Abstract
Background: Anastomotic leakage (AL) is one of the most feared complications in colorectal cancer (CRC) surgery. Although many series have reported the general risk factors for AL, published studies focusing on ileocolic anastomosis are scarce. The main aim of this study was to identify potential risk factors associated with ileocolic anastomosis dehiscence in surgery for CRC. Methods: A total of 188 patients who underwent primary ileocolic anastomosis after elective CRC surgery in Braga’s Hospital from November of 2018 to February of 2022 were included. A multivariate logistic regression analysis was carried out to identify independent risk factors for AL. Results: AL occurred in 13 patients (6.9%), and about three-fourths of these patients required surgical re-intervention. The mortality rate was 5.3%. Diabetes mellitus, ASA score of ≥3, laparotomy or conversion to laparotomy approach, postoperative blood transfusion, and postoperative hypoalbuminemia were associated with an increased risk of AL. In the multivariable analysis, postoperative hypoalbuminemia (p = 0.018; OR: 0.281; CI: 0.098; 0.806) and shorter operating time (p = 0.038; OR: 0.985; CI: 0.972; 0.999) were independent risk factors for AL. Conclusions: Postoperative hypoalbuminemia and shorter operating time are independent risk factors for AL after ileocolic anastomosis.
1. Introduction
On a global level, colorectal cancer (CRC) is the third most common cancer in male and female populations [1]. Considering that surgical treatment represents the only curative option [2], CRC surgery accounts for a noteworthy proportion of all the surgical procedures performed in general surgery.
Anastomotic leakage (AL) is one of the most feared complications in CRC surgery. Despite improvements in surgical technique and perioperative care, AL still occurs and is associated with a significant increase in 30-day morbimortality, length of hospital stay, and associated costs [3,4,5,6,7]. Some studies have also showed the adverse effect of AL on the late oncologic outcome in survivors, with a probability of a 5-year tumor-free survival significantly worse for patients with AL [6].
In spite of being a serious, heavily studied complication, it has yet to be completely understood. For instance, its incidence is not consensual among studies, varying from 1 [8] to 15.9% [9], probably due to the lack of a validated and universal definition that surgeons can agree upon [10], as well as confounding patient inclusion criteria.
There is a high prevalence of CRC located in the right colon; consequently, the oncologic right colectomy with ileocolic anastomosis is a common surgical procedure [11]. Although it is not the most common, AL after ileocolic anastomosis has been reported to be as high as 8.4% [12]. Regarding ileocolic anastomosis, most studies emphasize specific surgical details, such as the difference between stapled and hand sewn anastomosis, with divergent results. A Cochrane meta-analysis, including seven randomized trials and a total of 1125 patients, concluded that stapled anastomosis had a lower AL rate compared with hand-sewn anastomosis [13]. However, some recently published studies have shown contradictory results [12,14,15,16], while others found no difference between the two [16].
Similarly, regarding other risk factors for AL after ileocolic anastomosis, substantial results have been scarce. A prospective and multicenter study, including 1102 patients that underwent elective right colectomy, identified only preoperative nutritional status and the stapled anastomosis as independent risk factors for this complication [11]. More recently, other variables, such as male sex, hypertension (HTN) [17], and diabetes mellitus (DM) [14] have been suggested as possible factors associated with AL. Current smoking habits [8,14,18], a poor nutritional status [8,11], and perioperative blood transfusions [8,17] also seem to be variables associated with this complication.
Indeed, although there are numerous papers on this subject, the majority of the studies included a mixed cohort of patients with different pathologies, as well as different performed procedures. Depending on the type of anastomosis, the epidemiology, pathophysiology, and its impact and prognosis are different, and therefore should be studied independently.
Thus, the primary end point of this study was to identify the pre-, intra-, and post-operative risk factors associated with AL after ileocolic anastomosis in elective CRC surgery. The secondary end points included the assessment of the impact of AL on clinical management, length of hospital stay, and the 30-day morbimortality rate.
The knowledge of these risk factors may influence and aid surgeons in optimizing the preoperative management of well-known risk factors to tailor a surgical plan, as well as to intensify the postoperative monitoring in high-risk patients [8]. Such measures would hopefully reduce the incidence rate of this complication and allow early diagnosis and anticipation of its treatment, resulting in better oncologic and overall outcomes for these patients.
2. Results
2.1. Baseline Clinical and Preoperative Data
The patient demographic characteristics are shown in the Table 1, and their association with AL is reported in Table 2.

Table 1.
Patient demographic characteristics.

Table 2.
Association of baseline clinical and preoperative data with AL, expressed as number of patients, n (%), or median (IQR).
Of the 188 included patients, 120 (63.8%) were men, with a median age of 73 years; 22.4% of patients possessed a BMI in the obese category (BMI ≥ 30).
Of the preoperative medical conditions evaluated, HTN was the most common (67.6%), followed by DM (27.7%), and CPOD (5.3%). Only DM showed a significant relationship with AL (p = 0.009). Most patients exhibited an ASA score of II (58.0%) or III (34.0%). The ASA score had a significant association with AL (p = 0.046), with the statistical difference occurring in patients with a score ≥ IV, representing 23.1% of the AL group. None of the remaining preoperative variables analyzed showed an association with the occurrence of AL.
2.2. Surgical Procedure
The median operating time was 135 min, without a statistical difference between patients with and without AL (p = 0.050).
Regarding the surgical approach, the most frequent was the laparoscopic approach (86.7%). There was a 3.2% conversion rate to laparotomy surgery. Patients with a laparotomy, or converted to a laparotomy approach, exhibited a significantly higher risk of AL (53.8%), (p = 0.010).
Suture reinforcement was performed in 127 patients (67.6%); however, there was no difference (p = 0.553) between the two groups.
There was a very asymmetrical patient distribution noted in terms of anastomosis performed. The majority of the anastomoses were the stapled (98.4%) and side to side configurations (98.7%). Given the very few cases of handsewn and end-to-end anastomosis, these variables were not compared in terms of AL risk. When we analyzed the group with isoperistaltic Vs, anisoperistaltic (p = 0.163), and intracorporeal Vs, extracorporeal (p > 0.990), there were no significant differences in either variable in terms of AL rates.
Surgical data and their association with AL are reported in Table 3.

Table 3.
Association of surgical data with AL, expressed as number of patients, n (%), or median (IQR).
2.3. Postoperative Findings
The most frequent tumor location was the ascending colon (60.1%), followed by the transverse colon (18.1%), the hepatic flexure (13.8%), the caecum (5.3%), and the ileocecal appendix (2.7%).
The majority of the operated tumors were in a TNM stage of II (39.3%), followed by stage I (29.5%) and stage III (25.7%). Tumor location and TNM staging were similar between the two groups in terms of AL occurrence.
