Gut–Brain Axis, Microbiota and Probiotics—Current Knowledge on Their Role in Irritable Bowel Syndrome: A Review
Abstract
:1. Introduction
2. Pathogenetic Hypotheses in IBS
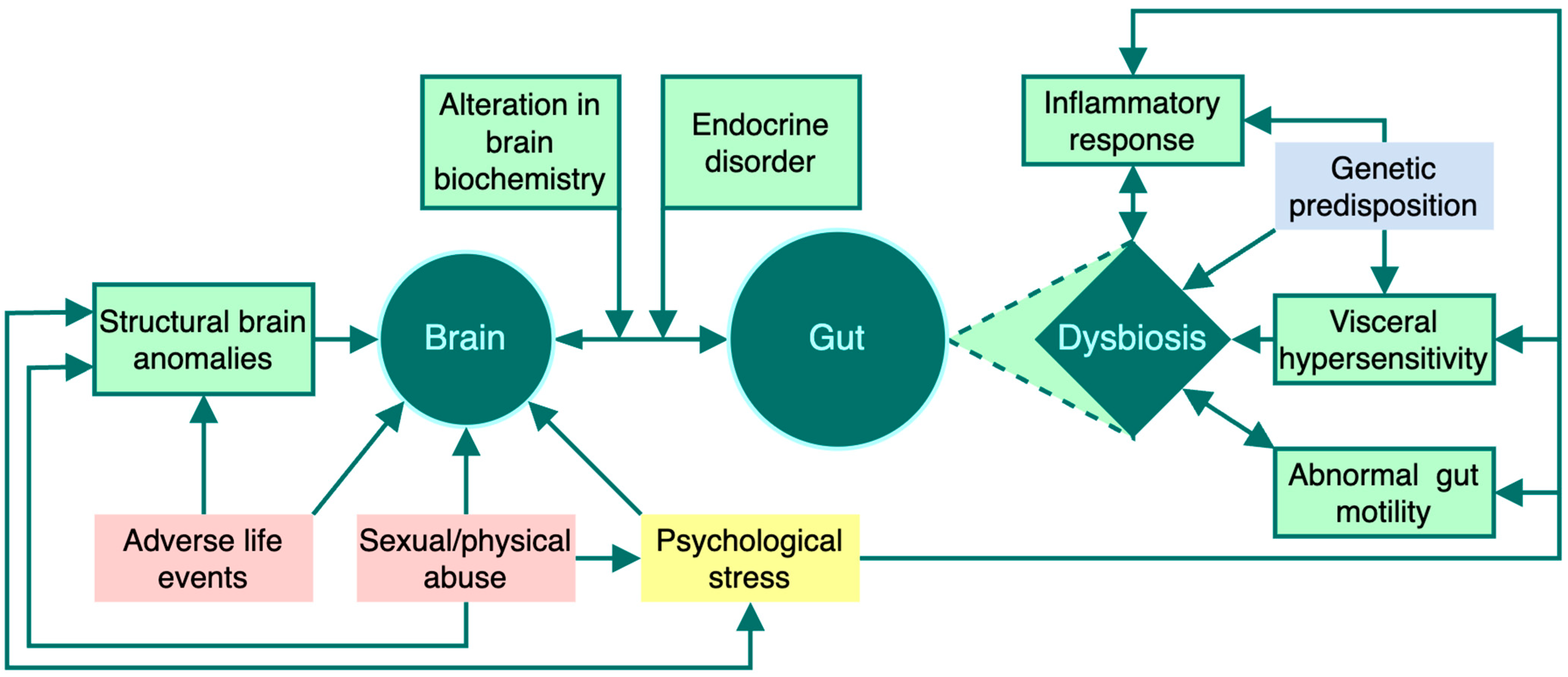
2.1. The Gut-Brain Axis
2.2. The Role of Genetic and Perinatal Factors in IBS Pathogenesis
2.3. Psychosocial Factors
2.4. The Role of Visceral Hypersensitivity in IBS
2.5. The Role of Hormonal Factors in IBS Pathogenesis
2.6. The Role of Mast Cells in IBS Pathogenesis
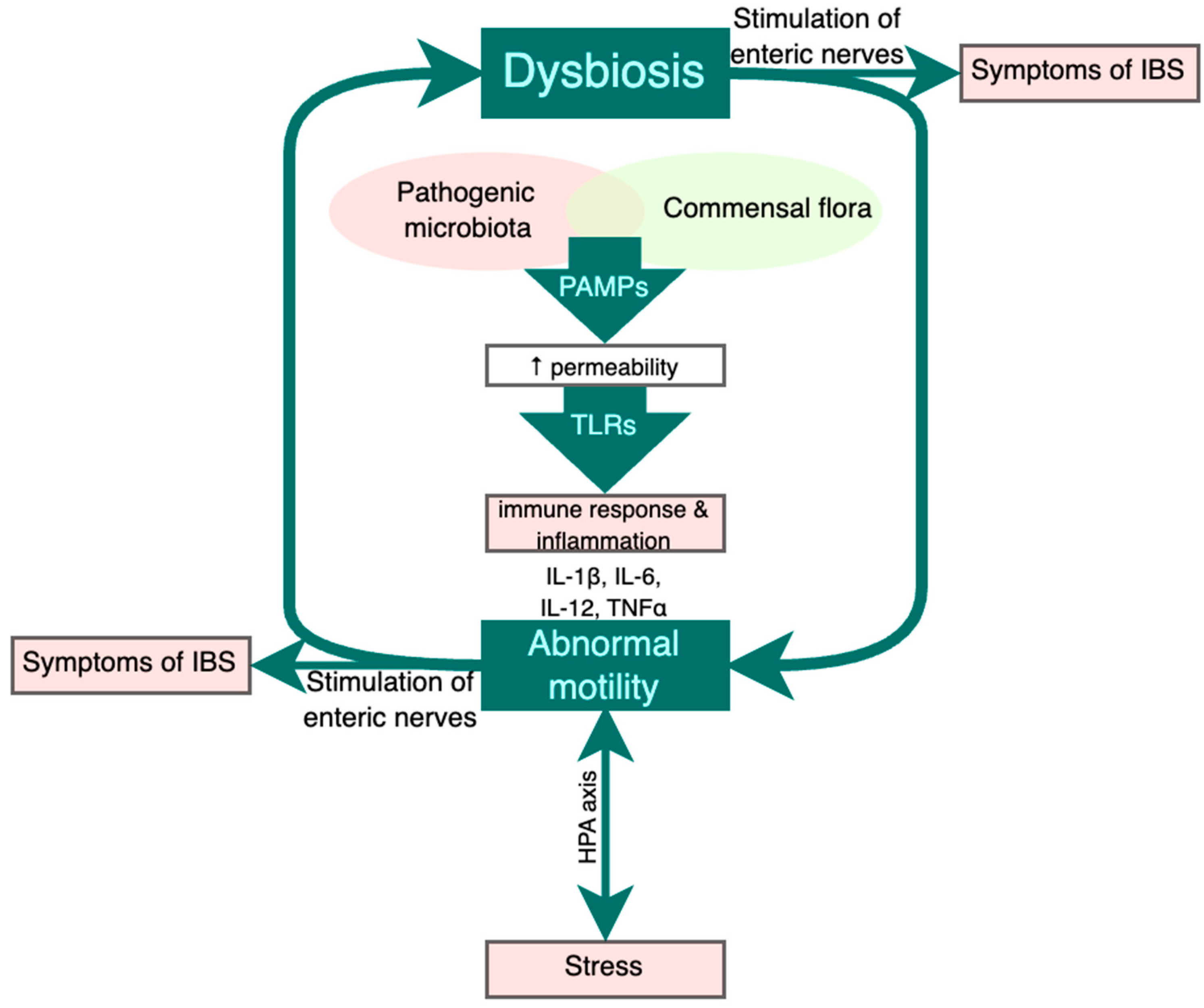
3. Postinfectious IBS
4. Role of Probiotics in IBS Pathogenesis and Management
4.1. Role of Probiotics in the Restoration of Microbiota Composition
4.2. Role of Probiotics in Improving Intestinal Motility
4.3. Role of Probiotics in Visceral Hypersensitivity
4.4. Probiotics and the Modulation of Inflammatory and Immune Processes
4.5. Role of Probiotics in Stress Response
5. Role of Prebiotics in IBS Pathogenesis and Management
6. Clinical Microbiota-Altering Treatment
7. Discussion
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sebastián Domingo, J.J. Síndrome del intestino irritable. Med. Clínica 2022, 158, 76–81. [Google Scholar] [CrossRef]
- Mearin, F.; Rey, E.; Santander, C. Síndrome del intestino irritable: Cómo mejorar la toma de decisiones en la práctica clínica. Med. Clínica 2018, 151, 489–497. [Google Scholar] [CrossRef]
- Schmulson, M.J.; Drossman, D.A. What Is New in Rome IV. J. Neurogastroenterol. Motil. 2017, 23, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Bonaz, B.; Bazin, T.; Pellissier, S. The Vagus Nerve at the Interface of the Microbiota-Gut-Brain Axis. Front. Neurosci. 2018, 12, 49. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, P.J.; Cryan, J.F.; Dinan, T.G.; Clarke, G. Irritable Bowel Syndrome: A Microbiome-Gut-Brain Axis Disorder? World J. Gastroenterol. 2014, 20, 14105–14125. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, J.A.; Blaser, M.J.; Caporaso, J.G.; Jansson, J.K.; Lynch, S.V.; Knight, R. Current Understanding of the Human Microbiome. Nat. Med. 2018, 24, 392–400. [Google Scholar] [CrossRef]
- Sommer, F.; Bäckhed, F. The Gut Microbiota—Masters of Host Development and Physiology. Nat. Rev. Microbiol. 2013, 11, 227–238. [Google Scholar] [CrossRef]
- Bastiaanssen, T.F.S.; Cowan, C.S.M.; Claesson, M.J.; Dinan, T.G.; Cryan, J.F. Making Sense of … the Microbiome in Psychiatry. Int. J. Neuropsychopharmacol. 2019, 22, 37–52. [Google Scholar] [CrossRef]
- Rousseaux, C.; Thuru, X.; Gelot, A.; Barnich, N.; Neut, C.; Dubuquoy, L.; Dubuquoy, C.; Merour, E.; Geboes, K.; Chamaillard, M.; et al. Lactobacillus Acidophilus Modulates Intestinal Pain and Induces Opioid and Cannabinoid Receptors. Nat. Med. 2007, 13, 35–37. [Google Scholar] [CrossRef] [PubMed]
- Popa, G.; Gheorghe, A.; Preda, M.; Popa, M.I. The Intestinal Microbiota Reconfigures the Boundaries of Knowledge. Infectio 2017, 49, 5–9. [Google Scholar] [CrossRef]
- Burns, G.; Carroll, G.; Mathe, A.; Horvat, J.; Foster, P.; Walker, M.M.; Talley, N.J.; Keely, S. Evidence for Local and Systemic Immune Activation in Functional Dyspepsia and the Irritable Bowel Syndrome: A Systematic Review. Am. J. Gastroenterol. 2019, 114, 429. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.; Mazmanian, S.K. Innate Immune Recognition of the Microbiota Promotes Host-Microbial Symbiosis. Nat. Immunol. 2013, 14, 668–675. [Google Scholar] [CrossRef] [PubMed]
- Shukla, R.; Ghoshal, U.; Ranjan, P.; Ghoshal, U.C. Expression of Toll-like Receptors, Pro-, and Anti-Inflammatory Cytokines in Relation to Gut Microbiota in Irritable Bowel Syndrome: The Evidence for Its Micro-Organic Basis. Neurogastroenterol. Motil. 2018, 24, 628–642. [Google Scholar] [CrossRef] [PubMed]
- Bashashati, M.; Rezaei, N.; Shafieyoun, A.; McKernan, D.P.; Chang, L.; Öhman, L.; Quigley, E.M.; Schmulson, M.; Sharkey, K.A.; Simrén, M. Cytokine Imbalance in Irritable Bowel Syndrome: A Systematic Review and Meta-Analysis. Neurogastroenterol. Motil. 2014, 26, 1036–1048. [Google Scholar] [CrossRef]
- Bennet, S.M.P.; Polster, A.; Törnblom, H.; Isaksson, S.; Capronnier, S.; Tessier, A.; Le Nevé, B.; Simrén, M.; Öhman, L. Global Cytokine Profiles and Association With Clinical Characteristics in Patients With Irritable Bowel Syndrome. Am. J. Gastroenterol. 2016, 111, 1165. [Google Scholar] [CrossRef]
- Mu, C.; Yang, Y.; Zhu, W. Crosstalk Between The Immune Receptors and Gut Microbiota. Curr. Protein Pept. Sci. 2015, 16, 622–631. [Google Scholar] [CrossRef]
- Ray, A.; Dittel, B.N. Interrelatedness between Dysbiosis in the Gut Microbiota Due to Immunodeficiency and Disease Penetrance of Colitis. Immunology 2015, 146, 359–368. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, Y.; Jin, T.; Yi, C.; Ocansey, D.K.W.; Mao, F. The Role of NOD2 in Intestinal Immune Response and Microbiota Modulation: A Therapeutic Target in Inflammatory Bowel Disease. Int. Immunopharmacol. 2022, 113, 109466. [Google Scholar] [CrossRef]
