Abstract
Serum specimens obtained from 680 individuals were examined to measure the amounts of pepsinogens 1 and 2, anti-CagA antibodies, and anti-Helicobacter pylori antibodies. We examined sera obtained from 610 Mongolian individuals living in the capital city, Ulaanbaatar. Seventy serum specimens were collected from Japanese people who were health-screened: These were stored at the gastroenterology laboratory of Jichi Medical University. The sera of the Japanese people were used as a control specimen. Two enzyme-linked immunosorbent assay (ELISA) kits, an E-plate ELISA kit from Eiken Chemical Co., Ltd. (Tokyo, Japan), and a Biohit ELISA kit from Biohit Oyj (Helsinki, Finland), were used for the detection of anti-H. pylori IgG antibodies in the sera of the 610 Mongolian people. An ELISA kit EIA-4138 from DRG Instruments GmbH (Germany) was used for the detection of anti-CagA IgG antibodies in the serum specimens. Serum pepsinogens were detected by an ELISA kit from Biohit Oyj. Of the 610 serum specimens, 385 specimens tested positive for the detection of anti-H. pylori antibodies using the two ELISA kits, and 47 tested negative. For the detection of anti-H. pylori antibodies by the Biohit ELISA kit, 560 and 50 specimens were positive and negative, respectively. The ratio of serum pepsinogen 1/2 was statistically lower (p < 0.0001) in the H. pylori-positive (560 specimens) than in the H. pylori-negative (50 specimens) specimens. However, the levels of serum pepsinogen 1 had no statistical significance (p = 0.465) between the specimens of the H. pylori-positive and -negative specimens. The ratio of serum pepsinogen 1/2 was 6.74 ± 0.12 in the H. pylori-positive specimens, whereas the ratio of serum pepsinogen 1/2 was 12.69 ± 1.02 in the H. pylori-negative specimens. This study demonstrated the high prevalence of H. pylori infection in Mongolian people, including young generations, and the people infected with H. pylori possessed low pepsinogen 1/2 ratios, indicating atrophic gastritis. The serological examinations by the two ELISA kits did not consistently reflect the prevalence of H. pylori infection in Mongolian people.
1. Introduction
Helicobacter pylori is a Gram-negative curved rod bacterium with polar flagella as the motility organs. This pathogen colonizes the human stomach: Approximately half of the world’s population has been infected with it. A number of epidemiological studies have demonstrated a close relationship between H. pylori infection and the occurrence of gastric cancer. Since 1994, H. pylori has been recognized as a class 1 carcinogen, as published by the International Agency for Research on Cancer [1]. H. pylori colonizes the stomach of more than 50% of the world’s population. The majority of people colonized by H. pylori develop asymptomatic gastritis. However, approximately 10% of individual patients infected with this pathogen develop peptic ulcers. Moreover, about 1% of H. pylori-infected patients develop gastric cancer, and less than 0.1% develop gastric MALT lymphoma [2,3].
Epidemiological studies have reported a high seroprevalence of H. pylori infection in patients with gastric carcinoma and have established a positive correlation between H. pylori infection and the occurrence of gastric cancer [4,5]. Gastric cancer is the third leading cause of cancer-related deaths in the world. High-risk areas of gastric cancer include Korea, Japan, China, and Mongolia, and the age standardized rate (ASR) for the occurrence is higher than 20 per 100,000 in those countries. The Cancer Registry at the Korean National Cancer Center determined in 2002 that the incidence rate of gastric cancer of Korean people was 65.6 per 100,000 for men and 25.8 per 100,000 for women [6]. Though the age-adjusted mortality rate from gastric cancer in Japanese people in 1980 was 69.9 per 100,000 and 34.5 per 100,000 for men and women, respectively, its mortality rate in 2003 reduced to 34.5 per 100,000 and 13.2 per 100,000 for men and women, respectively [7]. Intermediate risk areas (ASR: 11–19 per 100,000) for the occurrence of gastric cancer include Malaysia, Singapore, and Taiwan, while low-risk areas (ASR: <10 per 100,000) include Australia, New Zealand, India, and Thailand [8]. Meanwhile, the incidence rate of gastric cancer in Mongolian people increased from 19.5 per 100,000 in 2003 to 23.4 per 100,000 in 2011 [9,10].
Environmental factors, especially water supply and diet, contribute to the infection rate of H. pylori in people in developing and developed countries [11,12]. The prevalence rate of H. pylori infection varies from 20% to 50% in people in industrialized countries, whereas it is over 90% of people in developing countries, such as Ethiopia, Gambia, Libya, Egypt, Nigeria, and Bangladesh. In addition, the prevalence rate of H. pylori infection reaches about 82–88% of people in India, China, Siberia, Brazil, and Iran. In the group investigated in India, the prevalence rates of H. pylori infection were 88% in adults greater than 21 years old and 22% in children aged 0–4. In China, the prevalence of H. pylori infection is 88% in adults and 68% in children aged 3–12. In Kazakhstan, the H. pylori-seropositive rate is 86% in adults and 64% in children. The H. pylori-seropositive rate is 77% in people below 20 years old and 100% in people over 60 years old.
