Abstract
Cow urine is a rich source of mobile nutrients such as nitrate (NO3−) and potassium (K+). The aim of this experiment was to evaluate the wetting pattern distribution through soil profile of cow urine patch in an andisol. Two field experiments across two consecutive years were carried out to compare cow urine patches in relation to initial wetting pattern and volume of soil affected. Bromide (Br−) has successfully been used as an inert hydrologic tracer to indicate the movement of NO3− and K+ in soil–water systems. The distribution of Br− (used as a urine tracer) on the soil surface and down the profile was irregular in all the patches. Cow urine patches covered a surface area of 0.27 and 0.35 m2, respectively, and penetrated to a depth of 70 cm. The rapid downward movement of urine occurred through macropore flow but even so, between 27% and 40% of the applied Br− was detected in the 0–5 cm soil layer. Br− showed concentrations greater than 1500 mg kg−1 and up to 3000 mg kg−1, and as the concentration of Br− decreases, the frequency and depth of affected layers increases. Despite the differences in moisture and in the distribution of the Br− concentration in both years, the concentration frequency of 500 to 1500 mg kg−1 represented around 37% of the affected volume of soil (bulb of urine) in both years. Up to 40% of the bulb represented N equivalent rates between 187 and 975 kg N ha−1. These values can potentially be emitted in gases such as NH3, N2O, and N2. It is suggested that the presence of N in the volume of affected soil could vary due to the moisture content of the soil, and that in andisols of southern Chile under permanent grasslands there are a large number of macropores that would induce preferential flows.
1. Introduction
The excretions of cattle, mainly dung and urine, represent the most important source of nutrient recycling from grazing animals [1]. Between 55% and 90% of nutrients ingested by grazing animals are returned to grassland in the form of dung and urine [2,3]. The nutrients in the excreta are recycled by soil, absorbed by plants, or lost from soils through leaching, surface runoff or emission of gases into the atmosphere. The magnitude of losses depends on the nutrient content in the soil.
Cow excreta deposition typically covers a small area of soil, and the nutrients contained therein achieve high concentrations per unit area [4], reaching, for example, a contribution equivalent to 1000 kg N ha−1 per patch. Urine accounts for most of the mobile nutrients excreted, such as N, K and S [5], being transported through the soil profile through water movement [6,7]. Urine patches are the major source or “hot-spots” of N and can be lost by different pathways, for example nitrous oxide emissions [8,9], ammonia volatilization [10,11], pasture uptake [12,13], and leaching [14,15]. These pathways are especially dependent on differences in the physical properties of soils and moisture content [7]. Frequently, there is preferential water movement through the macropores that are connected to the soil surface, and it is produced when the deposition rate exceeds the infiltration rate of the soil, as occurs during some urination events [16], leading to the leaching of mobile nutrients rapidly, such as NO3− and K+ [6,17]. Bromide (Br−) has been used successfully as an inert hydrologic tracer to indicate the movement of NO3− and K+ in soil-water systems [6,17]. In addition, Br− has been used for decades to study leaching patterns in a representative range of soils, highlighting distinct differences in the hydrology of the soils. The main features of Br− are: (i) it is present in low background concentrations in soil, (ii) it rarely sorbs to soil particles, and (iii) it is not biologically degradable [18].
Williams and Haynes [6] compared cattle and sheep urine patches in relation to initial wetting pattern and volume of soil affected. The authors found that the distribution of Br− (used as a urine tracer) [19] across the soil surface and down the profile was irregular in both patches. Monaghan et al. [17] related the movement of the Br− of a urine patch through the profile of 10 different soils of New Zealand with physical properties of the soil such as hydraulic conductivity (Ks) and air permeability (Ka) and found that the Ks predicted the movement of urine about 20 cm depth in a better way.
Most soils in southern Chile are volcanic and include different taxonomic orders, such as Inceptisols, Andisols and Ultisols [20], and cover between 50% and 60% of the arable land of the country (5.4 million ha) [21]. Due to their andic properties [22,23], these soils have completely different characteristics than other types of soils throughout the world. Andisols have been described to have variable charge [22], high air and water holding capacity [24], high hydraulic conductivity [25], large total porosity, great depth, and a great shrinkage capacity [26,27]. In addition, these soils have low bulk density (<0.9 g cm3), elevated levels of organic carbon [28] and of allophane [29,30]. The specific differences of the andisols of southern Chile with respect to other andisols in other countries are that they do not have a Bt horizon, are deep derivatives of basaltic andeositic ash [31] and they are in agroecosystems of very high productivity [32].
