Abstract
The hydrolytic stability of thin poly(ethyl 2-cyanoacrylate), PECA, adhesive films on grit-blasted mild steel substrates was investigated using electrochemical impedance spectroscopy (EIS). Using this novel approach for such adhesive films, the effects of two additives, salicylic acid (SA) and phthalic anhydride (PA), were studied, specifically measuring their influence on polymer film/surface impedance and capacitance changes over a period of 14 days. Results indicate that SA decreased the polymer film hydrolytic stability rapidly, resulting in a substantial drop in impedance modulus from ~10 kΩcm2 to ~10 Ωcm2 at 100 Hz due to electrolyte ingress, whilst the PA-containing film modulus also diminished from ~4 MΩcm2 to ~1 kΩcm2 at 100 Hz. Furthermore, the capacitance values of the SA-containing films rose (up to ~100 µFcm−2), demonstrating the onset of a charge transfer (corrosion) process within the first 12 h exposure to a saline electrolyte. In contrast, the PA-containing film’s transition from a film-dominated capacitance (~0.01 µFcm−2) to a larger double-layer capacitance took (~1 µFcm−2) took several days and was accounted for by differences in the additive’s chemistry, demonstrating the ability of EIS to detect changes in both bulk film (e.g., moisture ingress and bond scission) and metal-film interfacial processes (e.g., onset of corrosion) in real time. Comparison was also made with a standard industry combined tensile test/hydrolytic accelerated ageing regime. Unlike, EIS this did not, however, give useful time-dependent information, although after 6 weeks a decrease in bond strength occurred in the order PA-containing film < PECA< SA-containing film in agreement with the EIS results, thus demonstrating the effectiveness of EIS for monitoring the degradation of such thin film adhesives.
1. Introduction
Alkyl 2-cyanoacrylates (CAs), such as ethyl 2-cyanocrylate (ECA), are fast-acting adhesives based on reactive monomers that are rapidly polymerized by nucleophiles (Lewis bases). Such short chain CAs are used in commercial instant adhesive formulations (“instant or super glue”), whose bond strength depends on the surface chemical and physical properties of the substrate material [1]. PECA, however, generally displays poor hydrolytic stability. which was reported in a recent study involving electrochemical impedance spectroscopy EIS [2]. Although EIS has long been used to investigate the performance of protective coatings employed for corrosion protection purposes [3,4,5,6,7], and, to a lesser extent, for non-CA adhesive bonds at the interface between metals and polymers [8,9,10,11,12], there are very few reports of impedance studies of CAs, exceptions being those reported in [2,13,14]. Unlike most adhesives, the polymerisation of CAs occurs predominantly on surfaces where Lewis bases such as hydroxyl ions are abundant.
Knowledge of the degradation mechanisms of CAs is somewhat limited, although rapid room temperature depolymerisation of high molecular weight PBCA “parent” polymer in the presence of a base (tetrabutylammonium hydroxide) has been previously reported [15]. This involved abstraction of the polymer chain end proton, followed by an unzipping reaction, resulting in rapid re-polymerisation of released monomer to “daughter” polymer. Remaining Lewis base species in the polymer matrix can also induce degradation at the polymer–metal interface. This depends, however, upon ester group size and pH [16]. For example, in a saline near-neutral environment, ester cleavage by side chain hydrolysis results in poly(cyanoacrylic acid) and alcohol formation. Alkaline hydrolysis on surfaces ultimately leads to loss of adhesion of the polymer on the substrate and ultimately bond failure.
EIS is a non-destructive technique enabling changes occurring in films to be examined as a function of time. Comparison is also made with a standard industry destructive test regime based on a tensile test (ASTM D1002) [17] performed in conjunction with an accelerated moisture test (ASTM D1151) [18]. This industry practice, however, is strictly limited in terms of the information it may provide during the hydrolytic degradation process. In contrast, the EIS technique provides useful information concerning the chemical and physical processes occurring during such a process. EIS can also detect changes at an earlier stage than the standard tensile test methods currently adopted by industry. Thus, EIS could well play an important role in future work concerning CA adhesive development and performance.