- Nagaishi, T.; Watabe, T.; Kotake, K.; Kumazawa, T.; Aida, T.; Tanaka, K.; Ono, R.; Ishino, F.; Usami, T.; Miura, T.; et al. Immunoglobulin A–Specific Deficiency Induces Spontaneous Inflammation Specifically in the Ileum. Gut 2022, 71, 487–496. [Google Scholar] [CrossRef]
- Pietrzak, B.; Tomela, K.; Olejnik-Schmidt, A.; Mackiewicz, A.; Schmidt, M. Secretory IgA in Intestinal Mucosal Secretions as an Adaptive Barrier against Microbial Cells. Int. J. Mol. Sci. 2020, 21, 9254. [Google Scholar] [CrossRef]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef] [PubMed]
- El-Salhy, M.; Hausken, T.; Gilja, O.H.; Hatlebakk, J.G. The Possible Role of Gastrointestinal Endocrine Cells in the Pathophysiology of Irritable Bowel Syndrome. Expert. Rev. Gastroenterol. Hepatol. 2017, 11, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Bär, F.; Von Koschitzky, H.; Roblick, U.; Bruch, H.P.; Schulze, L.; Sonnenborn, U.; Böttner, M.; Wedel, T. Cell-Free Supernatants of Escherichia Coli Nissle 1917 Modulate Human Colonic Motility: Evidence from an in Vitro Organ Bath Study. Neurogastroenterol. Motil. 2009, 21, 559-e17. [Google Scholar] [CrossRef] [PubMed]
- Levy, R.L.; Whitehead, W.E.; Von Korff, M.R.; Feld, A.D. Intergenerational Transmission of Gastrointestinal Illness Behavior. Am. J. Gastroenterol. 2000, 95, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Locke, G.R., 3rd; Zinsmeister, A.R.; Talley, N.J.; Fett, S.L.; Joseph Melton, L. Familial Association in Adults with Functional Gastrointestinal Disorders. Mayo Clin. Proc. 2000, 75, 907–912. [Google Scholar] [CrossRef]
- Czogalla, B.; Schmitteckert, S.; Houghton, L.A.; Sayuk, G.S.; Camilleri, M.; Olivo-Diaz, A.; Spiller, R.; Wouters, M.M.; Boeckxstaens, G.; Lorenzo Bermejo, J.; et al. A Meta-Analysis of Immunogenetic Case–Control Association Studies in Irritable Bowel Syndrome. Neurogastroenterol. Motil. 2015, 27, 717–727. [Google Scholar] [CrossRef]
- Cheung, C.K.; Wu, J.C. Genetic Polymorphism in Pathogenesis of Irritable Bowel Syndrome. World J. Gastroenterol. 2014, 20, 17693–17698. [Google Scholar] [CrossRef]
- Gazouli, M.; Wouters, M.M.; Kapur-Pojskić, L.; Bengtson, M.-B.; Friedman, E.; Nikčević, G.; Demetriou, C.A.; Mulak, A.; Santos, J.; Niesler, B. Lessons Learned—Resolving the Enigma of Genetic Factors in IBS. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 77–87. [Google Scholar] [CrossRef]
- Fouhy, F.; Watkins, C.; Hill, C.J.; O’Shea, C.-A.; Nagle, B.; Dempsey, E.M.; O’Toole, P.W.; Ross, R.P.; Ryan, C.A.; Stanton, C. Perinatal Factors Affect the Gut Microbiota up to Four Years after Birth. Nat. Commun. 2019, 10, 1517. [Google Scholar] [CrossRef]
- Waehrens, R.; Li, X.; Sundquist, J.; Sundquist, K.; Zöller, B. Perinatal and Familial Risk Factors for Irritable Bowel Syndrome in a Swedish National Cohort. Scand. J. Gastroenterol. 2018, 53, 559–566. [Google Scholar] [CrossRef]
- Jones, M.P.; Dilley, J.B.; Drossman, D.; Crowell, M.D. Brain-Gut Connections in Functional GI Disorders: Anatomic and Physiologic Relationships. Neurogastroenterol. Motil. 2006, 18, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Vanuytsel, T.; Bercik, P.; Boeckxstaens, G. Understanding Neuroimmune Interactions in Disorders of Gut–Brain Interaction: From Functional to Immune-Mediated Disorders. Gut 2023, 72, 787–798. [Google Scholar] [CrossRef] [PubMed]
- Hanning, N.; Edwinson, A.L.; Ceuleers, H.; Peters, S.A.; De Man, J.G.; Hassett, L.C.; De Winter, B.Y.; Grover, M. Intestinal Barrier Dysfunction in Irritable Bowel Syndrome: A Systematic Review. Therap Adv. Gastroenterol. 2021, 14, 1756284821993586. [Google Scholar] [CrossRef] [PubMed]
- Radovanovic-Dinic, B.; Tesic-Rajkovic, S.; Grgov, S.; Petrovic, G.; Zivkovic, V. Irritable Bowel Syndrome—From Etiopathogenesis to Therapy. Biomed. Pap. Med. Fac. Univ. Palacky. Olomouc Czech Repub. 2018, 162, 1–9. [Google Scholar] [CrossRef]
- Vanuytsel, T.; van Wanrooy, S.; Vanheel, H.; Vanormelingen, C.; Verschueren, S.; Houben, E.; Rasoel, S.S.; Tόth, J.; Holvoet, L.; Farré, R.; et al. Psychological Stress and Corticotropin-Releasing Hormone Increase Intestinal Permeability in Humans by a Mast Cell-Dependent Mechanism. Gut 2014, 63, 1293–1299. [Google Scholar] [CrossRef] [PubMed]
- Bassotti, G.; Bologna, S.; Ottaviani, L.; Russo, M.; Dore, M.P. Intestinal Manometry: Who Needs It? Gastroenterol. Hepatol. Bed Bench 2015, 8, 246–252. [Google Scholar]
- Bhattarai, Y.; Muniz Pedrogo, D.A.; Kashyap, P.C. Irritable Bowel Syndrome: A Gut Microbiota-Related Disorder? Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 312, G52–G62. [Google Scholar] [CrossRef]
