Abstract
Background: Electrodeposition of functional films from polyphenol-containing solutions has emerged as a new field of surface functionalization from bio-sourced molecules. There is, however, almost no knowledge about the chemical structure of such complex films. It is the aim of this research to use the known electrodeposition of films made from catechol and resorcinol, two isomers of dihydroxybenzene, to understand the electrodeposition of a more complex polyphenol, quercetin, which is constituted from a fused catechol and resorcinol moiety. The aim of this article is hence to introduce some methodology in the interpretation of the electrochemical behavior of complex polyphenols starting from their building blocks. Methods: Cyclic voltammetry (CV) is used to deposit films from quercetin and from equimolar blends of catechol and resorcinol on amorphous carbon and gold working electrodes. The main experimental parameter was the potential sweep rate used during the CVs. Results: The CV of quercetin is not the exact sum of the CV of the catechol + resorcinol blends, but the major features are conserved, namely the presence of two main oxidation peaks affiliated to those of catechol and resorcinol but shifted to less anodic potentials. In addition, the anodic electron transfer coefficients of the two oxidation waves of quercetin are higher than those measured in the catechol resorcinol blend. However, film deposition ability is reduced with quercetin compared to catechol + resorcinol blend in probable relationship to steric hindrance occurring during the non-electrochemical crosslinking of the deposit. The quercetin-based films deposited at 10 mV·s−1 on gold electrodes are conformal and display some antioxidant activity.
1. Introduction
The electrochemical deposition of organic films from soluble redox active monomers has become a versatile functionalization tool for conductive materials like metals or graphite-based materials. In particular, the electrodeposition of polyaniline [1], polythiophene [2], or polypyrrole [3] has become an interesting method for developing biosensing platforms or anticorrosive coatings. The possibility of depositing coatings through electrodeposition from phenol-containing solutions has been discovered by accident [4] because the electrochemical detection of iodide in the presence of phenol leads to the passivation of the electrode. This constitutes a drawback in analytical chemistry but an advantage in materials and surface science. It has hence been demonstrated that a catecholamine like dopamine can be electrodeposited [5,6,7,8] to yield a conformal film without the addition of an oxidant in solution. Indeed, the deposition from a dopamine solution in the presence of an oxidant not only leads to film deposition on surfaces but also to the formation of an insoluble and useless precipitate in solution. Phenol [4] and its hydroxylated [9] forms, namely the isomeric catechol [10,11,12], hydroquinone [13] and resorcinol [13], also display an electrochemical behavior, but only catechol and resorcinol lead to film deposition and consequently to electrode passivation due to the insulating character of the deposited films. Moreover, the oxidation of catechol on amorphous carbon electrodes leads to films containing crystalline domains when the cyclic voltammetry scans are performed at 20 mV·s−1 [12]. These findings highlight a strong structure-electrochemical-film deposition relationship. Many polyphenols are indeed made from a combination of different isomers of dihydroxyphenols and may also be tested for their ability to form films under the application of electric fields.
Among those molecules, quercetin (2-(3,4-Dihydroxyphenyl)-3,5,7-trihydroxy-4H-1-benzopyran-4-one) belongs to the family of flavonoids, a subclass of polyphenols [14]. It is a secondary metabolite present in many plants, like in red onions. Since many catecholamines and derivatives of phenol and polyphenols can undergo electrodeposition as thin films, it is of interest to investigate this possibility for quercetin, which can be considered as being made from a catechol and a resorcinol moiety fused together by a B cycle also containing an oxidable -OH group (Scheme 1). It has previously been shown that the interfacial electrochemical behavior of resveratrol, containing resorcinol and a phenol moiety, is dominated by the behavior of resorcinol [15]. This may, however, not be a general finding, and the electrodeposition of an A-B type compound where both A and B are electroactive may completely differ from the sum of the electrochemical behavior of A and B. This is the major question asked in the present investigation, where the electrochemical film deposition of quercetin using cyclic voltammetry will be compared to an equimolar mixture of catechol and resorcinol. The main experimental parameter will be the potential sweep rate at which the cyclic voltammetry experiments are performed.
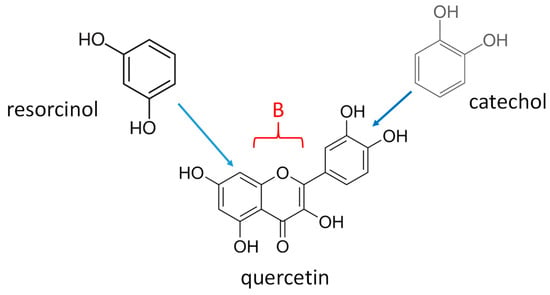
Scheme 1.
Structure of quercetin and its containing dihydroxybenzenes, catechol and resorcinol fused together through the B ring.
The main goal of this article is to provide some experimental basis able to relate the structure of the used polyphenols to their interfacial electrochemical properties. This experimental background is mandatory before addressing this structure-property relationship with theoretical methods like DFT calculations. Quercetin is a primary example for such studies because it is constituted from two different phenol derivatives with an already known electrochemical behavior [10,13].
