Abstract
Appendiceal tumors represent a large amalgam of different tumor lineages. The continuous evolution in their pathological classifications has led to some variable recommended attitudes over time. The aim of this study is to review the incidence, clinicopathological characteristics, therapeutic approach and oncological results in this type of tumor at our institution. This is a single-centre retrospective cohort study. Every pathologic report catalogued as an appendiceal specimen was reviewed for a time period of 5 years (2013–2017) at our institution. Demographic, clinical, pathological and oncologic follow-up data were recorded. A descriptive study of the sample was completed. A total of 1434 appendiceal specimens was analyzed. Appendiceal neoplasms incidence was 3.2%. Epithelial tumors were the predominant histological subtype, making up 68% of the cases. Low-grade appendiceal mucinous neoplasia and neuroendocrine tumors were the most frequent neoplasms with malignant potential, with 13 and 6 cases, respectively. In more than 80% of neoplasia cases, the definitive treatment was appendectomy. Mortality cases were related to tumors with a very poor prognosis and an advanced stage. All patients had adequate oncological follow-up. Although it is still quite rare, the incidence of appendiceal tumors is increasing with an epidemiological change in favor of mucinous neoplasms currently predominating. Therefore, it is necessary to know and use an updated anatomo-pathological classification in order to provide correct treatment in the first or second surgical stage, as well as the correct follow-up of patients.
1. Introduction
Appendiceal tumors are usually described as a rare entity, making up approximately 0.2 to 0.5 of gastrointestinal tumors. In most cases, preoperative clinical detection is difficult and very often its appearance comes as a casual finding after an emergency appendectomy due to acute appendicitis, which makes up around 2% approximately of the same [1].
Its histologic classification [2] as well as its epidemiology have suffered recent changes [3], and this implies that a lack of awareness regarding the treatment recommendations in each case could be a latent risk in our clinical practice. The correct alternative behavior could be very different from having a correct follow-up or having the possibility of surgery, sometimes a very aggressive one [4].
Historically, neuroendocrine tumors (NET) have been the main appendiceal tumor. However, there has been an epidemiological change and now epithelial neoplasms are more frequent [5], being currently the main reason for insisting on the correct use of its nomenclature and classification (Figure 1). For instance, terms such as mucocele, cistoadenoma or borderline tumors have disappeared and in the last classification they were included among the low-grade appendiceal mucinous neoplasms (LAMNs) [1].

Figure 1.
(a) Conventional appendiceal adenocarcinoma: the light is occupied by an epithelial proliferation of atypical cells that form glands and that focally infiltrate the wall. (HE × 20). (b) Mucinous adenocarcinoma: an epithelial proliferation of atypical cells that form glands which are well differentiated with mucus secretion in the light and that focally infiltrates the wall. (HE × 40). (c) Neuroendocrine carcinoma: infiltration of the appendix wall by a vague proliferation of a high degree without glandular differentiation, of isolated cells of solid aspect, infiltration of vascular structures that in immunohistochemistry studies were positive for neuroendocrine markers (chromogranin and synaptophysin) (HE × 100).
From a histologic point of view, appendiceal tumors are classified into epithelial and non-epithelial, and at the same time, the first group is subdivided into mucinous and non-mucinous. In this same division, we can find together benign and malignant neoplasms, which is another option when it comes to classifying them [2,6,7] (Table 1).

Table 1.
Appendiceal tumors classification based on the last update of the World Health Organization (WHO).
Considering this context, we have designed a study to establish the incidence of appendiceal tumors in our region, as well as to make a critical analysis of its histologic classification, follow-up and outcome from an oncological point of view.
2. Results
A total of 1434 patients with an appendectomy was finally reviewed, with a distribution of 791 men (55%) and 643 women (45%). The mean age of the appendectomy was 34.5 (IQR 18–52). From the total sample, 1375 cases (96%) were operated on an emergency basis with clinical suspicion of acute appendicitis. Pain (78%) and pain plus fever (16.6%) were the two main symptoms among this cohort. A total of 46 appendiceal neoplasms was definitely diagnosed in the present sample, which means an incidence of 3.2%; the mean age of patients with appendiceal tumors was 63 years (IQR 52–75).