It is necessary to determine the potential volatilization and denitrification losses in volcanic soils. These losses are dependent on the concentration of N. For this reason, it is important to know the variation in N concentration that occurs within the urine patches; we hypothesize that it has an uneven concentration distribution within the urine patch, and therefore, with spatial variability and with different potential points for N losses. The hypothesis of this pilot study is that the distribution of urine through the depth of the soil varies according to the physical characteristics of the soil that influence the movement of water. The objective was to evaluate the simulation of a patch of cow urine in distribution and Br− concentration at different depths of an Andisol, using bromide as a tracer.
2. Materials and Methods
2.1. Site and Soil Characteristics
The distribution of urine through the soil profile was temporally replicated twice, on 23 March 2017, and on 26 March 2018. No statistical replications were made, similar to Williams and Haynes [6], because the method is destructive, and it is very likely that the distribution of the urine patch in the soil solution be different in each case when considering the physical and chemical characteristics of the soil. Therefore, a descriptive image simulation software was used to visualize the movement of Br− concentration at the surface and through the depth of the soil of two temporal replications. The experiment was carried out on a permanent pasture commonly used for dairy production, consisting mainly of perennial ryegrass (Lolium perenne L.) and white clover (Trifolium repens L.) at the Vista Alegre research farm (Universidad Austral de Chile) located in Región de los Ríos, Valdivia, Chile (39°47′ S, 73°12′ W; Figure 1) at 20 m.a.s.l. The soil is of volcanic ash origin, classified as Duric Hapludand, which is characterized by being moderately deep, with a silty loamy texture in the first centimeters, which becomes thicker with the increase in depth [31]. Before starting the experiment, a sector with a flat slope was selected to avoid surface runoff when applying the marked solution. Additionally, a day in which there had been no precipitation for at least two previous days was selected for the application of the Br− solution in both temporal replications. The weather conditions prevailing during the experiment were obtained from Austral Meteorological Station, located in the Estación Experimental Austral (EEAA) [33].
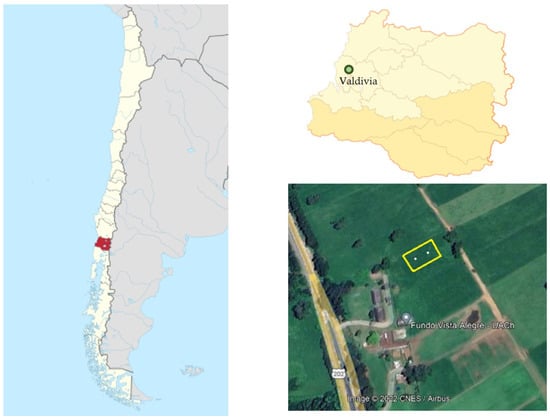
Figure 1.
Map of Chile. The red area represents Región de Los Ríos. Green circle represents the experimental location. Yellow rectangle and white points represent the experimental area experimental repetitions, respectively.
2.2. Description of the Study
Cow urine patches were created by the application of 2 L of solution containing 20 g of KBr L−1 of water. This solution was applied at the height of 1 m at the rate of 0.2 L s−1 [5,34]. This was equivalent to applying ~27 g of Br− in total to the soil as urine patch. This solution is equivalent to a concentration of 1.35% of N in the urine excreted by a cow. This concentration of N in the urine is typical of a dairy production system [5]. Collection of soil samples began 30 min after the simulated urination, which allowed time for preferential movement of the KBr through the soil profile. At the end of this time, there was no visible ponding of the urine on the soil surface. The soil cores were collected from the demarcation of a grid in the area of the urine patch. The sampling grid comprised 8 × 8 sections, with a spacing of 10 cm between cores, where each nucleus was divided into depths of 0–5, 5–10, 10–15, 15–20, 20–30, 30–40, 40–50, 50–60, 60–70 and 70–80 cm (Figure 2). The soil samples were taken manually with a 1 m long hollow still cylinder and 5 cm in diameter, which was marked with the depths of each sample for better accuracy. The concentration of Br− was analyzed individually for each sample, allowing to identify the distribution of Br− throughout the soil profile. In total, 640 samples of each year were analyzed. The surface area covered by the Br− solution was calculated from the number of samples in the 0–5 cm depth that contained Br−. Similarly, the depth to which the bromide solution penetrated was determined by the presence of Br− in the samples collected from the various depths. Systat SigmaPlot 12.5.0.88 software was used for the graphic representation of the different Br− concentrations in the different layers. The evaluation of Br− concentration distribution in the soil was carried out with descriptive statistics using GraphPad Prism 5.