Overall, it will be shown that the interfacial electrochemical behavior of quercetin reflects what is expected from the presence of its building blocks but with additional complexity related to the presence of the internal B ring.
2. Materials and Methods
2.1. Chemicals
Quercetin (ref. Q4951), catechol (ref. C9510), resorcinol (ref. 398047), tetramethylammonium chloride (ref. T19525) and potassium hexacyanoferrate (P9387) were purchased from Sigma-Aldrich (Saint-Quentin Fallavier, France) and used without further purification. Quercetin, catechol and resorcinol were dissolved in 70% ethanol in the presence of 50 mM N(CH3)4Cl as the supporting electrolyte. Quercetin was freshly dissolved at 0.5 mg/mL before the beginning of each experiment. This corresponds to a molar concentration of 1.6 × 10−3 M. The catechol and resorcinol solutions and their equimolar blend were prepared at the same molar concentration because one quercetin molecule contains one catechol and one resorcinol moiety (Scheme 1). Potassium hexacyanoferrate was dissolved at 1 mM in a 50 mM sodium acetate buffer at pH = 5.0. 2,2-diphyl-1-picrylhydfrazyl (ref. 257621) was used for assessing the antioxidant activity of quercetin-based electrodeposited films and was dissolved at 10−4 mol·L−1 in absolute ethanol.
2.2. Electrodes and Electrode Cleaning
The electrochemical experiments were performed in a three-electrode configuration using a PGSTAT 204 potentiostat (Metrohm, Paris, France). The working electrode, the reference electrode and the counter-electrode were an amorphous carbon (2 mm in diameter, ref. CHI 104 from CHInstruments, Austin, TX, USA), and Ag/AgCl (internal compartment filled with 3 M KCl, ref. CHI 111) and a Platinum wire (ref. CHI 115) respectively. The carbon working electrodes were polished just before each new experiment on a SiC cloth, followed by two alumina slurries with a particle size of 1 and 0.1 µm (Escil, Villeurbanne, France). Each polishing step was separated from the next one with ethanol (70% v/v) and distilled water (Direct-Q®3UV, Millipore, Frankfurt, Germany) washing steps. The working electrode was finally sonicated twice in distilled water for 2 min to remove adhering alumina particles. The influence of these polishing and ultrasonication steps on the working electrode was checked by performing cyclic voltammetry (CV) in the presence of 1 mM potassium hexacyanoferrate dissolved in 50 mM sodium acetate at pH = 5.0. The CV of potassium hexacyanoferrate is of a reversible nature and is a one-electron process: it should yield identical oxidation and reduction waves with peak potentials separated by 2.3RT/F = 59 mV at T = 298 K [16]. The electrode was considered satisfactory when the oxidation and reduction peaks were separated by less than 80 mV with oxidation-reduction currents equal to within 10%.
Some complementary experiments were performed on gold-coated (100 nm in nominal thickness) silica working electrodes cut in a size of 2 cm × 2 cm (ref. 643246, Sigma-Aldrich). Those electrodes were plasma cleaned in an air plasma for 2 min just before use (Harrick PDC plasma cleaner; Ithaca, NY, USA).
2.3. Electrochemical Deposition and Electrochemical Behavior of the Obtained Films
The quercetin solution and the catechol + resorcinol blended solution were freshly prepared before deposition by CV on freshly polished amorphous carbon electrodes. The potential was scanned between −0.5 and 1.2 V vs. Ag/AgCl and back to −0.5 V for at least 10 CV cycles (some experiments were performed up to 25 CV cycles). The potential sweep rate was changed between 5 and 1000 mV·s−1, but the major focus will be given to the experiments performed at 10, 100 and 1000 mV·s−1. After those CV cycles, the electrode was rinsed with the ethanol (70% v/v) containing 50 mM N(CH3)4Cl solution to check for the film capacitance and the presence of an eventual faradic response (manifested by an oxidation and /or reduction peak). To that aim, a CV scan at 100 mV·s−1 was performed and will be called CV1. Finally, the electrode was rinsed with the 50 mM sodium acetate buffer, and a CV (called CV2) was acquired at 100 mV·s−1 in the sodium acetate buffer containing 1 mM potassium hexacyanoferrate. In case of a totally impermeable film to this redox probe, CV1 and CV2 should superimpose.
2.4. Surface Properties of the Quercetin-Based Films
To characterize the quercetin-based films, they were deposited at a potential sweep rate of 10 mV·s−1 but on gold working electrodes (even if it is well known that the electron transfer process between solutes and electrodes is highly dependent on the used electrode) because they are easy to handle and to image by means of atomic force microscopy. The obtained films were rinsed with ethanol (70% v/v) and dried under a stream of filtered air. One of them was then imaged in the air using the ScanAsyst mode with a Bioscope Catalyst instrument (Bruker, Mannheim, Germany). The images (256 × 256 pixels) were acquired at a scan frequency of 0.5 Hz using Si3N4 cantilevers with a spring constant of 0.4 N·m−1 (as provided by the furnisher). The obtained topographies were flattened and analyzed for their RMS roughness using the Gwyddion 2.64 (GNU, Boston, MA, USA) software.
