Abstract
Stones of historical monuments exposed to the open air deteriorate over the course of time depending on physical, chemical, and biological factors acting in co-association. Among the biological factors, microorganisms play a key role in the deterioration process of stones. Detecting the level of microbial activity on stones is an essential step in diagnostic and monitoring studies of stone biodeterioration, and aids in controlling the performance of treatments applied to the stones. Therefore, this study aimed to develop a practical and rapid method for the determination of microbial activity on historical stones and use this method on the Mount Nemrut monuments (MNMs) (Adiyaman, Turkey). For that purpose, the fluorescein diacetate (FDA) hydrolysis method, frequently employed for soil environments, was adapted for the estimation and assessment of total microbial activity to understand whether microorganisms posed a potential risk for the biodeterioration of the limestones and sandstones of the MNMs. The traditional plate count method was also applied simultaneously to the same stone samples to compare and assist in the interpretation of the results of the FDA hydrolysis method, which relies on the quantitative determination of bacterial and fungal colonies in nutrient agar and malt extract agar medium, respectively. The results of the FDA hydrolysis and plate count methods showed consistency. The total microbial activity determined by the FDA hydrolysis method was low for both types of stone samples. In addition, the plate count method showed low bacterial and fungal counts on all of the samples. This revealed that microbial activity did not play an important role in the stone deterioration process on the MNMs, although different lichen species were frequently observed on both the sandstones and the limestones. Hence, further investigation must be undertaken for determination of their long-term behavior and effects on the stones of the MNMs. On the other hand, the results of the FDA hydrolysis and plate count methods showed correlation. Lower bacterial counts were observed when lower enzymatic activity was observed in the stone samples, and likewise, higher bacterial counts were observed when higher enzymatic activity was observed. Consequently, the application of the FDA hydrolysis method was determined to be reliable for the estimation of total microbial activity on historical stones. The method had obvious advantages in terms of its rapid measurement rate and sensitivity, even on small samples.
1. Introduction
While looking attentively at the stones of a historical building or monument, few stones appear as sound and little affected. As a matter of fact, one can easily observe gradual deterioration undergoing in the majority of stones, such as cracks, deformation, detachment, material loss, discoloration and deposit, and biological colonization as well []. Among these deterioration types, biological colonization, defined as the “colonization of stone by plants and microorganisms such as bacteria, cyanobacteria, algae, fungi and lichen”, has endangered the survival of historical monuments for many years [] (p.64). Biological colonization also includes influences by other organisms, such as animals nesting on and in the stones [], as well as insect pests [].
Environmental factors like rain, snow, wind, solar radiation, humidity, and temperature fluctuations are known to affect the habitation, growing, and expansion of microorganisms on rock surfaces [,]. Moreover, the existence of organic and inorganic energy sources together with optimum environmental conditions will favor the colonization of microorganisms in and on stone surfaces. The activities of these microorganisms could bring about different stone deterioration problems, such as powdering, scaling, biopitting, detachment, dissolution, solubilization, patina, and crust, which affects the durability of historic stone materials over the long-term [,,]
Recent research has revealed that biological agents may lead to stone deterioration, either acting alone or in co-association with other types of decay factors [,]. Therefore, obtaining information about the level of microbial activity is significant in terms of evaluating potential deterioration problems and taking preventive measures; however, when the level of microbial activity is high in stones, characterization of the species also becomes necessary in order to develop appropriate biocontrol procedures [,].
Since most of the methods conducted to determine the level of microbial activity were developed for the assessment of microorganisms from various environments and industrial areas such as soil, freshwater, food, medical, drinking water, etc. [], optimization of these methods is needed for their employment on the stones of historical monuments. Moreover, of these methods, few can be conducted with small amounts of samples [] and to date, none have been proven to be completely satisfactory for determination of the viability and total microbial activity of microorganisms in different types of stones [,].
This study aimed to develop a practical and rapid method for the measurement of total microbial activity in stones, so as to evaluate the contribution of microorganisms to existing stone deterioration problems in the case of the Mount Nemrut monuments (MNMs). Furthermore, this study was conducted to eliminate the need for a biocidal treatment in addition to other types of conservation treatments that were studied in the same case (http://nemrut.org.tr/en/aim-and-scope/).
