Abstract
Carbon mineralization has attracted great interest as a promising strategy to achieve a decarbonized pathway by 2050. Despite the significant environmental and economic promise associated with using industrial solid waste for carbon mineralization, the scale-up application of this approach is limited due to its low reactivity and relatively high cost. A clear understanding of the detailed mechanisms governing various carbonation techniques is needed to achieve high CO2 conversion efficiency. This review can provide valuable insight into carbon mineralization pathways, advantages and challenges, and potential feedstocks. Factors affecting reaction kinetics, and thereby carbonation efficiency, are also discussed. Then, we focus on the research progress of the most representative industrial solid wastes for CO2 mineralization, process conditions, and their carbonation potential. Lastly, a market analysis of the precipitated carbonate products is provided to assess economic feasibility for practical applications.
1. Introduction
Global industrialization and urbanization have increased greenhouse gas emissions, with CO2 levels reaching 419 ppm in 2023 and rising by 2.8 ppm annually [1]. This increase in CO2 concentration is a primary factor driving global warming, which has far-reaching consequences for human health, environmental stability, and economic prosperity [2]. The World Meteorological Organization predicts a 98% chance that global temperature will set new records between 2023 and 2027, with a substantial likelihood of exceeding the critical 1.5 °C threshold above pre-industrial levels for at least one year [3]. Moreover, industrial activities generate large volumes of byproducts, contributing to environmental pollution and biodiversity loss if not managed properly [4]. Sustainable management of these byproducts is essential to meet the Paris Agreement goals [5]. In response to these challenges, global efforts have been accelerated toward sustainability and carbon neutrality. Reducing CO2 emissions is critical for sustainable development. A significant milestone in this global endeavor was the “Glasgow Climate Pact”, established in the 26th UN Climate Change Conference, where 200 countries committed to addressing global warming and reducing greenhouse gas emissions [6].
Various carbon dioxide removal (CDR) technologies have been developed to reduce CO2 emissions (Table 1). While these approaches show promise, they face different challenges that hinder their widespread applications. For instance, chemical adsorption using solvents or adsorbents is considered an effective CO2 capture method; however, the main limitation of this technique is the release of harmful chemicals during solvent recovery which requires a high amount of energy [7]. Porous adsorbents, such as activated carbon and metal–organic frameworks, have demonstrated efficiency in CO2 sequestration. The primary challenges with these methods include their capacity, selectivity, and regeneration efficiency [8]. Membrane separation technology provides a selective approach to CO2 removal. Despite its potential, this method faces limitations in large-scale applications due to membrane fouling and high operational costs [9]. Biological CO2 removal techniques, such as algae-based adsorbents, offer a sustainable alternative. However, their scalability is limited by the extensive land and water resources required for implementation [10]. Addressing the technical, economic, and environmental challenges of these CDR techniques is essential for their broader application to achieve global climate goals. Carbon dioxide capture, utilization, and storage (CCUS) emerged as an effective and economically viable strategy for reducing CO2 emissions. It is estimated that this strategy can be widely applied in various industries and can capture more than 7 billion tons of CO2 annually by 2050, which is vital for achieving low-carbon sustainable development [11,12]. The International Energy Agency (IEA) estimates that the CCUS techniques can reduce 1.4 and 5.9 Gt of CO2 emissions by 2030 and 2050, respectively [13].

Table 1.
Carbon dioxide removal technologies.
Among CCUS technologies, carbon mineralization as a natural process of CO2 sequestration has attracted considerable attention due to its ability to permanently store CO2. The concept of CO2 mineralization was first proposed by Seifritz in 1990 [37], who suggested that a potential mechanism for CO2 storage is the weathering of silicate minerals by consuming CO2. This process involves a spontaneous reaction of alkaline minerals containing calcium (Ca) and magnesium (Mg) ions with CO2, which results in carbonates’ production, including CaCO3 and MgCO3. These solid carbonates are thermodynamically stable, meaning that their Gibbs free energy is much lower than CO2, resulting in permanent carbon storage [38].
Carbon mineralization technology can be generally divided into in situ and ex situ techniques [39]. In situ mineralization involves the direct injection of CO2 from the atmosphere into geological formations, containing Ca or Mg-rich natural minerals, to form solid carbonate. Conversely, in ex situ carbon mineralization, CO2 sequestration into solid carbonate can be conducted through a series of chemical steps above ground [40,41]. Unlike the in situ mineralization technology, which is limited by specific geographical and environmental conditions, the ex situ mineralization approach offers broader applicability and greater flexibility [42]. Moreover, there is are wide availability of potential feedstocks for ex situ mineralization, such as mine tailings from mining operations, and different types of industrial alkaline solid waste [43]. Another major benefit of ex situ mineralization is the substantial potential for the economic value derived from producing solid carbonates [44]. The main application of solid carbonates is within construction materials such as concrete, aggregates, and other building products [45,46,47]. These carbonate productions can also be beneficial in other applications, such as valuable chemical productions, pharmaceuticals, selective metal recycling, mineralization for power generation, paint, and paper [48,49]. Therefore, this approach can be considered as a novel strategy for CCUS, which not only addresses an environmental concern through carbon storage permanently but also generates commercially valuable products, which can help reduce the overall process cost [50,51].
Despite the environmental and economic benefits of carbon mineralization using industrial solid waste, the efficiency of mineralization techniques is limited by slow reaction rates and the kinetics of ion dissolution/extraction and carbonate precipitation [52]. The dissolution rate, which involves releasing Ca and Mg ions from feedstocks, is the slowest and most limiting factor in the carbonation process. Meanwhile, the precipitated rate relates to the formation of carbonates [53]. Based on the literature, the ion dissolution/extraction rate plays a critical role in controlling the overall efficiency of carbonation [53]. In this regard, adding chemical leaching agents with stepwise pH adjustments leads to higher dissolution rates and facilitates ion extraction from feedstocks [54]. However, the selection of chemicals is particularly important due to waste management challenges from both technical and economic perspectives [55]. In this regard employing recyclable reagents has been considered to mitigate operational cost resulting from chemicals’ consumption and their subsequent processing waste [56].
The reaction rate can also be accelerated via pretreatment strategies such as grinding minerals and/or controlling reaction conditions such as temperature and pressure. However, these strategies result in higher operational costs, leading to a trade-off between improved reaction kinetics and economic feasibility [57]. Moreover, the mineralization reaction rate is significantly affected by the type of feedstocks, their composition (richness of Ca and Mg) and reactivity, availability of CO2, carbonation routes (direct or indirect), and reaction pathways (wet or dry) [56]. In addition, operational factors, including the feedstock particle size, reaction temperature, CO2 pressure, pH value, reaction time, and solid-feedstock-to-liquid ratio, have great impacts on the reaction rate [58,59]. All these affecting factors need to be optimized and thoroughly considered to enhance the reaction rate and achieve higher carbonation efficiency [60,61].
The current review paper aims to provide clear insights into ex situ carbon mineralization using industrial solid wastes as feedstock. To this end, mineralization pathways, carbonation reactions, and the factors affecting reaction rate are comprehensively reviewed to achieve higher carbonation efficiency, resulting in high-purity products. The potential application of carbonated products as valuable byproducts of carbon mineralization techniques and their market analysis are also highlighted. Moreover, some pilot studies are discussed to understand the technical feasibility and environmental effects of ex situ carbon mineralization for widespread industrial adoption. Finally, the current challenges and future perspectives of ex situ mineralization using industrial solid wastes are briefly discussed.
2. Carbon Mineralization
Carbon mineralization technology has emerged as one of the most effective methods for CO2 sequestration in recent years [62]. This technology is a natural or engineered process in which the minerals containing divalent metals, particularly calcium (Ca) and magnesium (Mg), react with CO2 to form carbonates (e.g., calcite and magnesite) (Figure 1). The strong stability of these carbonates makes this technique a reliable and permanent CO2 storage method [63]. During the mineralization process at a standard temperature of 25 °C, the Gibbs free energy of the system decreases because carbonate minerals have a lower energy (60–180 kJ/mol) than that of CO2 molecules, making the reaction thermodynamically favorable or spontaneous (ΔG < 0) [60,64]. This energy difference drives the reaction forward, requiring no external energy sources, under appropriate conditions (optimal temperature and pressure). The common mineralization reactions of CO2 can be represented by Equations (1)–(4):
CaO + CO2 → CaCO3
MgO + CO2 → MgCO3
CaSiO3 + CO2 → CaCO3 + SiO2
Mg2SiO4 + 2CO2 → 2MgCO3 + SiO2
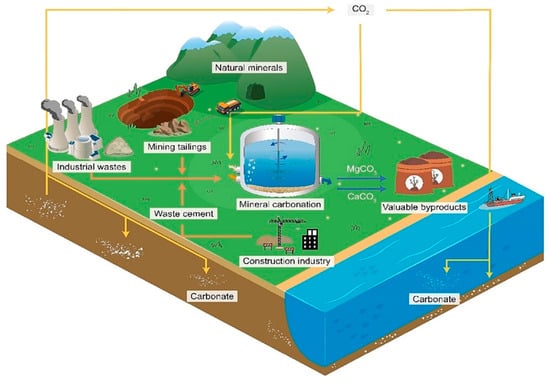
Figure 1.
The schematic illustration of carbon sequestration technology [11].
Carbon mineralization techniques, particularly in situ and ex situ mineralization, are emerging as durable CDR solutions with high Technology Readiness Levels (TRLs). Table 2 provides the technical and environmental comparison of these mineralization techniques.

Table 2.
Comparison of carbon mineralization techniques [2,53,65].
In situ mineralization has achieved a higher TRL compared to ex situ mineralization because it builds on established geological processes and has been successfully demonstrated in filed projects, such as the CarbFix project in Iceland, where injected CO2 rapidly mineralizes in basalt formations. Impressively, CarbFix has shown that over 95% of the injected CO2 can be permanently mineralized within just two years, offering a secure and scalable solution for long-term carbon storage [66]. Another significant in situ pilot is the Wallula project in Washington State, USA, where around 977 tons of supercritical CO2 was injected into deep basalt formations in the Columbia River. Subsequent analysis confirmed the formation of carbonate minerals, validating the potential for geological storage in these settings [67]. Therefore, the in situ technique can offer large storage potential and permanent sequestration using existing subsurface engineering techniques. However, their implementation is limited by the availability of suitable geological formations and uncertainties around scaling up to a gigaton level, as well as slow reaction time [68,69]. In contrast, ex situ mineralization is less mature (lower TRL) but is attracting more research and commercial interest. This technique provides better process control compared to in situ mineralization and produces valuable carbonates as a byproduct that can be used in cement, construction materials, pH reducers, and manufacturing paint and paper [53]. Despite higher cost and energy demand, ex situ mineralization is drawing attention due to flexibility, integration with industrial processes, and potential for value-added products. Moreover, this process allows for easier measurement and verification of CO2 removal, which is valuable for carbon markets and regulatory frameworks [70].
3. Ex Situ Carbon Mineralization
Ex situ carbon mineralization involves the surface mineralization of CO2 using a wide variety of feedstocks, including natural minerals (e.g., basalts, olivine, serpentinite, and wollastonite) or industrial solid waste (e.g., steel slags, cement waste, mining waste, coal ash, and paper) in controlled reactors [64]. As indicated in Figure 2, there are various approaches for ex situ carbon mineralization based on the potential solid feedstocks (natural minerals and industrial wastes), mineralization pathways (direct and indirect), and carbonation reaction (solid–gas or dry, and aqueous or wet).
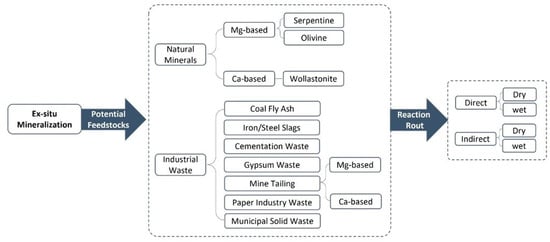
Figure 2.
The classification of ex situ carbon mineralization.
3.1. Potential Solid Feedstocks
3.1.1. Natural Mineral
Natural minerals are valuable feedstocks for CO2 mineralization, as they can be applied for both in situ and ex situ processes [71]. While these feedstocks are mainly used for in situ mineralization, the high content of mineral species in industrial waste has also made them applicable to ex situ mineralization [56]. Natural minerals can be categorized into two groups: magnesium-based feedstocks, containing a high Mg content (e.g., serpentine and olivine), and calcium-based feedstocks, which have a high Ca content (e.g., wollastonite). These minerals have been frequently assessed for carbon mineralization due to their chemical characteristics and CO2 reactivity [72,73]. Olivine is one of the most magnesium-rich reactive silicate minerals, with the general chemical formula Mg2SiO4. Olivine has been widely explored for CO2 sequestration due to its high magnesium content, with high reactivity with CO2, resulting in rapid kinetics to form stable magnesium carbonate like magnesite (MgCO3) [74]. Serpentine is a group of hydrated magnesium silicate minerals and has the general chemical formula Mg3(Si2O5)(OH)4 [75]. Serpentine has been the focus of significant studies due to its high magnesium content and its availability as a main component of different mine tailings and industrial wastes [75,76,77]. Wollastonite is one of the most calcium-rich silicate minerals and has the chemical formula CaSiO3. The high calcium content of wollastonite makes it a practically valuable feedstock for carbon mineralization [78].
3.1.2. Industrial Solid Waste
The global industry generates a significant amount of solid waste annually. The use of alkaline solid wastes as potential feedstocks for carbon mineralization has been rapidly adopted due to their high reactivity and availability near industrial sites [79,80,81]. A study by Pan et al. [82] revealed that China leads globally in CO2 reduction through mineralization using industrial solid wastes, at 132.2 MtCO2 annually; followed by Canada, with 29.4; and the United States, with 27.0 MtCO2 (Figure 3).
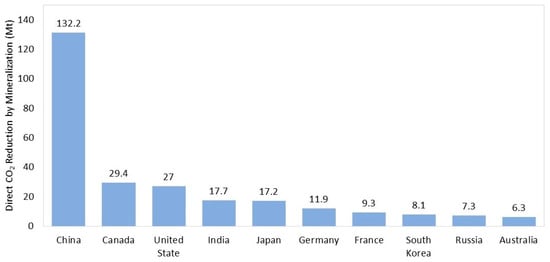
Figure 3.
Global direct CO2 reduction amounts due to mineralization (adapted from [82]).
Certain types of industrial alkaline solid waste, such as iron and steel slag [83,84,85,86], fly ash [87,88,89,90,91], cement [92,93,94,95], waste gypsum [96,97,98], and mine tailings [99,100,101,102,103], can be utilized in CO2 mineralization technology. The high concentrations of CaO and MgO in these waste residues contribute to their reactivity for mineralization. Additionally, as these wastes are produced by industrial processes, they are often located close to CO2 emission sources. This proximity offers significant potential for the efficient large-scale mineralization of CO2 using industrial alkaline solid waste [64]. Figure 4 indicates the potential contribution of different industrial solid wastes to CO2 emission reduction around the world. As can be seen in Figure 4a, the data reveal that CO2 mineral carbonation of industrial solid wastes can reduce total CO2 emissions by approximately 310 Mt, with iron and steel slags being the largest contributor (43.5%), followed by cement wastes, mining wastes, and coal combustion ashes. Based on Figure 4b, carbon mineralization using industrial wastes can also contribute to around 3700 Mt of CO2 emission reduction indirectly by using carbonated products instead of conventional carbon-intensive materials and eliminating emissions associated with their production [82]. Carbonated cement wastes contribute the most to CO2 emissions reduction indirectly, accounting for a 55.7% reduction (about 2 billion tons of CO2 emissions). Carbonated coal combustion products, iron and steel slags, and mining wastes also significantly contribute to indirect CO2 emission reduction [82].
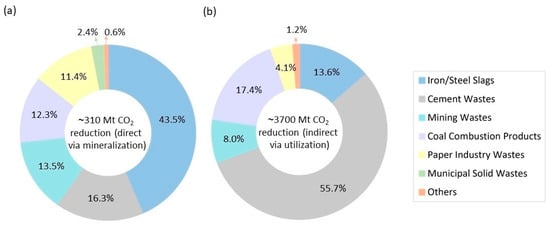
Figure 4.
CO2 emission reduction via (a) ex situ mineralization and (b) carbonated product utilization (adapted from [82]).
Therefore, iron/steel slags, cement wastes, and mining wastes are the most representative industrial solid wastes used for direct CO2 reduction via carbon mineralization due to their high alkali contents and reactivity, which will be discussed thoroughly in Section 5.
3.2. Mineralization Pathways
Ex situ CO2 mineralization can be divided into direct and indirect approaches based on different process pathways. Direct carbonation offers a single-step reaction of CO2 with calcium or magnesium-rich materials to form high-stability carbonates [57]. This technique is the simplest approach to CO2 mineralization, utilizing different feedstocks to facilitate reactions [104]. The main challenge of this method is slow reaction rates, leading to lower efficiency carbonation, which can be improved through different pretreatment strategies, known as mineral activation [64]. This process primarily involves mechanical activation, such as the initial crushing and grinding of raw mineral materials for particle size reduction. This step increases the surface area of the minerals, thereby enhancing their reactivity [105]. Another pretreatment method is thermal activation/treatment via increasing the temperature. Higher temperatures increase mineral surface reactivity to facilitate ion extraction, especially for less reactive feedstocks like serpentine [64]. Higher temperatures for the dissolution process (metal extraction) and higher pressure during the carbonation process can significantly accelerate reaction rates while resulting in higher energy costs [106]. In addition, adding chemicals can enhance the dissolution of minerals via metal extraction and facilitate the carbonation reaction. However, it is more often associated with indirect mineralization [107]. The indirect carbonation process offers a more efficient approach by dividing the process into two steps, including ion extraction from minerals using a chemical solvent (acids or ammonium salts), followed by carbonate formation in a controlled pH swing reactor to facilitate the carbonation reaction [64]. Figure 5 indicates a simple schematic of carbon mineralization through direct and indirect pathways.
