Abstract
Serpentinite soils represent extreme environments characterized by deficiencies in essential nutrients (Ca, K, P, N), an unfavorable Ca/Mg ratio, low water retention, and elevated concentrations of several geogenic potentially toxic elements (PTEs). In particular, the study site, located in Sassello (Liguria, Italy) within the serpentinites of the High-Pressure–Low-Temperature (HP–LT) metaophiolites of the Voltri Massif, exhibited concentrations of chromium, nickel and cobalt exceeding Italian legal thresholds by up to one order of magnitude. This study aimed to assess fungal diversity and to isolate culturable strains naturally adapted to these challenging conditions for potential use in bioremediation. Culturable-dependent analyses allowed for the isolation of viable fungal strains, with Penicillium (52%), Umbelopsis (17.9%), and Aspergillus (11.6%) found as dominant genera. Additionally, metabarcoding analyses provided a broader view of fungal community composition, revealing the presence and distribution of both culturable and non-culturable taxa. The combined approach highlighted the richness of the serpentinite soil mycobiota and its role as a reservoir of PTE-resistant organisms. These findings offer new insights into the ecology of metal-rich soils and identify promising candidates for sustainable remediation strategies in PTE-contaminated environments.
1. Introduction
Extreme environments, such as serpentinite soils, characterized by high concentrations of potentially toxic elements (PTEs) represent distinct ecological niches defined by specific abiotic stressors [1,2]. The elevated presence of these elements is a key factor that creates these harsh habitats [3,4,5]. Serpentinitic soils are a notable example of this environment type, exhibiting both chemical toxicity and nutrient imbalance [6]. These soils originate from the weathering of ultramafic bedrock (e.g., serpentinites, serpentineschists, peridotites), which is naturally characterized by the high level of various potentially toxic elements (PTEs) such as cobalt, chromium, and nickel [7,8]. Additionally, they exhibit low availability of essential nutrients (i.e., N, P, K, Ca) and a high Mg/Ca ratio [9,10,11]. The Voltri Massif (VM), located in the southern area of the Western Alps (Ligurian Alps, NW Italy), represents the largest ophiolite complex in the Italian Alps–Apennine system [12]. Covering approximately 800 km2, the VM represents a remnant of the Jurassic Ligurian Tethys [13]. The massif is composed of ultramafic rocks (partially serpentinized peridotites, serpentinites and serpentineschists), alongside metagabbros, metabasalts and eclogites. The ultramafic rocks display different degrees of serpentinization, varying from partially serpentinized lherzolites to massive serpentinites to strongly foliated serpentinites (serpentine schists) [14]. Within this geological context, the Beigua UNESCO Global Geopark (UGGp) hosts outcrops rich in PTEs, offering a unique setting to study serpentinized substrates. In these conditions, the bioavailable fraction of PTEs has been demonstrated to interfere with metabolic processes, thus inducing various physiological responses in organisms. The effects of PTEs are primarily mediated by their interaction with specific cellular binding sites, which promotes the excessive generation of reactive oxygen species (ROS). This oxidative stress triggers a sequence of reactions that extend from the cellular to the metabolic and physiological levels [15,16,17]. The resultant pro-inflammatory state leads to the oxidation of essential macromolecules, such as proteins and DNA, thereby compromising the efficiency of cellular repair systems [17,18].
In serpentinite soils, these chemical peculiarities impose strong selective pressures that drive speciation and the emergence of serpentine endemism [19,20]. Many of bacteria and fungi inhabiting these environments exhibit metal tolerance [21,22,23,24] which allows them to thrive in metal-rich conditions [25,26,27,28]. Over time, microbial communities have developed specific adaptations enabling them to colonize and persist in these inhospitable habitats [29]. These adaptations provide survival advantages and drive changes in community structure, functional diversity and ecosystem processes, such as soil weathering and nutrient cycling [27,28]. These distinctive features make these organisms excellent candidates for biotechnological applications, such as in the field of nature-based solutions [30]. Eco-friendly bioremediation approaches employ living organisms (i.e., prokaryotes, fungi, and plants) to remove or neutralize hazardous compounds in polluted sites [31,32]. These technologies exploit native organisms to detoxify and remove pollutants from soil, through their different metabolic capabilities: enzymes, organic acids, and secondary metabolites [16,33]. Concerning fungi, this ability depends on metallothioneins and phytochelatins, proteins that bind and deactivate potentially toxic elements [34]. Moreover, the extensive growth of hyphae in three-dimensional space allows them to act as macroorganisms at the microscale, increasing the surface area to efficiently detect pollutants and remediate contaminated soils in a sustainable manner [35,36,37].
Nonetheless, data on the structure of fungal communities in serpentinite soils remain scarce, and the roles of biotic and abiotic factors in serpentinite weathering, carbon sequestration, and soil evolution are still poorly understood [38].
Starting from this distinctive environmental substrate, the primary objectives of this study are as follows: i. to assess the mycodiversity present within PTE-rich serpentinite soil samples using both culturable and non-culturable methods, and to compare the resulting data; ii. to isolate, identify, and preserve fungal strains with potential applications in sustainable environmental remediation.
2. Materials and Methods
2.1. Sampling Points and Collection of Soil Samples
The studied soils are located adjacent to a sixty-meter-long road cut situated in the locality of Bric Gippone (Sassello, SV; 44°29′12.0″ N, 8°30′51.5″ E) at 585 m a.s.l. and with a south slope exposition [39]. Despite the limited extent of the outcrop, the soil profiles are developed on different ultramafic bedrock comprising partially serpentinized peridotites (PSPs), massive serpentinites (MSs), and foliated serpentinites (FSs). The soil is poorly developed and has been classified as Magnesic Leptic Skeletic Cambisols [40,41], with A horizon directly in contact with C horizon (AC soils) and with a centimetric organic horizon at the top of the profile (5–10 cm). Six sampling sites were selected according to the described bedrock heterogeneity (Figure 1) and in each site one soil sample (about 1.5 kg) was collected in October 2022 for chemical and mycological analyses by drilling up to a constant depth of 50 cm after removing the O horizon, following the criteria of GEMAS project [42].