Cytotoxin associated gene A (CagA) is the most important pathogenic factor of H. pylori and plays a crucial role in the occurrence of gastric cancer [13]. Earlier studies by other groups have reported that CagA-positive H. pylori strains were isolated from almost all patients with gastric cancer [14,15,16,17].
Pepsinogens are the proenzymes of the digestive enzyme pepsin, with two immunologically distinguishable isoforms (pepsinogens 1 and 2) [18]. Pepsinogen 1 is secreted from fundic mucosa cells and gastric chief cells, and pepsinogen 2 is secreted from similar cells in the pyloric glands in the antrum [19]. The levels of pepsinogen 1 decrease in atrophic stomachs of patients infected with H. pylori, thereby reducing the ratios of pepsinogen 1/2. Therefore, the detection of serum pepsinogen levels in patients infected with H. pylori is important in assessing the grade of atrophic gastritis and predicting the gastric cancer that will be caused by long-term colonization by this pathogen [20].
However, earlier investigations have provided negligible information as to the prevalence of H. pylori infection in Mongolian people. Therefore, we examined the levels of serum anti-H. pylori antibodies in Mongolian people using two ELISA kits and the levels of serum pepsinogen in people infected with H. pylori.
2. Results
2.1. Detection of Anti-H. pylori Antibodies in the Sera of Mongolian People by Two Different ELISA Kits
As described earlier, 43 and 27 serum specimens from the 70 Japanese people tested negative and positive, respectively, during the examination for anti-H. pylori IgG antibodies using a Japanese ELISA kit (an E-plate ELISA kit). However, 41 and 29 serum specimens from the same 70 people tested negative and positive, respectively, during the examination for anti-H. pylori IgG antibodies by a Finnish ELISA kit (a Biohit ELISA kit) (Table 1). In summary, no statistical significance was observed in the detection of anti-H. pylori antibodies in sera between the two ELISA kits via nonparametric tests of the 70 serum specimens (Wilcoxon, p = 0.157; McNemar’s test, p = 0.5).

Table 1.
Status of age and gender of the 610 sera collected from Mongolian people.
We next measured anti-H. pylori IgG antibodies in the 610 serum specimens from Mongolian people using either the E-plate ELISA kit or the Biohit ELISA kit to examine the H. pylori-positive rate. Of the 610 serum specimens, 385 aliquots tested H. pylori-positive and 47 aliquots tested H. pylori-negative in both ELISA kits. However, 175 serum aliquots tested H. pylori-positive using the Biohit ELISA kit, despite testing H. pylori-negative in the E-plate ELISA kit. In addition, 3 serum aliquots tested H. pylori-negative using the Biohit ELISA kit, but tested H. pylori-positive in the E-plate ELISA kit. The regression line, based on the serum concentrations of anti-H. pylori IgG antibodies detected in the 610 specimens using each ELISA kit, had a low correlation (R = 0.496) (Figure 1).
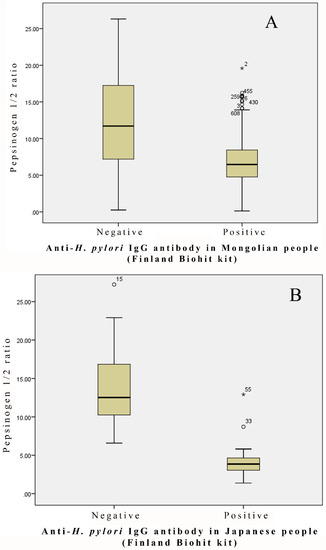
Figure 1.
Pepsinogen 1/2 ratio in the H. pylori-negative and -positive groups: (A) 610 Mongolian people; (B) 70 Japanese people.
As summarized in Table 2, the positive ratios of anti-H. pylori IgG antibodies in the 610 serum specimens measured by the Biohit ELISA kit were higher in all age groups than those of anti-H. pylori IgG antibodies in the same serum specimens measured by the E-plate ELISA kit. Anti-H. pylori IgG antibodies (positive ratio: 91.5%) were detected in 333 of the 364 serum specimens from Mongolian females investigated by the Biohit ELISA kit. Similarly, anti-H. pylori IgG antibodies (positive ratio: 92.3%) were detected in 227 of the 246 serum specimens from Mongolian males investigated by the same ELISA kit (data not shown). On this basis, no statistical significance was observed in the positive ratio of anti-H. pylori antibodies between males and females in Mongolia using the Biohit ELISA.

Table 2.
Detection rates of Helicobacter pylori-positive samples in the two ELISA kits.
2.2. Detection of Anti-CagA Antibodies in the Sera of Mongolian People
A DRG ELISA kit (for the detection of anti-CagA IgG antibodies) judged that 42 and 28 serum aliquots of the 70 serum specimens from Japanese people were anti-CagA IgG antibody-negative and -positive, respectively (Table 3). Meanwhile, 41 of the 42 antibody-negative serum specimens retained neither anti-H. pylori IgG antibodies nor anti-CagA IgG antibodies in the Biohit ELISA and DRG ELISA. However, of the 42 serum specimens, only one aliquot, in which the anti-CagA IgG antibodies were below the limits of detection, tested IgG antibody-positive against H. pylori via the Biohit ELISA. Of the same 70 serum specimens, 27 aliquots, in which anti-H. pylori IgG antibodies were detected by the E-plate ELISA, turned out to include anti-CagA IgG antibodies in the DRG ELISA. The 42 aliquots in which the anti-H. pylori IgG antibodies were below the limits of detection in the E-plate ELISA did not include anti-CagA IgG antibodies. However, only one aliquot in which anti-CagA IgG antibodies were detected anti-H. pylori IgG antibody-negative via the E-plate ELISA.