- Weaver, K.R.; Boulineaux, C.M.; Robinson, J.M.; Butler, K.; Heitkemper, M.M.; Henderson, W.A. Sex Hormones, BDNF, Leptin, and TGF-Β1 in Females With IBS: A Pilot Investigation. Biol. Res. Nurs. 2021, 23, 231–237. [Google Scholar] [CrossRef]
- Jeffery, I.B.; O’Toole, P.W.; Öhman, L.; Claesson, M.J.; Deane, J.; Quigley, E.M.M.; Simrén, M. An Irritable Bowel Syndrome Subtype Defined by Species-Specific Alterations in Faecal Microbiota. Gut 2012, 61, 997–1006. [Google Scholar] [CrossRef]
- Zamani, M.; Alizadeh-Tabari, S.; Zamani, V. Systematic Review with Meta-Analysis: The Prevalence of Anxiety and Depression in Patients with Irritable Bowel Syndrome. Aliment. Pharmacol. Ther. 2019, 50, 132–143. [Google Scholar] [CrossRef]
- Fairlie, T.; Shah, A.; Talley, N.J.; Chey, W.D.; Koloski, N.; Yeh Lee, Y.; Gwee, K.-A.; Jones, M.P.; Holtmann, G. Overlap of Disorders of Gut-Brain Interaction: A Systematic Review and Meta-Analysis. Lancet Gastroenterol. Hepatol. 2023, 8, 646–659. [Google Scholar] [CrossRef] [PubMed]
- Marano, G.; Mazza, M.; Lisci, F.M.; Ciliberto, M.; Traversi, G.; Kotzalidis, G.D.; De Berardis, D.; Laterza, L.; Sani, G.; Gasbarrini, A.; et al. The Microbiota–Gut–Brain Axis: Psychoneuroimmunological Insights. Nutrients 2023, 15, 1496. [Google Scholar] [CrossRef] [PubMed]
- Wheatcroft, J.; Wakelin, D.; Smith, A.; Mahoney, C.R.; Mawe, G.; Spiller, R. Enterochromaffin Cell Hyperplasia and Decreased Serotonin Transporter in a Mouse Model of Postinfectious Bowel Dysfunction. Neurogastroenterol. Motil. 2005, 17, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.D. Enteric Neuroimmunophysiology and Pathophysiology. Gastroenterology 2004, 127, 635–657. [Google Scholar] [CrossRef]
- Ludidi, S.; Conchillo, J.M.; Keszthelyi, D.; Van Avesaat, M.; Kruimel, J.W.; Jonkers, D.M.; Masclee, A.A.M. Rectal Hypersensitivity as Hallmark for Irritable Bowel Syndrome: Defining the Optimal Cutoff. Neurogastroenterol. Motil. 2012, 24, 729-e346. [Google Scholar] [CrossRef]
- Ludidi, S.; Mujagic, Z.; Jonkers, D.; Keszthelyi, D.; Hesselink, M.; Kruimel, J.; Conchillo, J.; Masclee, A. Markers for Visceral Hypersensitivity in Patients with Irritable Bowel Syndrome. Neurogastroenterol. Motil. 2014, 26, 1104–1111. [Google Scholar] [CrossRef]
- Farzaei, M.H.; Bahramsoltani, R.; Abdollahi, M.; Rahimi, R. The Role of Visceral Hypersensitivity in Irritable Bowel Syndrome: Pharmacological Targets and Novel Treatments. J. Neurogastroenterol. Motil. 2016, 22, 558–574. [Google Scholar] [CrossRef]
- Thijssen, A.Y.; Jonkers, D.M.; Leue, C.; van der Veek, P.P.J.; Vidakovic-Vukic, M.; van Rood, Y.R.; Clemens, C.H.M.; Masclee, A.A.M. Dysfunctional Cognitions, Anxiety and Depression in Irritable Bowel Syndrome. J. Clin. Gastroenterol. 2010, 44, e236. [Google Scholar] [CrossRef]
- Spiller, R.; Aziz, Q.; Creed, F.; Emmanuel, A.; Houghton, L.; Hungin, P.; Jones, R.; Kumar, D.; Rubin, G.; Trudgill, N.; et al. Guidelines on the Irritable Bowel Syndrome: Mechanisms and Practical Management. Gut 2007, 56, 1770–1798. [Google Scholar] [CrossRef]
- Choghakhori, R.; Abbasnezhad, A.; Hasanvand, A.; Amani, R. Inflammatory Cytokines and Oxidative Stress Biomarkers in Irritable Bowel Syndrome: Association with Digestive Symptoms and Quality of Life. Cytokine 2017, 93, 34–43. [Google Scholar] [CrossRef]
- O’Sullivan, M.; Clayton, N.; Breslin, N.P.; Harman, I.; Bountra, C.; McLaren, A.; O’Morain, C.A. Increased Mast Cells in the Irritable Bowel Syndrome. Neurogastroenterol. Motil. 2000, 12, 449–457. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, F.; Mao, L.; Feng, T.; Wang, K.; Xu, M.; Lv, B.; Wang, X. Bifico Relieves Irritable Bowel Syndrome by Regulating Gut Microbiota Dysbiosis and Inflammatory Cytokines. Eur. J. Nutr. 2023, 62, 139–155. [Google Scholar] [CrossRef]
- Gwee, K.A.; Collins, S.M.; Read, N.W.; Rajnakova, A.; Deng, Y.; Graham, J.C.; McKendrick, M.W.; Moochhala, S.M. Increased rectal mucosal expression of interleukin 1beta in recently acquired post-infectious irritable bowel syndrome. Gut 2003, 52, 523–526. [Google Scholar] [CrossRef]
- Balmus, I.-M.; Ilie, O.-D.; Ciobica, A.; Cojocariu, R.-O.; Stanciu, C.; Trifan, A.; Cimpeanu, M.; Cimpeanu, C.; Gorgan, L. Irritable Bowel Syndrome between Molecular Approach and Clinical Expertise—Searching for Gap Fillers in the Oxidative Stress Way of Thinking. Medicina 2020, 56, 38. [Google Scholar] [CrossRef] [PubMed]
