Dynamics of Pharmaceuticals in the Soil–Plant System: A Case Study on Mycorrhizal Artichoke
Abstract
1. Introduction
2. Materials and Methods
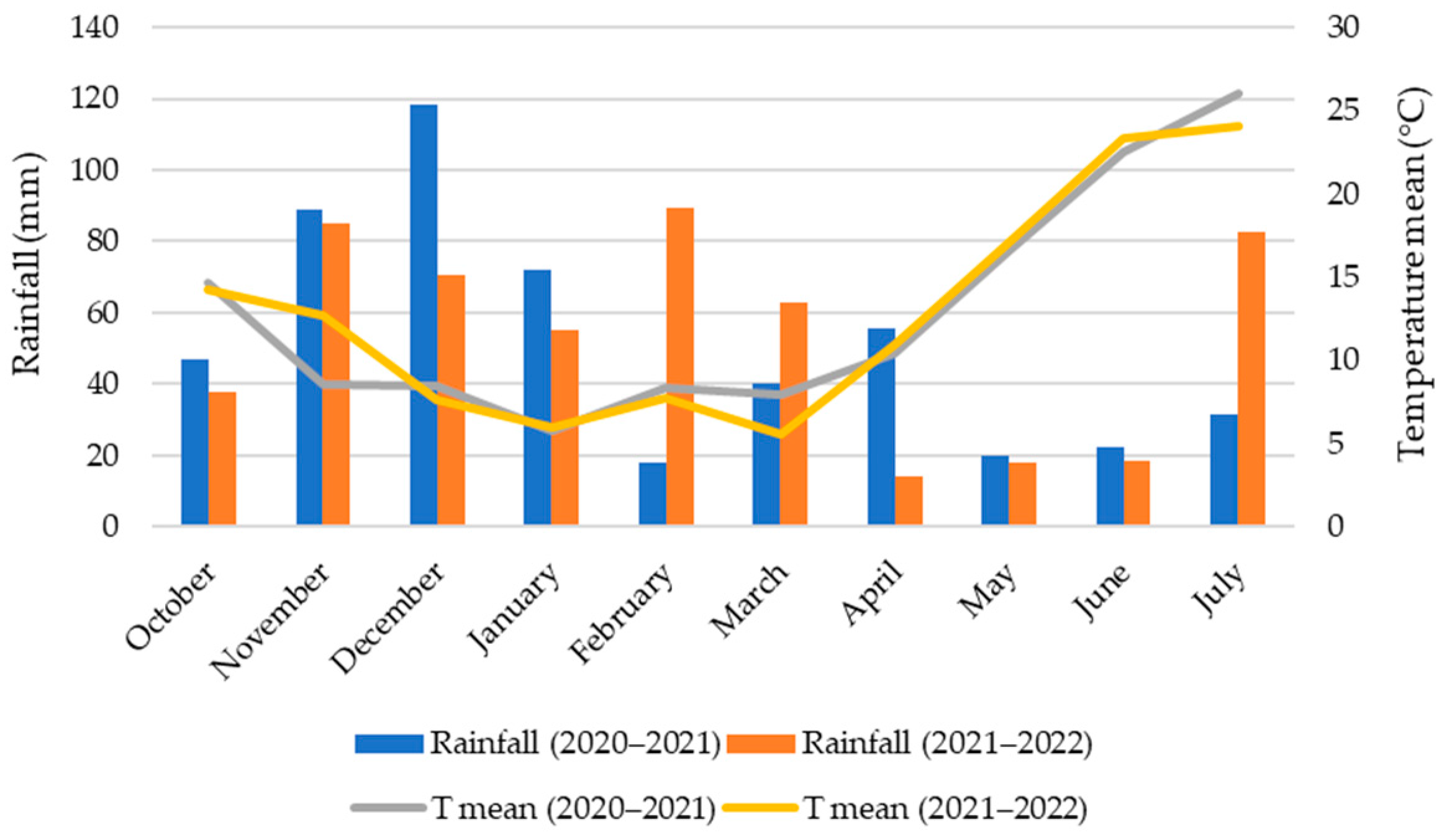
2.1. Site Description and Climate Conditions
2.2. Experimental Design and Crop Management
2.3. Water, Soil and Plant Sampling and Analysis
2.4. Pharmaceutical Active Compounds (PhACs) Uptake and Translocation Factors
2.5. Statistical Data Analysis
3. Results and Discussion
3.1. Assessment of Mycorrhizal Frequency
3.2. Characteristics of Soil and Water Used for Irrigation
3.2.1. Soil
3.2.2. Water
3.3. Pharmaceutical Active Compounds (PhACs) in Irrigation Water
3.4. Dynamics of the Pharmaceutical Active Compounds (PhACs) in the Soil–Plant System
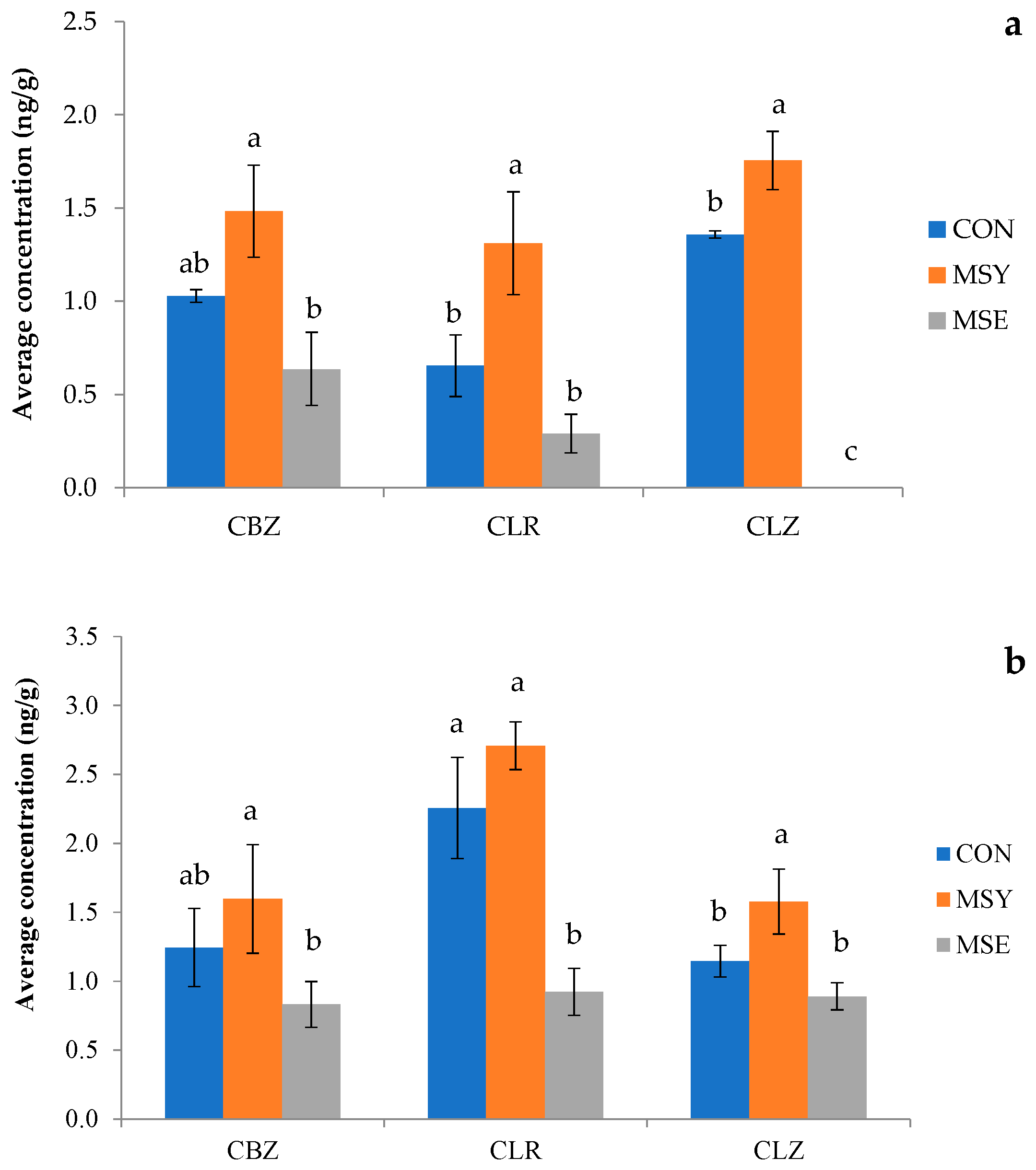
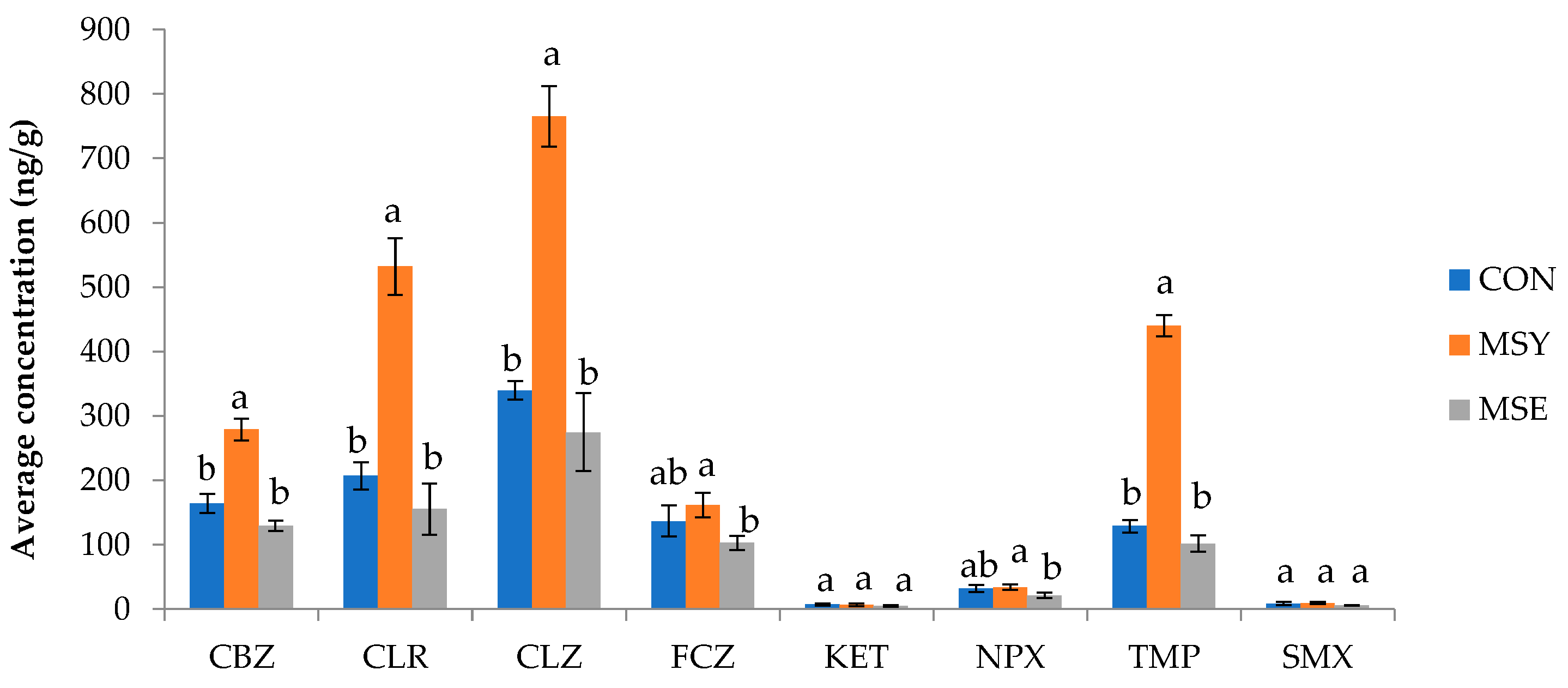
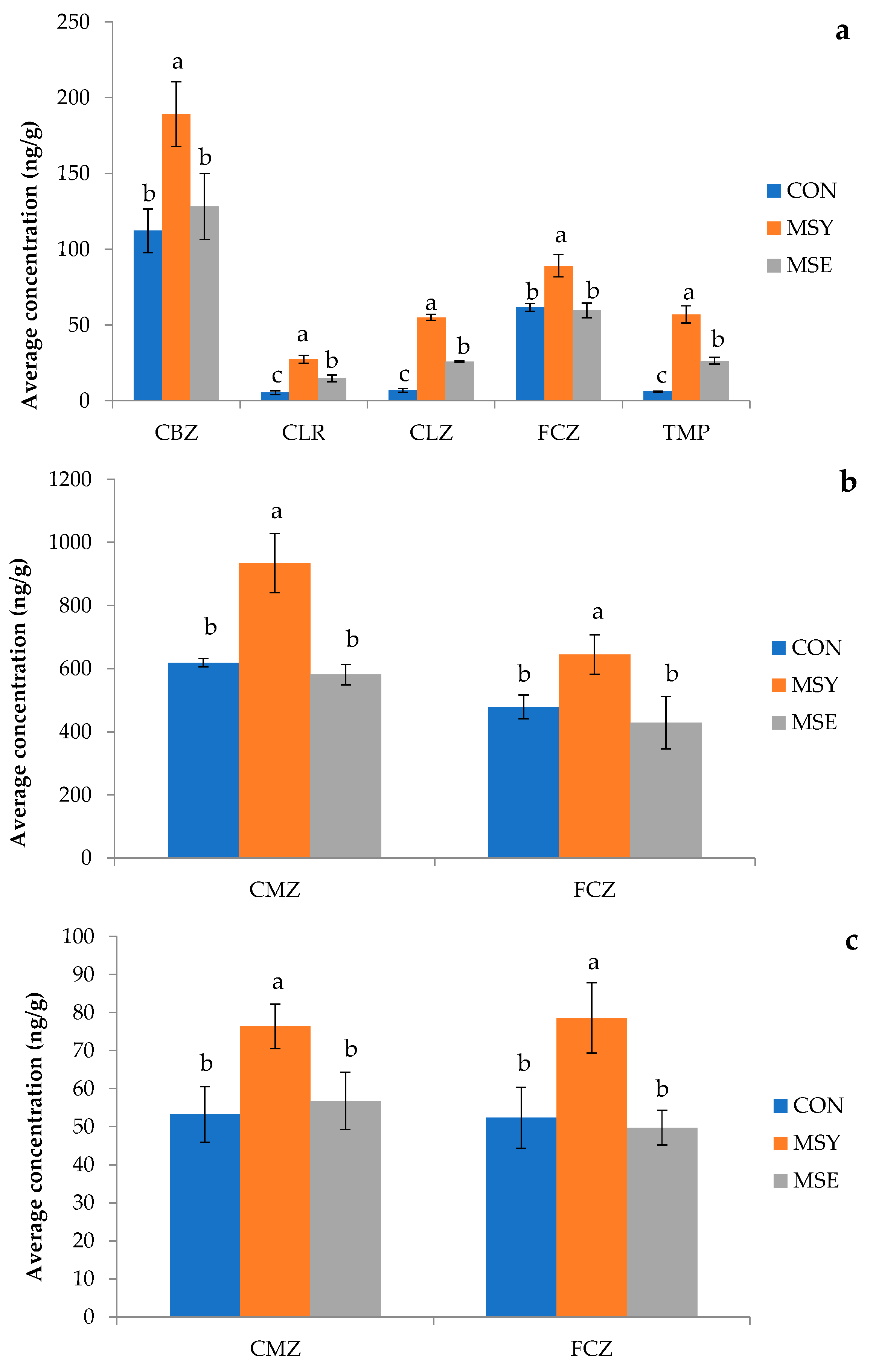
3.4.1. Pharmaceutical Active Compounds (PhACs) in Soil
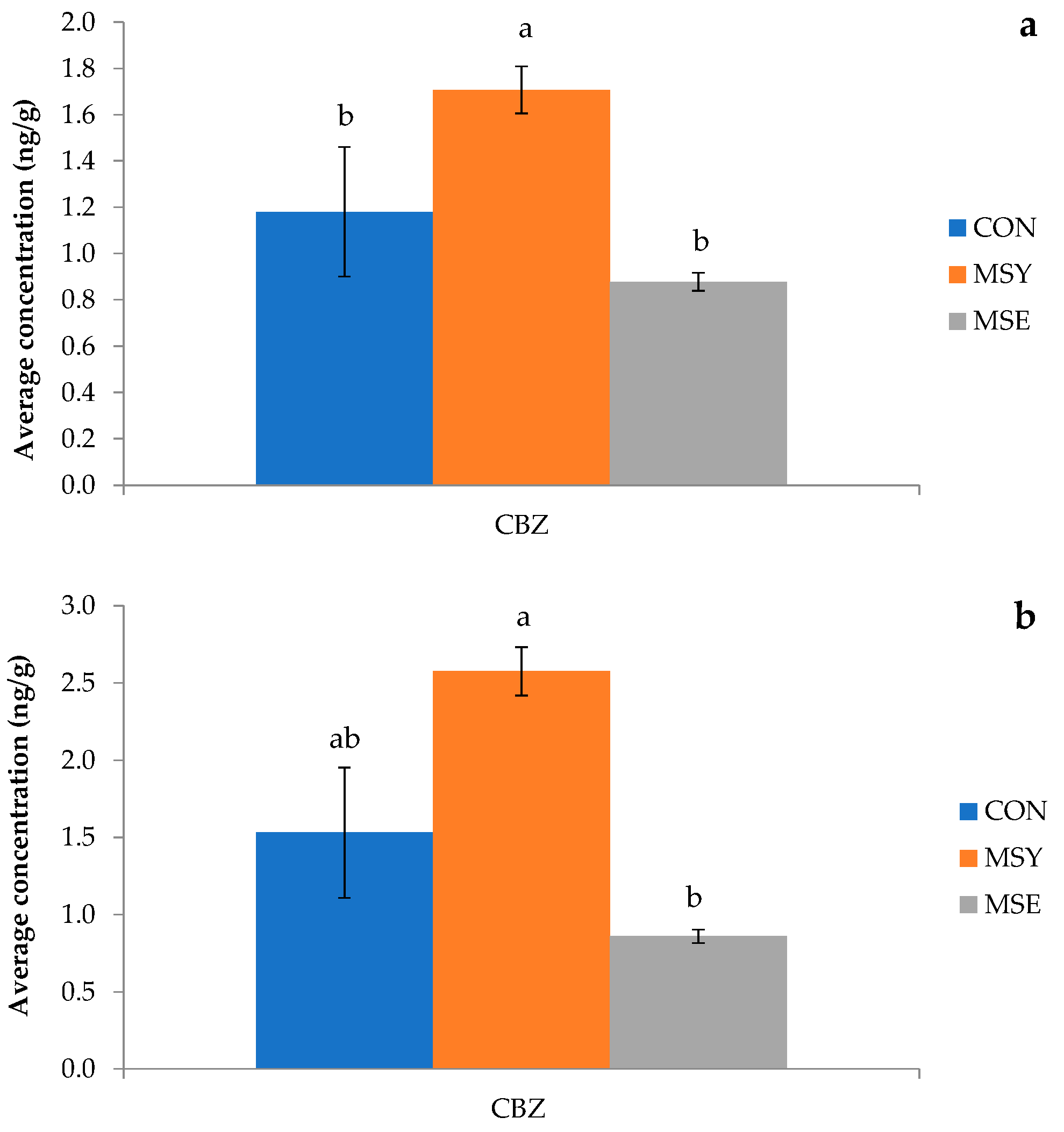
3.4.2. Pharmaceutical Active Compounds (PhACs) in Plant Organs
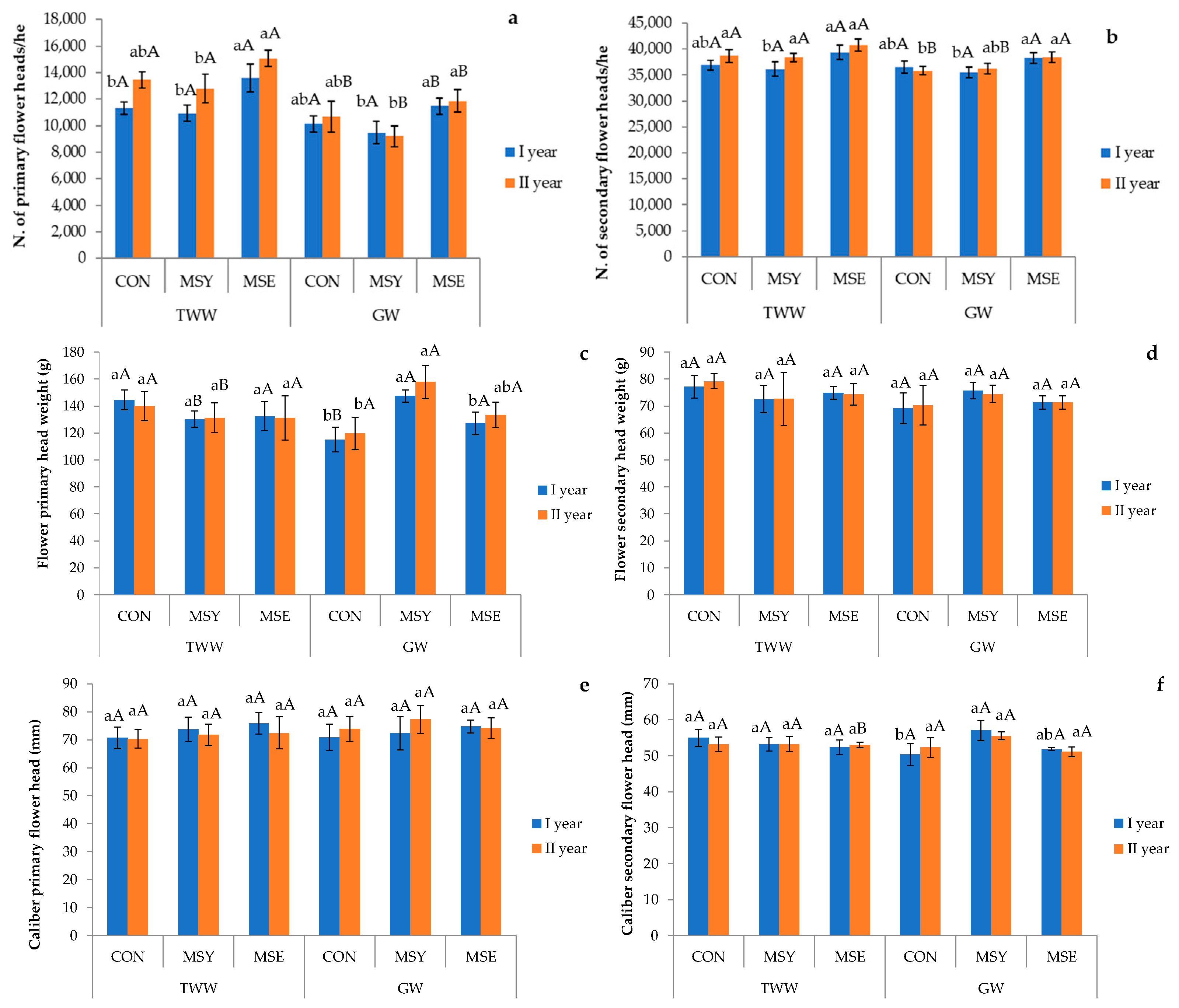
3.5. Artichoke Agronomic Parameters