Table 3.
Detection of anti-cytotoxin associated gene A (CagA) antibody status and cross-tabulation of the two anti-H. pylori IgG ELISA kits.
Next, we examined the anti-CagA IgG antibodies in sera obtained from Mongolian people using the same ELISA kit. Of the 560 anti-H. pylori IgG antibody-positive serum specimens (Biohit ELISA), 500 serum specimens tested positive for the detection of anti-CagA antibodies (Table 3). Six serum specimens tested positive for the detection of anti-H. pylori IgG-negative antibodies in the Biohit ELISA, but also tested negative for the detection of anti-CagA antibodies. Meanwhile, the positive ratio of anti-CagA IgG antibodies was 94.6% in the 388 serum specimens that tested positive for the detection of anti-H. pylori IgG antibodies using the E-plate ELISA. Intriguingly, of the 222 serum specimens, of which anti-H. pylori IgG antibodies were below the limits of detection in 139 during the examination using the E-plate ELISA kit, 139 aliquots (62.6%) included anti-CagA IgG antibodies.
2.3. Relationship between the Ratio of Pepsinogen 1/2 and H. pylori Infection
Figure 1 shows the ratio of pepsinogen 1/2 in the serum specimens from the 70 Japanese people, with 29 and 41 aliquots being positive and negative, respectively, in the examination for anti-H. pylori antibodies using the Biohit ELISA kit. The ratio of pepsinogen 1/2 was obviously lower in the H. pylori-positive serum specimens than in the H. pylori-negative serum specimens. The mean values of the ratio of pepsinogen 1/2 were 4.1 ± 0.41 and 14.55 ± 1.04 in the H. pylori-positive and -negative serum specimens, respectively.
We next examined the ratio of pepsinogen 1/2 in the serum specimens from the 610 Mongolian people, with 560 and 50 aliquots testing positive and negative, respectively, in the examination for anti-H. pylori antibodies using the Biohit ELISA kit. The ratio of pepsinogen 1/2 was statistically lower (p < 0.0001) in the H. pylori-positive serum specimens than in the H. pylori-negative serum specimens (Figure 2). However, the levels of serum pepsinogen 1 in the H. pylori-positive specimens were comparable to those of pepsinogen 1 in the H. pylori-negative specimens (p = 0.465). The mean values of the ratio of pepsinogen 1/2 were 6.74 ± 0.12 and 12.69 ± 1.02 in the H. pylori-positive and -negative serum specimens, respectively.
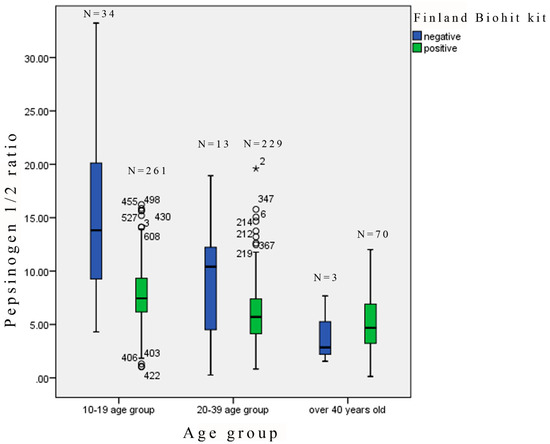
Figure 2.
Pepsinogen 1/2 ratio in each age group.
Table 4 shows the ratios of pepsinogen 1/2 in each age group of Mongolian people. The lowest values (below 3 or 5) of the pepsinogen 1/2 ratio were observed in the 204 serum specimens with positive antibodies against H. pylori, as detected by the Biohit ELISA kit, and the serum specimens were assumed to be associated with atrophic gastritis.

Table 4.
Cross-tabulation of the pepsinogen 1/2 ratio in each age group.
Of the 560 serum specimens in which the anti-H. pylori IgG antibodies were positive in the examination using the Biohit ELISA, 47 aliquots and 157 aliquots were below 3 and 5, respectively, for the values of the pepsinogen 1/2 ratio. The difference in the pepsinogen 1/2 ratio between the H. pylori-positive and -negative serum specimens was greater in the group aged 10 to 19 than in the other age groups (Figure 2). Of the 50 serum specimens in which the anti-H. pylori IgG antibodies were negative in the examination using the Biohit ELISA, 4 aliquots from the groups aged 20 to 39 and over 40 were less than 3 (for the pepsinogen 1/2 ratio). In addition, the ratio of pepsinogen 1/2 was statistically lower (p < 0.0001) in the anti-CagA IgG-positive serum specimens than in the anti-CagA IgG-negative serum specimens, all of which were positive in the examination for anti-H. pylori IgG antibodies by the Biohit ELISA (Figure 3). On the contrary, no difference in the levels of serum pepsinogens 1 and 2 was observed between the anti-H. pylori IgG-positive and -negative specimens which judged by the E-plate ELISA kit, and therefore the ratio of pepsinogen 1/2 in the anti-H. pylori IgG-positive serum specimens was comparable (no statistical significance) to that of pepsinogen 1/2 in the anti-H. pylori IgG-negative serum specimens.