2. Materials and Methods
2.1. Description of the Studied Sites
Sampling was carried out on the MNMs, located in the town of Kahta, Adiyaman (Turkey), which have particular importance as a result of being on the United Nations Educational, Scientific, and Cultural Organization World Heritage List since 1987 (https://whc.unesco.org/en/list/448/) (Figure 1).
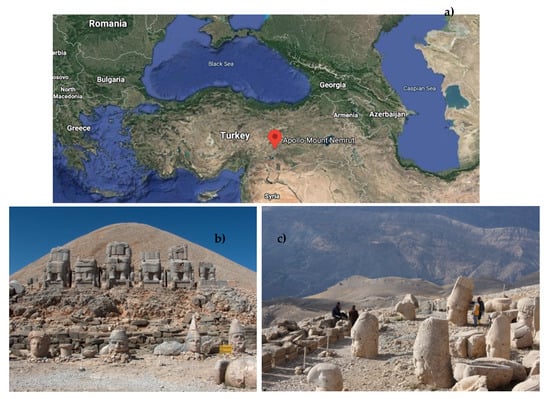
Figure 1.
(a) Location of the Mount Nemrut Monuments (MNMs) on a map of Turkey (Google Maps, accessed 29 Apr. 2020), (b) east terrace, and (c) west terrace of the MNMs (©K. Göze Akoğlu 2009).
King Antiochus I (69–31 BC), of the Commagene Kingdom, constructed monumental statues and a conical shape tumulus with crushed limestone pieces on the MNMs (N 0°1′4.27″, E 0°0′22.2536″). At an altitude of 2206 m, this site comprises 3 terraces—west, east, and north. There are giant limestone and sandstone statues and 2 rows of sandstone stelae on the east and west terraces (Figure 1) [,].
The MNMs suffer from prevailing harsh climatic conditions with wetting–drying and freezing–thawing processes that have led to the decay of the stones at the site []. The types of decay observed at the site comprise micro and macro karstic features, dissolution, pitting, biological colonization, and color change on the limestone statues [], and flaking/scaling, granular disintegration, cracking, back weathering, depositions, rounding/notching, and biological colonization on the sandstone statues and stelae [].
2.2. Sampling
Crustose, foliose, and fruticose lichens have been frequently observed on the stones of the MNMs []. Therefore, while investigating the level of microbial activity on stone surfaces that had no visible biological coverings, deteriorated limestones and sandstones with lichen on the surface were also sampled to prevent any loss of information (Figure 2).

Figure 2.
Representative photographs of the limestones and sandstones with lichen: (a) limestone and (b) sandstone on the east terrace (©Elif Sırt Çıplak 2009).
The number and size of the samples were arranged in a way so as to not to lead any type of damage to the monuments; therefore, they were limited to 17 samples (10 limestone, 7 sandstone), each less than 1 × 1 × 1 cm in size. Stone samples were taken using sterile equipment to avoid cross contamination and coded by numbering the first letter of the type of stone (L1, S1, etc.). Samples were stored at 4 °C until use.
2.3. Determination of Biological Activity
Since the 1980s, the fluorescein diacetate (FDA) hydrolysis method has been widely applied for the assessment of total microbial activity in soil ecosystems [,]. Although the first applications of the FDA hydrolysis method were mainly in the field of soil microbiology this method can now efficiently determine a wide range of microorganisms, such as bacteria, fungi, algae, and lichen, in other environments, such as freshwater, forest, and marine environments, as a result of subsequent modifications and improvements over the course of time [,,]. Since microbial diversity in a soil habitat is considered to have similar and even richer microbial diversity in comparison with stone surfaces, this method was adapted and applied to determine the level of microbial activity on stone surfaces as well [], as a response to the need for a practical and standardized method with high precision for the assessment of activities of microbial populations.
