritin concentrations from preoperative intravenous iron isomaltoside for iron deficiency anaemia in patients with colorectal cancer: A pilot randomised controlled trial. PLoS ONE 2022, 17, e0270640. [Google Scholar] [CrossRef] [PubMed]
- Jerath, A.; Austin, P.C.; Ko, D.T.; Wijeysundera, H.C.; Fremes, S.; McCormack, D.; Wijeysundera, D.N. Socioeconomic Status and Days Alive and Out of Hospital after Major Elective Noncardiac Surgery: A Population-based Cohort Study. Anesthesiology 2020, 132, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Jerath, A.; Austin, P.C.; McCormack, D.; Wijeysundera, D.N. Impact of postoperative intensive care unit utilization on postoperative outcomes in adults undergoing major elective noncardiac surgery. J. Clin. Anesth. 2020, 62, 109707. [Google Scholar] [CrossRef]
- Jerath, A.; Austin, P.; Wijeysundera, D.N. Days Alive and Out of Hospital: Validation of a Patient-centered Outcome for Perioperative Medicine. Anesthesiology 2019, 131, 84–93. [Google Scholar] [CrossRef]
- Bell, M.; Eriksson, L.I.; Svensson, T.; Hallqvist, L.; Granath, F.; Reilly, J.; Myles, P.S. Days at Home after Surgery: An Integrated and Efficient Outcome Measure for Clinical Trials and Quality Assurance. EClinicalMedicine 2019, 11, 18–26. [Google Scholar] [CrossRef]
- Jørgensen, C.C.; Petersen, P.B.; Kehlet, H.; Madsen, F.; Hansen, T.B.; Husted, H.; Laursen, M.; Hansen, L.T.; Kjærsgaard-Andersen, P.; Solgaard, S.; et al. Days alive and out of hospital after fast-track total hip and knee arthroplasty: An observational cohort study in 16 137 patients. Br. J. Anaesth. 2019, 123, 671–678. [Google Scholar] [CrossRef]
- McIsaac, D.I.; Fottinger, A.; Sucha, E.; McDonald, B. Association of frailty with days alive at home after cardiac surgery: A population-based cohort study. Br. J. Anaesth. 2021, 126, 1103–1110. [Google Scholar] [CrossRef]
- Miles, L.F.; Soo, V.P.; Braat, S.; Bade-Boon, J.; Heritier, S.; Klein, A.A.; Myles, P.S.; Richards, T.; Symons, J.; Burbury, K.L.; et al. Associations between non-anaemic iron deficiency and outcomes following elective cardiac surgery (IDOCS): A prospective cohort study. The Lancet. Haematology 2022, 9, e514–e522. [Google Scholar] [CrossRef] [PubMed]
- Myles, P.S.; Shulman, M.A.; Heritier, S.; Wallace, S.; McIlroy, D.R.; McCluskey, S.; Sillar, I.; Forbes, A. Validation of days at home as an outcome measure after surgery: A prospective cohort study in Australia. BMJ Open 2017, 7, e015828. [Google Scholar] [CrossRef] [PubMed]
- Schick, V.; Boensch, M.; van Edig, M.; Alfitian, J.; Pola, T.; Ecker, H.; Lindacher, F.; Shah-Hosseini, K.; Wetsch, W.A.; Riedel, B.; et al. Impaired vascular endothelial function as a perioperative risk predictor—A prospective observational trial. BMC Anesthesiol. 2021, 21, 190. [Google Scholar] [CrossRef] [PubMed]
