Abstract
Being two immune-mediated diseases (IMIDs), the association between multiple sclerosis (MS) and inflammatory bowel disease (IBD) is plausible, but data in the literature are conflicting. The aim of our study was to evaluate the possible association between IBD and MS in a cohort of patients with IBD. In a retrospective study, we examined the medical records of 5739 patients with a confirmed diagnosis of IBD followed in our clinic between 1978 and 2022. Among these patients, we identified 14 with MS, with a prevalence of 0.24%. The reported prevalence of MS in the general population in Northern Italy in 2021 was 0.18% (p = 0.24). For each of the patients with MS identified, more than ten patients without MS were analyzed. The 14 MS cases were then compared with 342 controls. From the 14 patients with MS, 12 (85.7%) were female and 2 (14.3%) were male, while in the control group, 158 (46.2%) were female and 184 (53.8%) were male (p = 0.004). As for therapy, significant differences were found in mesalazine (5 (41.7%) cases vs. 317 (92.7%) controls, p < 0.0001) and anti-TNF treatment (0% cases vs. 26.6% controls, p = 0.03, respectively) at the time of MS diagnosis. Moreover, a Kaplan–Meier curve analysis showed that the 20-year survival probability was 98.4% for patients with IBD, while for patients diagnosed with MS and IBD it was 82.1% (p = 0.02). In conclusion, patients with IBD have a similar risk of developing MS compared to the general population, but female sex appears to increase the risk. Indeed, life expectancy at 20 years for patients with IBD and MS is lower than for patients with IBD alone.
1. Introduction
Inflammatory Bowel Diseases (IBD) is a heterogeneous group of immune-mediated diseases of unknown etiology that can variably affect the digestive tract [1,2]. Traditionally, it is grouped into two entities, Crohn’s disease (CD) and ulcerative colitis (UC), whose differential diagnosis is based on clinical, histological, laboratory, and endoscopic evidence [1]. In addition to these, there is a third entity, unclassified IBD (IBD-U), which is not a disease entity in its own right, but rather a temporary diagnosis until sufficient elements are present to define whether it is CD or UC [3].
IBD is a disease that occurs most frequently in countries with high socio-economic development, with incidence and prevalence particularly high in Europe and North America [4,5,6,7]. Within Western countries, people in urban areas are more prone to IBD compared to those in rural areas, and this is probably due to differences in lifestyle, resulting in greater exposure to IBD risk factors [8].
IBD is a disease of unknown etiology, but appears to be the result of a continuous and inappropriate inflammatory response, involving the participation of genetic alterations, environmental factors, gut microbiota dysbiosis and immune dysregulation in susceptible individuals [1]. IBD can appear at any age, even if a major peak of incidence is observed between the second and fourth decades of life and a more moderate peak between the sixth and seventh decades [9]. Concerning sex, no statistical differences have been reported in the incidence of IBD between men and women. However, above the age of 45 years, men demonstrate a higher risk of CD than women [10]. Extraintestinal manifestations of these diseases can affect any organ, but most commonly involve the skin, eyes, oral cavity, joints, and liver [11]. Additionally, a link between IBD and multiple sclerosis (MS) has been reported, but results are still inconsistent [12].
MS is a chronic, predominantly immune-mediated disease of the central nervous system in which inflammation, demyelination, and axonal loss occur even in the early stages of the disease. The course of the disease can be extremely variable among individual patients, and it remains one of the most frequent causes of neurological disability in young people [13]. Environmental risk factors including Epstein–Barr virus infection, exposure to tobacco smoke and organic solvents, obesity in adolescence, limited sun exposure/low vitamin D, and night shift work are associated with an increased risk of MS onset. In contrast, oral tobacco, high coffee consumption, and alcohol consumption are associated with a lower risk of MS onset [14].
There are a large number of publications on the correlation between MS and immune-mediated disease, and there is a higher rate of certain autoimmune diseases in people with MS and their first-degree relatives [15,16]. However, only a limited number of publications are available in the literature regarding the possible association between MS and IBD [17,18,19]. The aim of our study was to evaluate the possible association between IBD and MS in a cohort of patients with IBD from Northern Italy.
2. Results
2.1. General Characteristics
In the first phase, we analyzed all 5739 patients followed in our clinic from 1978 to 2022 with a confirmed diagnosis of IBD: of these, 14 received a diagnosis of MS, with a prevalence of 0.24%. The prevalence of MS (total number of cases) estimated on 1 January 2021 in Northern Italy (source AISM) was 48,850 affected persons [20], while the total number of residents in Northern Italy (ISTAT data on population 2021) corresponds to 27,373,273 persons. The prevalence in Northern Italy is 0.18%, so there is a difference of + 0.06% in our population (p = 0.24). Considering the total number of patients we analyzed, out of 5739 patients, 2639 were diagnosed with CD, 2135 with UC, and 965 with IBD-U. Specifically, 8 out of 2639 (0.30%) patients with CD, 4 out of 2135 (0.19%) patients with UC, and 2 out of 965 (0.21%) patients with IBDU had a diagnosis of MS (p = 0.70).
