Abstract
Among diverse contemporary colour prints, silver dye bleach prints and chromogenic prints are difficult to differentiate. They share similar visual characteristics and can use identical supports and surface finishes. However, their image-forming dyes differ, resulting in disparate conservation and restoration needs. This study aimed to determine practical measures for unambiguously differentiating between these two print types. Identifying characteristics—referred to here as ‘identifiers’—were collected from popular conservation sources and a mixed-method questionnaire survey. The accuracy and feasibility of these identifiers were evaluated against known prints sets. Examinations made use of water droplets, various light sources, digital 3D microscopy, and spectrophotometry. Results dichotomised these identifiers into ‘definite’ or ‘indefinite’ with ‘definite identifiers’ being able to discriminate independently. Only five out of 23 entries were termed definite identifiers. Azo dyes—image dyes of silver dye bleach prints—were established as the only constant definite identifiers of this print type. These findings were integrated into a flowchart to guide differentiation with the main recommendation being to deviate from indefinite identifiers to save time and effort. Parts of this work have been submitted in partial fulfilment of the requirements for a Master of Science degree at the University of Amsterdam in 2020.
1. Introduction
Back in 2019, participants of a process identification workshop failed to differentiate between two types of contemporary colour prints: silver dye bleach and chromogenic [1]. A photographic process is a chemical operation of printing photographs. The participants consulted current conservation sources for identification characteristics, which this paper refers to as ‘identifiers’. Observations were made using the unaided eye, stereo and handheld digital microscopes with magnifications ranging from 20× to 220×, and handheld torches with different spectrum properties. Nevertheless, the same identifiers seemed to indicate both print types, and hence, differentiation was made difficult. A mixed-method questionnaire survey conducted the following year established that even senior photograph conservators had similar difficulties differentiating between the two [2] (pp. 175–223).
The background research of this study revealed that silver dye bleach prints and chromogenic prints share similar visual characteristics. They both use an integral tripack emulsion that displays a continuous tone with visible image structure [3,4], and both are often printed on identical supports, resulting in indistinguishable surface finishes (Figure 1). However, their image dyes differ, resulting in varied light versus dark stability behaviour [5] (pp. 121–125) and dissimilar reactions with water [6] (pp. 105–108). These types of prints, therefore, have distinct needs regarding conservation and restoration. Despite this, difficulties in differentiation still form one of the main reasons why these types of prints continue to be stored together in most of the institutions polled. The purpose of this research was, thus, to understand the underlying problem with this differentiation and determine practical methods and tools to differentiate between these types of prints.

Figure 1.
(a) Photographer unknown (ca., 2000). ILFOCHROME CPS.1K [Photograph]. A silver dye bleach sample print on voided polyester support. (b) Photographer unknown (ca., 2000) IlfoColor IL.1K [Photograph]. A chromogenic sample print (negative-positive) on voided polyester support. Ilford manufacturer sample print set. Courtesy of Martin Jürgens (Rijksmuseum), photo-documented by Suk Fong Chun under daylight 5000 K softboxes using a Canon EOS 5Ds fitted with a 24–70 mm lens. These two prints made using different processes have similar visual characteristics and identical supports and gloss.
Previous literature revealed that, ca., 99% of colour prints are chromogenic [7] (p. 411) and have more variants than silver dye bleach prints [8] (pp. 161, 207). As this research aimed only at differentiating between silver dye bleach prints and chromogenic prints, it was necessary only to identify one versus the other. Thus, for efficiency, as the number of silver dye bleach prints was limited, they became the primary focus. Within this print type, only Cibachrome (renamed ILFOCHROME in 1992) [9] was used as a reference, as it was the dominant commercial product [8] (p. 216).
The first objective of this study was to understand the material properties of each print type, collect as many identifiers of silver dye bleach prints as possible, and compare those identifiers for uniqueness against those of chromogenic prints. These unique identifiers were referred to as ‘definite identifiers’, as opposed to ‘indefinite identifiers’; whereas the former could discriminate, the latter were common to both print types. The conclusion was that using definite identifiers alone could effectively discriminate. The outcome was a flowchart to guide differentiation. Parts of this work have been submitted in partial fulfilment of the requirements for a Master of Science degree at the University of Amsterdam in 2020 [2].
2. Materials and Methods
2.1. Silver Dye Bleach vs. Chromogenic
The silver dye bleach prints produced under the trade name Cibachrome (1963) are known for image quality and stability, as a result of their distinctive component materials and chemical development [10] (p. 7). These prints have appealed to diverse sectors, from NASA [11] to artists such as Jeff Wall and Irving Penn [12].
Silver dye bleach is a ‘positive-positive’ process—meaning that a print is a positive reproduction of the original colour transparency. Silver dye bleach is the only colour print material that incorporates preformed image dyes (azo dyes with aromatic rings) in the gelatine emulsion before use (Figure 2). This emulsion has an integral tripack construction, in which each layer incorporates, from top to bottom, the corresponding azo dyes of one of the three subtractive primary colours (yellow, magenta, and cyan) [13] (pp. 134–136). Support materials are limited to cellulose triacetate, polyester, and resin-coated paper [14].
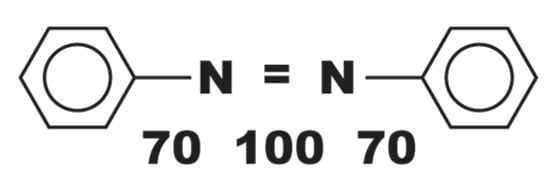
Figure 2.
Schematic of azo dye with aromatic rings showing bond energy in Kcal mol−1. Source: Figure by S. F. Chun based on a schematic from Krause P.; Shull, H. The Complete guide to Cibachrome printing, H.P.: Tucson, Arizona, USA, 1982, p. 148.
The silver dye bleach process works by reducing unwanted azo image dyes using metallic silver. In a developing bath, the exposed silver halides are reduced to metallic silver to form a negative black-and-white image. This step is followed by bleaching, in which the bleach catalyst shuttles back and forth between the silver atom and the azo dye, bleaching a ring-form halo in situ [15] (p. 542). Upon completion, the site becomes an emptied hole and no longer contains dyes or silver atoms, making it a definite identifier of silver dye bleach prints [16].