Patients without AL had a significantly shorter hospital stay (4 days) compared with those who had AL (11 days), (p = 0.001). Postoperative blood transfusions were required in 6 patients and were related to a higher risk of AL (p = 0.008).
The 30-day morbidity rate was 25.0%. The most frequent complication was AL (13 patients), with a median detection at 5 postoperative days. A total of 3 of the patients were treated with antibiotics only (AL Grade B), while the remaining 10 (76.9%) required surgical re-intervention (AL Grade C). According to the Clavien–Dindo Classification: 3 patients required treatment grade II, 6 patients required treatment grade IIIb, 1 exhibited multiorgan dysfunction (grade IV), and the remaining 3 had passed away at the 30-day mark (grade V).
The 30-day postoperative mortality rate was 5.3%, with a significant difference between the two groups in terms of AL occurrence (p = 0.024).
Postoperative findings and their association with AL are reported in Table 4.

Table 4.
Postoperative findings and their association with AL, expressed as number of patients, n (%), mean (SD), or median (IQR).
2.4. Association with AL
Multivariable logistics regression initially included all variables with a p value < 0.100. The variables were introduced in the following hierarchical order (modifiable risk factors followed by non-modifiable risk factors): postoperative albumin; preoperative hemoglobin, postoperative transfusion; surgical approach; DM; ASA score; operating time; postoperative CRP. From this first model, preoperative hemoglobin (p = 0.646), surgical approach (p = 0.983), the ASA score (p = 0.201), and postoperative CRP (p = 0.700) were removed, as they did not contribute to the model.
A second model was analyzed with the remaining variables. In this model, all variables made a statistically significant contribution; however, the variables postoperative transfusion and DM exhibited an OR with a very large CI (p = 0.009; OR: 29.028; CI: 2.2334; 361.081), (p = 0.046; OR: 4.227; CI: 1.027; 17.399), respectively, and were therefore removed from the model.
A third model was analyzed with the variables: postoperative albumin and operating time. This model was statistically significant (X2 (2) = 10.07, p = 0.007), but only explained 16% of the AL variance (Nagelkerke R2 = 0.160). Both variables were independent risk factors for AL: postoperative hypoalbuminemia (p = 0.018; OR: 0.281; CI: 0.098; 0.806) and shorter operating time (p = 0.038; OR: 0.985 IC: 0.972; 0.999) (Table 5). In this model, although the percentage of correctly classified cases was 90.2%, none of the AL cases were correctly classified (specificity of 100%, and sensitivity of 0%).

Table 5.
Multivariate analysis to identify independent risk factors for AL.
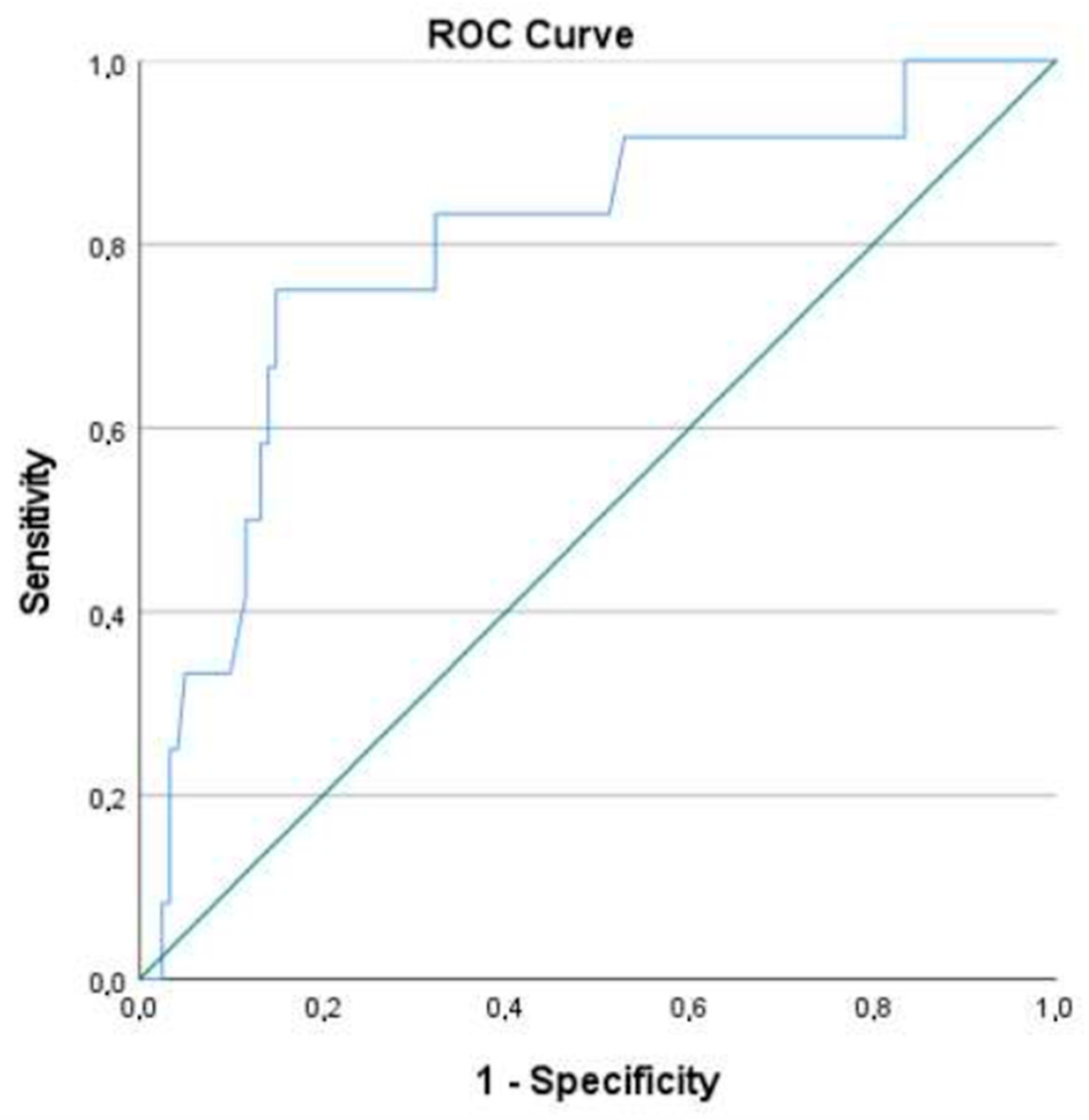
A ROC curve was then created, using the model’s predictive probabilities. The area under the curve (AUC) obtained was 0.795 (CI: 0.716; 0.860) (Figure 1). A cut-off point of 0.138 was chosen as the value that maximized both specificity and sensitivity. A second analysis was performed using the new cut-off point, resulting in a model that could correctly classify 84.2% of cases (with 75.0% sensitivity and 85.1% specificity).