The method fundamentally measures the amount of fluorescein that is formed as a result of FDA hydrolysis by the enzymatic activities of microorganisms []. In more detail, when FDA (3′, 6′ -diacetyl-fluorescein) is hydrolyzed by various enzymes, such as protease, lipase, and esterase, it releases fluorescein molecules, which form a colored end product []. These molecules can be measured successfully between 475–510 nm using a spectrophotometer [,,]. The FDA hydrolysis method has been assumed to estimate the level of total microbial activity on stone surfaces since the aforementioned enzymes, responsible for the conversion of FDA to fluorescein, are widespread in bacteria, fungi [], algae [], and lichens [].
The plate count method, on the other hand, has widespread application in the field as the traditional gold standard method, and it is also applicable for the determination of microbial activities on stone surfaces [,,]. In this study, the FDA hydrolysis and plate count methods were used; the FDA hydrolysis method was applied as the main analytical tool and the plate count method was used to assess the performance of the FDA hydrolysis method regarding its application in the field of stone biodeterioration. In fact, before choosing a methodology to be used as a support and verification tool for the FDA hydrolysis method, 3 criteria were defined. The first was the reliability of the method and wide area of usage, especially for environmental samples. Even though the plate count method has a disadvantage with regards to quantifying only culturable bacteria [], it is still the preferred choice for the routine determination of microbial numbers from food to environmental samples. The second was with regards to counting only living microorganisms, excluding dead ones and debris. The plate count method also met this criterion by quantifying colonies generated by active bacterial and fungal species. The last was regarding workability with any amount/concentration []. This criterion was crucial since the samples to be collected from historical monuments had to be as few and as small as possible. Eventually, the plate count method, adapted from the study of Jaton [], was conducted for determination of the level of microorganisms, as bacteria and fungi, colonizing on stone.
While using the plate count method as a verification tool for the FDA hydrolysis method, the interpretation and comparison of values, CFU per g of stone and µg fluorescein per g of stone, were made considering the limitations of both methodologies.
The FDA hydrolysis and plate count methods were conducted on 2 different types of stones, i.e., limestones and sandstones, located on the east and west terrace of the MNMs, to assess the suitability of the FDA hydrolysis method adapted for use on stones, and to determine whether the level of microbial activity was a potential risk for the eventual loss of integrity of historical materials.
2.3.1. Fluorescein Diacetate (FDA) Method
Buffer solution, FDA stock solution, and fluorescein stock solution were prepared accordingly [], and samples collected from the MNMs were crushed into a fine powder in a sterile agate mortar. Each powdered stone sample (in the range of 1 to 2 g) was placed into a glass flask and 15 mL of phosphate buffer (60 mM, pH of 7.5) was added. Next, 0.2 mL of FDA stock solution (1000 µg FDA/mL) was added to initiate the reaction. At the same time, blanks were prepared without the addition of FDA substrate. The samples were shaken thoroughly by hand and placed in an oven at 30 °C for 30 min. After this incubation period, the powdered samples were centrifuged (Rotofix 32A, Hettich Zentrifugen, Kirchlengern, Germany) at 4000 rpm for 3 min. The supernatants of these samples were filtered through Whatman no. 40 filter paper and the filtrates were measured at 490 nm using an Optima SP-3000 Plus UV–Vis spectrophotometer (Mettler Toledo, Columbus, OH, USA) [].
A terminator, used to stop the hydrolysis reaction at a specific time, was not used in this study, since the number of samples allowed for the spectrophotometric measurements to be conducted at one time []. Thus, a decrease in the amount of fluorescein with the addition of a terminator was prevented in the spectrophotometric measurements.
Following measurement of the absorbance of fluorescein released from the FDA, the concentration of fluorescein (µg fluorescein/mL) was calculated using the calibration graph produced from the standard 0 to 5 µg fluorescein/mL []. Next, the amount of fluorescein in 1 mL of solution was calculated for 1 g of stone in order to reach a comparable unit (µg fluorescein/g) with the plate count method.