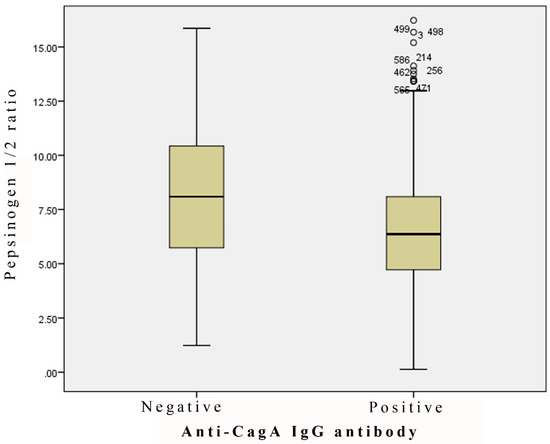
Figure 3.
Pepsinogen 1/2 ratio in the anti-CagA antibody-negative and -positive cases (p = 0.000063).
3. Discussion
Several studies have investigated the prevalence of H. pylori infection in Mongolia. In a study of H. pylori, HPV, HCV, HBV, and JC virus infection, anti-H. pylori antibodies were detected by the two antigens (NapA and GroEL) in 1022 dry-blood spot samples that were collected from Mongolian people. The prevalence of H. pylori infection in the samples was 45.5% in the detection by the NapA antigen and 86.0% in the detection by the GroEL antigen [21]. In addition, the detection of H. pylori genes through immunohistochemistry (IHC) analysis has suggested H. pylori infection in 76% of biopsy specimens obtained from 736 Mongolian people [22]. There are a number of methods for diagnosing an H. pylori infection. Among those methods, a serological examination is less accurate than a breath test, stool antigen test, and rapid urease test. However, serological examinations have still been reliable and are used widely in prevalence studies because other tests have difficulty examining H. pylori infections in various peoples within a relatively short time [23]. In the studies mentioned above, H. pylori infections were detected with unusual methods. Here, we present the prevalence of H. pylori infection in Mongolian people using a serological examination.
We performed this study using sera collected from Mongolian people who were over 11 years old, because it has been reported that some sera collected from children under 10 years old indicate a false-negative in serological examinations used to detect the anti-H. pylori IgG antibody.
In terms of sensitivity and specificity for the diagnosis of H. pylori infection, it has been reported that ELISA kits show some differences within a country. It is believed that an ELISA kit using a domestic representative strain as an antigen should be used in each country or area. In Japan, an ELISA kit that has been developed by a Japanese company is used for the diagnosis of H. pylori infection. Japanese ELISA kits such as the E-plate are higher in terms of sensitivity and specificity for the diagnosis of H. pylori infection in Japanese people than other ELISA kits that have been developed by other countries’ companies [24]. The Eiken E-plate ELISA kit is frequently used in Japan and is the most reliable in the detection of Japanese H. pylori strains. The E-plate ELISA kit is one of the most representative ELISA kits used worldwide [25] and adopts an HM-CAP antigen derived from a U.S. strain. ELISAs using the HM-CAP antigen have been reported to be 95% to 100% valid in the examination of H. pylori infection in Western countries [24,26,27]. Meanwhile, the Biohit ELISA kit is frequently used in European countries, especially Finland, Italy, and the United Kingdom. In sum, we used two ELISA kits that adopt different antigens in order to detect anti-H. pylori IgG antibodies: One kit is from Japan, and the other kit is from Finland.
In this study, we found a significant difference in the detection of serum anti-H. pylori antibodies between the two ELISA kits. Serum anti-H. pylori IgG antibodies were positive in 91.8% of 610 Mongolian people examined by the Finnish ELISA kit, whereas serum anti-H. pylori IgG antibodies were positive in 63.6% of the same Mongolian people examined by the Japanese ELISA kit. We found only a weak correlation between titers of the two ELISA kits. The antigens adopted by the two ELISA kits were qualitatively different, although we cannot confirm the antigens used in the Japanese ELISA kit (E-plate) and the Finnish ELISA kit (Biohit) in detail. The Japanese ELISA kit was incapable of detecting anti-H. pylori antibodies in the serum specimens obtained from 139 Mongolian people in spite of them being positive for anti-CagA antibodies. Meanwhile, the Finnish ELISA kit was capable of detecting anti-H. pylori antibodies in 133 serum specimens of the 139 serum specimens that were positive for anti-CagA antibodies. A statistical significance was observed in the pepsinogen 1/2 ratio between the positive and negative anti-H. pylori IgG antibodies detected by the Finnish ELISA kit, whereas no statistical significance was observed in the pepsinogen 1/2 ratio between the positive and negative anti-H. pylori IgG antibodies detected by the Japanese ELISA kit. These results suggest that the Finnish ELISA kit could be more reliable in the detection of serum anti-H. pylori antibodies in Mongolian people than the Japanese ELISA kit.
The detection rate of anti-H. pylori IgG antibodies by the Finnish ELISA kit was 91.8% and 88.5% in the total of all age groups and in the age group of 10–19, respectively. This means that H. pylori infection was prevalent in a young generation of Mongolians. These results are similar to the prevalence of H. pylori infection in people in developing countries.
