Selective Chemical Filters for VOF3: Tailoring MgF2 Filter Selectivity through Surface Chemistry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Filtering Capacities
2.3. Fluorination
2.4. Characterization
2.4.1. X-ray Powder Diffraction
2.4.2. Adsorption/Desorption Isotherms
2.4.3. Raman Spectroscopy
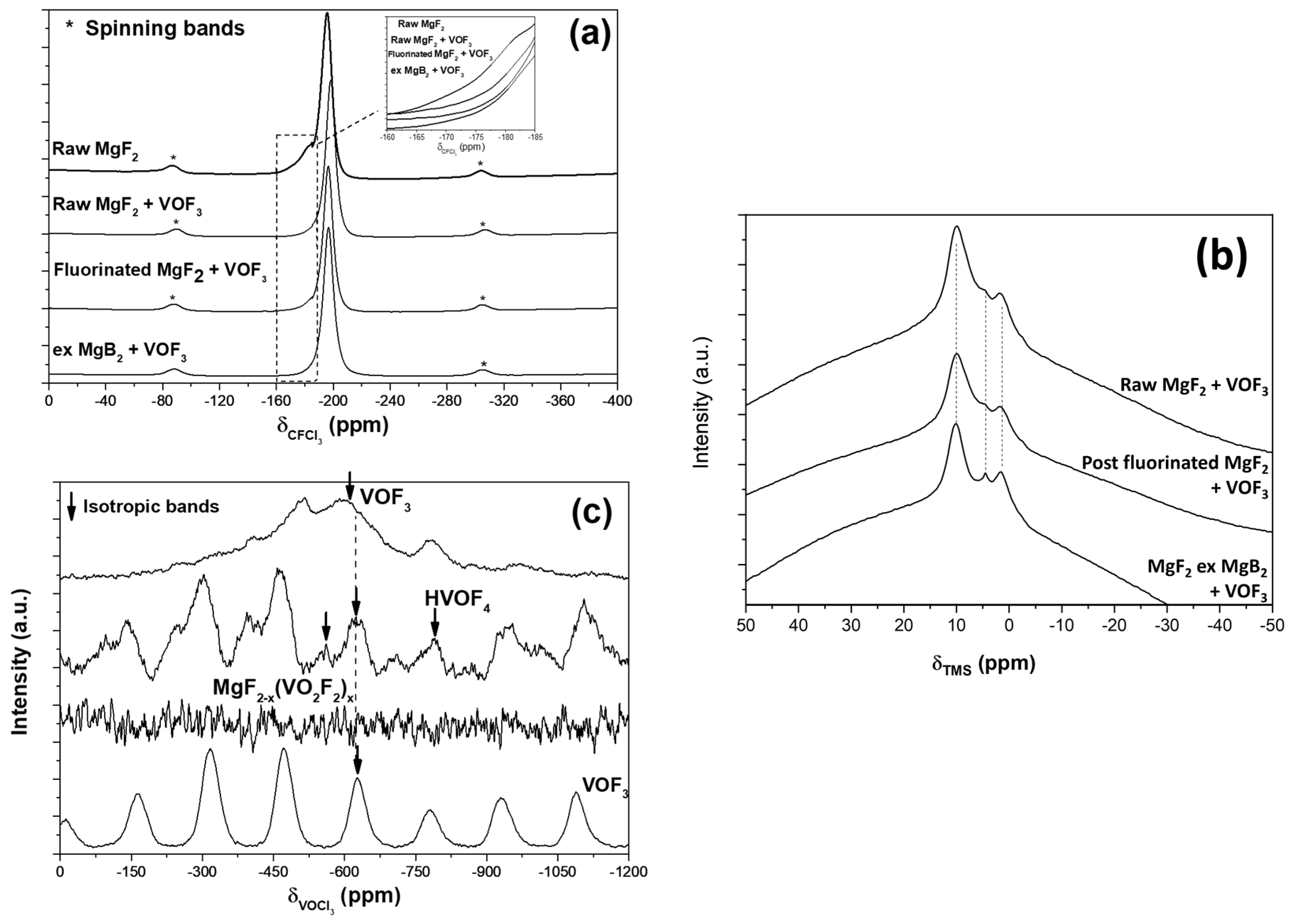
2.4.4. NMR Spectroscopy
3. Results and Discussion
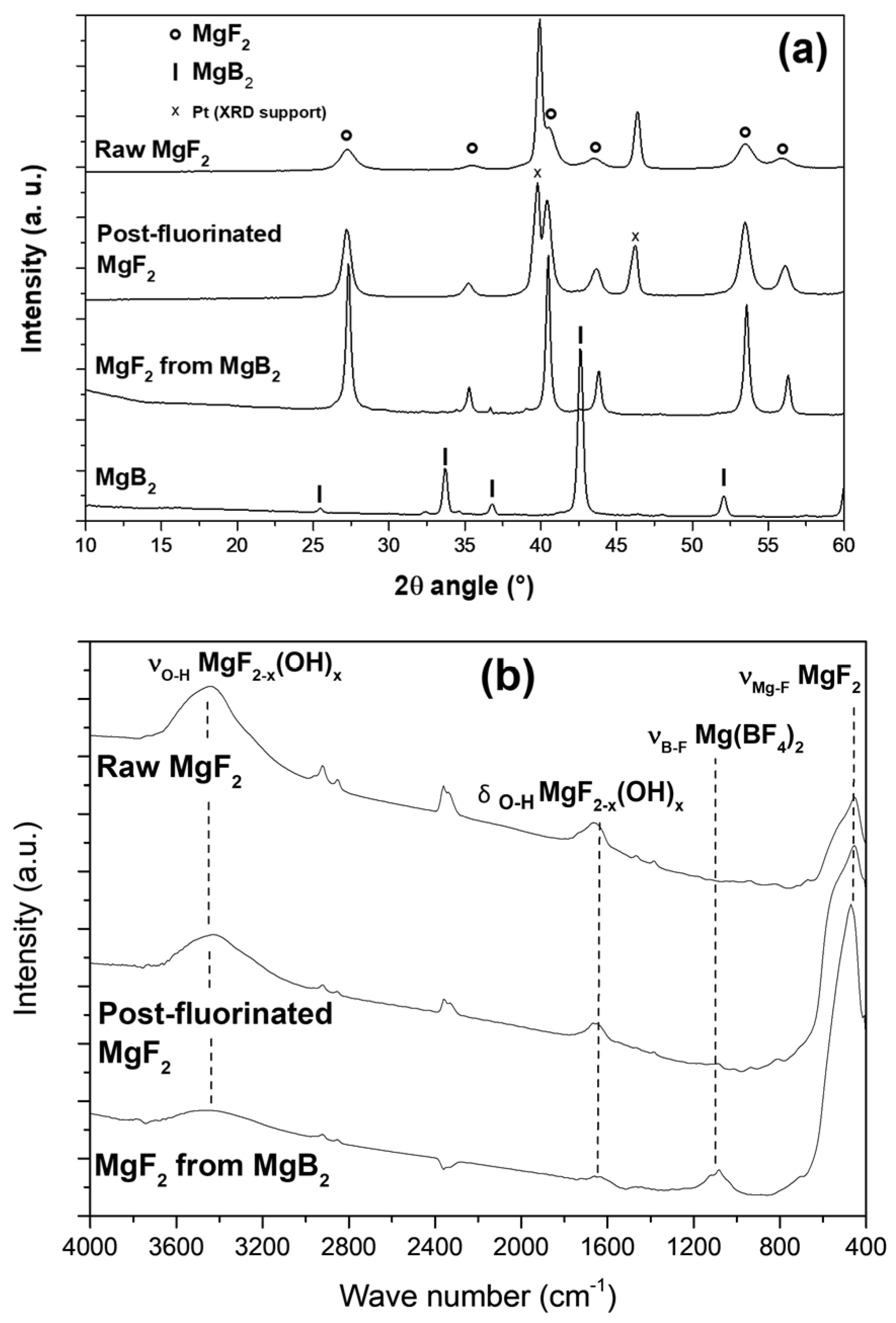
3.1. Fluorination to Tailor OH/F Ratio in MgF2−x(OH)x
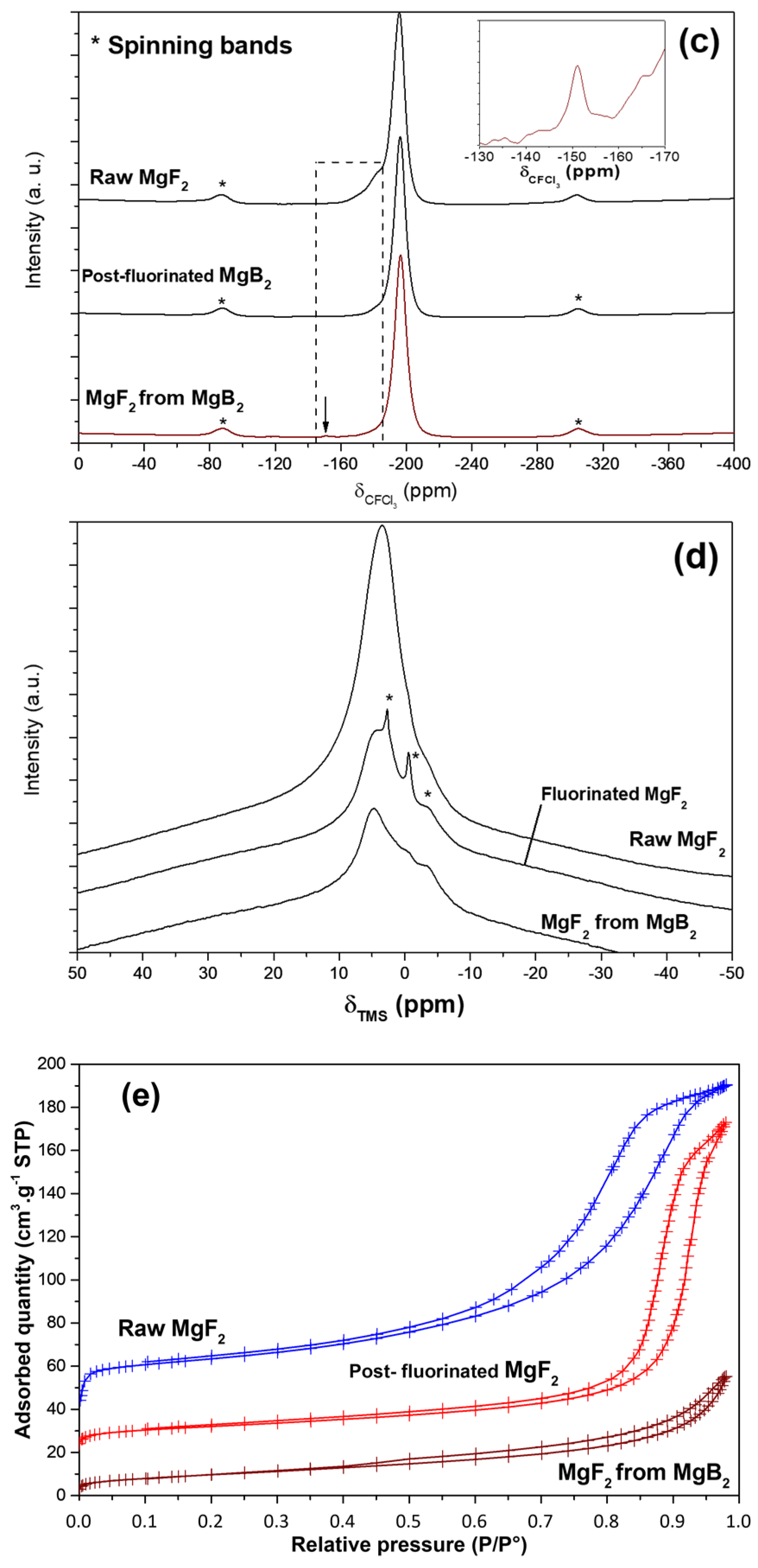
3.1.1. Conversion of MgB2 into Oxygen-Free MgF2
- The expected pore size, in accordance with the size of the released molecules (0.243, 0.320, 0.377 and 0.377 nm) for MgB2 (BF3), Mg3N2 (NF3), Mg2Si (SiF4) and Mg3P2 (PF5), respectively.
- The toxicity of the gases released at the completion of the first reaction item.
- The presence of solid products other than MgF2.
3.1.2. Tailoring of OH/F Ratio in Conventional MgF2
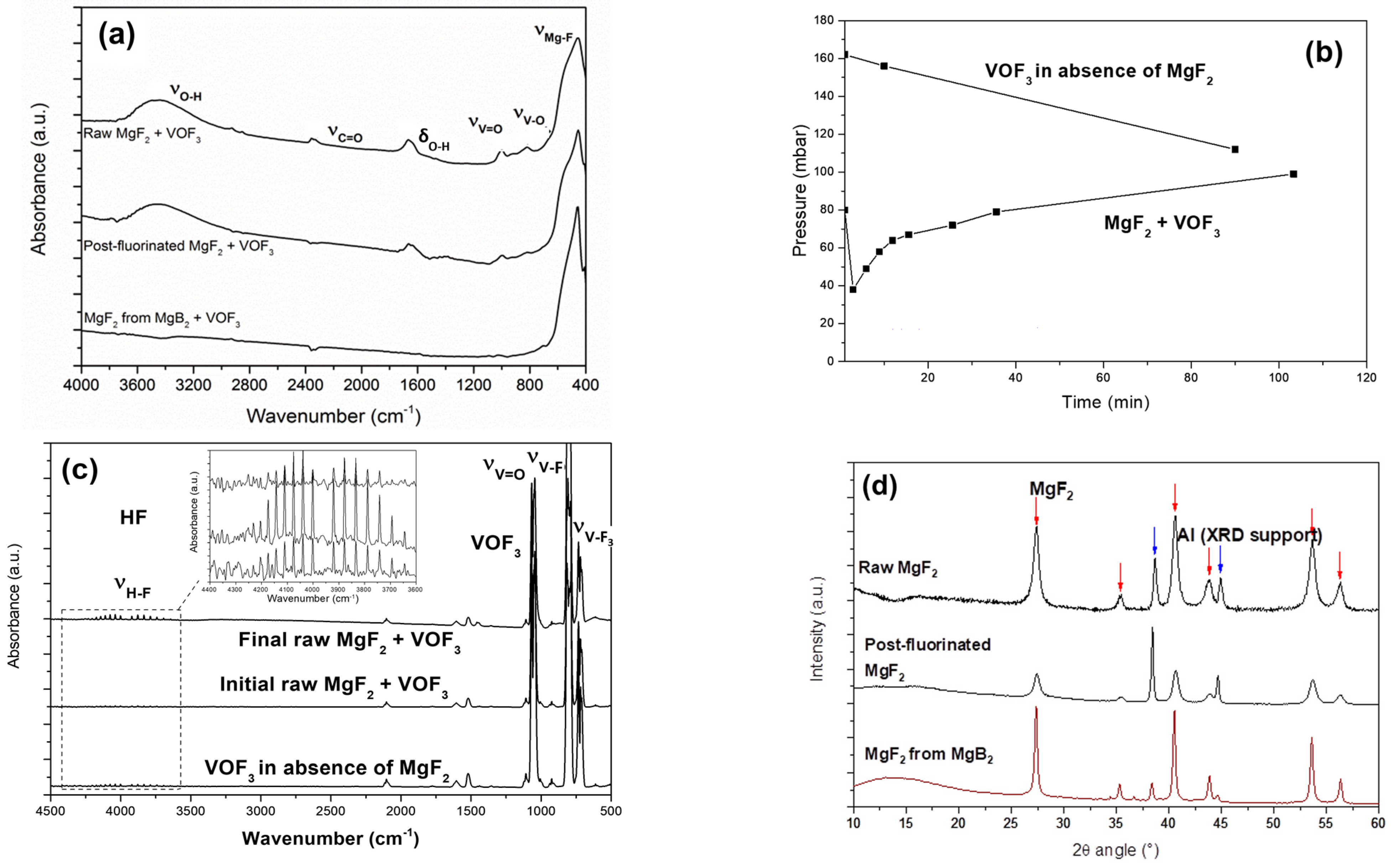
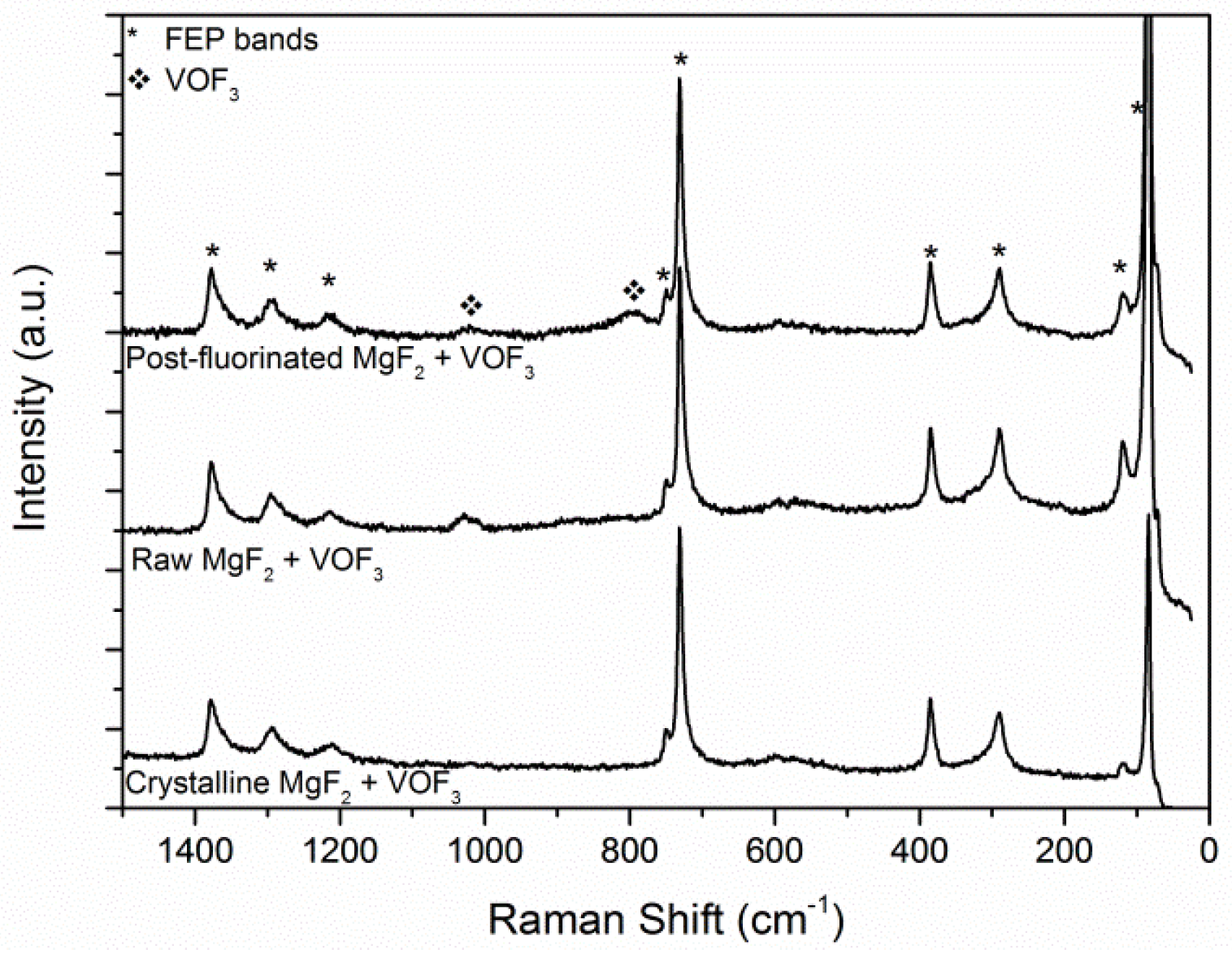
3.2. Sorption of VOF3 in MgF2−x(OH)x
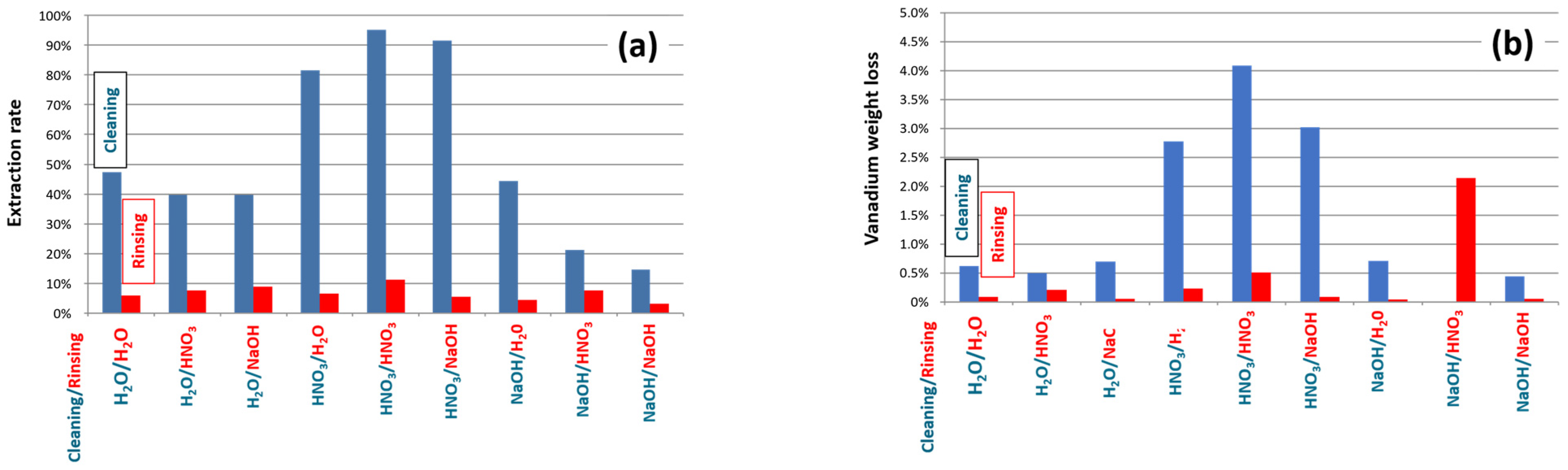
3.3. Regeneration of the Chemical Filter
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Langeslay, R.R.; Kaphan, D.M.; Marshall, C.L.; Stair, P.C.; Sattelberger, A.P.; Delferro, M. Catalytic Applications of Vanadium: A Mechanistic Perspective. Chem. Rev. 2019, 119, 2128–2191. [Google Scholar] [CrossRef] [PubMed]
- Landström, L.; Lu, J.; Heszler, P. Size-Distribution and Emission Spectroscopy of W Nanoparticles Generated by Laser-Assisted CVD for Different WF6/H2/Ar Mixtures. J. Phys. Chem. B 2003, 107, 11615–11621. [Google Scholar] [CrossRef]
