Abstract
Creation of alkaline bulk layers from mining waste is economically viable way to prevent the migration of toxic metals down the soil profile and revegetate heavy polluted soils over large areas. We have conducted perennial experiments on the revegetation of industrial barren located near the operating nonferrous smelter in humid subarctic climate. A vermiculite–lizardite material from closed phlogopite mining, containing 10% layered silicates, was used to create the alkaline sorption barrier on the sites with high level of Cu/Ni pollution and wide range of topographic wetness index (TWI). We have revealed the strong effect of TWI on metal accumulation by mineral material with the highest effectiveness for the most wet sites. At the same time, the stable Ca and Mg content over seasons revealed the prolonged material effect for the maintenance of alkalinity and macronutrient supply. Further, we demonstrate the potential of Festuca rubra, Festuca ovina, Achillea millefolium, Deschampsia cespitosa, Dactylis glomerata, Rumex acetosella, Silene suecica, and for the revegetation of mineral material in dry locations. We demonstrated the effectiveness of alkaline geochemical barrier for the accumulation of toxic metals and successful plant growth in a wide range of topographic units.
1. Introduction
Restoration of soils contaminated with heavy metals (HMs) is a relevant and multifaceted area of scientific research that requires a comprehensive approach and analysis. The introduction of alkaline materials is a promising method for reducing the mobility of heavy metals in the soil [1]. Stabilization of microelements by introducing alkaline materials in combination with planting pollution-resistant plant species contributes to the formation of a biogeochemical barrier that allows for immobilization of metals and limits their migration in the soil [2].
The most common and diverse materials in the immobilization of metals in soil systems are lime, clay minerals, phosphorus-containing, and calcium–silicon compounds, as well as metal oxides and organic residues [3,4]. Bentonite, kaolinite, attapulgite, sepiolite, montmorillonite, and vermiculite are minerals that are often used to clean soil contaminated with HMs [5,6,7]. These minerals have the cation-exchange properties, high adsorption and alkaline capacity, and also, due to the small size of the particles, a large specific surface.
Formed artificial and natural geochemical barriers allow for reducing the migration capacity of HMs due to chemical and biological transformations. The creation of geochemical barriers are currently active practical technologies for cleaning and protecting soils, underground and surface waters and the environment as a whole [8].
The industrial barren around the copper–nickel smelter (Monchegorsk, Russia) is one of the largest areas disturbed by aerial metal emissions in Europe [9]. In accordance with government and Kola MMC data, peak annual emissions were about 1800 t Cu and 2710 t Ni (1990), reducing to 700–900 t in 2003 and less than 500 t today; aerial technogenic deposition of HM from the smelter occur in forms of pentlandite (Ni,Fe)9S8, pyrrotite Fe7S8(Nix), chalcopyrite CuFeS2 along with chalcosite Cu2S, covellite CuS, cuprite Cu2O, tenorite CuO, and metal Cu and Ni [10].This area with a high level of soil pollution by HMs (Cu and Ni) and ecosystem degradation has been formed as a result of long-term (>70 yrs) aerial technogenic emissions [11]. In addition, this area is located in the subarctic conditions at the northern boundary of northern taiga with the highly vulnerable plant communities. In this regard, phytoremediation without sufficient improving soil conditions is not effective for this area, while the Technosol creation is the simplest way to restore the vegetation cover [12,13].
To limit the migration of heavy metals down the soil profile and provide economically viable landscaping technology over such a large area, the most effective solution is the formation of a bulk layer of alkaline mining waste [14]. When choosing the optimal waste, it is necessary to focus not only on its acidity but also on the macronutrient content, since the waste layer also serves as a provider of nutrients for the root systems at an early stages of ecosystem development. The use of overburden rocks formed during phlogopite mining (Kovdor, Russia) (Figure 1a) is shown to be promising for the revegetation over large areas. These rocks cover an area about 70,000 m2, the total volume of this material is estimated to be 120 million tons [15]. Given the mineral composition and location of waste disposal site of only 120 km from the industrial barren, the use of these materials is effective for the primary soil formation and is economically justified waste [14].
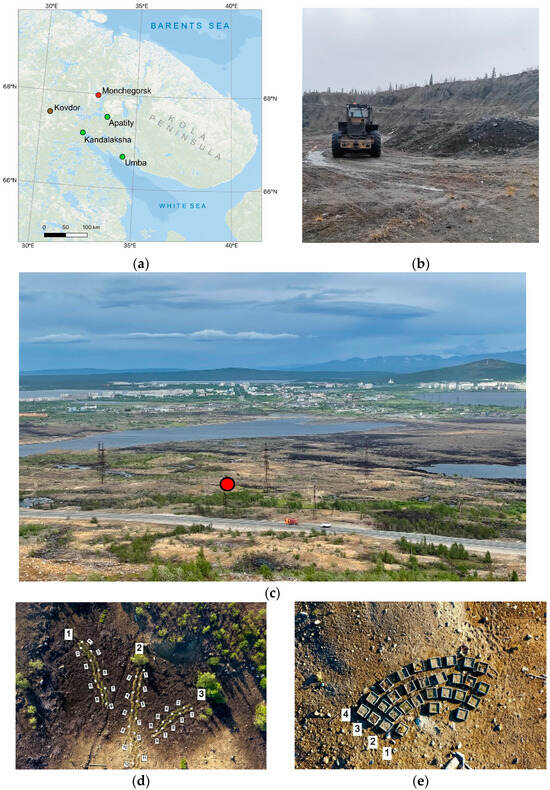
Figure 1.
Location (a); appearance of sampling point of the phlogopite overburden material (b) and the industrial barren (c); top view of experimental site 1 (d) and site 2 (e) in June 2022. Note: Green dots indicate the locations of plant seed collection, brown dots indicate the location of the overburden material, and red dots indicate the location of the experimental site. (d) White dots indicate the points of soil sampling.