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yahaya, S.M.; Mahmud, A.A.; Abdu, N. The Use of Wastewater for Irrigation: Pros and Cons for Human Health in Developing Countries. Total Environ. Res. Themes 2023, 6, 100044. [Google Scholar] [CrossRef]
- Karimidastenaei, Z.; Avellán, T.; Sadegh, M.; Kløve, B.; Haghighi, A.T. Unconventional Water Resources: Global Opportunities and Challenges. Sci. Total Environ. 2022, 827, 154429. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Regulation (EU) 2020/741 of the European Parliament and of the Council of 25 May 2020 on Minimum Requirements for Water Reuse. Off. J. Eur. Union 2020, 177, 32–55. [Google Scholar]
- Patel, M.; Kumar, R.; Kishor, K.; Mlsna, T.; Pittman Jr, C.U.; Mohan, D. Pharmaceuticals of Emerging Concern in Aquatic Systems: Chemistry, Occurrence, Effects, and Removal Methods. Chem. Rev. 2019, 119, 3510–3673. [Google Scholar] [CrossRef]
- Gallego, S.; Montemurro, N.; Béguet, J.; Rouard, N.; Philippot, L.; Pérez, S.; Martin-Laurent, F. Ecotoxicological Risk Assessment of Wastewater Irrigation on Soil Microorganisms: Fate and Impact of Wastewater-Borne Micropollutants in Lettuce-Soil System. Ecotoxicol. Environ. Saf. 2021, 223, 112595. [Google Scholar] [CrossRef]
- Akhter, S.; Bhat, M.A.; Hashem, A.; Abd_Allah, E.F.; Ahmed, S.; Siddiqi, W.A.; Kulsoom, I.; Un Nisa, F. Profiling of Antibiotic Residues in Soil and Vegetables Irrigated Using Pharmaceutical-Contaminated Water in the Delhi Stretch of the Yamuna River, India. Water 2023, 15, 4197. [Google Scholar] [CrossRef]
- Wei, H.; Tang, M.; Xu, X. Mechanism of Uptake, Accumulation, Transport, Metabolism and Phytotoxic Effects of Pharmaceuticals and Personal Care Products within Plants: A Review. Sci. Total Environ. 2023, 892, 164413. [Google Scholar] [CrossRef]
- Rottenberg, A. The Wild Gene Pool of Globe Artichoke. Israel J. Plant Sci. 2015, 62, 33–38. [Google Scholar] [CrossRef]
- Food and Agriculture Organisation of the United Nations. Crop Evapotranspiration: Guidelines for Computing Crop Water Requirements (FAO) 2013. Available online: http://faostat.fao.org (accessed on 20 November 2024).
