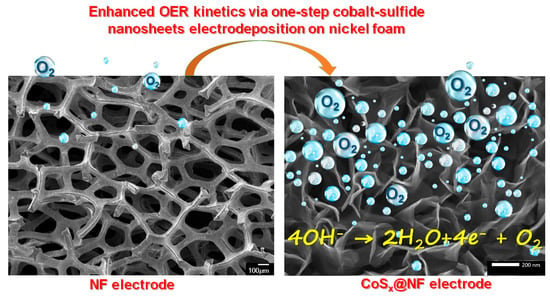
Highly Efficient Cobalt Sulfide Heterostructures Fabricated on Nickel Foam Electrodes for Oxygen Evolution Reaction in Alkaline Water Electrolysis Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Electrodeposition on NF Substrate
2.3. Structural and Morphological Characterization
2.4. Electrochemical Evaluation of the Fabricated Electrodes
3. Results and Discussion
3.1. Electrodeposition
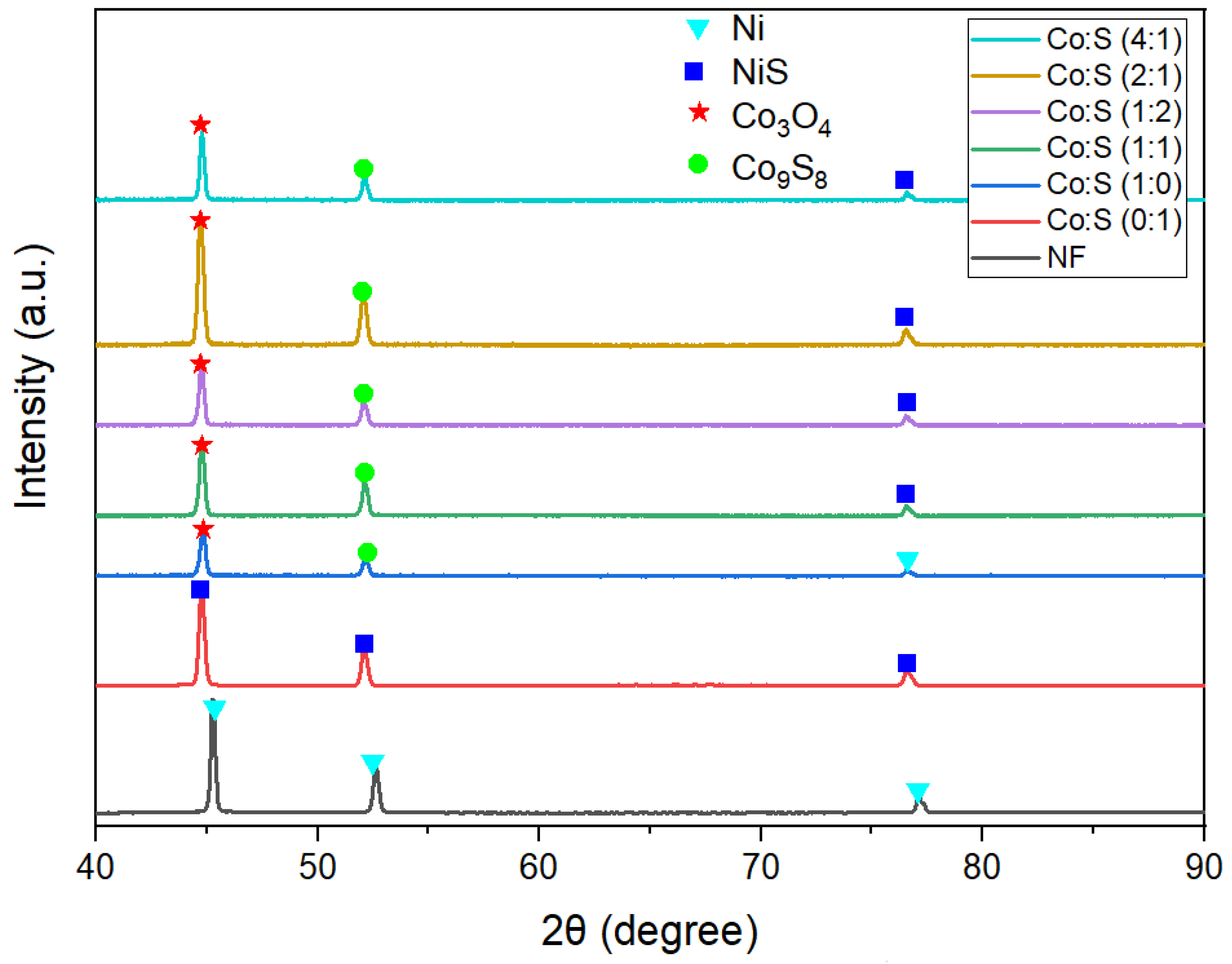
3.2. Structural and Morphological Evaluation
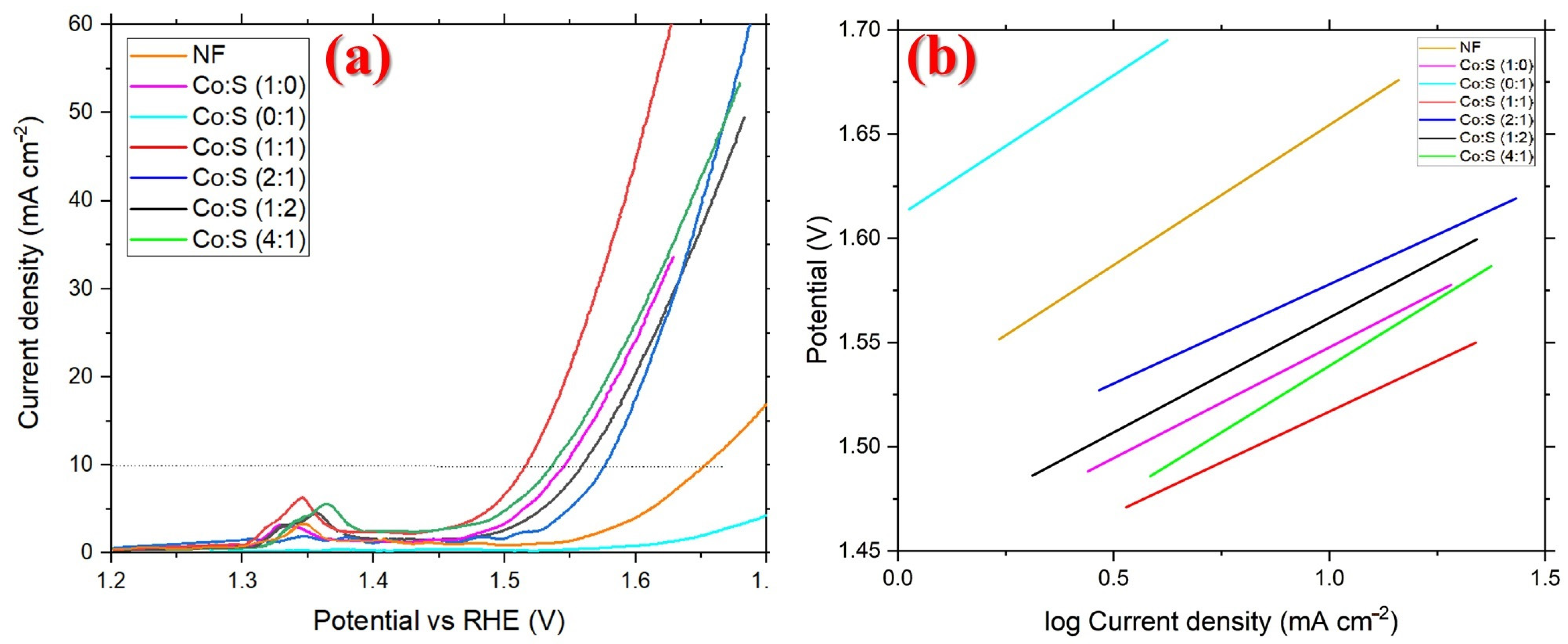
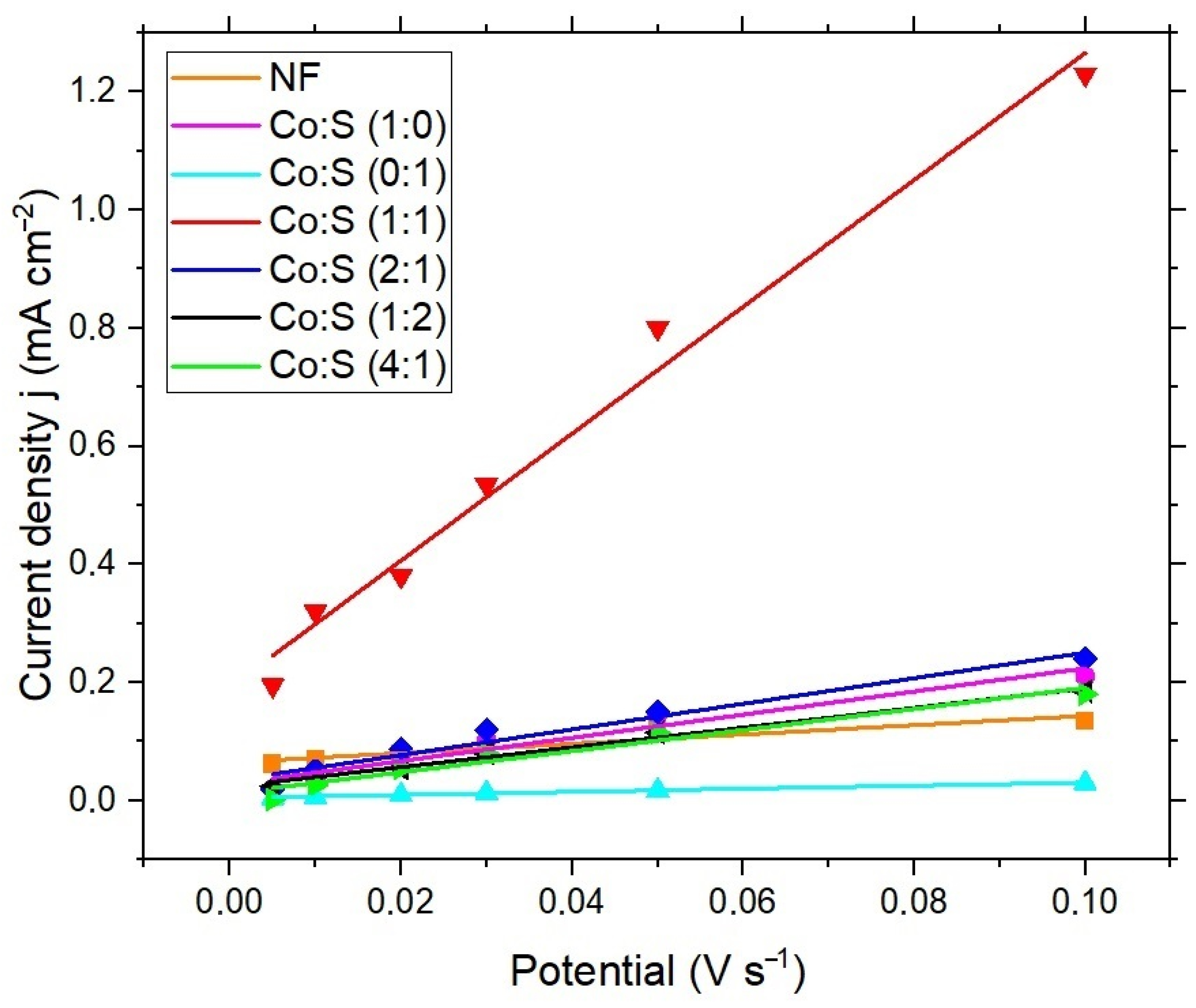
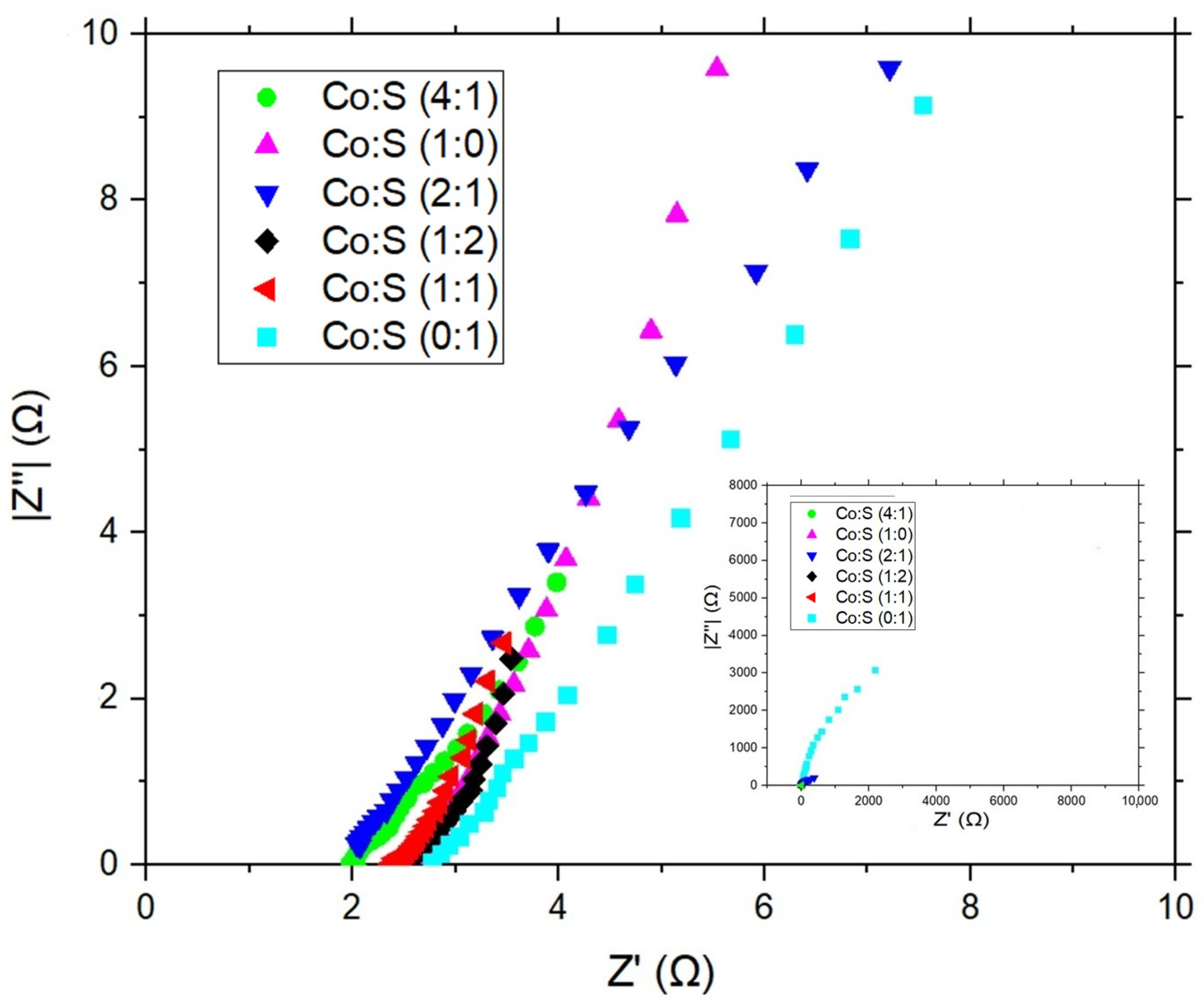
3.3. Electrochemical Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bockris, J.O.; Veziroglu, T.N. Estimates of the price of hydrogen as a medium for wind and solar sources. Int. J. Hydrogen Energy 2007, 32, 1605–1610. [Google Scholar] [CrossRef]
- Guo, Y.; Shang, C.; Li, J.; Wang, E. Recent development of hydrogen evolution, oxygen evolution and oxygen reduction reaction. Sci. Sin. Chim. 2018, 48, 926–940. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, M.; Fu, W.; Wei, L.; Zhao, X.; Zhou, X.; Ni, M.; Wang, H. Highly active and durable catalyst for hydrogen generation by the NaBH4 hydrolysis reaction: CoWB/NF nanodendrite with an acicular array structure. J. Alloys Compd. 2020, 836, 155429. [Google Scholar] [CrossRef]
- Poimenidis, I.A.; Papakosta, N.; Klini, A.; Farsari, M.; Konsolakis, M.; Loukakos, P.A.; Moustaizis, S. Electrodeposited Ni foam electrodes for increased hydrogen production in alkaline electrolysis. Fuel 2023, 342, 127798. [Google Scholar] [CrossRef]
- Guan, D.; Wang, B.; Zhang, J.; Shi, R.; Jiao, K.; Li, L.; Wang, Y.; Xie, B.; Zhang, Q.; Yu, J.; et al. Hydrogen society: From present to future. Energy Environ. Sci. 2023, 16, 4926–4943. [Google Scholar] [CrossRef]
- McCrory, C.C.L.; Jung, S.; Ferrer, I.M.; Chatman, S.M.; Peters, J.C.; Jaramillo, T.F. Benchmarking Hydrogen Evolving Reaction and Oxygen Evolving Reaction Electrocatalysts for Solar Water Splitting Devices. J. Am. Chem. Soc. 2015, 137, 4347–4357. [Google Scholar] [CrossRef]
- Han, G.-Q.; Shang, X.; Lu, S.-S.; Dong, B.; Li, X.; Liu, Y.-R.; Hu, W.; Zeng, J.-B.; Chai, Y.-M.; Liu, C.-G. Electrodeposited MoSx films assisted by liquid crystal template with ultrahigh electrocatalytic activity for hydrogen evolution reaction. Int. J. Hydrogen Energy 2017, 42, 5132–5138. [Google Scholar] [CrossRef]
- Wu, G.; Santandreu, A.; Kellogg, W.; Gupta, S.; Ogoke, O.; Zhang, H.; Wang, H.-L.; Dai, L. Carbon nanocomposite catalysts for oxygen reduction and evolution reactions: From nitrogen doping to transition-metal addition. Nano Energy 2016, 29, 83–110. [Google Scholar] [CrossRef]
- Kanan, M.W.; Nocera, D.G. In Situ Formation of an Oxygen-Evolving Catalyst in Neutral Water Containing Phosphate and Co2+. Science (1979) 2008, 321, 1072–1075. [Google Scholar] [CrossRef]