Within the industrial barren, areas that require the remediation most are peaty soils at meso-topographic depressions characterized by high moisture with high values of the topographic wetness index (TWI) [11,16]. At the same time, areas belonging to elevated convex micro-topographic forms are characterized by a lack of moisture during the seed germination period. Therefore, the very important task of effective remediation technology is the selection of plant assortments resistant to unfavorable moisture conditions with the high technogenic pressure in the Subarctic.
In 2021–2022, the field experiment of remediation using overburden rocks included sorption-active minerals (vermiculite and lizardite) was initiated in the local area of industrial barren with the wide range of TWI values. This article presents the results obtained during 2 years after the start of this long-term experiment. The hypothesis of our study was that this mineral material can act as an alkaline sorption barrier for heavy metals, improving the quality of the soil and creating the conditions for the development and growth of plants. The specific objectives of the current research were (i) to study the shifts in content of macronutrients and HMs in vermiculite–lizardite mineral material after 2 years of the field experiment; (ii) to reveal the TWI effect on the HMs accumulation and mobility in this mineral material; and (iii) to determine the list of native and commercial species perspective for plant cover creation on alkaline sorption barrier.
2. Materials and Methods
2.1. Characteristics of Vermiculite–Lizardite Material
Overburden rocks of closed at this phlogopite mining time (JSC “Kovdorsljuda” enterprise has been operated in 1965–2011) were used to create the bulk layer on the surface of polluted soil (Figure 1b). The mineral material used in the experiment consists of fine-grained particles not coarser than 10 mm; the proportion of such particles is about 80 wt.%. The share of sorption-active clay minerals—vermiculite and lizardite in this material (VL) is ~20 wt.% (~10 wt.% for each mineral).
The initial raw material is represented by a material containing pyroxenites and olivine–pyroxene rocks, alkaline rocks similar in composition to ijolites, and brown, ferruginous fragments of loose material (iddingsite), mainly in the form of intergrowths with lizardite and vermiculite [17].
Detailed mineralogical and technological studies have established that the main mineral phases in the composition of VL are (wt. %) as follows: lizardite: ~10, vermiculite: ~13, and sum of pyroxene and olivine: ~50. They also contain about 12% iddingsite, which is a microcrystalline mixture of minerals developing on olivine and pyroxene, among which the serpentine mineral antigorite and iron oxides predominate. Titanomagnetite, feldspar, quartz, nepheline, calcite, apatite, and melilite are present in small quantities. Lizardite in VL is present in the form of cryptocrystalline, dense, porcelain-like masses, thin-flaky clusters, and fibrous-curved worm-like aggregates. In classes larger than 3.0–5.0 mm, it is usually found in the form of veins (from 1–2 to 8–10 mm), smears, and spots of cream or light-yellow color against the background of brown iddingsited rocks. Also characteristic are fragments in which lizardite is a “matrix” containing relics of pyroxene, olivine, vermiculite, and titanomagnetite. Rare free grains of lizardite appear at a size of 3 mm; with a decrease in size, the proportion of exposed lizardite slowly increases. A characteristic feature of lizardite in VL is the presence of numerous intergrowths with vermiculite and inclusions of olivine, pyroxene, and other minerals, which limit the production of high-purity products at an acceptable cost level. At the same time, the use of lizardite-containing products as materials for environmental protection is limited only by safety requirements (i.e., the absence of toxic components) and a sufficient level of active components.
2.2. Design of Experiment
The study was conducted in 1.5 km from the operating non-ferrous plant in the middle of Kola peninsula, NE Europe (67.940050° N, 32.838117° E) (Figure 1a,c). Soils were represented by two types typical for the region: Podzol (zonal) and Histosol (intrazonal). However, soils there were extremely disturbed due to the long-term aerial–technogenic pollution and erosion and therefore were defined as Skeletic Leptic Entic Podzol (Arenic, Toxic) and Dystric Rheic Hemic Histosol (Toxic) soil correspondingly [18].
Two experimental sites located on the 25 m distance from each other were deployed in this study. In both sites, we constructed a 5 cm thick mineral layer from the VL material directly on the soil surface, which was pre-leveled and cleared of large stones. Then, we sowed seeds in this mineral layer and covered them with an additional 0.5 cm thick waste layer.
Experimental site 1 was organized in June 2022 in the soil catena with a 1.6 m difference in altitude to infer the suitability of the overburdened waste bulk layer to be a geochemical barrier in a wide range of soil humidities. We manually created three plots with 15, 25, and 17 m length here. For each, we deployed two 30 cm wide parallel zigzag strips with a distance of 30 cm between them. Then, we sowed seeds of Lolium multiflorum Lam. (Figure 1d).
Experimental site 2 was organized in October 2021 to test a plant assortment perspective for the revegetation of industrial barren under subarctic climatic conditions. This area is characterized by a flat surface belonging to the top of an elevated topographic form. Twenty-nine plots, 30 × 30 cm each, organized in four rows were deployed for sowing seeds (Figure 1e). The assortment included seeds of native plant species collected in July–September 2021 in the Murmansk Region (Figure 1a), as well as commercial seeds of species potentially suitable for preparing the reclamation mixtures. In June 2022, we planted the clumps of native plants in places where seeds could not sprout in the spring. In 2023, no overseeding or planting was carried out.
2.3. Methods of Analyses
The mineral material of alkaline barriers was sampled in September 2023–2024 using a plastic ring with diameter of 10 cm to a depth of 5 cm (total volume of each sample was 0.4 L). In parallel, the volumetric moisture content was measured using an SM 150 Kit soil moisture sensor (Delta-T Devices, Cambridge, UK) (n = 3). Then, the samples were transported to the lab, air-dried, homogenized, and 2 mm sieved.