In earlier studies, the clinical isolation ratio of cagA-positive H. pylori strains in total H. pylori strains has been 67% in the Netherlands [13], 94% in Brazil [14], 97% in Korea [15], 79% in Ethiopia [16], and 97.6% in Japan [17]. In this study, anti-CagA IgG antibodies were positive in 89.3% of all the H. pylori-positive sera of the Mongolian people examined. In sum, almost all H. pylori strains prevalent in Mongolia are suggested to have a cagA gene. In a previous study, seropositive CagA was significantly higher in patients with fundic atrophy than in healthy people without gastritis and gastric atrophy when the atrophic gastritis was assessed through the detection of serum pepsinogen and a histological examination [28]. In an epidemiological study, chronic atrophic gastritis was significantly associated with seropositive H. pylori, seropositive CagA, and serum pepsinogen levels [29]. This study showed that the pepsinogen 1/2 ratio was lower in the anti-CagA antibody-positive sera than in the anti-CagA antibody-negative sera. Further investigations will be necessary to identify the types of cagA genes in H. pylori strains in Mongolia and to clarify the role of CagA in the pathogenicity of Mongolian H. pylori strains.
The findings are consistent with reports that atrophic gastritis prevalence in the world is about 7% [30,31]. A definitive atrophy diagnosis through endoscopic and histological examinations requires invasive clinical intervention, although a histological examination using a gastric biopsy is one of the gold standards for the detection of atrophic gastritis and other diseases. In addition, an endoscopic examination for the detection of atrophic gastritis is invasive and uncomfortable, and it is unsuitable for various prevalence studies. Meanwhile, an examination of serum pepsinogen levels is non-invasive in the diagnosis of atrophic gastritis and is often carried out in European countries [32]. The test has a sensitivity of 82% (CI 95%, 70–93%) and a specificity of 98% (CI 95%, 97–99%) in the diagnosis of moderate or severe atrophic gastritis compared to the gold standard method, histopathological biopsy microscopy [33,34]. In this study, the pepsinogen 1/2 ratio was statistically lower in the H. pylori-positive sera than in the H. pylori-negative sera. When the pepsinogen 1/2 ratio was low (<3), the Gastrosoft (Biohit manufacturer) interpretation was “severe atrophic corpus gastritis” [35]. In a study by Nasrollahzadeh, D., et al., the advanced cutoff values in a gastric atrophy assessment were calculated by the pepsinogen 1/2 ratio (<5) (sensitivity: 75.0%, specificity: 91.0%), and this was more suitable for the diagnosis of atrophic gastritis than the manufacturer interpretation (pepsinogen 1/2 ratio: <3) [28]. In the present study, almost all of the Mongolian people were suspected of atrophic gastritis, according to that advanced cutoff value. Because most of the study population was under 40 years old, elderly people with an H. pylori infection could indicate a lower pepsinogen 1/2 ratio. The difference in the pepsinogen 1/2 ratio between H. pylori-positive and -negative groups was greater in the age group of 10–19 than in the other age groups. In the age group of 10–19, some people with serum anti-H. pylori positive antibodies clearly had gastric atrophy. These results suggest that an H. pylori infection could induce serious damage to the gastric mucosa of Mongolian people. The pepsinogen 1/2 ratio obviously decreased with an increase in age, regardless of the presence or absence of anti-H. pylori antibodies. Some risk factors, excluding an H. pylori infection, may have affected gastric atrophy. In addition, the pepsinogen 1/2 ratio in ages 10 to 39 was lower in the anti-H. pylori antibody-positive groups than in the anti-H. pylori antibody-negative groups. Meanwhile, the pepsinogen 1/2 ratio in ages over 40 was lower in the anti-H. pylori antibody-negative groups than in the anti-H. pylori antibody-positive groups. This may indicate that some other risk factors exist with the possibility of influencing gastric atrophy, such as alcohol consumption and high salt usage, at over 40 years of age. In addition, research limitations may have influenced the results of this study by our group, as only three people examined by the Finnish ELISA kit were negative for an H. pylori infection.
A study by another research group has suggested that the prediction of a high-risk status for gastric atrophy is defined as less than 3.1 for the pepsinogen 1/2 ratio, and that less than 2.2 for the pepsinogen 1/2 ratio and 28 ng/mL of pepsinogen 1 is defined as the best prediction of gastric cancers [36]. This study was mainly focused on the prediction of gastric cancers through the measurement of serum pepsinogen levels, but the prediction of gastric cancers could not be defined. In the present study, we demonstrated that an infection of H. pylori with positive CagA is a high risk for gastric atrophy in Mongolian people.
The limitation of this study was that it was impossible to compare the ELISA with a rapid urease test or an H. pylori culture using a gastric biopsy at the same time. It was difficult to involve relatively healthy individuals in the study because they refused involvement due to the invasive procedures of a gastric biopsy.