2.3.2. Plate Count Method
In this method, 1 g of powdered stone sample was put into a glass tube and 9 mL of sterile distilled water was added to obtain a dilution of 10:1. Once this dilution was obtained, serial dilutions (10–1 to 10–6) were produced by transferring 100 µL of the diluted solution into 900 µL of sterile distilled water, until obtaining a dilution of 10:6. Afterwards, triplicate mediums were inoculated with the 100 µL dilute solution from each dilution. The mediums used in this study were nutrient agar medium (8 g of nutrient broth and 7.5 g/L of agar powder) for the bacteria and malt extract agar medium (50 g of malt extract powder and 15 g/L of agar powder) for all of the types of fungi. The nutrient agar medium was incubated at 30 °C for 1 week and the malt extract agar medium was incubated at 24 °C for 4 days [].
After the incubation period, petri dishes containing between 30 and 300 colonies were counted, since less than 30 colonies was not acceptable statistically, and more than 300 colonies on a plate was likely to produce colonies that would be too close to each other to be distinguished as a distinct colony-forming unit (CFU). Following colony counting, the CFU in 1 g of the original sample was calculated as follows, and expressed as CFU/g of stone []:
- N = (C/3) × D × V
- N—CFU per gram or mL
- C—Total colony count of 3 petri dishes
- D—Dilution factor
- V—Transferred volume (mL)
3. Results
3.1. Spectrophotometric Measurements
Spectrophotometric measurements were performed on the powders of the deteriorated limestones and sandstones with and without lichens on their surfaces. The results of the fluorescein contents are given in Table 1 and Table 2.

Table 1.
Spectrophotometric measurement results of the samples without lichens on their surfaces.

Table 2.
Spectrophotometric measurement results of the samples with lichens on their surfaces.
When the results of the samples with and without lichens were compared, there was a significant difference between the values; the enzymatic activity for samples without lichens ranged from 3.14 to 9.32 µg fluorescein/g of stone, whereas samples with lichens had rather high values, ranging from 20.55 to 1134.90 µg fluorescein/g of stone (Table 1 and Table 2).
Considering the microbial activity in relation to the substrate type, samples without lichens (S1–S3 and L1–L5) on their surfaces had enzymatic activity values in the range of 3.14–7.36 µg fluorescein/g of stone for the sandstones and 4.34–9.32 µg fluorescein/g of stone for the limestones, as presented in Table 1.
On the other hand, samples with lichens (S4–S7 and L6–L10) had values in the range of 20.55–1134.90 µg fluorescein/g of stone for the limestones and 31.22–260 µg fluorescein/g of stone for the sandstones, as presented in Table 2.
3.2. Plate Count Method
Colonies of aerobic heterotrophic bacteria and fungi were counted in nutrient agar medium and malt extract agar medium, respectively. The total number of bacteria for samples without lichens ranged from 1.20 × 104 to 8.02 × 104 CFU/g of stone, whereas samples with lichens had relatively high values (except for sample S4), ranging from 7.24 × 104 to 20.57 × 104 CFU/g of stone (Table 3).

Table 3.
Determination of the aerobic heterotrophic bacteria as colony forming unit (CFU) per gram of stone in all of the samples.
On the other hand, the number of colonies of fungi on the malt extract agar medium was not in the range of the countable numbers (30–300), and were less than the lower limit. All of the samples had counts similar to <2 × 103 CFU/g of stone for both the sandstone and limestone samples.
Considering the type of stone, the bacterial counts ranged between 1.40 × 104 and 2.60 × 104 CFU/g for the sandstones, and 1.20 × 104 and 8.02 × 104 CFU/g of stone for the limestones without lichens, whereas samples with lichens had counts in the range of 0.6 × 104 to 13.5 × 104 CFU/g of stone for sandstones and 10.30 × 104 to 22.20 × 104 CFU/g of stone for the limestones (Table 3).
3.3. Enzymatic Activity/Total Microbial Activity and Total Number of Bacteria
4. Discussion
The FDA hydrolysis method was applied to determine the total microbial activity (fluorescein concentration) of natural microflora on deteriorated limestones and sandstones with and without lichens on their surfaces. Unlike in other methodologies [,,], a solvent for terminating the hydrolysis reaction was not used in this experimental procedure due to the number of samples that could be measured simultaneously. Therefore, any decrease in the amount of fluorescein concentration was accordingly prevented. Skipping the step of stopping the hydrolysis reaction was critical, especially in samples with low biological activity, because determination of the presence of microorganisms was important, even at a low concentration.