The total element content was analyzed using a SciAps X-550 X-ray fluorescence analyzer (Andover, MA, USA) (Geochemistry mode) after calibration using the A1316 steel coupon provided by the manufacturer. Further, the content of mobile metal fractions was analyzed using 0.01N and 0.1N HCl for metal extraction, as described in [19]. The integral description of fraction labels used in the current paper is presented in Table 1.

Table 1.
Labels and conditions for determining the metal fractions.
The concentration of elements in the solution was determined via the ICP AES method using ICPE-9000 (Shimadzu, Kyoto, Japan). Quality was controlled by analyzing the standard samples CRM-SOIL-A (Certified Reference Material Soil Solution A) and ICP Calibration Standard Surface Water IV-STOCK-10 (Table 2). For all samples, the standard error did not exceed 5%.

Table 2.
Measurement conditions for ICPE-9000.
The exchangeable ammonium content was determined after soil sample treatment with 1M KCl at soil: solution ratio 1:2.5 by a semi-quantitative colorimetric method in accordance with the State Standard [20].
The sorption properties of samples of monomineral lizardite (LK) and vermiculite (VK), as well as a material isolated from vermiculite–lizardite (VL) conglomerates by crushing them to the particle size of 2–5 mm, were studied in a laboratory experiment. Cu and Ni solutions with a concentration of 10 and 100 mg·L−1 were prepared using reagents—Cu and Ni sulfate crystal hydrates of chemically pure grade. Sorbents were added to the solutions in an amount of 10 g·L−1 and kept for 30 days to achieve chemical equilibrium. Then, the pH of the obtained suspensions was determined, suspensions were filtered, and solutions were analyzed using ICPE-9000.
Names of plant species are listed according to https://www.worldfloraonline.org/ (accessed on 20 March 2025). Life forms are briefly characterized according to the Raunkiær system [21,22] and the Serebryakov system [23,24,25]. Quantitative topographic information was derived from a very-high-resolution digital terrain model (DTM) that resulted from the stereo-photogrammetric processing of uncrewed aerial vehicle (UAV) survey data (2020) [16]. Statistical analysis was performed using Microsoft Excel 2016.
3. Results
3.1. Functioning of Sorption Alkaline Barrier
3.1.1. Wetness Conditions
The TWI is a derivative from the elevation, quantitatively characterizing the surface to be more or less prone for accumulating runoff [26]. This index is unitless and substantially differed in the range of 4–16 unitless within the experimental site (Figure 2b). Soil moisture at sampling points varied from 15 to 70 vol.% and corresponded to TWI values (Figure 2c).
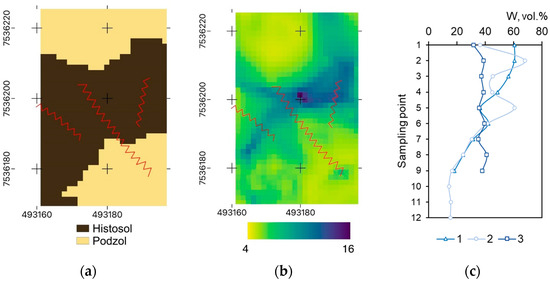
Figure 2.
Location of experimental area 1 within the units of predominant soil types (a), TWI map (b), and soil humidity (c). Note: (a,b): coordinates are given in Northing and Easting values (m), and the projection of spatial data is UTM Zone 36N; red lines indicate the location of strips. Numbers of strips are given from left to right.
The moisture of artificially created mineral strips reflects the variations in TWI conditions of the area. Thus, plot 3 with TWI 6–8 had the least differentiation in the soil moisture (32–41 vol.%). Plot 1 with wider TWI (4–10) had the wider range of soil moisture—18–61 vol.% as expected. The soil moisture of plot 2, which is located both on Podzol and Histosol soils, had a clear gradation into three zones: on moist peaty soil (40–60 vol.%), transitional from peat to Podzol (20–40 vol.%), and Podzol (less than 20 vol.%).
3.1.2. Total Content of Macronutrients and Heavy Metals
The concentrations of a major macronutrients in the initial mineral material were 12.6 wt.% Mg and 1.6 wt.% Ca. During the experiment, their total contents changed insignificantly: 11.0–14.3 wt.% for Mg and 1.1–2.0 wt.% for Ca (Figure 3a). This result indicates the stability of the Ca and Mg content in the bulk layer over two years. The K and P content in the initial VL was 1.7 and 2.4 g·kg−1, correspondingly. During the experiment, minor fluctuations in the K content (1.2–2.7 g·kg−1, mean 1.5 g·kg−1)) were observed in the samples, while the P content decreased (1.1–3.5 g·kg−1, mean 1.4 g·kg−1), which might be particularly associated with the plant nutrition.
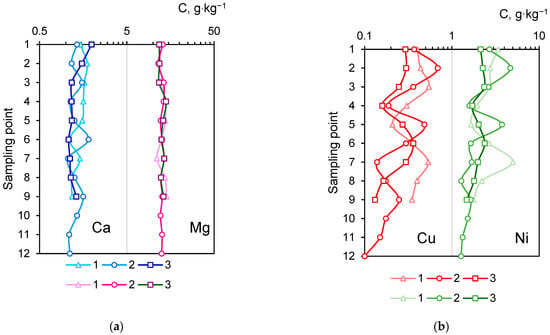
Figure 3.
The bulk concentrations of chemical elements (Ca, Mg (a), Cu, Ni (b) in the second and third summer seasons. Sampling points are shown on Figure 1c.