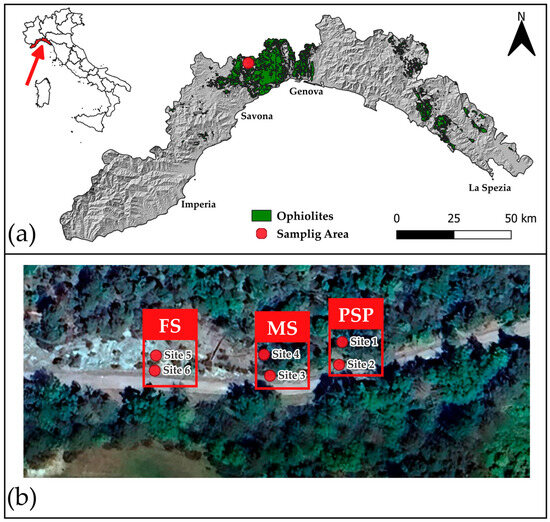
Figure 1.
(a) Location of the study area within the Voltri Massif, Liguria, Italy (region marked in red). The green area indicates the ophiolite formations (serpentinite soils); the red dot marks the specific sampling area (Source: Liguria Region Open Data) [43]. (b) Detailed view of the sampling area. The satellite image shows the sites according to their distinct bedrock (listed from right to left on the map): partially serpentinized peridotites (PSP: Site 1 and Site 2), massive serpentinites (MS: Site 3 and Site 4), and foliated serpentinite (FS: Site 5 and Site 6) [39].
2.2. Chemical Characterization
The PTE content of soils were determined on the granulometric fraction < 2 mm by means of field portable energy-dispersive X-ray fluorescence (EDXRF) and by inductively coupled plasma-atomic emission spectrometry (ICP-AES analysis). The EDXRF analyses were performed on 5 g pressed powder pellets using the X-MET7500 (Oxford Instruments, Abingdon, UK) spectrometer (GeoSpectra s.r.l.—Spin-Off company of the University of Genoa) with a Fundamental Parameter (FP) calibration built by factory.
The ICP–AES was performed at the Regional Agency for Environmental Protection of Liguria (ARPAL, Genova, Italy) using a Perkin Elmer–Optima 2100 DV spectrometer (PerkinElmer Inc., Waltham, MA, USA) on 3.5 g sample powder after total digestion of the sample by dissolution with aqua regia, prepared by mixing nitric acid (HNO3, 65%, ρ ≈ 1.42 g/mL) and hydrochloric acid (HCl, 37%, ρ ≈ 1.18 g/mL) in a 1:3 volume ratio. Both analytical techniques were used in the study. XRF was applied for the determination of major elements (>10,000 mg/kg, not reported in Table 1) and for minor elements (100 mg/kg < x < 10,000 mg/kg), while ICP-AES was employed for the analysis of trace elements.

Table 1.
The chemical composition of the studied soils (mg/kg). Summary statistics (mean, median, minimum, and maximum) are based on 18 analyses, encompassing both the current dataset (S1–S6) and previously reported data from the same sampling locations [14,41]. All measurements were performed using ICP-AES, yielding results comparable to those obtained by XRF.
2.3. Mycological Characterization Processing
The soil samples were plated on Malt Extract Agar medium added with chloramphenicol (MEA + C) (Sigma-Aldrich, St. Louis, MO, USA) and on Rose Bengal medium (RB) (Millipore, Burlington, MA, USA). Three replicates of MEA + C and three replicates of RB for each site were prepared. Samples were serially diluted in sterile water to reach a 1:10,000 dilution, from which 1 mL was inoculated in each Petri plate. The plates were incubated at 24 °C in the dark for 7 days and monitored for 7 days. After the period of incubation, fungi were enumerated as Colonies Forming Units (CFUs) and isolated colonies were used for the preparation of axenic cultures. Each strain was then scraped from the surface of pure colonies and preserved using cryovials with 50% nutrient liquid broth and 50% glycerol at 30%. The fungi were stored in the ColD UNIGE JRU MIRRI-IT collection, Laboratory of Mycology, University of Genoa (ColD). The most frequent fungi were identified morphologically and molecularly. The morphological identification of the colonies was conducted using a stereomicroscope and, subsequently, observing slides under an optical microscope, supported by comparison with the main taxonomic keys [44,45,46]. For molecular analysis, genomic DNA was extracted using modified CTAB method [47] from 100 mg of 7-day-old pure cultures biomass. PCRs were performed using the ITS and LSU region as primary barcode and genus-specific markers beta-tubulin BT and calmodulin CMD as secondary barcodes. PCR was set up in a volume of 24 μL: 15.875 μL Milli-Q water obtained using an Arium Mini water purification system (Sartorius AG, Göttingen, Germany), 1.5 μL MgCl2 50 mM, 5 μL Green GoTaq 5× buffer (Promega, Madison, WI, USA), 0.5 μL dNTP 10 mM, 0.5 μL 10 µM of each primer, 0.125 GoTaq 5 U/µL and 1 μL of extracted DNA. The PCR program was as follows: 5 min 95 °C, 35× (35 s 95 °C, 50 s 55 °C, 2 min 72 °C), 5 min 72 °C [48]. PCR products were purified and sequenced by Macrogen Europe, Milan, Italy. Amplicons were bidirectionally sequenced, assembled and compared to similar sequences using BLAST (Basic Local Alignment Search Tool, https://blast.ncbi.nlm.nih.gov/Blast.cgi, accessed on 18 November 2024) against sequences present in the sequence database of the National Center of Biotechnology Information (NCBI, http://blast.ncbi.nlm.nih.gov/Blast.cgi, accessed on 16 November 2024) (GenBank). The sequences of the isolated strains were deposited in GenBank with the following accession numbers: ITS sequences from PQ643985 to PQ643996; LSU sequences from PQ644004 to PQ644011; BT sequences from PQ654171 to PQ654175; CMD sequences from PQ654176 to PQ654178.
The composition of fungal communities associated with the six investigated sites was also assessed using a metabarcoding approach. Specifically, soil samples (500 mg each) from each site were sent to BMR Genomics (Padua, Italy) for DNA extraction and sequencing analysis. Genomic DNA was extracted by means of the DNeasy 96 PowerSoil Pro QIAcube HT Kit (Qiagen, Hilden, Germany). The ITS1 and ITS2 sequences were employed as reference markers for the DNA amplification. The sequences obtained from the Illumina sequencing were analyzed using QIIME2 software (Quantitative Insights Into Microbial Ecology 2, version 2019.7) [49]. The DADA2 plugin (version 1.16) was used for the purposes of quality control, denoising and the removal of chimeric sequences [50]. For the analysis of metabarcoding data, Amplicon Sequence Variants (ASVs) were used to ensure precise delineation of fungal taxa. Following bioinformatics analysis, a total of 167,718 high-quality sequences were retained, averaging 27,853 sequences per sample.