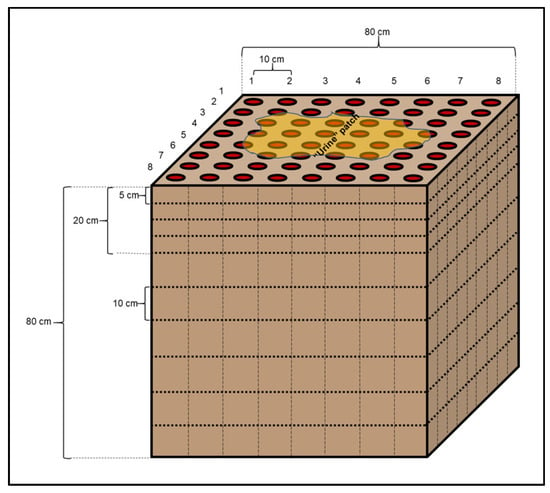
Figure 2.
Schematic design of the experiment into the soil. Each circle indicates the position of each soil sampled down to 80 cm of depth, divided into each layer. The yellow irregular spot indicates the patch after applied the KBr solution.
2.3. Bromide Analysis
The samples were dried at 65 °C for 24 h then ground to pass through a sieve with round holes 2 mm in diameter. The Br− was extracted from each soil core in distilled water using a soil:water ratio of 1:5. Sufficient 5 M sodium nitrate (as an ionic strength adjuster, ISA) was added to provide a 2% solution [35]. The Br− concentration in the solution was measured directly using a bromide-ion-specific electrode (Hanna Instruments HI-4102). The forage contained in each soil sample was included in the analysis; thus, any Br− that had adhered to the herbage from the application was also extracted.
3. Results
In year 1, there was no rainfall for 11 days before the application of the solution with KBr, including the date of the experiment, while in year 2, rainfall occurred for five consecutive days with an accumulated rainfall of approximately 48 mm three days before the experiment was carried out (Figure 3).
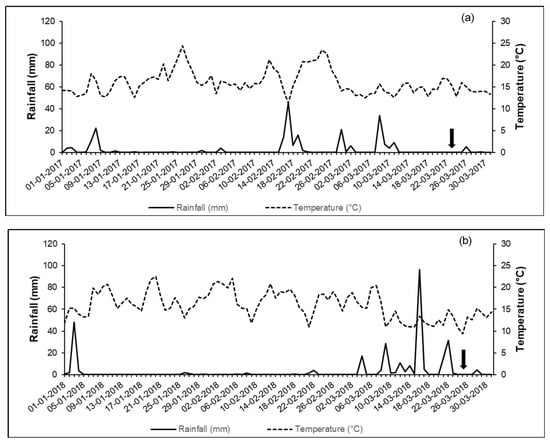
Figure 3.
Rainfall (mm) and temperature (°C) in year 1 (a) and year 2 (b). The arrows indicate the day of application of the KBr solution.
The distribution pattern of Br− concentrations on surface and across the soil profile was irregular, varying between the two years, with a decreased concentration as depth increased (Figure 4, Figure 5 and Figure 6). The patches of Br− solution applied to the soil for year 1 and year 2, covered areas of 0.27 and 0.35 m2, respectively (Figure 6a,b). Both patches reached 70 cm of depth through the soil (Figure 4). The volumes of the soil that were affected by the Br− solution in year 1 and 2 were 0.12 and 0.15 m3, respectively.
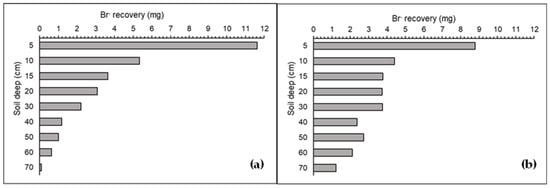
Figure 4.
Br− recovery (g) in the soil profile on year 1 (a) and year 2 (b) following simulation of dairy cow urinations using potassium bromide (KBr) solution.
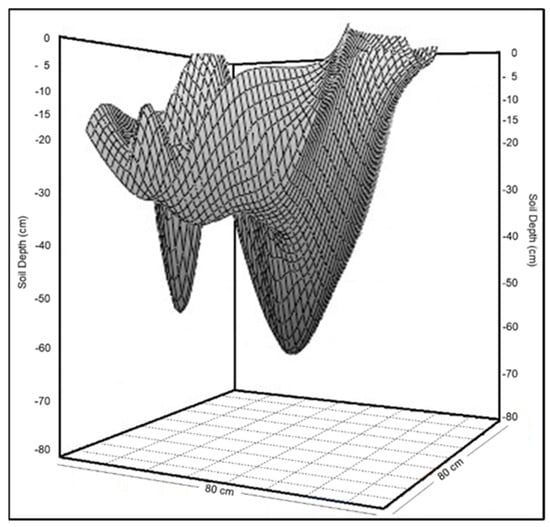
Figure 5.
Example 3D schematic representation of the bulb formed after the application of the KBr solution in year 1.
