Low Short-Chain-Fatty-Acid-Producing Activity of the Gut Microbiota Is Associated with Hypercholesterolemia and Liver Fibrosis in Patients with Metabolic-Associated (Non-Alcoholic) Fatty Liver Disease
Abstract
:1. Introduction
2. Results
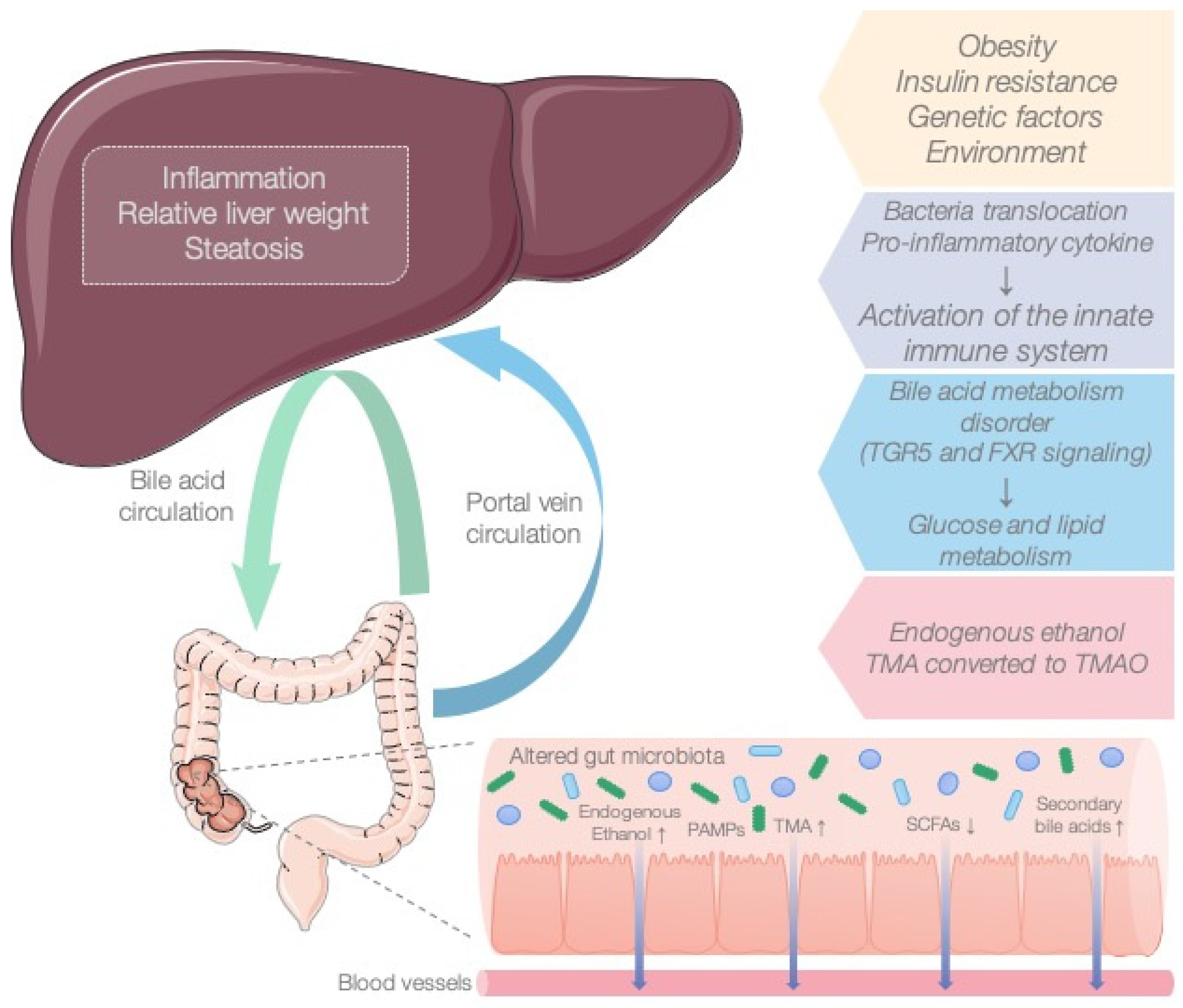
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ciaula, A.; Baj, J.; Garruti, G.; Celano, G.; Angelis, M.; Wang, H.; Di Palo, D.; Bonfrate, L.; Wang, D.; Portincasa, P. Liver Steatosis, Gut-Liver Axis, Microbiome and Environmental Factors. A Never-Ending Bidirectional Cross-Talk J. Clin. Med. 2020, 9, 2648. [Google Scholar] [CrossRef] [PubMed]
- Ge, H.; Wei, W.; Tang, L.; Tian, Y.; Zhu, Y.; Luo, Y.; Liu, S. CONSORT-Characteristics and metabolic phenotype of gut microbiota in NAFLD patients. Medicine 2022, 101, e29347. [Google Scholar] [CrossRef] [PubMed]
- Demir, M.; Lang, S.; Martin, A.; Farowski, F.; Wisplinghoff, H.; Vehreschild, M.; Krawczyk, M.; Nowag, A.; Scholz, C.; Kretzschmar, A.; et al. Phenotyping non-alcoholic fatty liver disease by the gut microbiota: Ready for prime time? J. Gastroenterol. Hepatol. 2020, 35, 1969–1977. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.C.; Liu, Y.Y.; Lin, C.C.; Wang, C.C.; Wu, Y.J.; Yong, C.C.; Chen, K.D.; Chuah, S.K.; Yao, C.C.; Huang, P.Y.; et al. Gut Microbiota Dysbiosis in Patients with Biopsy-Proven Nonalcoholic Fatty Liver Disease: A Cross-Sectional Study in Taiwan. Nutrients 2020, 12, 820. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Fan, J.-G. Microbial metabolites in non-alcoholic fatty liver disease. World J. Gastroenterol. 2019, 25, 2019–2028. [Google Scholar] [CrossRef]
- May, K.S.; Hartigh, L.J. Gut Microbial-Derived Short Chain Fatty Acids: Impact on Adipose Tissue Physiology. Nutrients 2023, 15, 272. [Google Scholar] [CrossRef]
- Hou, K.; Wu, Z.; Chen, X.; Wang, J.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef]
- Ji, Y.; Yin, Y.; Sun, L.; Zhang, W. The Molecular and Mechanistic Insights Based on Gut–Liver Axis: Nutritional Target for Non-Alcoholic Fatty Liver Disease (NAFLD) Improvement. Int. J. Mol. Sci. 2020, 21, 3066. [Google Scholar] [CrossRef]
- Bellanti, F.; Lo, B.A.; Vendemiale, G. Hepatic Mitochondria-Gut Microbiota Interactions in Metabolism-Associated Fatty Liver Disease. Metabolites 2023, 13, 322. [Google Scholar] [CrossRef]
- Ikeda, T.; Nishida, A.; Yamano, M.; Kimura, I. Short-chain fatty acid receptors and gut microbiota as therapeutic targets in metabolic, immune, and neurological diseases. Pharmacol. Ther. 2022, 239, 108273. [Google Scholar] [CrossRef]