4. Materials and Methods
4.1. Study Population
Serum specimens from 680 individuals were examined to measure the amounts of pepsinogens 1 and 2, anti-CagA antibodies, and anti-H. pylori antibodies. The specimens were collected from 610 Mongolian people living in the capital city, Ulaanbaatar, and were stored in the virology laboratory at Jichi Medical University in Japan. Here, 295 aliquots of the serum specimens were provided from blood donors over 20 years old. Another 315 aliquots were obtained from people (ages 10 to 19) who underwent a medical checkup. Seventy serum specimens were collected from Japanese people over 40 years old who took a health screening: They were stored in the gastroenterology laboratory at Jichi Medical University. The sera of the Japanese people were used as the control specimens. Of the 70 serum specimens, 43 aliquots were negative in the examination for anti-H. pylori antibodies by an E-plate ELISA kit (Eiken Chemical Co., Ltd., Tokyo, Japan). The other 27 aliquots were positive in the examination for these antibodies by the same ELISA kit.
We divided the serum specimens of the Mongolian people into the following three groups: A group of ages 10 to 19, ages 20 to 39, and over 40 (Table 1): 295 serum specimens were from the group aged 10 to 19, 242 serum specimens were from the group aged 20 to 39, and 73 serum specimens were from the over-40 age group. Those serum specimens included 364 serum specimens from females and 246 serum specimens from males. The mean age of the 610 Mongolian people (ranging from 11 to 66 years old) was 22.9 ± 11.5 years old (the median value: 20 years old).
4.2. Detection of Antibodies against H. pylori, CagA, and Pepsinogens in Serum Specimens
The two ELISA kits, an E-plate ELISA kit (Eiken Chemical Co., Ltd., Tokyo, Japan) and a Biohit ELISA kit (Biohit Oyj, Helsinki, Finland), were used to determine the concentration of anti-H. pylori IgG antibodies in the sera of the 610 Mongolian people. The protocol of the E-plate ELISA kit defines serum specimens as positive for anti-H. pylori IgG antibodies when antibodies are detected at a concentration greater than 10 U/mL. Meanwhile, the protocol of the Biohit ELISA kit defines serum specimens as positive for anti-H. pylori IgG antibodies when antibodies are detected at greater than 30 EIU.
An ELISA kit EIA-4138 from DRG Instruments GmbH (Marburg, Germany) was used to detect anti-CagA IgG antibodies in the serum specimens. The protocol of the ELISA kit defines the limits of detection of anti-CagA antibodies as below a concentration of 10 RU/mL.
Pepsinogens 1 and 2 in the serum specimens were measured using an ELISA kit (pepsinogen I and II, Biohit Oyj), and the ratios of pepsinogen 1/2 were calculated. Based on the instructions in the ELISA kit, gastric atrophic grades were assessed from the serum levels of the pepsinogens and the ratios of pepsinogen 1/2.
4.3. Ethics
The local ethics committees of both the Mongolian National University of Medical Sciences and Jichi Medical University in Japan approved the use of the above-mentioned sera in the investigations of this study. There is no information about patients written in this article. See ethical application ref: 2014/D/2014-11 (approved date: 6 November 2014).
4.4. Statistical Analysis
A computer program, SPSS version 20.0, was used to calculate statistical significances, regression line types, and mean values ± standard errors at a 95% confidence interval. Statistical evaluations comparing the groups were performed via an independent Student’s t-test and a regression line correlation analysis. A statistical comparison of nonparametric tests between the groups was evaluated via a Wilcoxon test and McNemar’s test.
5. Conclusions
In this study, we demonstrated the high retention rates of anti-H. pylori antibodies and the low pepsinogen 1/2 ratio in Mongolian people, indicating the prevalence of atrophic gastritis in Mongolian people. Furthermore, eradication therapy with triple drugs could be applied actively to people infected with H. pylori.
In the future, we will need to validate the gold standard for the diagnosis of H. pylori infection in Mongolia. The limitation of our study was that both serological test kits were not validated by comparing them with the gold standard in Mongolia, so we will need to determine the validity of these two kits in the future.