The fluorescein concentration values were observed to be significantly different for the samples with and without lichens. Much higher values were obtained in samples with lichens (20.55 to 1134.90 µg fluorescein/g). However, the difference was regarded as normal since the FDA hydrolysis reaction was known to also be catalyzed by symbiotic partners of lichens, fungi (mycobiont), and algae and/or cyanobacteria (photobiont) (Table 1 and Table 2) [,,,].
When examined in more detail, samples without lichens had almost the same or lower enzymatic activity, as an indication of the low biological activity uniformly distributed on the stone surfaces (Table 1) []. On the other hand, enzymatic activity values ranging from 20.55 to 1134.90 µg fluorescein/g in the samples with lichens referred to the variety of physiological states of the lichens, which could have been related with the type of partners in this symbiotic living form, their adaptation capacity to changing environmental conditions [], the type of stone [,] and their locations on these substrates as well (Table 2).
Regardless of the type of stone, i.e., limestones or sandstones, the samples without lichens had a low fluorescein concentration (3.14–7.36 µg fluorescein/g for the sandstones and 4.34–9.32 µg fluorescein/g for the limestones) (Table 1). However, the fluorescein concentration in the samples with lichens was higher in the limestone substrate, with the highest values as 899.46 (L7), 721.83 (L9), 708.75 (L8), and 1134.90 (L6) µg fluorescein/g (Table 2). This suggested that the lichens colonized on limestones that displayed higher metabolic activity as a consequence of their water content at the time of sampling, resulting in a maintained active metabolism until they were analyzed, because the enzymes responsible for FDA conversion are known to be easily effected by changing environmental conditions [].
The plate count method was conducted simultaneously with the FDA hydrolysis method to determine the quantity of aerobic heterotrophic bacteria and fungi on the same stone samples. The contribution of this method was significant in terms of assisting the interpretation of the results of the FDA hydroylsis method, determined as fluorecein per gram of stone, since few studies have measured the fluorescein concentration for the estimation of biological activity on stone surfaces [,,].
The results of the plate count method indicated that bacterial (0.6 × 104 – 22.2 × 104 CFU/g) and fungal (<2 × 103 CFU/g) activities were low on the stones of MNMs []. Even though bacterial and fungal counts were not significantly different in the samples with and without lichens, the results still varied remarkably within each sample group. Samples with lichens had relatively higher (except for S4) bacterial numbers, which could have been related to the lichens acting as a pioneer organism that favored the growth of bacteria species (Table 3) [,].
Sandstones had bacterial numbers that were relatively lower (1,40 × 104–2,60 × 104 CFU/g ) than the limestones, which displayed a slight increase (7.24 × 104–13.5 × 104 CFU/g, except S4) with the existance of lichens. Likewise, it was observed that the bacterial activity values on the limestones were the highest when covered with lichens (10.30 × 104–22.20 × 104 CFU/g) (Table 3). The relatively higher bacterial activity levels on limestone surfaces could have been related to the irregular surfaces of the limestones, as a result of weathering, because microcavities on limestones could facilitate the attachment, growth, distribution, and resistance to atmospheric conditions [,]. On the other hand, the material properties of stones has also been known to affect the preference and colonization of bacteria on different substrates. However, in the case of the MNMs, the mentioned differences in the quantity of bacteria between the limestones and sandstones were not so significant that it required further discussion.
The performance of the FDA hydrolysis method was examined by comparing its values with those of the plate count method. Quantitative differences in the results of these methods were evident, depending on the diversity of the microbial communities existing on the stone surfaces and the efficiency of the methods for the measurement of microbial activities on the stone substrates.
Since the plate count method is a culture-dependent method and does not provide information about the entire microbial community of the stone surfaces, it can only detect bacteria and fungi that are culturable under laboratory conditions. Therefore, when comparing the results of the FDA hydrolysis and plate count methods, they should not be considered to have a direct relationship. Obtaining high bacterial and fungal numbers in samples with high enzymatic activity, and also observing low enzymatic activity in samples with low bacterial and fungal counts, would verify the applicability of the FDA hydrolysis method for use on stones. However, it would not be suitable to always expect low enzymatic activity when bacterial and fungal counts are low, since the FDA hydrolysis method represents the total microbial activity, comprising algae, cyanobacteria, and lichens, in addition to bacteria and fungi.