The chromogenic process was introduced by Kodak and Agfa around 1936 [13] (p. 3). It is used in two manners: as a negative-positive process or a positive-positive (i.e., reversal) process. The former prints a positive image directly from a colour negative, and the latter prints a positive image from a slide using two exposures and two developments to reverse the process. Both negative-positive and reversal print materials lack colour dyes before use; colour dyes are formed only during processing, when the oxidised colour developer reacts with the colourless colour couplers (i.e., the dye-forming organic compounds) [13] (pp. 118–123, 129–130). The dyes formed are typically azomethine dyes; the colour-forming process results in signature dye clouds. However, the morphologies of dye clouds may vary in terms of product designs and processing conditions [15] (p. 279). As for an exposed, developed colour negative print, the order of dye layers from top to bottom is: cyan, magenta, and yellow. For an exposed, developed colour reversal print, from top to bottom, it is: yellow, magenta, and cyan [13] (pp. 120–121).
2.2. Methods and Tools
The material properties of contemporary colour prints are considered proprietary information. The first research method, archival research, therefore consulted patents [17,18,19,20,21] in addition to early product presentations [22] (pp. 90–97); manufacturer handbooks, such as Coote [23], Krause and Shull [24], Shanebrook [25]; scientific literature by Keller and Fujita; and light and dark stability data by Wilhelm and Brower [26]. This search was combined with an object-based study centred around an Ilford manufacturer sample set (ca., 2000) and the Andrew W. Mellon Foundation conservation digital print reference set (2006). The former contains, ca., 34 sheets (8 inches × 10 inches) of silver dye bleach prints (Cibachrome and ILFOCHROME) as well as chromogenic prints (IlfoColor).
The second method aimed to collect identifiers. Three popular photograph conservation sources were selected for collecting customised identifiers: Pénichon’s Twentieth Century Colour Photographs; Graphics Atlas; the Photographic Materials Group Wiki [27]. In addition, a mixed-method questionnaire survey was conducted to also unearth underused and unpublished identifiers. The questionnaire survey, distributed by email, targeted respondents from diverse professionals backgrounds, including art and heritage conservation, printing, auctions, and nanotechnology in Europe, the United Kingdom, and the United States. The key question therein was to ask the participants to propose identifiers they used, or would use, for identifying silver dye bleach prints and to prioritise the identifiers based on aptitude [2] (pp. 172–174).
The third method dichotomised the collected identifiers into ‘definite’ or ‘indefinite’. First, repeatedly proposed identifiers were combined as a single entry, and the final entries were then arranged in tables according to the frequency proposed and the priority perceived, respectively. This was followed by visual examination of the samples using the methods and tools described in the selected conservation sources, as well as those indicated in the survey responses. Diverse materials and instruments were used during the examinations, including water droplets, light sources (light-emitting diode, fluorescent, and incandescent), digital 3D microscopy (Hirox KH-7700, RH-2000), and spectrophotometry (GretagMacbeth Eye-One). Destructive, or non-destructive but invasive, experiments were attempted on donated test materials, while non-invasive examinations were conducted on the Ilford and Mellon sample sets and multiple private loans. Most examinations were single experiments, except for a redshift observation and the collection of spectra, which were conducted with multiple participants at a workshop [16].
3. Results
3.1. Positive Process and Order of Dye Layers
The archival and object-based research clarified that both silver dye bleach prints and chromogenic reversal prints used resin-coated paper with semi-gloss and glossy finishes, in addition to polyester and the superseded cellulose triacetate supports. Both print types were confirmed using the positive-positive process and had identical orders of dye layers.
3.2. Compatible Print Materials and Chemical Processes
Based on the previous sources and the Ilford manufacturer sample set, Ilford produced four silver dye bleach product lines between 1963 and 2012. Of these, there were, ca., 11 chemical processes compatible with, ca., 34 silver dye bleach print materials. However, previous inventories lacked either the chemical processes or their production end dates. For example, in The Focal Encyclopedia of Photography, an early print material, Cibachrome-A (ca., 1974–1980), and the chemical process P30 were mismatched [28] (p. 710); the latter was introduced only in, ca., 1980 [29].
3.3. Identifiers from Conservation Sources
Fourteen identifiers were found in the three selected conservation sources. Among them, 11 (78.6%) overlapped with the entries of the survey, causing a high rate of adoption of the identifiers from conservation sources. However, three entries were found to be exclusive to the conservation sources, including ‘redshift’, which is described further in Section 3.5.2.
3.4. Identifiers from Survey
The survey response rate was high (62.5%), as 20 out of 32 professionals participated. Responses abounded in tips and questions concerning differentiation, and some responses even arrived alongside additional long emails. After categorising, there were altogether 23 entries of identifiers. Among them, 11 (47.8%) overlapped with those from the conservation sources, and 12 (52.2%) were exclusive to the survey. A spider chart was used to demonstrate the popularity of proposed identifiers, ranging from 1 to 11 (Figure 3). They were composed of both definite and indefinite identifiers. The former was highlighted in orange.
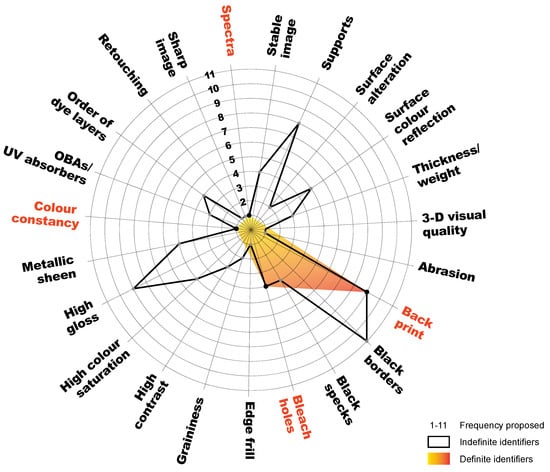
Figure 3.
Spider chart of definite and indefinite identifiers collected from a mixed-method questionnaire survey. Modified illustration of Chun, S. F. Cibachrome inside out: Identification of silver dye bleach prints. Master of Science in Conservation and Restoration of Cultural Heritage, University of Amsterdam, 2020; p. 73.