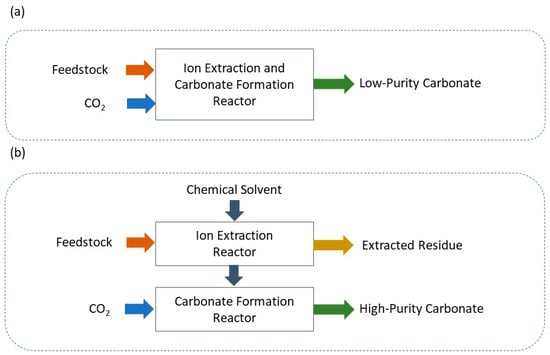
Figure 5.
Simple schematic of (a) direct and (b) indirect carbon mineralization routes [108].
The reactions in both direct and indirect carbonation can be conducted through gas–solid carbonation, referred to as the dry process, and aqueous carbonation, also known as the wet process. Mineralization through gas–solid (dry) carbonation involves the reaction of CO2 with minerals (calcium or magnesium) in the absence of water or with minimal moisture to form carbonates. Compared to the aqueous (wet) process, this method is more straightforward, as it bypasses the need for a liquid medium. However, the reaction rate in gas–solid carbonation is generally slower under natural conditions because the absence of a liquid medium reduces mineral reactivity. Higher temperature and pressure are typically required to enhance the reactivity of feedstocks, leading to a higher operational cost [53,64].
In the direct aqueous carbonation method, CO2 is dissolved in water, resulting in the formation of weak carbonic acid (H2CO3). This acid solution dissociates into hydrogen ions (H+) and bicarbonate ions (HCO3−) and gradually dissolves minerals [109]. The hydrogen ions facilitate the extraction of Ca and Mg ions from feedstocks, while the bicarbonate ions react with these extracted ions, resulting in the precipitation of carbonates [110]. This method leverages the solubility of CO2 in water to facilitate the reaction. In contrast, indirect aqueous carbonation involves a two-step approach with stepwise pH adjustments. In the first step, target metal ions (Ca2+ or Mg2+) are selectively extracted from natural minerals using specific extractants, including acids (e.g., HCl, HNO3, or acetic acid) and salts (e.g., magnesium chloride hydrates and ammonium salts), before the carbonation step [53]. Then, in the subsequent carbonation step, alkaline chemicals are introduced to facilitate the formation of carbonates [56,111]. This approach not only enhances carbonation efficiency but also yields high-purity byproducts, making it a highly effective method for CO2 mineralization [11]. Table 3 and Table 4 provide a summary of different ex situ carbon mineralization mechanisms and their advantages and challenges, respectively.

Table 3.
The mechanisms of various ex situ carbon mineralization routes.

Table 4.
Advantages and challenges of different carbon mineralization processes.
Without considering the application of mineral products, carbonation via the solid–gas reaction (dry mineralization) exhibits the slowest rate kinetics, which can be enhanced through energy-intensive pretreatments. These pretreatment strategies make the dry mineralization technique less favorable for large-scale applications due to higher operational costs. In contrast, aqueous or wet mineralization is more scalable due to faster reaction rates [64]. Water serves as a medium for dissolving CO2 and minerals in ionic forms, leading to better ion mobility in aqueous solutions. Faster mineralization by enhancing the rate kinetics results in cost-effective chemical processes. The application of the dry approach is primarily limited to highly reactive feedstocks (such as industrial alkaline wastes). Meanwhile, adding chemicals in the indirect wet mineralization method improves reaction rates, making it suitable for even less reactive feedstocks (e.g., serpentine). Moreover, separating dissolution and carbonation steps in an indirect wet approach leads to pure and high-value carbonate production [64]. The selection of effective leaching agents, including acid and/or ammonia salts to rapidly extract metals (Ca2+ and Mg2+) from minerals, is crucial to enhance the efficiency of carbon mineralization [58].
4. Carbon Mineralization Rate and Factors Affecting
The mineralization efficiency of CO2 is not only affected by CO2 availability and the richness of Ca and Mg ions in feedstocks but also depends on the reaction rates and the kinetics of dissolution and precipitation [52]. The dissolution rate is the rate of releasing Ca2+ or Mg2+ ions from the minerals, which is the slowest, rate-limiting step, while the precipitation rate is related to the formation of carbonates (CaCO3, MgCO3) [11,53]. Studies have verified that the dissolution of minerals is a key and dynamic factor in controlling the carbonation process [11].
The reaction rate (R%) and the carbonation rate (C%), indicating carbonation potential and CO2 sequestration efficiency, can be calculated using the following equations:
in which represents the weight percent of CO2 within the solid carbonate, and is the percent weight added if all the available ions convert to carbonates [74].
In addition to the carbonation pathways, the reaction rate is significantly influenced by the operational factors, which will be discussed in the following section. All these effective factors need to be optimized to balance carbonation efficiency with the economic feasibility for practical application [117,118].
4.1. Mineral Properties and Particle Size
The physical and chemical properties of minerals play a critical role in reaction rates, particularly the dissolution of Ca2+ and Mg2+ ions [119]. The activation energies of some minerals are so high that they require energy-intensive pretreatment, such as grinding or crushing, to increase the specific surface area of the minerals. Destroying the mineral structures into smaller particles (particularly those below 100 µm), which is also known as mechanical activation, increases the activity of materials and the carbonation rate significantly by providing more active sites to interact with CO2 [120]. For instance, fine grinding of feedstocks (as small as 48 µm) indicated more than three times the fraction of alkaline elements compared to feedstocks with a particle size between 150 and 300 µm [116]. However, achieving these fine particle size comes with practical limitations. Grinding materials below 38 µm can increase energy consumption by approximately 30% [121]. Moreover, particles smaller than 10 µm may pose operational challenges, such as aggregation and difficulties in handling within reactors [122]. Therefore, there is a trade-off between maximizing reactivity and considering energy and process costs, with optimal particle size ranges typically between 38 and 100 µm for industrial waste [116] and 10 and 100 µm for mine tailings [123].
4.2. Temperature
Increasing temperature enhances the kinetic of carbon mineralization by increasing the dissolution of Ca2+ and Mg2+ ions via improving the solubility of minerals in the aqueous solution, as expressed by Equation (7) [124,125]. Moreover, higher temperatures promote precipitation behavior through the formation of reactive carbonate species (HCO3− and CO32−), which are crucial for forming solid carbonates (CaCO3 and MgCO3), enabling the system to proceed more quickly [51].
where is the temperature-dependent dissolution rate constant, represents the independent rate constant; is the activation energy, is the universal gas constant, and is temperature.
On the other hand, elevated temperatures reduce CO2 solubility in water, limiting carbonate formation. Therefore, high temperatures often require compensating with increased pressure to offset reduced CO2 solubility, which escalates energy demands and complicates industrial scalability [51]. However, in gas–solid (dry) carbonation, the temperature enhances reaction kinetics without solubility constraints, but excessive heat triggers carbonate decomposition [126]. Based on the literature, the optimal temperature of 150–200 °C was recommended for the direct carbonation of minerals [126]. Meanwhile, the temperature of 25–80 °C was suggested for balancing cation dissolution and CO2 solubility for carbonation using industrial waste, since waste heat can be utilized for energy-efficient processing [127]. Overall, due to the complex effect of temperature, enhancing mineral reactivity while hindering CO2 solubility, specific optimization is needed based on feedstock reactivity, CO2 pressure, and carbonation pathways to balance reaction efficiency with energy costs, particularly for practical applications [58].
4.3. Pressure
Pressure significantly influences the kinetic and efficiency of carbon mineralization by enhancing both CO2 dissolution (the solubility of CO2 in an aqueous solution) based on Henry’s law (Equation (8)) and the subsequent carbonation reactions [128]. Elevated pressure can shift the equilibrium toward solid formation, promoting the precipitation of stable carbonates by reducing the energy barrier for precipitation [58]. This leads to faster carbonation rates and higher efficiency.
where is the partial pressure of CO2, represents Henry’s constant, and is the CO2 concentration dissolved in an aqueous solution. The linear relationship between the carbonation rate and CO2 pressure has been demonstrated in many studies [129,130,131]. Based on the literature, mineral carbonation using industrial waste is typically most effective at a CO2 pressure ranging from 0.1 to 2 MPa (1–20 bar) [72,132,133]. For mining waste, higher pressure, often more than 5 MPa (50 bar), is applied to achieve rapid carbonation, especially when combined with an elevated temperature [126]. However, using a high pressure also raises the energy demand and operational costs. Therefore, balancing these factors is critical for scalable applications [105,134].
4.4. pH Value
In carbonation, the pH value has an important influence on both the mineral dissolution rate and the precipitation rate. A critical aspect of the indirect mineralization process is the step-by-step pH adjustment, known as the pH swing technique [58,135]. This adjustment is necessary because the extraction of Ca2+ and Mg2+ occurs under acidic conditions (pH~2–5), while carbonate precipitation requires alkaline conditions (pH~8–12) [69,136]. As the pH value of the solution gradually declines by progressing the reactions, adding alkaline materials such as NaOH and NaHCO3 is required to facilitate carbonate formation [137]. This pH adjustment through adding acid and/or ammonium salt is considered as the activation method in indirect mineralization process, leading to a greater carbonation reaction compared to direct mineralization. Various types of acids and ammonium salts have been utilized for indirect carbon mineralization (Table 5).

Table 5.
Application of different types of acids and ammonium salts in indirect mineralization.
Generally, ammonium salts are promising due to their advantages, including (i) a high regeneration potential (up to 95%), and reducing waste and cost [145]; (ii) their mild acidity reduces corrosion and secondary waste generation [149]; and (iii) their ability to produce pure carbonate due to their high selectivity for calcium over impurities [121]. Strong acids such as HCl, H2SO4, and HNO3 are highly effective for Ca and Mg extraction but less sustainable due to regeneration challenges and waste generation [116,154]. Milder acidity of acetic acid, as an organic acid, reduces corrosion and waste generation compared to strong acids; however, it may not perform as well with magnesium-rich feedstocks [145].
4.5. Reaction Time
Reaction time is a key factor in carbon mineralization, determining the extent of the reaction and the conversion efficiency. As discussed earlier, the biggest challenge of direct mineralization is reaction time limitations due to being a single-step reaction [155]. Both longer reaction times and higher stirring rates promote carbonation kinetics through the deformation of the crystal morphology of minerals, resulting in particle size reductions [116]. While the initial reaction rate is high, it slows significantly over time due to the formation of passivation layers, such as dense carbonate coatings, which hinder the further diffusion of CO2 to reactive sites. This results in incomplete carbonation and requires extended reaction times or intensive conditions (e.g., high temperature and pressure) to achieve a higher conversion rate [156]. In contrast, indirect mineralization overcomes the reaction time limitation by separating dissolution and carbonation steps. A longer reaction time during the dissolution phase improves metal extraction from feedstocks and, subsequently, carbonation efficiency [157]. Generally, a longer reaction time increases the release of Ca2+ and Mg2+ ions from feedstocks, leading to a greater CO2 sequestration capacity for all types of feedstocks [158,159,160]. However, the effect decreases over time, meaning that there is an optimum reaction time. The ideal reaction time depends on the feedstock, reaction conditions (e.g., temperature, pressure, and pH), and the reactor system [161]. For instance, highly reactive materials, such as industrial alkaline waste, need a shorter reaction time (minutes to hours) under optimized conditions, whereas less reactive materials, such as natural silicates (olivine and serpentine), require extended durations (days to years) to achieve significant carbonation efficiency [58].
4.6. Liquid/Solid Ratio
The liquid to solid (L/S) ratio has been considered an important factor affecting the carbonation reaction. A low L/S ratio enhances the carbonation rates of Ca2+ and Mg2+ ions by increasing the availability of the reactive surface area relative to the liquid phase. However, a lower ratio may cause poor mixing and CO2 mass transfer due to increasing the viscosity of the solution, hindering the carbonation process [47]. Conversely, a higher L/S ratio improves mixing and CO2 dissolution but requires more water, raising the operational cost and energy demands for handling and recycling water. Moreover, excessively high L/S ratios can also act as a mass transfer barrier, and the low concentration of solids may lead to slower carbonation rates. Therefore, an optimal L/S ratio is required to balance ion availability with effective mixing to achieve the highest carbonation efficiency [58]. Based on the literature, the ideal range of L/S ratio for carbonation is generally between 10 and 20 mL/g [156,162,163], depending on the other parameters, such as temperature, pressure, feedstock reactivity, and economic feasibility.
Table 6 summarizes the effect of each factor on the carbonation reaction rate and its optimal ranges, considering different feedstocks.

Table 6.
Factors affecting carbon mineralization rate.
4.7. Reactor Design
In addition to all above-mentioned variables, reactor design critically influences the efficiency and scalability of ex situ carbon mineralization processes by controlling parameters such as temperature, pressure, reactant contact, and residence time.
Multi-stage reactor systems, such as Continuous Stirred-Tank Reactors (CSTRs) configured in series, enhance conversion rates by maintaining optimal conditions for mineral dissolution and carbonate precipitation. For example, Gomes et al. [164] indicated a configuration using two extraction and six carbonation reactors achieved 95% reaction efficiency by balancing capital costs and process intensity. Elevated temperatures (e.g., 150 °C) and CO2 pressures (10 bar) in pressurized reactors accelerate kinetics but increase energy demands, requiring trade-offs between reaction speed and operational costs [164]. Reactor designs that integrate grinding mechanisms or pre-treated mineral feedstocks (e.g., heat-activated serpentine) improve the surface area and reactive site accessibility, addressing slow diffusion limitations inherent in silicate mineral carbonation [165]. However, single-reactor systems face challenges in achieving complete conversion due to the limited residence time and mixing efficiency, underscoring the importance of staged configurations [164]. More advanced reactors, such as fixed beds, fluidized beds, and rotating packed beds, enhance reaction rates through continuous CO2 flow, particle abrasion, and heat recovery [166,167,168]. Fluidized beds outperform fixed beds by mitigating passivation layers via the continuous abrasion of surface layers and enabling energy reuse [169]. Moreover, fluidized beds provide superior mass transfer compared to fixed beds [170]. Rotating packed beds show promise for carbonation conversion compared to traditional reactors due to utilizing high centrifugal forces to significantly boost gas–liquid mass transfer efficiency [171].
Other advanced configurations, like the gravity pressure vessel (GPV), leverage the hydrostatic pressure (up to 150 bar) and integrated heat recovery to accelerate mineral carbonation while reducing energy demands, achieving >16% conversion for olivine at 120 bar and 185 °C [172]. Similarly, impinging-type reactors enhance gas–particle contact through computational fluid dynamics, optimizing inlets, prolonging dwell times, and boosting CO2 uptake in fly ash systems by 30–50% compared to conventional designs [173]. Lab-scale rocking autoclaves simulate GPV conditions, revealing that finer particle sizes (<20 µm) and higher pressures (>60 bar) double Mg leaching rates from ultramafic minerals like serpentine [172,174]. These designs prioritize turbulent mixing, heat integration, and phase separation to balance kinetics with energy costs, demonstrating that tailored reactor geometries and operational parameters are pivotal for scalable carbon sequestration [175]. Despite these innovations, scalability remains constrained by high energy costs and the need for large reactor volumes.
5. Carbon Mineralization Using Different Alkaline Solid Wastes
Among potential feedstocks for carbon mineralization, industrial solid wastes and mine tailings have been widely evaluated compared to natural minerals. Carbon mineralization using natural minerals is an energy-intensive process with some environmental issues [176]. Meanwhile, the carbonation of industrial wastes is more economical compared to natural minerals due to reduced mining and transportation expenses. Moreover, the higher reactivity and availability of alkaline wastes make them more suitable feedstock for CO2 mineralization [177]. Alkaline solid waste mineralization offers various advantages, such as lower energy consumption, a higher reaction rate, and higher carbonation efficiency compared to natural minerals [79]. More importantly, carbon mineralization using waste residues is not only beneficial for CO2 emission reduction but also can be considered as a waste management strategy. This strategy aligns well with circular economy principles by utilizing waste materials that would otherwise be discarded. It produces value-added products for new construction, thereby reducing the need for virgin raw materials [178]. As indicated earlier in Figure 4, among different alkaline solid wastes, iron and steelmaking slag, cement wastes, and mine tailings contribute greatly to CO2 reduction. In this section, we discuss the potential of these alkaline solid wastes through different carbonation routes under various conditions.
5.1. Iron/Steel Slags
The iron and steelmaking process is a complex, integrated sequence that transforms raw materials into high-quality steel products through chemical reactions, physical transformations, and metallurgical processes [179]. This process generates different byproducts, including slag, which is produced during both ironmaking and steelmaking stages [180]. Figure 6 indicates a schematic illustration of slag generation during iron and steelmaking processes.
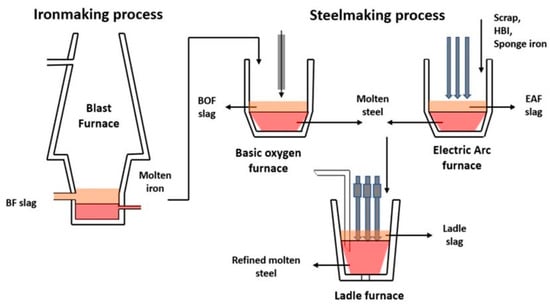
Figure 6.
Simple schematic of slag generation for iron and steel production [181].