In the case of the MNM stones, the relationship between the FDA hydrolysis and plate count method results was as anticipated—the stone samples showed lower enzymatic activity (S1, L4, and S2) when bacterial counts were low, and higher enzymatic activity (L6–L8) when bacterial counts were high (Table 1, Table 2 and Table 3). These correlations not only verified the sensitivity, but also increased the reliability of the FDA hydrolysis method for measurement of total microbial activity on stone surfaces.
The results obtained from both experiments showed that microbial activity was low on limestone and sandstone surfaces, but this does not mean that their activity will remain constant in the future [,]. Development of a practical and efficient method to use in long-term monitoring studies is important for this reason. It is especially crucial to detect microbial activity before it reaches a critical level in the case of the MNMs. On the other hand, lichens seemed to be responsible for the biodeterioration problem on the sandstones and limestones of the MNMs, as their microbial activity was proven by the fluorescein concentration measurements.
5. Conclusions
The level of microbial activity on stone surfaces was examined to understand if microorganisms played a role in the decay process of the stones of MNMs, and their stone deterioration problems were investigated.
Although the microbiological experiments began with the plate count method, its inefficiency for estimation of the total microbial activity on the stone surfaces led to the use of the FDA hydrolysis method, which is widely used in soil environments. The method was successfully conducted on the stones of the MNMs. With the joint interpretation of the results, it was concluded that microbial activity did not play an important role in the existing stone deterioration problems on the MNMs when the stone surfaces were not covered with lichens. On the other hand, lichens were frequently observed on both the limestones and sandstones of the MNMs. Therefore, investigation of the role of these organisms on stone deterioration and their long-term behavior remains crucial. In future studies, methods may be conducted for the performance assessment of potential biocidal treatments on lichens.
Herein, it was shown that FDA hydrolysis is a reliable method for estimation of the total microbial activity on stone substrates. It was able to determine the level of microbial activity on different types of stones, which is crucial for diagnostic and monitoring studies of stone biodeterioration in a range of historical monuments. The method had obvious advantages in terms of its rapid measurement rate and sensitivity, even in small samples. In addition, the practicality of this method makes it appropriate for cultural heritage conservators with minimum scientific equipment and consumables.
Author Contributions
Conceptualization, E.S.Ç. and K.G.A.; Investigation, E.S.Ç.; Methodology, E.S.Ç.; Writing—original draft, E.S.Ç. and K.G.A.; Writing—review & editing, E.S.Ç. and K.G.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external fundingAcknowledgements: This study was partially supported by the Commagene Nemrut Conservation and Development Program (CNCDP), which was developed by the Middle East Technical University (METU). The authors would like to thank Ing. Genevieve Orial from LRMH (France) for her kind guidance with her valuable expertise in discussions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cartwright, T.A.; Vergès-Belmin, V.; International Scientific Committee for Stone (Eds.) Illustrated Glossary on Stone Deterioration =: Glossaire Illustré Sur Les Formes D’altération de la Pierre; ICOMOS: Paris, France, 2008; p. 64. [Google Scholar]
