Abstract
The formation of atomically flat Si(111)–H surfaces was critical for molecular electronics, nanoscale device fabrication, and surface chemistry studies. We systematically investigated how initial oxide composition and dissolved oxygen affected terrace-formation kinetics during ammonium fluoride (NH4F) etching. N-type Si(111) was cleaned with either oxygen plasma or piranha solution to generate, respectively, a more uniform versus a chemically heterogeneous oxide, and then etched in NH4F containing 0–5% (w/v) ammonium sulfite (AS) as an oxygen scavenger. AFM acquired every 2 min over 20 min revealed that plasma-pretreated surfaces reached atomically flat terraces earlier and more reproducibly than piranha-pretreated surfaces. Increasing AS concentration suppressed oxygen-induced etch pits and promoted the earlier appearance of large, well-ordered terraces, whereas prolonged etching led to roughening. XPS and ATR-FTIR corroborated differences in the starting oxides and confirmed post-etch H-termination. Collectively, the results indicated that oxide uniformity together with oxygen scavenging correlated with faster attainment and greater persistence of low-roughness terraces, providing a practical framework for reproducibly preparing hydrogen-terminated Si(111)–H surfaces.
1. Introduction
Atomically flat silicon, characterized by its near-atomic smoothness and well-defined terraced structures, is an important silicon-based surface for the study of molecular interaction at the sub-atomic layer [1]. From an academic point of view, atomically flat Si(111)-H surfaces are very useful to elucidate the intrinsic step-by-step organic monolayer formation processes on the material surface due to the low electrical resistance, as well as possessing minimal physical defects [2]. Hence, atomically flat silicon is an ideal surface for studying various surface processes, from bond formation to electron transfer [3].
There are two primary methods for the formation of atomically flat silicon: (i) chemical etching and (ii) thermal annealing, and each has its distinct strengths and limitations. Chemical etching, particularly with NH4F [4,5,6], is ideal for n-type Si(111) doped with group V impurities (e.g., phosphorus), leveraging on low surface energy and reduced oxygen reactivity due to excess electrons, which stabilize hydrogen-terminated surfaces with uniform terraces [7,8,9]. Yet, precise control of etching time and temperature remains crucial to ensure optimal morphology, and NH4F is less effective for Si (100), the standard substrate for CMOS and MOSFET technologies, due to isotropic etching causing rougher surfaces [10]. In contrast, thermal annealing in UHV is preferred for p-type Si (100) doped with group III impurities (e.g., boron), as holes facilitate surface diffusion, enabling a simple 2 × 1 reconstruction with minimal defects [11,12,13]. This Si (100)-2 × 1 structure aligns with CMOS processes, unlike the complex Si(111)-7 × 7 reconstruction, which is valuable for surface science research [14,15]. But expensive setup and stringent requirements had rendered thermal annealing too impractical for industrial applications and had therefore limited its usage. Thus, NH4F etching’s scalability and cost-effectiveness make it more practical for Si(111)-H surfaces in research and niche applications.
Nonetheless, controlling the formation of atomically flat silicon with NH4F remains challenging, as each batch of silicon wafer offers slight differences in the composition, and these subtle differences may result in different outcomes. At the start of the chemical etching process to achieve atomically flat silicon surfaces, a pretreatment involving the cleaning of the surface with either piranha solution [16,17,18,19,20] or the RCA process [21,22] to effectively remove organic contaminants was typically deemed as a necessary step, and this results in a clean, hydrophilic surface characterized by a chemical oxide layer prior to acid etching. However, the uniformity of this oxide layer, though rarely addressed in studies of atomically flat silicon formation, is hypothesized to be critical for achieving high-quality Si(111)-H surfaces. How this uniform layer of pre-oxide on the silicon surface may affect the subsequent etching remains unclear. In principle, variations in the oxide layer’s thickness or consistency could potentially influence the formation of atomically flat surfaces, highlighting the importance of optimizing pretreatment conditions to ensure uniform oxide growth and enhance etching outcomes.
The method of surface cleaning can significantly influence the type and composition of silicon oxides formed at the initial stages, and this could subsequently influence the etching outcome. To address this question, this study systematically investigates how two commonly used pretreatments (piranha solution cleaning and oxygen plasma treatment) impact the NH4F etching process used to produce atomically flat Si(111)-H surfaces. Silicon substrates were pre-oxidized using piranha solution or oxygen plasma under controlled conditions, followed by NH4F etching for varying durations. The resulting surface morphology was characterized by atomic force microscopy (AFM), while X-ray photoelectron spectroscopy (XPS) was used to analyze the nature and chemical composition of the oxide layers. Fourier-transform infrared spectroscopy with attenuated total reflectance (FTIR-ATR) was employed to confirm the formation of surface Si–H bonds. By directly comparing the effects of these pretreatment methods, this study elucidates how oxide layer characteristics influence the kinetics and efficiency of NH4F etching. The findings aim to provide practical guidance for achieving reproducible, uniform, and atomically flat Si(111)-H surfaces for applications in surface chemistry, semiconductor processing, and nanoscale device fabrication. The graphical scheme describing this work is as presented in Figure 1.
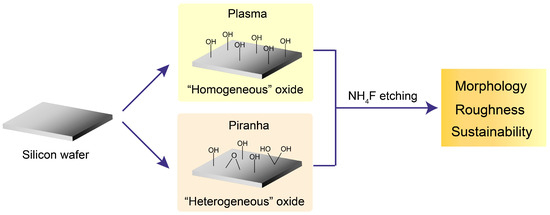
Figure 1.
Experimental scheme linking initial oxide composition to etching outcomes. Si(111) wafers were pretreated by oxygen plasma or piranha solution to generate, respectively, a more uniform or heterogeneous oxide. The samples were then etched in NH4F (±0–5% ammonium sulfite, where indicated) and evaluated for terrace morphology, RMS roughness, and the stability window of the low-roughness state.
2. Materials and Methods
Unless otherwise specified, all reagents were procured from Sigma-Aldrich (Burlington, MA, USA) and used without further purification. N-type (111) silicon wafers with a resistivity of 1–10 Ω·cm were obtained from Ruilong, Taiwan.
2.1. Preparation for Atomically Flat Surfaces
In the pretreatment, silicon wafers were cut into 10 mm × 10 mm pieces and cleaned sequentially with acetone, ethanol, and distilled water via sonication for 1 min each.