The initial stage is raw material preparation using primary input materials (e.g., iron ore, coal, and limestone). Then, the ironmaking stage follows as a crucial stage of steel production, requiring substantial energy input and generating significant CO2 emissions [181]. The main ironmaking process is transforming iron ore and other primary materials into molten iron (commonly referred to as hot metal) in blast furnace (BF) operations. Then, the obtained hot metals proceed to the steel refining stage through a basic oxygen furnace (BOF) or electric arc furnace (EAF), where impurities are removed, and the chemical composition is adjusted to create high-quality steel [105]. A ladle furnace (LF) is a critical processing unit used to refine the chemical composition of steel and remove impurities. During these stages, different byproducts, such as slag, sludge, dust, and gases, are generated. Slag is a significant byproduct of steelmaking processes, with an average amount of several hundred kilograms of slag generated per metric ton of steel production [182]. The amount of slag produced depends on different factors, such as the raw materials’ compositions and the specific steelmaking stages. Based on the literature, the global production of slag in 2022 reached 330–390 million tons through ironmaking and 190–290 million tons through steelmaking processes [112]. Myers et al. [182] estimated that between 2020 and 2100, the potential of slag-based CO2 mineralization in cumulative CO2 reduction is likely 26.4–41.9 gigatons CO2.
Researchers have investigated the potential of different slag types, including BF, BOF, EAF, and LF, as feedstocks for carbon mineralization due to their large quantity and highly alkaline contents. Table 7 indicates the composition of different types of slags. Accordingly, slag contains CaO and MgO contents of around 30–60% and 3–15%, respectively, along with silicate (SiO2), aluminates (Al2O3), ferrites (Fe2O3), and various trace minerals [105].

Table 7.
Chemical composition of different types of slags (wt%).
The chemical and mineral variability among slags leads to differences in their CO2 sequestration capacities. For instance, Elyasi Gomari et al. [184] compared the potential of BF and LF slags for carbon sequestration and found that LF slag achieves a higher CO2 capture efficiency than BF due to higher CaO and MgO contents. Moreover, Yu and Wang [190] found that EAF slag outperforms BOF slag in reactivity and CO2 storage, as the reaction rate of EAF is faster than BOF slag. In addition to the chemical composition, the reaction route and operational conditions also affect the carbonation efficiency. Table 8 provides a comprehensive overview of the carbon mineralization potential using various iron and steel slags through aqueous (wet) carbonation, gas–solid (dry) carbonation, and indirect carbonation under a wide range of process conditions.

Table 8.
Carbon mineralization using different types of slag.
As summarized in Table 8, limited studies have used direct gas–solid carbonation due to the low carbonation efficiency. Moon and Choi [135] reported that the CO2 storage capacity of AOD slag was only 18% at ambient temperature and pressure during 28 days of gas–solid carbonation reaction. To overcome this challenge, the gas–solid carbonation process needs to be carried out at high temperatures or pressures. For instance, Tian et al. [202] demonstrated that steel slag carbonation at a high temperature of 600 °C resulted in a higher carbonation conversion rate of 49.5–55.5% and CO2 storage capacity of 88.5 kg CO2/ton of slag. Yu and Wang [190] also confirmed that higher temperature (500 °C) could result in the higher Ca utilization of BOF and EAF slags. However, at a temperature of 550 °C, the Ca utilization is reduced, depending on the CO2 concentration. Therefore, direct gas–solid carbonation is not a suitable approach due to the slow reaction kinetics and gas diffusion, thereby limiting carbonation efficiency. In contrast, the direct aqueous approach has been widely applied for carbonation, as the presence of water leads to higher carbonation efficiency [212]. However, the slow kinetics remain a significant challenge of this technique, which can be improved by increasing the reaction time to 1 [193] or even 10 days [195] using a higher temperature of 100 °C [193] to 500 °C [190] or a higher CO2 pressure of 3 MPa [183], which resulted in higher operational costs.
Another approach for increasing carbonation efficiency using a direct aqueous route is enhancing the solubility of CO2 in the suspension via the introduction of salts, which impacts the CO2 concentration in the solution and changes the solution acidity [200]. To this end, Ren et al. [158] investigated the direct CO2 mineralization of BF slag by using saline solutions to enhance the reaction. Their study indicated that the CO2 mineralization capacity increased to 96.44 kg CO2/ton of slag by adding 1 mol/L NaCl, which is about 2.5 times more than the non-NaCl solution (37.59 kgCO2/ton of slag). Moreover, the NaCl solution resulted in a higher carbonation efficiency compared to the other saline solutions. The CO2 sequestration capacity reached 280 kg CO2/ton of slag by increasing the reaction time to 24 h [158]. In another study, Pan et al. [197] introduced an appealing process by incorporating BOF slag with cold rolling wastewater (CRW), which is produced in large volumes in steelmaking industries. They found that the high alkalinity of CRW (pH > 11) made it desirable for carbonation reactions. Moreover, CRW contains different inorganic ionic species, improving the dissolution of minerals and consequently increasing the carbonation conversion of BOF slag (83.8 kg CO2/ton of slag) [197]. In the other study, Pan et al. [198] achieved a greater carbonation efficiency (195 kg CO2/ton of slag) by increasing the reaction time to 8.5 min and using a smaller size of particles (<45 µm) [198]. They reported that the acceleration leaching/dissolution behavior of Ca ions from BOF slag into a solution is attributed to (i) using a smaller particle size and (ii) the presence of a high concentration of Na+ and Cl− in CRW. Chang et al. [199] also confirmed that the high pH of CRW enhanced the CO2 dissolving in the solution, thereby increasing the carbonation efficiency to 283 kg CO2/ton of slag [199].
As mentioned earlier, the direct aqueous carbonation resulted in low-purity carbonated products. To address this issue, the extraction rate of metal ions from feedstocks needs to be maximized by using extraction agents [54]. Therefore, adding chemical reagents (e.g., organic and inorganic acids and/or ammonium salts) has been suggested as the first step of indirect carbonation to selectively extract ions. Kodama et al. [208] compared three solvents of NH4Cl, CH3COOH, and HCl for carbonation BOF slag. The results indicated that the Ca extraction potential ranking was as follows: HCl > CH3COOH > NH4Cl. However, NH4Cl could offer better Ca selectivity of 99.6%, followed by HCl (90.2%) and CH3COOH (88%). The reason for this is attributed to the pH of solutions; for instance, the pH of NH4Cl solution was in the range of Ca extraction (8–10.5), while the pH of HCl and CH3COOH solutions was in the range of magnesium or manganese extraction (7–7.5) [208].
Generally, acids are highly effective at extracting metal ions, but they lack selectivity. They tend to extract multiple metal ions and impurities, which necessitate additional purification steps. In contrast, ammonium salts are generally less efficient for metal ion extraction, while offering better selectivity for certain metal ions [72,213,214]. Eloneva et al. [209] investigated Ca extraction from BOF using ammonium salt solutions, including NH4NO3, CH3COONH4, and NH4Cl. The results indicated that NH4NO3 resulted in greater Ca dissolution compared to the CH3COONH4 and NH4Cl, with insignificant differences, which aligns with their previous study [215]. Owais et al. [210] investigated the effect of NH4Cl molarity and particle size on Ca extraction from BOF slags. They found that by enhancing the molarity of the NH4Cl solvent, the Ca extraction rate increased linearly. Sun et al. [211] found that a high purity of CaCO3 (96 ± 2 wt%) could be achieved through carbonation of BOF slag by adding NH4Cl as an extraction agent under optimized conditions, with the CO2 capture capacity of 211 kg CO2/t of slag. Dri et al. [146] utilized NH4HSO4 for metal extraction from BOF slag and achieved 85% Mg extraction after 3 h of reaction at 90 °C. Liu et al. [207] obtained the CO2 capture capacity of 263 kg per ton of BF slag using recyclable NH4HSO4. They achieved 91% Mg and 97% Ca extraction from the BF, which were converted into carbonates, and 76% ammonium aluminum sulfate was obtained as a high-value-added byproduct with a purity of 99.6% via crystallization from the leaching solution.
According to the literature, direct aqueous carbonation using NaCl or CRW provides a simpler and more cost-effective approach, achieving a high CO2 capture capacity. This method is especially effective for BOF and EAF slags containing a high reactive CaO content. However, the direct carbonation route can be limited by slower kinetics and less control over impurities. Conversely, indirect carbonation, involving separate metal extraction and carbonation steps, provides greater selectivity and carbonate product purity. However, this technique requires more complex operations and a higher energy input due to the recovery of reagents. For industrial applications, a balance between operational simplicity and product purity is crucial. Therefore, direct aqueous carbonation of BOF and EAF slags using salts or CRW for providing higher ion dissolution is recommended as a cost-effective, large-scale CO2 capture technique. Meanwhile, indirect routes using ammonium salts and recyclable reagents are preferable when high-purity carbonate products are desired.
5.2. Cement Waste
Concrete ranks as the second most commonly consumed material globally after water by society [216]. Cement is a crucial binding material in concrete and is widely used as a construction material in buildings, infrastructure, and transportation systems worldwide [217]. Cement manufacturing is a significant contributor to global CO2 emissions, accounting for around 7% of human-caused CO2 emissions, and has the highest carbon intensity per unit of revenue among all industries [218]. Major emissions are from limestone calcination during clinker production, which accounts for 60% of total CO2 emissions [219]. Carbon mineralization using cement wastes is a promising approach for reducing CO2 emissions in the cement and concrete industries. Calcium-bearing phases in cement waste react with CO2 to form stable carbonate calcite. This process can potentially sequester most or all of the CO2 that was originally released during cement production [220].
Different types of cement wastes can be used for carbon mineralization, including cement kiln dust (CKD), cement bypass dust (CBD), recycled cement paste (RCP), and cement slurry waste (CSW). The effectiveness of carbon mineralization of these feedstocks depends on factors such as the waste composition, mineralization process, and intended application of the resulting product. Among these cement wastes, CKD appears to have the highest efficiency for carbon mineralization due to its high CaO content, fine particles, and abundance. The amount of CKD generated is estimated to be 15–20% of the total cement produced, which means that hundreds of millions of metric tons of CKD can be generated annually, which is associated with the global production of ordinary Portland cement (OPC) [221,222]. Therefore, the large volume of CKD generated has led to an increased focus on its potential application and management strategies in the cement industry [221]. CKD indicates significant potential for CO2 sequestration, with around 48–55 kg CO2 per ton of CKD [127]. As indicated in Table 9, CKD typically contains 38–50% CaO, making it a suitable feedstock for carbon mineralization [221,223]. Moreover, the presence of free lime (unreacted CaO) in CKD is particularly beneficial for CO2 sequestration, as it readily reacts with CO2 to form stable calcium carbonate [127]. The carbonation of CKD not only contributes to CO2 emission reduction but also reduces its alkalinity, decreasing the potential health hazards of CKD exposure, such as skin and eye irritation [224].

Table 9.
Chemical composition of different types of cement wastes (wt%).
Recycled cement paste (RCP) obtained from demolished concrete structures can also be utilized for carbon mineralization [220,229]. The carbonation process involves separating cement paste from aggregates in recycled concrete and exposing the cement paste to CO2 through direct or indirect carbonation. The resulting carbonated products contain calcium carbonate and amorphous alumina–silica gel. Carbonated cement waste can potentially be used as a supplementary cementitious material (SCM) in new concrete mixes, further reducing the carbon footprint of cement production [230]. Life-cycle assessments have shown that using carbonated cement waste as SCM can significantly reduce the carbon footprint of concrete production [231]. Moreover, the mineralized product incorporated into concrete mixes potentially improves certain properties of cement, such as strength and corrosion resistance [55]. For instance, CarbonCure, a Canadian company, recently developed a technology to inject CO2 into ready-mix concrete directly to embed CO2 as fine carbonates. Concrete containing carbon is estimated to indicate 7% higher compressive strength compared to conventional concrete produced with standard Portland cement [232].
As illustrated in Table 10, various research studies have focused on mineral carbonation using cement wastes through direct and indirect approaches under controlled conditions (temperature, pressure, etc.).

Table 10.
Carbon mineralization using different types of cement wastes.
Xuan et al. [228] investigated CSW carbonation through a direct gas–solid reaction. The results indicated that after 144 h of carbonation reaction, 110 kg CO2/ton of CSW could be captured under ambient temperature and pressure. Biava et al. [127] demonstrated that the direct aqueous carbonation of CKD in a pressurized reactor at 15 bar could yield 48 kg CO2/ton of CKD in 24 h reaction. Short et al. [236] achieved a higher CO2 sequestration potential of 162 kg CO2/ton of OPC through direct aqueous carbonation under higher temperatures (60 °C) and pressure (97 bar). As we can see, in addition to the carbonation route, carbon mineralization efficiency depends on the type of cement wastes due to their different compositions and process conditions, such as temperature and pressure. Researchers are exploring ways to optimize the carbon mineralization process. For instance, Kim et al. [227] applied indirect mineral carbonation to investigate the efficiency of Ca extraction from CKD, determining optimum reaction conditions and solvent type using different acids (hydrochloric acid and acetic acid) and ammonium salts (ammonium chloride and ammonium acetate). The optimum reaction conditions to achieve the highest extraction efficiency were a 30 min reaction time, 25 °C temperature, L/S ratio of 10:1, solvent concentration of 1.74 M, and stirring speed of 250 rpm. Among different solvents, using HCl resulted in the highest Ca extraction efficiency of around 95%, followed by CH3COOH, NH4Cl, NH4CH3CO2, and C6H5Na3O7 [227]. Therefore, the indirect route provides selective Ca extraction, which can then be converted into carbonates with high efficiency. However, due to the complexity and cost associated with using chemical reagents, direct aqueous carbonation remains a viable alternative for industrial applications owing to its simplicity, scalability, and cost. However, enhancing the reaction kinetics of direct aqueous carbonation through process optimization needs to be considered.
5.3. Mine Tailings
Mining industries produce large quantities of mine tailings or mining wastes during the extraction of economic metals from ultramafic deposits. Carbon mineralization using mine tailings is an innovative approach to sequester CO2 and reduce greenhouse gas emissions [126,238]. A finely ground form of mine tailings makes them suitable feedstocks for mineral carbonation by increasing their surface area and reaction potential [239]. Moreover, this approach can be beneficial for eliminating additional mining costs and environmental hazards of mining wastes [55,240]. This process utilizes the reactive minerals present in mine wastes, particularly magnesium silicates such as olivine (Mg2SiO4) and serpentine (Mg3Si2O5(OH)4), to react with CO2 and form stable carbonate minerals [106]. Magnesium silicates are preferred over calcium silicates for carbon mineralization due to their higher abundance and containing a higher weight concentration of MgO (up to 50%) compared to the CaO content in calcium silicates (typically 10%) (Table 11). This higher concentration of metal-bearing oxide groups enables magnesium silicates to capture and store more CO2 [50,126]. In this regard, Woodall et al. [123] investigated carbon mineralization of Stillwater tailings from the mining of nickel and platinum group metals (PGM) containing calcium-bearing plagioclase feldspar. Direct aqueous carbonation at an ambient temperature and pressure resulted in less than 1% Ca carbonation, with a CO2 capture capacity of only 1.79 kg CO2/t of stillwater tailing. The low carbonation efficiency was not only attributed to the tailing composition but also to the carbonation route. Therefore, further pretreatment activities, such as mechanical activation, are suggested to overcome the slow kinetics of direct aqueous carbonation by (i) reducing the particle size and increasing the surface area and (ii) disordering the crystal lattice and decreasing the activation energy [241].

Table 11.
Chemical composition of different types of mine tailings (wt%).
Using tailings for indirect mineral carbonation would be advantageous since it eliminates the need for mining and particle size reduction steps. This would result in reduced energy usage and lower environmental impact from these processes while also decreasing environmental liabilities [240].
Despite the potential of mine tailings for carbon mineralization, there is a significant research gap in the carbonation of mine tailings (Table 12). The indirect carbonation approach through a pH swing process has gained significant attention to overcome the challenge of the slow reaction time of the direct carbonation of tailings. For instance, Meyer et al. [241] achieved a better result for the carbonation of PGM tailings through the pH swing approach by adding HCl and NaOH under higher temperatures, with a shorter reaction time, compared to the study by Woodall et al. [123]. A net conversion of around 29.9, 2.9, and 8.9% was obtained for Ca, Mg, and Fe, respectively. The low conversion rates were attributed to low cation extraction, mainly due to the unreactive nature of orthopyroxene, as a major mineral in these tailings, containing 88% Mg content. The varied mineral compositions of tailings and waste rocks might reduce their reactivity in both direct and indirect carbonation processes [243]. For instance, Liu et al. [242] stated that using HCl and NH4OH through the pH swing method resulted in 82.33% Mg extraction from Mg-silicate tailings. Meanwhile, Meyer et al. [241] achieved only 5% Mg extraction from PGM tailings using HCl and NaOH.

Table 12.
Carbon mineralization using different mine tailings.
Based on the literature, mine tailings indicate a lower CO2 sequestration potential compared to cement wastes and slags, which might be due to their variable mineral composition, while rich in magnesium [160]. The lower reactivity is due to the highly crystalline nature of mine tailings, which require a longer reaction time or additional processing to achieve significant carbonation. For instance, Liu et al. [160] suggested using an external field (microwave power of 1000 W and ultrasound power of 500 W) to enhance Mg extraction from serpentine tailings. A Mg extraction efficiency of 66.64% was obtained in 120 min using CH3COOH and NH4OH to adjust the pH value during extraction and carbonation processes, respectively. The CO2 sequestration analysis indicated that high-level fixation of CO2 could be achieved in the form of MgCO3·3H2O with long-term stability. It should be noted that in the case of using extraction agents, they need to be effectively regenerated and recovered for the process to be economically viable [56]. Generally, mine tailing carbonation currently faces economic barriers but could become more viable with improved processing technologies, such as increasing the concentration of CO2 and optimizing tailings’ pore water saturation [69]. These approaches can result in value-added products that can also be integrated with the recovery of valuable metals like nickel and cobalt. However, finding the right balance between carbonation efficiency and operational practicality needs to be considered.