- Seghete, D.; Rayner, G.B., Jr.; Cavanagh, A.S.; Anderson, V.R.; George, S.M. Molybdenum Atomic Layer Deposition Using MoF6 and Si2H6 as the Reactants. Chem. Mater. 2011, 23, 1668–1678. [Google Scholar] [CrossRef]
- Wolden, C.A.; Pickerell, A.; Gawai, T.; Parks, S.; Hensley, J.; Way, J.D. Synthesis of β-Mo2C Thin Films. ACS Appl. Mater. Interfaces 2011, 3, 517–521. [Google Scholar] [CrossRef] [PubMed]
- Nikolaev, N.S.; Sadikova, A.T. Solubility of fluorides of certain elements in liquid uranium hexafluoride. Sov. Atom. Energy 1975, 39, 982–987. [Google Scholar] [CrossRef]
- Tananaev, I.V.; Nikolaev, N.S.; Luk’yanychev, Y.A.; Opalovskii, A.A. The chemistry of uranium fluorides. Russ. Chem. Rev. 1961, 30, 654–671. [Google Scholar] [CrossRef]
- Kemnitz, E.; Groß, U.; Rüdiger, S.; Shekar, C.S. Amorphous Metal Fluorides with Extraordinary High Surface Areas. Angew. Chem. Int. Ed. 2003, 42, 4251–4254. [Google Scholar] [CrossRef]
- Kemnitz, E.; Groß, U.; Rüdiger, S.; Shekar, C.S. Amorphe Metallfluoride mit außergewöhnlich großer spezifischer Oberfläche. Angew. Chem. 2003, 115, 4383–4386. [Google Scholar]
- Rüdiger, S.; Kemnitz, E. The fluorolytic sol–gel route to metal fluorides—A versatile process opening a variety of application fields. Dalton Trans. 2008, 9, 1117–1127. [Google Scholar] [CrossRef]
- Rüdiger, S.; Eltanamy, G.; Groß, U.; Kemnitz, E. Real sol-gel synthesis of catalytically active aluminium fluoride. J. Sol-Gel Sci. Technol. 2007, 41, 299–311. [Google Scholar] [CrossRef]