- Bhaskaralingam, A.; Sharma, G.; Wang, T.; Kumar, A.; Dhiman, P.; Kumar, D.; Shi, H. Bioremediation of Pharmaceuticals Waste and Pesticides Using Various Microorganisms: A Review. Process Saf. Environ. Prot. 2025, 194, 1116–1132. [Google Scholar] [CrossRef]
- Khan, M.F. Fungi for Sustainable Pharmaceutical Remediation: Enzymatic Innovations, Challenges, and Applications—A Review. Processes 2025, 13, 1034. [Google Scholar] [CrossRef]
- Bowles, T.M.; Barrios-Masias, F.H.; Carlisle, E.A.; Cavagnaro, T.R.; Jackson, L.E. Effects of Arbuscular Mycorrhizae on Tomato Yield, Nutrient Uptake, Water Relations, and Soil Carbon Dynamics under Deficit Irrigation in Field Conditions. Sci. Total Environ. 2016, 566–567, 1223–1234. [Google Scholar] [CrossRef] [PubMed]
- Rouphael, Y.; Franken, P.; Schneider, C.; Schwarz, D.; Giovannetti, M.; Agnolucci, M.; De Pascale, S.; Bonini, P.; Colla, G. Arbuscular Mycorrhizal Fungi Act as Biostimulants in Horticultural Crops. Sci. Hortic. 2015, 196, 91–108. [Google Scholar] [CrossRef]
- Bona, E.; Lingua, G.; Todeschini, V. Effect of Bioinoculants on the Quality of Crops. In Bioformulations: For Sustainable Agriculture; Arora, N.K., Mehnaz, S., Balestrini, R., Eds.; Springer: New Delhi, India, 2016; pp. 93–124. [Google Scholar] [CrossRef]
- Noceto, P.A.; Bettenfeld, P.; Boussageon, R.; Hériché, M.; Sportes, A.; van Tuinen, D.; Courty, P.E.; Wipf, D. Arbuscular Mycorrhizal Fungi, a Key Symbiosis in the Development of Quality Traits in Crop Production, Alone or Combined with Plant Growth-Promoting Bacteria. Mycorrhiza 2021, 31, 655–669. [Google Scholar] [CrossRef]
- Chen, M.; Arato, M.; Borghi, L.; Nouri, E.; Reinhardt, D. Beneficial Services of Arbuscular Mycorrhizal Fungi—From Ecology to Application. Front. Plant Sci. 2018, 9, 1270. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, G.; Ruta, C.; Traversa, A.; D’Ambruoso, G.; Tarraf, W.; De Mastro, F.; De Mastro, G.; Cocozza, C. Remediation of a Heavy Metals Contaminated Soil Using Mycorrhized and Non-Mycorrhized Helichrysum italicum (Roth) Don. Land Degrad. Dev. 2018, 29, 91–104. [Google Scholar] [CrossRef]
- Hu, B.; Hu, S.; Chen, Z.; Vymazal, J. Employ of Arbuscular Mycorrhizal Fungi for Pharmaceuticals Ibuprofen and Diclofenac Removal in Mesocosm-Scale Constructed Wetlands. J. Hazard. Mater. 2021, 409, 124524. [Google Scholar] [CrossRef]
- Richter, B.S.; Stutz, J.C. Mycorrhizal Inoculation of Big Sacaton: Implications for Grassland Restoration of Abandoned Agricultural Fields. Restor. Ecol. 2002, 10, 607–616. [Google Scholar] [CrossRef]
- Shi, Y.F.; Wang, S.M.; Guo, J.N.; Xu, Z.J.; Wang, S.G.; Sang, Y.M. Effects of Arbuscular Mycorrhizal Inoculation on the Phytoremediation of PAH-Contaminated Soil: A Meta-Analysis. Chemosphere 2022, 307, 136033. [Google Scholar] [CrossRef]
- Fan, X.X.; Chang, W.; Sui, X.; Liu, Y.F.; Song, G.; Song, F.Q.; Feng, F.J. Changes in Rhizobacterial Community Mediating Atrazine Dissipation by Arbuscular Mycorrhiza. Chemosphere 2020, 256, 127046. [Google Scholar] [CrossRef]
- Debiane, D.; Garçon, G.; Verdin, A.; Fontaine, J.; Durand, R.; Shirali, P.; Grandmougin-Ferjani, A.; Sahraoui, A.L.-H. Mycorrhization Alleviates Benzo[a]pyrene-Induced Oxidative Stress in an In Vitro Chicory Root Model. Phytochemistry 2009, 70, 1421–1427. [Google Scholar] [CrossRef]
- Langer, I.; Syafruddin, S.; Steinkellner, S.; Puschenreiter, M.; Wenzel, W.W. Plant Growth and Root Morphology of Phaseolus vulgaris L. Grown in a Split-Root System Is Affected by Heterogeneity of Crude Oil Pollution and Mycorrhizal Colonization. Plant Soil 2010, 332, 339–355. [Google Scholar] [CrossRef]
- Yu, X.Z.; Wu, S.C.; Wu, F.Y.; Wong, M.H. Enhanced Dissipation of PAHs from Soil Using Mycorrhizal Ryegrass and PAH-Degrading Bacteria. J. Hazard. Mater. 2011, 186, 1206–1217. [Google Scholar] [CrossRef] [PubMed]
- Małachowska-Jutsz, A.; Rudek, J.; Janosz, W. The Effect of Ribwort (Plantago lanceolata) and Its Mycorrhizas on the Growth of Microflora in Soil Contaminated with Used Engine Oil. Arch. Environ. Prot. 2011, 37, 99–113. [Google Scholar]
- Rajtor, M.; Piotrowska-Seget, Z. Prospects for Arbuscular Mycorrhizal Fungi (AMF) to Assist in Phytoremediation of Soil Hydrocarbon Contaminants. Chemosphere 2016, 162, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Alkobaisy, S.; Jamal, J. Factors Affecting Mycorrhizal Activity. In Arbuscular Mycorrhizal Fungi in Agriculture—New Insights; IntechOpen: London, UK, 2023. [Google Scholar] [CrossRef]
- Powell, C.L. Spread of Mycorrhizal Fungi through Soil. N. Z. J. Agric. Res. 1979, 22, 335–339. [Google Scholar] [CrossRef]
- De Mastro, F.; Brunetti, G.; De Mastro, G.; Ruta, C.; Stea, D.; Murgolo, S.; De Ceglie, C.; Mascolo, G.; Sannino, F.; Cocozza, C.; et al. Uptake of Different Pharmaceuticals in Soil and Mycorrhizal Artichokes from Wastewater. Environ. Sci. Pollut. Res. 2023, 30, 33349–33362. [Google Scholar] [CrossRef]
- Manasfi, R.; Brienza, M.; Ait-Mouheb, N.; Montemurro, N.; Perez, S.; Chiron, S. Impact of Long-Term Irrigation with Municipal Reclaimed Wastewater on the Uptake and Degradation of Organic Contaminants in Lettuce and Leek. Sci. Total Environ. 2021, 765, 142742. [Google Scholar] [CrossRef]
- Franco, A.; Fu, W.; Trapp, S. Influence of Soil pH on the Sorption of Ionizable Chemicals: Modeling Advances. Environ. Toxicol. Chem. 2009, 28, 458–464. [Google Scholar] [CrossRef]
- Carter, L.J.; Ryan, J.J.; Boxall, A.B. Effects of Soil Properties on the Uptake of Pharmaceuticals into Earthworms. Environ. Pollut. 2016, 213, 922–931. [Google Scholar] [CrossRef]
- Gielen, G.J.H.P.; van den Heuvel, M.R.; Clinton, P.W.; Greenfield, L.G. Factors Impacting on Pharmaceutical Leaching Following Sewage Application to Land. Chemosphere 2009, 74, 537–542. [Google Scholar] [CrossRef]
- Mininni, A.N.; Pietrafesa, A.; Calabritto, M.; Di Biase, R.; Brunetti, G.; De Mastro, F.; Murgolo, S.; De Ceglie, C.; Salerno, C.; Dichio, B. Uptake and Translocation of Pharmaceutically Active Compounds by Olive Tree (Olea europaea L.) Irrigated with Treated Municipal Wastewater. Front. Plant Sci. 2024, 15, 1382595. [Google Scholar] [CrossRef]