To quantify the alpha diversity, the Shannon Index was used using the function estimate_richness of the phyloseq R package. v.1.36.0 [51,52].
Pearson correlation were conducted using the MatLab R2023a Update 4 program [53]. The raw data used to generate the dataset for this study can be found in the NCBI Sequence Read Archive (SRA) under accession n° PRJNA1295429.
3. Results and Discussion
3.1. Chemical Characterization of Soil
The sampled soils contain a significant amount of unaltered or partially altered primary minerals, including antigorite, chrysotile, clinochlore, clinopyroxenes and spinels (mainly magnetite, chromite, ferrian-chromite and Cr-magnetite) as well as variable content of pedogenic minerals mainly represented by clay minerals and Fe-oxides (e.g., hematite) and oxyhydroxides (e.g., goethite), as reported by Marescotti et al. [41].
The results of the chemical analyses revealed that concentrations of Cr (mean: 1666 mg/kg), Ni (mean: 1065 mg/kg) and, to a lesser extent, Co (mean: 155 mg/kg) systematically exceed (up to an order of magnitude) the threshold limits established by Italian Legislative Decree No. 152 of 2006 [54] in all investigated soil types (i.e., PSP, MS and FS; Table 1). Among the different soil types, the most significant differences concern Cr and Ni contents, while Co contents are quite similar. In particular, a substantial increase in Cr and Ni concentrations is observed when moving from MS to PSP and FS soils.
Several works on the same sites [14,41] have shown that PTEs (Cr, Ni, and Co) are contained in primary minerals and their weathering products, thus confirming their natural origin. In particular, Ni is largely contained in the serpentine group minerals and to a lesser extent in clinochlore and in the pedogenic minerals (mainly hematite and goethite). Spinel-group minerals are, by far, the most important Cr-bearing primary minerals but no negligible Cr content is also present in clinochlore, clinopyroxenes, clay minerals, Fe-oxides and -oxyhydroxides. Spinels are the most important cobalt bearing minerals in all three soil types. Cobalt is also present in pedogenic minerals, with the highest contents occurring in amorphous Fe oxyhydroxides.
3.2. Mycological Characterization
The results regarding the culturable approach revealed the presence of viable fungi in concentration of 105 CFU g−1 dry soil. This concentration, despite the harsh conditions related to the elevated levels of potentially toxic elements, is a significant finding. A total of 691 fungal colonies were counted on the growing media and grouped into 23 species (Table 2). The most frequent fungi predominantly belonged to Penicillium, Umbelopsis, and Aspergillus genera.

Table 2.
Fungal colonies counted on the growing media (MEA + C and RB), grouped into 23 morphotypes. Sites 1, 2 correspond to Partially Serpentinized Peridotites (PSPs); Sites 3, 4 to Massive Serpentinites (MSs); and Sites 5,6 to Foliated Serpentinite (FS). The last column reports the values for Colony Forming Units (CFUs) expressed as a percentage.
The dominance of these three genera is in line with numerous studies focusing on metal-contaminated and extreme soil environments, which often report these taxa as robust, highly adaptable colonizers. Specifically, Penicillium emerged as the predominant genus, constituting 52% of the total, followed by Umbelopsis (17.9%) and Aspergillus (11.6%) (Figure 2). This clear hierarchy suggests a strong selective pressure favoring Penicillium species in this specific serpentinite soil environment. Penicillium citreonigrum represented the most abundant species of Penicillium spp. in each site. This fungus has been isolated from disparate environments, from marine sediments [55,56] to Atlantic Forest [57], and from rubber materials [58]. Bovio et al. [56] isolated P. citreonigrum from a marine contaminated polluted site, showing strong potential capacities of biodegradation of crude oil and good removal rates of aliphatic compounds (24%) [56]. This fungus is also well known for producing cytotoxic secondary metabolites [59] and metabolites of interest such as silver nanoparticles, exploiting antimicrobial activities [60,61]. The detection of this cosmopolitan, yet highly adaptable, species in our PTE-rich soil reinforces its known capabilities for survival and possible bioremediation in contaminated sites.
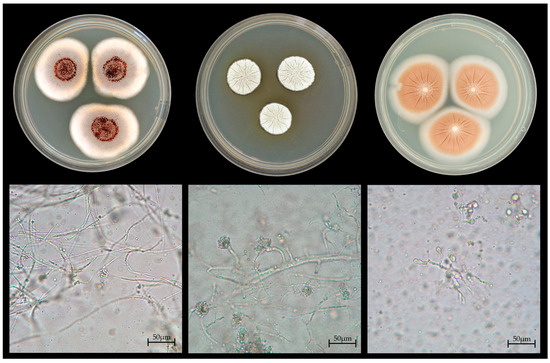
Figure 2.
Colonial and micromorphological features of key fungal isolates. (Top) row: colonies grown on MEA + C (from (left) to (right)): Penicillium citreonigrum, Aspergillus subnutans, Umbelopsis ramanniana. (Bottom) row ((left) to (right)): Micromorphological structures corresponding to the colonies above (scale bar: 50 µm).
The genus Umbelopsis comprises 26 species which are commonly isolated from leaf litter and soil [62]. The genus Umbelopsis was detected in asbestos contaminated soils in good percentage (11%) [63]. Our finding of Umbelopsis as the second most dominant genus (17.9%) is particularly noteworthy and potentially suggests a similar adaptation mechanism to the challenging mineralogy of serpentinite as observed in asbestos-contaminated environments. The ability of Umbelopsis isabellina to bioaccumulate heavy metals and xenobiotic compounds was investigated in an ecotoxicological study conducted by Janicki et al. [64]. U. isabellina demonstrated a noteworthy degree of tolerance in the presence of metals, with a maximum removal rate of up to 25% for lead [64]. Furthermore, U. isabellina and U. ramanniana were found to be oleaginous fungi, having the ability to produce large amounts of lipids. The presence of zinc and copper was found to have a positive effect on the production of linoleic acid by U. ramanniana. Such capabilities could be employed in the context of biorefineries [65]. The strong presence of Umbelopsis in our samples is thus highly relevant, not only indicating metal tolerance but also pointing to potential biotechnological applications, exploiting its metabolic capabilities for the management and remediation of metal-enriched soils. The genus Aspergillus has been highlighted in several studies for its environmental and biotechnological relevance. Daghino et al. [66] and Lu et al. [38] showed that Aspergillus fumigatus was one of the dominant species identified in asbestos-contaminated soils, showing remarkable tolerance to heavy metals and antimicrobial activity, also promoting the weathering of serpentinite minerals by acid dissolution [38,66].