The VL bulk layer has shown to be an artificial geochemical barrier for the accumulation of heavy metals (Cu and Ni). The soil concentration of Cu and Ni varied significantly depending on the location of the strip on the experimental site. The highest contents were observed in the moistest Histosol areas. Plot 1 showed the highest accumulation of toxic elements with concentrations of 0.21–0.54 g·kg−1 for Cu and 1.66–5 g·kg−1 for Ni (Figure 3b). In the second plot, the Cu content ranged from 0.09 to 0.69 g·kg−1, and Ni ranged from 1.28 to 4.68 g·kg−1. We emphasize that the element content varied depending on the soil type: at points 2/10–2/12, located on Podzols, we have found the lowest metal concentrations. The Cu concentration in the third plot ranged from 0.13 to 0.36 g·kg−1, and Ni ranged from 1.34 to 3.24 g·kg−1.
3.1.3. Metal Accumulation in Sorption Alkaline Barrier
The total metal content in the initial VL was 30 ± 1.5 and 600 ± 20 mg·kg−1 for Cu and Ni, respectively. Serpentine minerals and vermiculite are known as natural Ni concentrators [27,28]. Thus, in the initial VL, the total Ni content is about 600 ppm, which is an order of magnitude higher than the crustal abundance (Clarke) of this chemical element [29]. The Cu content in the initial VL is 30 ppm, which is close to the Clarke. The accumulation of metals in the mineral layer over two years of the experiment was 45–380 ppm for Cu and 40–1250 ppm for Ni.
The sorption properties of all studied materials in such parameters as sorption capacity and purification degree were similar (Table 3). The pH values of suspensions in experiments with lizardite varied from 8.0 to 8.3, with vermiculite: 4.5–8.1 and VL: 6.5–7.6. The purification degree was 97–100% for 10 mg·L−1 solution and 77–100% for 100 mg·L−1 solution. Between studied materials, lizardite had the highest neutralizing capacity and the highest purification degree (98–100%). The obtained data characterize VL as a material with alkaline and sorption properties.

Table 3.
Results of experiments on Cu and Ni sorption.
In the field experiment, we revealed that metal accumulation is affected by the location of the sampling point in the mesorelief (Figure 4a). In depressions with prolonged contact of VL material with the polluted Histosol, metals from the soil solution interact with the VL components to a greater extent compared to areas of less contaminated Podzol on the hill. For the entire set of soil samples, a linear correlation is observed between VL humidity and metal accumulation, with a higher approximation coefficient for Ni (R2 = 0.73) compared to Cu (R2 = 0.60). Such a correlation is quite high for the conditions of the field experiment with uncontrolled metal input from air and snow cover. Plot 2 is characterized by a wider change in humidity compared to the plots 1 and 3. The data for the plot 2 revealed a closer correlation between humidity and metal accumulation (Figure 4a).
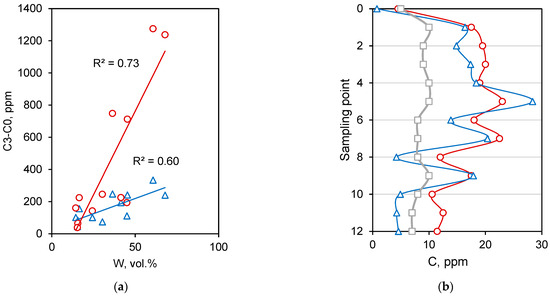
Figure 4.
Effect of humidity on accumulation of metals in plot 2 (a); change in the content of mobile form of metals and exchangeable ammonium along the plot 2 (b). Legend: ∆ Cu, ○ Ni, □ NH4+. Note: Point 0 is the content of chemical compounds in initial VL material.
An important characteristic of the geochemical barrier is its ability to retain toxic components when external conditions change. Two metal fractions were determined: mobile (C1) and sum of mobile and potentially mobile (C2). The influence of mesorelief on the metal accumulation is reflected in the separation of plot 2 into the depression zone with Histosol soil (points 1–5), a transition zone (6–9) and the elevation zone with Podzol soil (10–12) (Figure 4b). The first zone is characterized by stable high concentrations of the metal mobile fraction and also by higher exchangeable ammonium, which is due to its high content in Histosol (60 ppm).
The dependence of the content of mobile fractions on the total metal accumulation differed for Cu and Ni (Figure 5). In the recorded concentration range, the content of Cu forms increases linearly with an increase in its accumulation in VL material. The content of Ni fractions able to migrate stabilized with the accumulation above 500 ppm. Probably, at this sorption level, Ni enters the structure of minerals and does not pass into solution under the conditions in which migration fractions were determined.
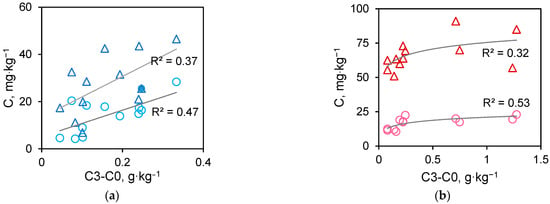
Figure 5.
The relationship between the total metal accumulation C3-C0 and the mobile C1 (○) or potentially mobile C2-C1 (∆) metal fractions for Cu (a) and Ni (b).
The mobility of toxic components is estimated based on two migration coefficients. The coefficient of short-term migration K1 was calculated as the ratio of the mobile and potentially mobile fractions, and the coefficient of potential migration K2 was calculated as the ratio of the sum of mobile fractions to the total metal accumulation (Table 4). Cu and Ni differed insignificantly in K2, while the short-term Cu were more mobile than Ni. It should be noted that the ability of VL material to fix Ni is its significant advantage, since Ni is more toxic than Cu [30].

Table 4.
Copper and nickel migration coefficients.