- Shaw, J.F.; Mulpuru, S.; Kendzerska, T.; Moloo, H.; Martel, G.; Eskander, A.; Lalu, M.M.; McIsaac, D.I. Association between frailty and patient outcomes after cancer surgery: A population-based cohort study. Br. J. Anaesth. 2022, 128, 457–464. [Google Scholar] [CrossRef]
- Wu, A.; Fahey, M.T.; Cui, D.; El-Behesy, B.; Story, D.A. An evaluation of the outcome metric ‘days alive and at home’ in older patients after hip fracture surgery. Anaesthesia 2022, 77, 901–909. [Google Scholar] [CrossRef]
- Plenge, U.; Parker, R.; Davids, S.; Davies, G.L.; Fullerton, Z.; Gray, L.; Groenewald, P.; Isaacs, R.; Kauta, N.; Louw, F.M.; et al. Quality of recovery after total hip and knee arthroplasty in South Africa: A national prospective observational cohort study. BMC Musculoskelet. Disord. 2020, 21, 721. [Google Scholar] [CrossRef]
| Author, Year | Study Characteristics | Study Design | Predictors Assessed |
|---|---|---|---|
| Bell, 2019 [20] | Age: ≥18 years (62.0) Gender: 42.3% male Country: Sweden Patients: 636,885 | Cohort study | Surgery type Age Sex Surgery duration Complications ASA physical status Charlson Comorbidity Index (CCI) |
| Fung, 2022 [16] | Age: ≥18 years (68.4 Iron therapy, 69.8 Usual care) Gender: Male (75.0% Iron therapy, 45.0% Usual care) Country: Hong Kong Patients: 40 | Randomised control trial | Iron therapy Usual care |
| Jerath, 2019 [19] | Age: ≥40 years (65.0) Gender: 37.7% male Country: Canada Patients: 540,072 | Cohort study | Age Gender Hospital Surgery duration Surgical volume Comorbidities |
| Jerath, 2020(a) [17] | Age: ≥40 years (65.0) Gender: 40.4% male Country: Canada Patients: 724,459 | Cohort study | Neighbourhood median household income quintile |
| Jerath, 2020(b) [18] | Age: ≥40 years (65.0) Gender: 53.3% male Country: Canada Patients: 101,385 | Cohort study | ICU admission Surgery type Age Gender Comorbidities |
| Jorgensen, 2019 [21] | Age: >18 years (69.0) Gender: 42.0% male Country: Denmark Patients: 16,137 | Cohort study | High risk groups |
| McIsaac, 2021 [22] | Age: >65 years (73.2 Frailty index > 0.21, 74.6 Frailty index < 0.21) Gender: Male (67.4% Frailty index > 0.21, 73.8% Frailty index < 0.21) Country: Canada Patients: 61,389 | Cohort study | Frailty |
| Miles, 2022 [23] | Age: ≥18 years (63.6) Gender: 80.0% male Country: Australia Patients: 480 | Cohort study | Iron deficient Iron replete |
| Myles, 2017 [24] | Age: ≥18 years (65.0) Gender: 67.7% male Country: Australia Patients: 2109 | Cohort study | Age Gender Smoking status Diabetes Heart failure ASA physical status Surgery type Surgery duration Complications |
| Plenge, 2020 [28] | Age: ≥18 years (62.0) Gender: 31.7% male Country: South Africa Patients: 186 | Cohort study | District and regional hospitals (DRH) Tertiary or central hospitals (TCH) |
| Reilly, 2022 [10] | Age: ≥18 years (62.0) Gender: 43.0% male Country: Australia Patients: 126,788 | Cohort study | Age Gender Location Public hospital Charlson Comorbidity Index (CCI) ASA physical status Surgery severity Complications Surgery duration Length of stay |