3.5. Definite Identifiers vs. Indefinite Identifiers
In total, 15 identifiers collected in both manners (sources and survey) were examined [2] (p. 39–62). Those identifiers concerning support materials, for instance, terms such as ‘high gloss’, were determined as indefinite due to the similarities already described between the two print types. ‘High colour saturation’, ‘high contrast’, and ‘sharpness’ were determined as indefinite identifiers because they were dependent on multiple factors, such as product design, the quality and subject of the original slides or negatives, and the processing conditions. As both silver dye bleach and chromogenic reversal use a positive-positive process and, hence, have identical orders of dye layers, any identifiers concerning process and layering—such as ‘black borders’, ‘black specks’, and ‘surface colour reflection’—were nullified. Further, the presence or absence of optical brightening agents and ultraviolet absorbers alone could not discriminate between silver dye bleach prints and chromogenic prints, because these print types have vast varieties, and additives are proprietary. For instance, a patent from as early as 1972 already suggested that ultraviolet absorbers could have been used in silver dye bleach prints [30]. ‘Edge frill’ and ‘Thickness/weight’ were not examined, due to inadequate resources.
Research found that five identifiers were definite, as opposed to 19 that were indefinite. Figure 3 demonstrated that the frequency proposed was much higher for indefinite identifiers than for definite identifiers. These five definite identifiers were ‘back print’, ‘bleach holes’, ‘colour constancy’, ‘spectra’, and ‘redshift’. However, this research found that back print (the product name and/or logo printed on the verso) was absent in both print types on polyester supports and was often missing in silver dye bleach prints on resin-coated paper. Therefore, back print was a definite identifier, although infrequently present. The other four definite identifiers are properties of the image azo dyes. The empirical experiments presented below were conducted to determine and evaluate these last four definite identifiers.
3.5.1. Bleach Holes
This experiment evaluated the practicality of observing bleach holes with high magnification. These were conducted on a silver dye bleach print on polyester support (model CCO.1K), a chromogenic print on polyester support (a Fuji high-gloss print), and a silver dye bleach print on resin-coated paper support (model RP L.44M).
The first observation used a Hirox digital microscope KH-7700 from the University of Amsterdam. At 160x, the bleach holes in the medium-density areas (red areas) of a silver dye bleach print were marginally identifiable (Figure 4a). Yet, similar ‘white holes’ were also seen in the medium-density areas (areas in a similar red colour) of a chromogenic print under the same magnification (Figure 4b). Bleach holes were more observable in the maximum-density areas (dark blue to black areas) of another silver dye bleach print (Figure 4c).
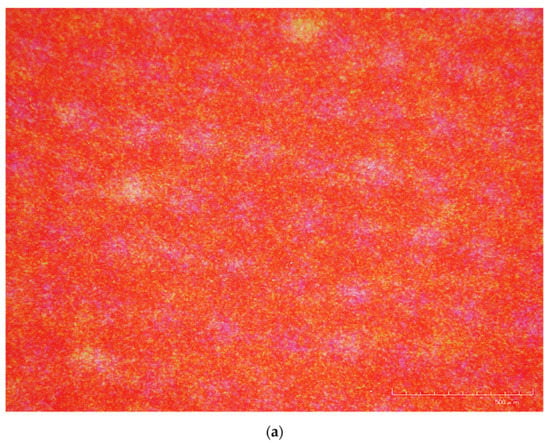
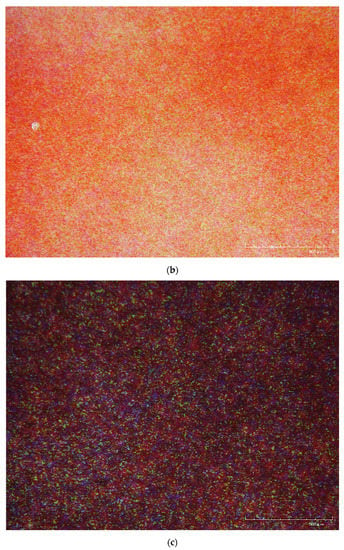
Figure 4.
(a) Magnification at 160X of a Cibacopy CCO.1K, a silver dye bleach sample print on voided polyester support with photographer unknown (ca., 2000). (b) 160X of a Fuji High Gloss, a chromogenic print on voided polyester support, photographer: Suk Fong Chun (ca., 2003). (c) 160X of a Rapid RL P.44M, a silver dye bleach sample print on resin-coated paper with photographer unknown (ca., 2000). These images were made using a Hirox digital microscope KH-7700 from the University of Amsterdam. All three images display ‘white holes’ at 160x but only those in 4a and 4c are bleach holes. Source: Chun, S.F. Cibachrome inside out: Identification of silver dye bleach prints. Master of Science in Conservation and Restoration of Cultural Heritage, University of Amsterdam, 2020; pp. 47–49.
For the second set of examinations, a Hirox model RH-2000 fitted with a revolver zoom lens from the Rijksmuseum was used at a slightly higher magnification. At 200x, bleach holes were evident in maximum-density areas of a silver dye bleach print only. Determination could not be made in medium areas. No images were recorded from this second observation. It is noteworthy to include that the support materials were different.
3.5.2. Redshift
Redshift is a visible colour change due to the de- and reaggregation of the cyan azo dyes. When wet, the cyan dyes fractionate into monomers (deaggregation), causing the absorption spectrum to shift to shorter wavelengths (blue/green). Thus, the image dye colour shifts to a complementary colour (i.e., red) and becomes less stable. Upon drying, reaggregation occurs, and the colour shifts back to gain stability. However, oxidation, which is irreversible, can occur during the deaggregation, resulting in part of the colour being unable to shift back [6] (pp. 105–108).
In this experiment, a strip of silver dye bleach print and a strip of chromogenic negative-positive print were used. Two drops of demineralised water were applied to the emulsion surface of each print and then air-dried. Observation under mixed lights (daylight through the window and the fluorescent cool white ceiling light) showed that the emulsion of the silver dye bleach print did not reverse to its original grey colour but dried to a pinkish tone. No effect was visible on the chromogenic print (Figure 5).

Figure 5.
(a) Before: The strip on the left is a silver dye bleach print on polyester support; the strip on the right is a chromogenic print strip (with steps) on polyester support. They were undergoing a redshift test using demineralised water droplets. (b) After: When dried, local discolouration occurred on the silver dye beach strip (left), while no change was on the chromogenic strip (right). Both strips were donated by Rita Hofmann-Sievert (BFH HKB), photo-documented by Suk Fong Chun under mixed indoor light using an iPhone 8. Source: Chun, S.F. Cibachrome inside out: Identification of silver dye bleach prints. Master of Science in Conservation and Restoration of Cultural Heritage, University of Amsterdam, 2020; p. 58.