It should be considered that:
- -
- For one of the fourteen patients with MS, no data on previous and current treatment were available.
- -
- For two of the fourteen patients with MS, only biographical data were available, including year of death, smoking status, type of IBD diagnosed, and date and age of IBD diagnosis and behavior, while all other elements analyzed were missing.
Considering the lack of these data, we obtained the following results: the mean age of MS diagnosis was 37.4 (11.9) years. MS was diagnosed after the diagnosis of IBD in 61.5% (8/13) of the cases under study, while in 38.5% (5/13) it was diagnosed before the diagnosis of IBD.
Regarding the lesions found in the MRI, considering as 1 = Encephalon, 2 = Medulla, and 3 = Encephalon + Medulla, 70% (7/10 = 70%) had lesions in Encephalon + Medulla (3), while 30% (3/10 = 30%) had lesions only in Encephalon. The type of MS diagnosed, MS-RR (relapsing–remitting multiple sclerosis), MS-SP (secondary progressive multiple sclerosis), and MS-PP (primary progressive multiple sclerosis) was considered. Of the cases under study, 91.7% (11/12 = 91.7%) were diagnosed with MS-RR and 8.3% (1/12 = 8.3%) were diagnosed with MSSP.
The current MS therapies prior to IBD diagnosis showed that 75% (9/12) of patients were not taking any MS medication prior to IBD diagnosis, 8.3% (1/12) of patients were taking dimethyl fumarate/teriflunomide, 8.3% (1/12) of patients were taking natalizumab, and 8.3% (1/12) of patients were taking interferon Beta 1a. On the other hand, regarding data on MS therapy after IBD diagnosis, 41.5% (5/12) are not currently receiving therapy; 8.3% receive therapy with ocrelizumab (1/12); 8.3% (1/12) receive therapy with teriflunomide; 8.3% (1/12) with cladribine; 8.3% (1/12) with methotrexate; 8.3% (1/12) with natalizumab; 8.3% (1/12) with PEG interferon, and 8.3% (1/12) receive therapy with interferon Beta 1a.
Regarding the total number of previous MS therapies, 25% (3/12) of the cases had no therapies; 8.3% (1/12) were receiving glatiramer acetate, teriflunomide, and azathioprine therapy; 8.3% (1/12) were receiving glatiramer acetate therapy; 8.3% (1/12) were receiving dimethyl fumarate and teriflunomide therapy; 8.3% (1/12) were receiving interferon-natalizumab therapy; 8.3% (1/12) were receiving treatment with interferon, glatiramer acetate, and ocrelizumab; 8.3% (1/12) were receiving treatment with natalizumab; 8.3% (1/12) were receiving treatment with interferon Beta 1a; 8.3% (1/12) were receiving treatment with interferon Beta 1a, glatiramer acetate, and rituximab and 8.3% (1/12) were receiving treatment with rituximab and natalizumab.
Moreover, the finding of oligoclonal bands in the cerebrospinal fluid was positive (OB+) in 50% (6/12) of the cases studied, while the remaining 50% were negative. Considering the onset symptom that later led to the diagnosis of MS in the cases of our study, 50% (5/10) had a sensory symptom, 20% (2/10) a motor symptom, and in 30% (3/10) the first symptom was retrobulbar optic neuritis (NORB). Furthermore, the Expanded Disability Status Scale value was available for 12 of the 14 patients with MS (2.5, IQR: 1.3–5.5).
The location of IBD lesions at diagnosis was 38.5% (5/13) IBD located in ileum-colon; 30.8% (4/13) extensive-colitis; 7.7% (1/13) in the right colon; 15.4% (2/13) in the left colon; and 7.7% (1/13) in the ileum.
Additionally, five patients diagnosed with MS were treated with thiopurines (azathioprine), four of whom were subsequently diagnosed with IBD. Furthermore, two patients were treated with anti-TNF. In the first patient, MS was diagnosed before starting anti-TNF therapy, but a relapse of MS was detected and treatment was discontinued. In the second patient, MS was diagnosed after anti-TNF treatment with a single relapse in 2020 and subsequent stabilization. Finally, another patient was treated with vedolizumab before the MS diagnosis and the other with ustekinumab after the MS diagnosis.