- Kim, K.N.; Yao, Y.; Ju, S.Y. Short Chain Fatty Acids and Fecal Microbiota Abundance in Humans with Obesity: A Systematic Review and Meta-Analysis. Nutrients 2019, 11, 2512. [Google Scholar] [CrossRef] [PubMed]
- Schwiertz, A.; Taras, D.; Schäfer, K.; Beijer, S.; Bos, N.A.; Donus, C.; Hardt, P.D. Microbiota and SCFA in lean and overweight healthy subjects. Obesity 2010, 18, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, H.; Howard, A.G.; Meyer, K.A.; Tsilimigras, M.C.B.; Avery, C.L.; Sha, W.; Sun, S.; Zhang, J.; Su, C.; et al. Circulating Short-Chain Fatty Acids Are Positively Associated with Adiposity Measures in Chinese Adults. Nutrients 2020, 12, 2127. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.Q.; An, Y.X.; Yu, C.G.; Ke, J.; Zhao, D.; Yu, K. The Association Between Fecal Short-Chain Fatty Acids, Gut Microbiota, and Visceral Fat in Monozygotic Twin Pairs. Diabetes, Metab. Syndr. Obesity Targets Ther. 2022, 15, 359–368. [Google Scholar] [CrossRef]
- Miranda, V.P.N.; Dos Santos Amorim, P.R.; Bastos, R.R.; de Faria, E.R.; de Castro Moreira, M.E.; do Carmo Castro Franceschini, S.; do Carmo Gouveia Peluzio, M.; de Luces Fortes Ferreira, C.L.; Priore, S.E. Abundance of Gut Microbiota, Concentration of Short-Chain Fatty Acids, and Inflammatory Markers Associated with Elevated Body Fat, Overweight, and Obesity in Female Adolescents. Mediat. Inflamm. 2019, 2019, 7346863. [Google Scholar] [CrossRef]
- Murugesan, S.; Ulloa-Martínez, M.; Martínez-Rojano, H.; Galván-Rodríguez, F.M.; Miranda-Brito, C.; Romano, M.C.; Piña-Escobedo, A.; Pizano-Zárate, M.L.; Hoyo-Vadillo, C.; García-Mena, J. Study of the diversity and short-chain fatty acids production by the bacterial community in overweight and obese Mexican children. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 1337–1346. [Google Scholar] [CrossRef]
- National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Final report. Circulation 2002, 106, 3143–3421. [Google Scholar] [CrossRef]
- Zeng, K.Y.; Bao, W.Y.; Wang, Y.H.; Liao, M.; Yang, J.; Huang, J.Y.; Lu, Q. Non-invasive evaluation of liver steatosis with imaging modalities: New techniques and applications. World J. Gastroenterol. 2023, 29, 2534–2550. [Google Scholar] [CrossRef]
- Visekruna, A.; Luu, M. The Role of Short-Chain Fatty Acids and Bile Acids in Intestinal and Liver Function, Inflammation, and Carcinogenesis. Front. Cell Dev. Biol. 2021, 9, 703218. [Google Scholar] [CrossRef]
- Anand, S.; Mande, S. Host-microbiome interactions: Gut-Liver axis and its connection with other organs. NPJ Biofilms Microbiomes 2022, 8, 89. [Google Scholar] [CrossRef]