For the plasma-treated surfaces, silicon substrates were cleaned using a DC 32G plasma system (115 V) operated at an RF power of 18 W for 2 min. High-purity argon was introduced during the plasma treatment to sustain a stable discharge and facilitate the removal of residual organic contaminants, thereby promoting the formation of a uniformly oxidized surface. For piranha-cleaned surfaces, the pieces were then treated with hot piranha solution (3:1 H2SO4:H2O2) for 30 min to remove organic and metallic contaminants, followed by thorough rinsing with distilled water to remove residual acid.
To achieve atomically flat silicon surfaces, the wafers were etched in a 40% (w/v) ammonium fluoride (NH4F) solution adjusted to pH 8 at room temperature in the dark and supplemented with 0%, 2% and 5% ammonium sulfite as an oxygen scavenger [23,24]. The etching solution was purged with argon for 15 min prior to use to minimize dissolved oxygen [25]. The wafers were etched in this solution for durations ranging from 1 to 20 min and subsequently rinsed with distilled water to remove residual etchant. Following a similar protocol to the one described above, the silicon surfaces were pretreated with plasma cleaning instead of piranha solution, and the ammonium sulfite concentration in the 40% ammonium fluoride (NH4F) etching solution was adjusted to 0%, 2% and 5% (w/v).
2.2. Atomic-Force Microscopy (AFM)
All Si(111) surfaces were characterized using atomic force microscopy (AFM) with a Multimode AFM system (Nanoview 1000, Utek Materials, Taiwan). Measurements were performed in tapping mode using a silicon cantilever with a resonant frequency of 150 kHz and a spring constant of 5 N/m. Images were acquired at a scan rate of 0.8 Hz with a resolution of 512 × 512 pixels, employing the manufacturer’s recommended automatic settings for integral and proportional gains. The scan area was set to 1 μm × 1 μm. Surface morphology data, including root mean square (RMS) roughness, were analyzed using Gwyddion software (version 2.69) for post-processing [26,27,28].
2.3. X-Ray Photoelectron Spectroscopy (XPS)
X-ray Photoelectron Spectroscopy (XPS) analysis of all grafted samples was conducted using a PHI 5000 VersaProbe (ULVAC-PHI) equipped with an Al Kα X-ray source (1486.6 eV). High-resolution spectra for Si2p were collected at a 45° angle relative to the sample surfaces and were fit with a spin–orbit split of 0.60 ± 0.02 eV (2p3/2:2p1/2 = 2:1), shared FWHM within chemical families, and a Shirley background. Binding energies were referenced to Si2p3/2 = 99.0 eV (substrate) with cross-checks to C 1s = 284.8 eV. Goodness-of-fit was evaluated by residuals and χ2. All spectra were processed with XPSpeak (ver 4.1) as well as CASAXPS (ver 2.3.25) to determine peak positions, areas, and full width at half maximum (FWHM) for enhanced clarity [29,30].
2.4. Fourier Transform Infrared Spectroscopy (FTIR)
Fourier Transform Infrared Spectroscopy (FTIR) with Attenuated Total Reflectance (ATR) was performed using a JASCO FT/IR-4700 spectrometer equipped with a liquid nitrogen-cooled Mercury Cadmium Telluride (MCT) detector. A VariGATR™ Grazing Angle Accessory (Harrick) with a 65°-angled germanium prism was used to identify chemical signals from Si(111) surfaces. Plasma and piranha pretreatment of n-type Si(111) surfaces served as controls for their respective etching surfaces. Spectra were collected over 200 scans at 8 cm−1 resolution using p-polarized light in the 2000–2200 cm−1 range to detect changes in silicon hydride (Si-H) bonds following surface modification. Subsequently, spectral data were normalized and processed using software Spectra Manager v2.15.01.
3. Results and Discussion
To investigate the impact of oxidation treatments on surface morphology and roughness, two distinct pretreatment forms (plasma and piranha) were applied to silicon substrates, with the objective of elucidating whether different oxidative processes result in variations in the formation of atomically flat surfaces. It is important to note that while both cleaning approaches on the silicon surface are efficient in the removal of adventitious carbon, the resulting surface hydroxyl species at the end of the cleaning process were different. It is this difference in hydroxyl presentation on the surface that forms the basis of our study here. Firstly, with cleaning through plasma etching, it is envisaged that the surface hydroxides would be more homogenous, while for solution-based piranha cleaning, the silicon surface would be decorated with a mixed population of Si-OH and Si-O-Si intermediates. It is hypothesized here that the differences in these oxides would ultimately result in structural differences in the ensuing NH4F etching for the formation of atomically flat silicon.
To confirm that the oxide species present on the silicon surface were indeed distinct, high-resolution XPS Si2p spectra were obtained for both plasma- and piranha-cleaned silicon surfaces. The Si2p spectra were first analyzed to verify the expected spin–orbit splitting and oxide-state assignments. The Si2p3/2 to Si2p1/2 area ratio was confirmed to be approximately 2:1 after deconvolution, consistent with standard spin–orbit coupling. As shown in Figure 2, the Si-O component of the piranha-treated sample required deconvolution into two distinct peaks: one centered at 103 eV and another at 101.8 eV. We had relied on binning of the Si-Ox region to determine the presence of asymmetry, and attempts to fit this region with a single Lorentzian peak produced unsatisfactory results, indicating that the oxide layer is heterogeneous. The higher-binding-energy peak at 103 eV is attributed to Si4+ (distal SiO2-like oxide), while the 101.8 eV feature corresponds to sub-oxide species (Si3+). In contrast, the plasma-cleaned surface exhibited a single Si-O peak at 103.2 eV, located 4.2 eV above the elemental Si2p3/2 signal at 99 eV, suggesting a more uniform oxide environment. Collectively, these results demonstrate that the two cleaning methods produce chemically distinct oxide layers, with piranha pretreatment yielding a mixture of oxide species, whereas plasma pretreatment results in a more homogeneous SiO2-like surface. Subsequent NH4F etching regenerated Si-H bonds centered at 2080 cm−1 was also confirmed by ATR-FTIR (Figure 2C,D).
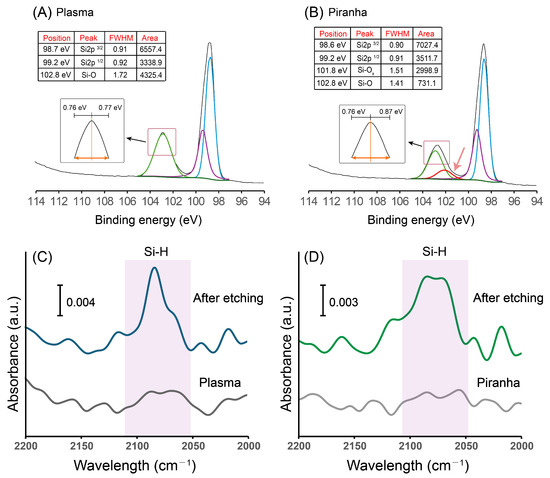
Figure 2.