The same kind of quercetin-based films deposited on gold working electrodes were evaluated for their antioxidant properties. To that aim, 1 mL of a DPPH solution at 10−4 mol·L−1 (in absolute ethanol) was deposited on the film-coated gold electrode. This volume was the minimum required to totally cover the whole surface of the electrode. After 40 min of contact with the quercetin-based film, the DPPH solution was removed, and its absorbance spectrum was measured between 400 and 600 nm using an Mc2 spectrophotometer (Safas, Monte Carlo, Monaco) with DPPH at 10−4 mol·L−1 in the reference cuvette. In the presence of an antioxidant, the DPPH solution undergoes discoloration, which is manifested by a reduction in the absorbance at 517 nm [17].
Some of the quercetin film-covered electrodes were also immersed in organic solvents like dimethylsulfoxide (DMSO), dichloromethane and N,N-dimethylacetamide during 1 h to evaluate (qualitatively) their solubility in such organic solvents.
3. Results
3.1. Deposition by CV
The CV curves of quercetin were compared to those of an equimolar blend of catechol and resorcinol at different potential sweep rates (Figure 1 and Figure S1 in the Supplementary Materials). The CVs of the catechol + resorcinol blends were also compared to those of catechol and resorcinol alone (at the same concentration as in the blend) and to the CV calculated by the sum of the CVs of the two used molecules (Figure 2).
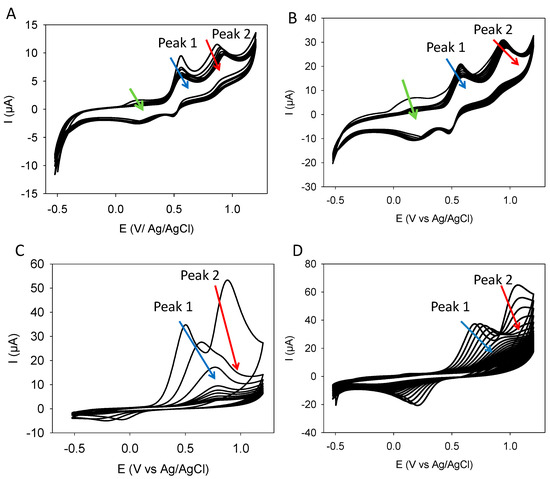
Figure 1.
CV curves of quercetin (panels (A,B)) and of equimolar catechol + resorcinol blends (panels (C,D)) at different potential sweep rates (10 mV·s−1 in panels (A,C) and 100 mV·s−1 in panels (B,D)). The arrows indicate the evolution of the oxidation peaks with the number of performed CV cycles.
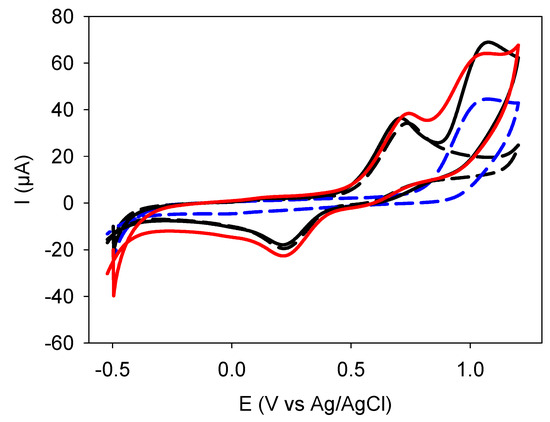
Figure 2.
Comparison of the CV curves performed at 100 mV·s−1 of catechol (black dashed line _ _ _), resorcinol (blue dashed line: _ _ _) and of their equimolar blend (black full line ____). The red full line (____) corresponds to the algebraic sum of the CV of catechol and resorcinol.
First, it appears that quercetin is characterized by the presence of 3 oxidation peaks, among them a first one (green arrows in Figure 1A,B) at around +0.2 V vs. Ag/AgCl, which is not observed in catechol, resorcinol, and the CVs obtained from their blends (Figure 1C,D). However, the two oxidation peaks of quercetin detected at around +0.5–0.6 V and +0.7–0.8 V are close to the oxidation peaks detected in the blend. Those peaks will now be called Peak 1 and Peak 2. Inspection of Figure 2 allows us to attribute Peak 1 and Peak 2 to catechol and resorcinol, respectively. However, these two peaks in quercetin do not exactly occur at the same potential as in the catechol + resorcinol blend: they are shifted to slightly lower potentials (about 0.1 V, as will be detailed later), suggesting that the oxidation of the catechol and resorcinol moieties in quercetin are facilitated in the fused quercetin structure. The first peak (labeled with a green arrow in the CVs of quercetin may well originate from the oxidation of the OH group in the central B ring of quercetin (Scheme 1). No further focus will be given to this peak since it rapidly vanished at all the investigated potential sweep rates (Figure 1A,B and Figure S1A in the Supplementary Materials). The intensity of Peaks 1 and 2 also decreases with the number of performed CV cycles but differently with quercetin and the catechol + resorcinol blend and in a potential sweep-rate-dependent manner.