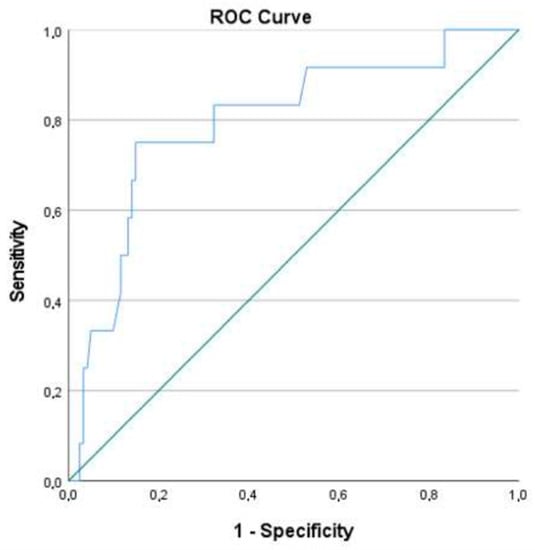
Figure 1.
ROC curve of the multivariate logistics regression model.
3. Materials and Methods
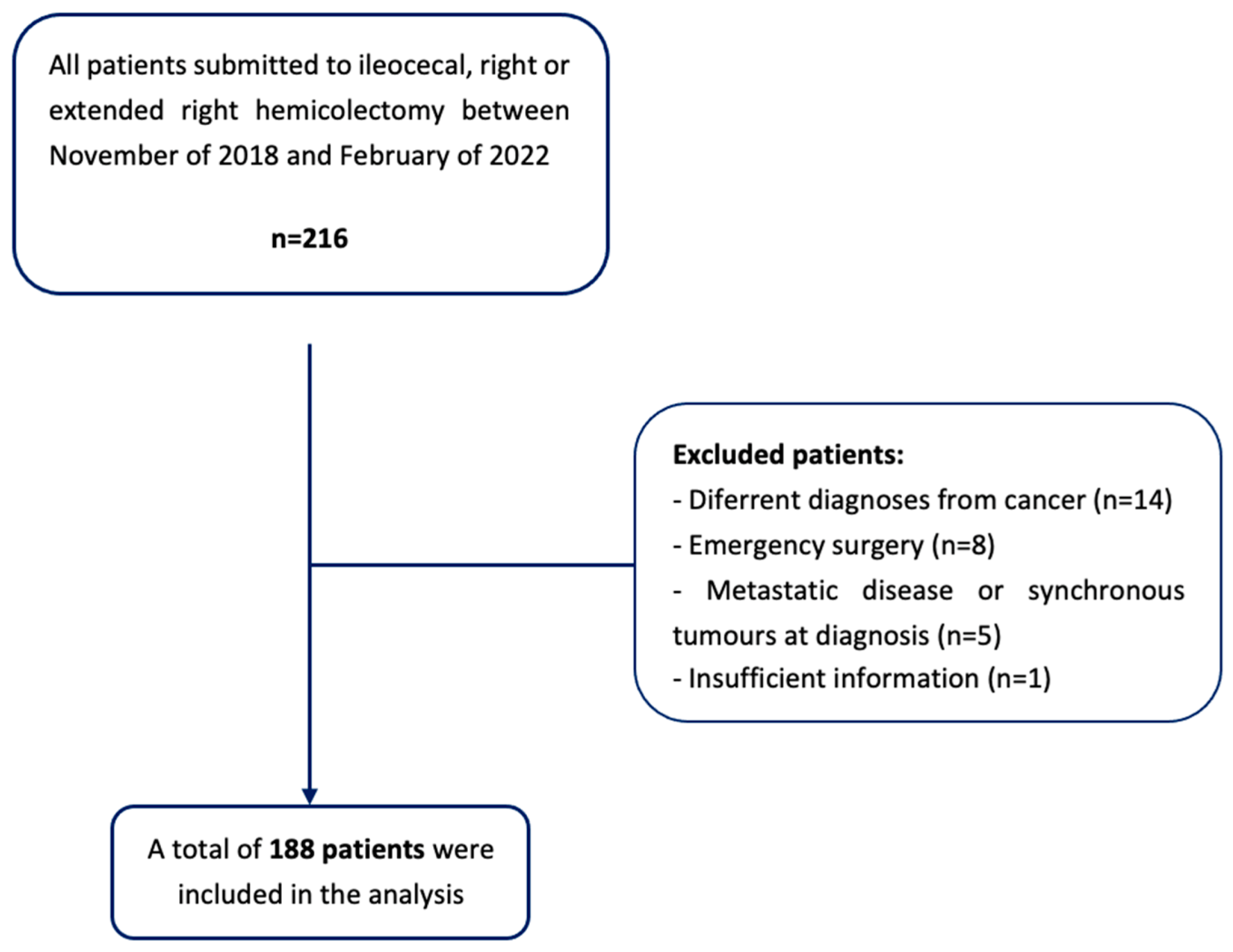
An observational, analytic, retrospective, and descriptive study was conducted. This study used a non-random convenience sample, and several inclusion and exclusion criteria were applied. The target population consisted of the patients submitted to primary ileocolic anastomosis after elective surgery for CRC, performed in Braga’s Hospital, between November of 2018 and February of 2022.
The exclusion criteria were as follows:
- Patients undergoing to emergency surgery or surgical re-intervention;
- Patients with a non-cancer diagnosis (i.e., inflammatory bowel disease or diverticular disease);
- The presence of metastatic disease at diagnosis, or synchronous tumors in other locations;
- The presence of a diverting stoma or a tumor resection surgery, with no ileocolic anastomosis;
- Insufficient information in the clinical process to determine the variables used in the study.
According to these criteria, a sample size of 188 patients was obtained (Figure 2).
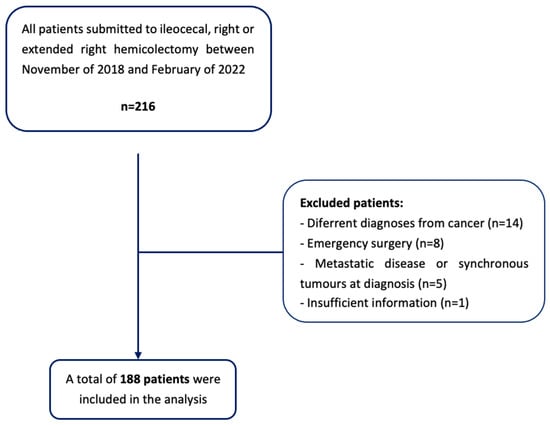
Figure 2.