The presence of Aspergillus in our study, while less dominant than Penicillium and Umbelopsis, confirms the established ecological role of this genus in chemically challenging soils, paralleling findings in other serpentinite and asbestos-contaminated areas.
Among the Aspergillus species isolated, Aspergillus persii has been previously isolated from soil samples and investigated for its antimicrobial properties [67]. To the best of our knowledge, this study represents the first instance of isolating this species from serpentinic soil. Research on strains isolated from serpentinite soils has demonstrated biosorption capabilities for metals such as cobalt [68]. A. parasiticus has shown the ability to uptake and tolerate high concentrations of potentially toxic elements in laboratory settings and regenerate biomass after treatment, making it a good candidate for heavy metal mycoremediation. Chromium is a constituent of serpentinites, with its presence being attributed to the presence of chromium-bearing minerals (e.g., chromite and other minerals of the spinel group) within the ultramafic rocks from which these serpentinites are derived. These resistant minerals either persist or undergo transformation during the serpentinization process, thereby contributing to the enrichment of high total Chromium content in serpentinite-derived soils [8,69,70]. The isolation of A. parasiticus is particularly compelling given the high chromium content characteristic of these serpentinitic soils, suggesting an intrinsic adaptation related to coping with this metalliferous environment. A. subnutans has been isolated from soil, though not specifically from serpentinite soil [71]. This species remains relatively understudied; however, its ability to develop in such an extreme environment suggests its potential significance for further research. Detailed investigations into its metabolic properties, particularly concerning the production of secondary metabolites or other bioactive compounds, could yield valuable insights.
The metabarcoding analysis confirmed the presence of the most frequently cultured fungal taxa. Notably, Penicillium citreonigrum, Umbelopsis ramanniana, and Aspergillus persii were identified at the species level. The distribution of fungal communities across the different sites is influenced by soil characteristics, which, in turn, vary depending on the underlying bedrock type. The sampled soils share common characteristics in their profile, characterized by a very thin O horizon (5–10 cm) and the A horizon is weakly developed and directly in contact with the C horizon (AC) soils. However, soils derived from the three different types of bedrock display varying profile thicknesses, increasing systematically from PSP soils (40–60 cm) to MS (50–70 cm) and FS (80–100 cm) [41]. The three soil types also differ in the degree of weathering of the bedrock; in fact, the weathering intensity and the content of newly formed authigenic minerals is generally higher in PSP with respect to MS and FS sites. In particular, the FS soil is mostly composed by unweathered or partially weathered primary minerals inherited from the parent rocks.
The differences in soil profiles and their degree of weathering significantly influence fungal communities, with evidence observed in part from the culture-based approach, and predominantly from metabarcoding data. Ascomycota and Basidiomycota were identified as the most prevalent phyla across all sites and exhibiting some variations, followed by the phylum Mucoromycota. The phyla under discussion have been identified as the most abundant in other serpentinite sites in the Western Alps [66]. The ASV for Ascomycota demonstrated a substantial increase across the sites, while Basidiomycota exhibited an 85% decrease (the table containing the ASV counts is reported in Table S1 in the Supplementary Materials). In sites 1–4, corresponding to the strongly weathered soils (PSP and MS), vegetation is abundant, and Basidiomycota dominate the fungal community. Conversely, in sites 5 and 6, located on less weathered soils derived from FS, there is a significant increase in Ascomycota (Figure 3). This distribution may be explained by metabolic adaptations and reproductive strategies. Basidiomycota are characterized by their ability to degrade complex polymers like organic matter and form mycorrhizal associations [72,73]. Moreover, their reliance on sexual reproduction enhances their resilience in stressful environments, as genetic diversity provides a greater capacity for adaptation [74]. In contrast, Ascomycota dominate under highly stressful conditions and in less developed soils due to their ability to tolerate nutrient-poor environments [38,75]. Their strategy of asexual reproduction enables rapid colonization, making them better suited to soils with limited organic matter and nutrients [76]. This transition reflects an ecological adaptation influenced by soil weathering and profiles, where highly weathered support more diverse and specialized fungal communities dominated by Basidiomycota, while soils with minimal weathering favor generalist and resilient strategies dominated by Ascomycota.
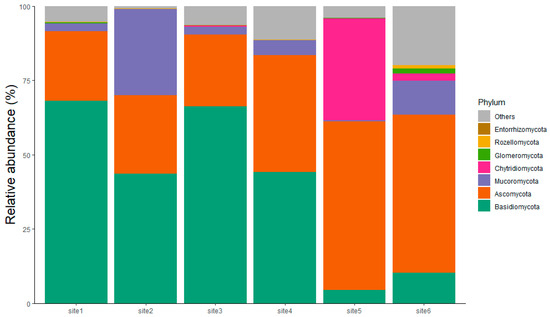
Figure 3.
Phylum-level taxonomic composition of fungal communities from the six sampled sites. as determined by metabarcoding analysis. The relative abundance (%) of the fungal phyla is shown for each site. It is noted that Site 1 and Site 2 are located within Partially Serpentinized Peridotites (PSPs); Site 3 and Site 4 within Massive Serpentinites (MSs); and Site 5 and Site 6 within Foliated Serpentinite (FS). Basidiomycota (green band) and Ascomycota (orange band) represent the most abundant phyla across the sites.
The Shannon index of each site was calculated from the metabarcoding data. The index value increased in correlation with the degree of soil alteration and the grade of serpentinization of the corresponding bedrock; that is, from more weathered and less serpentinized soils (PSP and MS) to less weathered and completely serpenitinized soil (FS) (Figure 4). This finding suggests a potential relationship between biodiversity and the structure and chemical/mineralogical composition of the soils. The phenomenon of the filter effect, attributable to chemical characteristics, was identified by the work of Botha et al. [77].
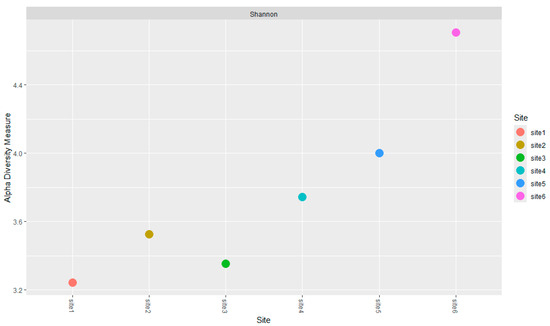
Figure 4.