The presence of a supplementary oxidation peak in quercetin, as well as the different evolutions of Peak 1 and Peak 2, is sufficient to demonstrate that the electrochemical behavior of catechol and resorcinol fused together in a flavonoid is not simply the sum of the catechol and resorcinol behavior which was more or less expected but not yet demonstrated. Note also that the first CV cycle of the catechol + resorcinol blend is also not exactly the sum of the CVs of catechol and resorcinol, particularly in the potential window between Peak 1 and Peak 2, where the theoretical CV obtained from the summation of the CV of the two selected molecules (red line in Figure 2) yields to a higher current than the experimental one (black full line in Figure 2). This means that when catechol starts to be oxidized and to undergo non electrochemical crosslinking at the electrode surface, it hinders the further deposition of resorcinol occurring at higher potentials. Note that the electrodeposition of catechol-based films [11,12] and resorcinol-based films [13] on amorphous carbon electrodes has been investigated previously.
When considering the evolution of the positions of Peak 1 and Peak 2 in quercetin and in the catechol + resorcinol blend, it appears visually that those peaks are anodically shifted upon faster potential sweep rates as expected for irreversible electrochemical processes (i.e., processes where the reduction current is different from the oxidation current due to the non-electrochemical transformation of the oxidation products) [18]. Note, however, that even if Peak 2 (affiliated to the oxidation of resorcinol) is of irreversible nature, this is not exactly true for Peak 1 (affiliated to the oxidation of catechol), which appears partially reversible, the more so, the higher the potential sweep rate (compare Figure 1C with Figure 1D and with Figure S1B in the Supplementary Materials). When plotting the oxidation peak potentials during the first CV cycle as a function of the logarithm of the potential sweep rate (v), the value of the electron transfer coefficients, a, are obtained according to [18]:
where T is the absolute temperature, and F is the Faraday constant.
If Equation (1) is applicable, the plots of the two peak potentials against lnv should yield a straight line. This is indeed the case (Figure 3) with fairly good linear regression coefficients. The slope of this straight line is inversely proportional to the electron transfer coefficient and the obtained values are gathered in Table 1. The electron transfer coefficients attributed to the two peaks of quercetin are higher than those attributed to the two peaks in the catechol + resorcinol blend, suggesting that the central B ring of quercetin promotes the electron transfer of the catechol and resorcinol moiety to the amorphous carbon electrode.
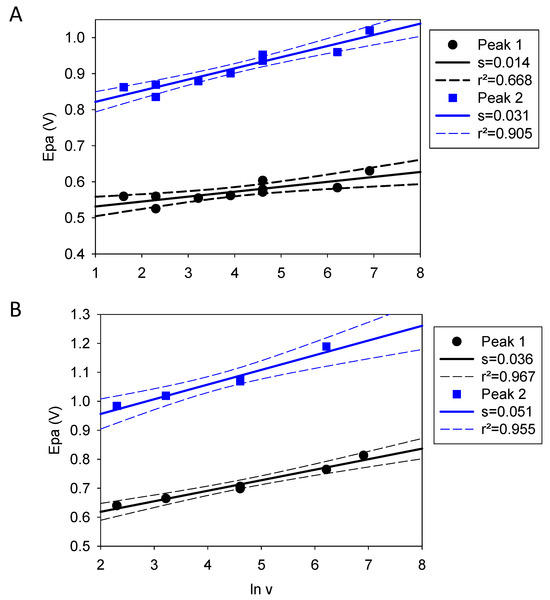
Figure 3.
Evolution of the two peak potentials of quercetin (panel (A)) and the catechol + resorcinol blend (panel (B)) as a function of the logarithm of the potential sweep rate. The black and blue symbols correspond to Peaks 1 and 2, respectively. The full and dashed lines correspond to the linear regression to the experimental data and to the limits of the 95% confidence intervals respectively.

Table 1.
Anodic electron transfer coefficients (a) for the two oxidation peaks of quercetin and of the catechol + resorcinol blend, as determined from the slopes of the straight lines in Figure 3 and using Equation (1).
The electron transfer coefficients obtained herein are higher than those reported by Nady et al. [19] for phenol, resorcinol and pyrogallol (between 0.146 and 0.431), suggesting a slower electron transfer than in these three systems. However, the experiments were performed in different conditions, such as a Pt working electrode at pH = 3.0.
According to Figure 1, the oxidation peaks and the oxidation peak currents undergo some shifts and some changes in intensity from cycle to cycle and in a potential sweep-rate-dependent manner. The data taken from Figure 1 are given in Figure 4 for the oxidation peak potentials and in Figure 5 for the oxidation peak currents. Quercetin is distinguished from the catechol + resorcinol blend in the sense that the two oxidation peak potentials only moderately increase from cycle to cycle (by about 20 mV between the first and the tenth cycles) when the CVs are performed at 10 and 100 mV−1. Surprisingly, at 1000 mV·s−1, the positions of Peaks 1 and 2 decrease upon an increase in the number of CV cycles, suggesting a facilitated oxidation process (Figure 4A,B). However, the peak positions of the catechol + resorcinol blend increase by about 300 mV for peak 1 for CVs performed at 10 mV·s−1 and by about 150–200 mV for CVs performed at 100 and 1000 mV·s−1 (Figure 4C). From Figure 4D, it can also be seen that the peak position of resorcinol (peak 2) saturates at 1.2 V for all the CV cycles. This comes from the fact that the potential was scanned only up to +1.2 V vs. Ag/AgCl because this corresponds to the limit of the electrochemical stability window of water. The resorcinol peak also totally disappears (as also seen in Figure 1C) after the first CV cycle performed at 10 mV·s−1 in agreement with previous findings [13].