- Fang, J.; Yu, C.-H.; Li, X.-J.; Yao, J.-M.; Fang, Z.-Y.; Yoon, S.-H.; Yu, W.-Y. Gut dysbiosis in nonalcoholic fatty liver disease: Pathogenesis, diagnosis, and therapeutic implications. Front. Cell. Infect. Microbiol. 2022, 12, 997018. [Google Scholar] [CrossRef]
- Reshetova, M.; Zolnikova, O.; Ivashkin, V.T.; Ivashkin, K.; Appolonova, S.A.; Lapina, T.L. Gut Microbiota and its Metabolites in Pathogenesis of NAFLD. Russ. J. Gastroenterol. Hepatol. Coloproctology 2022, 32, 75–88. [Google Scholar] [CrossRef]
- Zolnikova, O.; Reshetova, M.S.; Ivanova, M.N.; Ivashkin, V.T. Metabolomic profiles as a new understanding of disease processes. Russ. J. Gastroenterol. Hepatol. Coloproctology 2022, 32, 46–52. [Google Scholar] [CrossRef]
- La Cuesta-Zuluaga, D.; Mueller, N.T.; Álvarez-Quintero, R.; Velásquez-Mejía, E.P.; Sierra, J.A.; Corrales-Agudelo, V.; Carmona, J.A.; Abad, J.M.; Escobar, J.S. Higher fecal short-chain fatty acid levels are associated with gut microbiome dysbiosis, obesity, hypertension and cardiometabolic disease risk factors. Nutrients 2019, 11, 51. [Google Scholar] [CrossRef] [PubMed]
- Rahat-Rozenbloom, S.; Fernandes, J.; Gloor, G.B.; Wolever, T.M. Evidence for greater production of colonic short-chain fatty acids in overweight than lean humans. Int. J. Obes. 2014, 38, 1525–1531. [Google Scholar] [CrossRef]
- Ilyés, T.; Silaghi, C.N.; Crăciun, A.M. Diet-Related Changes of Short-Chain Fatty Acids in Blood and Feces in Obesity and Metabolic Syndrome. Biology 2022, 11, 1556. [Google Scholar] [CrossRef] [PubMed]
- Backhed, F.; Manchester, J.K.; Semenkovich, C.F.; Gordon, J.I. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc. Natl. Acad. Sci. USA 2007, 104, 979–984. [Google Scholar] [CrossRef]
- Jiao, A.; Yu, B.; He, J.; Yu, J.; Zheng, P.; Luo, Y.; Luo, J.; Mao, X.; Chen, D. Short chain fatty acids could prevent fat deposition in pigs via regulating related hormones and genes. Food Funct. 2020, 11, 1845–1855. [Google Scholar] [CrossRef]
- Iván, J.; Major, E.; Sipos, A.; Kovács, K.; Horváth, D.; Tamás, I.; Bay, P.; Dombrádi, V.; Lontay, B.; Smolková, K.; et al. The short–chain fatty acid propionate inhibits adipogenic differentiation of human chorion–derived mesenchymal stem cells through the free fatty acid receptor 2. Stem Cells Dev. 2017, 26, 1724–1733. [Google Scholar] [CrossRef]
- Aguilar, E.C.; da Silva, J.F.; Navia-Pelaez, J.M.; Leonel, A.J.; Lopes, L.G.; Menezes-Garcia, Z.; Ferreira, A.V.M.; Capettini, L.d.S.A.; Teixeira, L.G.; Lemos, V.S.; et al. Sodium butyrate modulates adipocyte expansion, adipogenesis, and insulin receptor signaling by upregulation of PPAR–gamma in obese Apo E knockout mice. Nutrition 2018, 47, 75–82. [Google Scholar] [CrossRef]