- Paz, A.; Tadmor, G.; Malchi, T.; Blotevogel, J.; Borch, T.; Polubesova, T.; Chefetz, B. Fate of Carbamazepine, Its Metabolites, and Lamotrigine in Soils Irrigated with Reclaimed Wastewater: Sorption, Leaching, and Plant Uptake. Chemosphere 2016, 160, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Doretto, K.M.; Rath, S. Sorption of Sulfadiazine on Brazilian Soils. Chemosphere 2013, 90, 2027–2034. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Knapp, H.V.; Arnold, J.G.; Demissie, M. Hydrological Modeling of the Iroquois River Watershed Using HSPF and SWAT. J. Am. Water Resour. Assoc. 2005, 41, 343–360. [Google Scholar] [CrossRef]
- Franco, A.; Trapp, S. Estimation of the Soil-Water Partition Coefficient Normalized to Organic Carbon for Ionisable Organic Chemicals. Environ. Toxicol. Chem. 2008, 27, 1995–2004. [Google Scholar] [CrossRef] [PubMed]
- American Public Health Association (APHA); American Water Works Association (AWWA); Water Environment Federation (WEF). Standard Methods for the Examination of Water and Wastewater, 24th ed.; Lipps, W., Braun-Howland, E., Baxter, T., Eds.; APHA Press: Washington, DC, USA, 2023. [Google Scholar]
- Montagna, M.T.; De Giglio, O.; Calia, C.; Pousis, C.; Triggiano, F.; Murgolo, S.; De Ceglie, C.; Bagordo, F.; Apollonio, F.; Diella, G.; et al. Microbiological and Chemical Assessment of Wastewater Discharged by Infiltration Trenches in Fractured and Karstified Limestone (SCA.Re.S. Project 2019–2020). Pathogens 2020, 9, 1010. [Google Scholar] [CrossRef]
- Swift, R.S. Method of Soil Analysis: Part 3. Chemical Methods; SSSA Book Series No. 5; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnston, C.T., Sumner, M.E., Eds.; ASA and SSSA: Madison, WI, USA, 1996; pp. 1011–1069. [Google Scholar]
- De Mastro, F.; Cocozza, C.; Traversa, A.; Cacace, C.; Mottola, F.; Mezzina, A.; Brunetti, G. Validation of a Modified QuEChERS Method for the Extraction of Multiple Classes of Pharmaceuticals from Soils. Chem. Biol. Technol. Agric. 2022, 9, 49. [Google Scholar] [CrossRef]
- Phillips, J.M.; Hayman, D.S. Improved Procedures for Clearing Roots and Staining Parasitic and Vesicular-Arbuscular Mycorrhizal Fungi for Rapid Assessment of Infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161, IN16–IN18. [Google Scholar] [CrossRef]
- Trouvelot, A.; Kough, J.L.; Gianinazzi-Pearson, V. Mesure du taux de mycorhization VA d’un système radiculaire. Recherche de méthodes d’estimation ayant une signification fonctionnelle. In Physiological and Genetical Aspects of Mycorrhizae; Gianinazzi-Pearson, V., Gianinazzi, S., Eds.; INRA: Paris, France, 1986; pp. 217–221. [Google Scholar]
- Carter, L.J.; Harris, E.; Williams, M.; Ryan, J.J.; Kookana, R.S.; Boxall, A.B. Fate and Uptake of Pharmaceuticals in Soil-Plant Systems. J. Agric. Food Chem. 2014, 62, 816–825. [Google Scholar] [CrossRef]
- Pérez, D.J.; Lombardero, L.R.; Doucette, W.J. Influence of Exposure Time, Physicochemical Properties, and Plant Transpiration on the Uptake Dynamics and Translocation of Pharmaceutical and Personal Care Products in the Aquatic Macrophyte Typha latifolia. Sci. Total Environ. 2023, 896, 165107. [Google Scholar] [CrossRef]
- Rusan, M.J.M.; Hinnawi, S.; Rousan, L. Long Term Effect of Wastewater Irrigation of Forage Crops on Soil and Plant Quality Parameters. Desalination 2007, 215, 143–152. [Google Scholar] [CrossRef]
- Romaneckas, K.; Buragienė, S.; Kazlauskas, M.; Steponavičius, D.; Naujokienė, V.; Bručienė, I.; Šarauskis, E. Effects of Soil Electrical Conductivity and Physical Properties on Seeding Depth Maintenance and Winter Wheat Germination, Development and Productivity. Agronomy 2023, 13, 190. [Google Scholar] [CrossRef]
- Li, S.; Tao, Z.; Liu, Y.; Li, S.; Kama, R.; Hu, C.; Fan, X.; Li, Z. Influence of Swine Wastewater Irrigation and Straw Return on the Accumulation of Selected Metallic Elements in Soil and Plants. Agriculture 2024, 14, 317. [Google Scholar] [CrossRef]
- Castro, E.; Mañas, M.P.; De Las Heras, J. Effects of Wastewater Irrigation on Soil Properties and Turfgrass Growth. Water Sci. Technol. 2011, 63, 1678–1688. [Google Scholar] [CrossRef] [PubMed]
- Alrajhi, A.; Beecham, S.; Nanthi, S.B.; Hassanli, A. Evaluation of Soil Chemical Properties Irrigated with Recycled Wastewater under Partial Root-Zone Drying Irrigation for Sustainable Tomato Production. Agric. Water Manag. 2015, 161, 127–135. [Google Scholar] [CrossRef]
- Bernal, A.D.; Ramos, S.M.C.; Tapia, N.T.; Portugal, V.O.; Hernandez, J.T.F.; Dendooven, L. Effects of Tanneries Wastewater on Chemical and Biological Soil Characteristics. Appl. Soil Ecol. 2006, 33, 269–277. [Google Scholar] [CrossRef]
- Lu, J.; Li, S.; Liang, G.; Wu, X.; Zhang, Q.; Gao, C.; Li, J.; Jin, D.; Zheng, F.; Zhang, M.; et al. The Contribution of Microorganisms to Soil Organic Carbon Accumulation under Fertilization Varies among Aggregate Size Classes. Agronomy 2021, 11, 2126. [Google Scholar] [CrossRef]
- Zhu, J.; Li, M.; Whelan, M. Phosphorus Activators Contribute to Legacy Phosphorus Availability in Agricultural Soils: A Review. Sci. Tot. Environ. 2018, 612, 522–537. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, M.; Liu, Y.; Zhang, F.; Hodge, A.; Feng, G. Carbon and Phosphorus Exchange May Enable Cooperation between an Arbuscular Mycorrhizal Fungus and a Phosphate-Solubilizing Bacterium. New Phytol. 2016, 210, 1022–1032. [Google Scholar] [CrossRef]
- Zhang, L.; Shi, N.; Fan, J.; Wang, F.; George, T.S.; Feng, G. Arbuscular Mycorrhizal Fungi Stimulate Organic Phosphate Mobilization Associated with Changing Bacterial Community Structure under Field Conditions. Environ. Microbiol. 2018, 20, 2639–2651. [Google Scholar] [CrossRef]
- Etesami, H.; Jeong, B.R. Contribution of Arbuscular Mycorrhizal Fungi, Phosphate-Solubilizing Bacteria, and Silicon to P Uptake by Plant: A Review. Front. Plant Sci. 2021, 12, 1355. [Google Scholar] [CrossRef] [PubMed]
- Behera, B.C.; Singdevsachan, S.K.; Mishra, R.R.; Dutta, S.K.; Thatoi, H.N. Diversity, Mechanism and Biotechnology of Phosphate Solubilising Microorganism in Mangrove: A Review. Biocatal. Agric. Biotechnol. 2014, 3, 97–110. [Google Scholar] [CrossRef]
- Mahanta, D.; Rai, R.K.; Dhar, S.; Varghese, E.; Raja, A.; Purakayastha, T.J. Modification of Root Properties with Phosphate Solubilizing Bacteria and Arbuscular Mycorrhiza to Reduce Rock Phosphate Application in Soybean-Wheat Cropping System. Ecol. Eng. 2018, 111, 31–43. [Google Scholar] [CrossRef]
- Santos, A.F.; Alvarenga, P.; Gando-Ferreira, L.M.; Quina, M.J. Urban Wastewater as a Source of Reclaimed Water for Irrigation: Barriers and Future Possibilities. Environments 2023, 10, 17. [Google Scholar] [CrossRef]