Author Contributions
Study concept and design, H.S., Y.H., and A.A.; acquisition of data, A.A.; analysis and interpretation of data, A.A.; drafting of manuscript, A.A. and H.S.; critical revision of the manuscript for important intellectual content and approval of the final version, A.A., T-O.B., K.S., H.O., H.S., and Y.H.; supervision, K.S., H.O., and Y.H.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- IARC. Schistosomes, Liver Flukes and Helicobacter Pylori; IARC: Lyon, France, 1994; Volume 61. [Google Scholar]
- Kuipers, E.J. Review article: Exploring the link between Helicobacter pylori and gastric cancer. Aliment. Pharmacol. Ther. 1999, 13, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Parsonnet, J.; Isaacson, P.G. Bacterial infection and MALT lymphoma. N. Engl. J. Med. 2004, 350, 213–215. [Google Scholar] [CrossRef]
- Ekstrom, A.M.; Held, M.; Hansson, L.E.; Engstrand, L.; Nyrén, O. Helicobacter pylori in gastric cancer established by CagA immunoblot as a marker of past infection. Gastroenterology 2001, 121, 784–791. [Google Scholar] [CrossRef]
- Uemura, N.; Okamoto, S.; Yamamoto, S.; Matsumura, N.; Yamaguchi, S.; Yamakido, M.; Taniyama, K.; Sasaki, N.; Schlemper, R.J. Helicobacter pylori infection and the development of gastric cancer. N. Engl. J. Med. 2001, 345, 784–789. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.R.; Won, Y.J.; Jung, K.W.; Kong, H.J.; Yim, S.H.; Lee, J.K.; Noh, H.I.; Lee, J.K.; Pisani, P.; Park, J.G.; et al. Nationwide cancer incidence in Korea, 1999~2001; first result using the national cancer incidence database. Cancer Res. Treat. 2005, 37, 325–331. [Google Scholar] [CrossRef]
- Nomura, K. (Ed.) Cancer Statistics in Japan 2005; Foundation for Promotion of Cancer Reseach (FPCR): Tokyo, Japan, 2005. [Google Scholar]
- Parkin, D.M.; Bray, F.; Ferlay, J.; Pisani, P. Global cancer statistics, 2002. CA-A Cancer J. Clin. 2005, 55, 74–108. [Google Scholar] [CrossRef]
- State Implementing Agency of Health, G.O.M. (Ed.) Health Indicators 2003; State Implementing Agency of Health, G.O.M.: Ulaanbaatar, Mongolia, 2003. [Google Scholar]
- State Implementing Agency of Health, G.O.M. (Ed.) Health Indicators 2011; State Implementing Agency of Health, G.O.M.: Ulaanbaatar, Mongolia, 2011. [Google Scholar]
- Frenck, R.W., Jr.; Clemens, J. Helicobacter in the developing world. Microb. Infect. 2003, 5, 705–713. [Google Scholar] [CrossRef]
- Rothenbacher, D.; Brenner, H. Burden of Helicobacter pylori and H. pylori-related diseases in developed countries: Recent developments and future implications. Microb. Infect. 2003, 5, 693–703. [Google Scholar] [CrossRef]
- van Doorn, L.J.; Figueiredo, C.; Sanna, R.; Plaisier, A.; Schneeberger, P.; de Boer, W.; Quint, W. Clinical relevance of the cagA, vacA, and iceA status of Helicobacter pylori. Gastroenterology 1998, 115, 58–66. [Google Scholar] [CrossRef]
- Ashour, A.A.; Magalhães, P.P.; Mendes, E.N.; Collares, G.B.; De Gusmão, V.R.; Queiroz, D.M.; Nogueira, A.M.M.; Rocha, G.A.; De Oliveira, C.A. Distribution of vacA genotypes in Helicobacter pylori strains isolated from Brazilian adult patients with gastritis, duodenal ulcer or gastric carcinoma. FEMS Immunol. Med. Microbiol. 2002, 33, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Woo, C.W.; Lee, Y.M.; Son, B.R.; Kim, J.W.; Chae, H.B.; Youn, S.J.; Park, S.M. Genotyping CagA, VacA subtype, IceA1, and BabA of Helicobacter pylori isolates from Korean patients, and their association with gastroduodenal diseases. J. Korean Med. Sci. 2001, 16, 579–584. [Google Scholar] [CrossRef]
- Asrat, D.; Nilsson, I.; Mengistu, Y.; Kassa, E.; Ashenafi, S.; Ayenew, K.; Wadström, T.; Abu-Al-Soud, W. Prevalence of Helicobacter pylori vacA and cagA genotypes in Ethiopian dyspeptic patients. J. Clin. Microbiol. 2004, 42, 2682–2684. [Google Scholar] [CrossRef]
- Azuma, T.; Yamakawa, A.; Yamazaki, S.; Fukuta, K.; Ohtani, M.; Ito, Y.; Dojo, M.; Yamazaki, Y.; Kuriyama, M. Correlation between variation of the 3′ region of the cagA gene in Helicobacter pylori and disease outcome in Japan. J. Infect. Dis. 2002, 186, 1621–1630. [Google Scholar] [CrossRef]
- Oishi, Y.; Kiyohara, Y.; Kubo, M.; Tanaka, K.; Tanizaki, Y.; Ninomiya, T.; Doi, Y.; Shikata, K.; Yonemoto, K.; Shirota, T.; et al. The serum pepsinogen test as a predictor of gastric cancer—The Hisayama study. Am. J. Epidemiol. 2006, 163, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Dinis-Ribeiro, M.; da Costa-Pereira, A.; Lopes, C.; Barbosa, J.; Guilherme, M.; Moreira-Dias, L.; Lomba-Viana, H.; Silva, R.; Abreu, N.; Lomba-Viana, R. Validity of serum pepsinogen I/II ratio for the diagnosis of gastric epithelial dysplasia and intestinal metaplasia during the follow-up of patients at risk for intestinal-type gastric adenocarcinoma. Neoplasia 2004, 6, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Bornschein, J.; Selgrad, M.; Wex, T.; Kuester, D.; Malfertheiner, P. Serological assessment of gastric mucosal atrophy in gastric cancer. BMC Gastroenterol. 2012, 12, 10. [Google Scholar] [CrossRef] [PubMed]