This short communication examines the use of EIS to study the effects of two additives, phthalic anhydride (PA) and salicylic acid (SA), which can modify the hydrolytic stability of PECA films [19]. In the past, EIS has not been utilised on CAs for such a purpose. This works follows on from a series of papers exploring the polymerisation of similar CAs using confocal Raman microspectroscopy [1], microcalorimetry [20], and, recently, EIS [2]. The intention of this study is to utilise the EIS method to examine the hydrolytic stability of a range of PECA films both with and without SA and PA additives, which has not been previously reported. In addition, the aim is to determine if EIS can detect the onset of corrosion of the grit-blasted mild steel, once the electrolyte and oxygen have permeated the layer.
2. Experimental Section
2.1. Chemicals and Materials
Analytical grade SA and PA were obtained from Sigma Aldrich Ltd. ECA monomer containing 5 ppm BF3 stabiliser and 800 ppm hydroquinone was supplied by Henkel Technologies, Ireland Ltd. Grit-blasted mild steel coupons cut to 1 mm × 25 mm × 100 mm were sourced from Q-Lab Ltd. [21] and were carefully wiped clean with iso-propyl alcohol, then air dried.
2.2. Electrochemical Equipment
Impedance (EIS) measurements were conducted using a Solartron SI 1287 Electrochemical Interface in conjunction with a Solartron 1255B Frequency Response Analyser (Solartron, Farnborough, UK). These were connected to a three-electrode electrochemical cell, controlled using a PC running ZPlot and ZView used in tandem with CorrWare software (all by Scribner and Associates), to input both experimental parameters and to capture and model data. The electrochemical cell arrangement is described more fully in reference [2]. EIS results are often interpreted in terms of electric circuit elements (resistance R, capacitance C, and inductance L). The circuit description code (CDC) is Rs(Cf[Rpo{Cdl(Rct[Cdif(Rdif)])}]), where Rs is the solution resistance, Cf the film capacitance, Rp the film pore resistance, Cdl the double-layer capacitance, Rct is the charge transfer resistance, Cdif the diffusion layer capacitance, and Rdif the diffusion layer resistance. The diffusion layer is a barrier layer formed from the metal surface reaction products including ferrous metal corrosion products [3,4,5,6,7,22]. The circuit is described in Scheme 1.
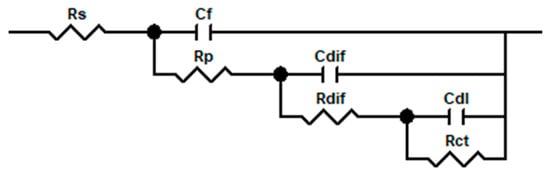
Scheme 1.
The circuit used in the EIS modelling.
2.3. Polymer Degradation Studies
The monomers were supplied by Henkel Ireland Ltd. Unless otherwise specified, a base formulation consisting of strong acid-stabilised ECA monomer was used for the investigations carried out in this study. Formulations were prepared by adding known amounts of powdered additives to 100 mL solution of ECA and placing them on an electronic shaker for 3 h. Formulations were stored at 3–4 °C and kept no longer than 2–3 days. ECA monomer was mixed to form a homogeneous solution with PA or SA additives, at concentrations that were initial trial amounts. Typically, ECA monomer (with or without additive) was deposited on the grit-blasted mild steel coupon to form a 1 µm thick film and allowed to cure at room temperature for three days. Next, the coupon was glued to a polypropylene sample bottle (with an exposed area of 2.54 cm2) with an (Araldite Rapid Adhesive) epoxy adhesive forming an electrolyte vessel for EIS studies. These were performed by following moisture ingress using a three-electrode setup comprising an Ag/AgCl (saturated KCl) reference electrode, a platinum wire mesh counter electrode, and the polymer-coated metal coupon working electrode and monitored over a period of time. A 10 mV AC perturbation was applied over a frequency range of 105 Hz to 0.1 Hz at the open circuit potential (Eocp) ~ −0.630 V (Ag/AgCl(saturated KCl) after an initial 6 h equilibration). Tests were conducted in triplicate. A 3.5 w/v% (0.6 M) NaCl solution served as the supporting electrolyte. This electrolyte is widely used to study corrosion processes on metallic materials [23].
2.4. Shear Strength Mechanical Testing
Adhesive shear strength tensile tests were conducted according to ASTM D-1002 [10] with a coupon overlap of 12.7 mm. Bonded samples were cured for three days before testing. The structural integrity of adhesively bonded interfaces was assessed after accelerated environmental ageing effects at 40 °C and 98% RH for 6 weeks according to ASTM D-1151 [11]. Tensile strength values were obtained from five samples at different times using a Zwick ProLine Z050TN (ZwickRoell, Kennesaw, GA, USA). A tensile load was applied until joint failure occurred at a rate of 1.27 mm/min. The post-cure treatment for PECA consisted of 24 h exposure at a constant temperature of 65 °C.