- Skelly, A.N.; Sato, Y.; Kearney, S.; Honda, K. Mining the microbiota for microbial and metabolite-based immunotherapies. Nat. Rev. Immunol. 2019, 19, 305–323. [Google Scholar] [CrossRef]
- Albillos, A.; Gottardi, A.; Rescigno, M. The gut-liver axis in liver disease: Pathophysiological basis for therapy. J. Hepatol. 2020, 72, 558–577. [Google Scholar] [CrossRef] [PubMed]
- Campisano, S.; Colla, A.L.; Echarte, S.M.; Chisari, A.N. Interplay between early-life malnutrition, epigenetic modulation of the immune function and liver diseases. Nutr. Res. Rev. 2019, 32, 128–145. [Google Scholar] [CrossRef]
- Li, M.; Wang, J.; Wang, F.; Strappe, P.; Liu, W.; Zheng, J.; Zhou, Z.; Zhang, Y. Microbiota fermentation characteristics of acylated starches and the regulation mechanism of short-chain fatty acids on hepatic steatosis. Food Funct 2021, 12, 8659–8668. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. Diabetologia 2016, 59, 1121–1140. [Google Scholar] [CrossRef]
- Associazione Italiana per lo Studio del Fegato (AISF); Società Italiana di Diabetologia (SID); Società Italiana dell’Obesità (SIO); Members of the guidelines panel; Coordinator; AISF Members; SID Members; SIO Members; Metodologists. Non-alcoholic fatty liver disease in adults 2021: A clinical practice guideline of the Italian Association for the Study of the Liver (AISF), the Italian Society of Diabetology (SID) and the Italian Society of Obesity (SIO). Dig. Liver Dis. 2022, 54, 170–182. [Google Scholar] [CrossRef]
- Ivashkin, V.T.; Maevskaya, M.V.; Zharkova, M.S.; Kotovskaya, Y.V.; Tkacheva, O.N.; Troshina, E.A.; Shestakova, M.V.; Maev, I.V.; Breder, V.V.; Gheivandova, N.I.; et al. Clinical Practice Guidelines of the Russian Scientific Liver Society, Russian Gastroenterological Association, Russian Association of Endocrinologists, Russian Association of Gerontologists and Geriatricians and National Society for Preventive Cardiology on Diagnosis and Treatment of Non-Alcoholic Liver Disease. Russ. J. Gastroenterol. Hepatol. Coloproctology 2022, 32, 104–140. [Google Scholar] [CrossRef]
- Cao, X.; Zolnikova, O.; Maslennikov, R.; Reshetova, M.; Poluektova, E.; Bogacheva, A.; Zharkova, M.; Ivashkin, V. Differences in Fecal Short-Chain Fatty Acids between Alcoholic Fatty Liver-Induced Cirrhosis and Non-alcoholic (Metabolic-Associated) Fatty Liver-Induced Cirrhosis. Metabolites 2023, 13, 859. [Google Scholar] [CrossRef]
- Sdwd. Available online: https://patents.google.com/patent/RU2220755C1/ru (accessed on 1 January 2021).