3.2. Plant Development on Sorption Alkaline Barrier
3.2.1. The TWI Effect on the Development of Lolium Multiflorum Lam
We have revealed that differences in the topography and soil moisture were the cause of different rates of L. multiflorum development in plot 2 (Figure 6). Thus, at the first season of the experiment, the plant growth was uniform with the large-scale formation of ears in August 2022. In the following summer seasons of 2023–2024 years, the higher soil moisture accelerated the growth of vegetative shoots in the spring and earlier drying out in the autumn. The biomass of aboveground parts of plants also depended on the TWI.

Figure 6.
The overview of experimental site 1 in June 2022 (a), September 2023 (b), and July 2024 (c).
3.2.2. The Selection of a Plant Assortment for Remediation
The developments of 29 species and one sub-species of plants from seven families were studied for three summer seasons (Table 5, Figure 7a).

Table 5.
Development of herb and grass plants in three seasons of the experiment.


Figure 7.
The overview of experimental site 2 in July 2023 (a) and plants from perspective assortment for revegetation of industrial wasteland: Bromopsis inermis Leyss (b), Dactylis glomerata L. (c), Festuca ovina L. (d), Festuca rubra L. (e), Achillea millefolium L. (f), Rumex acetosella L. (g), colonization of polluted soil of industrial barren by Silene suecica (Lodd.) Greuter & Burdet (h).
Commercial Species
The most effective for the landscaping were commercial seeds of species found in the flora of the region: Festuca ovina L., F. rubra L., Bromopsis inermis Leyss, and Dactylis glomerata L (Figure 7b–e). Representatives of the genus Festuca are widespread in all natural zones of the region and grow well on dry, light, and sandy soils. They have also demonstrated a high percentage of vegetative mass growth and seed maturation within the experiment. B. inermis gained vegetative mass well but did not bear fruit throughout the experiment. Most species of the Gramineae were in the vegetative phase, which may be due to their mass consumption by mammals. The most actively consumed vegetative organs belong to D. glomerata and Phleum pratense L. These species have therefore a low potential for seed dissemination in the local area around the plant. On the other hand, these species have high biomass and therefore are promising as a food source for animals that live in the area adjacent to industrial barren. Representatives of the Fabaceae have demonstrated low germination rates: Melilotus albus Medik. did not survive the winter, and Trifolium pratense L. had a low survival rate.
Native Species
Achillea millefolium L., Rumex acetosa L., and Silene suecica (Lodd.) Greuter & Burdet (all have shown good germination and vegetative mass gain among the native seeds (Figure 7f,g). A. millefolium has produced only the vegetative mass without generative organs. Despite the good results in monospecific grass stand and annual ripening of a large number of seeds under the disturbed substrates condition, S. suecica could not be recommended for use in grass mixtures, as it prefers unsodded spaces (Figure 7h). Representatives of the Caryophyllaceae have easily occupied open spaces and relatively large areas on dry, light substrates in the Murmansk Region. R. acetosa has proven to be resistant to insufficient moisture and has produced seeds. The biennial plant Plantago major L. has successfully germinated in the second growing season, although seeds did not sprout after winter. Among the native grasses, Deschampsia cespitosa (L.) P. Beauv. and Agrostis capillaris L. were the most promising grasses. D. cespitosa is rarely found in the assortment of commercial seeds but actively gains vegetative mass and produces seeds. A. capillaris has shown active seed maturation without a significant gain in vegetative mass.
4. Discussion
A two-year experiment studying the effect of the TWI on the functioning of a mineral layer as sorption alkaline geochemical barrier has shown that this layer has accumulated both major pollutants—Cu and Ni. We have found a relationship between the TWI and accumulation of heavy metals by artificial layer: the higher the wetness, the higher the metal accumulation. The sorption properties of the material used in the experiment are determined by the presence of layered silicates—vermiculite and the lizardite (serpentine-group mineral). Clay minerals act as ion-exchangers for heavy metals, and therefore materials containing them can be widely used for the remediation of metal-polluted soils due to their low cost, high abundance, easy manipulation, and harmlessness to the environment [31]. Ni-bearing clay minerals originate either from transformation of primary lizardite or from solution precipitation in cracks [32]. Despite its slow weathering rate, serpentine may buffer the acidity [33], which facilitates the precipitation of poorly soluble Cu compounds. Fine vermiculite particles form a clay-sized fraction of soil. Vermiculite participates in soil formation processes, fixing organic carbon [14,34] and when lizardite neutralizes excess acidity of soil solutions and atmospheric precipitation [35].
The conceptual scheme of functioning the alkaline sorption barriers on experimental plot 1 (Figure 8) shows the primary ways of toxic metals’ input and accumulation depending on relief position: deposition from air (industrial emissions and wind soil erosion) for all zones combined with input due to lateral flow (transition and accumulation zones) and immobilization from water-saturated Histosol in the lowest relief position.
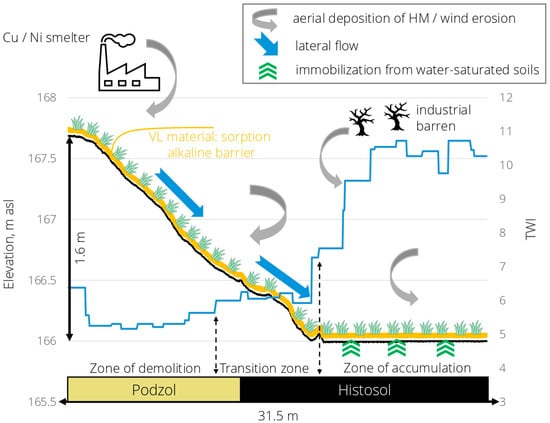
Figure 8.
The conceptual scheme of functioning the alkaline sorption barrier from VL material on industrial barren.