- Ayers, R.S.; Westcot, D.W. Water Quality for Agriculture; FAO Irrigation and Drainage Paper 29 Rev. 1; Food and Agriculture Organization of the United Nations: Rome, Italy, 1985. [Google Scholar]
- Muscarella, S.M.; Alduina, R.; Badalucco, L.; Capri, F.C.; Di Leto, Y.; Gallo, G.; Laudicina, V.A.; Paliaga, S.; Mannina, G. Water Reuse of Treated Domestic Wastewater in Agriculture: Effects on Tomato Plants, Soil Nutrient Availability and Microbial Community Structure. Sci. Total Environ. 2024, 928, 172259. [Google Scholar] [CrossRef]
- Urbano, V.R.; Mendonça, T.G.; Bastos, R.G.; Souza, C.F. Effects of Treated Wastewater Irrigation on Soil Properties and Lettuce Yield. Agric. Water Manag. 2017, 181, 108–115. [Google Scholar] [CrossRef]
- Zhang, Y.-L.; Lin, S.-S.; Dai, C.-M.; Shi, L.; Zhou, X.-F. Sorption–Desorption and Transport of Trimethoprim and Sulfonamide Antibiotics in Agricultural Soil: Effect of Soil Type, Dissolved Organic Matter, and pH. Environ. Sci. Pollut. Res. 2014, 21, 5827–5835. [Google Scholar] [CrossRef] [PubMed]
- Christou, A.; Karaolia, P.; Hapeshi, E.; Michael, C.; Fatta-Kassinos, D. Long-Term Wastewater Irrigation of Vegetables in Real Agricultural Systems: Concentration of Pharmaceuticals in Soil, Uptake and Bioaccumulation in Tomato Fruits and Human Health Risk Assessment. Water Res. 2017, 109, 24–34. [Google Scholar] [CrossRef]
- Cirelli, G.L.; Consoli, S.; Licciardello, F.; Aiello, R.; Giuffrida, F.; Leonardi, C. Treated Municipal Wastewater Reuse in Vegetable Production. Agric. Water Manag. 2012, 104, 163–170. [Google Scholar] [CrossRef]
- Ben Mordechay, E.; Mordehay, V.; Tarchitzky, J.; Chefetz, B. Pharmaceuticals in Edible Crops Irrigated with Reclaimed Wastewater: Evidence from a Large Survey in Israel. J. Hazard. Mater. 2021, 416, 126184. [Google Scholar] [CrossRef]
- Riemenschneider, C.; Al-Raggad, M.; Moeder, M.; Seiwert, B.; Salameh, E.; Reemtsma, T. Pharmaceuticals, Their Metabolites, and Other Polar Pollutants in Field-Grown Vegetables Irrigated with Treated Municipal Wastewater. J. Agric. Food Chem. 2016, 64, 5784–5792. [Google Scholar] [CrossRef] [PubMed]
- Rogowska, J.; Cieszynska-Semenowicz, M.; Ratajczyk, W.; Wolska, L. Micropollutants in Treated Wastewater. Ambio 2020, 49, 487–503. [Google Scholar] [CrossRef] [PubMed]
- Ternes, T.A.; Bonerz, M.; Herrmann, N.; Teiser, B.; Andersen, H.R. Irrigation of Treated Wastewater in Braunschweig, Germany: An Option to Remove Pharmaceuticals and Musk Fragrances. Chemosphere 2007, 66, 894–904. [Google Scholar] [CrossRef]
- Manallack, D.T.; Prankerd, R.J.; Yuriev, E.; Oprea, T.I.; Chalmers, D.K. The Significance of Acid/Base Properties in Drug Discovery. Chem. Soc. Rev. 2013, 42, 485–496. [Google Scholar] [CrossRef]
- Hai, F.I.; Yang, S.; Asif, M.B.; Sencadas, V.; Shawkat, S.; Sanderson-Smith, M.; Gorman, J.; Xu, Z.-Q.; Yamamoto, K. Carbamazepine as a Possible Anthropogenic Marker in Water: Occurrences, Toxicological Effects, Regulations and Removal by Wastewater Treatment Technologies. Water 2018, 10, 107. [Google Scholar] [CrossRef]
- Verliefde, A.R.; Heijman, S.; Cornelissen, E.; Amy, G.; Van der Bruggen, B.; Van Dijk, J. Rejection of Trace Organic Pollutants with High Pressure Membranes (NF/RO). Environ. Prog. 2008, 27, 180–188. [Google Scholar] [CrossRef]
- Sakshi; Singh, S.K.; Haritash, A.K. Polycyclic Aromatic Hydrocarbons: Soil Pollution and Remediation. Int. J. Environ. Sci. Technol. 2019, 16, 6489–6512. [Google Scholar] [CrossRef]
- Qin, Q.; Chen, X.J.; Zhuang, J. The Fate and Impact of Pharmaceuticals and Personal Care Products in Agricultural Soils Irrigated with Reclaimed Water. Crit. Rev. Environ. Sci. Technol. 2014, 45, 1379–1408. [Google Scholar] [CrossRef]
- Vazquez-Roig, P.; Andreu, V.; Blasco, C.; Picó, Y. Risk Assessment on the Presence of Pharmaceuticals in Sediments, Soils, and Waters of the Pego–Oliva Marshlands (Valencia, Eastern Spain). Sci. Total Environ. 2012, 440, 24–32. [Google Scholar] [CrossRef]
- Watkinson, A.J.; Murby, E.J.; Costanzo, S.D. Removal of Antibiotics in Conventional and Advanced Wastewater Treatment: Implications for Environmental Discharge and Wastewater Recycling. Water Res. 2007, 41, 4164–4176. [Google Scholar] [CrossRef]
- Kinney, C.A.; Furlong, E.T.; Werner, S.L.; Cahill, J.D. Presence and Distribution of Wastewater-Derived Pharmaceuticals in Soil Irrigated with Reclaimed Water. Environ. Toxicol. Chem. 2006, 25, 2956–2962. [Google Scholar] [CrossRef] [PubMed]
- De Mastro, F.; Cacace, C.; Traversa, A.; Pallara, M.; Cocozza, C.; Mottola, F.; Brunetti, G. Influence of Chemical and Mineralogical Soil Properties on the Adsorption of Sulfamethoxazole and Diclofenac in Mediterranean Soils. Chem. Biol. Technol. Agric. 2022, 9, 34. [Google Scholar] [CrossRef]
- Borgman, O.; Chefetz, B. Combined Effects of Biosolids Application and Irrigation with Reclaimed Wastewater on Transport of Pharmaceutical Compounds in Arable Soils. Water Res. 2013, 47, 3431–3443. [Google Scholar] [CrossRef]
- Grossberger, A.; Hadar, Y.; Borch, T.; Chefetz, B. Biodegradability of Pharmaceutical Compounds in Agricultural Soils Irrigated with Treated Wastewater. Environ. Pollut. 2014, 185, 168–177. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, S.; Zhang, H.; Shen, G.; Yuan, Z.; Zhang, W. Degradation Kinetics and Mechanism of Sulfadiazine and Sulfamethoxazole in an Agricultural Soil System with Manure Application. Sci. Total Environ. 2017, 607–608, 1348–1356. [Google Scholar] [CrossRef]
- Yang, S.L.; Bi, X.X.; Huang, B.; Xia, T.Y.; Deng, L.J.; Luo, X.Q.; Ren, Z. Screening and Transcriptomic Profiling of Tobacco Growth-Promoting Arbuscular Mycorrhizal Fungi. Plant Signal. Behav. 2025, 20, 2467935. [Google Scholar] [CrossRef]
- Cavagnaro, T.R.; Bender, S.F.; Asghari, H.R.; van der Heijden, M.G.A. The Role of Arbuscular Mycorrhizas in Reducing Soil Nutrient Loss. Trends Plant Sci. 2015, 20, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis, 3rd ed.; Academic Press: London, UK, 2008. [Google Scholar]
- Avio, L.; Maggini, R.; Ujvári, G.; Incrocci, L.; Giovannetti, M.; Turrini, A. Phenolics Content and Antioxidant Activity in the Leaves of Two Artichoke Cultivars are Differentially Affected by Six Mycorrhizal Symbionts. Sci. Hortic. 2020, 264, 109153. [Google Scholar] [CrossRef]
- Joner, E.J.; Corgié, S.; Amellal, N.; Leyval, C. Nutritional Constraints to PAH Degradation in a Rhizosphere Model. Soil Biol. Biochem. 2002, 34, 859–864. [Google Scholar] [CrossRef]