Among the above-mentioned alkaline solid wastes, mine tailings stand out as the most promising feedstock in terms of available stock and future potential. As mentioned earlier, iron and steel slag are significant byproducts of the steelmaking industry, with global production estimated at 330 to 390 million tons per year [112]. The Asia Pacific region, particularly China, dominates this market due to rapid urbanization and infrastructure growth. In the United States alone, about 16 million tons of slag was sold in 2023, primarily for use in construction and as a cement additive [244]. Steel slag, due to its high iron content, can also be recycled using magnetic separation. However, the utilization rate of iron slag remains relatively low, and regional supply imbalances can limit its broader adoption [245]. Cement waste, mainly generated from construction and demolition activities, is another major feedstock, with around 820 million tons produced annually in Europe and 191 million tons in the United States [246]. Concrete makes up a significant portion of this waste stream. While recycling cement waste as aggregate or supplementary cementitious material is technically feasible, contamination and the presence of mixed waste streams often hinder effective recycling. Much of this waste still ends up in landfills due to economic and infrastructural barriers [247]. In contrast, mine tailings represent an enormous and largely untapped resource. Their sheer volume far exceeds that of iron/steel slag and cement waste, offering a vast and long-term resource base [248]. Globally, there are approximately 282 billion tons of abandoned tailings in addition to 16 billion tons generated each year [249]. Among countries, China, Canada, and Australia have thousands of active and abandoned mine sites, making tailings a multi-billion-dollar opportunity for critical mineral recovery [250,251]. Moreover, mine tailings contain recoverable quantities of critical minerals essential for the green energy transition and digital technologies [252]. Reprocessing tailings not only reduces environmental risks such as acid mine drainage but also supports land reclamation and aligns with circular economy principles [253,254]. Although technical and economic challenges remain, ongoing advancements and increasing policy support are making mine tailings an attractive and strategic feedstock for the future [255].
6. Pilot Scale Carbon Mineralization Plants
Several pilot projects have been conducted or are underway to test the feasibility and scalability of ex situ carbon mineralization using industrial alkaline wastes. These projects focus on accelerating the natural process by which CO2 reacts with minerals in waste materials, capturing carbon in stable, solid forms. Below are notable case studies and pilot projects in this field:
Slag2PCC (Aalto University in Espoo, Finland): One notable example of a pilot-scale project for steel slag is the Slag2PCC process, which co-processes steel slag and CO2 to produce precipitated calcium carbonate (PCC) [209,256]. This process has been verified at pilot scale, with a plant designed to produce approximately 100 kg/h of PCC. In these experiments, almost 80% of the calcium in the slag was leached through a two-stage extraction process, and more than 70% of the CO2 was utilized and converted into PCC [257,258]. The process also demonstrated the ability to recycle the ammonium chloride solution used for extraction up to ten times, minimizing chemical consumption and improving sustainability [258]. However, challenges remain for large-scale industrialization, including optimizing the dissolution of CO2 in an aqueous solution, minimizing ammonia loss, and increasing the selective extraction yield of calcium from the slag, as much of it exists in less reactive mineral phases [63].
Capso (China): Capso completed the world’s first cement CCUS project in 2017, a pioneering pilot project using cement waste, in which captured CO2 was mineralized within cementitious materials. This project, which processed 50,000 tons per year of CO2, achieved a carbon fixation rate well above 12%. The mineralized cement not only sequestered CO2 permanently but also enhanced the material properties, increasing compressive strength by more than 40% and reducing curing times by 60% compared to conventional cement processes. The CO2 used in this process was captured and purified from a coal feedstock cement facility, meeting food-grade purity standards, and the pilot demonstrated both environmental and performance benefits [259].
Arca Climate Technologies & BHP (Mt Keith Nickel West, Australia): This project launched in late 2023 to test air-to-rock carbon mineralization using nickel mine tailings to permanently capture and store atmospheric CO2. To this end, rovers, surface manipulation technology, and machine learning were used to enhance and measure the rate of carbon mineralization in mine tailings [260]. The process aimed to capture and store CO2 in a single step by accelerating natural reactions between atmospheric CO2 and magnesium-rich minerals in the waste [261]. The project demonstrated integration with active mining operations, validated the scalability of the technology, and produced verifiable carbon removal credits. Arca’s work is further referenced in case studies and technical reports by the BC Centre for Innovation and Clean Energy (CICE) [262].
University of British Columbia (UBC)-Led Field Trials (Canada): This pilot is part of a broader initiative supported by Natural Resources Canada’s Clean Growth Program and involves collaborations with several mining companies and academic institutions. The project also includes a parallel field trial at a prospective nickel mine in British Columbia (BC), but the Northwest Territories (NWT) component is focused on the Gahcho Kué Diamond Mine. The project focuses on maximizing the reaction between CO2 and magnesium silicate-rich mine tailings (from nickel, diamond, and platinum mining), forming stable carbonate minerals to scale up laboratory-proven methods to field conditions. The successful field trials demonstrated that processed kimberlite tailings can effectively sequester CO2 under real mining conditions. If the waste produced from mining commodities in magnesium silicate rocks were completely carbonated, it could capture an estimated 100 to 200 million tons of CO2 annually at current mining rates [263].
Dumont Nickel Project (Quebec, Canada): Compared to other ultramafic mine wastes that undergo passive mineral carbonation, the Dumont mine’s tailings have the highest potential for CO2 storage due to their elevated brucite content (~11%), which is notably greater than the 2.5% brucite found in tailings from Australia’s Mount Keith mine. Despite this higher storage capacity, the carbonation rate measured in kinetic tests (2200 g of CO2 per square meter per year) is similar to rates reported at other sites. The primary constraint on carbonation in the Dumont tailings is that much of the material was nearly saturated [75].
In summary, pilot studies for mining waste mineralization have demonstrated technical viability, significant CO2 sequestration potential, and added value to the resulting construction materials, although further research and process optimization are needed for widespread industrial adoption. Pilot tests have also demonstrated that ex situ mineralization can be integrated with CO2 capture from industrial point sources, such as flue gases, and can utilize a wide range of industrial wastes as feedstocks. While laboratory and field trials have confirmed the technical viability of these processes, challenges remain regarding the cost, scalability, and logistics of handling large volumes of solid materials. Nonetheless, the environmental co-benefits, such as the stabilization of hazardous elements and the potential for beneficial reuse of treated waste—make ex situ carbon mineralization a compelling option for both carbon management and waste remediation.
7. Market Analysis
The global carbon capture utilization market was valued at USD 12 billion in 2024. By 2045, the market is expected to expand dramatically to around USD 239 billion in revenue [264]. Figure 7 indicates a clear transition from conventional CO2 utilization methods, primarily by enhanced oil recovery (EOR) in 2024, toward a broader range of applications, particularly in building and construction materials (e.g., carbonates and concretes) in 2045. This shift reflects the growing maturity of the CO2 utilization market [264].
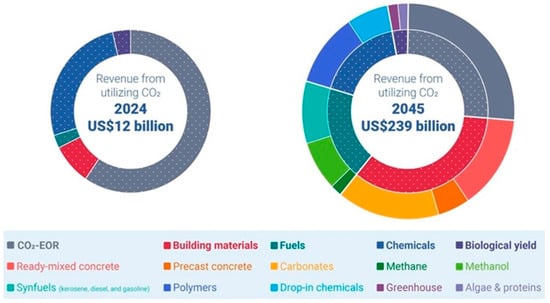
Figure 7.
The expected growth of the CO2 utilization market [264].
As mentioned earlier, carbon mineralization technologies can offer potential environmental benefits through effective waste management and permanent CO2 capture while simultaneously producing valuable byproducts. The formation of carbonated materials, including silica, various metal carbonates (magnesium, calcium, and iron), iron oxide, and other metals, can create benefits beyond carbon capture and storage [265]. In 2023, North America dominated the CO2 mineralization technology market, contributing about 40% of the global share, followed by Europe and the Asia—Pacific region, with approximately 30% and 20%, respectively. Latin America, the Middle East, and Africa collectively made up the remaining 10%. North America is also the fastest-growing region due to increasing focus on achieving carbon neutrality and scaling up industrial projects [266].
The carbonated products’ properties would significantly depend on the synthesis factors such as pH, temperature, retention time, ion concentration, additives, and stirring method, which were discussed previously. Based on the market needs and thermophysical properties, the products can be categorized into low-end, high-volume and high-end, low-volume uses [267]. The former application includes construction aggregates with a global demand of 22.5 Gt annually [122], fillers, soil acidity neutralizers, and land reclamation agents. These applications generally require large quantities but have less stringent specifications. In contrast, the high-end, low-volume uses need precise characteristics with specialized applications as catalysts, chromatography materials, ceramics, pigments, and pharmaceuticals [268]. Table 13 indicates the global market size of mineral carbonate applications for magnesium carbonate, calcium carbonate, and silica. As we can see, magnesium carbonate shows a global market size of USD 689 million, with an annual growth rate of 5.1% from 2022 to 2030. Meanwhile calcium carbonate production, with a much larger market size of USD 44,500 million, is growing at 5.4% annually. Silica, as a byproduct of carbonation, has the largest market, at USD 49,120 million, with the highest growth rate of 9.9%. All carbonated products indicate widespread applications across various industries [70].

Table 13.
Global market size of mineral carbonate products [70].
Energy consumption is a crucial factor in determining the commercial viability and environmental impact of CO2 mineralization technology. For this technology to be industrially applicable, the amount of CO2 captured must significantly exceed the CO2 emissions from energy use [269]. Various factors influence energy consumption, including process type, scale, operating parameters, and carbonation efficiency [146,270]. Due to limited large-scale applications, assessments are typically based on experimental research and simulations. Key energy-consuming processes include raw material grinding, CO2 compression, and material transport. While grinding is energy-intensive, it enhances raw material reactivity [271]. Therefore, optimizing the reaction time and CO2 absorption rate can reduce overall energy consumption. To make CO2 mineralization commercially viable and environmentally beneficial, it is essential to develop efficient, cost-competitive processes with low energy consumption. This may involve adapting to local conditions, improving process design, and utilizing renewable energy sources [82]. Generally, a comprehensive life-cycle assessment (LCA) framework is needed to accurately evaluate the net CO2 reduction potential of different mineralization processes while considering specific energy consumption and electricity generation methods [272,273].
8. Conclusions and Future Prospects
The carbon mineralization of industrial waste offers a promising strategy to reduce the carbon footprint of various industries while supporting circular economy principles. This technology has the potential to play a significant role in decarbonizing cement production and construction practices, with the benefit of producing valuable materials to use in construction. Based on the existing research on the carbon mineralization of industrial solid wastes, the following insights can be derived:
- While carbon mineralization shows great promise, several challenges must be addressed, including maintaining consistent product quality, optimizing large-scale processes, and developing appropriate standards and regulations for integrating carbonated products into construction practices.
- The effectiveness of CO2 mineralization varies depending on the composition of the industrial solid waste, with iron and steel slags showing particularly high potential due to their high alkali content and reactivity.
- Different carbonation methods are suitable for various types of waste. Direct aqueous carbonation and indirect pathways using reagents such as ammonium salts have shown promise for improving reaction kinetics and efficiency.
- The large-scale implementation of CO2 mineralization depends on various factors, including CO2 source characteristics, the availability of alkaline wastes, process scalability, and product market viability.
- Separating carbonated products as a final step of carbonation faces key challenges, including handling diverse product compositions, varying particle sizes, and energy and water demand, which need to be assessed to achieve efficient separation at scale while maintaining product purity.
- Future efforts should focus on large-scale demonstration projects, integration with existing industrial operations, and fundamental research to improve reaction control and efficiency. Moreover, ongoing research to improve process energy consumption is crucial for ensuring net CO2 reductions at industrial scales through life-cycle assessment and techno-economic analysis.
In conclusion, the carbon mineralization of industrial waste presents a promising approach for significantly reducing industrial CO2 emissions while generating valuable byproducts. To fully harness this potential, ongoing research, technological innovations, supportive policies, and international collaboration are essential to develop effective waste-to-resource supply chains. As the technology advances and becomes more economically viable, it could become integral to global initiatives aimed at mitigating climate change and transitioning toward a more sustainable, circular economy.
Author Contributions
Conceptualization, H.H. and J.S.; investigation, H.H.; writing—original draft preparation, H.H.; review and editing, G.G.-C. and J.S.; supervision, G.G.-C. and J.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors are thankful for the support from the National Research Council Canada under the Industrial Carbon Management program.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| BF | Blast furnace. |
| BOF | Basic oxygen furnace. |
| Ca | Calcium. |
| CBD | Cement bypass dust. |
| CCUS | Carbon dioxide capture, utilization, and storage. |
| CDR | Carbon dioxide removal. |
| CKD | Cement kiln dust. |
| CSTRs | Continuous Stirred-Tank Reactors. |
| CSW | Cement slurry waste. |
| DAC | Direct air capture. |
| EAF | Electric arc furnace. |
| EOR | Enhanced oil recovery. |
| GPV | Gravity pressure vessel. |
| IEA | International Energy Agency. |
| LCA | Life-cycle assessment. |
| LF | Ladle furnace. |
| Mg | Magnesium. |
| OPC | Ordinary Portland cement. |
| PCC | Precipitated calcium carbonate. |
| PGMs | Platinum group metals. |
| RCP | Recycled cement paste. |
| S/L | Solid/liquid ratio. |
| SCM | Supplementary cementitious material. |
| TRLs | Technology Readiness Levels. |
References
- Zhang, Z.; Zheng, K.; Chen, L.; Yuan, Q. Review on accelerated carbonation of calcium-bearing minerals: Carbonation behaviors, reaction kinetics, and carbonation efficiency improvement. J. Build. Eng. 2024, 86, 108826. [Google Scholar] [CrossRef]
- Gadikota, G. Carbon mineralization pathways for carbon capture, storage and utilization. Commun. Chem. 2021, 4, 23. [Google Scholar] [CrossRef] [PubMed]
- Neeraj; Yadav, S. Carbon storage by mineral carbonation and industrial applications of CO2. Mater. Sci. Energy Technol. 2020, 3, 494–500. [Google Scholar] [CrossRef]
- Lin, X.; Zhang, Y.; Liu, H.; Boczkaj, G.; Cao, Y.; Wang, C. Carbon dioxide sequestration by industrial wastes through mineral carbonation: Current status and perspectives. J. Clean. Prod. 2024, 434, 140258. [Google Scholar] [CrossRef]
- United Nations Framework Convention on Climate Change, The Paris Agreement; The Paris Agreement|UNFCCC: Paris, France, 2015.