Flowchart of patient selection.
3.1. Data Collection
Data was collected through access to the informatics clinical processes in the Glintt—Soluções Clínicas® and registered in a Microsoft Excel confidential document.
Variables related to patient demographic data (gender, age, and body mass index); presence of comorbidities, such as HTN, DM, or chronic obstructive pulmonary disease (COPD); American Society of Anesthesiology (ASA) score; smoking habits; pre-operatory data; value of hemoglobin, glucose, albumin, and serum protein levels; carcinoembryonic antigen (CEA) and carbohydrate antigen 19.9 (CA 19.9); preoperative blood transfusions; intra-operatory data; surgical procedures (such as ileocecal resection, right colectomy, and extended right colectomy; open vs. laparoscopic approach; surgical anastomosis performed, such as hand-sewn vs. stapled; side to side Vs, end to side; intra vs. extracorporeal; iso vs. anisoperistaltic; reinforcement of sutures; duration of surgery; intraoperative complication or unplanned event/finding and blood transfusions); and postoperative data, including value of hemoglobin, C-reactive protein (CRP), albumin and serum protein levels; blood transfusions; tumor location; and cancer staging were collected retrospectively.
The time for AL diagnosis, diagnostic criteria (clinical features, laboratory and imaging tests), impact of AL on clinical management, length of hospital stay, and the overall 30-day morbimortality were also registered.
3.2. Definitions
AL was defined using the proposed consensus from the International Study Group of Rectal Cancer (ISREC) in 2010 as a “communication between the intra and extraluminal compartments owing to a defect of the integrity of the intestinal wall at the anastomosis” [19,20,21]. Furthermore, a grading system was suggested, subdivided into three grades of AL severity, according to its impact on clinical management [19,20,21]:
- Grade A: Corresponds to the definition of a ‘‘radiologic leakage’’, used by several authors. This grade of AL is not associated with clinical symptoms or abnormal laboratory tests, and no active therapeutic intervention is required.
- Grade B: Patient’s clinical condition requires an active therapeutic intervention, such as the administration of antibiotics and/or radiologically guided drainage, that can be managed without operative reintervention.
- Grade C: Patients are often quite ill and require operative re-laparotomy.
The diagnostic criteria for AL were based on clinical features such as abdominal pain and distension, fever, signs of peritonitis or a purulent/fecal drainage from the wound or in their drains, markedly increased parameters of infection (leukocytosis and/or an increase in CRP), and imaging tests, in particular computed tomography, showing leakage of the endoluminally administered contrast agent into the extra intestinal space via the suture or staple line.
Tumor location was verified intraoperatively. Cancer staging was based on postoperative histopathologic evaluation, with TNM grading.
Tobacco use was registered as non-smoker, active smoker, or previous smoker. Blood transfusions were only registered as having occurred or not (number of units administered was not collected).
The ASA score was collected and divided in three categories for statistical analysis, considering the associated risk and the number of patients receiving each score: score of I and II in the first category, score of III in the second, and scores ≥ IV in the third category.
The 30-day morbidity was registered according to the Clavien–Dindo Classification of surgical complications [22].
3.3. Statistical Analysis
The statistical analysis was performed using the Statistical Package for Social Sciences (SPSS®) version 22.0.
The normality of the distribution of continuous variables was assessed using the Kolmogorov–Smirnov and the Shapiro–Wilk tests, skewness and kurtosis, and by analyzing the graphics and histogram. Not all variables showed a normal distribution; therefore, parametric and non-parametric tests were performed as needed. When normality was met, group comparisons were performed using the independent samples t-test. Homogeneity of variances was assessed using Levene’s test. The effect size reported was Cohen’s d. If the variable did not follow a normal distribution, the Mann–Whitney test was performed, and r was reported as effect size [23].
Categorical variables were compared with the Chi-squared test. If the percentage of cells with an expected count bellow 5 was greater than 20%, the Fisher’s exact test was performed. For effect size, when both variables were dichotomous, the Phi coefficient was used. For variables with more than one category Cramer’s V was reported [24].
For continuous variables, measures of central tendency and dispersion are presented as the mean and standard deviation, or median and interquartile range (if normality was not met), whereas categorical variables are reported as the total number and percentage.
To determine the independent risk factors related with AL, a multivariate logistic regression model was used. Variables with a p < 0.100 [25] in the univariate analysis were included. Variables were introduced in hierarchical order (primarily, the modifiable, and secondarily, the non-modifiable risk factors) and removed if they did not contribute to the model. Additionally, considering the small sample size of the outcome, some variables were removed when associated with a 95% confidence interval (CI) with a very large range. To assess the model’s goodness of fit, the Hosmer and Lemeshow test was used. The effect estimates are presented as odds ratio (OR), with 95% CI and two-sided p-values. A receiver operating characteristic (ROC) curve was then performed to analyze the model’s discriminative capacity. Statistical significance was defined at the level of p < 0.05.
3.4. Ethical Considerations
This project required the collection of confidential data regarding the patients included in the sample. They were properly codified, and their anonymity is guaranteed.
The study received the approval of all the necessary institutions: the Ethics Committee for Health of Braga’s Hospital (protocol code: 76_2022; date of approval: 22 June 2022), the Data Protection Office of Braga’s Hospital (protocol code: 20220057_CirGeral240522; date of approval: 24 May 2022) and the Ethics Committee for Research in Life and Health Sciences (protocol code: CEICVS 041/2022; date of approval: 6 June 2022).
4. Discussion
In this study the AL rate was 6.9%, within range of that described in the literature [11,13]. Similar to what has been reported in previous studies, the mortality rate was 5.3% and was significantly increased in patients with AL [8,14].
Analyzing the surgical technique is always important when considering anastomotic failure. As verified in our study, in which 83.5% of surgeries were laparoscopic, currently, the laparoscopic approach is considered the standard of care in almost all CRC surgeries [25,26], providing equivalent surgical [26,27] and long-term oncological outcomes when compared to the laparotomy approach [28,29].
In terms of AL occurrence, most studies have reported similar rates between the two approaches [30,31]. However, a recent study that included 25,097 patients experiencing CRC surgery, after adjusted analysis and exclusion of emergency surgeries, reported that patients who underwent laparotomy or conversion to laparotomy procedures had a two-fold increase in the occurrence of AL and were more than 2 times more likely to die [32]. Another study, focused on right hemicolectomy, also reported the laparotomy approach as an independent risk factor for AL [13]. This study revealed similar results, with a higher rate of AL in laparotomy and conversion to laparotomy procedures compared to the laparoscopic approach (p = 0.001) in univariate analysis. However, in the multivariate logistics regression model, lost statistical significance. A plausible explanation for these findings is that the surgeon’s choice of laparotomy approach may be a marker of operative difficulty and therefore, enhance the risk of AL [13].