2.2. Case–Control Study
For the purpose of the analysis, participants were split into those with MS (cases) and those without MS (controls). Among the 5739 patients with IBD, more than 10 controls with IBD without MS were randomly selected for each case of IBD with MS. General characteristics are presented in Table 1.

Table 1.
Comparison between cases and controls.
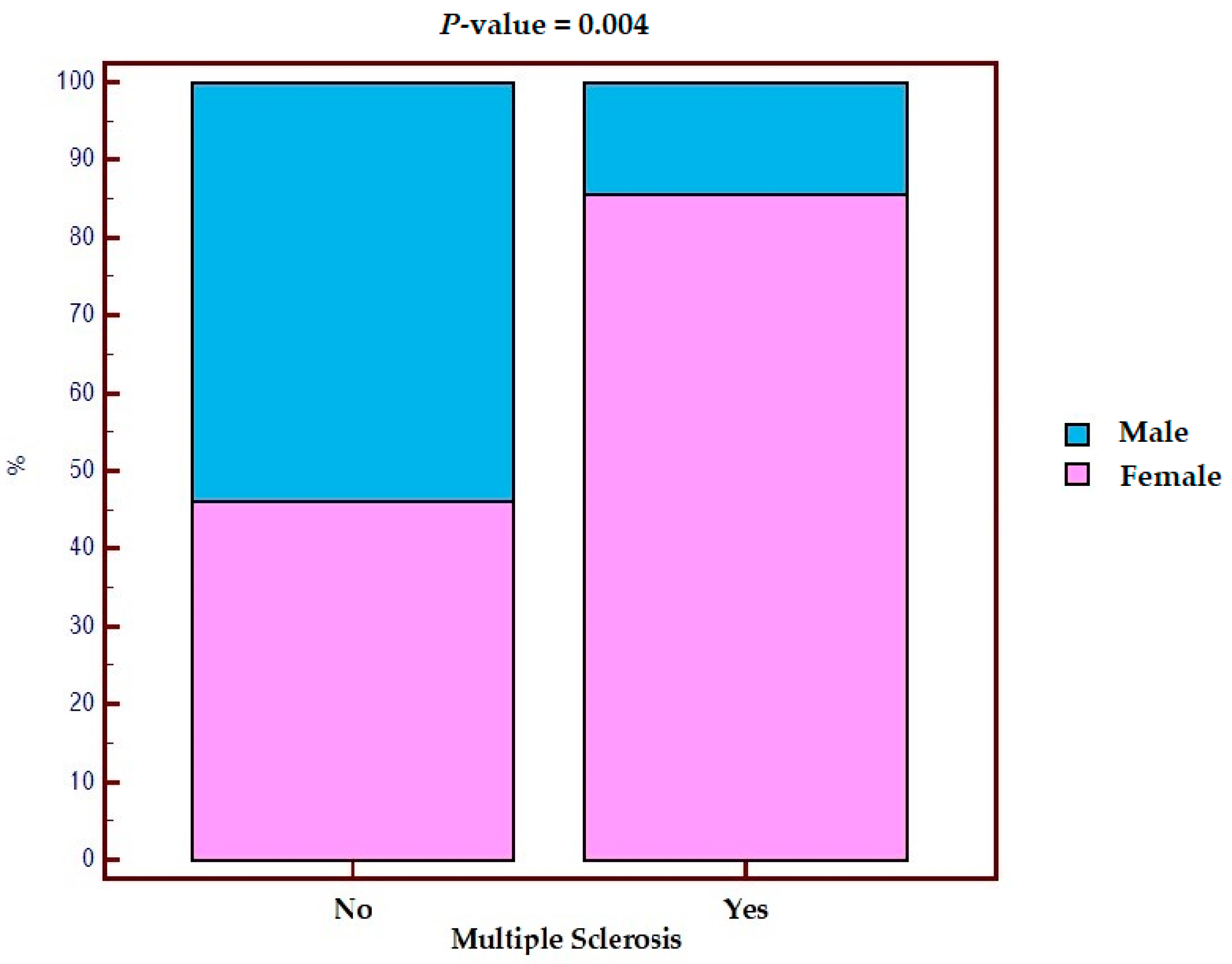
Out of 14 patients with MS, 12 (85.7%) were female and 2 (14.3%) were male, while in the control, out of 342 patients, 158 (46.2%) were female and 184 (53.8%) were male. After performing the chi-square test, we observed that the risk of MS in women was statistically significantly increased (Figure 1) (p = 0.004). In fact, female sex appears to be a risk factor associated with the development of MS in IBD (OR 8.43, 95% CI 1.86–38.19).