- Song, J.; Wei, C.; Huang, Z.-F.; Liu, C.; Zeng, L.; Wang, X.; Xu, Z. A review on fundamentals for designing oxygen evolution electrocatalysts. Chem. Soc. Rev. 2020, 49, 2196–2214. [Google Scholar] [CrossRef]
- Imran Anwar, M.; Manzoor, S.; Ma, L.; Asad, M.; Zhang, W.; Shafiq, Z.; Ashiq, M.-N.; Yang, G. Nitrogen-rich three-dimensional metal-organic framework microrods as an efficient electrocatalyst for oxygen evolution reaction and supercapacitor applications. Fuel 2023, 331, 125746. [Google Scholar] [CrossRef]
- Jung, S.; McCrory, C.C.L.; Ferrer, I.M.; Peters, J.C.; Jaramillo, T.F. Benchmarking nanoparticulate metal oxide electrocatalysts for the alkaline water oxidation reaction. J. Mater. Chem. A Mater. 2016, 4, 3068–3076. [Google Scholar] [CrossRef]
- Chi, J.-Q.; Shang, X.; Liang, F.; Dong, B.; Li, X.; Liu, Y.-R.; Yan, K.-L.; Gao, W.-K.; Chai, Y.-M.; Liu, C.-G. Facile synthesis of pyrite-type binary nickel iron diselenides as efficient electrocatalyst for oxygen evolution reaction. Appl. Surf. Sci. 2017, 401, 17–24. [Google Scholar] [CrossRef]
- Sarfraz, B.; Bashir, I.; Rauf, A. CuS/NiFe-LDH/NF as a Bifunctional Electrocatalyst for Hydrogen Evolution (HER) and Oxygen Evolution Reactions (OER). Fuel 2023, 337, 127253. [Google Scholar] [CrossRef]
- Shit, S.; Chhetri, S.; Jang, W.; Murmu, N.C.; Koo, H.; Samanta, P.; Kuila, T. Cobalt Sulfide/Nickel Sulfide Heterostructure Directly Grown on Nickel Foam: An Efficient and Durable Electrocatalyst for Overall Water Splitting Application. ACS Appl. Mater. Interfaces 2018, 10, 27712–27722. [Google Scholar] [CrossRef] [PubMed]
- Kale, S.B.; Lokhande, A.C.; Pujari, R.B.; Lokhande, C.D. Cobalt sulfide thin films for electrocatalytic oxygen evolution reaction and supercapacitor applications. J. Colloid Interface Sci. 2018, 532, 491–499. [Google Scholar] [CrossRef]
- Kuai, L.; Geng, J.; Chen, C.; Kan, E.; Liu, Y.; Wang, Q.; Geng, B. A Reliable Aerosol-Spray-Assisted Approach to Produce and Optimize Amorphous Metal Oxide Catalysts for Electrochemical Water Splitting. Angew. Chem. Int. Ed. 2014, 53, 7547–7551. [Google Scholar] [CrossRef]
- Smith, R.D.L.; Prévot, M.S.; Fagan, R.D.; Zhang, Z.; Sedach, P.A.; Siu, M.K.J.; Trudel, S.; Berlinguette, C. Photochemical Route for Accessing Amorphous Metal Oxide Materials for Water Oxidation Catalysis. Science 2013, 340, 60–63. [Google Scholar] [CrossRef]
- Borthakur, P.; Boruah, P.K.; Das, M.R.; Ibrahim, M.M.; Altalhi, T.; El-Sheshtawy, H.S.; Szunerits, S.; Boukherroub, R.; Amin, M. CoS2 Nanoparticles Supported on rGO, g-C3N4, BCN, MoS2, and WS2 Two-Dimensional Nanosheets with Excellent Electrocatalytic Performance for Overall Water Splitting: Electrochemical Studies and DFT Calculations. ACS Appl. Energy Mater. 2021, 4, 1269–1285. [Google Scholar] [CrossRef]