Of the 1375 patients operated in an emergency setting, all but 3 were just for appendectomies, 2 were for ileocecal resections including a diseased appendix, while another case required a right hemicolectomy. Of these, the two ileocecal resections were performed due to an intraoperative mass but not specifically due to suspicion of an appendiceal neoplasm. The histological analysis of the specimen revealed in both cases a LAMN. The case of the right hemicolectomy that was performed during emergency intervention was because of a preoperative radiological suspicion of peri-appendiceal plastron and an intraoperative judgment of a complicated cecal mass. The final pathologic report was just appendicitis with plastron and peritonitis.
There were an additional 57 cases that were managed conservatively and where surgical intervention was differed and scheduled. Of these cases, just one required a different intervention than an appendectomy; a right hemicolectomy was performed due to the suspicion of an appendiceal mucinous neoplasm as an incidental finding in an abdominal CT scan for other reasons. A radical oncological right hemicolectomy was performed through a laparoscopic approach. The final pathological report was a LAMN.
None of the operative reports described the presence of a free mucus.
When analyzing the macroscopic findings described by the surgeons during the intervention and the subsequent pathological report, we could not find a statistically significant interrelationship between all those criteria for clinical suspicion of appendiceal tumor by the surgeon during the intervention (appendiceal mass, free mucus, plastron or integrity of the appendiceal specimen) and the final histologic diagnosis. Differences between the presented symptoms and the final diagnosis of appendiceal neoplasm could not be identified either.
Table 2 summarizes demographic data, the type of primary approach and histological findings in the sample.

Table 2.
General characteristics of the sample. Demographic and clinical characteristics, technical details and anatomo-pathological findings of the sample.
According to their histologic classification, the epithelial tumors comprised 68.3% of the total, being the mucinous subtype more frequent than the non-mucinous one. The most frequent findings related to borderline neoplasms or with malignant potential were the LAMN tumors with 13 cases, followed by the neuroendocrine ones with 6 cases. Of these, all the cases were tumors < 1 cm and G1 (except 1 case G2), where the final treatment was an appendectomy. The patient with NET G2 was a pediatric patient who, after the appendectomy, continued with strict radiological monitoring without signs of a reappearance.
The most frequent benign neoplasm was an appendiceal hyperplasic polyp in nine patients, followed by a neuroma. The distribution of our findings, according to the histologic classification of appendiceal tumors, is summarized in Table 3.

Table 3.
Tumors distribution according to histologic subtype.
Based on histological findings, five cases (11%) of the patients with appendiceal tumors required a second procedure for completing the treatment with curative intent. All of them received a right hemicolectomy: two because of a mucinous adenocarcinoma pT3 (Figure 1), one of them with a free mucus; another two cases with LAMN pT3 (a free mucus in one of them); and finally, one patient with a locally advanced and metastatic mucinous neoplasm with uncertain malignant potential.
Additionally, during the follow-up, three more patients were diagnosed with local or peritoneal recurrences and salvage surgery procedures were performed: two right hemicolectomies and a cytoreductive surgery with multivisceral excision. All three cases had poor prognosis histological markers in the primary specimen such as signet ring cells (two cases) and peritoneal mucinous carcinomatosis (one case).
During the long-term follow-up, four cases of mortality were registered. They were the patients with neoplasms and a poor prognosis: two cases of signet ring cell adenocarcinoma (Figure 2), one patient with a mixed adenoneuroendocrine tumor (Figure 3) and a single case of peritoneal mucinous carcinomatosis.
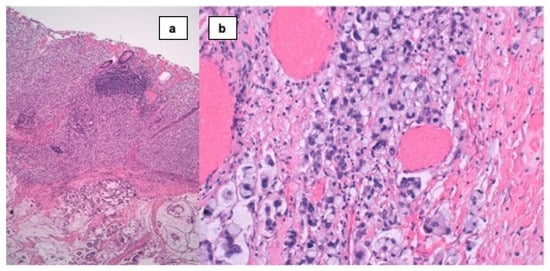
Figure 2.