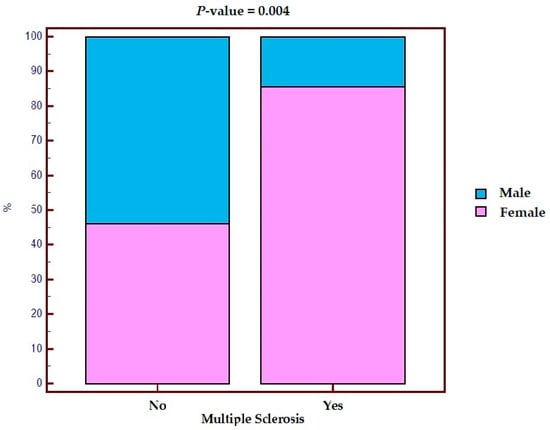
Figure 1.
Distribution by sex in the population with IBD with and without multiple sclerosis.
Furthermore, no differences were observed between active, former, or ex-smokers and the risk of developing MS (p = 0.98) (OR 0.85; 95% CI: 0.23–3.13). In terms of type of IBD, no significant differences were found when comparing cases vs. controls (p = 0.19). Similarly, no significant differences were found in age at IBD diagnosis (p = 0.93). Regarding surgical resection, of the 14 patients with MS, 2 (14.3%) underwent surgery, while 12 (85.7%) did not. On the other hand, in the control group, out of 342 patients, 107 (31.3%) underwent surgery, while 235 (68.7%) did not, so we did not observe significant differences between groups (p = 0.18).
For the therapy, taking into account the absence of specific data (discussed in the previous section) for 3 of the 14 patients, the following results were obtained: 5 patients (41.7%) were on mesalazine at the time of MS diagnosis, while in 317 (92.7%) of the control, this drug was used (p < 0.0001). In fact, even when considering the 8 patients who developed MS after diagnosis of IBD, the use of mesalazine in these patients was 62.5% (5/8) versus 92.7% (317/342) in the control group (p = 0.002). Regarding thiopurine treatment, of the 12 patients with MS, 1 (8.3%) was being treated with azathioprine at the time of MS diagnosis, while thiopurine were used in 91 (26.6%) patients in the control group (p = 0.16). Concerning the data on anti-TNF treatment, no patients at the time of MS diagnosis were reported, while 26.6% in the control group were receiving anti-TNF treatment (p = 0.03). Finally, for both anti-integrin and ustekinumab treatment, no significant differences were found in either group when comparing patients with MS versus the control group (p = 0.95 and p = 0.65, respectively).
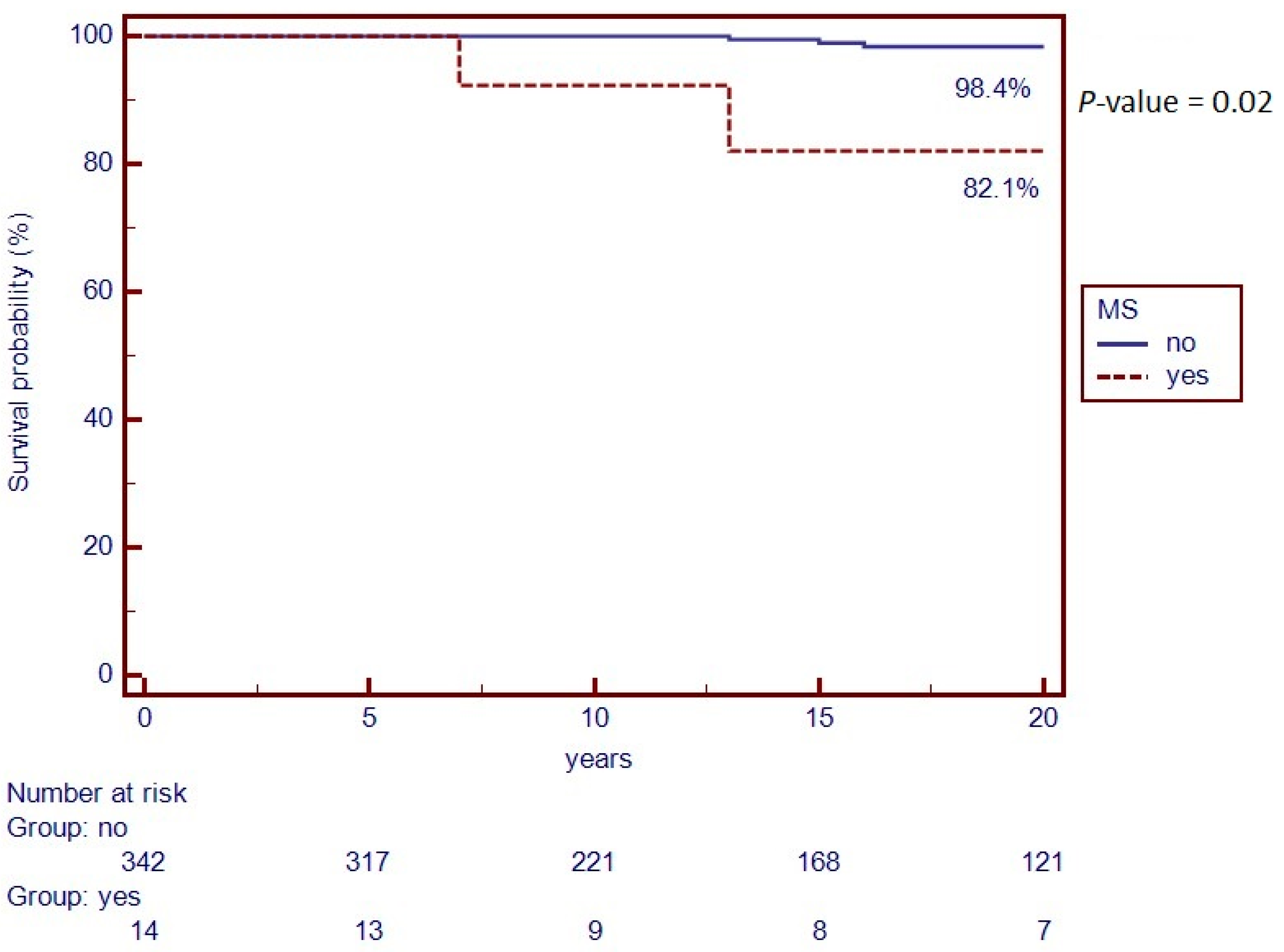