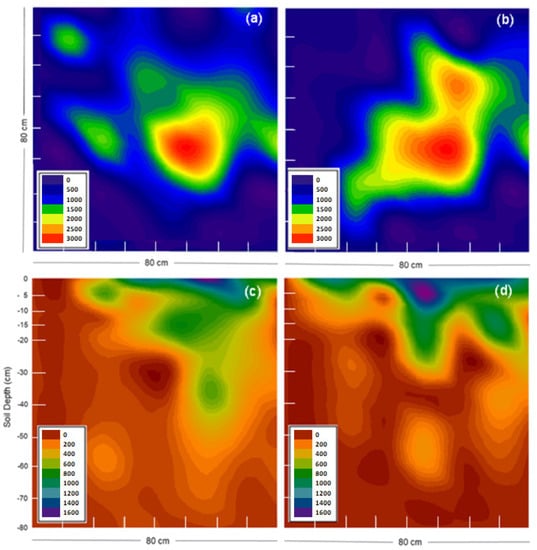
Figure 6.
Distribution pattern of Br− concentration on soil surface and soil profile following simulation of dairy cow urination on year 1 (a,c) and year 2 (b,d). Different colors represent different Br− concentrations (mg kg−1). Images c and d represent a layer in the middle of surface sampled into the deep.
Each “urine” patch showed different amounts of Br− recovered in each depth layer sampled; from total Br− applied, 104% of Br− was recovered in year 1 and 122% in year 2. In both years of the experiment, the distribution was irregular and decreased as the depth increased. The layer 0–5 cm was the layer that showed the highest amount of Br− recovered in both years, which showed 40% and 27% for year 1 and 2, respectively, while from the surface to 20 cm depth, 81% and 64% of Br− were recovered, respectively, with year 2 more presence of Br− at a greater depth (Figure 4).
Figure 7 shows the frequency of the distribution of Br− concentrations at different depth layers in the soil, for year 1 and year 2. For both years, 19% of the volume of the upper soil layer (0–5 cm) showed higher concentration of Br−, which ranged from 1500 mg kg−1 to 3000 mg kg−1. Then, Br− concentration decreased with the increase in soil depth. However, the concentrations of 500 and 1000 mg Br− kg−1 showed the highest frequency for the layers below 60 cm in year 2 (Figure 6b); in both years, the sum of cores of both concentrations represented 31% of the volume of soil affected by “urine”, and, if cores frequency of 1500 mg kg−1 were considered, it reached 37%. Logically, cores that were not marked with the KBr solution were those that were found in greater frequency per layer.
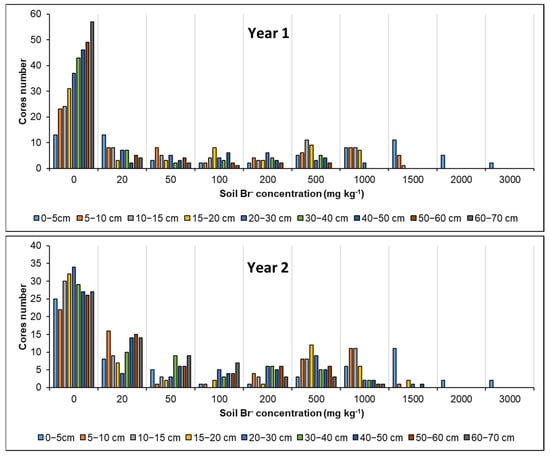
Figure 7.
The number of cores and frequency of different Br− concentrations (mg kg−1) in each layer of soil depth from 0 to 70 cm in year 1 and year 2. Different colors of each bar indicate each layer of soil depth.
4. Discussion
The irregular distribution pattern of KBr solution on the surface and through the soil profile presented in this study was also found by Williams and Haynes [6]. However, in our experiment, the maximum affected area was 0.35 m2 and the depth reached 70 cm, while the experiment of Williams and Haynes [6] showed a greater affected area ranging from 0.37 to 0.42 m2, while the depth reached was only 40 cm. This difference could be due to the Templeton silt loam soils in New Zealand, classified as Typic Ustochrept [36], are characterized by being moderately well drained and have slow permeability in some subsoil horizons. The surface that was affected in our study by the KBr solution agrees with the ranges of covered area evaluated by Haynes and Williams [37] and Nguyen and Goh [38], which range from 0.16 to 0.50 m2. However, in those studies, the spatial concentration distribution of Br− of the urine patches was not studied.