- Mete, R.; Tulubas, F.; Oran, M.; Yılmaz, A.; Avci, B.A.; Yildiz, K.; Turan, C.B.; Gurel, A. The Role of Oxidants and Reactive Nitrogen Species in Irritable Bowel Syndrome: A Potential Etiological Explanation. Med. Sci. Monit. 2013, 19, 762–766. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.C.; Farmer, A.D.; Ness, T.J.; Meerveld, B.G.-V. Critical Evaluation of Animal Models of Visceral Pain for Therapeutics Development: A Focus on Irritable Bowel Syndrome. Neurogastroenterol. Motil. 2020, 32, e13776. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, M.; Cerdà-Cuéllar, M.; Martínez, V. Antibiotic-Induced Dysbiosis Alters Host-Bacterial Interactions and Leads to Colonic Sensory and Motor Changes in Mice. Gut Microbes 2015, 6, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, M.; Vergara, P.; Martínez, V. Stress and Antibiotics Alter Luminal and Wall-Adhered Microbiota and Enhance the Local Expression of Visceral Sensory-Related Systems in Mice. Neurogastroenterol. Motil. 2013, 25, e515–e529. [Google Scholar] [CrossRef]
- Wei, L.; Singh, R.; Ro, S.; Ghoshal, U.C. Gut Microbiota Dysbiosis in Functional Gastrointestinal Disorders: Underpinning the Symptoms and Pathophysiology. JGH Open 2021, 5, 976–987. [Google Scholar] [CrossRef]
- Hegde, S.; Lin, Y.-M.; Fu, Y.; Savidge, T.; Shi, X.-Z. Precision Lactobacillus Reuteri Therapy Attenuates Luminal Distension-Associated Visceral Hypersensitivity by Inducing Peripheral Opioid Receptors in the Colon. Pain 2020, 161, 2737. [Google Scholar] [CrossRef]
- Anton, C.; Ciobica, A.; Doroftei, B.; Maftei, R.; Ilea, C.; Darii Plopa, N.; Bolota, M.; Anton, E. A Review of the Complex Relationship between Irritable Bowel Syndrome and Infertility. Medicina 2020, 56, 592. [Google Scholar] [CrossRef]
- Kim, Y.S.; Kim, N. Sex-Gender Differences in Irritable Bowel Syndrome. J. Neurogastroenterol. Motil. 2018, 24, 544–558. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M. Sex as a Biological Variable in Irritable Bowel Syndrome. Neurogastroenterol. Motil. 2020, 32, e13802. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M. Physiological Underpinnings of Irritable Bowel Syndrome: Neurohormonal Mechanisms. J. Physiol. 2014, 592, 2967–2980. [Google Scholar] [CrossRef] [PubMed]
- Lenhart, A.; Naliboff, B.; Shih, W.; Gupta, A.; Tillisch, K.; Liu, C.; Mayer, E.A.; Chang, L. Postmenopausal Women with Irritable Bowel Syndrome (IBS) Have More Severe Symptoms than Premenopausal Women with IBS. Neurogastroenterol. Motil. 2020, 32, e13913. [Google Scholar] [CrossRef]
- Han, B. Correlation between Gastrointestinal Hormones and Anxiety-depressive States in Irritable Bowel Syndrome. Exp. Ther. Med. 2013, 6, 715–720. [Google Scholar] [CrossRef]
- Osadchuk, M.A.; Burdina, V.O. Irritable bowel syndrome with extraintestinal manifestations from a position of neuroendocrine pathology. Eksp. Klin. Gastroenterol. 2015, 2, 29–34. [Google Scholar]
- Wang, L.; Fang, X.; Pan, G. Bacillary dysentery as a causative factor of irritable bowel syndrome and its pathogenesis. Gut 2004, 53, 1096–1101. [Google Scholar] [CrossRef]
- Barbara, G.; Stanghellini, V.; De Giorgio, R.; Corinaldesi, R. Functional Gastrointestinal Disorders and Mast Cells: Implications for Therapy. Neurogastroenterol. Motil. 2006, 18, 6–17. [Google Scholar] [CrossRef]
- Khoshbin, K.; Camilleri, M. Effects of Dietary Components on Intestinal Permeability in Health and Disease. Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 319, G589–G608. [Google Scholar] [CrossRef]
- Hillestad, E.M.R.; van der Meeren, A.; Nagaraja, B.H.; Bjørsvik, B.R.; Haleem, N.; Benitez-Paez, A.; Sanz, Y.; Hausken, T.; Lied, G.A.; Lundervold, A.; et al. Gut Bless You: The Microbiota-Gut-Brain Axis in Irritable Bowel Syndrome. World J. Gastroenterol. 2022, 28, 412–431. [Google Scholar] [CrossRef] [PubMed]
- Gasbarrini, A.; Lauritano, E.C.; Garcovich, M.; Sparano, L.; Gasbarrini, G. New Insights into the Pathophysiology of IBS: Intestinal Microflora, Gas Production and Gut Motility. Eur. Rev. Med. Pharmacol. Sci. 2008, 12 (Suppl. S1), 111–117. [Google Scholar] [PubMed]
- Liang, C.-M.; Hsu, C.-H.; Chung, C.-H.; Chen, C.-Y.; Wang, L.-Y.; Hsu, S.-D.; Chang, P.-K.; Hong, Z.-J.; Chien, W.-C.; Hu, J.-M. Risk for Irritable Bowel Syndrome in Patients with Helicobacter Pylori Infection: A Nationwide Population-Based Study Cohort Study in Taiwan. Int. J. Environ. Res. Public. Health 2020, 17, 3737. [Google Scholar] [CrossRef] [PubMed]
- Marginean, C.M.; Cioboata, R.; Olteanu, M.; Vasile, C.M.; Popescu, M.; Popescu, A.I.S.; Bondari, S.; Pirscoveanu, D.; Marginean, I.C.; Iacob, G.A.; et al. The Importance of Accurate Early Diagnosis and Eradication in Helicobacter Pylori Infection: Pictorial Summary Review in Children and Adults. Antibiotics 2023, 12, 60. [Google Scholar] [CrossRef]
- Chan, W.W.; Grover, M. The COVID-19 Pandemic and Post-Infection Irritable Bowel Syndrome: What Lies Ahead for Gastroenterologists. Clin. Gastroenterol. Hepatol. 2022, 20, 2195–2197. [Google Scholar] [CrossRef]
- Kamp, K.J.; Levy, R.L.; Munson, S.A.; Heitkemper, M.M. Impact of COVID-19 on Individuals With Irritable Bowel Syndrome and Comorbid Anxiety and/or Depression. J. Clin. Gastroenterol. 2022, 56, e149–e152. [Google Scholar] [CrossRef]