| Schick, 2021 [25] | Age: ≥18 years (64.0) Gender: 72.0% male Country: Germany Patients: 71 | Cohort study | Flow-mediated dilation ASA physical status Surgery type Surgery duration |
| Shaw, 2022 [26] | Age: ≥18 years Gender: Male (57.9% Frail pFI > 0.21, 53.0% Non-frail pFI ≤ 0.21) Country: Canada Patients: 52,012 | Cohort study | Frailty |
| Wu, 2022 [27] | Age: ≥70 years (84.7) Gender: 27.0% male Country: Australia Patients: 825 | Cohort study | Surgery type |
| Author, Year | Study Participation | Study Attrition | Prognostic Factor Measurement | Outcome Measurement | Study Confounding | Statistical Analysis | Overall ROB |
|---|---|---|---|---|---|---|---|
| Bell 2019 [20] | Low | Low | Low | Low | Low | Low | Low |
| Fung 2022 [16] | Low | Low | Low | Low | Moderate | Low | Low |
| Jerath 2019 [19] | Low | Low | Low | Moderate | Moderate | Low | Moderate |
| Jerath 2020(a) [17] | Low | Low | Low | Moderate | Low | Low | Low |
| Jerath 2020(b) [18] | Moderate | Low | Low | Moderate | Moderate | Moderate | Moderate |
| Jorgensen 2019 [21] | Low | Low | Low | High | Moderate | Low | Moderate |
| McIsaac 2021 [22] | Low | Low | Low | Moderate | Moderate | Moderate | Moderate |
| Miles 2022 [23] | Low | Low | Low | Low | Low | Low | Low |
| Myles 2017 [24] | High | High | Low | Moderate | High | Low | High |
| Plenge 2020 [28] | Low | Low | Low | Low | Low | Low | Low |
| Reilly 2022 [10] | Low | Low | Low | Low | Low | Low | Low |
| Schick 2021 [25] | High | Low | Low | Moderate | Low | Moderate | High |
| Shaw 2022 [26] | High | Low | Low | Low | Moderate | Low | Moderate |
| Wu 2022 [27] | Low | Low | Low | Moderate | High | Low | Moderate |
| Predictors | Author, Year | Scoring Method, Notes | Positively Associated | Negatively Associated | Results | Outcome/Comments |
|---|---|---|---|---|---|---|
| ASA physical status | Bell, 2019 [20] | Spearman’s correlations | Higher ASA score **a | N/A | 1, 28 (26 to 29) 2, 27 (24 to 29) 3, 24 (16 to 18) 4, 11 (0 to 22) | DAH30 |
| Myles, 2017 [24] | Multivariable analysis | Higher ASA score *b | N/A | 1, 25.9 (25.1 to 26.6) ^ 2, 24.4 (24.0 to 24.7) ^ 3, 23.6 (23.2 to 23.9) ^ 4, 23.0 (22.6 to 23.3) ^ | DAH30 (50–75th percentile) | |
| Reilly, 2022 [10] | Multivariate quintile regression | ASA 2, 3, 4 compared to ASA b | N/A | 2, 0.002 (−0.01 to −0.03) ^ 3, −0.47 (−0.52 to −0.42) ^ 4, −1.93 (−2.16 to −1.70) ^ | DAH30 (50–75th percentile) | |
| Schick, 2021 [25] | Multivariable linear regression | Higher ASA score * | N/A | −4.3 (−7.2 to −1.3) y | DAH30 | |
| Surgery duration | Jerath, 2019 [19] | Spearman rank correlation | Surgery duration (minutes) ** | N/A | 118 (95 to 151) 152 (110 to 228) | DAH30 above and below median, median surgical time associated being less than or greater than median DAH30 in cohort |