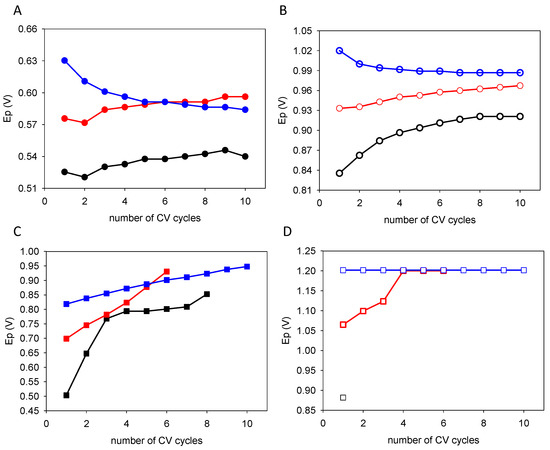
Figure 4.
Evolution of the peak potentials as a function of the number of performed CV cycles for quercetin (panels (A,B)) and for the equimolar catechol + resorcinol blends (panels (C,D)). The full symbols correspond to the first oxidation peak (panels (A,C)), whereas the open symbols correspond to the second oxidation peak (panels (B,D)). The black, red, and blue symbols correspond to experiments performed at 10, 100 and 1000 mV·s−1, respectively. The full lines (of the same color) are aimed to guide the eye.
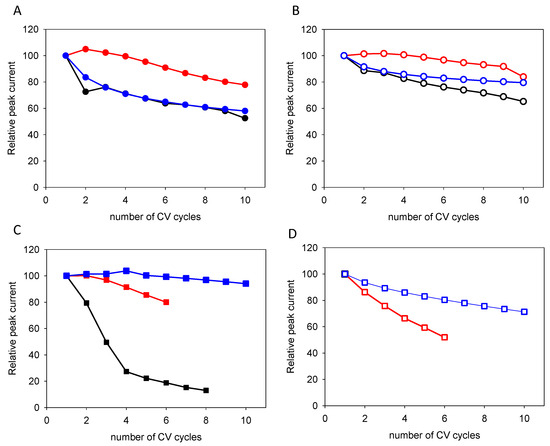
Figure 5.
Evolution of the relative peak currents as a function of the number of performed CV cycles for quercetin (panels (A,B)) and for the equimolar catechol + resorcinol blends (panels (C,D)). The full symbols correspond to the first oxidation peak (panels (A,C)), whereas the open symbols correspond to the second oxidation peak (panels (B,D)). The black, red and blue symbols correspond to experiments performed at 10, 100 and 1000 mV·s−1, respectively. The full lines (of the same color) are aimed to guide the eye and the arrows indicate the trend upon an increase in the potential sweep rate.
Concerning the peak currents, a potential sweep rate dependent decrease is observed (Figure 5) but is particularly marked in the case of the catechol + resorcinol blend when the CVs are performed at 10 mV·s−1 (Figure 5C,D) as was already apparent from Figure 1C. This current decrease is due to electrode passivation by a deposited film. In this case, the coatings should progressively become impermeable to a redox probe like potassium hexacyanoferrate.
3.2. Permeability and Film Properties of the Quercetin Based Films
The coatings made from resorcinol and catechol alone have been investigated for their permeability to potassium hexacyanoferrate in previous investigations: the coatings made from the electrodeposition of catechol become impermeable to this negatively charged redox probe after a few CV cycles, the faster this occurs, the slower the potential sweep rate during the CV cycles is [11]. However, the coating made from resorcinol becomes conformal and impermeable after a very small number of CV cycles, namely from 1 to 3 cycles [13], reflecting the efficient film-forming properties of this molecule. The blends behave similarly to resorcinol (Figure S2 in the Supplementary Materials), whereas quercetin-based coatings become impermeable to the redox probe (after 10 CV cycles) only at low potential sweep rates of 10 mV·s−1 (Figure 6A) some important residual permeability remaining when the films are deposited at higher potential sweep rates (Figure 6B).
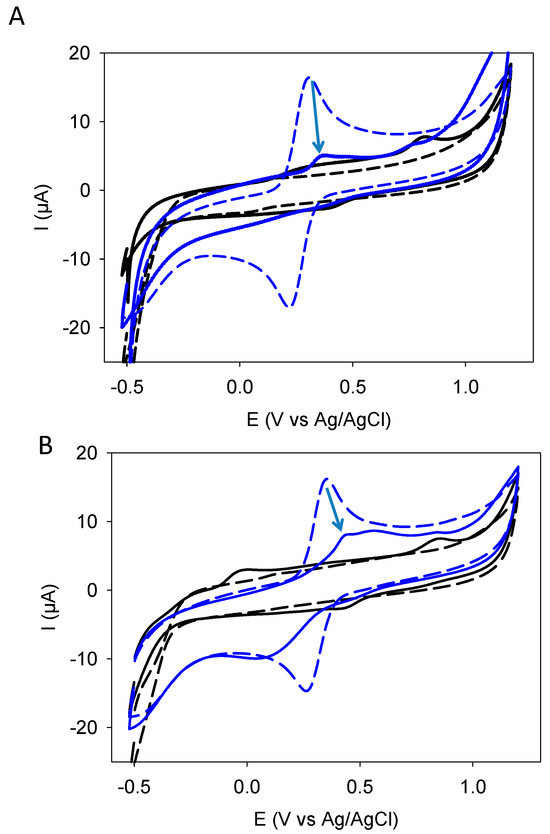
Figure 6.