If we consider a urine patch of an average size of 0.35 m2 and assume a stocking rate of 2 cows ha−1 day−1, a frequency of urine events of ten times per day, and that 85% of the urine is deposited within the grazed grassland [5] without overlapping patches, 21% of the grazed area could be covered in one year, i.e., the grassland area could be fully covered in approximately 5 years. These calculations can be applied to typical strip grazing systems of southern Chile, where the distribution of urine patches depositions is more homogeneous. However, in a continuous stocking method the spatial distribution pattern of the urine patches is not homogeneous, since there are zones of high density of urine depositions, such as under the shade of trees or near water troughs [5]; however, confirming this assumption would require long-term experimentation, measuring the distribution of urine patches. The surface of the grassland covered by a single urination event is influenced by several factors, including soil moisture content, the presence of macropores that open to the soil surface, the amount of herbage present, soil surface microtopography, vegetation cover, slope, and wind [2,5,6,34]. Whatever the area covered by urine, the affected area can depend on the lateral diffusion of nutrients and on the lateral component of root growth, and this may extend to two to five times the area usually covered [39].
It is widely recognized that soils derived from volcanic ash (andisols) have excellent physical properties [27,40,41]. Their physical behavior is associated with their high content of organic carbon (>10%) [29], which confers a low bulk density, and a high storage capacity and fluid conduction [29]. The functional aspects of the porous system are closely related to the hydraulic properties of the soil, which are related to the transmission of fluids [42,43]. These properties are linked to the structure [44]; they can present anisotropy (i.e., vertical hydraulic conductivity is less than horizontal), which was reported for andisols [45]. Furthermore, Zúñiga et al. [46] reported increases in hydraulic conductivity in volcanic soils under grasslands. Moreover, Zúñiga et al. [47] found increases in the functionality and interconnection of the porous system through the continuity indexes of pores in pastures that have not been tilled and that maintained their structure. Analogously, our experiment was conducted in a permanent grassland not submitted to tillage. The hydraulic properties would explain the depth of 70 cm reached by the “urine” solution. Hence, it could be predicted that a larger volume of urine excreted in a few seconds (1.6 to 2.8 L) [2,38] would cause a great depth reached by urine in the soil profile [5].
Regarding procedures, it is assumed that the deviation from 100% of Br− applied was due to the sampling error introduced during the field sampling and laboratory analysis of soil, such as the variation in temperature, which affects the measurement of the Br− concentration by the selective ion of Br− electrode. These differences are also reported in other studies such as Williams et al. [34], Monagan et al. [17] and Williams and Haynes [6].
Some studies have reported that 75% to 85% of the urine is retained in the first 20 cm of the soil [6,34,48], i.e., 15–25% were present below 20 cm. Our study showed that 19 and 36% of the was urine below 20 cm in year 1 and year 2, respectively. This could be explained by the contrasting meteorological conditions between year 1 and year 2, which would have caused more conduction of the solution to greater depth in year 2. The greater penetration depth in the soil caused by larger volume of urine excreted by cows tends to result in a greater concentration of nutrient ions in the zone affected by the urine, such as NH4+, NO3− and K+ [5]. The quantity of KBr that we applied to the solution was equivalent to a concentration of 1.35% of N in the urine excreted by a cow. This concentration is within the ranges shown in the studies of Ledgard et al. [49] and Safley et al. [50]. If urine with this concentration was applied to 0.35 m2 of soil (patch size of year 2), it would be equivalent to applying ~770 kg N ha−1, but only if the concentration within the affected area were homogeneous. However, it must be considered that the 27 g of Br− was irregularly distributed on the affected area and was diffused into the soil profile. However, Br− concentrations varied in each sampled layer and through depth. Considering that there were cores that had concentrations between 1500 and 3000 mg kg−1, mostly in the center of the affected area, these would be equivalent to N application rates between 488 and 975 kg ha−1, respectively, covering 19% of the area affected by the KBr solution and decreasing as it approaches the edge of the patch. Due to the visual and tactile difference in soil moisture in each repetition, and to the differences in rainfall patterns just before each repetition (i.e., 48 mm of rainfall three days before the KBr application in year 2 vs. no rainfall for 11 days before the application in year 1), it is suggested that there was more moisture in year 2 compared with year 1.
Overall, the concentration frequency of 500 to 1500 mg kg−1 represented ~37% of the affected volume into the soil and reached a depth of 70 cm in both years, suggesting that the differences in soil moisture would not have affected the proportion of the bulb where the concentrations are greater than 500 mg kg−1. In summary, up to 40% of the bulb would equal N application rates between 187 and 975 kg N ha−1. These values would represent “hotspots” that exceed N uptake by plants [51]. The amount of N that remains in the soil could potentially be emitted in gases such as NH3, N2O, and N2, since it has been found that the greater the N rates applied to the soil, as fertilizer or urine, the greater the N2O (265 times more polluting than the CO2) and N2 (harmless gas) emissions, especially under high moistures conditions. It has been reported that WFPS values above 90% could produce only N2 [52], as occurs with the application of urine [53,54,55].