- Poimenidis, I.A.; Lykaki, M.; Moustaizis, S.; Loukakos, P.; Konsolakis, M. One-step solvothermal growth of NiO nanoparticles on nickel foam as a highly efficient electrocatalyst for hydrogen evolution reaction. Mater. Chem. Phys. 2023, 305, 128007. [Google Scholar] [CrossRef]
- Surendran, S.; Shanmugapriya, S.; Lee, Y.S.; Sim, U.; Selvan, R.K. Carbon-Enriched Cobalt Phosphide with Assorted Nanostructure as a Multifunctional Electrode for Energy Conversion and Storage Devices. ChemistrySelect 2018, 3, 12303–12313. [Google Scholar] [CrossRef]
- Prabakaran, K.; Lokanathan, M.; Kakade, B. Three dimensional flower like cobalt sulfide (CoS)/functionalized MWCNT composite catalyst for efficient oxygen evolution reactions. Appl. Surf. Sci. 2019, 466, 830–836. [Google Scholar] [CrossRef]
- Poimenidis, I.A.; Papakosta, N.; Klini, A.; Farsari, M.; Moustaizis, S.D.; Konsolakis, M.; Loukakos, P. Ni foam electrodes decorated with Ni nanoparticles via pulsed laser deposition for efficient hydrogen evolution reaction. Mater. Sci. Eng. B 2024, 299, 116922. [Google Scholar] [CrossRef]
- Zhao, Y.; Adiyeri Saseendran, D.P.; Huang, C.; Triana, C.A.; Marks, W.R.; Chen, H.; Zhao, H.; Patzke, G.-R. Oxygen Evolution/Reduction Reaction Catalysts: From In Situ Monitoring and Reaction Mechanisms to Rational Design. Chem. Rev. 2023, 123, 6257–6358. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, L.; He, Y.; Zhu, H. Recent advances in transition-metal-sulfide-based bifunctional electrocatalysts for overall water splitting. J. Mater. Chem. A Mater. 2021, 9, 5320–5363. [Google Scholar] [CrossRef]
- Zhu, Y.; Song, L.; Song, N.; Li, M.; Wang, C.; Lu, X. Bifunctional and Efficient CoS 2 –C@MoS 2 Core–Shell Nanofiber Electrocatalyst for Water Splitting. ACS Sustain. Chem. Eng. 2019, 7, 2899–2905. [Google Scholar] [CrossRef]
- Elshahawy, A.M.; Li, X.; Zhang, H.; Hu, Y.; Ho, K.H.; Guan, C.; Wang, J. Controllable MnCo 2 S 4 nanostructures for high performance hybrid supercapacitors. J. Mater. Chem. A Mater. 2017, 5, 7494–7506. [Google Scholar] [CrossRef]
- Yu, Y. Study on Electrochemistry and Nucleation Process of Nickel Electrodeposition. Int. J. Electrochem. Sci. 2017, 12, 485–495. [Google Scholar] [CrossRef]
- Shi, J.; Li, X.; He, G.; Zhang, L.; Li, M. Electrodeposition of high-capacitance 3D CoS/graphene nanosheets on nickel foam for high-performance aqueous asymmetric supercapacitors. J. Mater. Chem. A Mater. 2015, 3, 20619–20626. [Google Scholar] [CrossRef]
- Sultana, U.K.; He, T.; Du, A.; O’Mullane, A.P. An amorphous dual action electrocatalyst based on oxygen doped cobalt sulfide for the hydrogen and oxygen evolution reactions. RSC Adv. 2017, 7, 54995–55004. [Google Scholar] [CrossRef]
- Jiang, S.P.; Chen, Y.Z.; You, J.K.; Chen, T.X.; Tseung, A.C.C. Reactive Deposition of Cobalt Electrodes: I. Experimental. J. Electrochem. Soc. 1990, 137, 3374–3380. [Google Scholar] [CrossRef]