High-resolution Si2p XPS and ATR-FTIR spectra of (A) plasma- and (B) piranha-pretreated Si(111) surfaces. Plasma treatment produced a single Si–O peak (103.2 eV), whereas piranha treatment yielded additional sub-oxide contributions (101.8 eV), indicating oxide heterogeneity (from the asymmetry of the Si-Ox peak). After ammonium fluoride etching, (C) plasma and (D) piranha-pretreated surfaces showed clear Si–H stretching bands (~2080 cm−1), confirming successful hydrogen termination. All assignments, as well as FWHM and area under the peak, were presented as insets above. The deconvolution was presented in different colors for better visualization.
The fabrication of atomically flat Si(111) surfaces is conventionally achieved through ammonium fluoride (NH4F) etching, as well-documented in prior literature. In this study, we hypothesize that the presence of surface sub-oxide species (Si+1–Si+3) may play a critical role in the initial stages of etching, potentially inducing transient surface roughening. Because these partially oxidized silicon species exhibit intermediate chemical reactivity compared to Si0 and Si4+, their non-uniform distribution could locally perturb the etching kinetics and delay the establishment of uniform terrace morphology. To investigate this hypothesis, we performed controlled etching using a 40% (w/v) NH4F solution, carefully adjusted to pH 8, on high-resistivity n-type Si(111) substrates. Etching was carried out for fixed intervals ranging from 2 to 20 min, with samples collected at each time point. The etched surfaces were subsequently analyzed by atomic force microscopy (AFM) under tapping mode to evaluate topographic evolution. This systematic approach allowed us to construct a time-resolved dataset, effectively cataloging the progression from the initially roughened surface state to the eventual formation of atomically flat, well-defined terraces. Through this methodology, we aim to elucidate the transition regime between sub-oxide-dominated surface chemistry and terrace stabilization, thereby shedding light on the kinetic interplay between surface oxide heterogeneity and step-flow etching. Understanding this relationship provides mechanistic insight into how surface chemistry dictates terrace formation and could inform optimized pre-cleaning strategies for achieving reproducibly smooth Si(111) surfaces for subsequent functionalization.
The plasma-pretreated samples displayed a distinct morphological evolution during the NH4F etching process. In the control group without ammonium sulfite (0%), subtle surface changes became apparent after approximately 6 min of etching, giving rise to a heterogeneous terrace structure overlaid with a densely packed oxide layer (see Figure 3). This dense configuration, likely sustained by oxygen adsorption from the ambient environment, remained largely unchanged with continued immersion up to 12 min. By 18 min, the surface underwent a clear reorganization, yielding an atomically flat morphology characterized by uniformly distributed, well-defined terraces. This stage represents the optimal balance between etching and surface smoothing, where step-flow processes dominate and surface roughness reaches its minimum. However, prolonging the etch to 20 min reversed this trend, producing a noticeably rougher morphology and partially eroding the previously achieved terrace uniformity. In the absence of ammonium sulfite as an oxygen scavenger, etch pits (kinks) emerged, reflecting the uncontrolled competition between etching and oxidation at the solid–liquid interface. This imbalance disrupted terrace stabilization and compromised the flatness of the surface. These observations underscore the crucial role of oxygen management in maintaining the kinetic window necessary for the reproducible formation of atomically flat Si(111) terraces.
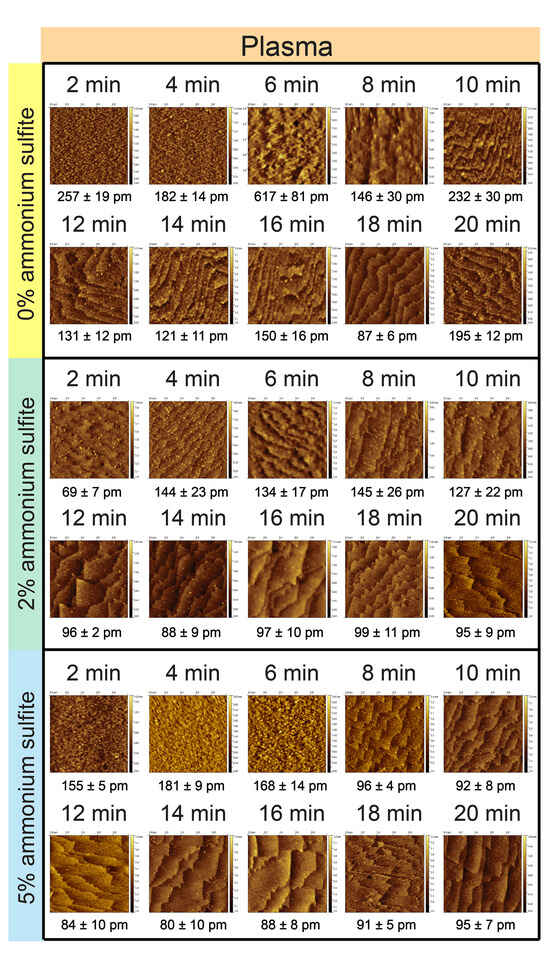
Figure 3.
Surface with plasma pretreatment and ammonium fluoride etching with 5% ammonium sulfite at intervals from 2 to 20 min. AFM images were scaled at 1 μm × 1 μm each and were obtained under tapping mode.
In the experimental group treated with 2% ammonium sulfite, the development of an atomically flat surface was markedly accelerated, as revealed by atomic force microscopy (AFM). Well-defined terrace structures began to emerge within just 2 min of etching, reflecting the rapid influence of ammonium sulfite as an oxygen scavenger that effectively suppresses interference from ambient oxygen. By 8 min, the surface reached an optimally reorganized state, exhibiting atomically flat terraces with exceptional uniformity and smoothness. Remarkably, these terraces retained their structural integrity throughout prolonged immersion, remaining stable for up to 20 min without significant degradation. Compared to the 0% ammonium sulfite control, which produced a denser but more irregular terrace network, the 2% ammonium sulfite treatment yielded significantly larger and flatter terraces. This outcome suggested that controlling the oxidation rate not only accelerates terrace nucleation but also promotes the growth of more expansive, well-ordered terraces, thereby enhancing the reproducibility and quality of atomically flat Si(111) surfaces.