In our cohort, the majority of the laparoscopic surgeries exhibited an intracorporeal anastomosis (78.2%). Despite its technical difficulty [33,34], this procedure has been increasingly implemented in our institution, considering its safety [35,36] and the benefits regarding postoperative outcomes, such as an early return of bowel function, a shorter hospital stay, and better cosmetic results, in comparison with extracorporeal anastomosis [33,34,37]. Nevertheless, no studies have been able to show the superiority of one of these techniques in terms of AL occurrence [26,34,37,38]. Similarly, this study found no significant differences between these two types of anastomosis. In terms of configuration, isoperistaltic was the most performed (91.7% vs. 8.3% of anisoperistaltic), without a statistical difference in AL risk. Our findings seem in accordance with those of a recent randomized trial that found comparable results using both approaches regarding postoperative complication rates. Nonetheless, it seemed that in the long term, patients with an anisoperistaltic anastomosis had a shorter intestinal transit time and a non-statistically significant tendency to increased chronic diarrhea [39].
The role of suture reinforcement in AL prevention has been conflicting [8,40,41]. In this study, anastomosis with reinforced sutures were not associated with significantly less AL. Our results are in agreement with a European Society of Coloproctology snapshot study that included 1347 patients undergoing right hemicolectomy or ileocolic resection, in which no differences in the oversewing of the anastomotic staple lines were found, in terms of reducing leak rates [40]. However these results are in contrast with a recently published study which showed that suture oversewing as anastomotic reinforcement was an independent protective factor for AL [8]. In the mentioned study, anastomotic reinforcement was defined as a seromuscular oversewing suture, either with a running suture or interrupted inverting stiches on the serosal layer of the entire anastomotic line. The results obtained showed a significant decrease in AL rate, as well as in severe AL (grade C). In this study, suture reinforcement was performed with running sutures, either using barbed or traditional sutures. Given the retrospective nature of this study, the type of suture reinforcement performed was chosen according to the surgeon’s preference, so it was not standardized, making the comparison of our results with those of the previous study less accurate.
Operating time has also been described to impact the occurrence of AL. A prolonged operating time has been related to the occurrence of AL [42]. However, in this study, we found contradicting results, with a shorter operating time being an independent risk factor for AL. Further studies should be conducted, with more complete operative records and more detailed operative times, in order to understand its true impact on the occurrence of this complication.
Perioperative variables have also been suggested as potential risk factors for AL. In this study, 52 patients had a previous diagnoses of DM. In the univariate analysis, there was an association between these patients and the occurrence of AL (p = 0.009). In the multivariate analysis, significance was maintained, but with an OR with a very large 95% CI range, and it was therefore removed from the model. A recent study focused on right hemicolectomy also found an association of AL with DM in the univariate and multivariate analysis. Similar to this study, in the multivariate analysis, the 95% CI reported was between 2.23 and 15.90, resulting in a need to interpret these results with caution [14].
Regarding postoperative variables, blood transfusions after CRC resections have been associated with AL and postoperative infections in a number of studies. This association is thought to be related to the immunosuppression effect caused by homologous blood transfusions [43,44,45,46,47]. In addition, severe postoperative anemia may lead to tissue hypoxia and, consequently, impair the anastomotic healing process [8]. This study is in agreement with previous findings, suggesting that the possible detrimental effect of blood transfusions is only a factor in intra- or postoperative transfusions [46,48]. Postoperative blood transfusions were associated with AL in the univariate analysis, but also in multivariate analysis, although with an OR with a very large CI range.
Serum albumin levels, as well as other hepatic proteins, correlate to the patient’s nutritional status and are indicators of risk for morbimortality in recovery from acute and chronic disease [49,50]. In major gastrointestinal surgery and CRC surgery, hypoalbuminemia has been associated with postoperative complications, in particular with the occurrence of AL [8,51,52,53,54,55]. While the exact pathophysiology by which low albumin levels can affect the correct healing of anastomosis is not fully understood, studies on experimental colonic anastomosis demonstrated an association with decreased collagen content, decreased values of tensile strength, and lower bursting pressures [56,57,58].
Postoperative hypoalbuminemia was significantly associated with AL in both the uni- and multivariate analysis. Note that in our institution, the postoperative levels of albumin were not routinely requested, with some patient’s showing missing information regarding this variable (53/188 patients), which could have impacted the observed results. In agreement with this study, Shimura et al. found only postoperative hypoalbuminemia to be significantly associated with AL [59]. The authors considered the low number of patients afflicted with advanced CRC with distant metastasis, or cancer cachexia (that would result in pre-operative hypoalbuminemia) in their cohort as a possible explanation for the absence of a relationship between AL and preoperative albumin levels. This explanation is also plausible to justify our results, since the majority of our patients possessed tumors at earlier stages (74.3% of the patients in our cohort had a TNM score ≤ 2).
One of the limitations of this study is its retrospective nature. This affected data collection, as most patients did not have information available for all the variables included in the study. A prospective study would increase the amount of information accessible for each variable and allow for a standardization of the surgical procedures being analyzed, contributing to more reliable and accurate results and allowing for future comparison with other studies. Another limitation was the single-center design. The sample size was relatively small, especially for the AL subgroup. This may explain why relatively consensual risk factors for ileocolic AL were not significant in the group comparison analysis. This also affected the number of variables that could be included and their possible significance in the multivariate regression model.
One of the strengths of this study was the rigorous exclusion criteria. By applying the exclusion criteria (mentioned in Materials and Methods section), we were able to eliminate potential confounding elements that could have affected the ability of the study to identify independent AL risk factors. Therefore, further studies should be conducted prospectively with larger samples, while maintaining stringent exclusion criteria in order to draw more accurate conclusions.
5. Conclusions
AL is one of the most devastating complications in CRC surgery, with serious impacts on morbidity and mortality.
Early diagnosis and prevention of AL based on the reliable identification of risk factors is and crucial. In this study, shorter operating time and postoperative hypoalbuminemia were independent risk factors for ileocolic anastomosis dehiscence.
Future studies should be performed, focusing on AL after ileocolic anastomosis in surgery for CRC, with larger sample sizes and standardized protocols. The continuation of research in this area, with a better understanding of the risk factors involved and their pathophysiology, could potentially improve the outcome of patients with AL, and hopefully reduce its occurrence.