- García-Valenzuela, J.A. Simple Thiourea Hydrolysis or Intermediate Complex Mechanism? Taking up the Formation of Metal Sulfides from Metal–Thiourea Alkaline Solutions. Comments Inorg. Chem. 2017, 37, 99–115. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, C.; Zhang, D.; Zhao, J.; Zheng, S.; Su, H.; Wei, F.; Yuan, B.; Fernandez, C. Facile Synthesis of a Nickel Sulfide (NiS) Hierarchical Flower for the Electrochemical Oxidation of H2O2 and the Methanol Oxidation Reaction (MOR). J. Electrochem. Soc. 2017, 164, B92–B96. [Google Scholar] [CrossRef]
- Liu, S.; Shi, Q.; Tong, J.; Li, S.; Li, M. Controlled synthesis of spherical α-NiS and urchin-like β-NiS microstructures. J. Exp. Nanosci. 2014, 9, 475–481. [Google Scholar] [CrossRef]
- Yan, K.-L.; Shang, X.; Li, Z.; Dong, B.; Chi, J.-Q.; Liu, Y.-R.; Gao, W.-K.; Chai, Y.-M.; Liu, C.-G. Facile synthesis of binary NiCoS nanorods supported on nickel foam as efficient electrocatalysts for oxygen evolution reaction. Int. J. Hydrogen Energy 2017, 42, 17129–17135. [Google Scholar] [CrossRef]
- Samal, R.; Mondal, S.; Gangan, A.S.; Chakraborty, B.; Rout, C.S. Comparative electrochemical energy storage performance of cobalt sulfide and cobalt oxide nanosheets: Experimental and theoretical insights from density functional theory simulations. Phys. Chem. Chem. Phys. 2020, 22, 7903–7911. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; He, Q.; Liu, M.; An, H.; Hou, L. Controlled synthesis of porous nanosheets-assembled peony-like cobalt nickel selenides for triiodide reduction in dye-sensitized solar cells. J. Alloys Compd. 2020, 818, 152817. [Google Scholar] [CrossRef]
- Shervedani, R.K.; Madram, A.R. Kinetics of hydrogen evolution reaction on nanocrystalline electrodeposited Ni62Fe35C3 cathode in alkaline solution by electrochemical impedance spectroscopy. Electrochim. Acta 2007, 53, 426–433. [Google Scholar] [CrossRef]
- Hosseini, M.G.; Momeni, M.M.; Faraji, M. Highly Active Nickel Nanoparticles Supported on TiO2 Nanotube Electrodes for Methanol Electrooxidation. Electroanalysis 2010, 22, 2620–2625. [Google Scholar] [CrossRef]
- Shaffer, D.W.; Xie, Y.; Concepcion, J.J. O–O bond formation in ruthenium-catalyzed water oxidation: Single-site nucleophilic attack vs. O–O radical coupling. Chem. Soc. Rev. 2017, 46, 6170–6193. [Google Scholar] [CrossRef]
- Craig, M.J.; Coulter, G.; Dolan, E.; Soriano-López, J.; Mates-Torres, E.; Schmitt, W.; Melchor, M.-G. Universal scaling relations for the rational design of molecular water oxidation catalysts with near-zero overpotential. Nat. Commun. 2019, 10, 4993. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Zheng, X.; Cao, C.; Lu, Q.; Zhang, J.; Wang, H.; Huang, Z.; Cao, Y.; Wang, Y.; Deng, Y. Lattice oxygen activation in disordered rocksalts for boosting oxygen evolution. Phys. Chem. Chem. Phys. 2023, 25, 4113–4120. [Google Scholar] [CrossRef]