- Petroff, D.; Blank, V.; Newsome, P.N.; Shalimar Voican, C.S.; Thiele, M.; de Lédinghen, V.; Baumeler, S.; Chan, W.K.; Perlemuter, G.; Cardoso, A.C.; et al. Assessment of hepatic steatosis by controlled attenuation parameter using the M and XL probes: An individual patient data meta-analysis. Lancet Gastroenterol. Hepatol. 2021, 6, 185–198. [Google Scholar] [CrossRef]
- Hsu, C.; Caussy, C.; Imajo, K.; Chen, J.; Singh, S.; Kaulback, K.; Le, M.D.; Hooker, J.; Tu, X.; Bettencourt, R.; et al. Magnetic Resonance vs Transient Elastography Analysis of Patients with Nonalcoholic Fatty Liver Disease: A Systematic Review and Pooled Analysis of Individual Participants. Clin. Gastroenterol. Hepatol. 2019, 17, 630–637. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines on non-invasive tests for evaluation of liver disease severity and prognosis—2021 update. J. Hepatol. 2021, 75, 659–689. [Google Scholar] [CrossRef] [PubMed]

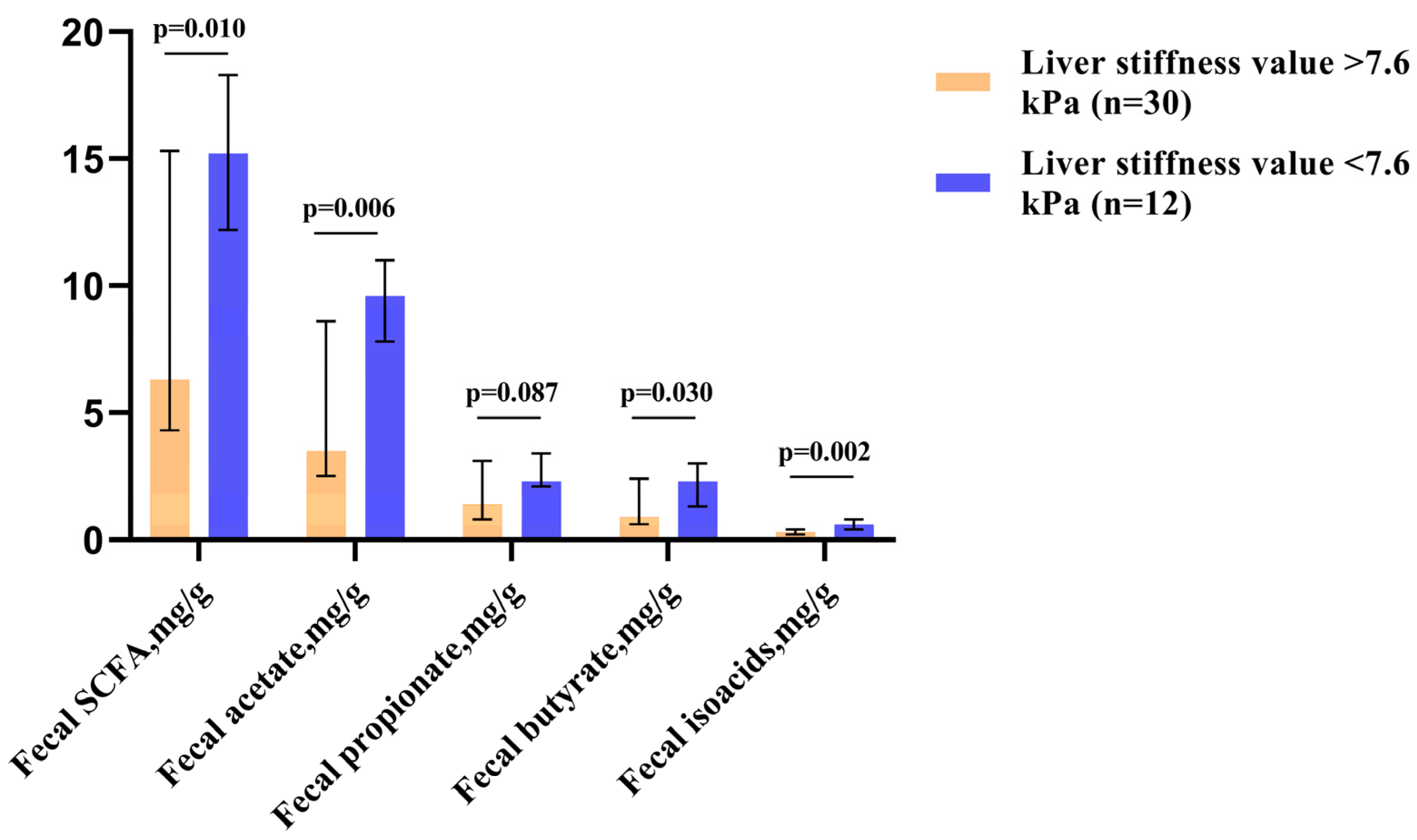
| Patients with Elevated Fecal SCFA Levels (n = 24) | Patients with Decreased Fecal SCFA Levels (n = 18) | p-Value | |
|---|---|---|---|
| Age, years | 49.5 [46–59.5] | 54 [52–60.3] | 0.073 |
| Male/Female | 13/11 | 4/14 | 0.057 |
| Body mass index, kg/m2 | 33.8 [30.3–36.7] | 31.4 [29.1–33.2] | 0.006 |
| Waist circumference, cm | 115 [107–120] | 111 [103–116] | 0.181 |
| Serum cholesterol, mmol/L | 4.9 [4.3–5.2] | 5.5 [5.3–5.7] | <0.001 |
| Serum HDL cholesterol, mmol/L | 0.92 [0.89–0.99] | 0.97 [0.82–1.04] | 0.970 |
| Serum LDL cholesterol, mmol/L | 3.2 [2.8–3.5] | 3.7 [3.6–3.9] | <0.001 |
| Serum triglycerides, mmol/L | 1.9 [1.6–2.4] | 1.9 [1.5–2.3] | 0.805 |
| Serum uric acid | 323 [300–382] | 370 [356–428] | 0.006 |
| Serum glucose, mmol/L | 5.4 [4.7–6.0] | 5.47 [5.50–5.60] | 0.298 |
| FIB-4 | 0.94 [0.69–1.25] | 1.65 [1.36–3.16] | <0.001 |
| Liver stiffness, kPa | 7.9 [6.6–9.4] | 11.6 [10.4–13.3] | <0.001 |
| Serum total protein, g/L | 71 [69–74] | 74 [72–74] | 0.034 |
| Serum albumin, g/L | 44 [42–45] | 44 [43–45] | 0.746 |
| Serum total bilirubin, μmol/L | 12.5 [10.9–15.7] | 9.96 [10.02–10.15] | <0.001 |
| Serum direct bilirubin, μmol/L | 2.3 [1.9–2.8] | 1.9 [1.7–2.0] | 0.001 |