Alpha Diversity (Shannon Index) of the fungal community across the sampling sites. Data were calculated from metabarcoding analysis. The X-axis represents the sites (from 1 to 6), and the Y-axis shows the calculated alpha diversity values.
The Pearson analysis also revealed a strong positive correlation (R: 0.9409, p-value: 0.0051) between fungal biodiversity, measured using Shannon’s index, and nickel concentration in the serpentinite sites. This significant relationship suggests that higher nickel concentrations are associated with greater fungal diversity.
In PTE-rich soils, an increase in diversity may be observed as a result of an increased number of species of resistant fungi [78]. The exposure to potentially toxic elements can elicit the emergence of tolerant organisms. The observed phenomenon may be due to the PTE tolerance, resistance and community adaptation. Fungal organisms possess the capacity to employ a variety of tolerance mechanisms, comprising both direct mechanisms, such as the intracellular transport of metals and the subsequent bioaccumulation of these elements, and indirect mechanisms, such as the precipitation and complexation of metal ions in the form of biominerals [79,80]. The results are consistent with the hypothesis that environmental stresses within serpentinite soils have driven the independent evolution of different genetic combinations conferring resistance to PTEs, including co-resistance and single-metal resistance [81].
4. Conclusions
This study of serpentinite soils allowed for the acquisition of deeper knowledge of the fungal community in this area through mycological characterization. The most prevalent fungi identified at a cultural level were found to belong to the genera Penicillium, Umbelopsis and Aspergillus. Metabarcoding data analysis validated the data from culturable techniques and provided a comprehensive assessment of the fungal community. The analysis identified the Ascomycota and Basidiomycota phyla as the most representative.
A positive correlation was identified between fungal biodiversity and nickel concentration, which confirms that these communities are not only tolerant but represent organisms with unique metabolic features, the product of specific evolutionary speciation driven by extreme edaphic stress. We therefore recognize these PTE-rich soils as a critical natural sink for this specialized genetic resource. Further colonization studies and bioaccumulation tests will be needed to clarify the role of fungi in the chemical and physical transformation of serpentinite rocks. Crucially, the establishment of a collection of indigenous strains will provide the necessary functional data to design bioinoculant formulations for effective, site-specific Nature-Based Solutions for PTE-contaminated soil remediation.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/soilsystems9040129/s1.
Author Contributions
Conceptualization, M.Z., P.M. and S.D.P.; methodology, G.C., M.Z., P.M. and S.D.P.; software, S.V.; validation M.Z. and P.M.; formal analysis, L.C., S.C. and S.V.; investigation, L.C., F.G. and S.C.; resources, M.Z. and P.M.; data curation, L.C., F.G., S.C. and S.V.; writing—original draft preparation, L.C.; writing—review and editing, L.C., S.V. and P.M.; visualization, L.C., G.C. and S.C.; supervision, M.Z., P.M. and S.V.; project administration, M.Z. and P.M.; funding acquisition, P.M. and M.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This article was funded by research funding of the Mycological Laboratory of DISTAV. Part of this work was also granted by the European Commission—NextGenerationEU, Project SUSMIRRI.IT “Strengthening the MIRRI Italian Research Infrastructure for Sustainable Bioscience and Bioeconomy”, code n. IR0000005.
Data Availability Statement
For any questions about data, please contact the corresponding author.
Acknowledgments
The authors would like to acknowledge Michele Brancucci (GeoSpectra s.r.l.) for chemical analyses by means of Field Portable XRF.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| FS | foliated serpentinite |
| MS | massive serpentinite |
| PSP | partially serpentinized peridotite |
| PTE | potentially toxic element |
References
- Shu, W.-S.; Huang, L.-N. Microbial Diversity in Extreme Environments. Nat. Rev. Microbiol. 2022, 20, 219–235. [Google Scholar] [CrossRef]
- Sepe, F.; Costanzo, E.; Ionata, E.; Marcolongo, L. Biotechnological Potential of Extremophiles: Environmental Solutions, Challenges, and Advancements. Biology 2025, 14, 847. [Google Scholar] [CrossRef]
- Rothschild, L.; Mancinelli, R.L. Insight Review Articles. Nature 2001, 409, 1092–1101. [Google Scholar] [CrossRef]
- Satyanarayana, T.; Raghukumar, C.; Shivaji, S. Extremophilic Microbes: Diversity and Perspectives. Curr. Sci. 2005, 89, 78–90. [Google Scholar]
- Jeong, S.W.; Choi, Y.J. Extremophilic Microorganisms for the Treatment of Toxic Pollutants in the Environment. Molecules 2020, 25, 4380. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.-W.; Yang, J.; Srimongkol, P.; Stadler, M.; Karnchanatat, A.; Ariyawansa, H.A. Fungal Frontiers in Toxic Terrain: Revealing Culturable Fungal Communities in Serpentine Paddy Fields of Taiwan. IMA Fungus 2025, 16, e155308. [Google Scholar] [CrossRef] [PubMed]
- Oze, C.; Fendorf, S.; Bird, D.K.; Coleman, R.G. Chromium Geochemistry of Serpentine Soils. Int. Geol. Rev. 2004, 46, 97–126. [Google Scholar] [CrossRef]
- Alexander, E.B. Serpentine Geoecology of Western North America: Geology, Soils, and Vegetation; Oxford University Press: New York, NY, USA, 2007. [Google Scholar]
- Proctor, J.; Woodell, S.R.J. The Ecology of Serpentine Soils. Adv. Ecol. Res. 1975, 9, 255–366. [Google Scholar] [CrossRef]
- Van Volkenburgh, E. Physiological Adaptations of Plants to Serpentine Soil. Plant Soil 2014, 382, 83–94. [Google Scholar]
- Branco, S.; Ree, R.H. Serpentine Soils Do Not Limit Mycorrhizal Fungal Diversity. PLoS ONE 2010, 5, e11757. [Google Scholar] [CrossRef]