- Zhu, Y.; Tahini, H.A.; Hu, Z.; Chen, Z.; Zhou, W.; Komarek, A.C.; Lin, Q.; Lin, H.-J.; Chen, C.-T.; Zhong, Y.; et al. Boosting Oxygen Evolution Reaction by Creating Both Metal Ion and Lattice-Oxygen Active Sites in a Complex Oxide. Adv. Mater. 2020, 32, 1905025. [Google Scholar] [CrossRef]
- Yoo, J.S.; Rong, X.; Liu, Y.; Kolpak, A.M. Role of Lattice Oxygen Participation in Understanding Trends in the Oxygen Evolution Reaction on Perovskites. ACS Catal. 2018, 8, 4628–4636. [Google Scholar] [CrossRef]
- Cao, D.; Liu, D.; Chen, S.; Moses, O.A.; Chen, X.; Xu, W.; Wu, C.; Zheng, L.; Chu, S.; Jiang, H.; et al. Operando X-ray spectroscopy visualizing the chameleon-like structural reconstruction on an oxygen evolution electrocatalyst. Energy Environ. Sci. 2021, 14, 906–915. [Google Scholar] [CrossRef]
- Fan, K.; Zou, H.; Lu, Y.; Chen, H.; Li, F.; Liu, J.; Sun, L.; Tong, L.; Toney, M.; Sui, M.; et al. Direct Observation of Structural Evolution of Metal Chalcogenide in Electrocatalytic Water Oxidation. ACS Nano 2018, 12, 12369–12379. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Dong, C.-L.; Huang, Y.-C.; Shen, S. Operando Spectral and Electrochemical Investigation into the Heterophase Stimulated Active Species Transformation in Transition-Metal Sulfides for Efficient Electrocatalytic Oxygen Evolution. ACS Catal. 2020, 10, 1855–1864. [Google Scholar] [CrossRef]
- Li, M.; Xu, Z.; Li, Y.; Wang, J.; Zhong, Q. In situ fabrication of cobalt/nickel sulfides nanohybrid based on various sulfur sources as highly efficient bifunctional electrocatalysts for overall water splitting. Nano Sel. 2022, 3, 147–156. [Google Scholar] [CrossRef]
- Poimenidis, I.A.; Papakosta, N.; Manousaki, A.; Klini, A.; Farsari, M.; Moustaizis, S.D.; Loukakos, P. Electrodeposited laser–nanostructured electrodes for increased hydrogen production. Int. J. Hydrogen Energy 2022, 47, 9527–9536. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, N.; Du, X.; Han, X.; Zhang, X. Transition metal atoms M (M = Mn, Fe, Cu, Zn) doped nickel-cobalt sulfides on the Ni foam for efficient oxygen evolution reaction and urea oxidation reaction. J. Alloys Compd. 2022, 893, 162269. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, M.; Wang, Y.; Chen, Z.; Yan, K. Facile synthesis of defect-rich ultrathin NiCo-LDHs, NiMn-LDHs and NiCoMn-LDHs nanosheets on Ni foam for enhanced oxygen evolution reaction performance. J. Alloys Compd. 2021, 852, 156949. [Google Scholar] [CrossRef]
- Qin, J.-F.; Yang, M.; Hou, S.; Dong, B.; Chen, T.-S.; Ma, X.; Xie, J.-X.; Zhou, Y.-N.; Nan, J.; Chai, Y.-M. Copper and cobalt co-doped Ni3S2 grown on nickel foam for highly efficient oxygen evolution reaction. Appl. Surf. Sci. 2020, 502, 144172. [Google Scholar] [CrossRef]