Author Contributions
Conceptualization, P.M.D.d.S., S.F.M. and J.C.P.; methodology, P.M.D.d.S. and S.F.M.; validation, P.M.D.d.S., S.F.M. and J.C.P.; formal analysis, P.M.D.d.S. and S.L.G.; investigation, P.M.D.d.S. and S.L.G.; resources, P.M.D.d.S. and S.L.G.; writing—original draft preparation, P.M.D.d.S. and S.L.G.; writing—review and editing, P.M.D.d.S. and S.F.M.; supervision, P.M.D.d.S. and S.F.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by all the necessary institutions: the Ethics Committee for Health of Braga’s Hospital (protocol code: 76_2022; date of approval: 22 June 2022), the Data Protection Office of Braga’s Hospital (protocol code: 20220057_CirGeral240522; date of approval: 24 May 2022), and the Ethics Committee for Research in Life and Health Sciences (protocol code: CEICVS 041/2022; date of approval: 6 June 2022).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to the protection of patient privacy.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Salibasic, M.; Pusina, S.; Bicakcic, E.; Pasic, A.; Gavric, I.; Kulovic, E.; Rovcanin, A.; Beslija, S. Colorectal Cancer Surgical Treatment, our Experience. Med. Arch. 2019, 73, 412–414. [Google Scholar] [CrossRef]
- Rentsch, M.; Schiergens, T.; Khandoga, A.; Werner, J. Surgery for Colorectal Cancer-Trends, Developments, and Future Perspectives. Visc. Med. 2016, 32, 184–1891. [Google Scholar] [CrossRef]
- Golub, R.W.; Cantu, R.; Stein, H.D. A multivariate analysis of factors contributing to leakage of intestinal anastomoses. J. Am. Coll. Surg. 1997, 184, 364–372. [Google Scholar]
- Alves, A.; Panis, Y.; Trancart, D.; Regimbeau, J.M.; Pocard, M.; Valleur, P. Factors associated with clinically significant anastomotic leakage after large bowel resection: Multivariate analysis of 707 patients. World J. Surg. 2002, 26, 499–502. [Google Scholar]
- Branagan, G.; Finnis, D. Prognosis After Anastomotic Leakage in Colorectal Surgery. Dis. Colon Rectum 2005, 48, 1021–1026. [Google Scholar] [CrossRef]
- Kube, R.; Mroczkowski, P.; Granowski, D.; Benedix, F.; Sahm, M.; Schmidt, U.; Gastinger, I.; Lippert, H. Anastomotic leakage after colon cancer surgery: A predictor of significant morbidity and hospital mortality, and diminished tumour-free survival. Eur. J. Surg. Oncol. EJSO 2010, 36, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Buchs, N.C.; Gervaz, P.; Secic, M.; Bucher, P.; Mugnier-Konrad, B.; Morel, P. Incidence, consequences, and risk factors for anastomotic dehiscence after colorectal surgery: A prospective monocentric study. Int. J. Color. Dis. 2007, 23, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Golda, T.; Lazzara, C.; Zerpa, C.; Sobrino, L.; Fico, V.; Kreisler, E.; Biondo, S. Risk factors for ileocolic anastomosis dehiscence; a cohort study. Am. J. Surg. 2019, 220, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, L.T.; Jørgensen, T.; Kirkeby, L.T.; Skovdal, J.; Vennits, B.; Wille-Jørgensen, P. Smoking and alcohol abuse are major risk factors for anastomotic leakage in colorectal surgery. Br. J. Surg. 1999, 86, 927–931. [Google Scholar] [CrossRef] [PubMed]
- Bruce, J.; Krukowski, Z.H.; Al-Khairy, G.; Russell, E.M.; Park, K.G.M. Systematic review of the definition and measurement of anastomotic leak after gastrointestinal surgery. Br. J. Surg. 2001, 88, 1157–1168. [Google Scholar] [CrossRef]
- Frasson, M.; ANACO Study Group; Granero-Castro, P.; Rodríguez, J.L.R.; Flor-Lorente, B.; Braithwaite, M.; Martínez, E.M.; Pérez, J.A.Á.; Cazador, A.C.; Espí, A. Risk factors for anastomotic leak and postoperative morbidity and mortality after elective right colectomy for cancer: Results from a prospective, multicentric study of 1102 patients. Eur. Soc. Coloproctol. Meet. 2014, 31, 105–114. [Google Scholar] [CrossRef]
- Choy, P.Y.G.; Bissett, I.P.; Docherty, J.G.; Parry, B.R.; Merrie, A.; Fitzgerald, A. Stapled versus handsewn methods for ileocolic anastomoses. Cochrane Database Syst. Rev. 2011, 9, CD004320. [Google Scholar] [CrossRef]
- European Society of Coloproctology Collaborating Group; Battersby, N.; Bhangu, A.; Chaudhri, S.; El-Hussuna, A.; Frasson, M.; Nepogodiev, D.; Singh, B.; Vennix, S.; Zmora, O.; et al. Relationship between method of anastomosis and anastomotic failure after right hemicolectomy and ileo-caecal resection: An international snapshot audit. Color. Dis. 2017, 19, e296–e311. [Google Scholar] [CrossRef]
- Jessen, M.; Nerstrøm, M.; Wilbek, T.E.; Roepstorff, S.; Rasmussen, M.S.; Krarup, P.-M. Risk factors for clinical anastomotic leakage after right hemicolectomy. Int. J. Color. Dis. 2016, 31, 1619–1624. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, P.; Jestin, P.; Gunnarsson, U.; Lindforss, U. Higher Frequency of Anastomotic Leakage with Stapled Compared to Hand-Sewn Ileocolic Anastomosis in a Large Population-Based Study. World J. Surg. 2015, 39, 1834–1839. [Google Scholar] [CrossRef]
- Jurowich, C.; Lichthardt, S.; Matthes, N.; Kastner, C.; Haubitz, I.; Prock, A.; Filser, J.; Germer, C.-T.; Wiegering, A. Effects of anastomotic technique on early postoperative outcome in open right-sided hemicolectomy. BJS Open 2018, 3, 203–209. [Google Scholar] [CrossRef]
- Sánchez-Guillén, L.; Frasson, M.; García-Granero, A.; Pellino, G.; Flor-Lorente, B.; Álvarez-Sarrado, E. Risk factors for leak, complications and mortality after ileocolic anastomosis: Comparison of two anastomotic techniques. Ann. R. Coll. Surg. Engl. 2019, 101, 571–578. [Google Scholar] [CrossRef]