3. Results and Discussion
3.1. Electrochemical Impedance Spectroscopy
Figure 1 shows Bode modulus plots for the thin PECA films (containing PA and SA) as well as post-cured PECA at 65 °C. In all cases, a progressive bulk polymer degradation is evident, resulting in a rapid decrease in the impedance modulus (or film resistance Rf) with time as electrolyte entered the film and the PECA bond scission process ensued. Most of the films, with the exception of the PECA containing SA, exhibited a high initial modulus (or Rf) ~ 109 Ωcm2 at 0.1 Hz. A slower drop in Rf, however, was observed over time with post-cured PECA and PA samples compared to those containing SA, reflecting that hydrolytic stability can be assessed by measuring this parameter.
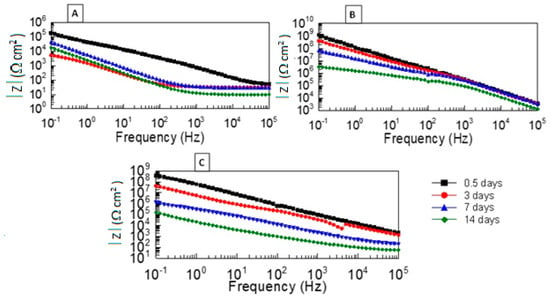
Figure 1.
Bode impedance magnitude (modulus) plots for PECA films aged in 3.5%wt/vol NaCl for up to 14 days: (A) salicylic acid, (B) post-cured PECA at 65 °C, and (C) phthalic anhydride.
Lower frequency 10−1–102 Hz data show considerable sensitivity to impedance changes during the course of exposure to the electrolyte. A pronounced decrease in the impedance was most likely due to ingress of high-dielectric-strength electrolyte, resulting in possible delamination of the polymeric film (and surface wetting) at the metal–polymer interface. This indicates that chemical degradation at the polymer–metal interface occurs faster than in the bulk polymer. In the latter case, bond scission is known to take place via a reverse Knoevenagel condensation reaction (i.e., hydrolysis reaction) as described in the scheme presented in reference [2]. The presence of two strongly electron-withdrawing groups in CA monomers such as ECA makes them highly reactive, and only trace amounts of the basic initiator are needed for polymerisation to proceed. The resulting polymer is, however, susceptible to degradation by a base that occurs primarily through hydrolysis. As a result, scission of the polymer backbone can occur, leading to lower molecular weight oligomers and other degradation products, including formaldehyde.
PECA films containing PA were slightly more resistant to degradation compared to the PECA, especially in the initial stages of electrolyte ingress, as shown in Figure 2. PA, however, has only a small effect on polymer molecular weight (MW) and does not significantly slow the polymerisation rate (curing process). It may also function as a post-cure water scavenger and the resultant dicarboxylic acid may cause polymer crosslinking and surface coupling reactions.
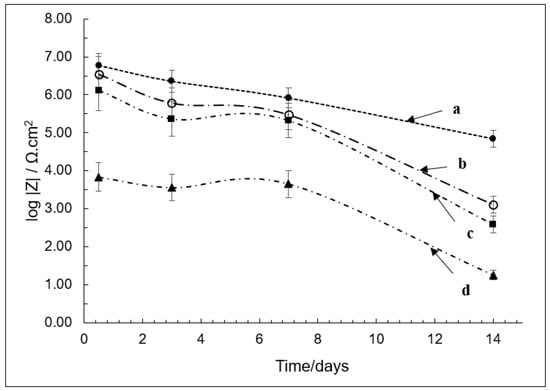
Figure 2.
Plot of impedance modulus (polymer film resistance (Rf)) against exposure time for PECA films under different conditions; (a) PECA post-cured at 65 °C, (b) PA/PECA, (c) uncured PECA, and (d) SA/PECA. SA/PECA; (d) performed the worst (with lowest impedance modulus) while post-cured PECA samples at 65 °C (a) performed the best (displaying highest impedance modulus). Values were taken at 100 Hz.