- Zhang, R.-L.; Duan, J.-J.; Feng, J.-J.; Mei, L.-P.; Zhang, Q.-L.; Wang, A.-J. Walnut kernel-like iron-cobalt-nickel sulfide nanosheets directly grown on nickel foam: A binder-free electrocatalyst for high-efficiency oxygen evolution reaction. J. Colloid Interface Sci. 2021, 587, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Min, K.; Yoo, R.; Kim, S.; Kim, H.; Shim, S.E.; Lim, D.; Baeck, S.-H. Facile synthesis of P-doped NiCo2S4 nanoneedles supported on Ni foam as highly efficient electrocatalysts for alkaline oxygen evolution reaction. Electrochim. Acta 2021, 396, 139236. [Google Scholar] [CrossRef]
- Gao, W.-K.; Qin, J.-F.; Wang, K.; Yan, K.-L.; Liu, Z.-Z.; Lin, J.-H.; Chai, Y.-M.; Liu, C.-G.; Dong, B. Facile synthesis of Fe-doped Co9S8 nano-microspheres grown on nickel foam for efficient oxygen evolution reaction. Appl. Surf. Sci. 2018, 454, 46–53. [Google Scholar] [CrossRef]
- Yin, X.; Sun, G.; Wang, L.; Bai, L.; Su, L.; Wang, Y.; Du, Q.; Shao, G. 3D hierarchical network NiCo2S4 nanoflakes grown on Ni foam as efficient bifunctional electrocatalysts for both hydrogen and oxygen evolution reaction in alkaline solution. Int. J. Hydrogen Energy 2017, 42, 25267–25276. [Google Scholar] [CrossRef]
- Wang, J.-Q.; Xi, C.; Wang, M.; Shang, L.; Mao, J.; Dong, C.-K.; Liu, H.; Kulinich, S.-A.; Du, X.-W. Laser-Generated Grain Boundaries in Ruthenium Nanoparticles for Boosting Oxygen Evolution Reaction. ACS Catal. 2020, 10, 12575–12581. [Google Scholar] [CrossRef]
- Shan, J.; Guo, C.; Zhu, Y.; Chen, S.; Song, L.; Jaroniec, M.; Zheng, Y.; Qiao, S.-Z. Charge-Redistribution-Enhanced Nanocrystalline Ru@IrOx Electrocatalysts for Oxygen Evolution in Acidic Media. Chem 2019, 5, 445–459. [Google Scholar] [CrossRef]
- Burke, L.D.; Twomey, T.A.M. Voltammetric behaviour of nickel in base with particular reference to thick oxide growth. J. Electroanal. Chem. Interfacial Electrochem. 1984, 162, 101–119. [Google Scholar] [CrossRef]
- Chandrasekaran, P.; Edison, T.N.J.I.; Sethuraman, M.G. Electrocatalytic study of carbon dots/Nickel iron layered double hydroxide composite for oxygen evolution reaction in alkaline medium. Fuel 2022, 320, 123947. [Google Scholar] [CrossRef]
- Navarro-Flores, E.; Chong, Z.; Omanovic, S. Characterization of Ni, NiMo, NiW and NiFe electroactive coatings as electrocatalysts for hydrogen evolution in an acidic medium. J. Mol. Catal. A Chem. 2005, 226, 179–197. [Google Scholar] [CrossRef]
- Li, X.; Liu, P.F.; Zhang, L.; Zu, M.Y.; Yang, Y.X.; Yang, H.G. Enhancing alkaline hydrogen evolution reaction activity through Ni-Mn3O4 nanocomposites. Chem. Commun. 2016, 52, 10566–10569. [Google Scholar] [CrossRef]
- McCrory, C.C.L.; Jung, S.; Peters, J.C.; Jaramillo, T.F. Benchmarking heterogeneous electrocatalysts for the oxygen evolution reaction. J. Am. Chem. Soc. 2013, 135, 16977–16987. [Google Scholar] [CrossRef]