To further investigate the influence of oxygen levels on surface morphology, the concentration of ammonium sulfite, an oxygen scavenger, was increased to 5% in the ammonium fluoride etching solution to examine terrace evolution under conditions of markedly reduced dissolved oxygen. Interestingly, unlike the lower-concentration groups, the surfaces treated under these conditions exhibited no obvious morphological changes during the early stages of etching. By 8 min of immersion, however, a distinct transformation was observed, with the surface developing exceptionally flat and well-defined terrace structures, as revealed by atomic force microscopy (AFM). These terraces displayed a high degree of uniformity and smoothness, reflecting a highly controlled etching process facilitated by the enhanced oxygen-scavenging capacity of the 5% ammonium sulfite solution. Furthermore, the terraces formed under these conditions were noticeably larger than those observed in the 2% ammonium sulfite group, suggesting that a more strongly reduced oxygen environment promoted the growth of expansive, atomically flat terraces. These findings underscore the critical role of oxygen modulation in dictating surface topography, demonstrating that higher ammonium sulfite concentrations effectively suppressed external oxygen interference and enabled more reproducible terrace formation during the etching process.
It was evident that ammonium sulfite played a pivotal role in facilitating the formation of atomically flat surfaces during ammonium fluoride etching of plasma-pretreated silicon substrates, exerting a significant influence on both the etching kinetics and the resulting surface morphology. The presence of ammonium sulfite, acting as an efficient oxygen scavenger, modulated the etching rate in the ammonium fluoride solution and enabled the development of larger and more expansive terrace structures compared to surfaces etched without this additive. In the absence of ammonium sulfite, atomically flat and homogeneous terrace structures were still attainable, but only after extended immersion times, as the etching process gradually smoothed initial surface irregularities. However, these terraces exhibited limited stability, with further immersion beyond the optimal duration leading to terrace degradation, increased roughness, and the loss of atomic flatness. Moreover, the presence of dissolved oxygen in the etching solution posed a significant challenge, as uncontrolled oxygen levels promoted the formation of etching pits, or kinks. These surface defects arose from an imbalance between the etching and oxidation rates, thereby disrupting the uniform development of terraces. By effectively controlling dissolved oxygen, the addition of ammonium sulfite minimized such imperfections and ensured the formation and long-term preservation of highly ordered, atomically flat terrace structures throughout the etching process.
To further investigate the influence of oxide composition on the formation of atomically flat surfaces, identical etching conditions were applied to silicon substrates that had been pretreated with piranha solution, enabling a direct comparison with plasma-pretreated surfaces (Figure 4). In the absence of ammonium sulfite, the piranha-pretreated substrates began to develop terrace structures only after 10 min of etching in ammonium fluoride solution, as revealed by atomic force microscopy (AFM). These surfaces exhibited larger etch pits, or kinks, which were more pronounced than those observed on plasma-pretreated surfaces under similar conditions. Furthermore, the terrace structures formed on the piranha-pretreated surfaces displayed a compact and densely packed configuration, closely resembling the terrace morphology observed on plasma-pretreated surfaces etched without ammonium sulfite. This observation suggested that the oxide composition, particularly the presence of silicon suboxides (SiOx) identified in prior XPS analysis, contributed to the formation of a more compact terrace arrangement. By 18 min of etching, the piranha-pretreated surfaces achieved an atomically flat morphology, characterized by highly uniform and smooth terraces, indicative of an etching process that gradually overcame the initial surface irregularities. This time-resolved evolution highlighted the significant role of pretreatment-induced oxide composition in dictating both the kinetics of terrace nucleation and the eventual attainment of atomically flat surfaces, with piranha pretreatment producing a slower but ultimately effective surface-flattening process compared to plasma pretreatment.
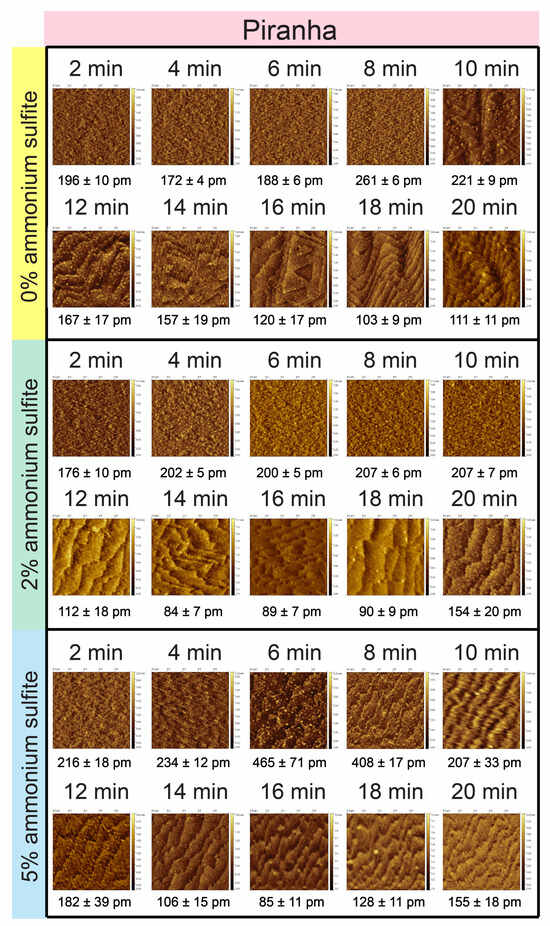
Figure 4.
Surface with piranha pretreatment and ammonium fluoride etching with 5% ammonium sulfite at intervals from 2 to 20 min. AFM images were scaled at 1 μm × 1 μm each and were obtained under tapping mode.
Remarkably, the application of 2% ammonium sulfite in the ammonium fluoride etching solution significantly mitigated the formation of etch pits, or kinks, on piranha-pretreated silicon surfaces, thereby improving overall surface quality relative to the untreated group. However, unlike the pronounced morphological transformations observed on plasma-pretreated surfaces under similar conditions, the piranha-pretreated surfaces did not display substantial changes in terrace morphology following the addition of 2% ammonium sulfite. The terraces retained a dense and compact configuration, a feature likely governed by the intrinsic silicon suboxide (SiOx) composition generated during piranha pretreatment, as previously confirmed by X-ray photoelectron spectroscopy (XPS). This configuration remained stable throughout the etching process. The ability of 2% ammonium sulfite to suppress etch pit formation without significantly altering terrace size or arrangement highlighted its function as an efficient oxygen scavenger that controlled surface defect formation while preserving the structural characteristics imparted by pretreatment. These findings underscored the interplay between oxide composition and oxygen management in achieving high-quality, atomically flat surfaces.