It is worth noting that all these PECA films are very much thinner (typically less than 1 µm) than most comparable adhesive and protective coating films, which are invariably considerably thicker (often more than 10 µm). Hence, electrolyte ingress, which can cause corrosion of the grit-blasted mild steel layer, is much more likely to be rapid in PECA films.
The poor hydrolytic stability associated with SA may be due to acid termination of propagating polymer chains, resulting in slower, initially incomplete polymerization and formation of monomer-plasticised higher free volume. A more water-permeable low MW polymer forms as a result. Other effects such as surface acid adsorption, salicylate salt formation, and acid/metal ion-catalysed polymer hydrolysis may also favour ingress of bulk and interfacial electrolyte/water. The low Rf of SA-containing films (dropping from only ~10 kΩcm2 to about 10 Ωcm2) is a reflection of these processes.
The time-dependence of the capacitance Cf and Cdl on the hydrolytic stability of PECA is illustrated in Figure 3. This figure indicates that post-cured PECA maintains the lowest capacitance throughout the test period, with decreased electrolyte ingress into the film. In this case, the capacitance is mainly associated with the film (Cf) and increases in a gradual linear fashion. Post-curing at elevated temperature reduces polymer film porosity and, thus, decelerates electrolyte ingress, thereby maintaining low Cf values throughout the test period. Less mobile monomer within the film also increases its inherent resistance. In contrast, the SA/PECA film displays an initial high capacitance, typical of values normally associated with double-layer Cdl processes, such as those involved in interfacial electron transfer, and remains high, increasing in a linear manner over time [24]. However, it has been found that accelerated ageing at 50 °C for 7 weeks can yield small molecules, due to unzipping of the polymer [25].
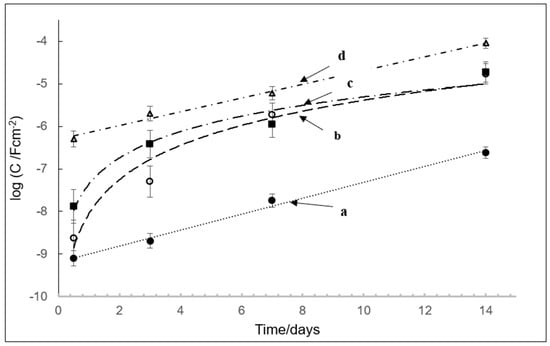
Figure 3.
Plot of logarithm of capacitance (Cdl or Cf) against exposure time for PECA films under different conditions; (a) PECA post-cured at 65 °C, (b) phthalic anhydride, PA/PECA, (c) PECA, and (d) salicylic acid, SA/PECA. Triplicate samples were tested.
The other two films studied (PECA and PA films) show an initial low capacitance of the order of 10 nFcm−2, reflective of values associated with a polymer film (Cf). Over a period of a few days, this changes, as demonstrated in Figure 3, where a rapid increase in capacitance values to those suggestive of Cdl, such as that occurring during corrosion of the metal substrate, is evident. Their capacitance values increase markedly, eventually approaching values displayed by the SA-containing films. Thus, the changing capacitance values indicate a transition from those typical of a polymer film (Cf ~ nFcm−2) to an interfacial charge transfer process, such as that of a metal corrosion reaction (Cdl).
3.2. Shear Strength Tensile Testing
A summary of tensile results is presented in Table 1, where it is evident that the PA had a less deleterious effect on the polymer film’s shear strength, following exposure to moisture at an elevated temperature. Changes in adhesive bond strength determined after 6 weeks exposure reflect the ingress of moisture through the polymer film to the underlying metal surface, resulting in some loss of adhesion in conjunction with possible formation of ferrous metal corrosion products.

Table 1.
Percentage decrease in bond strength of PECA prepared from various ECA formulations after 6 weeks aging at 40 °C and 98% RH on grit-blasted mild steel.
PA acts as a beneficial film modifier, whereas SA acts as a Lewis base scavenger and has a negative influence on polymer hydrolytic stability. Both have a low impact on ECA monomer viscosity. The PA/PECA film displayed a lower decrease in bond strength after accelerated ageing. In contrast, the SA had a detrimental effect (i.e., a greater decrease in adhesive bond strength) than the PA/PECA film and the PECA itself. These results confirm the EIS findings with the film bond strength decrease following the order PA/PECA < PECA< SA/PECA film. Once again, the PA film performs better than the PECA and the SA. As indicated by the EIS results, the reasons for this order are likely to be the same (i.e., hydrolytic-induced depolymerisation and the onset of interfacial delamination) but unlike the EIS approach, results are not accessible during the course of the 6 week test period.