- Poimenidis, I.A.; Tsanakas, M.D.; Papakosta, N.; Klini, A.; Farsari, M.; Moustaizis, S.D.; Loukakos, P.A. Enhanced hydrogen production through alkaline electrolysis using laser-nanostructured nickel electrodes. Int. J. Hydrogen Energy 2021, 46, 37162–37173. [Google Scholar] [CrossRef]
- Symes, D.; Taylor-Cox, C.; Holyfield, L.; Al-Duri, B.; Dhir, A. Feasibility of an oxygen-getter with nickel electrodes in alkaline electrolysers. Mater. Renew. Sustain. Energy 2014, 3, 27. [Google Scholar] [CrossRef]
- Edison, T.N.J.I.; Atchudan, R.; Karthik, N.; Ganesh, K.; Xiong, D.; Lee, Y.R. A novel binder-free electro-synthesis of hierarchical nickel sulfide nanostructures on nickel foam as a battery-type electrode for hybrid-capacitors. Fuel 2020, 276, 118077. [Google Scholar] [CrossRef]
- Huang, H.; Deng, X.; Yan, L.; Wei, G.; Zhou, W.; Liang, X.; Guo, J. One-Step Synthesis of Self-Supported Ni3S2/NiS Composite Film on Ni Foam by Electrodeposition for High-Performance Supercapacitors. Nanomaterials 2019, 9, 1718. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Z.; Mu, J.; Ai, B.; Zhang, T.; Ge, W.; Zhang, L. Enhanced lithium storage capability enabled by metal nickel dotted NiO–graphene composites. J. Mater. Sci. 2019, 54, 1475–1487. [Google Scholar] [CrossRef]
- Siddiqui, S.-E.-T.; Rahman, M.d.A.; Kim, J.-H.; Sharif, S.B.; Paul, S. A Review on Recent Advancements of Ni-NiO Nanocomposite as an Anode for High-Performance Lithium-Ion Battery. Nanomaterials 2022, 12, 2930. [Google Scholar] [CrossRef]
- Chia, X.; Ambrosi, A.; Sofer, Z.; Luxa, J.; Pumera, M. Catalytic and Charge Transfer Properties of Transition Metal Dichalcogenides Arising from Electrochemical Pretreatment. ACS Nano 2015, 9, 5164–5179. [Google Scholar] [CrossRef]
- Agudosi, E.S.; Abdullah, E.C.; Numan, A.; Mubarak, N.M.; Aid, S.R.; Benages-Vilau, R.; Romero, P.-G.; Khalid, M.; Omar, N. Fabrication of 3D binder-free graphene NiO electrode for highly stable supercapattery. Sci. Rep. 2020, 10, 11214. [Google Scholar] [CrossRef] [PubMed]
- Conway, B.E.; Birss, V.; Wojtowicz, J. The role and utilization of pseudocapacitance for energy storage by supercapacitors. J. Power Source 1997, 66, 1–14. [Google Scholar] [CrossRef]
- Ge, J.; Wu, J.; Fan, L.; Bao, Q.; Dong, J.; Jia, J.; Guo, Y.; Lin, J. Hydrothermal synthesis of CoMoO4/Co1−xS hybrid on Ni foam for high-performance supercapacitors. J. Energy Chem. 2018, 27, 478–485. [Google Scholar] [CrossRef]
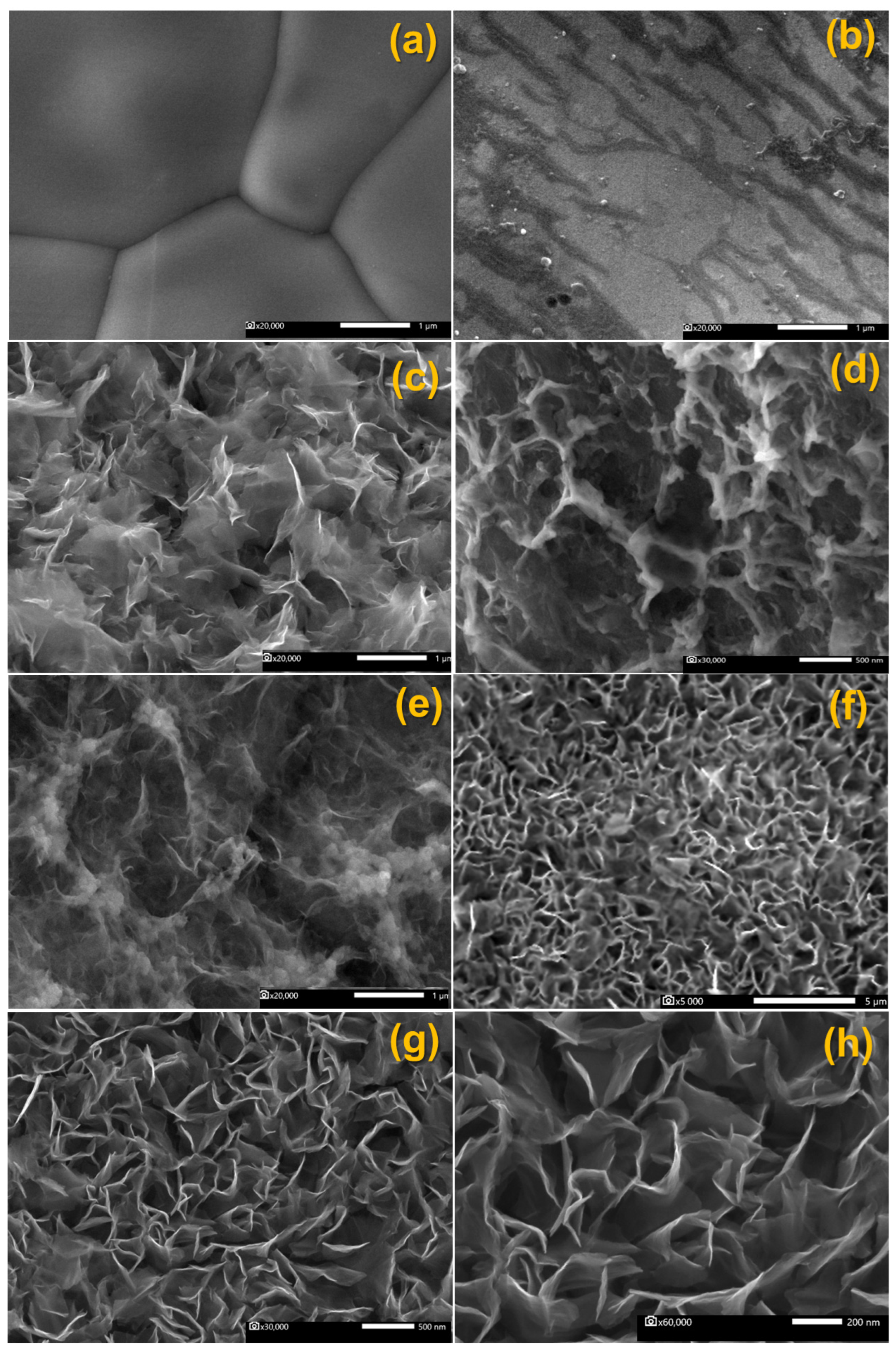
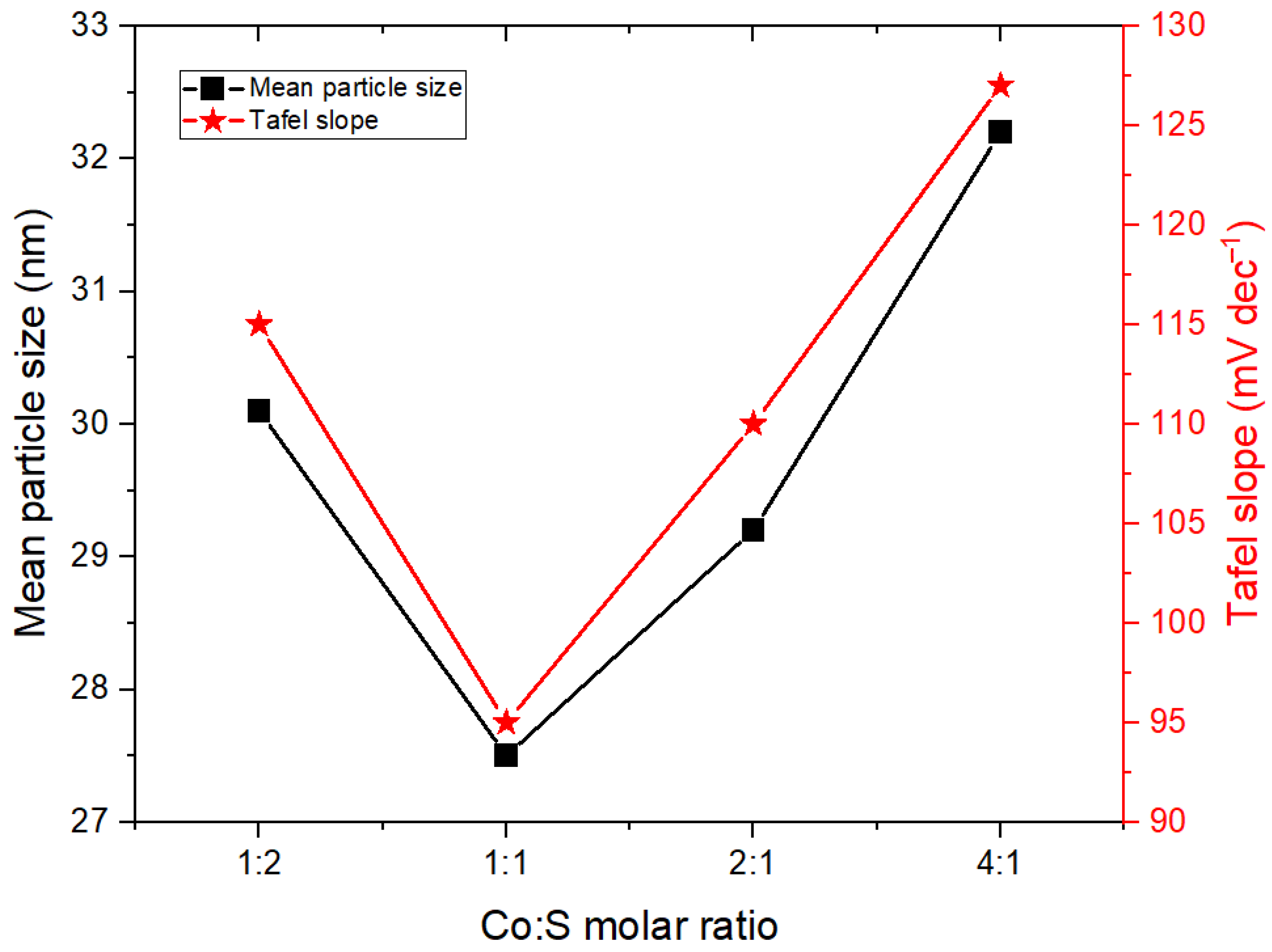
| Electrode | Mean Particle Size (nm) |
|---|---|
| NF | 35.3 |
| Co:S (1:0) | 28.1 |
| Co:S (0:1) | 31.5 |
| Co:S (1:2) | 30.1 |
| Co:S (2:1) | 29.2 |
| Co:S (4:1) | 32.2 |
| Co:S (1:1) | 27.5 |
| Electrode | |η10| (V) | Tafel Slope (mV dec−1) |
|---|---|---|
| NF | 0.42 | 134 ± 0.01 |
| Co:S (1:0) | 0.31 | 106 ± 0.7 |