5. Conclusions and Future Directions
Under the conditions of our pilot study, the irregular distribution of the Br− concentration in the surface and through the soil profile in a permanent grassland, the penetration up to 70 cm in depth, and the variation in the volume of the bulb in the soil depth would be affected by the moisture content of the soil. These patterns are determined by the water input and the soil properties, indicating that in andisols of southern Chile under permanent grasslands there are a large number of macropores that would induce preferential flows and that would presumably affect the mobility of nutrients, such as N. Our pilot study also suggests that under different soil moisture conditions, zones of high concentration of mobile nutrients (>500 mg Br− kg−1) can reach 40% of the total volume of soil affected by a patch of urine. Even though there was variation between years, a regular pattern was found.
This is the first study carried out in a permanent grassland of volcanic soils in southern Chile; however, more evaluations of the site-specific physical properties of the soil should have been introduced in this study to better explain our results. It is necessary to carry out more studies where the evaluation of the different localized physical properties of the soil where the urine is applied are included to explain the variables that would affect the irregular distribution of nutrients using cow urine in more detail. Additionally, carrying out an experiment including the spatial variability factor with the application of the solution in different points of a grassland should be considered, so that the distribution of urine can be interpreted in other regions.
Author Contributions
Conceptualization, D.E.P. and M.A.R.-S.; methodology, D.E.P. and M.A.R.-S.; formal analysis, M.A.R.-S.; investigation, M.A.R.-S.; resources, D.E.P.; data curation, M.A.R.-S.; writing—original draft preparation, M.A.R.-S.; writing—review and editing, D.E.P. and M.J.R.; visualization, M.A.R.-S.; supervision, D.E.P. and M.J.R.; project administration, D.E.P.; funding acquisition, D.E.P. and M.J.R. All authors have read and agreed to the published version of the manuscript.
Funding
Agencia Nacional de Investigación y Desarrollo (ANID; Scholarship N° 21160644) funded the doctoral studies of M.A.R-S. Support in writing up the work was greatly received by the Biotechnology and Biological Sciences Research Council (BBSRC) through the strategic program Soil to Nutrition (S2N; BBS/E/C/000I0320) at Rothamsted Research (M.J.R.). The contributions by M.J.R. were also funded by the Natural Environment Research Council (NERC) under research Programme NE/W005050/1 AgZero+: Towards sustainable, climate-neutral farming. AgZero+ is an initiative jointly supported by NERC and BBSRC.
Data Availability Statement
Data will be available from the corresponding author upon request.
Acknowledgments
Thank to José Verdejo for his collaboration and technical support in this study.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Bardgett, R.D.; Wardle, D.A. Herbivore-mediated linkages between aboveground y belowground communities. Ecology 2003, 84, 2258–2268. [Google Scholar] [CrossRef]
- Haynes, R.J.; Williams, P.H. Nutrient cycling y soil fertility in the grazed pasture ecosystem. Adv. Agron. 1993, 49, 119–199. [Google Scholar]
- Dijkstra, J.; Reynolds, C.K.; Kebreab, E.; Bannink, A.; Ellis, J.L.; France, J.; van Vuuren, A.M. Challenges in ruminant nutrition: Towards minimal nitrogen losses in cattle. In Energy and Protein Metabolism and Nutrition Is Sustainable Animal Protection; Oltjen, J.W., Kebreab, E., Lapierre, H., Eds.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2013; Volume 134, pp. 47–58. [Google Scholar]
- Ramírez-Sandoval, M.; Pinochet, D.; Rivero, M.J. Soil Dynamics and Nitrogen Absorption by a Natural Grassland under Cow Urine and Dung Patches in an Andisol in Southern Chile. Agronomy 2022, 12, 719. [Google Scholar] [CrossRef]
- Whitehead, D.C. Nutrient Elements in Grassland: Soil-Plant-Animal Relationships; CABI: Surrey, UK, 2000; 384p. [Google Scholar]