Finally, the Kaplan–Meier curve was used to compare survival between the two groups. Out of the 14 patients with MS, 2 (14.3%) died, while 12 (85.7%) are still alive. Regarding the two deceased cases, for one patient the year of death was less than 10 years after the diagnosis of MS, while for the other patient there are no data on this. On the other hand, in the control, out of 342 patients, 7 (2.1%) died, while 335 (97.9%) are still alive. When performing the Kaplan–Meier analysis, a statistically significant difference between groups was observed (p = 0.02). Specifically, in the control group (n = 342), the 20-year survival rate was 98.4%, while in the cases (n = 14) it was of 82.1% (Figure 2).
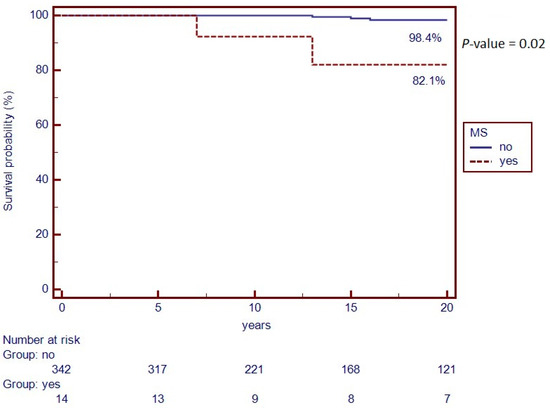
Figure 2.
Kaplan–Meier Curve of survival rate from IBD diagnosis to last follow-up visit.
3. Discussion
A limited number of publications are available in the literature regarding the association between IBD and MS. In this sense, a study examining patients who underwent total colectomy showed a higher prevalence of MS in the CD subgroup compared to the reference population [21]. Another study reported an association between UC and MS [22]. In addition, a recent study reported that the prevalence of MS in patients with IBD was found to be 0.2%, while the prevalence of IBD in patients with MS was 0.6% [19]. Also, there are conflicting opinions on the occurrence of IBD in relatives of patients with MS: some studies state that there is an increased risk of IBD in patients with MS, but no increased risk of IBD in relatives of people with MS [12,16], while others point out that first-degree relatives of patients with MS have an increased risk of CD and UC, as well as Addison’s disease and polyarteritis nodosa [23].
Moreover, when analyzing our data, female sex appears to be a risk factor associated with the development of MS in IBD (OR 8.43; 95% CI: 1.86–38.19). In this sense, to date, no association between female sex and the likelihood of developing MS and IBD has been reported. A plausible hypothesis for this association could be related to the greater susceptibility of women to the development of dysimmune diseases such as MS [13], although, with respect to the presentation of IBD, there are generally no statistical differences between the sexes [10]. As for age at IBD diagnosis, the data were similar in cases and controls (31.5 years (IQR 27.0–40.0) and 33.0 years (IQR 24.0–48.0), respectively), which is also in line with epidemiological data concerning IBD [14].