| Serum indirect bilirubin, μmol/L | 11.9 [9.4–13.3] | 8.5 [8.0–8.7] | <0.001 |
| Alanine aminotransferase, U/L | 49 [36–55] | 48 [38–82] | 0.628 |
| Aspartate aminotransferase, U/L | 31 [30–34] | 38 [33–50] | 0.037 |
| Gamma glutamyl transferase, U/L | 48 [33–57] | 30 [26–35] | 0.001 |
| Alkaline phosphatase, U/L | 74 [69–83] | 83 [73–90] | 0.114 |
| C-reactive protein, mg/L | 2.7 [1.2–5.3] | 3.2 [2.5–3.8] | 0.492 |
| Creatinine, μmol/L | 83 [77–88] | 86 [78–101] | 0.328 |
| Hemoglobin, g/L | 144 [136–152] | 140 [137–145] | 0.161 |
| White blood cells, 109/L | 6.8 [5.8–7.8] | 6.8 [6.0–7.7] | 0.999 |
| Platelets, 109/L | 294 [256–336] | 192 [170–223] | <0.001 |
| Fecal SCFA, mg/g | 15.36 [13.56–18.09] | 4.67 [3.36–5.81] | <0.001 |
| Fecal acetate, mg/g | 9.32 [7.90–11.02] | 2.72 [2.07–3.30] | <0.001 |
| Fecal propionate, mg/g | 2.76 [2.25–3.54] | 0.87 [0.61–1.32] | <0.001 |
| Fecal butyrate, mg/g | 2.49 [1.77–3.14] | 0.66 [0.43–0.82] | <0.001 |
| Fecal isoacids, mg/g | 0.57 [0.31–0.75] | 0.23 [0.17–0.31] | <0.001 |
| Fecal SCFA | Fecal Acetate | Fecal Propionate | Fecal Butyrate | |
|---|---|---|---|---|
| Body mass index | r = 0.363; p = 0.018 | NS | r = 0.496; p = 0.001 | r = 0.391; p = 0.010 |
| Waist circumference | NS | NS | NS | NS |
| Serum cholesterol | r = −0.534; p < 0.001 | r = −0.578; p < 0.001 | r = −0.435; p = 0.004 | r = −0.546; p < 0.001 |
| Serum HDL cholesterol | NS | NS | NS | NS |
| Serum LDL cholesterol | r = −0.561; p < 0.001 | r = −0.614; p < 0.001 | r = −0.382; p = 0.013 | r = −0.549; p < 0.001 |
| Serum triglycerides | NS | NS | NS | NS |
| Serum uric acid | NS | NS | NS | NS |
| Serum glucose | NS | NS | NS | NS |
| FIB-4 | r = −0.733; p < 0.001 | r = −0.739; p < 0.001 | r = −0.590; p < 0.001 | r = −0.720; p < 0.001 |
| Liver stiffness | r = −0.576; p < 0.001 | r = −0.622; p < 0.001 | r = −0.443; p = 0.003 | r = −0.527; p < 0.001 |
| Serum total protein | NS | NS | NS | NS |
| Serum albumin | NS | NS | NS | NS |
| Serum total bilirubin | r = 0.513; p = 0.001 | r = 0.488; p = 0.001 | r = 0.542; p < 0.001 | r = 0.423; p = 0.005 |
| Alanine aminotransferase | NS | NS | NS | NS |