- Rüdiger, S.; Groß, U.; Kemnitz, E. Non-aqueous sol–gel synthesis of nano-structured metal fluorides. J. Fluorine Chem. 2007, 128, 353–368. [Google Scholar] [CrossRef]
- Demourgues, A.; Penin, N.; Dambournet, D.; Clarenc, R.; Tressaud, A.; Durand, E. About MX3 and MX2 (Mn+ = Mg2+, Al3+, Ti4+, Fe3+; Xp− = F−, O2−, OH−) nanofluorides. J. Fluorine Chem. 2012, 134, 35–43. [Google Scholar] [CrossRef]
- Dambournet, D.; Demourgues, A.; Martineau, C.; Durand, E.; Majimel, J.; Legein, C.; Buzaré, J.Y.; Fayon, F.; Vimont, A.; Leclerc, H.; et al. Microwave Synthesis of an Aluminum Fluoride Hydrate with Cationic Vacancies: Structure, Thermal Stability, and Acidic Properties. Chem. Mater. 2008, 20, 7095–7106. [Google Scholar] [CrossRef]
- Dambournet, D.; Demourgues, A.; Martineau, C.; Durand, E.; Majimel, J.; Vimont, A.; Leclerc, H.; Lavalley, J.C.; Daturi, M.; Legein, C.; et al. Structural investigations and acidic properties of high surface area pyrochlore aluminium hydroxyfluoride. J. Mater. Chem. 2008, 18, 2483–2492. [Google Scholar] [CrossRef]
- Dambournet, D.; Demourgues, A.; Martineau, C.; Majimel, J.; Feist, M.; Legein, C.; Buzaré, J.Y.; Fayon, F.; Tressaud, A. Nanostructured Al-based Fluoride-Oxide Materials with a Core-Shell Morphology. J. Phys. Chem. C 2008, 112, 12374–12380. [Google Scholar] [CrossRef]
- Dambournet, D.; Eltanamy, G.; Vimont, A.; Lavalley, J.C.; Goupil, J.M.; Demourgues, A.; Durand, E.; Majimel, J.; Rüdiger, S.; Kemnitz, E.; et al. Coupling sol-gel synthesis and microwave-assisted techniques: A new route from amorphous to crystalline high-surface-area aluminium fluoride. Chem. Eur. J. 2008, 14, 6205–6212. [Google Scholar] [CrossRef]