3.5.3. Colour Constancy
Colour constancy refers to changes in the colour of a material as the light source varies, caused by the spectral properties of the colourants used [31] (p. 106). This change is evident when the light sources have distinctive spectral compositions and is most observable near neutral grey [32].
In this experiment, three known sample prints were selected from the ‘Contemporary Photography: Digital Prints’ sample set by the Andrew W. Mellon Foundation—namely, a silver halide gelatine print (Ilford multigrade warm tone, fibre base, 2006); a chromogenic print (Kodak Endura Supra, resin-coated, 2006); a silver dye bleach print (ILFOCHROME Classic Deluxe, polyester, 2006). The silver gelatine print served as a neutral grey reference, while the chromogenic print and the silver dye bleach print were used to provide a comparison.
Incandescent light (sunlight through clear window glass) and fluorescent light (compact fluorescent lamp, Walsun PL 9W, 6400 K) were chosen for their distinctive spectral compositions, while the grey areas of the three sample prints were placed adjacent to each other to facilitate visual comparison. They were observed with the unaided eye, viewing from a straight angle (45°). As the print moved from the windowpane towards the lamp, the grey in the silver dye bleach print became slightly reddish. Any change evident in the chromogenic print was negligible (Figure 6).
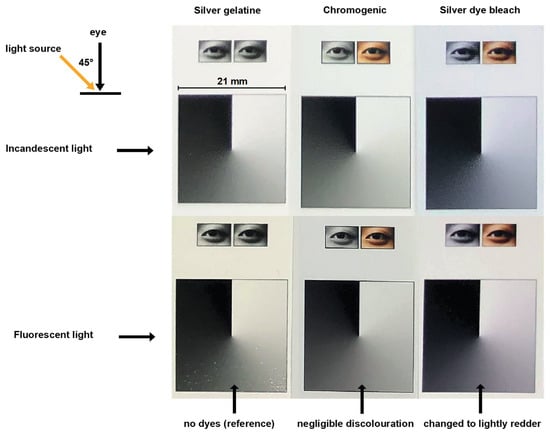
Figure 6.
Left to right: Ilford multigrade warm tone, fibre base, 2006, a silver gelatine print; Kodak Endura Supra, 2006, a chromogenic resin-coated print; ILFOCHROME Classic Deluxe, polyester, 2006, a silver dye bleach print. These are sample prints from the ‘Contemporary Photography: Digital Prints’ sample set of the Andrew W. Mellon Foundation, donated by Richard Jackson, photo-documented by Suk Fong Chun under mixed indoor light. The silver dye bleach print changed to slight reddish as light sources changed from incandescent to fluorescent. Source: Chun, S.F. Cibachrome inside out: Identification of silver dye bleach prints. Master of Science in Conservation and Restoration of Cultural Heritage, University of Amsterdam, 2020; p. 77.
3.5.4. Reflectance Spectra and Reflection Density Spectra
Many approaches can identify the colour of a surface as incident light strikes. The ratio between the incident intensity of light and the reflected intensity of light as a function of wavelengths is a reflectance spectrum. A reflectance spectrum is a fingerprint of a colourant [33] (pp. 5–14). Another way to represent dye spectra is to convert the reflectance spectra into reflection density spectra. The reflection density is the negative logarithm of the reflectance [33] (pp. 149–154). It sometimes shows more details in wavelength ranges of lower absorption. The reflection density spectra of the azo image dyes (silver dye bleach prints) have typical peak forms, evidently different from the azomethines dyes (chromogenic prints) [32].
The dye spectra were collected using a hand-held spectrophotometer, GretagMacbeth Eye-One, and three known prints—namely, a silver dye bleach print (ILFOCHROME Classic CPS.1K), a chromogenic print (Kodak Professional Paper), and another chromogenic print (Fuji Crystal Archive Pro Type C) as a control (Figure A1). All the prints were test strips laminated and dated 1998. Measurements were conducted without using any overlay with registration marks.
First, the magenta patch (at the highest density) was measured of each of the three prints. The yellow curve (dotted triangles) corresponds to the Kodak print, blue curve (dotted diamonds) to the Fuji print, and magenta curve (dotted squares) to the ILFOCHROME. The magenta azomethine dyes have a typical gaussian graph (symmetric bell-shape), while the magenta azo dyes have a typical steep slope, featuring a maximum peak at a longer wavelength than the magenta azomethine dyes. The difference in wavelength is marked by two blue vertical lines (Figure 7a).
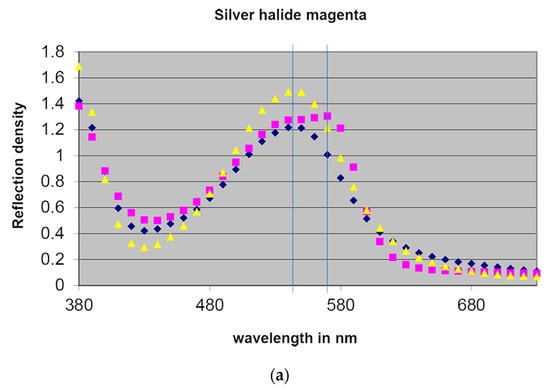
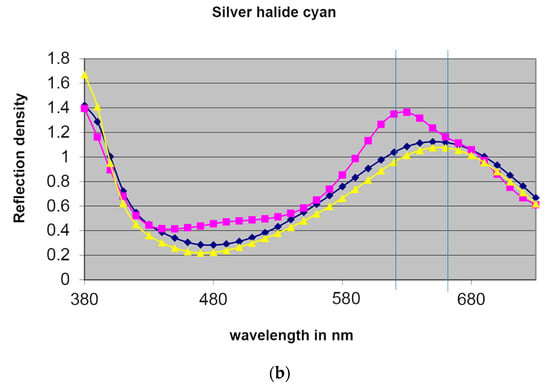
Figure 7.
(a) Reflection density spectra of the magenta image dyes of a silver dye bleach print (dotted magenta squares) and two chromogenic prints (dotted yellow triangles and dotted blue diamonds). (b) Reflection density spectra of the cyan image dyes interpreted in the same manner. Source: Hofmann-Sievert, R. ‘Contemporary Silver Halide Colour Photography’, workshop demonstration, University of Amsterdam, 4 December 2019. Modified illustration of Chun S. F. Cibachrome inside out: Identification of silver dye bleach prints. Master of Science in Conservation and Restoration of Cultural Heritage, University of Amsterdam, 2020; p. 79.