In the experimental group utilizing a 5% ammonium sulfite concentration within the ammonium fluoride etching solution, piranha-pretreated silicon surfaces exhibited distinct morphological changes at early stages, as observed through atomic force microscopy (AFM). Terrace structure formation commenced after 10 min of etching, indicating a relatively rapid response to the enhanced oxygen-scavenging environment provided by the higher ammonium sulfite concentration. By 14 min, these surfaces achieved an ideal atomically flat morphology, characterized by highly uniform and smooth terrace structures, suggesting an optimized etching process that effectively minimizes surface irregularities and etch pits, such as kinks. However, the stability of these atomically flat terraces was limited, as prolonged etching beyond 14 min led to structural degradation. Specifically, after 20 min of etching, the surface morphology began to deteriorate, with the terrace structures collapsing and resulting in increased surface roughness. This loss of structural integrity is likely attributable to the interplay between the etching kinetics and the high ammonium sulfite concentration, which, while effective in reducing oxygen-related defects initially, may disrupt the balance required to sustain the atomically flat configuration over extended etching durations. These findings highlight the critical role of ammonium sulfite concentration in modulating the formation and stability of terrace structures on piranha-pretreated surfaces, underscoring the need for precise control of etching conditions to maintain optimal surface morphology. In contrast, it is evident that plasma-pretreated surfaces exhibit significantly smoother surface roughness across various etching conditions compared to piranha-pretreated surfaces. Notably, all plasma-pretreated surfaces at 2 and 5 percent ammonium sulfite demonstrate roughness values below 100 picometers, suggesting that the presence of a more uniform and homogeneous oxide layer on these surfaces plays a critical role in promoting the formation of an atomically flat topography.
The rough profiles of surfaces subjected to plasma and piranha pretreatments revealed a noteworthy and reproducible trend. Surfaces etched with 2% or 5% ammonium sulfite exhibited elevated roughness during the initial phase of etching (2–8 min), which subsequently decreased to much lower values after 10 min or more of etching. This behavior suggested the presence of a transient kinetic overshoot, likely arising from the instability introduced by higher ammonium sulfite concentrations in the etching system. Excessive scavenging of dissolved oxygen by ammonium sulfite appeared to accelerate the etching rate relative to systems without the additive, producing an initial surge in surface roughness. As etching progressed, the reaction system stabilized, allowing step-flow mechanisms to dominate and yielding smoother surfaces with significantly reduced roughness values. These observations emphasized the importance of considering the chemical interactions between ammonium fluoride and the silicon surface to explain why distinct oxide pretreatments led to substantial variations in terrace evolution and surface morphology. Based on these observations, the RMS roughness evolution of the surfaces is summarized in Figure 5. The stable RMS region of the plasma-treated surfaces is highlighted to emphasize the contrast with the more fluctuating profiles observed for piranha-pretreated samples, which required longer etching durations to achieve comparable smoothness. These results underscore the synergistic effects of oxide uniformity and oxygen scavenging in accelerating terrace formation and minimizing surface roughness.
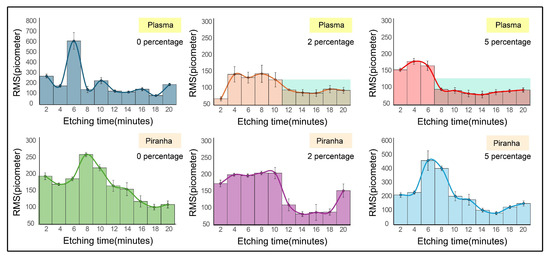
Figure 5.
RMS roughness of the plasma and piranha treatment surfaces from 2 to 20 min, and the plasma pretreatment surfaces had exhibited a smoother profile over time compared to the piranha treatment. All measurements were taken from n = 5.
Understanding the underlying mechanisms was therefore essential, particularly the initial surface reaction in the ammonium fluoride solution [31,32], which played a pivotal role in governing the overall etching behavior and could be represented by the following chemical equation:
In a 40% ammonium fluoride solution incorporating ammonium sulfite as an oxygen scavenger and adjusted to pH 8 with ammonium hydroxide, the concentrations of F− and OH− can be effectively controlled [33,34,35], resulting in a reduced etch rate that promotes the formation of atomically flat surfaces. As previously noted, the mechanism of ammonium fluoride solution in achieving atomically flat surfaces can be divided into two key reactions: oxidation and etching. These reactions can be represented as follows [36]:
- Oxidation:
- Etching:
To achieve ideal atomically flat surfaces, precise control of the oxidation rate is critical. The concentration of OH− plays a pivotal role in modulating the formation rate of Si-OH [37]. According to Equation (2), the pH of a 40% ammonium fluoride solution increases upon the addition of ammonium hydroxide. This buffered solution was expected to reduce the etch rate, thereby facilitating the formation of a more homogeneous and atomically flat surface structure.
Based on detailed observations from atomic force microscopy (AFM) images, a compelling hypothesis was formulated: a uniform oxide layer on the silicon surface enabled a more uniform and synchronous etching front, thereby accelerating the appearance of well-defined terraces, thereby accelerating the formation of well-defined atomic terraces. In contrast, surfaces characterized by heterogeneous oxide compositions, which often contain suboxides with differing chemical reactivities, required a longer etching duration to resolve these compositional inconsistencies. This extended processing time was essential for producing hydrogen-terminated surfaces suitable for applications that demanded atomically flat topographies. The presence of dissolved oxygen in the etching solution also played a critical role in the development of atomically flat silicon surfaces. Dissolved oxygen initiated secondary oxidation reactions during etching, introducing surface defects or etch pits and delaying the establishment of an ideal terrace morphology. Notably, plasma-pretreated surfaces etched in solutions containing lower concentrations of ammonium sulfite (e.g., 2% or 5%) exhibited enhanced terrace formation at earlier stages compared to piranha-pretreated surfaces. This observation underscored the superior ability of plasma pretreatment to generate a chemically uniform oxide layer that promoted rapid and reproducible formation of well-ordered terraces.