- Brouwer, F.M.; Vissers, R.L.M.; Lamb, W.M. Metamorphic History of Eclogitic Metagabbro Blocks from a Tectonic Mélange in the Voltri Massif, Ligurian Alps, Italy. Ofioliti 2002, 27, 1–16. [Google Scholar]
- UENC for, E. Structural and Metamorphic Signature of Alpine Tectonics in the Voltri Massif (Ligurian Alps, North-Western Italy). Geol. J. 2009, 44, 132–145. [Google Scholar] [CrossRef]
- Fornasaro, S.; Comodi, P.; Crispini, L.; Malatesta, C.; Zucchini, A.; Marescotti, P. Potentially Toxic Elements Distribution in the Serpentinized and Deformed Ultramafic Rocks from the Voltri Massif (NW, Italy). Period. Mineral 2023, 88, 259–276. [Google Scholar] [CrossRef]
- Abd Elnabi, M.K.; Elkaliny, N.E.; Elyazied, M.M.; Azab, S.H.; Elkhalifa, S.A.; Elmasry, S.; Mouhamed, M.S.; Shalamesh, E.M.; Alhorieny, N.A.; Abd Elaty, A.E.; et al. Toxicity of Heavy Metals and Recent Advances in Their Removal: A Review. Toxics 2023, 11, 580. [Google Scholar] [CrossRef]
- Kumar, V.; Dwivedi, S.K. Mycoremediation of Heavy Metals: Processes, Mechanisms, and Affecting Factors. Environ. Sci. Pollut. Res. 2021, 28, 2785–2805. [Google Scholar] [CrossRef]
- Fu, Z.; Xi, S. The Effects of Heavy Metals on Human Metabolism. Toxicol. Mech. Methods 2020, 30, 167–176. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy Metal Toxicity and the Environment. EXS 2012, 101, 133–164. [Google Scholar] [CrossRef]
- Harrison, S.P.; Rajakaruna, N. Serpentine: The Evolution and Ecology of a Model System; University of California Press: Oakland, CA, USA, 2011; pp. 80–83. [Google Scholar]
- Hysa, A.; Teqja, Z.; Bani, A.; Libohova, Z.; Cerda, A. Assessing Wildfire Vulnerability of Vegetated Serpentine Soils in the Balkan Peninsula. J. Nat. Conserv. 2022, 68, 126217. [Google Scholar] [CrossRef]
- Mengoni, A.; Barzanti, R.; Gonnelli, C.; Gabbrielli, R.; Bazzicalupo, M. Characterization of nickel-resistant bacteria isolated from serpentine soil. Environ. Microbiol. 2001, 3, 691–698. [Google Scholar] [CrossRef]
- Abou-Shanab, R.A.I.; van Berkum, P.; Angle, J.S. Heavy metal resistance and genotypic analysis of metal resistance genes in gram-positive and gram-negative bacteria present in Ni-rich serpentine soil and in the rhizosphere of Alyssum murale. Chemosphere 2007, 68, 360–367. [Google Scholar] [CrossRef]
- Iram, S.; Parveen, K.; Usman, J.; Nasir, K.; Akhtar, N.; Arouj, S.; Ahmad, I. Heavy metal tolerance of filamentous fungal strains isolated from soil irrigated with industrial wastewater. Biologija 2012, 58, 107–116. [Google Scholar] [CrossRef]
- Álvarez-López, V.; Prieto-Fernández, Á.; Becerra-Castro, C.; Monterroso, C.; Kidd, P.S. Rhizobacterial communities associated with the flora of three serpentine outcrops of the Iberian Peninsula. Plant Soil 2016, 403, 233–252. [Google Scholar] [CrossRef]
- Gadd, G.M. Interactions of fungi with toxic metals. New Phytol. 1993, 124, 25–60. [Google Scholar] [CrossRef]
- de Lima e Silva, A.A.; Ribeiro de Carvalho, M.A.; L de Souza, S.A.L.; Teixeira Dias, P.M.; da Silva Filho, R.G.; de Meirelles Saramago, C.S.; de Melo Bento, C.A.; Hofer, E. Heavy metal tolerance (Cr, Ag and Hg) in bacteria isolated from sewage. Braz. J. Microbiol. 2012, 43, 1620–1631. [Google Scholar] [CrossRef]
- Liao, M.; Xie, X.M. Effect of heavy metals on substrate utilization pattern, biomass, and activity of microbial communities in a reclaimed mining wasteland of red soil area. Ecotoxicol. Environ. Saf. 2007, 66, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.J.; Iii, R.L.T. Effects of Heavy Metal Contamination and Remediation on Soil Microbial Communities in the vicinity of a Zinc smelter. J. Environ. Qual. 1998, 27, 609–617. [Google Scholar] [CrossRef]
- Coleine, C.; Stajich, J.E.; Selbmann, L. Fungi are key players in extreme ecosystems. Trends Ecol. Evol. 2022, 37, 517–528. [Google Scholar] [CrossRef]
- Orellana, R.; Macaya, C.; Bravo, G.; Dorochesi, F.; Cumsille, A.; Valencia, R.; Rojas, C.; Seeger, M. Living at the Frontiers of Life: Extremophiles in Chile and Their Potential for Bioremediation. Front. Microbiol. 2018, 9, 2309. [Google Scholar] [CrossRef] [PubMed]
- Cecchi, G.; Vagge, G.; Cutroneo, L.; Greco, G.; Di Piazza, S.; Faga, M.; Zotti, M. Fungi as potential tool for polluted port sediment remediation. Environ. Sci. Pollut. Res. 2019, 26, 35602–35609. [Google Scholar] [CrossRef]
- Azubuike, C.C.; Chikere, C.B.; Okpokwasili, G.C. Bioremediation of Industrial Waste for Environmental Safety; Springer: Singapore, 2020. [Google Scholar]
- Pandey, N.; Chandrakar, V.; Keshavkant, S. Mitigating Arsenic Toxicity in Plants: Role of Microbiota. In Mechanisms of Arsenic Toxicity and Tolerance in Plants; Springer: Singapore, 2018; pp. 191–218. [Google Scholar] [CrossRef]
- Robinson, J.R.; Isikhuemhen, O.S.; Anike, F.N. Fungal–Metal Interactions: A Review of Toxicity and Homeostasis. J. Fungi 2021, 7, 225. [Google Scholar] [CrossRef]
- Harms, H.; Schlosser, D.; Wick, L.Y. Untapped potential: Exploiting fungi in bioremediation of hazardous chemicals. Nat. Rev. Microbiol. 2011, 9, 177–192. [Google Scholar] [CrossRef]
- Liang, X.; Gadd, G.M. Metal and metalloid biorecovery using fungi. Microb. Biotechnol. 2017, 10, 1199–1205. [Google Scholar] [CrossRef]
- Cecchi, G.; Marescotti, P.; Di Piazza, S.; Zotti, M. Native fungi as metal remediators: Silver myco-accumulation from metal contaminated waste-rock dumps (Libiola Mine, Italy). J. Environ. Sci. Health Part B 2017, 52, 191–195. [Google Scholar] [CrossRef]
- Lu, M.; Wang, X.; Li, Y.; Liu, H.; An, X.; Lian, B. Soil microbial community structure and environmental effects of serpentine weathering under different vegetative covers in the serpentine mining area of Donghai County, China. Sci. Total Environ. 2022, 835, 155452. [Google Scholar] [CrossRef]
- Google Earth. Imagery of Sampling Area on Voltri Massif. Available online: https://earth.google.com (accessed on 4 September 2023).