An important result of this study is the shown stability of Ca and Mg content throughout the study period. This content did not depend on the soil moisture, which indicates their slow dissolution potential and, hence, a prolonged maintenance of the alkalinity level necessary for the metal deposition, as well as the possibility of long-term use by plants as a source of macronutrients. The use of substrates as ameliorants requires limiting the particle size to 20 mm. Data on the granulometric composition of a representative VL sample show that only 10% of the material does not meet this requirement [18]. Thus, the allocation of a fraction of less than 20 mm will make it possible to obtain an ameliorant for phytoremediation, while the degree of waste utilization will be approximately 90%.
Even in the local experimental area, the TWI affected the plant development significantly. Results of a three-season experiment on the development of plants from commercial and native seeds on VL material under low soil moisture conditions have shown that the choice of plant assortment is an extremely important part of the reclamation strategy. In conditions of elevated topography covered by dry sandy soils, F. rubra, F. ovina, A. millefolium, D. cespitosa, D. glomerata, R. acetosella, and S. suecica have been found to be most promising for the creation of sustainable plant communities from native flora. Outside of grass mixtures, native S. suecica is also a promising plant due to its active regeneration and ability to grow in conditions of elevated copper concentrations in soils [36,37]. At the same time, long-term observations of plants growing in a single-species experiment have shown that the composition of plant communities for the reclamation of industrial barren should be developed based on both the general requirements of species for soil conditions and moisture and competitive relationships between species.
B. inermis is found in meadow and riverine communities in the south of the region and in disturbed areas up to the Rybachy Peninsula [38]. Despite this, it should be used with caution for reclamation in grass mixtures: B. inermis was used as a component of a grass mixture (1/9 part, together with F. rubra, F. pratensis and x Festulolium) in the experiment started in 2010 at the Podzol site near the current experiment location, where it ultimately formed a dense single-component turf.
The use of mixtures of different grass species is a more sustainable and efficient method for growing plants, which will reduce the risks associated with monoculture cultivation. The productivity of a polyculture consisting of complementary crops is usually higher than that of the most productive component in a monoculture [39,40]. Sowing perennial grass mixtures of Fabaceae and Gramineae crops is an effective way to restore disturbed soils, as they improve the physicochemical properties, nutrient regime, aeration and water permeability, increase the resistance to soil pollution, and provide an anti-erosion effect due to the developed root system and powerful grass stand, nitrogen fixation, large mass of plant cover, etc. [41]. The use of species from the native flora to create grass mixtures is also important, as they have high adaptability to climatic and soil conditions, which led to the formation of more sustainable plant communities in harsh technogenic environments [42]. However, in our experiment, Fabaceae had a low survival rate even in monospecific sowing. In this regard, it is necessary to continue research on the selection of seed material capable of reproduction in the Subarctic conditions.
In our research, we created a barrier with a thickness of 5 cm. The VL materials have a high alkalinity, which gives hope for a prolonged effect for decades. Our previous studies prove these expectations [14]. But we assume that the thickness rise could raise the metal accumulation on the areas with high TWI. Therefore, our future research directions will include the study of improving the barrier efficiency for the metal accumulation by increasing the thickness of the bulk layer on Histosol sites. The selection of hot spots for reclamation based on remotely sensed (UAV) TWI is a promising approach to localizing pollution in the area of industrially disturbed ecosystems.
5. Conclusions
A three-seasons field experiment of the creation the alkaline sorption barrier from vermiculite–lizardite mineral material from overburden rocks on the metal-polluted area under the humid climate of the Subarctic has demonstrated the prolonged effect of the mineral material containing layered silicates (vermiculite and lizardite) for the reclamation of a local site of industrial barren with automorphic and hydromorphic soils and a wide range of the TWI.
The main results of the experiment are as follows:
- (1)
- The content of macronutrients (Ca, Mg, N-NH4) was stable after 2 years of the field experiment and were not affected by TWI;
- (2)
- HMs were accumulated in the vermiculite–lizardite mineral material, and TWI was the primary factor that determined the values of HMs’ accumulation and mobility in this mineral material, as well as the rate of onset of phenological phases in the second and third vegetation seasons;
- (3)
- TWI is crucial factor of plant development on alkaline mineral material in dry locations; seven species (Festuca rubra, Festuca ovina, Achillea millefolium, Deschampsia cespitosa, Dactylis glomerata, Rumex acetosella, and Silene suecica) from native and commercial seeds can be recommended for the plant cover’ creation in such conditions.
These studies are important for the development of cost-effective technology for greening areas with low self-restoration rate.
Author Contributions
Conceptualization, M.S., Y.D., L.I. and I.K.; methodology, Y.D. and I.K.; software, Y.D.; validation, T.I. and A.P.; formal analysis, A.S., A.N. and I.K.; investigation, M.S., T.I., A.P., A.S. and A.N.; resources, A.N., I.K. and M.S.; data curation, A.S. and Y.D.; writing—original draft preparation, M.S., E.K., A.P. and I.K.; writing—review and editing, T.I., Y.D. and L.I.; visualization, Y.D., A.P. and I.K.; supervision, Y.D.; project administration, L.I. and I.K.; funding acquisition, M.S. All authors have read and agreed to the published version of the manuscript.