- Lennan, M.; Morgera, E. The Glasgow Climate Conference (COP26). Int. J. Mar. Coast. Law 2022, 37, 137–151. [Google Scholar] [CrossRef]
- Babamohammadi, S.; Shamiri, A.; Aroua, M.K. A review of CO2 capture by absorption in ionic liquid-based solvents. Rev. Chem. Eng. 2015, 31, 383–412. [Google Scholar] [CrossRef]
- Zhou, D.-D.; Zhang, X.-W.; Mo, Z.-W.; Xu, Y.-Z.; Tian, X.-Y.; Li, Y.; Chen, X.-M.; Zhang, J.-P. Adsorptive separation of carbon dioxide: From conventional porous materials to metal–organic frameworks. EnergyChem 2019, 1, 100016. [Google Scholar] [CrossRef]
- Shah, S.; Shah, M.; Shah, A.; Shah, M. Evolution in the membrane-based materials and comprehensive review on carbon capture and storage in industries. Emergent Mater. 2020, 3, 33–44. [Google Scholar] [CrossRef]
- Paul, S.; Bera, S.; Dasgupta, R.; Mondal, S.; Roy, S. Review on the recent structural advances in open and closed systems for carbon capture through algae. Energy Nexus 2021, 4, 100032. [Google Scholar] [CrossRef]
- Lin, X.; Li, X.; Liu, H.; Boczkaj, G.; Cao, Y.; Wang, C. A review on carbon storage via mineral carbonation: Bibliometric analysis, research advances, challenges, and perspectives. Sep. Purif. Technol. 2024, 338, 126558. [Google Scholar] [CrossRef]
- Kramer, G.J.; Haigh, M. No quick switch to low-carbon energy. Nature 2009, 462, 568–569. [Google Scholar] [CrossRef] [PubMed]
- IEA. Net Zero by 2050; IEA: Paris, France, 2021. [Google Scholar]
- Sodiq, A.; Abdullatif, Y.; Aissa, B.; Ostovar, A.; Nassar, N.; El-Naas, M.; Amhamed, A. A review on progress made in direct air capture of CO2. Environ. Technol. Innov. 2023, 29, 102991. [Google Scholar] [CrossRef]
- Ozkan, M.; Nayak, S.P.; Ruiz, A.D.; Jiang, W. Current status and pillars of direct air capture technologies. iScience 2022, 25, 103990. [Google Scholar] [CrossRef]
- ElementEnergy. Global Assessment of Direct Air Capture Costs; IEAGHG Technical Report; Global Assessment of Direct Air Capture Costs–IEAGHG: Cheltenham, UK, 2021; p. 97. [Google Scholar]
- Lebling, K.; Leslie-Bole, H.; Byrum, Z.; Bridgwater, L. 6 Things to Know About Direct Air Capture; World Resources Institute: Washington, DC, USA, 2022. [Google Scholar]
- Gutsch, M.; Leker, J. Co-assessment of costs and environmental impacts for off-grid direct air carbon capture and storage systems. Commun. Eng. 2024, 3, 14. [Google Scholar] [CrossRef]
- Nielsen, A.S.E.; Plantinga, A.J.; Alig, R.J. New Cost Estimates for Carbon Sequestration Through Afforestation in the United States; U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station: Corvallis, OR, USA, 2014. [Google Scholar]
- Lefebvre, D.; Williams, A.G.; Kirk, G.J.D.; Paul; Burgess, J.; Meersmans, J.; Silman, M.R.; Román-Dañobeytia, F.; Farfan, J.; Smith, P. Assessing the carbon capture potential of a reforestation project. Sci. Rep. 2021, 11, 19907. [Google Scholar] [CrossRef] [PubMed]
- Franklin, S.L., Jr.; Pindyck, R.S. A Supply Curve for Forest-Based CO2 Removal; MIT CEEPR, Center for Energy and Environmental Policy Research: Cambridge, MA, USA, 2024. [Google Scholar]
- Torres, A.B.; Marchant, R.; Lovett, J.C.; Smart, J.C.R.; Tipper, R. Analysis of the carbon sequestration costs of afforestation and reforestation agroforestry practices and the use of cost curves to evaluate their potential for implementation of climate change mitigation. Ecol. Econ. 2010, 69, 469–477. [Google Scholar] [CrossRef]
- Lal, R.; Negassa, W.; Lorenz, K. Carbon sequestration in soil. Curr. Opin. Environ. Sustain. 2015, 15, 79–86. [Google Scholar] [CrossRef]
- Don, A.; Seidel, F.; Leifeld, J.; Kätterer, T.; Martin, M.; Pellerin, S.; Emde, D.; Seitz, D.; Chenu, C. Carbon sequestration in soils and climate change mitigation-Definitions and pitfalls. Glob. Change Biol. 2024, 30, e16983. [Google Scholar] [CrossRef] [PubMed]
- Sperow, M. What might it cost to increase soil organic carbon using no-till on U.S. cropland? Carbon Balance Manag. 2020, 15, 26. [Google Scholar] [CrossRef]
- Bach, L.T.; Gill, S.J.; Rickaby, R.E.M.; Gore, S.; Renforth, P. CO2 Removal With Enhanced Weathering and Ocean Alkalinity Enhancement: Potential Risks and Co-benefits for Marine Pelagic Ecosystems. Front. Clim. 2019, 1, 7. [Google Scholar] [CrossRef]
- Strefler, J.; Amann, T.; Bauer, N.; Kriegler, E.; Hartmann, J. Potential and costs of carbon dioxide removal by enhanced weathering of rocks. Environ. Res. Lett. 2018, 13, 034010. [Google Scholar] [CrossRef]
- Breunig, H.M.; Fox, P.; Domen, J.; Kumar, R.; Alves, R.J.E.; Zhalnina, K.; Voigtländer, A.; Deng, H.; Arora, B.; Nico, P. Life cycle impact and cost analysis of quarry materials for land-based enhanced weathering in Northern California. J. Clean. Prod. 2024, 476, 143757. [Google Scholar] [CrossRef]
- Spence, E.; Cox, E.; Pidgeon, N. Exploring cross-national public support for the use of enhanced weathering as a land-based carbon dioxide removal strategy. Clim. Change 2021, 165, 23. [Google Scholar] [CrossRef]
- Rickels, W.; Rehdanz, K.; Oschlies, A. Economic prospects of ocean iron fertilization in an international carbon market. Resour. Energy Econ. 2012, 34, 129–150. [Google Scholar] [CrossRef]
- Lebling, K.; Northrop, E.; McCormick, C. Ocean-Based Carbon Dioxide Removal: 6 Key Questions, Answered; World Resources Institute: Washington, DC, USA, 2022. [Google Scholar]
- Emerson, D.; Sofen, L.E.; Michaud, A.B.; Archer, S.D.; Twining, B.S. A Cost Model for Ocean Iron Fertilization as a Means of Carbon Dioxide Removal That Compares Ship- and Aerial-Based Delivery, and Estimates Verification Costs. Earth’s Future 2024, 12, e2023EF003732. [Google Scholar] [CrossRef]
- Williamson, P.; Wallace, D.W.R.; Law, C.S.; Boyd, P.W.; Collos, Y.; Croot, P.; Denman, K.; Riebesell, U.; Takeda, S.; Vivian, C. Ocean fertilization for geoengineering: A review of effectiveness, environmental impacts and emerging governance. Process Saf. Environ. Prot. 2012, 90, 475–488. [Google Scholar] [CrossRef]
- Dziejarski, B.; Krzyżyńska, R.; Andersson, K. Current status of carbon capture, utilization, and storage technologies in the global economy: A survey of technical assessment. Fuel 2023, 342, 127776. [Google Scholar] [CrossRef]
- Baylin-Stern, A.; Berghout, N. Is Carbon Capture too Expensive? IEA: Paris, France, 2021. [Google Scholar]
- Nath, F.; Mahmood, M.N.; Yousuf, N. Recent advances in CCUS: A critical review on technologies, regulatory aspects and economics. Geoenergy Sci. Eng. 2024, 238, 212726. [Google Scholar] [CrossRef]
- Seifritz, W. CO2 disposal by means of silicates. Nature 1990, 345, 486. [Google Scholar] [CrossRef]
- Rosa, L.; Becattini, V.; Gabrielli, P.; Andreotti, A.; Mazzotti, M. Carbon dioxide mineralization in recycled concrete aggregates can contribute immediately to carbon-neutrality. Resour. Conserv. Recycl. 2022, 184, 106436. [Google Scholar] [CrossRef]
- Sandalow, D.; Aines, R.; Friedmann, J.; Kelemen, P.; McCormick, C.; Power, I.; Schmidt, B.; Wilson, S. Carbon Mineralization Roadmap; ICEF Innovation Roadmap Project: Tokyo, Japan, 2021. [Google Scholar]
- Kelemen, P.B.; McQueen, N.; Wilcox, J.; Renforth, P.; Dipple, G.; Vankeuren, A.P. Engineered carbon mineralization in ultramafic rocks for CO2 removal from air: Review and new insights. Chem. Geol. 2020, 550, 119628. [Google Scholar] [CrossRef]
- Kelemen, P.B.; Aines, R.; Bennett, E.; Benson, S.M.; Carter, E.; Coggon, J.A.; de Obeso, J.C.; Evans, O.; Gadikota, G.; Dipple, G.M.; et al. In situ carbon mineralization in ultramafic rocks: Natural processes and possible engineered methods. Energy Procedia 2018, 146, 92–102. [Google Scholar] [CrossRef]
- Thonemann, N.; Zacharopoulos, L.; Fromme, F.; Nühlen, J. Environmental impacts of carbon capture and utilization by mineral carbonation: A systematic literature review and meta life cycle assessment. J. Clean. Prod. 2022, 332, 130067. [Google Scholar] [CrossRef]
- Zhang, S.; DePaolo, D.J. Rates of CO2 Mineralization in Geological Carbon Storage. Acc. Chem. Res. 2017, 50, 2075–2084. [Google Scholar] [CrossRef]
- Zhang, Y.; Ying, Y.; Xing, L.; Zhan, G.; Deng, Y.; Chen, Z.; Li, J. Carbon dioxide reduction through mineral carbonation by steel slag. J. Environ. Sci. 2025, 152, 664–684. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.-W.; Pan, S.-Y.; Chen, C.-T.; Chen, Y.-H.; Chiang, P.-C. High-gravity carbonation of basic oxygen furnace slag for CO2 fixation and utilization in blended cement. J. Clean. Prod. 2016, 124, 350–360. [Google Scholar] [CrossRef]
- Pan, S.-Y.; Chung, T.-C.; Ho, C.-C.; Hou, C.-J.; Chen, Y.-H.; Chiang, P.-C. CO2 Mineralization and Utilization using Steel Slag for Establishing a Waste-to-Resource Supply Chain. Sci. Rep. 2017, 7, 17227. [Google Scholar] [CrossRef]
- Liu, G.; Schollbach, K.; van der Laan, S.; Tang, P.; Florea, M.V.A.; Brouwers, H.J.H. Recycling and utilization of high volume converter steel slag into CO2 activated mortars—The role of slag particle size. Resour. Conserv. Recycl. 2020, 160, 104883. [Google Scholar] [CrossRef]
- Miras, O.M.; Natalia, O. Pilot-Scale Experimental Work on Sustainable Process Method of Production of Precipitated Calcium Carbonate from Steel Slag and Carbon Dioxide. Master Thesis, Aalto University, Espoo, Finland, 2016. [Google Scholar]
- Teir, S.; Kotiranta, T.; Pakarinen, J.; Mattila, H.-P. Case study for production of calcium carbonate from carbon dioxide in flue gases and steelmaking slag. J. CO2 Util. 2016, 14, 37–46. [Google Scholar] [CrossRef]
- Li, J.; Luo, M.; Wang, K.; Li, G.; Zhang, G. Review of carbon dioxide mineralization of magnesium-containing materials. Carbon Neutralization 2023, 2, 574–584. [Google Scholar] [CrossRef]
- Li, L.; Yu, H.; Zhou, S.; Dao, V.; Chen, M.; Ji, L.; Benhelal, E. Activation and utilization of tailings as CO2 mineralization feedstock and supplementary cementitious materials: A critical review. Mater. Today Sustain. 2023, 24, 100530. [Google Scholar] [CrossRef]
- Al Baroudi, H.; Awoyomi, A.; Patchigolla, K.; Jonnalagadda, K.; Anthony, E.J. A review of large-scale CO2 shipping and marine emissions management for carbon capture, utilisation and storage. Appl. Energy 2021, 287, 116510. [Google Scholar] [CrossRef]
- Kirmani, F.U.D.; Raza, A.; Ahmad, S.; Arif, M.; Mahmoud, M. A holistic overview of the in-situ and ex-situ carbon mineralization: Methods, mechanisms, and technical challenges. Sci. Total Environ. 2024, 943, 173836. [Google Scholar] [CrossRef]
- Azdarpour, A.; Asadullah, M.; Mohammadian, E.; Hamidi, H.; Junin, R.; Karaei, M.A. A review on carbon dioxide mineral carbonation through pH-swing process. Chem. Eng. J. 2015, 279, 615–630. [Google Scholar] [CrossRef]
- Yadav, S.; Mehra, A. A review on ex situ mineral carbonation. Environ. Sci. Pollut. Res. 2021, 28, 12202–12231. [Google Scholar] [CrossRef]
- Zhou, S.; Li, L.; Ji, L.; Dai, B.; Wang, Z.; Benhelal, E.; Bolan, N.S.; Feron, P.; Yu, H. Progress in recyclable chemicals for sustainable ex-situ CO2 mineralisation. Green Energy Resour. 2024, 2, 100087. [Google Scholar] [CrossRef]
- Schinnerl, F.; Sattler, T.; Noori-Khadjavi, G.; Lehner, M. Direct aqueous mineral carbonation of secondary materials for carbon dioxide storage. J. CO2 Util. 2024, 88, 102942. [Google Scholar] [CrossRef]
- Guo, L.; Peng, X.; Wang, Q.; Zhao, Y.; Xu, L.; Wu, S. Research progress on carbon dioxide mineralization sequestration technology by tailings. Green Smart Min. Eng. 2024, 1, 307–321. [Google Scholar] [CrossRef]
- Ostovari, H.; Sternberg, A.; Bardow, A. Rock ‘n’ use of CO2: Carbon footprint of carbon capture and utilization by mineralization. Sustain. Energy Fuels 2020, 4, 4482–4496. [Google Scholar] [CrossRef]
- Yan, Z.; Wang, Y.; Yue, H.; Liu, C.; Zhong, S.; Ma, K.; Liao, W.; Tang, S.; Liang, B. Integrated Process of Monoethanolamine-Based CO2 Absorption and CO2 Mineralization with SFGD Slag: Process Simulation and Life-Cycle Assessment of CO2 Emission. ACS Sustain. Chem. Eng. 2021, 9, 8238–8248. [Google Scholar] [CrossRef]
- Ncongwane, M.S.; Broadhurst, J.L.; Petersen, J. Assessment of the potential carbon footprint of engineered processes for the mineral carbonation of PGM tailings. Int. J. Greenh. Gas Control 2018, 77, 70–81. [Google Scholar] [CrossRef]
- Di Maria, A.; Snellings, R.; Alaerts, L.; Quaghebeur, M.; Van Acker, K. Environmental assessment of CO2 mineralisation for sustainable construction materials. Int. J. Greenh. Gas Control 2020, 93, 102882. [Google Scholar] [CrossRef]
- Zevenhoven, R. Metals Production, CO2 Mineralization and LCA. Metals 2020, 10, 342. [Google Scholar] [CrossRef]
- Huan, Q.; Wibowo, H.; Yan, M.; Song, M. A review of CO2 mineral storage: Current processes, typical applications, and life cycle assessment. J. Environ. Chem. Eng. 2024, 12, 114785. [Google Scholar] [CrossRef]
- Bukar, A.M.; Asif, M. Technology readiness level assessment of carbon capture and storage technologies. Renew. Sustain. Energy Rev. 2024, 200, 114578. [Google Scholar] [CrossRef]
- Matter, J.M.; Broecker, W.S.; Gislason, S.R.; Gunnlaugsson, E.; Oelkers, E.H.; Stute, M.; Sigurdardóttir, H.; Stefansson, A.; Alfreðsson, H.A.; Aradóttir, E.S.; et al. The CarbFix Pilot Project–Storing carbon dioxide in basalt. Energy Procedia 2011, 4, 5579–5585. [Google Scholar] [CrossRef]
- Snæbjörnsdóttir, S.Ó.; Sigfússon, B.; Marieni, C.; Goldberg, D.; Gislason, S.R.; Oelkers, E.H. Carbon dioxide storage through mineral carbonation. Nat. Rev. Earth Environ. 2020, 1, 90–102. [Google Scholar] [CrossRef]
- The National Academies of Sciences·Engineering·Medicine. Negative Emissions Technologies and Reliable Sequestration: A Research Agenda; The National Academies Press: Washington, DC, USA, 2019; p. 510. [Google Scholar]
- Kelemen, P.; Benson, S.M.; Pilorgé, H.; Psarras, P.; Wilcox, J. An Overview of the Status and Challenges of CO2 Storage in Minerals and Geological Formations. Front. Clim. 2019, 1, 9. [Google Scholar] [CrossRef]
- Milani, D.; McDonald, R.; Fawell, P.; Weldekidan, H.; Puxty, G.; Feron, P. Ex-situ mineral carbonation process challenges and technology enablers: A review from Australia’s perspective. Miner. Eng. 2025, 222, 109124. [Google Scholar] [CrossRef]
- Wang, X.; Maroto-Valer, M.M. Integration of CO2 Capture and Mineral Carbonation by Using Recyclable Ammonium Salts. ChemSusChem 2011, 4, 1291–1300. [Google Scholar] [CrossRef]
- Wang, S.; Wang, M.; Liu, F.; Song, Q.; Deng, Y.; Ye, W.; Ni, J.; Si, X.; Wang, C. A Review on the Carbonation of Steel Slag: Properties, Mechanism, and Application. Materials 2024, 17, 2066. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.I.; Yaqoob, Z.; Mujtaba, M.A.; Fayaz, H.; Saleel, C.A. Developments in mineral carbonation for Carbon sequestration. Heliyon 2023, 9, e21796. [Google Scholar] [CrossRef]
- Kumari, P.; Yahmadi, R.; Mumtaz, F.; Vega, L.F.; Ceriani, A.; Tribuzio, R.; Dumée, L.F.; Decarlis, A. CO2 capture via subsurface mineralization geological settings and engineering perspectives towards long-term storage and decarbonization in the Middle East. Carbon Capture Sci. Technol. 2024, 13, 100293. [Google Scholar] [CrossRef]