- Williams, P.H.; Haynes, R.J. Comparison of initial wetting pattern, nutrient concentrations in soil solution and the fate of 15N-labelled urine in sheep y cattle urine patch areas of pasture soil. Plant Soil 1994, 162, 49–59. [Google Scholar] [CrossRef]
- Selbie, D.R.; Buckthought, L.E.; Shepherd, M. The challenge of the urine patch for managing nitrogen in grazed pasture systems. Adv. Agron. 2015, 129, 229–292. [Google Scholar]
- Anger, M.; Hoffmann, C.; Kuhbauch, W. Nitrous oxide emissions from artificial urine patches applied to different N-fertilized swards and estimated annual N2O emissions for differently fertilized pastures in an upland location in Germany. Soil Use Manag. 2003, 19, 104–111. [Google Scholar] [CrossRef]
- Di, H.J.; Cameron, K.C. Mitigation of nitrous oxide emissions in spray-irrigated grazed grassland by treating the soil with dicyandiamide, a nitrification inhibitor. Soil Use Manag. 2003, 19, 284–290. [Google Scholar] [CrossRef]
- Jarvis, S.; Pain, B. Ammonia volatilisation from agricultural land. Proc. Int. Fertil. Soc. 1990, 298, 1–35. [Google Scholar]
- Ryden, J.; Whitehead, D.; Lockyer, D.; Thompson, R.; Skinner, J.; Garwood, E. Ammonia emission from grassland and livestock production systems in the UK. Environ. Pollut. 1987, 48, 173–184. [Google Scholar] [CrossRef]
- Ledgard, S.F.; Steele, K.W.; Saunders, W.H.M. Effects of cow urine and its major constituents on pasture properties. N. Z. J. Agric. Res. 1982, 25, 61–68. [Google Scholar]
- Williams, P.H.; Haynes, R.J. Transformations and plant uptake of urine N and S in long and short-term pastures. Nutr. Cycl. Agroecosyst. 2000, 56, 109–116. [Google Scholar] [CrossRef]
- Di, H.J.; Cameron, K.C. Nitrate leaching losses and pasture yields as affected by different rates of animal urine nitrogen returns and application of a nitrification inhibitor—A lysimeter study. Nutr. Cycl. Agroecosyst. 2007, 79, 281–290. [Google Scholar] [CrossRef]
- Ledgard, S.F.; Luo, J.; Monaghan, R.M. Managing mineral N leaching in grassland systems. In Grassland Productivity and Ecosystem Services; Lemaire, G., Hodgson, J., Chabbi, A., Eds.; CABI: Wallingford, UK; Oxfordshire, UK, 2011; pp. 83–91. [Google Scholar]
- Cameron, K.; Haynes, R. Retention and Movement of Nitrogen in Soils. In Mineral Nitrogen in the Plant-Soil System; Haynes, R.J., Ed.; Academic Press: Cambridge, UK, 1986; pp. 166–241. [Google Scholar]
- Monaghan, R.M.; Carey, P.A.; Metherell, K.; Singleton, P.L.; Drewry, J.; Addison, B. Depth distribution of simulated urine in a range of soils soon after deposition. N. Z. J. Agric. Res. 1999, 42, 501–511. [Google Scholar] [CrossRef]
- Davis, G.; Thompson, H.; Bentley, G. Stiles Ground-water tracers—A short review. Groundwater 1980, 18, 14–23. [Google Scholar] [CrossRef]
- Levy, B.S.; Chambers, R.M. Bromide as a conservative tracer for soil-water studies. Hydrol. Process. 1987, 1, 385–389. [Google Scholar] [CrossRef]
- Luzio, W. Suelos de Chile; Universidad de Chile: Santiago, Chile, 2010; 360p. [Google Scholar]
- Besoain, E. Minerales secundarios. In Suelos Volcánicos de Chile; Tosso, J., Ed.; INIA: Santiago, Chile, 1985; pp. 23–106. [Google Scholar]
- Shoji, S.; Nanzyo, M.; Dahlgren, R. Volcanic Ash Soils: Genesis, Properties and Utilization; Elsevier Science: Amsterdam, The Netherlands, 1994; 288p. [Google Scholar]
- Soil Survey Staff. Keys to Soil Taxonomy, 11th ed.; USDA-Natural Resources Conservation Services: Washington DC, USA, 2010; 346p. [Google Scholar]
- Armas-Espinel, S.; Hernández-Moreno, J.; Muñoz-Carpena, R.; Regalado, C. Physical properties of “sorriba”—Cultivated volcanic soils from Tenerife in relation to andic diagnostic parameters. Geoderma 2003, 117, 297–311. [Google Scholar] [CrossRef]
- Ellies, A.; Grez, R.; Ramírez, C. La conductividad hidráulica en fase saturada como herramienta para el diagnóstico de la estructura del suelo. Agro Sur 1997, 25, 51–56. [Google Scholar] [CrossRef]
- Dörner, J.; Dec, D.; Peng, X.; Horn, R. Change of shrinkage behavior of an Andisol in southern Chile: Effects of land use and wetting/drying cycles. Soil Tillage Res. 2009, 106, 45–53. [Google Scholar] [CrossRef]