Comparison of CV1 (_____ black full line, measured after film deposition but in the absence of the redox probe) and CV2 (_____ blue full line, measured after film deposition but in the presence of 1 mM potassium hexacyanoferrate in the presence of 50 mM sodium acetate at pH = 5) in the case of film deposition (10 CV cycles) performed at 10 mV·s−1 (panel (A)) and at 100 mV·s−1 (panel (B)). The arrows indicate the decrease in the oxidation current of potassium hexacyanoferrate between the pristine electrode (_ _ _ blue dashed line) and after film deposition (CV2). The black dashed lines (_ _ _) correspond to CVs measured in the absence of a redox probe on the pristine and polished electrode.
Hence, the film-forming ability of quercetin is efficient in the sense of getting impermeable films when the deposition by CV is performed at sufficiently low potential sweep rates. In this sense, quercetin behaves as other polyphenols, like catechol, for which the film-forming ability is the combination of electrode-triggered oxidation followed by subsequent non-electrochemical reactions of adsorbed and oxidized species, probably a kind of radical crosslinking as suggested from electrodeposition experiments performed at different pH values [11].
Since the film-forming ability of quercetin on amorphous carbon is more efficient at 10 mV·s−1 than at higher potential sweep rates, we also performed CV deposition on gold working electrodes in these conditions (Figure S3 in the Supplementary Materials) in order to characterize the film morphology by means of AFM (Figure 7). When compared to the pristine gold electrode, the quercetin-based deposit (10 CV cycles at 10 mV·s−1) appears conformal and grainy with a root mean squared roughness of 3.3 nm for an average film thickness of 9.5 ± 0.5 nm as obtained by scanning the AFM tip through a needle-induced scratch on the film. The AFM surface topographies of catechol and resorcinol-based films deposited under the same conditions (10 CV cycles performed at 10 mV·s−1) on gold electrodes are displayed in Figure S4 in the Supplementary Materials. They are much rougher than the quercetin-based film, with RMS roughness values of 31 and 14 nm for the catechol and resorcinol-based coatings. In addition, the catechol-based coating contains large grains or domains in the µm size domain in addition to smaller particles, whereas the resorcinol-based film is more homogeneous, displaying only particles in the 100 nm range or less, as on the quercetin-based coating. Hence, the surface morphology of the quercetin-based electrodeposited films seems to be similar to that of the resorcinol-based films. Note, however, that the AFM topographies displayed in Figure 7 and in Figure S4 of the Supplementary Materials were not acquired in the same size range: 10 µm × 10 µm for quercetin film and 4 µm × 4 µm for the films made from the catechol and resorcinol building blocks. This should, however, not change the global interpretation proposed here.
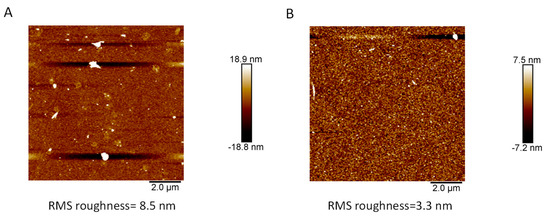
Figure 7.
AFM surface topography of the pristine gold electrode (panel (A)) and of a quercetin-based film (panel (B)) obtained on a gold electrode after 10 CV cycles performed at 10 mV·s−1.
The solubility of the quercetin-based films deposited on gold electrodes was investigated by immerging them for 1 h in organic solvents of various polarity, namely DMSO, ethanol, dichloromethane, N,N-dimethylformamide. No apparent solubilization was observed in all cases because the gold-coated electrode kept its brownish appearance.
In addition, the film deposited after 10 CV cycles on a 2 × 2 cm2 gold electrode (10 mV·s−1) was immersed in 1 mL of a DPPH solution at 10−4 mol·L−1. After 40 min of reaction, the absorbance of the DPPH solution was measured against the original DPPH solution (present in the reference cuvette of the spectrophotometer) and was found to be decreased by 0.18 absorbance units. This value was used to calculate the equivalent concentration of quercetin, leading to the same quenching of DPPH. To that aim, a calibration curve of DPPH quenching by ethanol-dissolved quercetin was used (A = −9 × 10−4 − 65.7C, r2 = 0.995, 6 points in the calibration curve, C being the quercetin concentration in mg·mL−1) and yielded an equivalent antioxidant concentration of 2.8 × 10−3 mg·mL−1 or 9.3 × 10−6 mol·L−1 on the electrodeposited quercetin film.