- Kandji, E.H.B.; Plante, B.; Bussière, B.; Beaudoin, G.; Dupont, P.-P. Kinetic testing to evaluate the mineral carbonation and metal leaching potential of ultramafic tailings: Case study of the Dumont Nickel Project, Amos, Québec. Appl. Geochem. 2017, 84, 262–276. [Google Scholar] [CrossRef]
- Power, I.M.; Paulo, C.; Long, H.; Lockhart, J.A.; Stubbs, A.R.; French, D.; Caldwell, R. Carbonation, Cementation, and Stabilization of Ultramafic Mine Tailings. Environ. Sci. Technol. 2021, 55, 10056–10066. [Google Scholar] [CrossRef]
- Vogeli, J.; Reid, D.L.; Becker, M.; Broadhurst, J.; Franzidis, J.P. Investigation of the potential for mineral carbonation of PGM tailings in South Africa. Miner. Eng. 2011, 24, 1348–1356. [Google Scholar] [CrossRef]
- Ding, W.; Fu, L.; Ouyang, J.; Yang, H. CO2 mineral sequestration by wollastonite carbonation. Phys. Chem. Miner. 2014, 41, 489–496. [Google Scholar] [CrossRef]
- Liu, W.; Teng, L.; Rohani, S.; Qin, Z.; Zhao, B.; Xu, C.C.; Ren, S.; Liu, Q.; Liang, B. CO2 mineral carbonation using industrial solid wastes: A review of recent developments. Chem. Eng. J. 2021, 416, 129093. [Google Scholar] [CrossRef]
- Capelo-Avilés, S.; Tomazini de Oliveira, R.; Gallo Stampino, I.I.; Gispert-Guirado, F.; Casals-Terré, A.; Giancola, S.; Galán-Mascarós, J.R. A thorough assessment of mineral carbonation of steel slag and refractory waste. J. CO2 Util. 2024, 82, 102770. [Google Scholar] [CrossRef]
- Ostovari, H.; Müller, L.; Skocek, J.; Bardow, A. From Unavoidable CO2 Source to CO2 Sink? A Cement Industry Based on CO2 Mineralization. Environ. Sci. Technol. 2021, 55, 5212–5223. [Google Scholar] [CrossRef]
- Pan, S.-Y.; Chen, Y.-H.; Fan, L.-S.; Kim, H.; Gao, X.; Ling, T.-C.; Chiang, P.-C.; Pei, S.-L.; Gu, G. CO2 mineralization and utilization by alkaline solid wastes for potential carbon reduction. Nat. Sustain. 2020, 3, 399–405. [Google Scholar] [CrossRef]
- Chu, G.; Li, C.; Liu, W.; Zhang, G.; Yue, H.; Liang, B.; Wang, Y.; Luo, D. Facile and cost-efficient indirect carbonation of blast furnace slag with multiple high value-added products through a completely wet process. Energy 2019, 166, 1314–1322. [Google Scholar] [CrossRef]
- Crouzet, C.; Brunet, F.; Montes-Hernandez, G.; Recham, N.; Findling, N.; Ferrasse, J.-H.; Goffé, B. Hydrothermal Valorization of Steel Slags—Part I: Coupled H2 Production and CO2 Mineral Sequestration. Front. Energy Res. 2017, 5, 29. [Google Scholar] [CrossRef]
- Hu, G.; Rohani, S.; Jiang, X.; Li, J.; Liu, Q.; Liu, W. CO2 Mineral Sequestration and Faujasite Zeolite Synthesis by Using Blast Furnace Slag: Process Optimization and CO2 Net-Emission Reduction Evaluation. ACS Sustain. Chem. Eng. 2021, 9, 13963–13971. [Google Scholar] [CrossRef]
- Chen, T.-L.; Shu, B.-K.; Chen, Y.-H.; Chiang, P.-C. Simultaneously comparing various CO2-mineralized steelmaking slags as supplementary cementitious materials via high gravity carbonation. J. CO2 Util. 2024, 90, 102985. [Google Scholar] [CrossRef]
- Miao, E.; Du, Y.; Zheng, X.; Zhang, X.; Xiong, Z.; Zhao, Y.; Zhang, J. Kinetic analysis on CO2 sequestration from flue gas through direct aqueous mineral carbonation of circulating fluidized bed combustion fly ash. Fuel 2023, 342, 127851. [Google Scholar] [CrossRef]
- Ding, Z.; Zhang, X.; Cheng, T.; Shen, Y.; Cai, W.-J.; Arthur Chen, C.-T.; Yang, Z.; Liu, T.; Xiao, J.; Xia, M.; et al. Unlocking high carbonation efficiency: Direct CO2 mineralization with fly ash and seawater. Chem. Eng. Sci. 2023, 282, 119349. [Google Scholar] [CrossRef]
- Miao, E.; Du, Y.; Zheng, X.; Zhang, X.; Xiong, Z.; Zhao, Y.; Zhang, J. CO2 sequestration by direct mineral carbonation of municipal solid waste incinerator fly ash in ammonium salt solution: Performance evaluation and reaction kinetics. Sep. Purif. Technol. 2023, 309, 123103. [Google Scholar] [CrossRef]
- Ćwik, A.; Casanova, I.; Rausis, K.; Zarębska, K. Utilization of high-calcium fly ashes through mineral carbonation: The cases for Greece, Poland and Spain. J. CO2 Util. 2019, 32, 155–162. [Google Scholar] [CrossRef]
- Hosseini, T.; Haque, N.; Selomulya, C.; Zhang, L. Mineral carbonation of Victorian brown coal fly ash using regenerative ammonium chloride—Process simulation and techno-economic analysis. Appl. Energy 2016, 175, 54–68. [Google Scholar] [CrossRef]
- Jin, F.; Zhao, M.; Xu, M.; Mo, L. Maximising the benefits of calcium carbonate in sustainable cements: Opportunities and challenges associated with alkaline waste carbonation. npj Mater. Sustain. 2024, 2, 1. [Google Scholar] [CrossRef]
- Medas, D.; Cappai, G.; De Giudici, G.; Piredda, M.; Podda, S. Accelerated carbonation by cement kiln dust in aqueous slurries: Chemical and mineralogical investigation. Greenh. Gases Sci. Technol. 2017, 7, 692–705. [Google Scholar] [CrossRef]
- Ye, J.; Fang, J.; Sun, Y.; Shi, X.; Chen, G.; Ma, T.; Zhi, X. CO2 mineralization of cement-based materials by accelerated CO2 mineralization and its mineralization degree: A review. Constr. Build. Mater. 2024, 444, 137712. [Google Scholar] [CrossRef]
- Andersson, R.; Stripple, H.; Gustafsson, T.; Ljungkrantz, C. Carbonation as a method to improve climate performance for cement based material. Cem. Concr. Res. 2019, 124, 105819. [Google Scholar] [CrossRef]
- Córdoba, P.; Rojas, S. Carbon sequestration through mineral carbonation: Using commercial FGD-gypsum from a copper smelter for sustainable waste management and environmental impact mitigation. J. Environ. Chem. Eng. 2024, 12, 112510. [Google Scholar] [CrossRef]
- Rahmani, O. An experimental study of accelerated mineral carbonation of industrial waste red gypsum for CO2 sequestration. J. CO2 Util. 2020, 35, 265–271. [Google Scholar] [CrossRef]
- Rahmani, O. CO2 sequestration by indirect mineral carbonation of industrial waste red gypsum. J. CO2 Util. 2018, 27, 374–380. [Google Scholar] [CrossRef]
- Wilson, S.; Harrison, A.L.; Dipple, G.M.; Power, I.M.; Barker, S.L.L.; Ulrich Mayer, K.; Fallon, S.J.; Raudsepp, M.; Southam, G. Offsetting of CO2 emissions by air capture in mine tailings at the Mount Keith Nickel Mine, Western Australia: Rates, controls and prospects for carbon neutral mining. Int. J. Greenh. Gas Control 2014, 25, 121–140. [Google Scholar] [CrossRef]
- Krevor, S.C.M.; Lackner, K.S. Enhancing serpentine dissolution kinetics for mineral carbon dioxide sequestration. Int. J. Greenh. Gas Control 2011, 5, 1073–1080. [Google Scholar] [CrossRef]
- Li, H.; Bian, Y.; Liu, M.; Lin, J.; Dai, M.; Xie, H.; Yu, H.; Chen, B.; Xue, M.; Li, Z.; et al. CO2 mineralization and utilization by tailings sand in China for potential carbon sinks and spatial project layout. Resour. Conserv. Recycl. 2024, 206, 107598. [Google Scholar] [CrossRef]
- Hou, S.; Guo, Y.; Sun, J.; Jiang, J.; Gao, H.; Liu, J. Prospect of gold tailings as a new mineral admixture: Effect on hydration, pore structure and mechanical properties of concrete. Mater. Today Sustain. 2025, 29, 101078. [Google Scholar] [CrossRef]
- Pei, T.; Zheng, Y.; Wang, Y.; Zhang, D.; Zhang, P.; Cui, S.; Zheng, Y.; Zhao, S. Utilization of copper tailings in the preparation of low-calcium Portland cement clinker and carbonation-hardening mechanism. Constr. Build. Mater. 2024, 457, 139362. [Google Scholar] [CrossRef]
- Wang, B.; Chen, S.; Feng, X.; Yuan, Z.; Chen, W.; Liu, W.; He, M.; Liu, Q. CO2 sequestration by indirect mineral carbonation of serpentine with (NH4)2SO4 as a recyclable extractant. Process Saf. Environ. Prot. 2024, 191, 2082–2094. [Google Scholar] [CrossRef]
- Moon, S.; Kim, E.; Noh, S.; Triwigati, P.T.; Choi, S.; Park, Y. Carbon mineralization of steel and iron-making slag: Paving the way for a sustainable and carbon-neutral future. J. Environ. Chem. Eng. 2024, 12, 112448. [Google Scholar] [CrossRef]
- Mälkki, M.; Mäkikouri, S. Extraction of magnesium from mine tailings for carbon dioxide mineralization: A preliminary study of the effect of ammonium sulfate to tailings ratio on products and yield. Hydrometallurgy 2024, 225, 106268. [Google Scholar] [CrossRef]
- Ma, Y.; Yi, S.; Wang, M. Biomimetic mineralization for carbon capture and sequestration. Carbon Capture Sci. Technol. 2024, 13, 100257. [Google Scholar] [CrossRef]
- Izumi, Y.; Iizuka, A.; Ho, H.-J. Calculation of greenhouse gas emissions for a carbon recycling system using mineral carbon capture and utilization technology in the cement industry. J. Clean. Prod. 2021, 312, 127618. [Google Scholar] [CrossRef]
- Qin, C.; Jiang, Y.; Zhou, J.; Zuo, S.; Chen, S.; Liu, Z.; Yin, H.; Li, Y. Influence of supercritical CO2 exposure on water wettability of shale: Implications for CO2 sequestration and shale gas recovery. Energy 2022, 242, 122551. [Google Scholar] [CrossRef]
- Romanov, V.; Soong, Y.; Carney, C.; Rush, G.E.; Nielsen, B.; O’Connor, W. Mineralization of Carbon Dioxide: A Literature Review. ChemBioEng Rev. 2015, 2, 231–256. [Google Scholar] [CrossRef]
- Huang, Y.; Zheng, X.; Wei, Y.; He, Q.; Yan, S.; Ji, L. Protonated amines mediated CO2 mineralization of coal fly ash and polymorph selection of CaCO3. Chem. Eng. J. 2022, 450, 138121. [Google Scholar] [CrossRef]
- Wang, S.; Kim, J.; Qin, T. Mineral carbonation of iron and steel by-products: State-of-the-art techniques and economic, environmental, and health implications. J. CO2 Util. 2024, 81, 102707. [Google Scholar] [CrossRef]
- Zhao, Y.; Itakura, K.-i. A State-of-the-Art Review on Technology for Carbon Utilization and Storage. Energies 2023, 16, 3992. [Google Scholar] [CrossRef]
- Rackley, S.A. (Ed.) 10—Mineral carbonation. In Carbon Capture and Storage, 2nd ed.; Butterworth-Heinemann: Boston, MA, USA, 2017; pp. 253–282. [Google Scholar]
- Ambarita, M.; Pusparizkita, Y.M.; Schmahl, W.W.; Reswara, G.; Putra, M.M.; Pratama Lamura, M.D.; Jamari, J.; Bayuseno, A.P. Using selective HCl and H2SO4 acids to extract calcium from extracted asphalt waste residues in preparation for subsequent indirect mineral carbonation. Case Stud. Chem. Environ. Eng. 2024, 10, 100998. [Google Scholar] [CrossRef]
- Walker, I.; Bell, R.; Rippy, K. Mineralization of alkaline waste for CCUS. npj Mater. Sustain. 2024, 2, 28. [Google Scholar] [CrossRef]
- Giannoulakis, S.; Volkart, K.; Bauer, C. Life cycle and cost assessment of mineral carbonation for carbon capture and storage in European power generation. Int. J. Greenh. Gas Control 2014, 21, 140–157. [Google Scholar] [CrossRef]
- Lu, X.; Carroll, K.J.; Turvey, C.C.; Dipple, G.M. Rate and capacity of cation release from ultramafic mine tailings for carbon capture and storage. Appl. Geochem. 2022, 140, 105285. [Google Scholar] [CrossRef]
- Ofori, K.A.; Hanson, W.; Huang, K.; Pan, L. Sulfate-activated mineral carbonation of olivine minerals with mechanisms explained by shrinking core models and by machine learning algorithm. Miner. Eng. 2024, 219, 109058. [Google Scholar] [CrossRef]
- Tang, Y.; Yang, R.; Bian, X. A review of CO2 sequestration projects and application in China. Sci. World J. 2014, 2014, 381854. [Google Scholar] [CrossRef]
- Molahid, V.L.M.; Mohd Kusin, F.; Syed Hasan, S.N.; Ramli, N.A.; Abdullah, A.M. CO2 Sequestration through Mineral Carbonation: Effect of Different Parameters on Carbonation of Fe-Rich Mine Waste Materials. Processes 2022, 10, 432. [Google Scholar] [CrossRef]
- Woodall, C.M.; McQueen, N.; Pilorgé, H.; Wilcox, J. Utilization of mineral carbonation products: Current state and potential. Greenh. Gases Sci. Technol. 2019, 9, 1096–1113. [Google Scholar] [CrossRef]
- Woodall, C.M.; Lu, X.; Dipple, G.; Wilcox, J. Carbon Mineralization with North American PGM Mine Tailings—Characterization and Reactivity Analysis. Minerals 2021, 11, 844. [Google Scholar] [CrossRef]
- Jo, H.; Lee, M.-G.; Park, J.; Jung, K.-D. Preparation of high-purity nano-CaCO3 from steel slag. Energy 2017, 120, 884–894. [Google Scholar] [CrossRef]
- Daval, D.; Hellmann, R.; Martinez, I.; Gangloff, S.; Guyot, F. Lizardite serpentine dissolution kinetics as a function of pH and temperature, including effects of elevated pCO2. Chem. Geol. 2013, 351, 245–256. [Google Scholar] [CrossRef]
- Stokreef, S.; Sadri, F.; Stokreef, A.; Ghahreman, A. Mineral carbonation of ultramafic tailings: A review of reaction mechanisms and kinetics, industry case studies, and modelling. Clean. Eng. Technol. 2022, 8, 100491. [Google Scholar] [CrossRef]
- Biava, G.; Zacco, A.; Zanoletti, A.; Sorrentino, G.P.; Capone, C.; Princigallo, A.; Depero, L.E.; Bontempi, E. Accelerated Direct Carbonation of Steel Slag and Cement Kiln Dust: An Industrial Symbiosis Strategy Applied in the Bergamo–Brescia Area. Materials 2023, 16, 4055. [Google Scholar] [CrossRef] [PubMed]
- Kemache, N.; Pasquier, L.-C.; Cecchi, E.; Mouedhen, I.; Blais, J.-F.; Mercier, G. Aqueous mineral carbonation for CO2 sequestration: From laboratory to pilot scale. Fuel Process. Technol. 2017, 166, 209–216. [Google Scholar] [CrossRef]
- Azdarpour, A.; Afkhami Karaei, M.; Hamidi, H.; Mohammadian, E.; Honarvar, B. CO2 sequestration through direct aqueous mineral carbonation of red gypsum. Petroleum 2018, 4, 398–407. [Google Scholar] [CrossRef]
- Pasquier, L.-C.; Mercier, G.; Blais, J.-F.; Cecchi, E.; Kentish, S. Parameters optimization for direct flue gas CO2 capture and sequestration by aqueous mineral carbonation using activated serpentinite based mining residue. Appl. Geochem. 2014, 50, 66–73. [Google Scholar] [CrossRef]
- Pasquier, L.-C.; Mercier, G.; Blais, J.-F.; Cecchi, E.; Kentish, S. Reaction Mechanism for the Aqueous-Phase Mineral Carbonation of Heat-Activated Serpentine at Low Temperatures and Pressures in Flue Gas Conditions. Environ. Sci. Technol. 2014, 48, 5163–5170. [Google Scholar] [CrossRef]
- Sousa, V.; Nogueira, R.; Meireles, I.; Silva, A. Managing carbon waste in a decarbonized industry: Assessing the potential of concrete mixing storage. Environ. Sci. Pollut. Res. Int. 2024, 31, 17804–17821. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, H.; Liu, M.; Fu, Y.; Si, Z.; Zhang, X.; Zhong, Q. The characterization and mechanism of carbonated steel slag and its products under low CO2 pressure. Mater. Today Commun. 2023, 35, 105827. [Google Scholar] [CrossRef]
- Wang, K.; Zheng, H.; Li, S.; Sun, Y.; Ba, H.; Ma, J. Study on carbonation behavior and carbon footprint of steel slag-calcium carbide slag-desulfurization gypsum composite system. Sci. Rep. 2025, 15, 15199. [Google Scholar] [CrossRef] [PubMed]
- Moon, E.-J.; Choi, Y.C. Development of carbon-capture binder using stainless steel argon oxygen decarburization slag activated by carbonation. J. Clean. Prod. 2018, 180, 642–654. [Google Scholar] [CrossRef]
- Triwigati, P.T.; Noh, S.; Sim, G.; Lee, J.; Kim, E.; Moon, S.; Park, Y. Investigation of pH swing carbon mineralization for valuable element recovery and CO2 sequestration using steelmaking slag. J. Ind. Eng. Chem. 2025, 147, 658–667. [Google Scholar] [CrossRef]
- Ravi, M.; Rathore, S.K.; Sivalingam, M. Performance evaluation of CO2 capture on using potential adsorbents in a CI engine exhaust–An experimental investigation. Greenh. Gases Sci. Technol. 2024, 14, 138–151. [Google Scholar] [CrossRef]