Regarding exposure to cigarette smoke, based on the data obtained, there is no statistically significant increased risk of MS in active smoking patients compared to non-smokers or former smokers (OR 0.85; 95% CI: 0.23–3.13). However, according to some studies, there is a clear dose–response relationship, in which cumulative smoking dose correlates with an increased risk of developing MS, whereas a previous history of smoking is associated with a worse prognosis [14].
On the other hand, according to the diagnoses of CD, IBD-U, and UC in the patients with MS, no significant differences were found when comparing cases vs. controls (p = 0.190). In contrast, an association between UC and MS has been reported in the literature, but not between CD and MS [22,23]. It should also be noted that these studies do not consider the difference in diagnosis between CD, IBD-U, and UC, but only between CD and UC. In this sense, the prevalence of MS in Northern Italy is 0.18%, with an absolute difference of +0.06% in our population (p = 0.24). In view of these results, and comparing the prevalence we have obtained with the prevalence of MS in Northern Italy, it is possible to state that IBD does not increase the risk of developing MS.
Regarding therapy, there are no studies in the literature concerning mesalazine in patients with IBD and MS. In this regard, in our data, no increased risk of MS was reported in patients treated with mesalazine. Therefore, these data may reassure us about its use, since, after diagnosis of IBD, mesalazine is one of the first attack drugs used to extinguish intestinal inflammation in the early stages of the disease, and the same is true for maintenance therapy, at least in UC.
As for other therapies, such as thiopurine or anti-integrin treatments, at the time of IBD diagnosis, only one patient for each of the mentioned drugs with a diagnosis of MS was receiving treatment, so no significant differences could be found. However, one review highlights the fact that azathioprine seems to reduce the relapse rate in patients with MS, although its effect on the progression of disability has not been demonstrated [24]. On the other hand, after MS diagnosis, five patients were treated with thiopurine (azathioprine), with different disease evolutions: in one of the patients examined, a relapse of MS was reported a few years after exposure to azathioprine, while in another patient a relapse was reported in the same year of treatment with azathioprine, although this was interrupted due to intolerance. Moreover, no differences were found in relation to anti-TNF treatment at the time of MS diagnosis, as none of the patients with MS were taking this drug. In contrast, in our cases, two patients were treated with anti-TNFs. In one of the two patients, anti-TNF therapy was administered after the diagnosis of MS and two relapses of MS were reported, one of which was concomitant with infliximab administration, which was therefore discontinued. In this sense, administering anti-TNF to a patient with a known MS diagnosis is contraindicated [25]. In one study, the use of anti-TNF drugs in patients with CD was associated with a statistically significant increase in the incidence of MS, although it was pointed out that this effect was lost when controlling for age/sex [25]. In relation to ustekinumab exposure at the time of IBD diagnosis, no significant values were found, as no patient diagnosed with MS was receiving treatment with it.
Lastly, in our study, out of 14 patients with MS, 2 died. In contrast, in patients diagnosed with IBD alone, 7 out of 342 patients died. According to our data, 2.1% of the controls died, compared to 14.3% of the cases investigated. Moreover, the Kaplan–Meier curve showed that the probability of survival at 20 for the controls was 98.4%, while for the cases it was lower, 82.1%, in line with the epidemiological data of the population [26].
Our study has some limitations: first, as this was a retrospective study, some patient data were missing or unavailable. Similarly, the selection of the patients for the control group and the impossibility of comparing all of them represents another limitation of the study. In addition, the longtime window in which data were collected may have led to uneven collection over time. However, the patients were followed up by the same physician at the same center, reducing the possibility of having inconsistent data. Finally, the sample size is lower compared to studies involving multicenter studies, even if our study is the largest in the literature with data from a single center.
4. Materials and Methods
We retrospectively reviewed the medical records of all patients with IBD followed in our gastroenterology clinic between 1978 and 2022, with the aim of assessing the possible correlation between IBD and MS and comparing it with that of the general population in the same country. The clinical and anamnestic data of the patients were also collected. Patients were included in the ‘IBD cohort’ (Comitato Etico Interaziendale A.O.U. Città della Salute e della Scienza di Torino—A.O. Ordine Mauriziano—A.S.L. Città di Torino, No. 0056924). All patients with a confirmed diagnosis of IBD according to the European Crohn’s and Colitis Organization (ECCO) [27] were included in the study. On the other hand, the lack of data on the presence of symptoms related to MS was considered as an exclusion criterion for the follow-up analysis.
For each of the patients diagnosed with MS (cases), more than ten patients with IBD who were not diagnosed with MS (controls) were randomly selected (in alphabetical order) from the medical records of all patients with IBD followed in our gastroenterology clinic between 1978 and 2022.