(a) Signet ring cell carcinoma: infiltration of the appendix wall by a vague proliferation (superior half) of isolated cells of solid aspect, with mucus secretion areas and glandular differentiation (inferior half). (HE × 40). (b) Signet ring cell carcinoma: infiltration of the appendix wall by a vague proliferation of isolated cells of solid aspect, that present cytoplasm with an only vacuole and rejected nucleus when in a huge increase.

Figure 3.
(a) High-grade appendiceal mucinous neoplasm (HAMN): glandular proliferation by neoplasm with cytological changes of high grade, with invasion of infiltrative type and hypocellular mucinous deposits. (HE × 40). (b) Mixed adeno-neuroendocrine carcinoma (MANEC): tumor that presents two types of differentiation: one area of glandular proliferation with mucus secretion and another more solid area, more differentiated, corresponding to the neuroendocrine component (HE × 20).
Every patient with an appendiceal neoplasm was adequately followed up during the study period, including clinical, analytical, endoscopic and radiological surveillance. The average follow-up time in the present population was 17 months.
3. Discussion
The current incidence of appendiceal tumors described at our institution is slightly higher than the ones that have been previously described in other studies. In a recent systematic review from Bastiaenen et al. [8], an incidence of appendiceal neoplasms of 0.71% was reported. Likewise, in an analysis of 4545 pieces of appendectomy, the group of Lohsiriwat et al. [9] found a rate of tumors of 0.97%. However, there are reports in other studies with incidences of neoplasms of up to 2.5%, similar to our findings [10,11]. Our findings can be added to others described in the literature, where an increase of up to 54% in the incidence of appendiceal tumors in recent decades is described [12].
The mean age of 63 years (IQR 52–75) of the patients with neoplasm in our sample is confirmed as in some studies being older than 50 was considered as a risk factor for appendiceal tumors [10,13]. Even some recent studies with samples from up to 3293 patients have found an increase in the risk of appendiceal neoplasm and appendiceal diameter > 10 mm seen by CT [14] of up to three times.
Given that many of these tumors are small and asymptomatic, it is possible that small variations in their prevalence may be related to the different rates of blank appendectomy, given that those institutions with a higher rate of blank appendectomies are also the most prone to the discovery of pauci-symptomatic appendiceal tumors. In this regard, the rate of blank appendectomies that we have obtained in our institution is around 8%, which can be considered high at the current time, and perhaps this is the reason for the relatively high rate of appendiceal neoplasms at our institution in comparison to other series. Although not specifically discussing the number of blank appendectomies, in a similar vein Orchard et al. demonstrated an increased rate of appendectomies at the same time that they observed an increase in appendiceal neoplasms incidence [3].
In the histologic distribution of neoplasms in our study, epithelial neoplasms, and within this group LAMNs, predominate over neuroendocrine tumors. This finding confirms the epidemiologic change seen in recent years where neuroendocrine tumors, previously known as carcinoids, are not any more the most frequent ones [1]. In the period analyzed by Marmor et al. [12], an increase in epithelial tumors can be observed without a decrease in the incidence of neuroendocrine ones. Recently, Naar et al. [14], with more than 3000 patients registered, confirmed a change in the tendency in favor of the epithelial neoplasms.
In general, the treatment of the LAMN is an appendectomy with a free margin. They usually have a low recurrence rate after the appendectomy and in the subgroups of patients, where the opposite has been observed, they are those with a perforated appendix before surgery or those with an intraoperative finding of a periappendiceal free mucus. In the case of a ruptured appendix, it has been associated with a recurrence of up to 65% compared to 17% in those appendixes which were not ruptured. Some authors have even proposed cytoreductive surgery and intraperitoneal hyperthermic chemotherapy in cases of perforated LAMNs [7]. Generally, LAMNs have a good prognosis with 5-year survival rates of about 93% according to recent reports [15].
In the case of NETs smaller than 1 cm with a free margin, the appendectomy itself is sufficient treatment. In the case of tumors of 1–2 cm where there is not a free meso-appendiceal margin bigger than 3 mm, multifocality, or vascular invasion, a right hemicolectomy would be indicated [1]. In our study, a pediatric patient with a neuroendocrine tumor of grade 2 did not present the previously mentioned characteristics and accepted enough treatment with an appendectomy with strict follow-up. In the case of NETs bigger than 2 cm or grade 3, a right hemicolectomy is recommended [7].