4. Discussion
The electrodeposition of quercetin, in which catechol and resorcinol are fused together with an electroactive B ring, was compared to the electrodeposition of an equimolar catechol + resorcinol blend. The oxidation of the B ring in quercetin produced a weak current and was measured only for a limited number of CV cycles. The CV of quercetin is dominated by two oxidation peaks appearing at slightly lower potentials (about 0.1 V, see Figure 3) than the oxidation peak of catechol and resorcinol measured alone and in their blend. This strongly suggests that fusing the two isomers of benzenediol together facilitates their oxidation. This is not surprising because all the C=C double bonds in quercetin are conjugated (Scheme 1), and this can explain that the probable radical species formed during the oxidation steps are stabilized by resonance. From a semi-quantitative point of view, the anodic electron transfer coefficients of quercetin are higher than those of catechol and resorcinol measured in their blend (Table 1), which is in agreement with the finding that the oxidation of the catechol and resorcinol moieties in quercetin requires less energy (occurs at lower potentials) than for the same chemicals in their blend.
On the other hand, the passivation of the electrode with a film is more efficient for the catechol + resorcinol blend than for quercetin. Indeed, after 10 CV deposition cycles, the quercetin-based films are only impermeable to the hexacyanoferrate anionic redox probe when the deposition is performed at 10 mV·s−1 (Figure 6A). When the deposition is performed at 100 mV·s−1 the film is highly permeable and not conformal but island-like. However, concerning the catechol + resorcinol blend, the film obtained after 10 CV cycles is almost totally impermeable to the same redox probe even when the deposition is performed at a potential sweep rate as high as 1000 mV·s−1 (Figure S2 in the Supplementary Materials). These findings suggest that even if the catechol and resorcinol moieties of quercetin are easier to oxidize than catechol and resorcinol, the film-forming ability from quercetin is hindered probably by steric hindrance effects. Indeed, when a smaller resorcinol molecule is oxidized, it is easier for it to find a close and well-oriented partner at the interface to form a dimer than when quercetin is oxidized. In addition, in the case of quercetin, it is not yet known if dimer formation occurs through preferential resorcinol-resorcinol binding or through resorcinol-catechol binding. In the former case, the oligomerization of quercetin should be limited to dimers, whereas in the latter case, longer oligomers should be formed. Future structural characterizations should address this point.
The easier film-forming ability of the catechol + resorcinol blend when compared to quercetin is also in agreement with the faster current decrease from cycle to cycle in the mixture (Figure 5). The same trend holds true when considering the evolution of the oxidation peaks from cycle to cycle (Figure 4): a marked increase in these peak potentials is found for the blend (Figure 4C,D) when compared to quercetin (Figure 4A,B).
In forthcoming investigations, the structure of films made from complex polyphenols like quercetin needs to be investigated thoroughly by combining transmission electron microscopy, X-ray photoelectron spectroscopy, FTIR and Raman spectroscopy. Previous research showed that this is a particularly challenging task [12]. This study allowed us to show the possibility of depositing conformal coatings on carbon and gold electrodes from a flavonoid like quercetin using cyclic voltammetry. It will be extended to other flavonoids like catechin, epicatechin, etc., on gold or other metal electrodes to allow deeper spectroscopic characterization of the eventually obtained coatings, notably using Raman, Fourier transformed infrared spectroscopy and X-ray photoelectron spectroscopy. The final aim will be to find structure-properties relationships for such 2D materials made from molecules available from plant extracts using green chemistry methods.
5. Conclusions
In this report, it was shown that the CVs of quercetin made from catechol and a resorcinol electroactive unit fused together with a B cycle in a flavonoid structure are not strictly identical to those of a catechol and resorcinol blend. This is not surprising because the global structure of quercetin is highly conjugated and renders the catechol and resorcinol moiety not independent from each other. Nevertheless, the electrochemical behavior of quercetin keeps the main signature of catechol and resorcinol, with two main oxidation peaks attributed to catechol and resorcinol. On the one hand, the oxidation process is facilitated in the fused quercetin structure, but on the other hand, the film-forming ability is lower, probably due to steric hindrance occurring during the non-electrochemical reactions following the oxidation step in a kind of EC (electrochemical-chemical) process.
When the CVs of quercetin are performed at 10 mV·s−1 (and also at lower potential sweep rates), conformal films in the few nanometers thickness range are obtained on gold working electrodes and those coatings exhibit an interesting antioxidant behavior by quenching (and discoloring) the stable DPPH radical. This suggests that not all the OH groups of quercetin are oxidized in quinones, which are not antioxidants. Of course, this assumption needs to be confronted with the data obtained from X-ray photoelectron spectroscopy.
This investigation paves the way for future investigations on the electrochemical interfacial behavior of complex polyphenols, having acquired semi-quantitative data on their building blocks. In the future, DFT calculations should also be undertaken to allow us to make assumptions on the crosslinking network of such complex films, which are insoluble in most common organic solvents. This is a major advantage to get stable and robust coatings but is redhibitory for detailed structural investigations.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/surfaces7030042/s1, Figure S1: CV curves of quercetin and of an equimolar catechol + resorcinol blend at a potential sweep rate of 1000 mV·s−1; Figure S2: permeability toward hexacyanoferrate of a film made after 10 CV cycles from a catechol + resorcinol blend at a potential sweep rate of 1000 mV·s−1; Figure S3: CV deposition (10 mV·s−1) of quercetin on a gold electrode. Figure S4: surface topographies of films deposited from catechol and resorcinol (10 cycles performed at 10 mV·s−1).