Statistical Analysis
The normal distribution of continuous variables was assessed using the D’Agostino-Pearson test. Continuous variables not normally distributed were reported as median and interquartile range (IQR), and continuous variables normally distributed were reported as mean ± standard deviation (SD). Categorical variables were reported as numbers and percentages. The Mann–Whitney test and the chi-square test were used to compare continuous and categorical variables, respectively. Multivariate analysis (logistic regression) was then carried out by inserting the variables of clinical interest. The Kaplan–Meier test was used to compare mortality in the two groups.
A p-value of less than 0.05 was considered statistically significant. All statistical analyses were performed using MedCalc® Statistical Software version 20.104 (MedCalc Software Ltd., Ostend, Belgium).
5. Conclusions
In conclusion, our study shows that patients with IBD have a similar risk of developing MS compared to the general population. Despite this, female sex appears to be a risk factor associated with the development of the disease, and life expectancy at 20 years for patients with IBD and MS is lower than for patients with IBD alone.
Furthermore, our study reassures us about the use of mesalazine in the treatment of IBD, as it does not significantly increase the risk of developing MS, while the use of anti-TNF in patients with MS seems to be unclear, as it might be associated with disease recurrence. However, longitudinal and larger-sample-size studies should be conducted to corroborate these data.
Author Contributions
Conceptualization, M.V. and P.C.; methodology, G.P.C., G.L.P., M.V. and P.C.; software, G.P.C.; validation, E.B. and A.C.; formal analysis, G.L.P.; investigation, D.G.R.; resources, E.B. and A.C.; data curation, F.S., V.S., A.A. and D.P.; writing—original draft preparation, N.P.-D.-d.-C. and G.L.P.; writing—review and editing, D.G.R.; visualization, A.A. and D.P.; supervision, E.B. and A.C.; project administration, M.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki. Patients were included in the “IBD cohort” (Comitato Etico Interaziendale A.O.U. Città della Salute e della Scienza di Torino—A.O. Ordine Mauriziano—A.S.L. Città di Torino, No. 0056924).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data was collected anonymously. Anonymous data can be requested in case of need.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Molodecky, N.A.; Soon, I.S.; Rabi, D.M.; Ghali, W.A.; Ferris, M.; Chernoff, G.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Barkema, H.W.; et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 2012, 142, 46–54.e42. [Google Scholar] [CrossRef] [PubMed]
- Actis, G.C.; Pellicano, R.; Fagoonee, S.; Ribaldone, D.G. History of Inflammatory Bowel Diseases. J. Clin. Med. 2019, 8, 1970. [Google Scholar] [CrossRef] [PubMed]
- Thurgate, L.E.; Lemberg, D.A.; Day, A.S.; Leach, S.T. An Overview of Inflammatory Bowel Disease Unclassified in Children. Inflamm. Intest. Dis. 2019, 4, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef]
- Alatab, S.; Sepanlou, S.G.; Ikuta, K.; Vahedi, H.; Bisignano, C.; Safiri, S.; Sadeghi, A.; Nixon, M.R.; Abdoli, A.; Abolhassani, H.; et al. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Fornari, C.; Madotto, F.; Fiorino, G.; Ardizzone, S.; Bortoli, A.; Caprioli, F.; Cestari, R.; Conti, S.; Cortelezzi, C.; Mantovani, L.; et al. Inflammatory bowel diseases in Italy: Incidence trends and patients’ characteristics. Value Health 2013, 16, A501. [Google Scholar] [CrossRef]
- Caviglia, G.P.; Garrone, A.; Bertolino, C.; Vanni, R.; Bretto, E.; Poshnjari, A.; Tribocco, E.; Frara, S.; Armandi, A.; Astegiano, M.; et al. Epidemiology of Inflammatory Bowel Diseases: A Population Study in a Healthcare District of North-West Italy. J. Clin. Med. 2023, 12, 641. [Google Scholar] [CrossRef]
- Kuenzig, M.E.; Nguyen, G.C.; Benchimol, E.I. Rural and Urban Differences in the Risk of Inflammatory Bowel Disease and Subsequent Health Services Utilization in Ontario. Healthc. Q. 2019, 22, 6–9. [Google Scholar] [CrossRef]