| Myles, 2017 [24] | Multivariable analysis | Surgery duration (hours) *b | N/A | <2.0, 25.6 (25.2 to 26.0) ^ 2.0–3.99, 24.0 (23.7 to 24.3) ^ 3.0–3.99 23.1 (22.7 to 23.4) ^ ≥4.0, 22.0 (21.6 to 22.5) ^ | DAH30 (50–75th percentile) | |
| Reilly, 2022 [10] | Multivariate quintile regression | Surgery duration (minutes) b | Surgery duration (minutes) b | 30–60, 0.27 (0.22 to 0.32) >120, −1.00, (−1.06 to −0.94) | DAH30 (50–75th percentile) | |
| Bell, 2019 [20] | Spearman’s correlations | Surgery duration (minutes) ** | N/A | <59, 28 (25 to 29) ≥60, 26 (22 to 28) | DAH30 | |
| Schick, 2021 [25] | Multivariable regression analysis | Surgery duration (minutes) * | N/A | −0.02 (−0.03 to −0.01) | DAH30 | |
| Surgery type | Jerath, 2020(b) [18] | N/A | Surgery performed | N/A | Nephrectomy, 26 (24 to 27) Lower gastrointestinal surgery, 23 (20 to 25) Peripheral arterial disease, 24 (20–27) | DAH30 |
| Myles, 2017 [24] | Multivariable analysis | Surgery performed b | N/A | Vascular, 26.0 (24.3 to 27.3) ^ Ear, nose, throat, 25.8 (24.9 to 27.0) ^ Orthopaedic, 21.9 (21.2 to 22.6) ^ Cardiac, 22.8 (22.6 to 22.9) ^ Neurosurgery, 22.8 (22.2 to 23.5) ^ | Median (95% CI) | |
| Bell, 2019 [20] | Spearman’s correlations | Surgery performed **a | N/A | Nervous system, 25 (15 to 28) Endocrine, Breast, 29 (28 to 29) Eyes, 29 (28 to 29) Ear, Nose, Throat, Jaw, 29 (28 to 29) Heart, Major vessels, 23 (16 to 29) Lung, Trachea, 22 (11 to 26) Gastrointestinal, 27 (21 to 29) Urology, Sex organs, 28 (26 to 29) Obstetrics, 27 (26 to 28) Musculoskeletal, 25 (20 to 27) Peripheral vessels, Lymphatics, 27 (22 to 29) Other surgeries, 27 (17 to 29) | DAH30 | |
| Elective surgery | Reilly, 2022 [10] | Multivariate quintile regression | N/A | Emergency admission b | Emergency admission, −2.19 (−2.32 to −2.06) | DAH30 (50–75th percentile) |
| Surgery severity | Reilly, 2022 [10] | Multivariate quintile regression | Surgical severity of intermediate when compared to minor b | Surgical severity of major and complex major when compared to Minor b | Intermediate, 0.18 (0.10 to 0.25) Major, −1.07 (−1.15 to −0.99) Complex major, −1.10 (−1.19 to −1.02) | DAH30 (50–75th percentile) |
| Surgical volume | Jerath, 2019 [19] | Spearman’s correlations | Greater or equal to median DAH30 3276 (1613 to 5828) ** | Less than median DAH30 2271 (878 to 5208) ** | Median, 3276 (1613 to 5828) 2271 (878 to 5208) | DAH30 |
| Hospital location | Plenge, 2020 [28] | Mann–Whitney U-test | N/A | Tertiary and central hospitals compared to district and regional hospitals * | District and regional hospitals, 27 (26 to 27) Tertiary and central hospitals, 26 (24 to 27) | DAH30 |
| Comorbidities | Bell, 2019 [20] | Spearman’s correlations | CCI **a | N/A | CCI 1 year including cancer, 0p, 27 (25 to 29) 1p, 26 (20 to 28) 2–3p, 27 (22 to 29) 4p–, 24 (15 to 28) CCI 1 year excluding cancer, 0p, 27 (25 to 29) 1p, 26 (20 to 28) 2–3p, 26 (20 to 29) 4p–, 24 (15 to 28) CCI 5 years including cancer, 0p, 28 (25 to 29) 1p, 26 (21 to 28) 2–3p, 27 (22 to 29) 4p–, 25 (16 to 28) CCI 5 years excluding cancer, 0p, 28 (25 to 29) 1p, 26 (21 to 28) 2–3p, 26 (21 to 29) 4p−, 25 (16 to 28) | DAH30 |