| Gamma glutamyl transferase | r = −0.341; p = 0.027 | r = −0.305; p = 0.050 | NS | r = −0.344; p = 0.026 |
| Gamma glutamyl transferase | r = 0.403; p = 0.008 | r = 0.432; p = 0.004 | r = 0.493; p = 0.001 | r = 0.409; p = 0.007 |
| Alkaline phosphatase | NS | NS | NS | NS |
| Creatinine | r = −0.438; p = 0.004 | r = −0.349; p = 0.024 | r = −0.422; p = 0.005 | r = −0.476; p = 0.001 |
| Hemoglobin | NS | NS | NS | NS |
| Platelets | r = 0.782; p < 0.001 | r = 0.742; p < 0.001 | r = 0.750; p < 0.001 | r = 0.704; p < 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, X.; Zolnikova, O.; Maslennikov, R.; Reshetova, M.; Poluektova, E.; Bogacheva, A.; Zharkova, M.; Ivashkin, V. Low Short-Chain-Fatty-Acid-Producing Activity of the Gut Microbiota Is Associated with Hypercholesterolemia and Liver Fibrosis in Patients with Metabolic-Associated (Non-Alcoholic) Fatty Liver Disease. Gastrointest. Disord. 2023, 5, 464-473. https://doi.org/10.3390/gidisord5040038
Cao X, Zolnikova O, Maslennikov R, Reshetova M, Poluektova E, Bogacheva A, Zharkova M, Ivashkin V. Low Short-Chain-Fatty-Acid-Producing Activity of the Gut Microbiota Is Associated with Hypercholesterolemia and Liver Fibrosis in Patients with Metabolic-Associated (Non-Alcoholic) Fatty Liver Disease. Gastrointestinal Disorders. 2023; 5(4):464-473. https://doi.org/10.3390/gidisord5040038
Chicago/Turabian StyleCao, Xinlu, Oksana Zolnikova, Roman Maslennikov, Maria Reshetova, Elena Poluektova, Arina Bogacheva, Maria Zharkova, and Vladimir Ivashkin. 2023. "Low Short-Chain-Fatty-Acid-Producing Activity of the Gut Microbiota Is Associated with Hypercholesterolemia and Liver Fibrosis in Patients with Metabolic-Associated (Non-Alcoholic) Fatty Liver Disease" Gastrointestinal Disorders 5, no. 4: 464-473. https://doi.org/10.3390/gidisord5040038
APA StyleCao, X., Zolnikova, O., Maslennikov, R., Reshetova, M., Poluektova, E., Bogacheva, A., Zharkova, M., & Ivashkin, V. (2023). Low Short-Chain-Fatty-Acid-Producing Activity of the Gut Microbiota Is Associated with Hypercholesterolemia and Liver Fibrosis in Patients with Metabolic-Associated (Non-Alcoholic) Fatty Liver Disease. Gastrointestinal Disorders, 5(4), 464-473. https://doi.org/10.3390/gidisord5040038