Funding
Chemical analyses and manuscript preparation was funded by Russian Science Foundation, grant number 24-77-10055. Spatial analysis and mapping were performed in accordance with the governmental assignment FSSF-2024-0023. Work of Yury Dvornikov is supported by RUDN University Strategic Academic Leadership Program.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
We thank Nikita, Alexander and Serafim Nifakin for their hard work on the formation of experimental sites, Viktoria Maksimova–for the seed collection in Kandalaksha area. We also thank Olga Korytnaya for the support with lab analyses.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| TWI | Topographic wetness index |
| VL | Vermiculite–lizardite material |
| UAV | Uncrewed aerial vehicle |
References
- Cui, W.; Li, X.; Duan, W.; Xie, M.; Dong, X. Heavy metal stabilization remediation in polluted soils with stabilizing materials: A review. Environ. Geochem. Health 2023, 45, 4127–4163. [Google Scholar] [CrossRef] [PubMed]
- Kumpiene, J.; Lagerkvist, A.; Maurice, C. Stabilization of As, Cr, Cu, Pb and Zn in soil using amendments—A review. Waste Manag. 2008, 28, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.H.; Zhu, X.; Liu, H.C.; Wang, L.L.; Chen, J. Effect of inorganic amendments on the stabilization of heavy metals in contaminated soils. Huan Jing Ke Xue 2013, 34, 3722–3726. [Google Scholar]
- Huang, L.M.; Yu, G.W.; Cai, X.; Long, X.X. Immobilization of Pb, Cd, Cu and Zn in a Multi-Metal Contaminated Acidic Soil using Inorganic Amendment Mixtures. Int. J. Environ. Res. 2017, 11, 425–437. [Google Scholar] [CrossRef]
- Sun, Y.; Zhao, D.; Xu, Y.; Wang, L.; Liang, X.; Shen, Y. Effects of sepiolite on stabilization remediation of heavy metal-contaminated soil and its ecological evaluation. Front. Environ. Sci. Eng. 2016, 10, 85–92. [Google Scholar] [CrossRef]
- Malandrino, M.; Abollino, O.; Buoso, S.; Giacomino, A.; La Gioia, C.; Mentasti, E. Accumulation of heavy metals from contaminated soil to plants and evaluation of soil remediation by vermiculite. Chemosphere 2011, 82, 169–178. [Google Scholar] [CrossRef]
- Lan, M.M.; Liu, C.; Liu, S.J.; Qiu, R.L.; Tang, Y.T. Phytostabilization of Cd and Pb in highly polluted farmland soils using ramie and amendments. Int. J. Environ. Res. Public Health 2020, 17, 1661. [Google Scholar] [CrossRef] [PubMed]
- Vodyanitskii, Y.N. Artificial permeable redox barriers for purification of soil and ground water: A review of publications. Eurasian Soil Sci. 2014, 47, 1058–1068. [Google Scholar] [CrossRef]
- Kozlov, M.V.; Zvereva, E.L. Industrial barrens: Extreme habitats created by non-ferrous metallurgy. Rev. Environ. Sci. Biotechnol. 2007, 6, 231–259. [Google Scholar] [CrossRef]
- Barcan, V. Leaching of nickel and copper from soil contaminated by metallurgical dust. Environ. Int. 2002, 28, 63–68. [Google Scholar] [CrossRef]
- Kashulina, G.M. Extreme pollution of soils by emissions of the copper–nickel industrial complex in the Kola Peninsula. Eurasian Soil Sci. 2017, 50, 837–849. [Google Scholar] [CrossRef]
- Koptsik, G.N. Problems and prospects concerning the phytoremediation of heavy metal polluted soils: A review. Eurasian Soil Sci. 2014, 47, 923–939. [Google Scholar] [CrossRef]
- Koptsik, G.N.; Koptsik, S.V.; Smirnova, I.E.; Sinichkina, M.A. Remediation of Technogenic Barren Soils in the Kola Subarctic: Current State and Long-Term Dynamics. Eurasian Soil Sci. 2021, 54, 619–630. [Google Scholar] [CrossRef]
- Slukovskaya, M.V.; Vasenev, V.I.; Ivashchenko, K.V.; Dolgikh, A.V.; Novikov, A.I.; Kremenetskaya, I.P.; Ivanova, L.A.; Gubin, S.V. Organic matter accumulation by alkaline-constructed soils in heavily metal-polluted area of Sub-arctic zone. J. Soils Sediments 2021, 21, 2071–2088. [Google Scholar] [CrossRef]
- Kremenetskaya, I.P.; Alekseeva, S.A.; Slukovskaya, M.V.; Mosendz, I.A.; Drogobuzhskaya, S.V.; Ivanova, L.A. Expanded vermiculite-reached product obtained from mining waste: The effect of roasting temperature on the agronomic properties. Physicochem. Probl. Miner. Process. 2020, 56, 102–112. [Google Scholar] [CrossRef]
- Dvornikov, Y.A.; Slukovskaya, M.V.; Yaroslavtsev, A.M.; Meshalkina, J.L.; Sarzhanov, D.A.; Vasenev, V.I. High-resolution mapping of soil pollution by Cu and Ni at a polar industrial barren area using proximal and remote sensing. Land Degrad. Dev. 2022, 33, 1731–1744. [Google Scholar] [CrossRef]
- Kremenetskaya, I.P.; Alekseeva, S.A.; Rukhlenko, E.D.; Lashchuk, V.V.; Bastrygina, S.V.; Ivanova, L.A.; Teresh-chenko, S.V. Materials of the Nature-Conservative Indentation from the Phlogopite Mining Waste. Ecol. Ind. Russ. 2015, 19, 18–23. Available online: https://www.ecology-kalvis.ru/jour/article/view/535 (accessed on 20 March 2025).
- Food and Agriculture Organization of the United Nations. Available online: https://www.fao.org/soils-portal/data-hub/soil-classification/world-reference-base/en/ (accessed on 20 March 2025).
- Slukovskaya, M.V.; Petrova, A.G.; Ivanova, L.A.; Ivanova, T.K.; Mosendz, I.A.; Novikov, A.I.; Kremenetskaya, I.P. Serpentine Overburden Products–Nature-Inspired Materials for Metal Detoxification in Industrially Polluted Soil. Toxics 2023, 11, 957. [Google Scholar] [CrossRef]
- Soils. Determination of Exchangeable Ammonium by CINAO Method. Available online: https://star-pro.ru/gost/26489-85 (accessed on 8 March 2025).