- Mizutani, T.; Ishizaka, A.; Koga, M.; Ikeuchi, K.; Saito, M.; Adachi, E.; Yamayoshi, S.; Iwatsuki-Horimoto, K.; Yasuhara, A.; Kiyono, H.; et al. Correlation Analysis between Gut Microbiota Alterations and the Cytokine Response in Patients with Coronavirus Disease during Hospitalization. Microbiol. Spectr. 2022, 10, e0168921. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Rodrigues, C.F.; Stojanović-Radić, Z.; Dimitrijević, M.; Aleksić, A.; Neffe-Skocińska, K.; Zielińska, D.; Kołożyn-Krajewska, D.; Salehi, B.; Milton Prabu, S.; et al. Probiotics: Versatile Bioactive Components in Promoting Human Health. Medicina 2020, 56, 433. [Google Scholar] [CrossRef]
- Islam, M.T.; Quispe, C.; Martorell, M.; Docea, A.O.; Salehi, B.; Calina, D.; Reiner, Ž.; Sharifi-Rad, J. Dietary Supplements, Vitamins and Minerals as Potential Interventions against Viruses: Perspectives for COVID-19. Int. J. Vitam. Nutr. Res. 2022, 92, 49–66. [Google Scholar] [CrossRef]
- Distrutti, E.; Monaldi, L.; Ricci, P.; Fiorucci, S. Gut Microbiota Role in Irritable Bowel Syndrome: New Therapeutic Strategies. World J. Gastroenterol. 2016, 22, 2219–2241. [Google Scholar] [CrossRef]
- Berg, L.K.; Goll, R.; Fagerli, E.; Ludviksen, J.K.; Fure, H.; Moen, O.S.; Sørbye, S.W.; Mollnes, T.E.; Florholmen, J. Intestinal Inflammatory Profile Shows Increase in a Diversity of Biomarkers in Irritable Bowel Syndrome. Scand. J. Gastroenterol. 2020, 55, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Satish Kumar, L.; Pugalenthi, L.S.; Ahmad, M.; Reddy, S.; Barkhane, Z.; Elmadi, J. Probiotics in Irritable Bowel Syndrome: A Review of Their Therapeutic Role. Cureus 2022, 14, e24240. [Google Scholar] [CrossRef]
- Eskesen, D.; Jespersen, L.; Michelsen, B.; Whorwell, P.J.; Müller-Lissner, S.; Morberg, C.M. Effect of the Probiotic Strain Bifidobacterium Animalis Subsp. Lactis, BB-12®, on Defecation Frequency in Healthy Subjects with Low Defecation Frequency and Abdominal Discomfort: A Randomised, Double-Blind, Placebo-Controlled, Parallel-Group Trial. Br. J. Nutr. 2015, 114, 1638–1646. [Google Scholar] [CrossRef] [PubMed]
- Soltani, N.; Abbasi, S.; Baghaeifar, S.; Taheri, E.; Farhoudi Sefidan Jadid, M.; Emami, P.; Abolhasani, K.; Aslanshirzadeh, F. Antibacterial and Antibiofilm Activity of Lactobacillus Strains Secretome and Extraction against Escherichia Coli Isolated from Urinary Tract Infection. Biotechnol. Rep. 2022, 36, e00760. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Laitila, A.; Ouwehand, A.C. Bifidobacterium Animalis Subsp. Lactis HN019 Effects on Gut Health: A Review. Front. Nutr. 2021, 8, 790561. [Google Scholar] [CrossRef] [PubMed]
- Morovic, W.; Roos, P.; Zabel, B.; Hidalgo-Cantabrana, C.; Kiefer, A.; Barrangou, R. Transcriptional and Functional Analysis of Bifidobacterium Animalis Subsp. Lactis Exposure to Tetracycline. Appl. Environ. Microbiol. 2018, 84, e01999-18. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.; Houghton, L.A.; Morris, J.; Reilly, B.; Guyonnet, D.; Goupil Feuillerat, N.; Schlumberger, A.; Jakob, S.; Whorwell, P.J. Clinical Trial: The Effects of a Fermented Milk Product Containing Bifidobacterium Lactis DN-173 010 on Abdominal Distension and Gastrointestinal Transit in Irritable Bowel Syndrome with Constipation. Aliment. Pharmacol. Ther. 2009, 29, 104–114. [Google Scholar] [CrossRef]
- Chen, Y.-M.; Li, Y.; Wang, X.; Wang, Z.-L.; Hou, J.-J.; Su, S.; Zhong, W.-L.; Xu, X.; Zhang, J.; Wang, B.-M.; et al. Effect of Bacillus Subtilis, Enterococcus Faecium, and Enterococcus Faecalis Supernatants on Serotonin Transporter Expression in Cells and Tissues. World J. Gastroenterol. 2022, 28, 532–546. [Google Scholar] [CrossRef]
- Szajewska, H.; Hojsak, I. Health Benefits of Lactobacillus Rhamnosus GG and Bifidobacterium Animalis Subspecies Lactis BB-12 in Children. Postgrad. Med. 2020, 132, 441–451. [Google Scholar] [CrossRef]
- Tillisch, K.; Labus, J.; Kilpatrick, L.; Jiang, Z.; Stains, J.; Ebrat, B.; Guyonnet, D.; Legrain-Raspaud, S.; Trotin, B.; Naliboff, B.; et al. Consumption of Fermented Milk Product with Probiotic Modulates Brain Activity. Gastroenterology 2013, 144, 1394–1401.e4. [Google Scholar] [CrossRef]
- Zhou, L.; Foster, J.A. Psychobiotics and the Gut-Brain Axis: In the Pursuit of Happiness. Neuropsychiatr. Dis. Treat. 2015, 11, 715–723. [Google Scholar] [CrossRef]
- Martín, R.; Chain, F.; Miquel, S.; Natividad, J.M.; Sokol, H.; Verdu, E.F.; Langella, P.; Bermúdez-Humarán, L.G. Effects in the Use of a Genetically Engineered Strain of Lactococcus Lactis Delivering in Situ IL-10 as a Therapy to Treat Low-Grade Colon Inflammation. Hum. Vaccines Immunother. 2014, 10, 1611–1621. [Google Scholar] [CrossRef] [PubMed]