- Dambournet, D.; Demourgues, A.; Martineau, C.; Pechev, S.; Lhoste, J.; Majimel, J.; Vimont, A.; Lavalley, J.C.; Legein, C.; Buzaré, J.Y.; et al. Nanostructured Aluminium Hydroxyfluorides Derived from β-AlF3. Chem. Mater. 2008, 20, 1459–1469. [Google Scholar] [CrossRef]
- Wojciechchova, M.; Zielinski, M.; Pietrowski, M. MgF2 as a non-conventional catalyst support. J. Fluorine Chem. 2003, 120, 1–11. [Google Scholar] [CrossRef]
- Wang, Y.; Gohari-Bajestani, Z.; Lhoste, J.; Auguste, S.; Hémon-Ribaud, A.; Body, M.; Legein, C.; Maisonneuve, V.; Guiet, A.; Brunet, S. The Effects of Various Parameters of the Microwave-Assisted Solvothermal Synthesis on the Specific Surface Area and Catalytic Performance of MgF2 Nanoparticles. Materials 2020, 13, 3566. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, H.; Wang, J.; Wilkinson, S.J. Durable MgO–MgF2 Composite Film for Infrared Anti-Reflection Coatings. European Patent EP2715412, 2012. [Google Scholar]
- Lisitsyn, V.M.; Lisitsyna, L.A.; Popov, A.I.; Kotomin, E.A.; Abuova, F.U.; Akilbekov, A.; Maier, J. Stabilization of primary mobile radiation defects in MgF2 crystals. Nucl. Instrum. Methods Phys. Res. Sect. B 2016, 374, 24–28. [Google Scholar] [CrossRef]
- Cappellini, G.; Furthmüller, J.; Bechstedt, F.; Botti, S. Electronic and Optical Properties of Alkaline Earth Metal Fluoride Crystals with the Inclusion of Many-Body Effects: A Comparative Study on Rutile MgF2 and Cubic SrF2. Symmetry 2023, 15, 539. [Google Scholar] [CrossRef]