- Sterflinger, K.; Piñar, G. Microbial deterioration of cultural heritage and works of art—Tilting at windmills? Appl. Microbiol. Biotechnol. 2013, 97, 9637–9646. [Google Scholar] [CrossRef] [PubMed]
- Caneva, G.; Nugari, M.P.; Salvadori, O. Plant Biology for Cultural Heritage; Getty Conservation Institute: Los Angeles, CA, USA, 2008. [Google Scholar]
- Gorbushina, A.A. Life on the rocks: Life on the rocks. Environ. Microbiol. 2007, 9, 1613–1631. [Google Scholar] [CrossRef] [PubMed]
- Warscheid, T.; Braams, J. Biodeterioration of stone: A review. Int. Biodeterior. Biodegrad. 2000, 46, 343–368. [Google Scholar] [CrossRef]
- Flores, M.; Lorenzo, J.; Gómez-Alarcón, G. Algae and bacteria on historic monuments at Alcala de Henares, Spain. Int. Biodeterior. Biodegrad. 1997, 40, 241–246. [Google Scholar] [CrossRef]
- Suihko, M.-L.; Alakomi, H.-L.; Gorbushina, A.; Fortune, I.; Marquardt, J.; Saarela, M. Characterization of aerobic bacterial and fungal microbiota on surfaces of historic Scottish monuments. Syst. Appl. Microbiol. 2007, 30, 494–508. [Google Scholar] [CrossRef] [PubMed]
- Sanmartín, P.; DeAraujo, A.; Vasanthakumar, A. Melding the Old with the New: Trends in Methods Used to Identify, Monitor, and Control Microorganisms on Cultural Heritage Materials. Microb. Ecol. 2018, 76, 64–80. [Google Scholar] [CrossRef]
- Mihajlovski, A.; Seyer, D.; Benamara, H.; Bousta, F.; di Martino, P. An overview of techniques for the characterization and quantification of microbial colonization on stone monuments. Ann. Microbiol. 2015, 65, 1243–1255. [Google Scholar] [CrossRef]
- Şahin Güçhan, N. “Conservation of relationship between place and context: Mount Nemrut Tumulus (keynote presentation)”. 2011; pp. 151–167. [Google Scholar]
- Topal, T.; Deniz, B.E.; Güçhan, N.Ş. Decay of Limestone Statues at Mount Nemrut (Adiyaman, Turkey). Int. J. Archit. Herit. 2015, 9, 244–264. [Google Scholar] [CrossRef]
- Akoğlu, K.G.; Caner-Saltık, E.N. Hydric dilation of Mount Nemrut sandstones and its control by surfactants. J. Cult. Herit. 2015, 16, 276–283. [Google Scholar] [CrossRef]
- Schnürer, J.; Rosswall, T. Fluorescein Diacetate Hydrolysis as a Measure of Total Microbial Activity in Soil and Litter. Appl. Environ. Microbiol. 1982, 43, 1256–1261. [Google Scholar] [CrossRef]
- Tayler, S.; May, E. Investigations of the localisation of bacterial activity on sandstone from ancient monuments. Int. Biodeterior. Biodegrad. 2000, 46, 327–333. [Google Scholar] [CrossRef]
- Green, V.S.; Stott, D.E.; Diack, M. Assay for fluorescein diacetate hydrolytic activity: Optimization for soil samples. Soil Biol. Biochem. 2006, 38, 693–701. [Google Scholar] [CrossRef]
- Sánchez-Monedero, M.A.; Mondini, C.; Cayuela, M.L.; Roig, A.; Contin, M.; de Nobili, M. Fluorescein diacetate hydrolysis, respiration and microbial biomass in freshly amended soils. Biol. Fertil. Soils 2008, 44, 885–890. [Google Scholar] [CrossRef]
- Da Silva Aragão, O.O.; de Oliveira-Longatti, S.M.; de Castro Caputo, P.S.; Rufini, M.; Carvalho, G.R.; de Carvalho, T.S.; de Souza Moreira, F.M. Microbiological indicators of soil quality are related to greater coffee yield in the Brazilian Cerrado region. Ecol. Indic. 2020, 113, 106205. [Google Scholar] [CrossRef]
- Adam, G.; Duncan, H. Development of a sensitive and rapid method for the measurement of total microbial activity using ¯uorescein diacetate (FDA) in a range of soils. Soil Biol. Biochem. 2001, 33, 943–951. [Google Scholar] [CrossRef]
- Ciplak, E.S.; Gozen, A.C.; Saltık, E.C. The contribution of traditional techniques to new technology to evaluate the potential risk of stone deterioration by microorganisms. Sci. Art: A Future Stone 2016, 1, 57–65. [Google Scholar]