- World Reference Base for Soil Resources 2014: International Soil Classification System for Naming Soils and Creating Legends for Soil Maps. Available online: https://www.researchgate.net/publication/266065065_World_Reference_Base_for_soil_resources_2014_international_soil_classification_system_for_naming_soils_and_creating_legends_for_soil_maps (accessed on 10 September 2025).
- Marescotti, P.; Comodi, P.; Crispini, L.; Gigli, L.; Zucchini, A.; Fornasaro, S. Potentially Toxic Elements in Ultramafic Soils: A Study from Metamorphic Ophiolites of the Voltri Massif (Western Alps, Italy). Minerals 2019, 9, 502. [Google Scholar] [CrossRef]
- Albanese, S.; Sadeghi, M.; Lima, A.; Cicchella, D.; Dinelli, E.; Valera, P.; Falconi, M.; Demetriades, A.; De Vivo, B.; Andersson, M.; et al. GEMAS: Cobalt, Cr, Cu and Ni Distribution in Agricultural and Grazing Land Soil of Europe. J. Geochem. Explor. 2015, 154, 81–93. [Google Scholar] [CrossRef]
- Geoportale Regione Liguria. Available online: https://geoportal.regione.liguria.it (accessed on 14 October 2025).
- Pitt, J.I.; Hocking, A.D. Fungi and Food Spoilage, 3rd ed.; Springer: New York, NY, USA, 2009. [Google Scholar]
- Chen, A.J.; Hubka, V.; Frisvad, J.C.; Visagie, C.M.; Houbraken, J.; Meijer, M.; Varga, J.; Demirel, R.; Jurjević, Z.; Kubátová, A.; et al. Polyphasic Taxonomy of Aspergillus Section Aspergillus (Formerly Eurotium), and Its Occurrence in Indoor Environments and Food. Stud. Mycol. 2017, 88, 37–135. [Google Scholar] [CrossRef] [PubMed]
- Bissett, J. A Revision of the Genus Trichoderma. I. Section Longibrachiatum Sect. Nov. Can. J. Bot. 1984, 62, 924–931. [Google Scholar] [CrossRef]
- Doyle, J.J.; Doyle, J.L. A Rapid DNA Isolation Procedure for Small Quantities of Fresh Leaf Tissue. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]
- Di Piazza, S.; Houbraken, J.; Meijer, M.; Cecchi, G.; Kraak, B.; Rosa, E.; Zotti, M. Thermotolerant and Thermophilic Mycobiota in Different Steps of Compost Maturation. Microorganisms 2020, 8, 880. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science Using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Shannon, C.E. A Mathematical Theory of Communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- McMurdie, P.J.; Holmes, S. phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef]
- Pernet, C.R.; Wilcox, R.; Rousselet, G.A. Robust Correlation Analyses: False Positive and Power Validation Using a New Open Source MATLAB Toolbox. Front. Psychol. 2013, 3, 606. [Google Scholar] [CrossRef]
- Legislative Decree No. 152 of April 3, 2006, Annex 5, Part IV, Title V: Remediation of Contaminated Sites (Official Gazette No. 88, Ordinary Supplement No. 96 of April 14, 2006). Available online: https://www.gazzettaufficiale.it/dettaglio/codici/materiaAmbientale (accessed on 5 November 2022).
- Huang, J.N.; Zou, Q.; Chen, J.; Xu, S.H.; Luo, D.; Zhang, F.G.; Lu, Y.Y. Phenols and Diketopiperazines Isolated from Antarctic-Derived Fungi, Penicillium citreonigrum SP-6. Phytochem. Lett. 2018, 27, 114–118. [Google Scholar] [CrossRef]
- Bovio, E.; Gnavi, G.; Prigione, V.; Spina, F.; Denaro, R.; Yakimov, M.; Calogero, R.; Crisafi, F.; Varese, G.C. The Culturable Mycobiota of a Mediterranean Marine Site after an Oil Spill: Isolation, Identification and Potential Application in Bioremediation. Sci. Total Environ. 2017, 576, 310–318. [Google Scholar] [CrossRef]
- Cruz, R.; Santos, C.; de Lima, J.S.; Moreira, K.A.; de Souza-Motta, C.M. Diversity of Penicillium in Soil of Caatinga and Atlantic Forest Areas of Pernambuco, Brazil: An Ecological Approach. Nova Hedwigia 2013, 97, 543–556. [Google Scholar] [CrossRef]
- Levinskaitė, L. Biodegradation Potential of Penicillium Isolated from Synthetic Polymeric Materials. J. Environ. Eng. 2018, 144, 06018002. [Google Scholar] [CrossRef]
- Uchiyama, Y.; Takino, M.; Noguchi, M.; Shiratori, N.; Kobayashi, N.; Sugita-Konishi, Y. The In Vivo and In Vitro Toxicokinetics of Citreoviridin Extracted from Penicillium citreonigrum. Toxins 2019, 11, 360. [Google Scholar] [CrossRef]
- Ali, F.T.; El-Sheikh, H.H.; El-Hady, M.M.; Elaasser, M.M.; El-Agamy, D.M. Silver Nanoparticles Synthesized by Penicillium citreonigrum and Fusarium moniliforme Isolated from El-Sharkia, Egypt. Int. J. Sci. Eng. Res. 2014, 5, 181–192. [Google Scholar]
- Hamad, M.T. Biosynthesis of Silver Nanoparticles by Fungi and Their Antibacterial Activity. Int. J. Environ. Sci. Technol. 2019, 16, 1015–1024. [Google Scholar] [CrossRef]
- Hou, D.; Tang, D.; Wang, Y.; Zhu, J.; Luo, R.; Liu, Z.; Lu, Y.; Sun, T.; Ma, Y.; Zhang, Y.; et al. Molecular Phylogenetics of the Umbelopsis Genus—Identification of New Species and Evaluation of Their Oil Application Value. J. Appl. Microbiol. 2024, 135, lxae065. [Google Scholar] [CrossRef]