- Golliher, W.R. Process for Separation and Recovery of Volatile Fluoride Impurities from Uranium Hexafluoride Containing the Same. U.S. Patent 3165376, 12 January 1962. [Google Scholar]
- Watanabe, D.; Sasakira, A.; Hoshino, K.; Kawamura, F. Adsorption of molybdenum hexafluoride on magnesium difluoride for uranium purification in FLUOREX reprocessing. J. Nucl. Sci. Technol. 2011, 48, 1413–1419. [Google Scholar] [CrossRef]
- Seregin, M.B.; Mikhalichenko, A.A.; Kuznetsov, A.Y.; Sokovin, G.S.; Chekmarev, A.M. Sorption of gaseous RuF5 on granulated fluoride sorbents. Radiochemistry 2011, 53, 288–291. [Google Scholar] [CrossRef]
- Wuttke, S.; Vimont, A.; Lavalley, J.-C.; Daturi, M.; Kemnitz, E. Infrared Investigation of the Acid and Basic Properties of a Sol-Gel Prepared MgF2. J. Phys. Chem. C 2010, 114, 5113–5120. [Google Scholar] [CrossRef]
- Rodriguez-Carvajal, J. Recent advances in magnetic structure determination by neutron powder diffraction. Phys. B Condens. Matter 1993, 192, 55–69. [Google Scholar] [CrossRef]
- Dhaoudadi, H.; Chaabane, H.; Touati, F. Nanorods Synthesized by A Facile. Hydrothermal Method in the Presence of CTAB. Nano-Micro Lett. 2011, 3, 153–159. [Google Scholar] [CrossRef]
- Hunt, G.R.; Perry, C.H.; Ferguson, J. Far-Infrared reflectance and transmittance of Potassium Magnesium fluoride and magnesium fluoride. Phys. Rev. A 1964, 134, A688–A691. [Google Scholar] [CrossRef]