- Raunkiaer, C.C. Types biologiques pour la géographiebotanique. Bull. Acad. R. Sci. 1905, 5, 347–437. [Google Scholar]
- Raunkiaer, C.C. Plant Life Forms; Clarendon Press: Oxford, UK, 1937; p. 104. [Google Scholar]
- Serebryakov, I.G. Ecologiclal Morphology of Plants: Life Forms of Angiosperms and Conifers; Higher School: Moscow, Russia, 1962; p. 378. (In Russian) [Google Scholar]
- Zhmylev, P.Y.; Alekseev, Y.E.; Morozova, O.V. Diversity of Plant Life Forms in Moscow Region; Dubna State University: Dubna, Russia, 2017; p. 325. (In Russian) [Google Scholar]
- Sekretareva, N.A. Vascular plants of the Russian Arctic and adjacent territories; KMK Scientific Press Ltd.: Moscow, Russia, 2004; pp. 1–131. ISBN 978-954-642-069-5. (In Russian) [Google Scholar]
- Beven, K.J.; Kirkby, M.J. A physically based, variable contributing area model of basin hydrology/Un modèle à base physique de zone d’appel variable de l’hydrologie du bassin versant. Hydrol. Sci. Bull. 1979, 24, 43–69. [Google Scholar] [CrossRef]
- Brindley, G.W. The structure and chemistry of hydrous nickel-containing silicate and nickel-aluminium hydroxy minerals. Bull. Mineral. 1980, 103, 161–169. [Google Scholar] [CrossRef]
- Siebecker, M.G.; Chaney, R.L.; Sparks, D.L. Nickel speciation in several serpentine (ultramafic) topsoils via bulk synchrotron-based techniques. Geoderma 2017, 298, 35–45. [Google Scholar] [CrossRef]
- Hu, Z.; Gao, S. Upper crustal abundances of trace elements: A revision and update. Chem. Geol. 2008, 253, 205–221. [Google Scholar] [CrossRef]
- Tarasova, E.; Drogobuzhskaya, S.; Tapia-Pizarro, F.; Morev, D.V.; Brykov, V.A.; Dovletyarova, E.A.; Slukovskaya, M.V.; Navarro-Villarroel, C.; Paltseva, A.A.; Neaman, A. Vermiculite-lizardite industrial wastes promote plant growth in a peat soil affected by a Cu/Ni smelter: A Case Study at the Kola Peninsula, Russia. J. Soil Sci. Plant Nutr. 2020, 20, 1013–1018. [Google Scholar] [CrossRef]
- Raval, N.P.; Shah, P.U.; Shah, N.K. Adsorptive removal of nickel (II) ions from aqueous environment: A review. J Environ. Manag. 2016, 179, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Reddy, B.J.; Frost, R.L.; Dickfos, M.J. Characterisation of Ni silicate-bearing minerals by UV–vis–NIR spectroscopy Effect of Ni substitution in hydrous Ni–Mg silicates. Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 2009, 71, 1762–1768. [Google Scholar] [CrossRef]
- Heikkinen, P.M.; Räisänen, M.L. Mineralogical and geochemical alteration of Hiturasulphide mine tailings with emphasis on nickel mobility and retention. J. Geochem. Explor. 2008, 97, 1–20. [Google Scholar] [CrossRef]
- Kremenetskaya, I.; Vasilieva, T.; Semushin, V.; Slukovskaya, M.; Ivanova, I.; Chislov, M.; Zvereva, I.; Marchevskaya, V. Physicochemical transformation of expanded vermiculite after long-term use in hydroponics. Appl. Clay Sci. 2020, 198, 1–7. [Google Scholar] [CrossRef]
- Slukovskaya, M.V.; Kremenetskaya, I.P.; Mosendz, I.A.; Ivanova, T.K.; Drogobuzhskaya, S.V.; Ivanova, L.A.; Novikov, A.I.; Shirokaya, A.A. Thermally activated serpentine materials as soil additives for copper and nickel immobilization in highly polluted peat. Environ. Geochem. Health 2022, 45, 67–83. [Google Scholar] [CrossRef]
- Ivanova, L.A.; Slukovskaya, M.V.; Kremenetskaya, I.P.; Alekseeva, S.A.; Neaman, A.A. Ornamental plant cultivation using vermiculite-lizardite mining waste in the industrial zone of the Subarctic. In Proceedings of the Green Technologies and Infrastructure to Enhance Urban Ecosystem Services, Moscow, Russia, 23–26 May 2018; pp. 199–204. [Google Scholar] [CrossRef]
- Nagy, L. Biological flora of the British Isles: Silene suecica. J. Ecol. 2013, 101, 532–544. [Google Scholar] [CrossRef]
- Ramenskaya, M.L. Analysis of the Flora of the Murmansk Region and Karelia; Nauka Publishing House: Leningrad, Russia, 1983; p. 215. (In Russian) [Google Scholar]
- Willey, R.W. Intercropping—Its importance and research needs. I. Competition and yield advantages. Agronomy and Research Approaches. Field Crop Abstract 1979, 32, 1–10. [Google Scholar]
- Gebru, H. A review on the comparative advantages of intercropping to mono-cropping system. J. Biol. Agric. Healthc. 2015, 5, 1–13. [Google Scholar]
- Semahegn, Z. Intercropping of cereal with legume Crops. Int. J. Res. Agron. 2022, 5, 26–31. [Google Scholar] [CrossRef]
- Simmons, M.; Bertelsen, M.; Windhager, S.; Zafian, H. The performance of native and non-native turfgrass mono-cultures and native turfgrass polycultures: An ecological approach to sustainable lawns. Ecol. Eng. 2011, 37, 1095–1103. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).