- Wong, L.C.; Rodenburg, U.; Leite, R.R.; Korthals, G.W.; Pover, J.; Koerten, H.; Kuramae, E.E.; Bodelier, P.L.E. Exploring Microbial Diversity and Interactions for Asbestos Modifying Properties. Sci. Total Environ. 2024, 951, 175577. [Google Scholar] [CrossRef] [PubMed]
- Janicki, T.; Długoński, J.; Krupiński, M. Detoxification and Simultaneous Removal of Phenolic Xenobiotics and Heavy Metals with Endocrine-Disrupting Activity by the Non-Ligninolytic Fungus Umbelopsis isabellina. J. Hazard. Mater. 2018, 360, 661–669. [Google Scholar] [CrossRef]
- Zhu, S.; Bonito, G.; Chen, Y.; Du, Z.Y. Oleaginous Fungi in Biorefineries. Ref. Modul. Life Sci. 2020, 2, 577–589. [Google Scholar] [CrossRef]
- Daghino, S.; Murat, C.; Sizzano, E.; Girlanda, M.; Perotto, S. Fungal Diversity Is Not Determined by Mineral and Chemical Differences in Serpentine Substrates. PLoS ONE 2012, 7, e44233. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Yu, N.H.; Jeon, S.J.; Lee, H.W.; Bae, C.H.; Yeo, J.H.; Lee, H.B.; Kim, I.S.; Park, H.W.; Kim, J.C. Antibacterial Activities of Penicillic Acid Isolated from Aspergillus persii against Various Plant Pathogenic Bacteria. Lett. Appl. Microbiol. 2016, 62, 488–493. [Google Scholar] [CrossRef]
- Pal, A.; Ghosh, S.; Paul, A.K. Biosorption of Cobalt by Fungi from Serpentine Soil of Andaman. Bioresour. Technol. 2005, 96, 1335–1342. [Google Scholar] [CrossRef] [PubMed]
- Shugaba, A.; Wuyep, P.A.; Nok, A.J.; Ameh, D.A.; Lori, J.A. Bioremediation of Hexavalent Chromium and Tannic Acid in Synthetic Tannery Wastewater Using Free and Calcium Alginate-Immobilized Spores and Mycelia of Aspergillus niger and Aspergillus parasiticus. Bioremediat. J. 2010, 14, 142–149. [Google Scholar] [CrossRef]
- McClain, C.N.; Fendorf, S.; Webb, S.M.; Maher, K. Quantifying Cr(VI) Production and Export from Serpentine Soil of the California Coast Range. Environ. Sci. Technol. 2017, 51, 141–149. [Google Scholar] [CrossRef]
- Chen, A.J.; Varga, J.; Frisvad, J.C.; Jiang, X.Z.; Samson, R.A. Polyphasic Taxonomy of Aspergillus Section Cervini. Stud. Mycol. 2016, 85, 65–89. [Google Scholar] [CrossRef][Green Version]
- Wubet, T.; Christ, S.; Schöning, I.; Boch, S.; Gawlich, M.; Schnabel, B.; Fischer, M.; Buscot, F. Differences in Soil Fungal Communities between European Beech (Fagus sylvatica L.) Dominated Forests Are Related to Soil and Understory Vegetation. PLoS ONE 2012, 7, e47500. [Google Scholar] [CrossRef]
- Zhong, Z.; Zhang, X.; Wang, X.; Fu, S.; Wu, S.; Lu, X.; Ren, C.; Han, X.; Yang, G. Soil Bacteria and Fungi Respond Differently to Plant Diversity and Plant Family Composition during the Secondary Succession of Abandoned Farmland on the Loess Plateau, China. Plant Soil 2020, 448, 183–200. [Google Scholar] [CrossRef]
- Wallen, R.M.; Perlin, M.H. An Overview of the Function and Maintenance of Sexual Reproduction in Dikaryotic Fungi. Front. Microbiol. 2018, 9, 503. [Google Scholar] [CrossRef]
- Egidi, E.; Delgado-Baquerizo, M.; Plett, J.M.; Wang, J.; Eldridge, D.J.; Bardgett, R.D.; Maestre, F.T.; Singh, B.K. A Few Ascomycota Taxa Dominate Soil Fungal Communities Worldwide. Nat. Commun. 2019, 10, 2369. [Google Scholar] [CrossRef] [PubMed]
- Camenzind, T.; Weimershaus, P.; Lehmann, A.; Aguilar-Trigueros, C.; Rillig, M.C. Soil Fungi Invest into Asexual Sporulation under Resource Scarcity, but Trait Spaces of Individual Isolates Are Unique. Environ. Microbiol. 2022, 24, 2962–2978. [Google Scholar] [CrossRef]
- Botha, D.; Barnard, S.; Claassens, S.; Rajakaruna, N.; Venter, A.; Ismail, A.; Allam, M.; Siebert, S.J. Soil Type and Precipitation Level Have a Greater Influence on Fungal than Bacterial Diversity in Serpentine and Non-Serpentine Biological Soil Crusts. Ecol. Res. 2024, 39, 862–878. [Google Scholar] [CrossRef]
- Pan, X.; Zhang, S.; Zhong, Q.; Gong, G.; Wang, G.; Guo, X.; Xu, X. Effects of Soil Chemical Properties and Fractions of Pb, Cd, and Zn on Bacterial and Fungal Communities. Sci. Total Environ. 2020, 715, 136904. [Google Scholar] [CrossRef] [PubMed]
- Kapahi, M.; Sachdeva, S. Bioremediation Options for Heavy Metal Pollution. J. Health Pollut. 2019, 9, 191203. [Google Scholar] [CrossRef]
- Newsome, L.; Falagán, C. The Microbiology of Metal Mine Waste: Bioremediation Applications and Implications for Planetary Health. GeoHealth 2021, 5, e2020GH000319. [Google Scholar] [CrossRef]
- Kazakou, E.; Dimitrakopoulos, P.G.; Baker, A.J.M.; Reeves, R.D.; Troumbis, A.Y. Hypotheses, Mechanisms and Trade-Offs of Tolerance and Adaptation to Serpentine Soils: From Species to Ecosystem Level. Biol. Rev. 2008, 83, 495–508. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).