| Co:S (0:1) | 0.53 | 135 ± 0.7 |
| Co:S (1:2) | 0.32 | 113 ± 0.6 |
| Co:S (2:1) | 0.34 | 110 ± 0.4 |
| Co:S (4:1) | 0.3 | 127 ± 0.7 |
| Co:S (1:1) | 0.28 | 95 ± 0.3 |
| Electrodes | Method | Tafel Slope mV dec−1 | Electrolyte | Reference |
|---|---|---|---|---|
| NiCo2S4/Ni3S2 | Hydrothermal | 137 | 1 M KOH | [50] |
| NiCo-LDHs | Hydrothermal | 118 | 1 M KOH | [51] |
| Co-Ni3S2/NF | Hydrothermal method–liquid-phase vulcanization | 176 | 1 M KOH | [52] |
| CoNiSx/NF | Sulfuration process | 107 | 1 M KOH | [53] |
| NiCo2S4/NF | Hydrothermal | 95 | 1 M KOH | [54] |
| Co9S8 NM/NF | Hydrothermal | 150 | 1 M KOH | [55] |
| NiCo2S4-NF | Hydrothermal | 91 | 1 M NaOH | [56] |
| Ru nanoparticles | Laser-generated | 70 | 0.5 M H2SO4 | [57] |
| Ru@IrOx | Charge redistribution | 69 | 0.05 M H2SO4 | [58] |
| Co:S (1:1)@NF | Electrodeposition | 95 | 1 M KOH | This work |
| Electrode | CDL Value (mF cm−2) | ECSA (cm2) |
|---|---|---|
| NF | 0.76 ± 0.13 | 38 ± 0.5 |
| Co:S (1:0) | 1.96 ± 0.13 | 98 ± 0.4 |
| Co:S (0:1) | 0.26 ± 0.01 | 13 ± 0.3 |
| Co:S (1:2) | 1.67 ± 0.09 | 83.5 ± 0.5 |
| Co:S (2:1) | 2.16 ± 0.23 | 108 ± 0.8 |
| Co:S (4:1) | 1.78 ± 0.19 | 89 ± 0.4 |
| Co:S (1:1) | 10.74 ± 0.71 | 537 ± 1.1 |
| Electrode | Rs (Ω) | Rct (Ω) |
|---|---|---|
| Co:S (1:0) | 2.1 ± 0.003 | 39.4 ± 9.5 |
| Co:S (0:1) | 2.8 ± 0.004 | 155,566 ± 3449 |
| Co:S (1:2) | 2.5 ± 0.004 | 1.9 ± 0.2 |
| Co:S (2:1) | 2 ± 0.009 | 521 ± 7 |
| Co:S (4:1) | 2.3 ± 0.01 | 0.75 ± 0.03 |
| Co:S (1:1) | 2.2 ± 0.008 | 0.000006 ± 0.008 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poimenidis, I.; Papakosta, N.; Loukakos, P.A.; Marnellos, G.E.; Konsolakis, M. Highly Efficient Cobalt Sulfide Heterostructures Fabricated on Nickel Foam Electrodes for Oxygen Evolution Reaction in Alkaline Water Electrolysis Cells. Surfaces 2023, 6, 493-508. https://doi.org/10.3390/surfaces6040033
Poimenidis I, Papakosta N, Loukakos PA, Marnellos GE, Konsolakis M. Highly Efficient Cobalt Sulfide Heterostructures Fabricated on Nickel Foam Electrodes for Oxygen Evolution Reaction in Alkaline Water Electrolysis Cells. Surfaces. 2023; 6(4):493-508. https://doi.org/10.3390/surfaces6040033
Chicago/Turabian StylePoimenidis, Ioannis, Nikandra Papakosta, Panagiotis A. Loukakos, George E. Marnellos, and Michalis Konsolakis. 2023. "Highly Efficient Cobalt Sulfide Heterostructures Fabricated on Nickel Foam Electrodes for Oxygen Evolution Reaction in Alkaline Water Electrolysis Cells" Surfaces 6, no. 4: 493-508. https://doi.org/10.3390/surfaces6040033
APA StylePoimenidis, I., Papakosta, N., Loukakos, P. A., Marnellos, G. E., & Konsolakis, M. (2023). Highly Efficient Cobalt Sulfide Heterostructures Fabricated on Nickel Foam Electrodes for Oxygen Evolution Reaction in Alkaline Water Electrolysis Cells. Surfaces, 6(4), 493-508. https://doi.org/10.3390/surfaces6040033