Finally, future work involving EIS measurements aimed at determining the effects of other additives, metal substrates, moisture, and heat on small-bond-gap CA metal bonds is worth pursuing.
4. Conclusions
The additives PA and SA were deliberately chosen for their positive and negative effects on PECA adhesive bond strength, respectively. Results confirm that EIS can be used to effectively to investigate the hydrolytic stability of PECA layers, both modified with surface active (SA) and surface inactive (PA) additives, as well as on unmodified films. This decrease in the bond integrity is due to a combination of the PECA degradation and the onset of the grit-blasted mild steel corrosion. It is notable that EIS measurements taken, even after 12 h, demonstrate a correlation between the influence of the additives and the mechanical bond strength, which is normally only measured over a much longer time period using the current tensile/accelerated ageing method, which is slow and costly. Future work could use EIS to explore a wide range of additives and the effects of different surfaces and heat treatments on CA adhesive performance, both in terms of curing and hydrolytic stability.
Author Contributions
Conceptualization, B.R. and A.B.; methodology, K.R.; software, K.R.; validation, K.R., B.R. and A.B.; formal analysis, K.R.; investigation, K.R.; resources, B.R.; data curation, K.R.; writing—original draft preparation, K.R.; writing—review and editing, A.B. and J.C.; visualization, K.R.; supervision, A.B. and B.R.; project administration, A.B. and J.C.; funding acquisition, B.R. All authors have read and agreed to the published version of the manuscript.
Funding
The FOCAS Institute is funded under the National Development Plan 2000–2006 with the assistance of the European Development Fund.
Data Availability Statement
Much of the data is contained in internal reports for Henkel and is confidential.
Acknowledgments
The authors gratefully acknowledge the award of a TU Dublin/Henkel Enterprise Scholarship and are grateful to Hugh Byrne and the FOCAS Institute for use of their facilities.
Conflicts of Interest
BR was employed by Henkel, Ireland. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Raheem, K.; Cassidy, J.; Betts, A.; Ryan, B. Use of confocal Raman microscopy to characterise ethyl cyanoacrylate adhesive depth curing. Phys. Chem. Chem. Phys. 2020, 22, 23899–23907. [Google Scholar] [CrossRef] [PubMed]
- Raheem, K.; Cassidy, J.; Ryan, B.; Betts, A. Monitoring the curing, degradation and moisture ingress into alkyl 2-cyanoacrylate adhesives using electrochemical impedance spectroscopy. J. Solid State Electrochem. 2024, 28, 4029–4040. [Google Scholar] [CrossRef]
- Amirudin, A.; Thieny, D. Application of electrochemical impedance spectroscopy to the study of polymer-coated electrodes. Prog. Org. Coat. 1995, 26, 1. Available online: http://refhub.elsevier.com/S0013-4686(20)31118-X/sbref0021 (accessed on 4 June 2024). [CrossRef]
- Mansfeld, F. Use of electrochemical impedance spectroscopy for the study of corrosion protection by polymer coatings. J. Appl. Electrochem. 1995, 25, 187–202. [Google Scholar] [CrossRef]
- Macdonald, D.D. Reflections on the history of electrochemical impedance spectroscopy. Electrochim. Acta 2006, 51, 1376–1388. [Google Scholar] [CrossRef]
- Lvovich, V.F. Impedance Spectroscopy Applications to Electrochemical and Dielectric Phenomena; Wiley: Hoboken, NJ, USA, 2012. [Google Scholar]
- Lasia, A. Electrochemical Impedance Spectroscopy and Its Applications; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Davis, G.D.; Mani, S.; Rich, M.J.; Drzal, L.T. Electrochemical impedance spectroscopy inspection of composite adhesive joints. J. Adhes. Sci. Technol. 2005, 19, 467–492. [Google Scholar] [CrossRef]