- Lindahl, B.D.; Stenlid, J.; Olsson, S.; Finlay, R.D. Translocation of 32P between Interacting Mycelia of a Wood-Decomposing Fungus and Ectomycorrhizal Fungi in Microcosm Systems. New Phytol. 1999, 144, 183–193. [Google Scholar] [CrossRef]
- Yan, Q.; Feng, G.; Gao, X.; Sun, C.; Guo, J.; Zhu, Z. Removal of Pharmaceutically Active Compounds (PhACs) and Toxicological Response of Cyperus alternifolius Exposed to PhACs in Microcosm Constructed Wetlands. J. Hazard. Mater. 2016, 301, 566–575. [Google Scholar] [CrossRef] [PubMed]
- Denora, M.; Candido, V.; Brunetti, G.; De Mastro, F.; Murgolo, S.; De Ceglie, C.; Salerno, C.; Gatta, G.; Giuliani, M.M.; Mehmeti, A.; et al. Uptake and Accumulation of Emerging Contaminants in Processing Tomato Irrigated with Tertiary Treated Wastewater Effluent: A Pilot-Scale Study. Front. Plant Sci. 2023, 14, 1238163. [Google Scholar] [CrossRef]
- Miller, E.L.; Nason, S.L.; Karthikeyan, K.G.; Pedersen, J.A. Root Uptake of Pharmaceuticals and Personal Care Product Ingredients. Environ. Sci. Technol. 2016, 50, 525–541. [Google Scholar] [CrossRef]
- Shi, Q.; Xiong, Y.; Kaur, P.; Sy, N.D.; Gan, J. Contaminants of Emerging Concerns in Recycled Water: Fate and Risks in Agroecosystems. Sci. Total Environ. 2022, 814, 152527. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, M.; Shenker, M.; Chefetz, B. Insights into the Uptake Processes of Wastewater-Borne Pharmaceuticals by Vegetables. Environ. Sci. Technol. 2014, 48, 5593–5600. [Google Scholar] [CrossRef] [PubMed]
- Chuang, Y.-H.; Liu, C.-H.; Sallach, J.B.; Hammerschmidt, R.; Zhang, W.; Boyd, S.A.; Li, H. Mechanistic Study on Uptake and Transport of Pharmaceuticals in Lettuce from Water. Environ. Int. 2019, 131, 104976. [Google Scholar] [CrossRef]
- Kodesová, R.; Klement, A.; Golovko, O.; Féř, M.; Kočárek, M.; Nikodem, A.; Grabic, R. Soil Influences on Uptake and Transfer of Pharmaceuticals from Sewage Sludge Amended Soils to Spinach. J. Environ. Manage. 2019, 250, 109407. [Google Scholar] [CrossRef] [PubMed]
- Shenker, M.; Harush, D.; Ben-Ari, J.; Chefetz, B. Uptake of Carbamazepine by Cucumber Plants—A Case Study Related to Irrigation with Reclaimed Wastewater. Chemosphere 2011, 82, 905–910. [Google Scholar] [CrossRef]
- Winker, M.; Clemens, J.; Reich, M.; Gulyas, H.; Otterpoh, R. Ryegrass Uptake of Carbamazepine and Ibuprofen Applied by Urine Fertilization. Sci. Total Environ. 2010, 408, 1902–1908. [Google Scholar] [CrossRef]
- García-Valcárcel, A.I.; Loureiro, I.; Escorial, C.; Molero, E.; Tadeo, J.L. Uptake of Azoles by Lamb’s Lettuce (Valerianella locusta L.) Grown in Hydroponic Conditions. Ecotoxicol. Environ. Saf. 2016, 124, 138–146. [Google Scholar] [CrossRef]
- Lobell, D.B.; Schlenker, W.S.; Costa-Roberts, J. Climate Trends and Global Crop Production Since 1980. Science 2011, 333, 616–620. [Google Scholar] [CrossRef]
- Iizumi, T.; Yokozawa, M.; Sakurai, G.; Travasso, M.I.; Romanenkov, V.; Oettli, P.; Newby, T.; Ishigooka, Y.; Furuya, J. Historical Changes in Global Yields: Major Cereal and Legume Crops from 1982 to 2006. Glob. Ecol. Biogeogr. 2014, 23, 346–357. [Google Scholar] [CrossRef]
- Rezaei, E.E.; Webber, H.; Asseng, S.; Boote, K.; Durand, J.L.; Ewert, F.; Martre, P.; MacCarthy, D.S. Climate Change Impacts on Crop Yields. Nat. Rev. Earth Environ. 2023, 4, 831–846. [Google Scholar] [CrossRef]
- Ofori, S.; Abebrese, D.K.; Růžičková, I.; Wanner, J. Reuse of Treated Wastewater for Crop Irrigation: Water Suitability, Fertilization Potential, and Impact on Selected Soil Physicochemical Properties. Water 2024, 16, 484. [Google Scholar] [CrossRef]
- Du, Z.; Zhao, S.; She, Y.; Zhang, Y.; Yuan, J.; Rahman, S.U.; Qi, X.; Xu, Y.; Li, P. Effects of Different Wastewater Irrigation on Soil Properties and Vegetable Productivity in the North China Plain. Agriculture 2022, 12, 1106. [Google Scholar] [CrossRef]
- Ierna, A.; Mauro, R.P.; Mauromicale, G. Improved Yield and Nutrient Efficiency in Two Globe Artichoke Genotypes by Balancing Nitrogen and Phosphorus Supply. Agron. Sustain. Dev. 2012, 32, 773–780. [Google Scholar] [CrossRef]
- Shaheen, A.M.; Fatma, F.; Rizk, A.; Elbassiony, A.M.; El-Shal, Z.S.A. Effect of Ammonium Sulphate and Agricultural Sulphur on the Artichoke Plant Growth, Head Yield, and Its Some Physical and Chemical Properties. Res. J. Agric. Biol. Sci. 2007, 3, 82–90. [Google Scholar]
- Ancona, S.; De Mastro, G.; Jenderek, M.M.; Ruta, C. Micropropagation Supports Reintroduction of an Apulian Artichoke Landrace in Sustainable Cropping Systems. Agronomy 2021, 11, 1169. [Google Scholar] [CrossRef]
- Campanelli, A.; Ruta, C.; Tagarelli, A.; Morone-Fortunato, I.; De Mastro, G. Effectiveness of Mycorrhizal Fungi on Globe Artichoke (Cynara cardunculus L. var. scolymus) Micropropagation. J. Plant Interact. 2013, 9, 100–106. [Google Scholar] [CrossRef]
- Gatta, G.; Libutti, A.; Beneduce, L.; Gagliardi, A.; Disciglio, G.; Lonigro, A.; Tarantino, E. Reuse of Treated Municipal Wastewater for Globe Artichoke Irrigation: Assessment of Effects on Morpho-Quantitative Parameters and Microbial Safety of Yield. Sci. Hortic. 2016, 213, 55–65. [Google Scholar] [CrossRef]



| PhACs | Mw (g mol−1) | Chemical Structure | Chemical Class | Water Solubility (mg L−1) | KOW | pKa | Literature Kd (Reference) |
|---|---|---|---|---|---|---|---|
| Carbamazepine (CBZ) | 236.27 |  | anti- depressants | 18 at 25 °C | 2.45 | 13.9 | 12–20 [30] 0.53–16.7 [31]; 0.43 [32]; 0.08 [33] |
| Clarithromycin (CLR) | 748 | 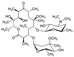 | antibiotic | 1.693 at 25 °C | 3.16 | 8.99 | 262–400 [30] |
| Climbazole (CLZ) | 292.76 | 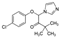 | antifungal | 58 at 25 °C | 3.76 | 6.49 | 123–200 [30] |
| Fluconazole (FCZ) | 306.27 |  | antifungal | 4.363 at 25 °C | 0.25 | 2.27 | 16.04 [34] |
| Ketoprofene (KET) | 254.28 | 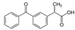 | anti- inflammatory | 51 at 22 °C | 3.12 | 4.45 | 0.09–9.59 [31]; 1.26–8.24 [35] |
| Naproxen (NPX) | 230.26 | 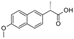 | anti- inflammatory | 15.9 at 25 °C | 3.18 | 4.15 | 0.23–17.5 [31]; 2.39–4.4 [36] |
| Sulfamethoxazole (SMX) | 253.28 | 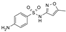 | antibiotic | 610 at 37 °C | 0.89 | 1.6 | 0.6–4.9 [30] |
| Trimethoprim (TMP) | 290.32 | 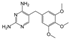 | antibiotic | 400 at 25 °C | 0.91 | 7.12 | 1.16 [32]; 7.42 [37]; 7.06–9.2 [38] |