| Reilly, 2022 [10] | Multivariate quintile regression | CCI 1, 2 and ≥3 compared to CCI 0 b | N/A | 1, −0.14 (−0.18 to −0.10) 2, −0.14 (−0.23 to −0.05) ≥3, −2.81 (−3.25 to −2.36) | DAH30 (50–75th percentile) | |
| Myles, 2017 [24] | Quasi-likelihood ratio test | Diabetes *b Heart failure *b | N/A | Diabetes, Yes, 23.0 (22.4 to 23.6) ^ No, 23.8 (23.8 to 23.9) ^ Heart Failure, Yes, 22.9 (22.4 to 23.4) ^ No, 23.8 (23.7 to 23.9) ^ | DAH30 (50–75th percentile) | |
| Risk | Jorgensen, 2019 [21] | Mann–Whitney U-test | Low-risk patients * | High-risk patients * | High-risk patients, 27 (26 to 28) Low-risk patients, 28 (27 to 28) | DAH30 |
| Age | Jerath, 2019 [19] | Spearman’s correlations | Age 63 (53–71) ** | Age 69 (60–77) ** | Median age (years), 63 (53 to 71) 69 (60 to 77) | DAH30 above and below median, median ages associated being less than or greater than median DAH30 in cohort |
| Myles, 2017 [24] | Quasi-likelihood ratio test | Age *b | N/A | <50, 24.8 (24.4 to 25.2) ^ 50–60, 24.4 (24.0 to 24.9) ^ 60–70, 24.0 (23.6 to 24.3) ^ 70–80, 23.0 (22.7 to 23.4) ^ >79, 22.2 (21.7 to 22.7) ^ | DAH30 (50–75th percentile) | |
| Sex | Bell, 2019 [20] | Spearman’s correlations | Patient sex **a | N/A | Male, 27 (22 to 29) Female, 27 (24 to 29) | DAH30 |
| Reilly, 2022 [10] | Multivariate quintile regression | Patient sex b | N/A | Female, −0.44 (−0.46 to −0.41) | DAH30 (50th percentile) | |
| Neighborhood median Household hncome quintile | Jerath, 2020(a) [17] | Multivariable quantile regression models | N/A | Quintile **b | Quintile 1, 26 (24 to 27) Quintile 2, 26 (24 to 27) Quintile 3, 26 (25 to 27) Quintile 4, 26 (25 to 27) Quintile 5, 26 (25 to 27) | DAH30 (50th percentile) |
| Frailty | McIsaac, 2021 [22] | Sensitivity analysis | N/A | Frailty ** | Ratio of means, 0.80 (0.79 to 0.81) | DAH30 |
| Shaw, 2022 [26] | Two-tailed, absolute standardised differences | N/A | Frailty b | Frail pFI, 22.0 (64) Non-frail pFI, 18.6 (8.5) | DAH30, mean (SD) | |
| Complications | Bell, 2019 [20] | Mann–Whitney/Kruskal–Wallis | N/A | AKI V ARDS V Arrhythmia V Cardiac arrest V DVT V Delirium V Infection V Stroke V MI V Pneumonia V Paralytic ileus V Pulmonary embolism V Pulmonary oedema V ICD10 = T81 V Any major complication V | AKI, 11.00 (10.79 to 11.22) ARDS, 12.94 (12.34 to 13.54) Arrhythmia, 1.00 (0.81 to 1.19) Cardiac arrest, 10.32 (10.01 to 10.64) DVT, 4.30 (3.90 to 4.69) Delirium, 5.84 (5.61 to 6.06) Infection, 6.89 (6.51 to 7.28) Stroke, 8.40 (8.22 to 8.58) MI, 4.83 (4.66 to 5.00) Pneumonia, 8.95 (8.83 to 9.06) Paralytic ileus, 4.46 (4.32 to 4.59) Pulmonary embolism, 7.57 (7.36 to 7.78) Pulmonary oedema, 12.41 (12.14 to 12.69) ICD10 = T81, 4.71 (4.65 to 4.78) Any major complication, 7.03 (6.97 to 7.10) | DAH30 |