Additionally, the concentration of ammonium sulfite was a critical parameter in dictating the morphology of atomically flat surfaces. Experimental evidence showed that plasma-pretreated surfaces etched in ammonium fluoride solutions containing 2% or 5% ammonium sulfite developed significantly larger terrace structures compared to their piranha-pretreated counterparts. Ammonium sulfite functioned as a highly effective oxygen scavenger, efficiently capturing dissolved oxygen in the etching solution. This scavenging action was crucial, as it mitigated the formation of etch pits—microscopic surface defects that compromised the smoothness and uniformity of the silicon surface. Despite rigorous efforts to remove oxygen by purging the solution with argon for 15 min before etching, trace levels of oxygen persisted. Consequently, the presence of ammonium sulfite was indispensable for minimizing the detrimental effects of residual oxygen, thereby facilitating the formation of smoother and more homogeneous terrace structures across the silicon surface.
Herein, it is once again necessary to discuss the precise role of ammonium sulfite. The mechanistic role of ammonium sulfite during NH4F etching can be rationalized through its oxygen-scavenging function, which stabilizes the surface chemistry by minimizing parasitic oxidation. Dissolved oxygen in the etching solution is known to reoxidize the Si–H surface, generating etch pits that disrupt terrace propagation. The addition of AS mitigates these side reactions by converting dissolved O2 into less reactive species (SO32− + ½O2 + H2O → SO42− + 2H+), thereby maintaining a reductive environment favorable for uniform terrace growth. This mechanism does not necessarily accelerate the intrinsic etch rate of silicon but instead promotes a more spatially synchronized etch front by suppressing localized reoxidation events. The resulting reduction in surface defect density allows a more coherent advancement of terrace formation, as evidenced by AFM observations showing earlier appearance of well-defined terraces (≈8 min with 5% AS in plasma-pretreated samples). Consequently, ammonium sulfite enhances etching efficiency primarily through defect suppression and surface stabilization rather than direct rate enhancement, underscoring the interplay between oxygen management and terrace morphology evolution.
Table 1 compares the effects of pretreatment method and ammonium sulfite concentration on terrace formation kinetics, sustainability, uniformity, and terrace width. Plasma-pretreated surfaces formed terraces faster (10 min) than piranha-pretreated surfaces (12 min), and the addition of 2% or 5% ammonium sulfite further accelerated terrace formation to 8 min in both cases. However, only plasma-pretreated surfaces showed sustainable and uniform terrace growth when ammonium sulfite was present, with the highest uniformity and widest terraces (251 ± 53 pm) observed at 5% ammonium sulfite. In contrast, piranha-pretreated surfaces exhibited poor uniformity and no sustainable terrace growth, even with AS addition, and their terrace widths remained significantly narrower. These results confirm that oxide uniformity and effective oxygen scavenging are essential for producing reproducible, well-ordered, and wide terraces, with plasma pretreatment combined with 5% ammonium sulfite offering the most favorable outcome.

Table 1.
Comparison of initial terrace formation time, sustainability, uniformity, and average width for silicon wafers pre-treated with plasma and piranha, respectively, with and without 2% and 5% ammonium sulfite (AS) additives. Note: Initial terrace formation = first time point with monatomic steps and RMS ≤ 100 pm (mean ± SEM, n ≥ 5 areas from ≥3 wafers). Sustainability = persistence of RMS ≤ 100 pm for ≥10 min after first attainment. Uniformity = qualitative rubric from AFM (Excellent/Good/Average/Poor) based on terrace width CV (<10%/10–20%/20–35%/>35%). Average width = mean terrace width from line profiles (1 μm scans; ≥50 step intervals per map).
By reducing oxygen-induced secondary reactions, ammonium sulfite not only enhances the etching efficiency but also contributes to the early-stage development of well-defined terrace structures, particularly in plasma-pretreated samples. This synergistic interplay between plasma pretreatment, ammonium sulfite concentration, and controlled etching conditions underscores the importance of optimizing chemical and environmental factors to achieve superior surface morphology in silicon processing.
4. Conclusions
In this work, we fabricated atomically flat silicon starting from surfaces prepared by simple cleaning steps. Based on detailed observations from atomic force microscopy (AFM) images, we formulated a compelling hypothesis: a chemically uniform oxide layer on the silicon surface enabled a more uniform and synchronous etching front, thereby accelerating the appearance of well-defined atomic terraces under ammonium fluoride etcgubg, thereby accelerating the formation of well-defined atomic terraces. In contrast, surfaces characterized by heterogeneous oxide compositions, which often contained suboxides with varying chemical reactivities, required prolonged etching to resolve these compositional inconsistencies and to achieve hydrogen-terminated surfaces.
The presence of dissolved oxygen in the etching solution also played a pivotal role in determining surface quality. Dissolved oxygen initiated secondary oxidation reactions during etching, which introduced surface defects or etch pits and consequently delayed the development of the desired terrace morphology. Notably, plasma-pretreated surfaces etched in solutions containing 2% or 5% ammonium sulfite exhibited accelerated terrace formation compared to piranha-pretreated surfaces, highlighting the superior ability of plasma pretreatment to generate a chemically uniform oxide layer that promoted rapid and reproducible surface reorganization.
The concentration of ammonium sulfite was identified as a critical parameter governing terrace morphology. Experimental evidence demonstrated that plasma-pretreated surfaces etched in ammonium fluoride solutions containing 2% or 5% ammonium sulfite developed significantly larger and more expansive terraces than their piranha-pretreated counterparts. Ammonium sulfite acted as an efficient oxygen scavenger, capturing dissolved oxygen and mitigating the formation of etch pits that otherwise compromised terrace smoothness and uniformity. Even with rigorous efforts to purge oxygen by argon bubbling for 15 min prior to etching, trace amounts of oxygen persisted in the solution. The inclusion of ammonium sulfite was therefore indispensable, as it minimized the deleterious effects of residual oxygen, facilitated the creation of smoother and more homogeneous terraces, and promoted early-stage terrace nucleation. Importantly, this additive did not increase the intrinsic chemical etch rate of silicon but rather enabled a more simultaneous and spatially uniform initiation of etching across the surface. By suppressing oxygen-induced secondary reactions and reducing localized reoxidation, ammonium sulfite facilitated coherent terrace propagation, thereby enhancing the reproducibility and efficiency of atomically flat surface formation. It is also necessary to note that we did not quantify dissolved-oxygen dynamics or sulfur residues, and oxygen-scavenging effects are inferred from morphology and prior literature [38,39].