The second scans measured the cyan patches in the same manner as above. The cyan azomethine dyes have a typical gaussian graph, while the cyan azo dyes have a distinct steep slope, with the maximum peak at a shorter wavelength than the cyan azomethine dyes, as marked by two blue vertical lines (Figure 7b). In this way, the distinctions between the two types of image dyes are evident.
4. Discussion
The Ilford manufacturer sample set allows for an object-based comparison between silver dye bleach and chromogenic print materials, many of which display indifferentiable visual characteristics. Previous sources, such as Pénichon′s Twentieth Century Colour Photographs and Graphics Atlas, mention the similarities between silver dye bleach prints and chromogenic reversal prints in the order of dye layers and the use of direct processing. In this study, for the first time, any identifier correlating to processing or layering is inferred to be unable to discriminate and thus defined as indefinite identifiers.
Over the years, the same chemical process was designed to be compatible with multiple print materials [32]. This one-to-many relationship has not yet been described comprehensively in current sources, leaving room for improvement. In this study, four pairing tables are compiled to present these relationships per product line in chronological order (Table A1, Table A2, Table A3 and Table A4). Different Cibachrome and ILFOCHROME print materials have different light-sensitivity, granularity, sharpness, contrast, colour saturation, and chemical stability. These findings coincide with the uncertainty raised during attempts at differentiation.
The high response rate of the survey and the carefully written responses imply that participants show interest in this conservation and restoration issue. The rate of participants either using, or who would use, the identifiers collected from the sources is very high, suggesting that the three popular conservation sources influenced the choice of identifiers made by the survey participants. Much fewer definite identifiers than indefinite identifiers were used, or would be used. This also explains why differentiation between the two print types has hitherto been uncertain
Redshift was tested as a definite identifier, but the change in colour can be destructive and, therefore, is not recommended. As a last resort, spot tests should be constrained to imperceptible areas. The observability of bleach holes depends on the scale of the magnification and the density of the image, while the colour constancy test needs neutral grey references. However, non-sample prints may not contain a near-grey image colour; thus, visual comparison can be impractical. In comparison with observing bleach holes or colour constancy, observing the reflection density spectra is definite, objective, and readily achievable. Although the application of a spectrophotometer is instrumental, it is non-destructive and, thus, is still practical.
All these findings are integrated into a Silver Dye Bleach Identification Flowchart to highlight the relationships between the choice of identifiers and the destined determinations (Figure 8). To begin, once a print is determined to be colour and has no misregistration, photomechanical processes [34] (pp. 231–263), tricolour pigment processes [8] (pp. 80–125), and dye imbibition processes [8] (pp. 126–159) can be excluded. If the print has a continuous tone with clear image structure and texture, then the dye diffusion processes [8] (pp. 232–273) can be excluded (e.g., Polaroid). From there, it may be assumed that the print is either a silver dye bleach print or a chromogenic print. At this point, there are three diversions to access: processing, layering, or dyes.
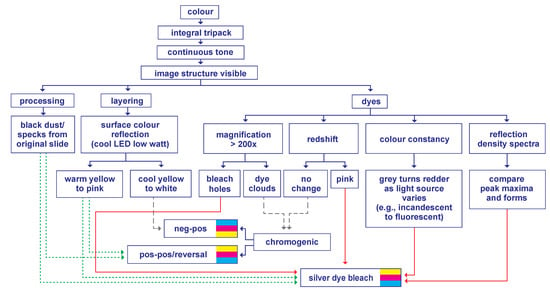
Figure 8.
Silver Dye Bleach Identification Flowchart. © Suk Fong Chun. Modified illustration of Chun, S. F. Cibachrome inside out: Identification of silver dye bleach prints. Master of Science in Conservation and Restoration of Cultural Heritage, University of Amsterdam, 2020; p. 81.
The dense green dotted lines link one identifier to two colour processes, which means this identifier is ambiguous. The loose grey dotted lines lead to the chromogenic process. The solid orange lines lead to the silver dye bleach process. In short, this flowchart shows that the identifiers related to processing and layering will not provide a definite identification of silver dye bleach prints, but the identifiers related to image dyes will.
5. Conclusions
The close similarity between silver dye bleach prints and chromogenic reversal prints significantly narrows options for identifiers. For the first time, the identifiers of silver dye bleach prints are dichotomised into definite or indefinite, with the latter common to both print types and hence unable to discriminate. The main recommendation made here is to deviate from indefinite identifiers to save time and effort. However, even though this proposal is supported by the results drawn from empirical examinations, it is still a fundamentally novel approach and needs a clear introduction. In this regard, a step-by-step disseminating plan may be helpful. As an early method of illustration, this Silver Dye Bleach Identification Flowchart could be used, which illustrates the relationships between the choice of identifiers and the resulting determinations at a glance.
TThe next step would be to panel-test this model extensively. As most of the examinations were single experiments conducted solely by the first author, double-blind experiments with more participants would be essential to build a larger dataset to improve validity. Of the four definite identifiers, redshift was destructive, colour constancy was tool-free but image-colour-dependent, and bleach holes and reflection density spectra were tool-dependent. Therefore, further research for more ways of identifying azo dyes is desirable. The existing layout of this Silver Dye Bleach Identification Flowchart is readily extendable to facilitate additional definite identifiers once they have been established in future studies. The same is true for the pairing tables once more product information has been confirmed.
As the photograph conservation field has a growing interest in the identification of colour processes, this Silver Dye Bleach Identification Flowchart may be considered the main contribution of this research because it shall guide a definite differentiation between silver dye bleach prints and chromogenic prints. The pairing tables may be seen as additional contributions as they can improve the accuracy in dating silver dye bleach print materials.
Author Contributions
Conceptualisation, S.F.C.; methodology, S.F.C.; validation, S.F.C. and R.H.-S.; investigation, S.F.C. and R.H.-S.; resources, S.F.C., R.H.-S. and S.S.; data curation, S.F.C.; writing—original draft preparation, S.F.C.; writing—review and editing, S.F.C., R.H.-S. and S.S.; visualisation, S.F.C. and R.H.-S.; supervision, S.S.; project administration, S.F.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available at UvA Scripties, University of Amsterdam at https://scripties.uba.uva.nl/search?id=c1010112;setlang=en (accessed on 1 November 2022).