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The author is ready to provide additional data (CV curves at potential sweep rates different from 10, 100 or 1000 mV·s−1) on request.
Conflicts of Interest
The author declares no conflicts of interest.
References
- Musiani, M.M.; Mengoli, G.; Furlanetto, F. Improved polyaniline coatings by electropolymerization. J. Appl. Polym. Sci. 1984, 29, 4433–4438. [Google Scholar] [CrossRef]
- Wang, X.; Shi, G.; Liang, Y. Low potential electropolymerization of thiophene at a copper oxide electrode. Electrochem. Comm. 1999, 1, 536–539. [Google Scholar] [CrossRef]
- Saidman, S.B.; Vela, M.E. Electroplymerization of pyrrole onto aluminium from alkaline solutions containing a surfactant. Thin Solid Films 2005, 493, 96–103. [Google Scholar] [CrossRef]
- Bejerano, T.; Forgacs, C.; Gileadi, E. Selective inhibition of electrode reactions by organic compounds. Electroanal. Chem. Interf. Electrochem. 1970, 2, 69–79. [Google Scholar] [CrossRef]
- Li, S.; Wang, H.; Young, M.; Xu, F.; Cheng, G.; Cong, H. Properties of electropolymerized dopamine and its analogues. Langmuir 2019, 35, 1119–1125. [Google Scholar] [CrossRef] [PubMed]
- Eom, T.; Lee, J.; Lee, S.; Ozlu, B.; Kim, S.; Martin, D.C.; Shim, B.S. Highly conductive polydopamine coatings by direct electrochemical synthesis on Au. ACS Appl. Polym. Mater. 2022, 4, 5319–5329. [Google Scholar] [CrossRef]
- Szewczyk, J.; Aguilar-Ferrer, D.; Coy, E. Polydopamine films: Electrochemical growth and sensing applications. Eur. Polym. J. 2022, 174, 111346. [Google Scholar] [CrossRef]
- Schindler, S.; Bechtold, T. Mechanistic insights into the electrochemical oxidation of dopamine by cyclic voltammetry. J. Electroanal. Chem. 2019, 836, 94–101. [Google Scholar] [CrossRef]
- Huynh, M.T.; Anson, C.W.; Cavell, A.C.; Stahl, S.S.; Hammes-Schiffer, S. Quinone 1e− and 2e−/2H+ reduction potentials: Identification and analysis of deviations from systematic scalling relationships. J. Am. Chem. Soc. 2016, 138, 15903–15910. [Google Scholar] [CrossRef] [PubMed]
- Enache, T.A.; Oliveira-Brett, A.M. Phenol and para-substituted phenols electrochemical oxidation pathways. J. Electroanal. Chem. 2011, 655, 9–16. [Google Scholar] [CrossRef]
- Barham, A.S.; Kennedy, B.M.; Cunnane, V.J.; Daous, M.A. The electrochemical polymerization of 1,2-dihydroxybenzee and 1,2-dihydroxybenzene alcohol prepared in different solution media. Electrochim. Acta 2014, 147, 19–24. [Google Scholar] [CrossRef]
- Ortiz Peña, N.; Ihiawakrim, D.; Ball, V.; Stanescu, S.; Rastei, M.; Sanchez, C.; Portehault, D.; Ersen, O. Correlative microscopy insight on electrodeposited ultrathin graphite oxide films. Phys. Chem. Lett. 2020, 11, 9117–9122. [Google Scholar] [CrossRef]
- Ball, V.; Alfieri, M.L.; Ziegler, K.; Arntz, Y.; d’Ischia, M. Structure-controlled electrodeposition and electrochemical behavior of films from isomeric diphenols at the solid-liquid interface. Surf. Interf. 2022, 30, 101841. [Google Scholar] [CrossRef]
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouységu, L. Plant polyphenols: Chemical properties, biological activities, and synthesis. Angew. Chem. Int. Ed. 2011, 50, 586–621. [Google Scholar] [CrossRef]
- Ball, V. Electrodeposition of antioxidant films from resveratrol. Colloids Surf. A Physicochem. Eng. Asp. 2024, 686, 133374. [Google Scholar] [CrossRef]
- Kissinger, P.T.; Heineman, W.R. Cyclic voltammetry. J. Chem. Educ. 1983, 60, 702–706. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxydant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Gileadi, E. Physical Electrochemistry: Fundamentals, Techniques and Applications; Wiley-VCH: Weinheim, Germany, 2011; Chapters 15 and 16. [Google Scholar]
- Nady, H.; El-Rabiei, M.M.; Abd El-Hafez, G.M. Electrochemical Oxidation Behavior of Some Hazardous Phenolic Compounds in Acidic Solution. Egypt. J. Pet. 2017, 26, 669–678. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).