In summary, our work here provided new mechanistic insight into how initial oxide composition and dissolved oxygen collectively governed the kinetics of terrace formation during ammonium fluoride etching of Si(111) surfaces. By systematically comparing plasma- and piranha-pretreated substrates under controlled etching conditions, we demonstrated that plasma pretreatment produced a more chemically uniform oxide layer, enabling faster and more reproducible formation of atomically flat terraces. Furthermore, by tuning the ammonium sulfite concentration as an oxygen scavenger, we identified an effective strategy to suppress oxygen-induced surface defects, such as etch pits, and to promote early-stage terrace nucleation. These findings advance the fundamental understanding of surface preparation and etching chemistry while also offering a practical framework for achieving reproducibly smooth, hydrogen-terminated Si(111) surfaces. Such surfaces are crucial for applications in molecular electronics, surface functionalization, and nanoscale device fabrication, where atomic-level control of surface morphology directly impacts performance and reproducibility.
Author Contributions
Conceptualization, Y.L.K.; methodology, P.-M.C.; validation, Y.L.K.; formal analysis, P.-M.C.; investigation, P.-M.C.; resources, Y.L.K.; data curation, P.-M.C.; writing—original draft, P.-M.C. and Y.L.K.; supervision, Y.L.K.; project administration, Y.L.K. All authors have read and agreed to the published version of the manuscript.
Funding
The work was carried out with funding from China Medical University grant (CMU113-MF-82) as well as a grant under the Ministry of Science and Technology in Taiwan (NSTC 114-2221-E-039-015).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Higashi, G.S.; Chabal, Y.J.; Trucks, G.W.; Raghavachari, K. Ideal hydrogen termination of the Si (111) surface. Appl. Phys. Lett. 1990, 56, 656–658. [Google Scholar] [CrossRef]
- Higashi, G.S.; Becker, R.S.; Chabal, Y.J.; Becker, A.J. Comparison of Si(111) surfaces prepared using aqueous solutions of NH4F versus HF. Appl. Phys. Lett. 1991, 58, 1656–1658. [Google Scholar] [CrossRef]
- Sato, Y.; Haze, M.; Nemoto, R.; Qian, W.; Yoshizawa, S.; Uchihashi, T.; Hasegawa, Y. Squeezed Abrikosov-Josephson Vortex in Atomic-Layer Pb Superconductors Formed on Vicinal Si(111) Substrates. Phys. Rev. Lett. 2023, 130, 106002. [Google Scholar] [CrossRef]
- Jakob, P.; Chabal, Y.J. Chemical etching of vicinal Si(111): Dependence of the surface structure and the hydrogen termination on the pH of the etching solutions. J. Chem. Phys. 1991, 95, 2897–2909. [Google Scholar] [CrossRef]
- Bae, S.-E.; Youn, Y.-S.; Lee, C.-W. Potential Dependence of Electrochemical Etching Reaction of Si(111) Surface in a Fluoride Solution Studied by Electrochemical and Scanning Tunneling Microscopic Techniques. J. Electrochem. Sci. Technol. 2020, 11, 330–335. [Google Scholar] [CrossRef]
- Lyu, X.; Macgregor, M.; Darwish, N.; Ciampi, S. Silicon-based tribovoltaic nanogenerators: Surface chemistry isotope effect on device performance and durability. Friction 2025, 13, 9440939–94409391. [Google Scholar] [CrossRef]
- Dumas, P.; Chabal, Y.J. Electron-energy-loss characterization of the H-terminated Si(111) and Si(100) surfaces obtained by etching in NH4F. Chem. Phys. Lett. 1991, 181, 537–543. [Google Scholar] [CrossRef]
- Dumas, P.; Chabal, Y.J. Electron energy loss spectroscopy of H-terminated Si(111) and Si(100) prepared by chemical etching. J. Vac. Sci. Technol. A 1992, 10, 2160–2165. [Google Scholar] [CrossRef]
- DeBenedetti, W.J.I.; Li, T.L.; Hines, M.A. Half-flat vs. atomically flat: Alkyl monolayers on morphologically controlled Si(100) and Si(111) have very similar structure, density, and chemical stability. J. Chem. Phys. 2016, 146, 052804. [Google Scholar] [CrossRef]
- Dumas, P.; Chabal, Y.J.; Jakob, P. Morphology of hydrogen-terminated Si(111) and Si(100) surfaces upon etching in HF and buffered-HF solutions. Surf. Sci. 1992, 269–270, 867–878. [Google Scholar] [CrossRef]
- Li, X.; Teramoto, A.; Suwa, T.; Kuroda, R.; Sugawa, S.; Ohmi, T. Formation speed of atomically flat surface on Si (100) in ultra-pure argon. Microelectron. Eng. 2011, 88, 3133–3139. [Google Scholar] [CrossRef]
- Teramoto, A. Evaluation of Low-Frequency Noise in MOSFETs Used as a Key Component in Semiconductor Memory Devices. Electronics 2021, 10, 1759. [Google Scholar] [CrossRef]
- Goto, T.; Kuroda, R.; Akagawa, N.; Suwa, T.; Teramoto, A.; Li, X.; Obara, T.; Kimoto, D.; Sugawa, S.; Ohmi, T.; et al. Atomically flattening of Si surface of silicon on insulator and isolation-patterned wafers. Jpn. J. Appl. Phys. 2015, 54, 04DA04. [Google Scholar] [CrossRef]
- Kuroda, R.; Suwa, T.; Teramoto, A.; Hasebe, R.; Sugawa, S.; Ohmi, T. Atomically Flat Silicon Surface and Silicon/Insulator Interface Formation Technologies for (100) Surface Orientation Large-Diameter Wafers Introducing High Performance and Low-Noise Metal–Insulator–Silicon FETs. IEEE Trans. Electron Devices 2009, 56, 291–298. [Google Scholar] [CrossRef]
- Mori, K.; Samata, S.; Mitsugi, N.; Teramoto, A.; Kuroda, R.; Suwa, T.; Hashimoto, K.; Sugawa, S. Influence of silicon wafer surface roughness on semiconductor device characteristics. Jpn. J. Appl. Phys. 2020, 59, SMMB06. [Google Scholar] [CrossRef]