- Heimer, N.E.; Del Sesto, R.E.; Meng, Z.; Wilkes, J.S.; Carper, W.R. Vibrational spectra of imidazolium tetrafluoroborate ionic liquids. J. Mol. Liq. 2006, 124, 84–95. [Google Scholar] [CrossRef]
- Wuttke, S.; Scholtz, G.; Rudiger, S.; Kemnitz, E. Variation of sol-gel synthesis parameters and their consequence for the surface area and structure of magnesium fluoride. J. Mat. Chem. 2007, 17, 4980–4988. [Google Scholar] [CrossRef]
- Scholtz, G.; Stosiek, C.; Noack, J.; Kemnitz, E. Local fluorine environments in nanoscopic magnesium hydr(oxide) fluorides studied by F-19 MAS NMR. J. Fluor. Chem. 2011, 132, 1079–1085. [Google Scholar] [CrossRef]
- Zidan, M.D.; Allaf, A.W. The gas-phase on-line production of vanadium oxytrihalides, VOX3 and their identification by infrared spectroscopy. Spectrochim. Acta Part A 2000, 56, 2693–2698. [Google Scholar] [CrossRef] [PubMed]
- Beattie, I.R.; Livingston, K.; Reynolds, D.J.; Ozin, G.A. Vibrational spectra of some oxide halides of the transition elements with particular reference to gas-phase and single-crystal Raman spectroscopy. J. Chem. Soc. A 1970, 1210–1216. [Google Scholar] [CrossRef]
- Clark, R.J.H.; Rippon, D.M. The vapour phase Raman spectra, Raman band contour analyses, Coriolis constants, force constants, and values for thermodynamic functions of the symmetric top molecules POF3, POCl3, VOF3, VOCl3, PSCl3 and FClO3. Mol. Phys. 1974, 28, 305–319. [Google Scholar] [CrossRef]
- Davis, M.F.; Jura, M.; Leung, A.; Levason, W.; Littlefield, B.; Reid, G.; Webster, M. Synthesis, chemistry and structures of complexes of the dioxovanadium(v) halides VO2F and VO2Cl. Dalton Trans. 2008, 44, 6265–6273. [Google Scholar] [CrossRef] [PubMed]