| Reilly, 2022 [10] | Multivariate quintile regression | N/A | HDU/ICU admission b Mechanical ventilation b Unplanned theatre event b | HDU/ICU admission, −6.79 (−7.10 to −6.48) Mechanical ventilation, −14.5 (−14.8 to −14.1) Unplanned theatre event, −0.63 (−0.82 to −0.44) | DAH30 (50–75th percentile) | |
| Myles, 2017 [24] | Quasi-likelihood ratio test | N/A | Myocardial infarction (120 (6.5%)) *b Stroke (13 (0.7%)) *b Pulmonary embolism (7 (0.4%)) b Surgical-site infection (129(7.0%)) *b Any of the listed complications (263 (14.2%)) *b Hospital readmission (150(7.1%)) *b | Myocardial infarction Yes (20.8(19.2 to 22.4)) ^ No (23.8 (23.7 to 23.9)) ^ Stroke Yes (10.1 (2.5 to 17.7)) ^ No (23.8 (23.5 to 24.0)) ^ Pulmonary embolism Yes (17.1 (8.4 to 25.9)) ^ No (23.7 (23.5 to 23.9)) ^ Cardiac arrest Yes (17.7 (0.9 to 34.5)) ^ No (23.7 (23.5 to 24.0)) ^ Surgical-site infection Yes (21. (19.0 to 23.0)) ^ No (23.8 (23.7 to 23.9)) ^ Any of the listed complications Yes (20.5 (19.1 to 21.9)) ^ No (23.9 (23.8 to 23.9)) ^ Hospital readmission Yes (17.9 (16.3 to 19.5)) ^ No (23.9 (23.8 to 23.9)) ^ | DAH30 (50–75th percentile) | |
| Intervention | Miles, 2022 [23] | Simultaneous-quantile regression | N/A | Iron-deficient patients compared to iron-replete patients (p = 0.70) | −0·11 (−0·66 to 0·45) ^ | DAH30 |
| Fung, 2022 [16] | Mann–Whitney U-test | N/A | Iron therapy compared to usual care (days) (p = 0.461) | Iron therapy, 20 (10 to 25) Usual care, 23 (20 to 25) | DAH30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartyn, J.; Morkaya, J.; Karunaratne, S.; Chen, T.Y.; Solomon, M.; Koh, C.; Sandroussi, C.; Steffens, D. Factors Associated with Days Alive and at Home within 30 Days (DAH30) Scores Following Surgery: A Systematic Review. Gastrointest. Disord. 2024, 6, 816-831. https://doi.org/10.3390/gidisord6040057
Bartyn J, Morkaya J, Karunaratne S, Chen TY, Solomon M, Koh C, Sandroussi C, Steffens D. Factors Associated with Days Alive and at Home within 30 Days (DAH30) Scores Following Surgery: A Systematic Review. Gastrointestinal Disorders. 2024; 6(4):816-831. https://doi.org/10.3390/gidisord6040057
Chicago/Turabian StyleBartyn, Jenna, James Morkaya, Sascha Karunaratne, Tian You Chen, Michael Solomon, Cherry Koh, Charbel Sandroussi, and Daniel Steffens. 2024. "Factors Associated with Days Alive and at Home within 30 Days (DAH30) Scores Following Surgery: A Systematic Review" Gastrointestinal Disorders 6, no. 4: 816-831. https://doi.org/10.3390/gidisord6040057
APA StyleBartyn, J., Morkaya, J., Karunaratne, S., Chen, T. Y., Solomon, M., Koh, C., Sandroussi, C., & Steffens, D. (2024). Factors Associated with Days Alive and at Home within 30 Days (DAH30) Scores Following Surgery: A Systematic Review. Gastrointestinal Disorders, 6(4), 816-831. https://doi.org/10.3390/gidisord6040057