We did not find an interrelationship between the macroscopic findings of the surgeon and the anatomopathological reports of the tumors. The previous result does not seem to be an infrequent result: in the work of Bolmers et al. [16], there was a correct intraoperative identification in only 3 out of 30 cases of appendiceal neoplasms. In another retrospective study with a sample of 3554 appendectomies, there was a correct suspicion of appendiceal neoplasm in 2 out of 20 patients [2], so a very similar number to the one obtained by our group. In the review of Bastiaenen et al. [8], seven studies analyzed this parameter and there was a correct interrelationship between the findings of the surgeon and the pathologist in only one of them; in all the rest, none of the neoplasia had an intraoperative suspicion.
In most of cases, an appendectomy is sufficient treatment for the found tumors. In our study, only eight patients underwent a second surgery, and this was mostly because of malignant tumors such as adenocarcinomas, locally advanced tumors or a metastasic disease. In the study done by Marmor et al. [12], it was found that 74% of the patients with appendiceal carcinomas have regional local metastases (39%) or distant ones (35%) at the time of being diagnosed, highlighting the mucinous carcinoma and the signet ring cell adenocarcinoma as the ones with greater probability. Likewise, a clear 5-year drop in survival is described in those patients diagnosed with regional metastases (60%) and distant ones (33%). In another recent study of Japanese origin, a 5-year survival rate of 83% was found for the mucinous adenocarcinoma and of 62% for the non-mucinous adenocarcinoma; most of the patients did not present lymphatic or distant metastases. In the case of being present, the prognosis was significantly poorer and the patients presented a drop in the 5-year survival rate from up to 52% in mucinous adenocarcinomas and 25% in the non-mucinous ones [15].
Two out of the four cases of mortality in our study were cases of signet ring cell adenocarcinoma that is highlighted as the histologic subtype with the poorest prognosis and 5-year survival rates lower than 30% [1,12].
Further research in the field would be quite advisable, mostly in order to find out what can be considered the risks and environmental or dietary exposition agents that have been modified and that have brought a subsequent change in this type of tumor’s histology, from neuroendocrine tumors to epithelial ones, with the implicit change in prognosis for patients and also possible treatment evolutions [5,17].
This study presents some limitations such as its retrospective design and being a single = center series. In this sense, although presenting some limitations, reporting of single-center series can be considered valuable in order to obtain cumulative data and knowledge about appendiceal neoplasms. However, this study has some strengths as it was carried out in a tertiary referral center for emergency surgery where almost the entirety of inhabitants of its sanitary area are evaluated and treated in the context of emergencies, which makes it exceptional that important losses of this kind of patient may happen.
Several practical implications can be drawn from the current study. First of all, it has been shown that the vast majority of patients with an incidental finding of an appendiceal neoplasm were just treated with appendectomy alone without the further necessity of completing any more extensive resection. This should also be taken into account in the context of the emergency surgery when it may be required, in order to avoid more risky interventions different from an appendectomy as it is hardly indicated when the final specimen analysis is available.
4. Materials and Methods
4.1. Study Design, Patients and Variables
A retrospective cohort study was carried out. Every pathological report from all the appendectomy pieces in our centre was compiled between January 2013 and December 2017 according to previous calculations of the sample size.
All the appendectomy cases have been included in the study: both elective and emergency ones, incorporating both children and adults. Our centre is the only one which has an Emergency Service, so all patients with an acute surgical abdominal pathology in this sanitary area come to our institution for these health services, in which, logically, the acute abdominal pain indicative of appendicitis is found.
We recorded demographic data, health information about the type and characteristics of the episode (scheduled/urgent surgery, associated symptoms, and analytical variables), the type of surgery performed and the findings during the same histopathologic diagnosis, progress after surgery and the oncological follow-up. In particular, we also recorded the need to finish the surgical treatment, having completed recommended complementary studies, the correct follow-up of patients and, finally, local recurrence, any other peritoneal relapse or distant metastases during follow-up, determining disease-free survival and overall survival.