- Hibbert, R.C. A 51V and 19F n.m.r. study of vanadium(V) oxide fluoride complexes displaying vanadium–fluorine coupling. J. Chem. Soc. Chem. Commun. 1985, 317–318. [Google Scholar] [CrossRef]
- Howell, J.A.S.; Moss, K.C. Nuclear magnetic resonance studies on fluorine-containing compounds. Part III. The tetrafluoro-oxovandate(V) ion. J. Chem. Soc. A 1971, 270–272. [Google Scholar] [CrossRef]
- Senchyk, G.A.; Bukhanko, V.O.; Lysenko, A.B.; Krautscheid, H.; Rusanov, E.B.; Chernega, A.N.; Karbowiak, M.; Domasevitch, K.V. AgI/VV Heterobimetallic Frameworks Generated from Novel-Type {Ag2(VO2F2)2(triazole)4} Secondary Building Blocks: A New Aspect in the Design of SVOF Hybrids. Inorg. Chem. 2012, 51, 8025–8033. [Google Scholar] [CrossRef]
- Chen, C.; Jiang, M.; Luo, X.; Tai, H.; Jiang, Y.; Yang, M.; Xie, G.; Su, Y. Ni-Co-P hollow nanobricks enabled humidity sensor for respiratory analysis and human-machine interfacing. Sens. Actuators B Chem. 2022, 370, 132441. [Google Scholar] [CrossRef]
- Chen, C.; Xie, G.; Dai, J.; Li, W.; Cai, Y.; Li, J.; Zhang, Q.; Tai, H.; Jiang, Y.; Su, Y. Integrated core-shell structured smart textiles for active NO2 concentration and pressure monitoring. Nano Energy 2023, 116, 108788. [Google Scholar] [CrossRef]
- Zegebreal, L.T.; Tegegne, N.A.; Hone, F.G. Recent progress in hybrid conducting polymers and metal oxide nanocomposite for room-temperature gas sensor applications: A review. Sens. Actuators A Phys 2023, 359, 114472. [Google Scholar] [CrossRef]