- Barone, V.; Puglisi, I.; Fragalà, F.; Stevanato, P.; Baglieri, A. Effect of living cells of microalgae or their extracts on soil enzyme activities. Arch. Agron. Soil Sci. 2019, 65, 712–726. [Google Scholar] [CrossRef]
- Bölter, M. Environmental conditions and microbiological properties from soils and lichens from Antarctica (Casey Station, Wilkes Land). Polar Biol. 1992, 11, 8. [Google Scholar] [CrossRef]
- Jaton, C. Aspects microbiologiques des alterations des pierres de monuments. In Proceedings of the Colloque International Sur la Deterioration des Pierres en Oeuvre. 1er. International Symposium on the Deterioration of Building Stones, La rochelle, France, 11–16 September 1972; pp. 149–154. [Google Scholar]
- Scheerer, S.; Ortega-Morales, O.; Gaylarde, C. Chapter 5 Microbial Deterioration of Stone Monuments—An Updated Overview. Adv. Appl. Microbiol. 2009, 66, 97–139. [Google Scholar]
- Amann, R. Who is out there? Microbial Aspects of Biodiversity. Syst. Appl. Microbiol. 2000, 23, 1–8. [Google Scholar] [CrossRef]
- Hazan, R.; Que, Y.-A.; Maura, D.; Rahme, L.G. A method for high throughput determination of viable bacteria cell counts in 96-well plates. BMC Microbiol. 2012, 12, 259. [Google Scholar] [CrossRef] [PubMed]
- Fontvieille, D.A.; Outaguerouine, A.; Thevenot, D.R. Fluorescein diacetate hydrolysis as a measure of microbial activity in aquatic systems: Application to activated sludges. Environ. Technol. 1992, 13, 531–540. [Google Scholar] [CrossRef]
- Scheerer, S. Microbial Biodeterioration of Outdoor Stone Monuments: Assessment Methods and Control Strategies; ProQuest LLC: Ann Arbor, Ml, USA, 2008; p. 256. [Google Scholar]
- Kappen, L.; Schroeter, B.; Scheidegger, C.; Sommerkorn, M.; Hestmark, G. Cold resistance and metabolic activity of lichens below 0 °C. Adv. Space Res. 1996, 18, 119–128. [Google Scholar] [CrossRef]
- Lisci, M.; Monte, M.; Pacini, E. Lichens and higher plants on stone: A review. Int. Biodeterior. Biodegrad. 2003, 51, 1–17. [Google Scholar] [CrossRef]
- Pouneva, I. Evaluation of Algal Culture Viability and Physiological State by Fluorescent Microscopic Methods. Bulg. J. Plant Physiol 1997, 23, 67–76. [Google Scholar]
- Prieto, B.; Silva, B.; Lantes, O. Biofilm quantification on stone surfaces: Comparison of various methods. Sci. Total Environ. 2004, 333, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.; Höftberger, M.; Green, T.G.A.; Türk, R. Photosynthesis of lichens from lichen-dominated communities in the alpine/nival belt of the Alps—II: Laboratory and field measurements of CO2 exchange and water relations. Flora Morphol. Distrib. Funct. Ecol. Plants 2008, 203, 34–46. [Google Scholar] [CrossRef]
- Dakal, T.C.; Cameotra, S.S. Microbially induced deterioration of architectural heritages: Routes and mechanisms involved. Environ. Sci. Eur. 2012, 24, 36. [Google Scholar] [CrossRef]
- Tayler, S.; May, E. A Comparison of Methods for the Measurement of Microbial Activity on Stone. Stud. Conserv. 1995, 40, 163. [Google Scholar] [CrossRef]
- Rajkowska, K.; Otlewska, A.; Kozirog, A.; Piotrowska, M.; Nowicka-Krawczyk, P.; Hachulka, M.; Wolski, G.J.; Kunicka-Styczynska, A.; Gutarowska, B.; Zydzik-Bialek, A. Assessment of biological colonization of historic buildings in the former Auschwitz II-Birkenau concentration camp. Ann. Microbiol. 2014, 64, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, P.; Eckhardt, F.E.W.; Palmer, R.J. Methods for the study of rock-inhabiting microorganisms—A mini review. J. Microbiol. Methods 1995, 23, 143–167. [Google Scholar] [CrossRef]
- Urzi, C. Microbial Deterioration of Rocks and Marble Monuments of the Mediterranean Basin: A Review. Corros Rev. 2004, 22, 441–458. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).