4.2. Sample Size Calculation
Taking into account an incidence rate of approximately 3% of the samples of appendectomy, an accuracy of 1% and a confidence interval of 95% (CI-95%), the sample size to analyze would correspond to 1242 patients.
4.3. Statistical Methods
The qualitative variables are presented with their frequency distribution. The quantitative variables can be summarized as mean and standard deviation (SD) or median and interquartile range (IQR) in the case of asymmetry. The association among qualitative variables was studied through the χ2 test or Fisher’s exact test, when these corresponded. The behavior of the quantitative variables towards the corresponding independent ones was analyzed through a Mann–Whitney U test.
All the calculations were carried out with the statistical program Package for Social Sciences (SPSS version 20.0.0; SPSS Corp.; Chicago, IL, USA).
5. Conclusions
Appendiceal neoplasms can appear in up to 3% of patients operated because of an appendectomy, which on most occasions is carried out without any pre- or postoperative suspicion of a tumor of any type. In most cases, the histologic diagnosis shows the presence of tumors, mostly of a low degree and with a behavior which is not very aggressive, so the appendectomy is usually sufficient treatment. Nevertheless, in case the appendectomy is not considered sufficient, conducting a final second surgery does not seem to endanger the oncological results in this context.
Author Contributions
C.C.-S.: study design, interpreting the data and writing the manuscript. F.D.G.-B.: data collection, interpreting the data and writing the manuscript. N.M.M.: study design, data collection and writing the manuscript. L.M.C.: interpreting the data and writing the manuscript. M.M.R.: study design and interpreting the data. J.A.T.F.: study design, data collection and interpreting the same. J.E.S.G.: critical review and final approval of the manuscript. J.J.O.K.: critical review and final approval of the manuscript. The authors C.C.-S. and F.D.G.-B. equally contributed to the current study. All authors have read and agreed to the published version of the manuscript.
Funding
The current study has not received specific funding from public agencies, private ones or non-profit institutions.
Institutional Review Board Statement
Ethical review and approval were waived for this study due to its retrospective design.
Informed Consent Statement
Patient consent was waived due to its retrospective design.
Data Availability Statement
Present data are not uploaded to any data repository. They will be available on demand, always for research purposes.
Conflicts of Interest
The authors declare no conflict of interest. The present project has not received any funding.
References
- Hatch, Q.M.; Gilbert, E.W. Appendiceal Neoplasms. Clin. Colon Rectal. Surg. 2018, 31, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Nagtegaal, I.D.; Odze, R.D.; Klimstra, D.; Paradis, V.; Rugge, M.; Schirmacher, P.; Washington, K.M.; Carneiro, F.; Cree, I.A.; The WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology 2020, 76, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Orchard, P.; Preece, R.; Thomas, M.G.; Dixon, S.W.; Wong, N.A.C.S.; Chambers, A.C.; Messenger, D.E. Demographic trends in the incidence of malignant appendiceal tumours in England between 1995 and 2016: Population-based analysis. BJS Open 2022, 6, zrac103. [Google Scholar] [CrossRef] [PubMed]
- Kunduz, E.; Bektasoglu, H.K.; Unver, N.; Aydogan, C.; Timocin, G.; Destek, S. Analysis of Appendiceal Neoplasms on 3544 Appendectomy Specimens for Acute Appendicitis: Retrospective Cohort Study of a Single Institution. Med. Sci. Monit. 2018, 24, 4421–4426. [Google Scholar] [CrossRef]