- Taheri, P.; de Wit, J.H.W.; Terryn, H.; Mol, J.M.C. In situ study of buried metal–polymer interfaces exposed to an aqueous solution by an integrated ATR-FTIR and electrochemical impedance spectroscopy system. J. Phys. Chem. C 2013, 117, 20826–20832. [Google Scholar] [CrossRef]
- Njoku, D.I.; Cui, M.; Xiao, H.; Shang, B.; Li, Y. Understanding the anticorrosive protective mechanisms of modified epoxy coatings with improved barrier, active and self-healing functionalities: EIS and spectroscopic techniques. Sci. Rep. 2017, 7, 15597. [Google Scholar] [CrossRef] [PubMed]
- Davis, G.D.; Pethrick, R.A.; Doyle, J. Detection of moisture in adhesive bonds using electrochemical impedance and dielectric spectroscopies. J. Adhes. Sci. Technol. 2009, 23, 507–528. [Google Scholar] [CrossRef]
- Han, Y.; Wang, J.; Zhang, H.; Zhao, S.; Ma, Q.; Wang, Z. Electrochemical impedance spectroscopy (EIS): An efficiency method to monitor resin curing processes. Sens. Actuators A Phys. 2016, 250, 78–86. [Google Scholar] [CrossRef]
- Chauffaille, S.; Devos, O.; Jumel, J.; Shanahan, M.E.R. Liquid diffusion in polymeric adhesives by electrochemical impedance spectroscopy (EIS). Int. J. Adhes. Adhes. 2010, 30, 602–608. [Google Scholar] [CrossRef]
- Radhakrishnan, S.; Saini, D.R. Dielectric monitoring of the curing process in cyanoacrylate resin. Polym. Sci. Eng. 1993, 33, 125–131. [Google Scholar] [CrossRef]
- Ryan, B.; McCann, G. Novel sub-ceiling temperature rapid depolymerization-repolymerization reactions of cyanoacrylate polymers. Macromol. Rapid Commun. 1996, 17, 217–227. [Google Scholar] [CrossRef]
- Vezin, W.R.; Florence, A.T. In vitro heterogeneous degradationof poly(n-alkyl α-cyanoacrylates). J. Biomed. Mater. Res. 1980, 14, 93–106. [Google Scholar] [CrossRef] [PubMed]
- ASTM D1002; Standard Test Method for Apparent Shear Strength of Single-Lap-Joint Adhesively Bonded Metal Specimens by Tension Loading (Metal-to-Metal). ASTM International: West Conshohocken, PA, USA, 2010.
- ASTM D 1151-00; Standard Practice for Effect of Moisture and Temperature on Adhesive Bonds. ASTM International: West Conshohocken, PA, USA, 2022. Available online: https://www.astm.org/d1151-00r22.html (accessed on 4 June 2024).
- Raheem, K. Investigation of Cyanoacrylate Adhesive Bond Curing and Durability Using Spectroscopic and Thermochemical Techniques. Ph.D. Thesis, Technological University of Dublin, Dublin, Ireland, 2020. [Google Scholar]
- Raheem, K.; Cassidy, J.; Betts, A.; Ryan, B. A simple microcalorimetry system to determine the adsorption behaviour of acids in large adhesive bond gaps using base-initiated solution polymerisation of ethyl-2-cyanoacrylate. Int. J. Adhes. Adhes. 2023, 125, 103424. [Google Scholar] [CrossRef]
- Grit Blasted Mild Steel q Panel Brochure. Available online: https://www.q-lab.com/products/q-panel-standard-substrates/q-panels (accessed on 4 June 2024).
- Lazanas, A.C.; Prodromidis, M.I. Electrochemical Impedance Spectroscopy—A tutorial. ACS Meas. Sci. 2023, 3, 162. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Lu, Y.; Guo, Z.; Gu, J.; Gu, C. Corrosion behaviour of a quenched and partitioned medium carbon steel in 3.5 wt.% NaCl solution. Corros. Sci. 2018, 130, 64–75. [Google Scholar] [CrossRef]
- Brett, C.M.A. Electrochemical Impedance Spectroscopy in the Characterisation and Application of Modified Electrodes for Electrochemical Sensors and Biosensors. Molecules 2022, 27, 1497. [Google Scholar] [CrossRef] [PubMed]
- Vouvoudi, E.C.; Tamias, G.A.; Chatzicharistou, E.A.; Achilias, D.S. Thermal Treatment of a Commercial Polycyanoacrylate Adhesive Addressed for Instant Glass Restoration, for Investigating Its Ageing Tolerance. Macromol 2023, 3, 636–652. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).