- Zhang, N.; Moment, A. Upcycling Construction and Demolition Waste into Calcium Carbonates: Characterization of Leaching Kinetics and Carbon Mineralization Conditions. ACS Sustain. Chem. Eng. 2023, 11, 866–879. [Google Scholar] [CrossRef]
- Zhang, N.; Huang, H.D.; Yang, R.; Zheng, A.; Moment, A. Aqueous metal ion leaching processes from high sulfur coal fly ash for carbon mineralization: The importance of pH control on cation extraction, carbonate purity, and silicon Q structure. Chem. Eng. J. 2023, 474, 145968. [Google Scholar] [CrossRef]
- Highfield, J.; Lim, H.; Fagerlund, J.; Zevenhoven, R. Mechanochemical processing of serpentine with ammonium salts under ambient conditions for CO2 mineralization. RSC Adv. 2012, 2, 6542–6548. [Google Scholar] [CrossRef]
- Lammers, L.N.; Duan, Y.; Anaya, L.; Koishi, A.; Lopez, R.; Delima, R.; Jassby, D.; Sedlak, D.L. Electrolytic Sulfuric Acid Production with Carbon Mineralization for Permanent Carbon Dioxide Removal. ACS Sustain. Chem. Eng. 2023, 11, 4800–4812. [Google Scholar] [CrossRef]
- Hong, S.; Park, A.-H.A.; Park, Y. Evaluation of elemental leaching behavior and morphological changes of steel slag in both acidic and basic conditions for carbon sequestration potential. Korean J. Chem. Eng. 2021, 38, 2279–2285. [Google Scholar] [CrossRef]
- Saran, R.; Arora, V.; Yadav, S. CO2 Sequestration by Mineral Carbonation: A Review. Glob. NEST J. 2018, 20, 497–503. [Google Scholar]
- Luo, Y.; He, D. Indirect Carbonation by a Two-Step Leaching Process Using Ammonium Chloride and Acetic Acid. JOM 2022, 74, 1958–1968. [Google Scholar] [CrossRef]
- Williams, J.M.; Zhang, N.; Moment, A.J. Assessment of Ammoniacal Leaching Agents for Metal Cation Extraction from Construction Wastes in Mineral Carbonation. ACS Omega 2024, 9, 29776–29788. [Google Scholar] [CrossRef] [PubMed]
- Dri, M.; Sanna, A.; Maroto-Valer, M.M. Dissolution of steel slag and recycled concrete aggregate in ammonium bisulphate for CO2 mineral carbonation. Fuel Process. Technol. 2013, 113, 114–122. [Google Scholar] [CrossRef]
- Wang, X.; Maroto-Valer, M. Integration of CO2 capture and storage based on pH-swing mineral carbonation using recyclable ammonium salts. Energy Procedia 2011, 4, 4930–4936. [Google Scholar] [CrossRef]
- Mun, M.; Cho, H.; Kwon, J.; Kim, K.; Kim, R. Investigation of the CO2 Sequestration by Indirect Aqueous Carbonation of Waste Cement. Am. J. Clim. Change 2017, 6, 132–150. [Google Scholar] [CrossRef]
- Sanna, A.; Dri, M.; Maroto-Valer, M. Carbon dioxide capture and storage by pH swing aqueous mineralisation using a mixture of ammonium salts and antigorite source. Fuel 2013, 114, 153–161. [Google Scholar] [CrossRef]
- Kohitlhetse, I.C.; Manono, M.S.; Motsetse, C.K.; Mendonidis, P.M. A Step towards CO2 Sequestration through Mineral Carbonation: Using Ammonium-Based Lixiviants for the Dissolution of Calcium from Iron-Making Blast Furnace Slag. Minerals 2024, 14, 695. [Google Scholar] [CrossRef]
- He, L.; Yu, D.; Lv, W.; Wu, J.; Xu, M. A Novel Method for CO2 Sequestration via Indirect Carbonation of Coal Fly Ash. Ind. Eng. Chem. Res. 2013, 52, 15138–15145. [Google Scholar] [CrossRef]
- Stasiulaitiene, I.; Vajegaite, V.; Martuzevicius, D.; Denafas, G.; Sliaupa, S.; Fagerlund, J.; Zevenhoven, R. Parameters affecting Mg(OH)2 extraction from serpentinites in lithuania for the purpose of CO2 reduction by mineral carbonation. Environ. Prog. Sustain. Energy 2014, 33, 512–518. [Google Scholar] [CrossRef]
- Arce Ferrufino, G.L.A.; Okamoto, S.; Dos Santos, J.C.; de Carvalho, J.A.; Avila, I.; Romero Luna, C.M.; Gomes Soares Neto, T. CO2 sequestration by pH-swing mineral carbonation based on HCl/NH4OH system using iron-rich lizardite 1T. J. CO2 Util. 2018, 24, 164–173. [Google Scholar] [CrossRef]
- Tong, Z.; Ma, G.; Zhou, D.; Yang, G.; Peng, C. The Indirect Mineral Carbonation of Electric Arc Furnace Slag Under Microwave Irradiation. Sci. Rep. 2019, 9, 7676. [Google Scholar] [CrossRef] [PubMed]
- Heřmanská, M.; Voigt, M.J.; Marieni, C.; Declercq, J.; Oelkers, E.H. A comprehensive and consistent mineral dissolution rate database: Part II: Secondary silicate minerals. Chem. Geol. 2023, 636, 121632. [Google Scholar] [CrossRef]
- DiGiovanni, C.; Hisseine, O.A.; Awolayo, A.N. Carbon dioxide sequestration through steel slag carbonation: Review of mechanisms, process parameters, and cleaner upcycling pathways. J. CO2 Util. 2024, 81, 102736. [Google Scholar] [CrossRef]
- Kim, K.; Kim, D.; Na, Y.; Song, Y.; Wang, J. A review of carbon mineralization mechanism during geological CO2 storage. Heliyon 2023, 9, e23135. [Google Scholar] [CrossRef]
- Ren, E.; Tang, S.; Liu, C.; Yue, H.; Li, C.; Liang, B. Carbon dioxide mineralization for the disposition of blast-furnace slag: Reaction intensification using NaCl solutions. Greenh. Gases Sci. Technol. 2020, 10, 436–448. [Google Scholar] [CrossRef]
- Tiwari, S.K.; Giri, B.S.; Thivaharan, V.; Srivastava, A.K.; Kumar, S.; Singh, R.P.; Kumar, R.; Singh, R.S. Sequestration of simulated carbon dioxide (CO(2)) using churning cementations waste and fly-ash in a thermo-stable batch reactor (TSBR). Environ. Sci. Pollut. Res. 2020, 27, 27470–27479. [Google Scholar] [CrossRef]
- Liu, J.; Chen, H.; Zhang, M. Mg2+ leaching and CO2 sequestration of magnesium tailings enhanced by external field coupled with pretreatment. J. Environ. Chem. Eng. 2024, 12, 113613. [Google Scholar] [CrossRef]
- Nduagu, E.; Romão, I.; Fagerlund, J.; Zevenhoven, R. Performance assessment of producing Mg(OH)2 for CO2 mineral sequestration. Appl. Energy 2013, 106, 116–126. [Google Scholar] [CrossRef]
- Rushendra Revathy, T.D.; Palanivelu, K.; Ramachandran, A. Direct mineral carbonation of steelmaking slag for CO2 sequestration at room temperature. Environ. Sci. Pollut. Res. 2016, 23, 7349–7359. [Google Scholar] [CrossRef]
- Matus, C.F.; Stopić, S.R.; Friedrich, B.G. Carbonation of minerals and slags under high pressure in an autoclave. Vojnoteh. Glas. /Mil. Tech. Cour. 2021, 69, 486–498. [Google Scholar] [CrossRef]
- Gomes, K.V.; Woodall, C.M.; Pilorgé, H.; Psarras, P.; Wilcox, J. Techno-economic analysis of indirect carbonation processes for carbon sequestration using mining waste. Energy Adv. 2025, 4, 435–446. [Google Scholar] [CrossRef]
- Gerdemann, S.; Dahlin, D.; Oconnor, W.; Penner, L.; Rush, G.E. Ex-Situ and In-Situ Mineral Carbonation as a Means to Sequester Carbon Dioxide; U.S. Department of Energy: Washington, DC, USA, 2004. [Google Scholar]
- Puthiya Veetil, S.; Hitch, M. Recent developments and challenges of aqueous mineral carbonation: A review. Int. J. Environ. Sci. Technol. 2020, 17, 4359–4380. [Google Scholar] [CrossRef]
- Zhang, D.; Ghouleh, Z.; Shao, Y. Review on carbonation curing of cement-based materials. J. CO2 Util. 2017, 21, 119–131. [Google Scholar] [CrossRef]
- Ho, H.-J.; Iizuka, A.; Shibata, E. Chemical recycling and use of various types of concrete waste: A review. J. Clean. Prod. 2021, 284, 124785. [Google Scholar] [CrossRef]
- Bhardwaj, R.; van Ommen, J.R.; Nugteren, H.W.; Geerlings, H. Accelerating Natural CO2 Mineralization in a Fluidized Bed. Ind. Eng. Chem. Res. 2016, 55, 2946–2951. [Google Scholar] [CrossRef]
- Chang, E.E.; Chen, C.H.; Chen, Y.H.; Pan, S.Y.; Chiang, P.C. Performance evaluation for carbonation of steel-making slags in a slurry reactor. J.Hazard. Mater. 2011, 186, 558–564. [Google Scholar] [CrossRef]
- Chang, E.E.; Pan, S.-Y.; Chen, Y.-H.; Tan, C.-S.; Chiang, P.-C. Accelerated carbonation of steelmaking slags in a high-gravity rotating packed bed. J. Hazard. Mater. 2012, 227–228, 97–106. [Google Scholar] [CrossRef]
- Santos, R.M.; Knops, P.C.M.; Rijnsburger, K.L.; Chiang, Y.W. CO2 Energy Reactor—Integrated Mineral Carbonation: Perspectives on Lab-Scale Investigation and Products Valorization. Front. Energy Res. 2016, 4, 5. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, C.; Xuan, T.; Zhang, X.; Wang, L.; Tian, Y.; Zhu, J. Simulation design and optimization of reactors for carbon dioxide mineralization. Energy Storage Sav. 2024, 3, 209–217. [Google Scholar] [CrossRef]
- Santos, R.M.; Verbeeck, W.; Knops, P.; Rijnsburger, K.; Pontikes, Y.; Van Gerven, T. Integrated Mineral Carbonation Reactor Technology for Sustainable Carbon Dioxide Sequestration: ‘CO2 Energy Reactor’. Energy Procedia 2013, 37, 5884–5891. [Google Scholar] [CrossRef]
- Biava, G.; Depero, L.E.; Bontempi, E. Accelerated Carbonation of Steel Slag and Their Valorisation in Cement Products: A Review. Span. J. Soil Sci. 2024, 14, 12908. [Google Scholar] [CrossRef]
- Cao, R.; Miller, Q.R.S.; Davidson, C.L.; Gallin, W.; Reidel, S.P.; Jiao, Z.; McLaughlin, J.F.; Nienhuis, E.T.; Schaef, H.T. Gigaton commercial-scale carbon storage and mineralization potential in stacked Columbia River basalt reservoirs. Int. J. Greenh. Gas Control 2024, 137, 104206. [Google Scholar] [CrossRef]
- Li, G.; Yang, J.; Li, H.; Liew, J.; Huang, J. Enhancing sustainable utilization of iron-based alkaline solid wastes for carbon mineralization: Insights into CO2 transport and adsorption dynamics. Dev. Built Environ. 2025, 21, 100595. [Google Scholar] [CrossRef]
- Winnefeld, F.; Leemann, A.; German, A.; Lothenbach, B. CO2 storage in cement and concrete by mineral carbonation. Curr. Opin. Green Sustain. Chem. 2022, 38, 100672. [Google Scholar] [CrossRef]
- Said, A.; Laukkanen, T.; Järvinen, M. Pilot-scale experimental work on carbon dioxide sequestration using steelmaking slag. Appl. Energy 2016, 177, 602–611. [Google Scholar] [CrossRef]
- Leventaki, E.; Couto Queiroz, E.; Krishnan Pisharody, S.; Kumar Siva Kumar, A.; Hoang Ho, P.; Andersson-Sarning, M.; Haase, B.; Baena-Moreno, F.M.; Cuin, A.; Bernin, D. Aqueous mineral carbonation of three different industrial steel slags: Absorption capacities and product characterization. Environ. Res. 2024, 252, 118903. [Google Scholar] [CrossRef] [PubMed]
- Baras, A.; Li, J.; Ni, W.; Hussain, Z.; Hitch, M. Evaluation of Potential Factors Affecting Steel Slag Carbonation. Processes 2023, 11, 2590. [Google Scholar] [CrossRef]
- Myers, C.A.; Nakagaki, T.; Akutsu, K. Quantification of the CO2 mineralization potential of ironmaking and steelmaking slags under direct gas-solid reactions in flue gas. Int. J. Greenh. Gas Control 2019, 87, 100–111. [Google Scholar] [CrossRef]
- Ukwattage, N.L.; Ranjith, P.G.; Li, X. Steel-making slag for mineral sequestration of carbon dioxide by accelerated carbonation. Measurement 2017, 97, 15–22. [Google Scholar] [CrossRef]
- Elyasi Gomari, K.; Rezaei Gomari, S.; Hughes, D.; Ahmed, T. Exploring the potential of steel slag waste for carbon sequestration through mineral carbonation: A comparative study of blast-furnace slag and ladle slag. J. Environ. Manag. 2024, 351, 119835. [Google Scholar] [CrossRef] [PubMed]
- Palankar, N.; Ravi Shankar, A.U.; Mithun, B.M. Durability studies on eco-friendly concrete mixes incorporating steel slag as coarse aggregates. J. Clean. Prod. 2016, 129, 437–448. [Google Scholar] [CrossRef]
- Pang, B.; Zhou, Z.; Hou, P.; Du, P.; Zhang, L.; Xu, H. Autogenous and engineered healing mechanisms of carbonated steel slag aggregate in concrete. Constr. Build. Mater. 2016, 107, 191–202. [Google Scholar] [CrossRef]
- Wang, Q.; Yan, P.; Yang, J.; Zhang, B. Influence of steel slag on mechanical properties and durability of concrete. Constr. Build. Mater. 2013, 47, 1414–1420. [Google Scholar] [CrossRef]
- Menad, N.E.; Kana, N.; Seron, A.; Kanari, N. New EAF Slag Characterization Methodology for Strategic Metal Recovery. Materials 2021, 14, 1513. [Google Scholar] [CrossRef]
- Kriskova, L.; Pontikes, Y.; Cizer, Ö.; Mertens, G.; Veulemans, W.; Geysen, D.; Jones, P.T.; Vandewalle, L.; Van Balen, K.; Blanpain, B. Effect of mechanical activation on the hydraulic properties of stainless steel slags. Cem. Concr. Res. 2012, 42, 778–788. [Google Scholar] [CrossRef]
- Yu, J.; Wang, K. Study on Characteristics of Steel Slag for CO2 Capture. Energy Fuels 2011, 25, 5483–5492. [Google Scholar] [CrossRef]
- You, K.-s.; Lee, S.-H.; Hwang, S.-H.; Kim, H.-s.; Ahn, J.-W. CO2 Sequestration via a Surface-Modified Ground Granulated Blast Furnace Slag Using NaOH Solution. Mater. Trans. 2011, 52, 1972–1976. [Google Scholar] [CrossRef]
- Ghacham, A.B.; Pasquier, L.-C.; Cecchi, E.; Blais, J.-F.; Mercier, G. CO2 sequestration by mineral carbonation of steel slags under ambient temperature: Parameters influence, and optimization. Environ. Sci. Pollut. Res. 2016, 23, 17635–17646. [Google Scholar] [CrossRef]
- Baciocchi, R.; Costa, G.; Di Gianfilippo, M.; Polettini, A.; Pomi, R.; Stramazzo, A. Thin-film versus slurry-phase carbonation of steel slag: CO2 uptake and effects on mineralogy. J. Hazard. Mater. 2015, 283, 302–313. [Google Scholar] [CrossRef]
- Omale, S.O.; Choong, T.S.Y.; Abdullah, L.C.; Siajam, S.I.; Yip, M.W. Utilization of Malaysia EAF slags for effective application in direct aqueous sequestration of carbon dioxide under ambient temperature. Heliyon 2019, 5, e02602. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Mehra, A. Experimental study of dissolution of minerals and CO2 sequestration in steel slag. Waste Manag. 2017, 64, 348–357. [Google Scholar] [CrossRef]
- Su, T.-H.; Yang, H.-J.; Shau, Y.-H.; Takazawa, E.; Lee, Y.-C. CO2 sequestration utilizing basic-oxygen furnace slag: Controlling factors, reaction mechanisms and V–Cr concerns. J. Environ. Sci. 2016, 41, 99–111. [Google Scholar] [CrossRef]
- Pan, S.-Y.; Chiang, P.-C.; Chen, Y.-H.; Tan, C.-S.; Chang, E.E. Ex Situ CO2 Capture by Carbonation of Steelmaking Slag Coupled with Metalworking Wastewater in a Rotating Packed Bed. Environ. Sci. Technol. 2013, 47, 3308–3315. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.-Y.; Chiang, P.-C.; Chen, Y.-H.; Chen, C.-D.; Lin, H.-Y.; Chang, E.E. Systematic Approach to Determination of Maximum Achievable Capture Capacity via Leaching and Carbonation Processes for Alkaline Steelmaking Wastes in a Rotating Packed Bed. Environ. Sci. Technol. 2013, 47, 13677–13685. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.E.; Chiu, A.-C.; Pan, S.-Y.; Chen, Y.-H.; Tan, C.-S.; Chiang, P.-C. Carbonation of basic oxygen furnace slag with metalworking wastewater in a slurry reactor. Int. J. Greenh. Gas Control 2013, 12, 382–389. [Google Scholar] [CrossRef]
- Li, H.; Tang, Z.; Li, N.; Cui, L.; Mao, X.-z. Mechanism and process study on steel slag enhancement for CO2 capture by seawater. Appl. Energy 2020, 276, 115515. [Google Scholar] [CrossRef]
- Zhang, S.; Ghouleh, Z.; Mucci, A.; Bahn, O.; Provençal, R.; Shao, Y. Production of cleaner high-strength cementing material using steel slag under elevated-temperature carbonation. J. Clean. Prod. 2022, 342, 130948. [Google Scholar] [CrossRef]
- Tian, S.; Jiang, J.; Chen, X.; Yan, F.; Li, K. Direct Gas–Solid Carbonation Kinetics of Steel Slag and the Contribution to In situ Sequestration of Flue Gas CO2 in Steel-Making Plants. ChemSusChem 2013, 6, 2348–2355. [Google Scholar] [CrossRef]
- Bonenfant, D.; Kharoune, L.; Sauve’, S.; Hausler, R.; Niquette, P.; Mimeault, M.; Kharoune, M. CO2 Sequestration Potential of Steel Slags at Ambient Pressure and Temperature. Ind. Eng. Chem. Res. 2008, 47, 7610–7616. [Google Scholar] [CrossRef]