- Gad, M.; Ravn, P.; Søborg, D.A.; Lund-Jensen, K.; Ouwehand, A.C.; Jensen, S.S. Regulation of the IL-10/IL-12 Axis in Human Dendritic Cells with Probiotic Bacteria. FEMS Immunol. Med. Microbiol. 2011, 63, 93–107. [Google Scholar] [CrossRef] [PubMed]
- Sabaté, J.-M.; Iglicki, F. Effect of Bifidobacterium Longum 35624 on Disease Severity and Quality of Life in Patients with Irritable Bowel Syndrome. World J. Gastroenterol. 2022, 28, 732–744. [Google Scholar] [CrossRef] [PubMed]
- Konturek, T.J.; Martinez, C.; Niesler, B.; van der Voort, I.; Mönnikes, H.; Stengel, A.; Goebel-Stengel, M. The Role of Brain-Derived Neurotrophic Factor in Irritable Bowel Syndrome. Front. Psychiatry 2021, 11, 531385. [Google Scholar] [CrossRef]
- Nagpal, R.; Wang, S.; Ahmadi, S.; Hayes, J.; Gagliano, J.; Subashchandrabose, S.; Kitzman, D.W.; Becton, T.; Read, R.; Yadav, H. Human-Origin Probiotic Cocktail Increases Short-Chain Fatty Acid Production via Modulation of Mice and Human Gut Microbiome. Sci. Rep. 2018, 8, 12649. [Google Scholar] [CrossRef]
- Miller, L.E.; Ouwehand, A.C. Probiotic Supplementation Decreases Intestinal Transit Time: Meta-Analysis of Randomized Controlled Trials. World J. Gastroenterol. 2013, 19, 4718–4725. [Google Scholar] [CrossRef]
- Choi, C.H.; Kwon, J.G.; Kim, S.K.; Myung, S.-J.; Park, K.S.; Sohn, C.-I.; Rhee, P.-L.; Lee, K.J.; Lee, O.Y.; Jung, H.-K.; et al. Efficacy of Combination Therapy with Probiotics and Mosapride in Patients with IBS without Diarrhea: A Randomized, Double-Blind, Placebo-Controlled, Multicenter, Phase II Trial. Neurogastroenterol. Motil. 2015, 27, 705–716. [Google Scholar] [CrossRef]
- Ki Cha, B.; Mun Jung, S.; Hwan Choi, C.; Song, I.-D.; Woong Lee, H.; Joon Kim, H.; Hyuk, J.; Kyung Chang, S.; Kim, K.; Chung, W.-S.; et al. The Effect of a Multispecies Probiotic Mixture on the Symptoms and Fecal Microbiota in Diarrhea-Dominant Irritable Bowel Syndrome: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Clin. Gastroenterol. 2012, 46, 220–227. [Google Scholar] [CrossRef]
- Dimidi, E.; Christodoulides, S.; Fragkos, K.C.; Scott, S.M.; Whelan, K. The Effect of Probiotics on Functional Constipation in Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Am. J. Clin. Nutr. 2014, 100, 1075–1084. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, C.; Zhang, J.; Sun, F.; Duan, L. Efficacy of Probiotics for Irritable Bowel Syndrome: A Systematic Review and Network Meta-Analysis. Front. Cell Infect. Microbiol. 2022, 12, 859967. [Google Scholar] [CrossRef]
- Mayer, E.A.; Savidge, T.; Shulman, R.J. Brain-Gut Microbiome Interactions and Functional Bowel Disorders. Gastroenterology 2014, 146, 1500–1512. [Google Scholar] [CrossRef]
- Johnson, A.C.; Greenwood-Van Meerveld, B.; McRorie, J. Effects of Bifidobacterium Infantis 35624 on Post-Inflammatory Visceral Hypersensitivity in the Rat. Dig. Dis. Sci. 2011, 56, 3179–3186. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, S.C.; Barbara, G.; Buurman, W.; Ockhuizen, T.; Schulzke, J.-D.; Serino, M.; Tilg, H.; Watson, A.; Wells, J.M. Intestinal Permeability--a New Target for Disease Prevention and Therapy. BMC Gastroenterol. 2014, 14, 189. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M.; Lasch, K.; Zhou, W. Irritable Bowel Syndrome: Methods, Mechanisms, and Pathophysiology. The Confluence of Increased Permeability, Inflammation, and Pain in Irritable Bowel Syndrome. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, G775–G785. [Google Scholar] [CrossRef] [PubMed]
- Mitselou, A.; Grammeniatis, V.; Varouktsi, A.; Papadatos, S.S.; Katsanos, K.; Galani, V. Proinflammatory Cytokines in Irritable Bowel Syndrome: A Comparison with Inflammatory Bowel Disease. Intest. Res. 2020, 18, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Bashashati, M.; Rezaei, N.; Bashashati, H.; Shafieyoun, A.; Daryani, N.E.; Sharkey, K.A.; Storr, M. Cytokine Gene Polymorphisms Are Associated with Irritable Bowel Syndrome: A Systematic Review and Meta-Analysis. Neurogastroenterol. Motil. 2012, 24, 1102-e566. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Shukla, R.; Ranjan, P.; Kumar, A. Interleukin-10: A Compelling Therapeutic Target in Patients With Irritable Bowel Syndrome. Clin. Ther. 2017, 39, 632–643. [Google Scholar] [CrossRef] [PubMed]
- Le Morvan de Sequeira, C.; Kaeber, M.; Cekin, S.E.; Enck, P.; Mack, I. The Effect of Probiotics on Quality of Life, Depression and Anxiety in Patients with Irritable Bowel Syndrome: A Systematic Review and Meta-Analysis. J. Clin. Med. 2021, 10, 3497. [Google Scholar] [CrossRef]
- Pusceddu, M.M.; Murray, K.; Gareau, M.G. Targeting the Microbiota, From Irritable Bowel Syndrome to Mood Disorders: Focus on Probiotics and Prebiotics. Curr. Pathobiol. Rep. 2018, 6, 1–13. [Google Scholar] [CrossRef]