- Johansson, J.; Andersson, R.E.; Landerholm, K.; Redéen, S. Incidence of Appendiceal Malignancies in Sweden Between 1970 and 2012. Mol. Med. 2020, 44, 4086–4092. [Google Scholar] [CrossRef] [PubMed]
- Carr, N.J.; Cecil, T.D.; Mohamed, F.; Sobin, L.H.; Sugarbaker, P.H.; Gonzalez-Moreno, S. A Consensus for Classification and Pathologic Reporting of Pseudomyxoma Peritonei and Associated Appendiceal Neoplasia: The Results of the Peritoneal Surface Oncology Group International (PSOGI) Modified Delphi Process. World J. Surg. 2016, 40, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Govaerts, K.; Lurvink, R.; De Hingh, I.; Van der Speeten, K.; Villeneuve, L.; Kusamura, S.; Kepenekian, V.; Deraco, M.; Glehen, O.; Moran, B.; et al. Appendiceal tumours and pseudomyxoma peritonei: Literature review with PSOGI/EURACAN clinical practice guidelines for diagnosis and treatment. Eur. J. Surg. Oncol. 2021, 47, 11–35. [Google Scholar] [CrossRef] [PubMed]
- Bastiaenen, V.P.; Allema, W.M.; Klaver, C.E.; van Dieren, S.; Koens, L.; Tanis, P.J.; Bemelman, W.A. Routine histopathologic examination of the appendix after appendectomy for presumed appendicitis: Is it really necessary? A systematic review and meta-analysis. Surgery 2020, 168, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Lohsiriwat, V.; Vongjirad, A.; Lohsiriwat, D. Value of Routine Histopathologic Examination of Three Common Surgical Specimens: Appendix, Gallbladder, and Hemorrhoid. World J. Surg. 2009, 33, 2189–2193. [Google Scholar] [CrossRef] [PubMed]
- Loftus, T.J.; Raymond, S.L.; Sarosi, G.A.J.; Croft, C.A.; Smith, R.S.; Efron, P.A.; Moore, F.A.; Brakenridge, S.C.M.; Mohr, A.M.; Jordan, J.R. Predicting appendiceal tumors among patients with appendicitis. J. Trauma Acute Care Surg. 2017, 82, 771–775. [Google Scholar] [CrossRef] [PubMed]
- Tajima, T.; Tajiri, T.; Mukai, M.; Sugiyama, T.; Hasegawa, S.; Yamamoto, S.; Sadahiro, S.; Shimada, H.; Makuuchi, H. Single-center analysis of appendiceal neoplasms. Oncol. Lett. 2018, 15, 6393–6399. [Google Scholar] [CrossRef]
- Marmor, S.; Portschy, P.R.; Tuttle, T.M.; Virnig, B.A. The Rise in Appendiceal Cancer Incidence: 2000–2009. J. Gastrointest. Surg. 2015, 19, 743–750. [Google Scholar] [CrossRef]
- Todd, R.D.; Sarosi, G.A.; Nwariaku, F.; Anthony, T. Incidence and predictors of appendiceal tumors in elderly males presenting with signs and symptoms of acute appendicitis. Am. J. Surg. 2004, 188, 500–504. [Google Scholar] [CrossRef] [PubMed]
- Naar, L.; Kim, P.; Byerly, S.; Vasileiou, G.; Zhang, H.; Yeh, D.D.; Kaafarani, H.M.; Alouidor, R.; Hing, K.K.; Sharp, V.; et al. Increased risk of malignancy for patients older than 40 years with appendicitis and an appendix wider than 10 mm on computed tomography scan: A post hoc analysis of an EAST multicenter study. Surgery 2020, 168, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Matsui, S.; Murata, K.M.; Fukunaga, Y.M.; Takeda, T.; Fujii, M.; Yamaguchi, T.M.; Kagawa, Y.M.; Mizushima, T.M.; Ohno, Y.; Yao, T.M.; et al. Analysis of Clinicopathological Characteristics of Appendiceal Tumors in Japan: A Multicenter Collaborative Retrospective Clinical Study—A Japanese Nationwide Survey. Dis. Colon Rectum 2020, 63, 1403–1410. [Google Scholar] [CrossRef]
- Bolmers, M.D.M.; de Jonge, J.; van Rossem, C.C.; van Geloven, A.A.W.; Bemelman, W.A.; van Acker, G.J.; Akkermans, B.; Akkersdijk, G.J.; Algie, G.D.; Allema, J.H.; et al. Appendicular neoplasms and consequences in patients undergoing surgery for suspected acute appendicitis. Int. J. Color. Dis. 2020, 35, 2065–2071. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Geng, Y.; Hu, W. Survival and prognostic factors for postoperative primary appendiceal cancer: A retrospective cohort study based on the Surveillance, Epidemiology, and End Results database. J. Gastrointest. Oncol. 2022, 13, 1433–1443. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).