- Lee, S.; Kim, J.-W.; Chae, S.; Bang, J.-H.; Lee, S.-W. CO2 sequestration technology through mineral carbonation: An extraction and carbonation of blast slag. J. CO2 Util. 2016, 16, 336–345. [Google Scholar] [CrossRef]
- Mun, M.; Cho, H. Mineral Carbonation for Carbon Sequestration with Industrial Waste. Energy Procedia 2013, 37, 6999–7005. [Google Scholar] [CrossRef]
- Bang, J.-H.; Lee, S.-W.; Jeon, C.; Park, S.; Song, K.; Jo, W.J.; Chae, S. Leaching of Metal Ions from Blast Furnace Slag by Using Aqua Regia for CO2 Mineralization. Energies 2016, 9, 996. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, W.; Hu, J.; Wang, L.; Gao, J.; Liang, B.; Yue, H.; Zhang, G.; Luo, D.; Li, C. Energy-efficient mineral carbonation of blast furnace slag with high value-added products. J. Clean. Prod. 2018, 197, 242–252. [Google Scholar] [CrossRef]
- Kodama, S.; Nishimoto, T.; Yamamoto, N.; Yogo, K.; Yamada, K. Development of a new pH-swing CO2 mineralization process with a recyclable reaction solution. Energy 2008, 33, 776–784. [Google Scholar] [CrossRef]
- Eloneva, S.; Said, A.; Fogelholm, C.-J.; Zevenhoven, R. Preliminary assessment of a method utilizing carbon dioxide and steelmaking slags to produce precipitated calcium carbonate. Appl. Energy 2012, 90, 329–334. [Google Scholar] [CrossRef]
- Owais, M.; Järvinen, M.; Taskinen, P.; Said, A. Experimental study on the extraction of calcium, magnesium, vanadium and silicon from steelmaking slags for improved mineral carbonation of CO2. J. CO2 Util. 2019, 31, 1–7. [Google Scholar] [CrossRef]
- Sun, Y.; Yao, M.-S.; Zhang, J.-P.; Yang, G. Indirect CO2 mineral sequestration by steelmaking slag with NH4Cl as leaching solution. Chem. Eng. J. 2011, 173, 437–445. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, C.; Huang, P.; Zhang, Y.; Sun, J. Modeling and response surface methodology optimization of reaction parameters for aqueous mineral carbonation by steel slag. Carbon Capture Sci. Technol. 2024, 12, 100229. [Google Scholar] [CrossRef]
- Lee, Y.H.; Eom, H.; Lee, S.M.; Kim, S.S. Effects of pH and metal composition on selective extraction of calcium from steel slag for Ca(OH)2 production. RSC Adv. 2021, 11, 8306–8313. [Google Scholar] [CrossRef]
- Ragipani, R.; Bhattacharya, S.; Akkihebbal, S.K. Understanding dissolution characteristics of steel slag for resource recovery. Waste Manag. 2020, 117, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Eloneva, S.; Teir, S.; Revitzer, H.; Salminen, J.; Said, A.; Fogelholm, C.-J.; Zevenhoven, R. Reduction of CO2 Emissions from Steel Plants by Using Steelmaking Slags for Production of Marketable Calcium Carbonate. Steel Res. Int. 2009, 80, 415–421. [Google Scholar] [CrossRef]
- Ho, H.-J.; Izumi, Y.; Iizuka, A. A CO2 removal technology based on mineral carbonation and the stability of product carbon storage in a cement matrix. Environ. Technol. Innov. 2024, 34, 103623. [Google Scholar] [CrossRef]
- Wu, S.; Shao, Z.; Andrew, R.M.; Bing, L.; Wang, J.; Niu, L.; Liu, Z.; Xi, F. Global CO2 uptake by cement materials accounts 1930–2023. Sci. Data 2024, 11, 1409. [Google Scholar] [CrossRef]
- Strunge, T.; Renforth, P.; Van der Spek, M. Towards a business case for CO2 mineralisation in the cement industry. Commun. Earth Environ. 2022, 3, 59. [Google Scholar] [CrossRef]
- Pedraza, J.; Zimmermann, A.; Tobon, J.; Schomäcker, R.; Rojas, N. On the road to net zero-emission cement: Integrated assessment of mineral carbonation of cement kiln dust. Chem. Eng. J. 2021, 408, 127346. [Google Scholar] [CrossRef]
- Skocek, J.; Zajac, M.; Ben Haha, M. Carbon Capture and Utilization by mineralization of cement pastes derived from recycled concrete. Sci. Rep. 2020, 10, 5614. [Google Scholar] [CrossRef]
- Al-Bakri, A.Y.; Ahmed, H.M.; Hefni, M.A. Cement Kiln Dust (CKD): Potential Beneficial Applications and Eco-Sustainable Solutions. Sustainability 2022, 14, 7022. [Google Scholar] [CrossRef]
- Lee, Y.H.; Yang, H.; Lee, S.M.; Kim, S.S. Surface Properties of Cement Kiln Dust with Water Treatment for Selective Extraction of Calcium and Potassium. ACS Omega 2020, 5, 24351–24355. [Google Scholar] [CrossRef]
- Huntzinger, D.N.; Gierke, J.S.; Sutter, L.L.; Kawatra, S.K.; Eisele, T.C. Mineral carbonation for carbon sequestration in cement kiln dust from waste piles. J. Hazard. Mater. 2009, 168, 31–37. [Google Scholar] [CrossRef]
- Huntzinger, D.N.; Eatmon, T.D. A life-cycle assessment of Portland cement manufacturing: Comparing the traditional process with alternative technologies. J. Clean. Prod. 2009, 17, 668–675. [Google Scholar] [CrossRef]
- Gunning, P.J.; Hills, C.D.; Carey, P.J. Accelerated carbonation treatment of industrial wastes. Waste Manag. 2010, 30, 1081–1090. [Google Scholar] [CrossRef]
- Irfan, M.F.; Hossain, S.M.Z.; Tariq, I.; Khan, N.A.; Tawfeeqi, A.; Goeva, A.; Wael, M. Modeling and Optimization of Aqueous Mineral Carbonation for Cement Kiln Dust Using Response Surface Methodology Integrated with Box-Behnken and Central Composite Design Approaches. Min. Metall. Explor. 2020, 37, 1367–1383. [Google Scholar] [CrossRef]
- Kim, M.-J.; Pak, S.Y.; Kim, D.; Jung, S. Optimum conditions for extracting Ca from CKD to store CO2 through indirect mineral carbonation. KSCE J. Civ. Eng. 2017, 21, 629–635. [Google Scholar] [CrossRef]
- Xuan, D.; Zhan, B.; Poon, C.S.; Zheng, H.; Ka, K.; Kwok, W. Carbon Dioxide Sequestration of Cement Slurry Waste and Valorisation of FRCAs in Eco-Construction Products by Carbonation. In Proceedings of the 14th International Congress on the Chemistry of Cement (ICCC 2015), Beijing, China, 13–16 October 2015. [Google Scholar]
- Zajac, M.; Skocek, J.; Skibsted, J.; Ben Haha, M. CO2 mineralization of demolished concrete wastes into a supplementary cementitious material—A new CCU approach for the cement industry. RILEM Tech. Lett. 2021, 6, 53–60. [Google Scholar] [CrossRef]
- Zajac, M.; Skocek, J.; Ben Haha, M.; Deja, J. CO2 Mineralization Methods in Cement and Concrete Industry. Energies 2022, 15, 3597. [Google Scholar] [CrossRef]
- Driver, J.G.; Bernard, E.; Patrizio, P.; Fennell, P.S.; Scrivener, K.; Myers, R.J. Global decarbonization potential of CO2 mineralization in concrete materials. Proc. Natl. Acad. Sci. USA 2024, 121, e2313475121. [Google Scholar] [CrossRef] [PubMed]
- Snokman, S. CarbonCure Ready Mix Technology Case Studies; CarbonCure Technologies: Dartmouth, NS, Canada, December 2016. [Google Scholar]
- Teramura, S.; Isu, N.; Inagaki, K. New Building Material from Waste Concrete by Carbonation. J. Mater. Civ. Eng. 2000, 12, 288–293. [Google Scholar] [CrossRef]
- Iizuka, A.; Fujii, M.; Yamasaki, A.; Yanagisawa, Y. Development of a New CO2 Sequestration Process Utilizing the Carbonation of Waste Cement. Ind. Eng. Chem. Res. 2004, 43, 7880–7887. [Google Scholar] [CrossRef]
- Johnson, D.C. Accelerated carbonation of waste calcium silicate materials. SCI Lect. Pap. Ser. 2000, 108, 1–10. [Google Scholar]
- Short, N.R.; Purnell, P.; Page, C.L. Preliminary investigations into the supercritical carbonation of cement pastes. J. Mater. Sci. 2001, 36, 35–41. [Google Scholar] [CrossRef]
- Huntzinger, D.N.; Gierke, J.S.; Kawatra, S.K.; Eisele, T.C.; Sutter, L.L. Carbon Dioxide Sequestration in Cement Kiln Dust through Mineral Carbonation. Environ. Sci. Technol. 2009, 43, 1986–1992. [Google Scholar] [CrossRef] [PubMed]
- Mawire, G.; McDonald, R.; Austin, P.; Mukherjee, A.; Esteban, L.; Dhami, N.K. Recycling of mine tailings as supplementary cementitious material: Impact of mine tailings’ mineralogy on hydration behaviour and phase assemblage of Ordinary Portland cement blends. Clean. Mater. 2025, 15, 100288. [Google Scholar] [CrossRef]
- Entezari Zarandi, A.; Larachi, F.; Beaudoin, G.; Plante, B.; Sciortino, M. Ambient mineral carbonation of different lithologies of mafic to ultramafic mining wastes/tailings—A comparative study. Int. J. Greenh. Gas Control 2017, 63, 392–400. [Google Scholar] [CrossRef]
- Vieira, K.R.M.; Arce, G.L.A.F.; Luna, C.M.R.; Facio, V.O.; Carvalho, J.A.; Neto, T.G.S.; Ávila, I. Understanding the acid dissolution of Serpentinites (Tailings and waste rock) for use in indirect mineral carbonation. S. Afr. J. Chem. Eng. 2022, 40, 154–164. [Google Scholar] [CrossRef]
- Meyer, N.A.; Vögeli, J.U.; Becker, M.; Broadhurst, J.L.; Reid, D.L.; Franzidis, J.P. Mineral carbonation of PGM mine tailings for CO2 storage in South Africa: A case study. Miner. Eng. 2014, 59, 45–51. [Google Scholar] [CrossRef]
- Liu, S.; Gu, H.; Yang, K.; Guo, J.; Wu, K.; Guo, L.; Yang, Z.; Xu, L. Preparation activated tailings by pH swing process: Towards yielding cemented tailings backfill and in-situ CO2 mineralization. Cem. Concr. Compos. 2024, 154, 105767. [Google Scholar] [CrossRef]
- Wang, F.; Dreisinger, D.; Jarvis, M.; Hitchins, T. Kinetic evaluation of mineral carbonation of natural silicate samples. Chem. Eng. J. 2021, 404, 126522. [Google Scholar] [CrossRef]
- USGS. Mineral Commodity Summaries 2024; U.S. Geological Survey: Reston, VA, USA, 2024; p. 212. [Google Scholar]
- Zhang, X.; Chen, J.; Jiang, J.; Li, J.; Tyagi, R.D.; Surampalli, R.Y. The potential utilization of slag generated from iron- and steelmaking industries: A review. Environ. Geochem. Health 2020, 42, 1321–1334. [Google Scholar] [CrossRef]
- Ma, M.; Tam, V.W.; Le, K.N.; Butera, A.; Li, W.; Wang, X. Comparative analysis on international construction and demolition waste management policies and laws for policy makers in China. J. Civ. Eng. Manag. 2023, 29, 107–130. [Google Scholar] [CrossRef]
- CEMBUREAU. Cement, Concrete & the Circular Economy; The European Cement Association: Brussels, Belgium, 2016. [Google Scholar]
- Sarker, S.K.; Haque, N.; Bhuiyan, M.; Bruckard, W.; Pramanik, B.K. Recovery of strategically important critical minerals from mine tailings. J. Environ. Chem. Eng. 2022, 10, 107622. [Google Scholar] [CrossRef]
- Bentham, J. Clean TeQ Accelerates Expansion into Global Mine Tailings Management; Global Mining Review: Farnham, UK, 2023. [Google Scholar]
- Wilson, S.; Dipple, G.M.; Power, I.M.; Thom, J.M.; Anderson, R.G.; Raudsepp, M.; Gabites, J.E.; Southam, G. Carbon Dioxide Fixation within Mine Wastes of Ultramafic-Hosted Ore Deposits: Examples from the Clinton Creek and Cassiar Chrysotile Deposits, Canada. Econ. Geol. 2009, 104, 95–112. [Google Scholar] [CrossRef]
- Mining Association of Canada. Towards Sustainable Mining; The Mining Association of Canada: Ottawa, ON, Canada, 2007. [Google Scholar]
- Fikru, M.G.; Koppera, S. Public perceptions of mineral criticality and preferences for energy transition strategies in the United States. Commun. Earth Environ. 2024, 5, 768. [Google Scholar] [CrossRef]
- Chowdhury, M.O.; Talan, D. From Waste to Wealth: A Circular Economy Approach to the Sustainable Recovery of Rare Earth Elements and Battery Metals from Mine Tailings. Separations 2025, 12, 52. [Google Scholar] [CrossRef]
- Abbadi, A.; Mucsi, G. A review on complex utilization of mine tailings: Recovery of rare earth elements and residue valorization. J. Environ. Chem. Eng. 2024, 12, 113118. [Google Scholar] [CrossRef]
- Berthet, E.; Lavalley, J.; Anquetil-Deck, C.; Ballesteros, F.; Stadler, K.; Soytas, U.; Hauschild, M.; Laurent, A. Assessing the social and environmental impacts of critical mineral supply chains for the energy transition in Europe. Glob. Environ. Change 2024, 86, 102841. [Google Scholar] [CrossRef]
- Mattila, H.-P.; Zevenhoven, R. Design of a Continuous Process Setup for Precipitated Calcium Carbonate Production from Steel Converter Slag. ChemSusChem 2014, 7, 903–913. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, C.; Mei, X.; Saxén, H.; Zevenhoven, R. Research progress of steel slag-based carbon sequestration. Fundam. Res. 2025, 5, 282–287. [Google Scholar] [CrossRef]
- Zhao, Q.; Chu, X.; Mei, X.; Meng, Q.; Li, J.; Liu, C.; Saxén, H.; Zevenhoven, R. Co-treatment of Waste From Steelmaking Processes: Steel Slag-Based Carbon Capture and Storage by Mineralization. Front. Chem. 2020, 8, 571504. [Google Scholar] [CrossRef]
- Capso. Cement Mineralization. Available online: https://www.capso.com/methodologies/cement-mineralization/ (accessed on 12 March 2025).
- Stutt, A. Arca Climate Technologies Launches Pilot Project with BHP to Capture CO2 from Mine Waste; MINING.COM: Toronto, ON, Canada, 2023. [Google Scholar]
- ARCA. Industrial-Scale Carbon Removal. Available online: https://arcaclimate.com/what-we-do/ (accessed on 12 March 2025).
- CICE. The Arca Carbon Sandbox Project; The B.C. Centre for Innovation and Clean Energy: Vancouver, BC, Canada, 2024. [Google Scholar]
- Wickramasinghe, S. UBC-Led Project Combats Emissions by Locking Carbon Dioxide in Mine Waste; The University of British Columbia: Kelowna, BC, Canada, 2019. [Google Scholar]
- Eve, P. Carbon Dioxide Utilization 2025–2045: Technologies, Market Forecasts, and Players. IDTechEx. Available online: https://www.idtechex.com/en/research-report/carbon-dioxide-utilization/1028 (accessed on 12 March 2025).
- Yuen, Y.T.; Sharratt, P.N.; Jie, B. Carbon dioxide mineralization process design and evaluation: Concepts, case studies, and considerations. Environ. Sci. Pollut. Res. 2016, 23, 22309–22330. [Google Scholar] [CrossRef]
- Verified Market Reports. CO2 Mineralization Technology Market; 717846. Available online: https://www.verifiedmarketreports.com/product/co2-mineralization-technology-market/ (accessed on 12 March 2025).
- Alturki, A. The Global Carbon Footprint and How New Carbon Mineralization Technologies Can Be Used to Reduce CO2 Emissions. ChemEngineering 2022, 6, 44. [Google Scholar] [CrossRef]
- Sanna, A.; Uibu, M.; Caramanna, G.; Kuusik, R.; Maroto-Valer, M.M. A review of mineral carbonation technologies to sequester CO2. Chem. Soc. Rev. 2014, 43, 8049–8080. [Google Scholar] [CrossRef] [PubMed]
- Naraharisetti, P.K.; Yeo, T.Y.; Bu, J. New classification of CO2 mineralization processes and economic evaluation. Renew. Sustain. Energy Rev. 2019, 99, 220–233. [Google Scholar] [CrossRef]
- Khoo, H.H.; Sharratt, P.N.; Bu, J.; Yeo, T.Y.; Borgna, A.; Highfield, J.G.; Björklöf, T.G.; Zevenhoven, R. Carbon Capture and Mineralization in Singapore: Preliminary Environmental Impacts and Costs via LCA. Ind. Eng. Chem. Res. 2011, 50, 11350–11357. [Google Scholar] [CrossRef]
- Wang, C.; Jiang, H.; Miao, E.; Wang, Y.; Zhang, T.; Xiao, Y.; Liu, Z.; Ma, J.; Xiong, Z.; Zhao, Y.; et al. Accelerated CO2 mineralization technology using fly ash as raw material: Recent research advances. Chem. Eng. J. 2024, 488, 150676. [Google Scholar] [CrossRef]
- Lee, J.; Ryu, K.H.; Ha, H.Y.; Jung, K.-D.; Lee, J.H. Techno-economic and environmental evaluation of nano calcium carbonate production utilizing the steel slag. J. CO2 Util. 2020, 37, 113–121. [Google Scholar] [CrossRef]
- Tiefenthaler, J.; Braune, L.; Bauer, C.; Sacchi, R.; Mazzotti, M. Technological Demonstration and Life Cycle Assessment of a Negative Emission Value Chain in the Swiss Concrete Sector. Front. Clim. 2021, 3, 729259. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).