- Cosnes, J.; Gower-Rousseau, C.; Seksik, P.; Cortot, A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology 2011, 140, 1785–1794. [Google Scholar] [CrossRef]
- Rustgi, S.D.; Kayal, M.; Shah, S.C. Sex-based differences in inflammatory bowel diseases: A review. Ther. Adv. Gastroenterol. 2020, 13, 1756284820915043. [Google Scholar] [CrossRef]
- Ribaldone, D.G.; Pellicano, R.; Actis, G.C. The gut and the inflammatory bowel diseases inside-out: Extra-intestinal manifestations. Minerva Gastroenterol. Dietol. 2019, 65, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Kosmidou, M.; Katsanos, A.H.; Katsanos, K.H.; Kyritsis, A.P.; Tsivgoulis, G.; Christodoulou, D.; Giannopoulos, S. Multiple sclerosis and inflammatory bowel diseases: A systematic review and meta-analysis. J. Neurol. 2017, 264, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; Vidal-Jordana, A.; Montalban, X. Multiple sclerosis: Clinical aspects. Curr. Opin. Neurol. 2018, 31, 752–759. [Google Scholar] [CrossRef] [PubMed]
- Alfredsson, L.; Olsson, T. Lifestyle and Environmental Factors in Multiple Sclerosis. Cold Spring Harb. Perspect. Med. 2019, 9, a028944. [Google Scholar] [CrossRef] [PubMed]
- Broadley, S.A.; Deans, J.; Sawcer, S.J.; Clayton, D.; Compston, D.A. Autoimmune disease in first-degree relatives of patients with multiple sclerosis. A UK survey. Brain 2000, 123 Pt 6, 1102–1111. [Google Scholar] [CrossRef] [PubMed]
- Dobson, R.; Giovannoni, G. Autoimmune disease in people with multiple sclerosis and their relatives: A systematic review and meta-analysis. J. Neurol. 2013, 260, 1272–1285. [Google Scholar] [CrossRef]
- Sonnenberg, A.; Ajdacic-Gross, V. Similar birth-cohort patterns in Crohn’s disease and multiple sclerosis. Mult. Scler. 2018, 24, 140–149. [Google Scholar] [CrossRef]
- Yang, Y.; Musco, H.; Simpson-Yap, S.; Zhu, Z.; Wang, Y.; Lin, X.; Zhang, J.; Taylor, B.; Gratten, J.; Zhou, Y. Investigating the shared genetic architecture between multiple sclerosis and inflammatory bowel diseases. Nat. Commun. 2021, 12, 5641. [Google Scholar] [CrossRef]
- Wang, X.; Wan, J.; Wang, M.; Zhang, Y.; Wu, K.; Yang, F. Multiple sclerosis and inflammatory bowel disease: A systematic review and meta-analysis. Ann. Clin. Transl. Neurol. 2022, 9, 132–140. [Google Scholar] [CrossRef]
- Istat.it Malattie. Available online: https://www.istat.it/it/archivio/malattie (accessed on 19 June 2023).
- Rang, E.H.; Brooke, B.N.; Hermon-Taylor, J. Association of ulcerative colitis with multiple sclerosis. Lancet 1982, 2, 555. [Google Scholar] [CrossRef]
- Pokorny, C.S.; Beran, R.G.; Pokorny, M.J. Association between ulcerative colitis and multiple sclerosis. Intern. Med. J. 2007, 37, 721–724. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, N.M.; Frisch, M.; Rostgaard, K.; Wohlfahrt, J.; Hjalgrim, H.; Koch-Henriksen, N.; Melbye, M.; Westergaard, T. Autoimmune diseases in patients with multiple sclerosis and their first-degree relatives: A nationwide cohort study in Denmark. Mult. Scler. 2008, 14, 823–829. [Google Scholar] [CrossRef] [PubMed]
- Fernández, O.; Fernández, V.; De Ramón, E. Azathioprine and methotrexate in multiple sclerosis. J. Neurol. Sci. 2004, 223, 29–34. [Google Scholar] [CrossRef]
- Avasarala, J.; Guduru, Z.; McLouth, C.J.; Wilburn, A.; Talbert, J.; Sutton, P.; Sokola, B.S. Use of anti-TNF-α therapy in Crohn’s disease is associated with increased incidence of multiple sclerosis. Mult. Scler. Relat. Disord. 2021, 51, 102942. [Google Scholar] [CrossRef] [PubMed]
- Koch-Henriksen, N.; Sørensen, P.S. The changing demographic pattern of multiple sclerosis epidemiology. Lancet Neurol. 2010, 9, 520–532. [Google Scholar] [CrossRef]
- Maaser, C.; Sturm, A.; Vavricka, S.R.; Kucharzik, T.; Fiorino, G.; Annese, V.; Calabrese, E.; Baumgart, D.C.; Bettenworth, D.; Borralho Nunes, P.; et al. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 1: Initial diagnosis, monitoring of known IBD, detection of complications. J. Crohns Colitis 2019, 13, 144–164. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).