| MgF2 Treatment | Bulk Weight Uptake (%) | Vanadium Weight Uptake (%) | Vanadium ICP Analysis (%) |
|---|---|---|---|
| Raw | 8.7 | 3.6 | 3.4 |
| Post-fluorinated at 240 °C | 5.1 | 2.1 | 2.0 |
| Synthesized from MgB2 | 3.1 | 1.3 | 1.2 |
| Process Step | Starting Material | After Filtering | After NaOH Regeneration | |
|---|---|---|---|---|
| SSABET (m²·g−1) | First run | 72 | 37 | 64 |
| Second run | 64 | 38 | 51 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jouffret, L.; Hiltbrunner, J.-M.; Petit, E.; Selmi, A.; Morel, B.; Dubois, M. Selective Chemical Filters for VOF3: Tailoring MgF2 Filter Selectivity through Surface Chemistry. Surfaces 2023, 6, 480-492. https://doi.org/10.3390/surfaces6040032
Jouffret L, Hiltbrunner J-M, Petit E, Selmi A, Morel B, Dubois M. Selective Chemical Filters for VOF3: Tailoring MgF2 Filter Selectivity through Surface Chemistry. Surfaces. 2023; 6(4):480-492. https://doi.org/10.3390/surfaces6040032
Chicago/Turabian StyleJouffret, Laurent, Jean-Michel Hiltbrunner, Elodie Petit, Ania Selmi, Bertrand Morel, and Marc Dubois. 2023. "Selective Chemical Filters for VOF3: Tailoring MgF2 Filter Selectivity through Surface Chemistry" Surfaces 6, no. 4: 480-492. https://doi.org/10.3390/surfaces6040032
APA StyleJouffret, L., Hiltbrunner, J.-M., Petit, E., Selmi, A., Morel, B., & Dubois, M. (2023). Selective Chemical Filters for VOF3: Tailoring MgF2 Filter Selectivity through Surface Chemistry. Surfaces, 6(4), 480-492. https://doi.org/10.3390/surfaces6040032