| Parameters | TWW | GW | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1st Year | 2nd Year | 1st Year | 2nd Year | |||||||||
| CON | MSY | MSE | CON | MSY | MSE | CON | MSY | MSE | CON | MSY | MSE | |
| pH (H2O) | 7.6 ± 0.2 | 7.4 ± 0.1 | 7.6 ± 0.2 | 7.9 ± 0.0 | 7.4 ± 0.3 | 7.5 ± 0.1 | 7.9 ± 0.2 | 7.4 ± 0.2 | 7.6 ± 0.0 | 7.5 ± 0.1 | 7.6 ± 0.0 | 7.4 ± 0.3 |
| pH (KCl) | 5.7 ± 0.1 | 6.5 ± 0.1 | 6.5 ± 0.2 | 7.1 ± 0.1 | 6.7 ± 0.2 | 6.9 ± 0.3 | 7.0 ± 0.0 | 6.6 ± 0.3 | 6.7 ± 0.1 | 6.4 ± 0.0 | 6.7 ± 0.1 | 6.4 ± 0.0 |
| EC (μS cm−1) | 1769.0 ± 32 | 1844.0 ± 45 | 1708.0 ± 25.9 | 1863.9 ± 56.9 | 1863.4 ± 42.8 | 1695.4 ± 72.5 | 629.5 ± 20.9 | 789.6 ± 21.8 | 678.0 ± 15.2 | 601.9 ± 12.9 | 741.9 ± 41.1 | 628.1 ± 28.0 |
| CaCO3tot (%) | 3.0 ± 0.4 | 2.2 ± 0.30 | 2.4 ± 0.10 | 3.1 ± 0.1 | 2.1 ± 0.2 | 2.3 ± 0.1 | 3.0 ± 0.30 | 2.4 ± 0.40 | 2.3 ± 0.50 | 2.9 ± 0.2 | 2.0 ± 0.3 | 2.2 ± 0.2 |
| OC (g kg−1) | 7.8 ± 1.1 | 7.7 ± 1.1 | 6.7 ± 0.8 | 8.2 ± 1.2 | 8.0 ± 0.9 | 7.7 ± 1.1 | 7.5 ± 1.0 | 7.2 ± 0.2 | 5.7 ± 1.5 | 7.4 ± 0.6 | 7.3 ± 0.5 | 6.0 ± 1.1 |
| OM (%) | 13.5 ± 1.3 | 13.3 ± 1.3 | 11.6 ± 1.1 | 14.1 ± 1.1 | 13.8 ± 0.9 | 13.3 ± 1.2 | 13.0 ± 1.3 | 12.4 ± 1.2 | 9.8 ± 1.9 | 12.8 ± 0.8 | 12.6 ± 0.8 | 10.3 ± 1.6 |
| Ntot (g kg−1) | 1.3 ± 0.1 | 0.9 ± 0.4 | 1.1 ± 0.1 | 1.7 ± 0.2 | 1.3 ± 0.3 | 1.4 ± 0.2 | 0.8 ± 0.4 | 0.6 ± 0.1 | 0.7 ± 0.2 | 0.9 ± 0.2 | 0.5 ± 0.2 | 0.7 ± 0.1 |
| Pava (mg kg−1) | 73.7 ± 4.4 | 95.5 ± 3.7 | 68.9 ± 5.9 | 82.4 ± 2.3 | 124.6 ± 2.1 | 76.2 ± 1.6 | 49.5 ± 4.7 | 86.8 ± 4.6 | 48.2 ± 6.4 | 53.8 ± 1.3 | 79.6 ± 2.2 | 49.8 ± 5.2 |
| Parameters | TWW | GW | Recommended Value | ||||
|---|---|---|---|---|---|---|---|
| 1st Year | 2nd Year | 1st Year | 2nd Year | Italy [60] | EU [3] | FAO [61] | |
| Temperature (°C) | 19.88 ± 5.75 | 20.1 ± 5.8 | 22.30 ± 0.14 | 21.80 ± 0.10 | - | - | - |
| pH | 7.22 ± 0.27 | 7.30 ± 0.20 | 7.50 ± 0.06 | 7.48 ± 0.02 | 6.0–9.5 | 6.50–8.50 | |
| EC (dS m−1) | 1.08 ± 0.10 | 0.90 ± 0.10 | 0.35 ± 0.00 | 0.32 ± 0.10 | 3.0 | (0.7–3) SM | |
| TSSs (mg L−1) | 2.36 ± 1.63 | 2.0 ± 0.50 | 0.83 ± 0.8 | 0.75 ± 0.10 | 10 | 35 | <50.00 |
| Turbidity (NTU) | 1.22 ± 0.72 | 1.11 ± 0.10 | 1.39 ± 015 | 1.32 ± 0.08 | - | - | |
| N-NH4+ (mg L−1) | 2.24 ± 4.86 | 2.10 ± 0.20 | 0.04 ± 0.02 | 0.03 ± 0.02 | |||
| N-NO2− (mg L−1) | 0.79 ± 1.77 | 0.80 ± 0.0 | ND | ND | |||
| N-NO3− (mg L−1) | 5.89 ± 2.44 | 6.10 ± 1.20 | 0.93 ± 0.31 | 0.88 ± 0.18 | |||
| Ntot (mg L−1) | 12.65 ± 7.16 | 13.10 ± 1.90 | 2.89 ± 0.25 | 2.77 ± 0.20 | 15 | - | |
| Ptot (mg L−1) | 4.75 ± 1.88 | 5.10 ± 1.90 | 0.03 ± 0 | 0.03 ± 0 | 2 | - | |
| COD (mg O2 L−1) | 22.57 ± 7.25 | 20.10 ± 3.3 | 0.70 ± 0.20 | 0.60 ± 0.20 | 100 | ||
| BOD5 (mgO2 L−1) | 9.80 ± 1.04 | 10.80 ± 1.0 | ND | ND | 20 | 25 | <30 |
| Free Chlorine (mg L−1) | 0.13 ± 0.24 | 0.10 ± 0.0 | ND | ND | |||
| Tot. Chlorine (mg L−1) | 1.07 ± 2.50 | 1.10 ± 0.0 | 0.1 ± 0.0 | ND | |||
| TC (MPN 100 mL−1) | 1556.19 ± 752.32 | 1456.20 ± 485.2 | ND | ND | |||
| E. coli (MPN 100 mL−1) | 596.69 ± 230.58 | 606.10 ± 189.47 | ND | ND | 100 | 1000 | <200 |
| Pharmaceuticals | Formula | GW | TWW | TWW |
|---|---|---|---|---|
| I and II Years | I Year | II Year | ||
| µg/L | ||||
| Clarithromycin | C38H69NO13 | <LOQ | 0.8 ± 0.001 | <LOQ |
| Sulfamethoxazole | C10H11N3O3S | <LOQ | <LOQ | 0.28 ± 0.001 |
| Trimethoprim | C14H18N4O3 | <LOQ | <LOQ | <LOQ |
| Ketoprofen | C16H14O3 | <LOQ | 0.2 ± 0.01 | <LOQ |
| Carbamazepine | C15H12N2O | <LOQ | 0.54 ± 0.04 | 0.51 ± 0.01 |
| Fluconazole | C13H12F2N6O | <LOQ | 0.07 ± 0.002 | 0.25 ± 0.001 |
| Climbazole | C15H17ClN2O2 | <LOQ | 0.03 ± 0.001 | 0.1 ± 0.001 |
| Naproxen | C14H14O3 | <LOQ | 0.21 ± 0.02 | <LOQ |
| PhACs | Inoculation | Plant Organs | ||
|---|---|---|---|---|
| Roots | Leaves and Stems | Head | ||
| CBZ | CON | 0.69 ± 0.10 | 3.80 ± 0.34 | 0.33 ± 0.04 |
| MSY | 0.68 ± 0.05 | 3.37 ± 0.48 | 0.27 ± 0.02 | |
| MSE | 0.99 ± 0.15 | 4.51 ± 0.50 | 0.44 ± 0.03 | |
| CLR | CON | 0.03 ± 0.01 | <LOQ | <LOQ |
| MSY | 0.05 ± 0.00 | <LOQ | <LOQ | |
| MSE | 0.10 ± 0.01 | <LOQ | <LOQ | |
| CLZ | CON | 0.02 ± 0.00 | <LOQ | <LOQ |
| MSY | 0.07 ± 0.00 | <LOQ | <LOQ | |
| MSE | 0.10 ± 0.02 | <LOQ | <LOQ | |
| FCZ | CON | 0.46 ± 0.06 | 3.55 ± 0.49 | 0.39 ± 0.08 |
| MSY | 0.56 ± 0.05 | 4.03 ± 0.59 | 0.49 ± 0.10 | |
| MSE | 0.59 ± 0.10 | 4.16 ± 0.43 | 0.49 ± 0.02 | |
| TMP | CON | 0.05 ± 0.00 | <LOQ | <LOQ |
| MSY | 0.13 ± 0.01 | <LOQ | <LOQ | |
| MSE | 0.26 ± 0.06 | <LOQ | <LOQ | |
| Experimental Factor | Number of Heads (n. ha−1) | Heads Mean Weight (g) | Diameter Heads (cm) | |||
|---|---|---|---|---|---|---|
| Main | Secondary | Main | Secondary | Main | Secondary | |
| Growing seasons | ** | * | n.s. | n.s. | n.s. | n.s. |
| First | 11,155.56 b | 37,111.11 b | 132.92 a | 73.48 a | 73.08 a | 53.31 a |
| Second | 12,177.78 a | 38,066.67 a | 135.62 a | 73.74 a | 73.34 a | 53.08 a |
| Irrigation treatment | *** | *** | n.s. | n.s. | n.s. | n.s. |
| GW | 10,466.67 b | 36,800.00 b | 133.53 a | 72.07 a | 73.91 a | 53.06 a |
| TWW | 12,866.67 a | 38,377.78 a | 135.01 a | 75.16 a | 72.51 a | 53.33 a |
| Inoculum | *** | *** | n.s. | n.s. | n.s. | n.s. |
| CON | 11,400 b | 37,000.00 b | 129.93 a | 73.97 a | 71.49 a | 52.72 a |
| MSY | 10,600 b | 36,566.67 b | 141.76 a | 73.87 a | 73.79 a | 53. 66 a |
| MSE | 13,000 a | 39,200.00 a | 131.12 a | 72.99 a | 74.35 a | 53.20 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Mastro, F.; Brunetti, G.; Cocozza, C.; Murgolo, S.; Mascolo, G.; Salerno, C.; Ruta, C.; De Mastro, G. Dynamics of Pharmaceuticals in the Soil–Plant System: A Case Study on Mycorrhizal Artichoke. Soil Syst. 2025, 9, 51. https://doi.org/10.3390/soilsystems9020051
De Mastro F, Brunetti G, Cocozza C, Murgolo S, Mascolo G, Salerno C, Ruta C, De Mastro G. Dynamics of Pharmaceuticals in the Soil–Plant System: A Case Study on Mycorrhizal Artichoke. Soil Systems. 2025; 9(2):51. https://doi.org/10.3390/soilsystems9020051
Chicago/Turabian StyleDe Mastro, Francesco, Gennaro Brunetti, Claudio Cocozza, Sapia Murgolo, Giuseppe Mascolo, Carlo Salerno, Claudia Ruta, and Giuseppe De Mastro. 2025. "Dynamics of Pharmaceuticals in the Soil–Plant System: A Case Study on Mycorrhizal Artichoke" Soil Systems 9, no. 2: 51. https://doi.org/10.3390/soilsystems9020051
APA StyleDe Mastro, F., Brunetti, G., Cocozza, C., Murgolo, S., Mascolo, G., Salerno, C., Ruta, C., & De Mastro, G. (2025). Dynamics of Pharmaceuticals in the Soil–Plant System: A Case Study on Mycorrhizal Artichoke. Soil Systems, 9(2), 51. https://doi.org/10.3390/soilsystems9020051