- Waterboer, T.; Dondog, B.; Michael, K.M.; Michel, A.; Schmitt, M.; Vaccarella, S.; Franceschi, S.; Clifford, G.; Pawlita, M. Dried blood spot samples for seroepidemiology of infections with human papillomaviruses, Helicobacter pylori, Hepatitis C Virus, and JC Virus. Cancer Epidemiol. Biomark. Prev. 2012, 21, 287–293. [Google Scholar] [CrossRef]
- Khasag, O.; Boldbaatar, G.; Tegshee, T.; Duger, D.; Dashdorj, A.; Uchida, T.; Matsuhisa, T.; Yamaoka, Y. The prevalence of Helicobacter pylori infection and other risk factors among Mongolian dyspeptic patients who have a high incidence and mortality rate of gastric cancer. Gut Pathog. 2018, 10, 14. [Google Scholar]
- Dunn, B.E.; Cohen, H.; Blaser, M.J. Helicobacter pylori. Clin. Microbiol. Rev. 1997, 10, 720–741. [Google Scholar] [CrossRef]
- Obata, Y.; Kikuchi, S.; Miwa, H.; Yagyu, K.; Lin, Y.; Ogihara, A. Diagnostic accuracy of serological kits for Helicobacter pylori infection with the same assay system but different antigens in a Japanese patient population. J. Med. Microbiol. 2003, 52, 889–892. [Google Scholar] [CrossRef] [PubMed]
- Marchildon, P.A.; Sugiyama, T.; Fukada, Y.; Peacock, J.S.; Asaka, M.; Shimoyama, T.; Graham, D.Y. Evaluation of the effects of strain-specific antigen variation on the accuracy of serologic diagnosis of Helicobacter pylori infection. J. Clin. Microbiol. 2003, 41, 1480–1485. [Google Scholar] [CrossRef]
- Megraud, F.; Lehours, P. Helicobacter pylori detection and antimicrobial susceptibility testing. Clin. Microbiol. Rev. 2007, 20, 280–322. [Google Scholar] [CrossRef]
- Okuda, M.; Miyashiro, E.; Koike, M.; Tanaka, T.; Bouoka, M.; Okuda, S.; Yoshikawa, N. Serodiagnosis of Helicobacter pylori infection is not accurate for children aged below 10. Pediatr. Int. 2002, 44, 387–390. [Google Scholar] [CrossRef] [PubMed]
- Nasrollahzadeh, D.; Aghcheli, K.; Sotoudeh, M.; Shakeri, R.; Persson, E.C.; Islami, F.; Kamangar, F.; Abnet, C.C.; Boffetta, P.; Engstrand, L.; et al. Accuracy and cut-off values of pepsinogens I, II and gastrin 17 for diagnosis of gastric fundic atrophy: Influence of gastritis. PLoS ONE 2011, 6, e26957. [Google Scholar] [CrossRef]
- Weck, M.N.; Gao, L.; Brenner, H. Helicobacter pylori infection and chronic atrophic gastritis: Associations according to severity of disease. Epidemiology 2009, 20, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Varis, K.; Sipponen, P.; Laxen, F.; Samloff, I.M.; Huttunen, J.K.; Taylor, P.R.; Heinonen, O.P.; Albanes, D.; Sande, N.; Virtamo, J.; et al. Implications of serum pepsinogen I in early endoscopic diagnosis of gastric cancer and dysplasia. Helsinki Gastritis Study Group. Scand. J. Gastroenterol. 2000, 35, 950–956. [Google Scholar]
- Vannella, L.; Lahner, E.; Annibale, B. Risk for gastric neoplasias in patients with chronic atrophic gastritis: A critical reappraisal. World J. Gastroenterol. 2012, 18, 1279–1285. [Google Scholar] [CrossRef] [PubMed]
- Broutet, N.; Plebani, M.; Sakarovitch, C.; Sipponen, P.; Megraud, F. Pepsinogen A, pepsinogen C, and gastrin as markers of atrophic chronic gastritis in European dyspeptics. Br. J. Cancer 2003, 88, 1239–1247. [Google Scholar] [CrossRef]
- Vaananen, H.; Vauhkonen, M.; Helske, T.; Kääriäinen, I.; Rasmussen, M.; Tunturi-Hihnala, H.; Koskenpato, J.; Sotka, M.; Turunen, M.; Sandström, R.; et al. Non-endoscopic diagnosis of atrophic gastritis with a blood test. Correlation between gastric histology and serum levels of gastrin-17 and pepsinogen I: A multicentre study. Eur. J. Gastroenterol. Hepatol. 2003, 15, 885–891. [Google Scholar] [CrossRef]
- Dinis-Ribeiro, M.; Yamaki, G.; Miki, K.; Costa-Pereira, A.; Matsukawa, M.; Kurihara, M. Meta-analysis on the validity of pepsinogen test for gastric carcinoma, dysplasia or chronic atrophic gastritis screening. J. Med. Screen 2004, 11, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Telaranta-Keerie, A.; Kara, R.; Paloheimo, L.; Härkönen, M.; Sipponen, P. Prevalence of undiagnosed advanced atrophic corpus gastritis in Finland: An observational study among 4,256 volunteers without specific complaints. Scand. J. Gastroenterol. 2010, 45, 1036–1041. [Google Scholar] [CrossRef] [PubMed]
- Gantuya, B.; Oyuntsetseg, K.; Bolor, D.; Erdene-Ochir, Y.; Sanduijav, R.; Davaadorj, D.; Tserentogtokh, T.; Uchida, T.; Yamaoka, Y. Evaluation of serum markers for gastric cancer and its precursor diseases among high incidence and mortality rate of gastric cancer area. J. Castric. Cancer 2019, 22, 104–112. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).