- Hsiao, G.S.; Virtanen, J.A.; Penner, R.M. Scanning tunneling microscopy investigations of the Si(111) topography produced by etching in 40% NH4F: Observation of an optimum etch duration. Appl. Phys. Lett. 1993, 63, 1119–1121. [Google Scholar] [CrossRef]
- Allongue, P.; Henry de Villeneuve, C.; Morin, S.; Boukherroub, R.; Wayner, D.D.M. The preparation of flat H–Si(111) surfaces in 40% NH4F revisited. Electrochim. Acta 2000, 45, 4591–4598. [Google Scholar] [CrossRef]
- Sawada, Y.; Tsujino, K.; Matsumura, M. Hydrogen Evolution from Atomically Flat Si(111) Surfaces Exposed to 40% NH4F, Oxygen-Free Water, or Wet Gas. J. Electrochem. Soc. 2006, 153, C854. [Google Scholar] [CrossRef]
- Bae, S.-E.; Yoon, J.-H.; Lee, C.-W.J.; Jeon, I.C. In situ EC-STM studies of n-Si(111):H in 40% NH4F solution at pH 10. Electrochim. Acta 2008, 53, 6178–6183. [Google Scholar] [CrossRef]
- Bae, S.-E.; Oh, M.-K.; Min, N.-K.; Paek, S.-H.; Hong, S.-I.; Lee, C.-W. Preparation of Atomically Flat Si(111)-H Surfaces in Aqueous Ammonium Fluoride Solutions Investigated by Using Electrochemical, In Situ EC-STM and ATR-FTIR Spectroscopic Methods. Bull. Korean Chem. Soc. 2004, 25, 1822–1828. [Google Scholar] [CrossRef]
- Kaji, K.; Yau, S.L.; Itaya, K. Atomic scale etching processes of n-Si(111) in NH4F solutions: In situ scanning tunneling microscopy. J. Appl. Phys. 1995, 78, 5727–5733. [Google Scholar] [CrossRef]
- Angermann, H.; Rappich, J.; Korte, L.; Sieber, I.; Conrad, E.; Schmidt, M.; Hübener, K.; Polte, J.; Hauschild, J. Wet-chemical passivation of atomically flat and structured silicon substrates for solar cell application. Appl. Surf. Sci. 2008, 254, 3615–3625. [Google Scholar] [CrossRef]
- Bae, S.-E.; Yoon, J.-H.; Lee, C.-W.J. Slow etching of triangular pits on atomically flat monohydride terminated Si(111) surface in 40% NH4F solution. Surf. Sci. 2008, 602, 1185–1190. [Google Scholar] [CrossRef]
- Fukidome, H.; Matsumura, M. Electrochemical study of atomically flattening process of silicon surface in 40% NH4F solution. Appl. Surf. Sci. 1998, 130–132, 146–150. [Google Scholar] [CrossRef]
- Sasahara, Y.; Miyake, Y.; Kumaki, J. Preparation of a Si(111) Atomically Flat Substrate via Wet Etching and Evaluation as an AFM Substrate for Observations of Isolated Chains, Crystals, and Crystallization of Isotactic Poly(methyl methacrylate) at the Molecular Level. Langmuir 2020, 36, 7494–7504. [Google Scholar] [CrossRef]
- Hsiao, Y.-S.; Chen, P.-M.; Khung, Y.L. Thermal reactivity of pyrrole and its methyl derivatives on silicon (111) hydride surfaces. Appl. Surf. Sci. 2023, 613, 156005. [Google Scholar] [CrossRef]
- Rusli, S.; Lee, C.-H.; Wu, P.-C.; Khung, Y.L. The evaluation of DNA aptamer monolayers on silicon surface for the promotion of cell-surface interaction. Surf. Interfaces 2024, 46, 104146. [Google Scholar] [CrossRef]
- Hung, T.-C.; Wu, Y.-C.; Khung, Y.L. The Influence of Structural Changes in Sub-Nanometer Monolayers on Gold Surfaces toward Cell Morphology. Appl. Surf. Sci. 2025, 686, 162151. [Google Scholar] [CrossRef]
- Zida, S.I.; Lin, Y.-D.; Khung, Y.L. Sonochemical Reaction of Bifunctional Molecules on Silicon (111) Hydride Surface. Molecules 2021, 26, 6166. [Google Scholar] [CrossRef]
- Khung, Y.L.; Rusli, S.; Hsiao, Y.-S. Thermal grafting of aniline derivatives to silicon (111) hydride surfaces. Appl. Surf. Sci. 2022, 580, 152257. [Google Scholar] [CrossRef]
- Flidr, J.; Huang, Y.-C.; Newton, T.A.; Hines, M.A. Extracting site-specific reaction rates from steady state surface morphologies: Kinetic Monte Carlo simulations of aqueous Si(111) etching. J. Chem. Phys. 1998, 108, 5542–5553. [Google Scholar] [CrossRef]
- Hidayat, R.; Khumaini, K.; Kim, H.-L.; Chowdhury, T.; Mayangsari, T.R.; Cho, S.; Cho, B.; Park, S.; Jung, J.; Lee, W.-J. Gas-phase etching mechanism of silicon oxide by a mixture of hydrogen fluoride and ammonium fluoride: A density functional theory study. J. Vac. Sci. Technol. A 2023, 41, 032604. [Google Scholar] [CrossRef]
- Kim, B.; Lee, W.; Lim, S. Understanding the contributions of F−, HF, and HF2− to the etching of SiO2 and unveiling the reaction kinetics to represent etching behavior of SiO2 up to pH 5. Appl. Surf. Sci. 2024, 657, 159829. [Google Scholar] [CrossRef]
- Houbertz, R.; Memmert, U.; Behm, R.J. On the potential-dependent etching of Si(111) in aqueous NH4F solution. Surf. Sci. 1998, 396, 198–211. [Google Scholar] [CrossRef]
- Allongue, P.; Kieling, V.; Gerischer, H. Etching mechanism and atomic structure of H-Si(111) surfaces prepared in NH4F. Electrochim. Acta 1995, 40, 1353–1360. [Google Scholar] [CrossRef]
- Hines, M.A. The picture tells the story: Using surface morphology to probe chemical etching reactions. Int. Rev. Phys. Chem. 2001, 20, 645–672. [Google Scholar] [CrossRef]
- Newton, T.A.; Huang, Y.-C.; Lepak, L.A.; Hines, M.A. The site-specific reactivity of isopropanol in aqueous silicon etching: Controlling morphology with surface chemistry. J. Chem. Phys. 1999, 111, 9125–9128. [Google Scholar] [CrossRef]
- Lian, Z.; Zhu, C.; Zhang, S.; Ma, W.; Zhong, Q. Study on the synergistic oxidation of sulfite solution by ozone and oxygen: Kinetics and mechanism. Chem. Eng. Sci. 2021, 242, 116745. [Google Scholar] [CrossRef]
- Peng, J.; Yang, Z.; Lian, P. Kinetics of uncatalyzed oxidation of ammonium sulfite from wet ammonia desulfurization. Int. J. Chem. Kinet. 2023, 55, 238–246. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).