Acknowledgments
This research would have been impossible without the generous support from Martin Jürgens of Rijksmuseum; printmaker Richard Jackson; Cibachrome photographer and printer Douglas Vincent; retired hand-printer Michael Talbert and site owner Maurice Fisher of Photomemorabilia.co.uk; and Cibachrome Association. Special thanks to Ella Hendriks, Maarten van Bommel, Maartje-Stols Witlox, Rene Peschar, Clara von Waldthausen, Katrin Pietsch, and fellow students of the Conservation and Restoration of Cultural Heritage programme of the University of Amsterdam; Rosina Herrera Garrido and Magdalena Pilko of Rijksmusem; Han Neevel and Suzan de Groot of Rijksdienst voor het Cultureel Erfgoed; Bertrand Lavédrine of Muséum National d'Histoire Naturelle; Monica Marchesi of Stedelijk Museum Amsterdam; Anne Ruygt of Fotomuseum Antwerpen; Kayleigh van der Gulik of Stichting Behoud Moderne Kunst Project Collectiekennis 2.0 Fotografie; Virginia Morant Gisbert of Nederlands Fotomuseum; Jessica Keister of Steel City Art Conservation; Sylvie Pénichon of Art Institute of Chicago; Nora W. Kennedy of The Metropolitan Museum of Art; Lee Ann Daffner of The Museum of Modern Art; Teresa Mesquit of Moderna Museet; Sarah Freeman of J. Paul Getty Museum; photographic conservator Susie Clark; Daniel Heikens of Dialogue Vintage Photography Foundation; landscape photographer Christopher Burkett; Ellen Dosse and Freek Baars of Spaarnestad Photo; Joanna Phillips, Jessica Morhard, and colleagues of the Restaurierungszentrum Düsseldorf. We thank Olivia Brum of LIMA Art Platform for proofreading this text.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Figure A1.
Chromogenic test strips used for measuring reflection density spectra: a Kodak Professional Paper and a Fuji Crystal Archive Pro Type C. The white dotted circles indicate the locations being measured. Source: Hofmann-Sievert, R. ‘Contemporary Silver Halide Colour Photography’, workshop demonstration, University of Amsterdam, 4 December 2019.

Table A1.
Chronology of Cibachrome and ILFOCHROME Systems (Professional).
Table A1.
Chronology of Cibachrome and ILFOCHROME Systems (Professional).
| Start Date (Ca.) | End Date (Ca.) | Process | Model Name | Code | Type | Support | Finish | Contrast | Maximum Size |
|---|---|---|---|---|---|---|---|---|---|
| 1959 | First Cibachrome laboratory print | Cellulose triacetate, verso painted white using gelatine and barium sulphate | High gloss | High | 16 × 22 mm | ||||
| 1963 | Cibachrome Display print | CCP | White-pigmented triacetate | ||||||
| 1964 | 1967 | P-7A | Cilchrome | 90 × 130 mm | |||||
| 1967 | 1973 | Cibachrome print material | CCP | ||||||
| 1969 | 1973 | Cibachrome transparency material | CCT-D 661 | CCT | Transparent cellulose triacetate | High gloss | High | ||
| 1973 | 1974 | P-10 | Cibachrome print material | CCP-D 182 | CCP | White-pigmented polyester | |||
| 1980 | Cibachrome transparency material | CCT-D 661 | CCT | Transparent polyester | |||||
| 1974 | 1980 | P-18 | Cibachrome print material | CCP-D 182 | CCP | White-pigmented polyester | |||
| 1980 | 1991 | P-3 | Cibachrome II | CPS.1K | CPS II | White polyester (voided polyester) | Sheet: 30 × 40 in Roll: 50 in × 30 m (98 ft) | ||
| CRC.44M | CRC II | Resin-coated (RC) paper | Pearl | ||||||
| CTD.F7 | CTD II | Transparent polyester | High gloss | ||||||
| 1987 | 1991 | CF.1K | CF | Voided polyester | Low | ||||
| CL.F7 | Transparent polyester | ||||||||
| 1991 | 2007 | P-3X P-3 P-30 | ILFOCHROME Classic Deluxe | CF.1K | CF | Voided polyester | Super glossy | ||
| 2011 | CPS.1K | CPS II | High (1992) | 268 gsm | |||||
| Normal (2003) | |||||||||
| 2012 | P-3X | CL M.1K (SP 761s) | Medium | (CL M2.1K 267 gsm) | |||||
| 2004 | ILFOCHROME Classic Film | COH.F7 (SP 760s) Overhead film | Transparent polyester | Sheet: 8.5 × 11 in Roll: 50 in × 65 ft | |||||
| 2012 | P-3X P-3 P-30 | CC.F7 (SP 759s) | Sheet: 20 × 24 in Roll: 50 in × 30 m (98 ft) (CC2.F7 296 gsm) | ||||||
| 2012 | CT.F7 (SP 806s) | Translucent polyester | 0.18 mm (CT2.F7 298 gsm) | ||||||
| P-3X | CT.F7.L (laser) | ||||||||
| 2004 | P-3X P-3 P-30 | ILFOCHROME Classic RC Paper | CP H.1M (SP 756s) | RC paper | Glossy | High | 180 gsm Sheet: 30 × 40 in Roll: 50 in × 30 m (98 ft) | ||
| CP M.1M (SP 804s) | Medium | ||||||||
| CP M.44M (SP 805s) | Pearl | ||||||||
| P-3X | CP M.44M.L (laser) | ||||||||
| End of 1990s | 2012 | A new range of ILFOCHROME were marketed to be compatible with the latest processors: CC2.F7, CLM2.1K, CPS.1K, CT2.F7 | |||||||

Table A2.
Chronology of Cibachrome-A and Cibachrome-A II Systems (Amateur).
Table A2.