- Messaoudi, M.; Violle, N.; Bisson, J.-F.; Desor, D.; Javelot, H.; Rougeot, C. Beneficial Psychological Effects of a Probiotic Formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in Healthy Human Volunteers. Gut Microbes 2011, 2, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Messaoudi, M.; Lalonde, R.; Violle, N.; Javelot, H.; Desor, D.; Nejdi, A.; Bisson, J.-F.; Rougeot, C.; Pichelin, M.; Cazaubiel, M.; et al. Assessment of Psychotropic-like Properties of a Probiotic Formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in Rats and Human Subjects. Br. J. Nutr. 2011, 105, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Moloney, R.D.; Johnson, A.C.; O’Mahony, S.M.; Dinan, T.G.; Greenwood-Van Meerveld, B.; Cryan, J.F. Stress and the Microbiota–Gut–Brain Axis in Visceral Pain: Relevance to Irritable Bowel Syndrome. CNS Neurosci. Ther. 2016, 22, 102–117. [Google Scholar] [CrossRef] [PubMed]
- Francavilla, R.; Miniello, V.; Magistà, A.M.; De Canio, A.; Bucci, N.; Gagliardi, F.; Lionetti, E.; Castellaneta, S.; Polimeno, L.; Peccarisi, L.; et al. A Randomized Controlled Trial of Lactobacillus GG in Children With Functional Abdominal Pain. Pediatrics 2010, 126, e1445–e1452. [Google Scholar] [CrossRef] [PubMed]
- Raskov, H.; Burcharth, J.; Pommergaard, H.C.; Rosenberg, J. Irritable Bowel Syndrome, the Microbiota and the Gut-brain Axis. Gut Microbes 2016, 7, 365–383. [Google Scholar] [CrossRef] [PubMed]
- Benarroch, E.E. Brain-Derived Neurotrophic Factor: Regulation, Effects, and Potential Clinical Relevance. Neurology 2015, 84, 1693–1704. [Google Scholar] [CrossRef]
- Qi, Q.; Chen, F.; Zhang, W.; Wang, P.; Li, Y.; Zuo, X. Colonic N-Methyl-d-Aspartate Receptor Contributes to Visceral Hypersensitivity in Irritable Bowel Syndrome. J. Gastroenterol. Hepatol. 2017, 32, 828–836. [Google Scholar] [CrossRef]
- Distrutti, E.; O’Reilly, J.-A.; McDonald, C.; Cipriani, S.; Renga, B.; Lynch, M.A.; Fiorucci, S. Modulation of Intestinal Microbiota by the Probiotic VSL#3 Resets Brain Gene Expression and Ameliorates the Age-Related Deficit in LTP. PLoS ONE 2014, 9, e106503. [Google Scholar] [CrossRef]
- Savignac, H.M.; Tramullas, M.; Kiely, B.; Dinan, T.G.; Cryan, J.F. Bifidobacteria Modulate Cognitive Processes in an Anxious Mouse Strain. Behav. Brain Res. 2015, 287, 59–72. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert Consensus Document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef]
- Wilson, B.; Whelan, K. Prebiotic Inulin-Type Fructans and Galacto-Oligosaccharides: Definition, Specificity, Function, and Application in Gastrointestinal Disorders. J. Gastroenterol. Hepatol. 2017, 32 (Suppl. S1), 64–68. [Google Scholar] [CrossRef] [PubMed]
- O’Keefe, S.J.D.; Li, J.V.; Lahti, L.; Ou, J.; Carbonero, F.; Mohammed, K.; Posma, J.M.; Kinross, J.; Wahl, E.; Ruder, E.; et al. Fat, Fibre and Cancer Risk in African Americans and Rural Africans. Nat. Commun. 2015, 6, 6342. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B.; Rossi, M.; Dimidi, E.; Whelan, K. Prebiotics in Irritable Bowel Syndrome and Other Functional Bowel Disorders in Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Am. J. Clin. Nutr. 2019, 109, 1098–1111. [Google Scholar] [CrossRef] [PubMed]
- Silk, D.B.A.; Davis, A.; Vulevic, J.; Tzortzis, G.; Gibson, G.R. Clinical Trial: The Effects of a Trans-Galactooligosaccharide Prebiotic on Faecal Microbiota and Symptoms in Irritable Bowel Syndrome. Aliment. Pharmacol. Ther. 2009, 29, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Azpiroz, F.; Dubray, C.; Bernalier-Donadille, A.; Cardot, J.-M.; Accarino, A.; Serra, J.; Wagner, A.; Respondek, F.; Dapoigny, M. Effects of ScFOS on the Composition of Fecal Microbiota and Anxiety in Patients with Irritable Bowel Syndrome: A Randomized, Double Blind, Placebo Controlled Study. Neurogastroenterol. Motil. 2017, 29, e12911. [Google Scholar] [CrossRef] [PubMed]
- Niv, E.; Halak, A.; Tiommny, E.; Yanai, H.; Strul, H.; Naftali, T.; Vaisman, N. Randomized Clinical Study: Partially Hydrolyzed Guar Gum (PHGG) versus Placebo in the Treatment of Patients with Irritable Bowel Syndrome. Nutr. Metab. 2016, 13, 10. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Ren, Y.; Lu, J.; Bartlett, M.; Chen, L.; Zhang, Y.; Guo, X.; Liu, C. A Novel Prebiotic Blend Product Prevents Irritable Bowel Syndrome in Mice by Improving Gut Microbiota and Modulating Immune Response. Nutrients 2017, 9, 1341. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, K.; Reed, D.E.; Schneider, T.; Dang, F.; Keshteli, A.H.; Palma, G.D.; Madsen, K.; Bercik, P.; Vanner, S. FODMAPs Alter Symptoms and the Metabolome of Patients with IBS: A Randomised Controlled Trial. Gut 2017, 66, 1241–1251. [Google Scholar] [CrossRef]