- Kwak, H.D.; Kim, S.-H.; Kang, D.W.; Baek, S.-J.; Kwak, J.M.; Kim, J. Risk Factors and Oncologic Outcomes of Anastomosis Leakage After Laparoscopic Right Colectomy. Surg. Laparosc. Endosc. Percutaneous Tech. 2017, 27, 440–444. [Google Scholar] [CrossRef]
- Ellis, C.T.; Maykel, J.A.; Surgery, C.; Polk, H.C. Defining Anastomotic Leak and the Clinical Relevance of Leaks Definition of Large Bowel Anastomotic Leak Definition. Clin. Colon Rectal Surg. 2021, 34, 359–365. [Google Scholar] [PubMed]
- Kulu, Y.; Ulrich, A.; Bruckner, T.; Contin, P.; Welsch, T.; Rahbari, N.N.; Büchler, M.W.; Weitz, J. Validation of the International Study Group of Rectal Cancer definition and severity grading of anastomotic leakage. Surgery 2013, 153, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Rahbari, N.N.; Weitz, J.; Hohenberger, W.; Heald, R.J.; Moran, B.; Ulrich, A.; Holm, T.; Wong, W.D.; Tiret, E.; Moriya, Y.; et al. Definition and grading of anastomotic leakage following anterior resection of the rectum: A proposal by the International Study Group of Rectal Cancer. Surgery 2010, 147, 339–351. [Google Scholar] [CrossRef]
- Clavien, P.A.; Barkun, J.; de Oliveira, M.L.; Vauthey, J.N.; Dindo, D.; Schulick, R.D.; Graf, R.; Cameron, J.; Padbury, R.; Masatoshi, M.D.; et al. The Clavien- Dindo classification of surgical complications: Five-year experience. Ann. Surg. 2009, 250, 187–196. [Google Scholar] [CrossRef]
- Field, A.P. Discovering Statistics Using IBM SPSS Statistics, 5th ed.; Sage: London, UK, 2017. [Google Scholar]
- Bursac, Z.; Gauss, C.H.; Williams, D.K.; Hosmer, D.W. Purposeful selection of variables in logistic regression. Source Code Biol. Med. 2008, 3, 17. [Google Scholar] [CrossRef]
- Bracale, U.; Melillo, P.; Lazzara, F.; Andreuccetti, J.; Stabilini, C.; Corcione, F.; Pignata, G. Single-Access Laparoscopic Rectal Resection Versus the Multiport Technique. Surg. Innov. 2014, 22, 46–53. [Google Scholar] [CrossRef]
- Sciuto, A.; Merola, G.; De Palma, G.D.; Sodo, M.; Pirozzi, F.; Bracale, U. Predictive factors for anastomotic leakage after laparoscopic colorectal surgery. World J. Gastroenterol. 2018, 24, 2247–2260. [Google Scholar] [CrossRef]
- Guillou, P.J.; Quirke, P.; Thorpe, H.; Walker, J.; Jayne, D.G.; Smith, A.M.; Heath, R.M.; Brown, J.M. Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): Multicentre, randomised controlled trial. Lancet 2005, 365, 1718–1726. [Google Scholar] [CrossRef]
- Dubose, A.C.; Kuy, S. A Comparison of Laparoscopically Assisted and Open Colectomy for Colon Cancer. In 50 Studies Every Surgeon Should Know; Oxford Medicine Online: Oxford, UK, 2017. [Google Scholar]
- Lacy, A.M.; Delgado, S.; García-Valdecasas, J.C.; Castells, A.; Piqué, J.M.; Grande, L.; Fuster, J.; Targarona, E.M.; Pera, M.; Visa, J. Port site metastases and recurrence after laparoscopic colectomy. Surg. Endosc. 1998, 12, 1039–1042. [Google Scholar] [CrossRef]
- Arezzo, A.; Passera, R.; Scozzari, G.; Verra, M.; Morino, M. Laparoscopy for rectal cancer reduces short-term mortality and morbidity: Results of a systematic review and meta-analysis. Surg. Endosc. 2012, 27, 1485–1502. [Google Scholar] [CrossRef] [PubMed]
- Abraham, N.S.; Young, J.M.; Solomon, M. Meta-analysis of short-term outcomes after laparoscopic resection for colorectal cancer. Br. J. Surg. 2004, 91, 1111–1124. [Google Scholar] [CrossRef] [PubMed]
- Mungo, B.; Papageorge, C.M.; Stem, M.; Molena, D.; Lidor, A.O. The Impact of Operative Approach on Postoperative Complications Following Colectomy for Colon Cancer. World J. Surg. 2017, 41, 2143–2152. [Google Scholar] [CrossRef] [PubMed]
- Milone, M.; Elmore, U.; Allaix, M.E.; Bianchi, P.P.; Biondi, A.; Boni, L.; Bracale, U.; Cassinotti, E.; Ceccarelli, G.; Corcione, F.; et al. Fashioning enterotomy closure after totally laparoscopic ileocolic anastomosis for right colon cancer: A multicenter experience. Surg. Endosc. 2019, 34, 557–563. [Google Scholar] [CrossRef]
- Carnuccio, P.; Jimeno, J.; Parés, D. Laparoscopic right colectomy: A systematic review and meta-analysis of observational studies comparing two types of anastomosis. Tech. Coloproctol. 2013, 18, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Nors, J.; Sommer, T.; Wara, P. Leakage Rate After Laparoscopic Ileocolic Intracorporeal Anastomosis. J. Laparoendosc. Adv. Surg. Tech. 2018, 28, 1287–1293. [Google Scholar] [CrossRef] [PubMed]
- Kornmann, V.N.N.; Hagendoorn, J.; Van Koeverden, S.; Van Ramshorst, B.; Smits, A.B. Totally Laparoscopic Right Hemicolectomy with Intracorporeal Anastomosis is a Technically and Oncologically Safe Procedure. Acta Chir. Belg. 2013, 113, 439–443. [Google Scholar] [CrossRef]
- Biondi, A.; Santocchi, P.; Pennestrì, F.; Santullo, F.; D’ugo, D.; Persiani, R. Totally laparoscopic right colectomy versus laparoscopically assisted right colectomy: A propensity score analysis. Surg. Endosc. 2017, 31, 5275–5282. [Google Scholar] [CrossRef] [PubMed]
- Magistro, C.; Di Lernia, S.; Ferrari, G.; Zullino, A.; Mazzola, M.; De Martini, P.; De Carli, S.; Forgione, A.; Bertoglio, C.L.; Pugliese, R. Totally laparoscopic versus laparoscopic-assisted right colectomy for colon cancer: Is there any advantage in short-term outcomes? A prospective comparative assessment in our center. Surg. Endosc. 2013, 27, 2613–2618. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez, N.; Abrisqueta, J.; Luján, J.; Hernández, Q.; Rufete, M.D.; Parrilla, P. Isoperistaltic versus antiperistaltic ileocolic anastomosis. Does it really matter? Results from a randomised clinical trial (ISOVANTI). Surg. Endosc. 2018, 33, 2850–2857. [Google Scholar] [CrossRef]