Chronology of Cibachrome-A and Cibachrome-A II Systems (Amateur).
| Start Date (Ca.) | End Date (Ca.) | Process | Model Name | Code | Type | Support | Finish | Contrast | Sheet Size (Inch) |
|---|---|---|---|---|---|---|---|---|---|
| 1974 (UK) 1975 (USA) 1976 (Europe) | 1980 | P-12 | Cibachrome-A Kit | CCP-A 182E | A | White-pigmented acetate base | High gloss | Very high | 8 × 10 11 × 14 16 × 20 (USA, Canada) 12 × 16 (outside USA, Canada) |
| 1977 | Cibachrome Discovery Kit (USA) Cibachrome Starter Kit (UK) | CCP-A 182U | 4 × 5 (USA) 8 × 10 (UK) | ||||||
| 1979 | Resin-coated (RC) paper | Pearl | |||||||
| 1980 | 1989 | P-30 Liquid | Cibachrome-A II De Luxe Glossy (Initial type) | CPSA.1K | A II | White polyester (voided polyester) | Super glossy | High | 5 × 7 8 × 10 |
| Cibachrome-A II Paper (Initial type) | CRCA.44M | RC paper | Pearl | ||||||
| Cibachrome-A II Transparency (Initial type) | CTD.F7 | Transparent polyester | Super glossy | ||||||
| 1989 | 1991 | P-30P Powders | Cibachrome-A II De Luxe Glossy (Improved type) | CPSA.1K | Voided polyester | ||||
| Cibachrome-A II Paper (Improved type) | CRCA.44M | RC paper | Pearl | ||||||
| Cibachrome-A II Transparency (Improved type) | CTD.F7 | Transparent polyester | Super glossy |

Table A3.
Chronology of Cibacopy and ILFOCHROME Rapid Systems (Graphics).
Table A3.
Chronology of Cibacopy and ILFOCHROME Rapid Systems (Graphics).
| Start Date (Ca.) | End Date (Ca.) | Process | Model Name | Code | Type | Support | Finish | Contrast | Maximum Size |
|---|---|---|---|---|---|---|---|---|---|
| 1978 | 1991 | P-22 | Cibacopy | CCO.1K | CCO | White-pigmented polyester | High gloss | High | Roll: 11 in × 30 m (98 ft) |
| CCO.1M | Resin-coated (RC) paper | Glossy | Roll: 11 in x 60 m (196 ft) | ||||||
| CCO.44M | RC paper | Pearl | |||||||
| CCO.44L | RC paper Lightweight | ||||||||
| CTR.F7 | CTR | Translucent polyester | High gloss | Roll: 11 in × 60 m (196 ft) | |||||
| 1991 | 2004 | P-4 | ILFOCHROME Rapid | RP L.1K | RL | White polyester (voided polyester) | Super glossy | Low | Sheet: 30 × 40 in Roll: 50 in × 30 m (98 ft) |
| RP L.1M | RC paper | Glossy | |||||||
| RL L.44M | RC paper | Pearl | |||||||
| CTR.F7 | Translucent polyester | Super glossy | 8.5 × 11 in |

Table A4.
Chronology of Cibachrome and ILFOCHROME Micrographic Systems (Archival).
Table A4.
Chronology of Cibachrome and ILFOCHROME Micrographic Systems (Archival).
| Start Date (Ca.) | End Date (Ca.) | Process | Model Name | Code | Type | Support | Finish | Contrast | Thick-ness (Mil) | Maximum Size |
|---|---|---|---|---|---|---|---|---|---|---|
| 1984 | 1991 | P-5 | Cibachrome Micrographic Film | CMM.F4 | M (Master film) | Polyester | Super glossy | High | 4 | Roll: 16 mm and 35 mm × 30 m Sheet: 10.5 × 14.8 cm Roll: 10.5 cm × 30 m |
| CMM.F7 | 7 | Sheet: 24 × 30 cm, 21 × 29.7 cm | ||||||||
| CMP.F4 | P (Print film) | Low | 4 | Roll: 35 mm × 30 m and 300 m | ||||||
| CMP.F7 | 7 | Sheet: 10.5 × 14.8 cm, 18 × 24 cm, 21 × 29.7 cm | ||||||||
| 1991 | 2004 | ILFOCHROME Micrographic Film | Same as above | |||||||
Table A1, Table A2, Table A3 and Table A4. These tables are compiled based on the information from Pénichon, S. Twentieth Century Colour Photographs: The Complete Guide to Processes, Identification & Preservation [8], Cibachrome Association [9], Wilhelm, H; Brower C. The Permanence and Care of Color Photographs: Traditional and Digital Color Prints, Color Negatives, Slides, and Motion Pictures [26], Peres, M.R. (Ed.) Focal Encyclopedia of Photography [27], Graphics Atlas [29], Photomemorabilia.co.uk [35], and ebay listings. Source: Modified tables of Chun S. F. Cibachrome inside out: Identification of silver dye bleach prints. Master of Science in Conservation and Restoration of Cultural Heritage, University of Amsterdam, 2020; pp. 100–105.
References
- Van der Gulik, K. Workshop ID Color Photography, Object Based Practical 3; University of Amsterdam, 2019. [Google Scholar]
- Chun, S.F. Cibachrome Inside Out: Identification of Silver Dye Bleach Prints. Master of Science in Conservation and Restoration of Cultural Heritage; University of Amsterdam: Amsterdam, The Netherlands, 2020; pp. 39–62, 172–223. Available online: https://scripties.uba.uva.nl/search?id=c1010112 (accessed on 1 November 2022).
- Graphics Atlas: Silver Dye Bleach Magnification. Available online: http://www.graphicsatlas.org/media/images/id/silver_dye_bleach_magnification_image1_fullscreen.jpg (accessed on 1 November 2022).
- Graphics Atlas: Chromogenic Magnification. Available online: http://www.graphicsatlas.org/media/images/id/chromogenic_magnification_image1_fullscreen.jpg (accessed on 1 November 2022).
- Meyer, A.; Bermane, D. The stability and permanence of Cibachrome images. J. App. Photog. Eng. 1983, 9, 121–125. [Google Scholar]
- Bermane, D. Influence of azo-dye aggregation on the dark stability of Cibachrome images. J. Imag. Technol. 1985, 11, 105–108. [Google Scholar]
- Fenech, A.; Strlič, M.; Cassar, M. The past and the future of chromogenic colour photographs: Lifetime modelling using near-infrared spectroscopy & enhancement using hypoxia. App. Phys. A Mat. 2012, 106, 411. [Google Scholar] [CrossRef]
- Pénichon, S. Twentieth Century Colour Photographs: The Complete Guide to Processes, Identification & Preservation; Thames & Hudson: London, UK, 2013; pp. 80–159, 161, 216, 207, 232–273. [Google Scholar]
- Cibachrome Association: Patrimoine: Technologie Cibachrome. Available online: https://association-cibachrome.com/patrimoine/technologie/ (accessed on 1 November 2022).