- Hustoft, T.N.; Hausken, T.; Ystad, S.O.; Valeur, J.; Brokstad, K.; Hatlebakk, J.G.; Lied, G.A. Effects of Varying Dietary Content of Fermentable Short-Chain Carbohydrates on Symptoms, Fecal Microenvironment, and Cytokine Profiles in Patients with Irritable Bowel Syndrome. Neurogastroenterol. Motil. 2017, 29, e12969. [Google Scholar] [CrossRef]
- Cui, J.; Lin, Z.; Tian, H.; Yang, B.; Zhao, D.; Ye, C.; Li, N.; Qin, H.; Chen, Q. Long-Term Follow-Up Results of Fecal Microbiota Transplantation for Irritable Bowel Syndrome: A Single-Center, Retrospective Study. Front. Med. 2021, 8, 710452. [Google Scholar] [CrossRef]
- Sender, R.; Fuchs, S.; Milo, R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef] [PubMed]
- de Vos, W.M.; de Vos, E.A. Role of the Intestinal Microbiome in Health and Disease: From Correlation to Causation. Nutr. Rev. 2012, 70, S45–S56. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; Dinan, T.G. Mind-Altering Microorganisms: The Impact of the Gut Microbiota on Brain and Behaviour. Nat. Rev. Neurosci. 2012, 13, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Tap, J.; Derrien, M.; Törnblom, H.; Brazeilles, R.; Cools-Portier, S.; Doré, J.; Störsrud, S.; Le Nevé, B.; Öhman, L.; Simrén, M. Identification of an Intestinal Microbiota Signature Associated with Severity of Irritable Bowel Syndrome. Gastroenterology 2017, 152, 111–123.e8. [Google Scholar] [CrossRef] [PubMed]
- Vahora, I.S.; Tsouklidis, N.; Kumar, R.; Soni, R.; Khan, S.; Vahora, I.S.; Tsouklidis, N.; Kumar, R.; Soni, R.; Khan, S. How Serotonin Level Fluctuation Affects the Effectiveness of Treatment in Irritable Bowel Syndrome. Cureus 2020, 12, e9871. [Google Scholar] [CrossRef]
- Krammer, L.; Sowa, A.S.; Lorentz, A. Mast Cells in Irritable Bowel Syndrome: A Systematic Review. J. Gastrointestin Liver Dis. 2019, 28, 463–472. [Google Scholar] [CrossRef]
| Genus | Lactobacillus | Bifidobacterium | Other |
|---|---|---|---|
| Species | Lactobacillus acidophilus | Bifidobacterium animalis spp. Lactis | Bacillus coagulans |
| Lactobacillus casei | Bifidobacterium breve | Enterococcus faecalis | |
| Lactobacillus helveticus | Bifidobacterium infantis spp. Lactis | Saccharomyces boulardii | |
| Lactobacillus johnsonii | Bifidobacterium longum | Streptococcus thermophilus | |
| Lactobacillus paracasei | |||
| Lactobacillus plantarum | |||
| Lactobacillus reuteri | |||
| Lactobacillus rhamnosus |
| Benefit | Probiotics | References |
|---|---|---|
| Pathogenic microbiota development inhibition | Bifidobacterium spp., Lactobacillus spp. | [83,84] |
| Intestinal motility improvement | Bifidobacterium lactis, Bifidobacterium breve, Bifidobacterium longum, Bacillus subtilis, Streptococcus faecium, Streptococcus thermophilus, Lactobacillus acidophilus, Lactobacillus plantarum, Lactobacillus rhamnosus | [85,86,87,88,89] |
| Antinociception | Bifidobacterium lactis, Streptococcus thermophiles, Lactobacillus bulgaricus, Lactococcus lactis | [90,91] |
| Decrease in inflammatory and immune response | Bifidobacterium infantis, Lactobacillus lactis, Lactobacillus acidophilus, E. coli Nissle | [92,93] |
| Stress response improvement | Bifidobacterium longum, Bifidobacterium breve, Bifidobacterium infantis, Streptococcus casei, Lactobacillus rhamnosus, Lactobacillus helveticus, Lactobacillus casei, Lactobacillus acidophilus, Lactobacillus plantarum, Lactobacillus bulgaricus | [94,95] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marginean, C.M.; Popescu, M.; Drocas, A.I.; Cazacu, S.M.; Mitrut, R.; Marginean, I.C.; Iacob, G.A.; Popescu, M.S.; Docea, A.O.; Mitrut, P. Gut–Brain Axis, Microbiota and Probiotics—Current Knowledge on Their Role in Irritable Bowel Syndrome: A Review. Gastrointest. Disord. 2023, 5, 517-535. https://doi.org/10.3390/gidisord5040043
Marginean CM, Popescu M, Drocas AI, Cazacu SM, Mitrut R, Marginean IC, Iacob GA, Popescu MS, Docea AO, Mitrut P. Gut–Brain Axis, Microbiota and Probiotics—Current Knowledge on Their Role in Irritable Bowel Syndrome: A Review. Gastrointestinal Disorders. 2023; 5(4):517-535. https://doi.org/10.3390/gidisord5040043
Chicago/Turabian StyleMarginean, Cristina Maria, Mihaela Popescu, Andrei Ioan Drocas, Sergiu Marian Cazacu, Radu Mitrut, Iulia Cristina Marginean, George Alexandru Iacob, Marian Sorin Popescu, Anca Oana Docea, and Paul Mitrut. 2023. "Gut–Brain Axis, Microbiota and Probiotics—Current Knowledge on Their Role in Irritable Bowel Syndrome: A Review" Gastrointestinal Disorders 5, no. 4: 517-535. https://doi.org/10.3390/gidisord5040043
APA StyleMarginean, C. M., Popescu, M., Drocas, A. I., Cazacu, S. M., Mitrut, R., Marginean, I. C., Iacob, G. A., Popescu, M. S., Docea, A. O., & Mitrut, P. (2023). Gut–Brain Axis, Microbiota and Probiotics—Current Knowledge on Their Role in Irritable Bowel Syndrome: A Review. Gastrointestinal Disorders, 5(4), 517-535. https://doi.org/10.3390/gidisord5040043