- 2015 European Society of Coloproctology Collaborating Group; Glasbey, J.C.; Nepogodiev, D.; Battersby, N.; Bhangu, A.; El-Hussuna, A.; Frasson, M.; Singh, B.; Vennix, S.; Zmora, O.; et al. The impact of stapling technique and surgeon specialism on anastomotic failure after right- sided colorectal resection: An international multicentre, prospective audit. Color. Dis. 2018, 20, 1028–1040. [Google Scholar]
- Fleetwood, V.A.; Gross, K.; Alex, G.C.; Cortina, C.S.; Smolevitz, J.B.; Sarvepalli, S.; Bakhsh, S.R.; Myers, J.A.; Singer, M.A.; Orkin, B.A. Common side closure type but not stapler brand or oversewing influences side-to-side anastomotic leak rates. J. Am. Coll. Surg. 2015, 221, 590–595. [Google Scholar] [CrossRef]
- Vasiliu, E.C.Z.; Zarnescu, N.O.; Costea, R.; Neagu, S. Review of Risk Factors for Anastomotic Leakage in Colorectal Surgery. Chirurgia 2015, 110, 319–326. [Google Scholar]
- Ozben, V.; Stocchi, L.; Ashburn, J.; Liu, X.; Gorgun, E. Impact of a restrictive vs liberal transfusion strategy on anastomotic leakage and infectious complications after restorative surgery for rectal cancer. Color. Dis. 2017, 19, 772–780. [Google Scholar] [CrossRef]
- Park, J.S.; Choi, G.-S.; Kim, S.H.; Kim, H.R.; Kim, N.K.; Lee, K.Y.; Kang, S.B.; Kim, J.Y.; Lee, K.Y.; Kim, B.C.; et al. Multicenter Analysis of Risk Factors for Anastomotic Leakage After Laparoscopic Rectal Cancer Excision. Ann. Surg. 2013, 257, 665–671. [Google Scholar] [CrossRef]
- Tartter, P.I.; Quintero, S.; Barron, D.M. Perioperative blood transfusion associated with infectious complications after colorectal cancer operations. Am. J. Surg. 1986, 152, 479–482. [Google Scholar] [CrossRef]
- Tang, R.; Chen, H.H.; Wang, Y.L.; Changchien, C.R.; Chen, J.-S.; Hsu, K.-C.; Chiang, J.-M.; Wang, J.-Y. Risk Factors for Surgical Site Infection After Elective Resection of the Colon and Rectum: A Single-Center Prospective Study of 2,809 Consecutive Patients. Ann. Surg. 2001, 234, 181–189. [Google Scholar] [CrossRef]
- Ydy, L.R.A.; Slhessarenko, N.; de Aguilar-Nascimento, J.E. Effect of Perioperative Allogeneic Red Blood Cell Transfusion on the Immune-Inflammatory Response After Colorectal Cancer Resection. World J. Surg. 2007, 31, 2044–2051. [Google Scholar] [CrossRef] [PubMed]
- Ford, C.D.; Vanmoorleghem, G.; Menlove, R.L. Blood transfusions and postoperative wound infection. Surgery 1993, 113, 603–607. [Google Scholar] [PubMed]
- Gibbs, J.; Cull, W.; Henderson, W.; Daley, J.; Hur, K.; Khuri, S.F. Preoperative Serum Albumin Level as a Predictor of Operative Mortality and Morbidity Results from the National VA Surgical Risk Study. Arch Surg. 1999, 134, 36–42. [Google Scholar] [CrossRef]
- Fuhrman, M.P.; Charney, P.; Mueller, C.M. Hepatic proteins and nutrition assessment. J. Am. Diet. Assoc. 2004, 104, 1258–1264. [Google Scholar] [CrossRef]
- Kudsk, K.; Tolley, E.; DeWitt, R.; Janu, P.; Blackwell, A.; Yeary, S.; King, B. Preoperative albumin and surgical site identify surgical risk for major postoperative complications. J. Parenter. Enter. Nutr. 2003, 27, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kuzu, M.A.; Terzioğlu, H.; Genç, V.; Erkek, A.B.; Özban, M.; Sonyürek, P.; Elhan, A.H.; Torun, N. Preoperative Nutritional Risk Assessment in Predicting Postoperative Outcome in Patients Undergoing Major Surgery. World J. Surg. 2006, 30, 378–390. [Google Scholar] [CrossRef] [PubMed]
- Garth, A.K.; Newsome, C.M.; Simmance, N.; Crowe, T.C. Nutritional status, nutrition practices and post-operative complications in patients with gastrointestinal cancer. J. Hum. Nutr. Diet. 2010, 23, 393–401. [Google Scholar] [CrossRef]
- Lohsiriwat, V.; Lohsiriwat, D.; Boonnuch, W.; Chinswangwatanakul, V.; Akaraviputh, T.; Lert-Akayamanee, N. Pre-operative hypoalbuminemia is a major risk factor for postoperative complications following rectal cancer surgery. World J. Gastroenterol. 2008, 14, 1248–1251. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.-C.; You, J.-F.; Yeh, C.-Y.; Chen, J.-S.; Tang, R.; Wang, J.-Y.; Chin, C.-C. Low preoperative serum albumin in colon cancer: A risk factor for poor outcome. Int. J. Color. Dis. 2010, 26, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Irvin, T.T.; Hunt, T.K. Effect of Malnutrition on Colonic Healing. Ann. Surg. 1974, 180, 765–772. [Google Scholar] [CrossRef]
- Gonçalves, C.G.; Groth, A.K.; Ferreira, M.; Matias, J.E.F.; Coelho, J.C.U.; Campos, A.C.L. Influence of Preoperative Feeding on the Healing of Colonic Anastomoses in Malnourished Rats. J. Parenter. Enter. Nutr. 2008, 33, 83–89. [Google Scholar] [CrossRef]
- Ward, M.W.N.; Danzi, M.; Lewin, M.R.; Rennie, M.J.; Clark, C.G. The effects of subclinical malnutrition and refeeding on the healing of experimental colonic anastomoses. Br. J. Surg. 1982, 69, 308–310. [Google Scholar] [CrossRef] [PubMed]
- Shimura, T.; Toiyama, Y.; Hiro, J.; Imaoka, H.; Fujikawa, H.; Kobayashi, M.; Ohi, M.; Inoue, Y.; Mohri, Y.; Kusunoki, M. Monitoring perioperative serum albumin can identify anastomotic leakage in colorectal cancer patients with curative intent. Asian J. Surg. 2018, 41, 30–38. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).