- Reilly, J.M. Storage Guide for Color Photographic Materials: Caring for Color Slides, Prints, Negatives, and Movie Films; University of the State of New York: Albany, NY, USA, 1998; p. 7. [Google Scholar]
- United States. NASA Contractor Report No. NASA-CR-141629, 1974. Available online: https://ntrs.nasa.gov/api/citations/19750008729/downloads/19750008729.pdf (accessed on 1 November 2022).
- Metropolitan Museum of Art: The Collection: Photographers: Jeff Wall; Irving Penn. Available online: https://www.metmuseum.org/art/collection/search/286725,https://www.metmuseum.org/art/collection/search/714753?ft=silver+dye+bleach%2c+Iriving+Penn&offset=0&rpp=40&pos=1 (accessed on 1 November 2022).
- Keller, K. (Ed.) Science and Technology of Photography; VCH: Weinheim, Germany, 1993; pp. 3, 118–123, 129–130, 134–136. [Google Scholar]
- Cibachrome Association: Cibachrome II. Available online: https://association-cibachrome.com/2019/03/13/cibachrome-ii/ (accessed on 1 November 2022).
- Fujita, S. Organic Chemistry of Photography; Springer: Berlin, Germany, 2004; pp. 279, 542. [Google Scholar]
- Hofmann-Sievert, R. Workshop Contemporary Silver Halide Colour Photography, Object Based Practical 3; University of Amsterdam: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Anderau, W.; Von Wartburg, R.; Piller, B. Photographic Material for The Silver Dyestuff Bleaching Process. United States Patent Office Patent no. 3,394,004, 1968. Available online: https://patentimages.storage.googleapis.com/df/77/66/216b6f53175187/US3394004.pdf. (accessed on 1 November 2022).
- Dreyfuss, P. Method of Producing Color Photographic Pictures. United States Patent Office Patent No. 3,156,561, 1964. Available online: https://patentimages.storage.googleapis.com/10/4c/f5/c1a5f754b906ba/US3156561.pdf. (accessed on 1 November 2022).
- Marthaler, M.; Jan, G. Method for Processing Silver Dye-Bleach Materials. United States Patent Office Patent No. 4,304,846, 1981. Available online: https://patentimages.storage.googleapis.com/47/ad/67/d83929222c348a/US4304846.pdf. (accessed on 1 November 2022).
- Steiger, R.; Brugger, P. Process for The Spectral Sensitisation of Photographic Silver Halide Emulsions and Products Thereof. European Patent Office Patent No. EP0428334B1, 1991. Available online: https://patentimages.storage.googleapis.com/b9/b7/8a/bf6196cafae777/EP0428334B1.pdf. (accessed on 1 November 2022).
- Steiger, R.; Schellenberg, M. Photographic Material for The Silver-Dye Bleaching Process. European Patent Office Patent No. EP0233152A2, 1987. Available online: https://patents.google.com/patent/EP0233152A2/en. (accessed on 1 November 2022).
- Meyer, A. Some Features of the Silver-Dye Bleach Process. J. Photog. Sci. 1965, 13, 90–97. [Google Scholar] [CrossRef]
- Coote, J.H. The Focalguide to Cibachrome; Focal Press: London, UK, 1978; p. 25. [Google Scholar]
- Krause, P.; Shull, H. The Complete Guide to Cibachrome Printing; H.P.: Tucson, AZ, USA, 1982. [Google Scholar]
- Shanebrook, R.L. Making Kodak Film: The Illustrated Story of State-of-the-Art Photographic Film Manufacturing; Robert, L., Ed.; Shanebrook: Rochester, NY, USA, 2010. [Google Scholar]
- Wilhelm, H.; Brower, C. The Permanence and Care of Color Photographs: Traditional and Digital Color Prints, Color Negatives, Slides, and Motion Pictures; Preservation Pub Co: Grinnell, IA, USA, 1993; pp. 135–136, 139, 142–144, 185–186, 194, 198–200. [Google Scholar]
- American Institute for Conservation Wiki: Photographic Materials Group Preservation of Traditional Color Photographic Materials: Silver Dye Bleach. Available online: https://www.conservation-wiki.com/wiki/PMG_Preservation_of_Traditional_Color_Photographic_Materials#Silver_Dye_Bleach (accessed on 1 November 2022).
- Peres, M.R. (Ed.) Focal Encyclopedia of Photography: Digital Imaging, Theory and Applications, History, and Science, 4th ed.; Focal Press: Burlington, MA, USA, 2007; p. 710. [Google Scholar]
- Graphics Atlas: Materials and Processing Chart. Available online: http://www.graphicsatlas.org/media/images/id/cibrachrome_materials_processing.pdf (accessed on 1 November 2022).
- Marthaler, M.; Oetiker, A. Silver Dye Bleach Photographic Elements and Processes for Their Use. United States Patent Office Patent No. 3,650,739, 1972. Section 3, Lines 49–53. Available online: http://patentimages.storage.googleapis.com/1f/4f/c4/99676d1b3e8be3/US3650739.pdf (accessed on 1 November 2022).
- Berns, R.S. Color Science and the Visual Arts: A Guide for Conservators, Curators and the Curious; The Getty Conservation Institute: Los Angeles, CA, USA, 2016; p. 106. [Google Scholar]
- Hofmann-Sievert, R.; (Bern University of the Arts, Bern, Switzerland). Personal communication (email), 10 June 2020.
- Johnston-Feller, R. Color Science in the Examination of Museum Objects: Nondestructive Procedures; The Getty Conservation Institute: Los Angeles, CA, USA, 2001; pp. 5–14, 149–154. [Google Scholar]
- Van Dijk, J. Handboek Herkennen Fotografische En Fotomechanische Procedés: Historische En Moderne Procedés En Digitale Afdruktechnieken; Primavera Pers: Leiden, The Netherlands, 2011; pp. 231–263. [Google Scholar]
- Cibachrome (the Silver Dye Bleach Process). Available online: https://www.photomemorabilia.co.uk/Ilford/Cibachrome.html (accessed on 1 November 2022).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).