- Dörner, J.; Dec, D.; Feest, E.; Vásquez, N.; Díaz, M. Dynamics of soil structure and pore functions of a volcanic ash soil under tillage. Soil Tillage Res. 2012, 125, 52–60. [Google Scholar] [CrossRef]
- Matus, F.; Amigo, X.; Kristiansen, S. Aluminium stabilization controls organic carbon levels in Chilean volcanic soils. Geoderma 2006, 132, 158–168. [Google Scholar] [CrossRef]
- Dörner, J.; Dec, D.; Peng, X.; Horn, R. Effect of land use change on the dynamic behaviour of structural properties of an Andisol in southern Chile under saturated and unsaturated hydraulic conditions. Geoderma 2010, 159, 189–197. [Google Scholar] [CrossRef]
- Dörner, J.; Dec, D.; Peng, X.; Horn, R. Effect of land use on structural stability and pore functions of an andisol (Typic hapludand) in Southern Chile. J. Soil. Sci. Plant Nutr. 2009, 9, 190–209. [Google Scholar]
- Centro de Información de Recursos Naturales (CIREN). Estudio Agrológico X Región. Descripción de Suelos, Materiales y Símbolos; CIREN: Santiago, Chile, 2003; 412p. [Google Scholar]
- Balocchi, O.; López, I.G. Herbage production, nutritive value and grazing preference of diploid and tetraploid perennial ryegrass cultivars (Lolium perenne L.). Chil. J. Agric. Res. 2009, 69, 331–339. [Google Scholar] [CrossRef]
- Red Agrometeorológica del Instituto de Investigaciones Agropecuarias. Available online: https://agrometeorologia.cl (accessed on 11 March 2021).
- Williams, P.H.; Gregg, P.E.H.; Hedley, M.J. Use of potassium bromide solutions to stimulate dairy cow urine flow and retention in pasture soils. N. Z. J. Agric. Res. 1990, 33, 489–495. [Google Scholar] [CrossRef]
- Abdalla, N.A.; Lear, B. Determination of inorganic bromide in soils and plant tissues with a bromide selective-ion electrode. Commun. Soil Sci. Plant Anal. 1975, 6, 489–494. [Google Scholar] [CrossRef]
- Kear, B.; Gibbs, H.; Miller, R. Soils of the Downs and Plains of Canterbury and North Otago, New Zealand; Bulletin N° 14; Soil Bureau: Wellington, New Zealand, 1967. [Google Scholar]
- Williams, P.H.; Haynes, R.J. Forms of sulphur in sheep excreta and their fate after application on to pasture soil. J. Sci. Food Agric. 1993, 62, 323–329. [Google Scholar] [CrossRef]
- Nguyen, M.L.; Goh, K.M. Distribution, transformations and recovery of urinary sulphur and sources of plant-available soil sulphur in irrigated pasture soil-plant systems treated with 35sulphur-labelled urine. J. Agric. Sci. 1994, 122, 91–105. [Google Scholar] [CrossRef]
- Lantinga, E.A.; Keuning, J.A.; Groenwold, J.; Deenen, P.A.G. Distribution of excreted nitrogen by grazing cattle and its effects on sward quality, herbage production and utilization. In Animal Manure on Grassland and Fodder Crops: Fertilizer or Waste; Van der Meer, H.G., Unwin, R.J., van Dijk, T.A., Ennik, G.C., Eds.; Martinus Nijhoff: Dordrecht, The Netherlands, 1987; pp. 103–117. [Google Scholar]
- Ellies, A.; Horn, R.; Smith, R. Effect of management of a volcanic ash soil on structural properties. Int. Agrophysics 2000, 14, 377–384. [Google Scholar]
- Dec, D.; Dörner, J.; Balocchi, O.; López, I. Temporal dynamics of hydraulic and mechanical properties of an Andosol under grazing. Soil Tillage Res. 2012, 125, 44–51. [Google Scholar] [CrossRef]
- Dörner, J.; Horn, R. Anisotropy of pore functions in structured Stagnic Luvisols in the Weichselien moraine region in N Germany. J. Plant Nutr. Soil Sci. 2006, 169, 213–220. [Google Scholar] [CrossRef]
- Cameron, K.C.; Di, H.J.; Moir, J.L. Nitrogen losses from the soil/plant system: A review. Ann. Appl. Biol. 2013, 162, 145–173. [Google Scholar] [CrossRef]
- Hartge, K.H.; Horn, R. Die Physikalische Untersuchung von Bóden, 3rd ed.; Ferdinand Enke: Stuttgart, Germany, 1992; 176p. [Google Scholar]
- Zúñiga, F.; Dec, D.; Valle, S.R.; Dörner, J. Anisotropía de las propiedades hidráulicas de un Andisol del Sur de Chile. Agro Sur 2019, 44, 79–86. [Google Scholar] [CrossRef]
- Zúñiga, F.; Dec, D.; Valle, S.R.; Dörner, J.; MacDonald, R. Estabilidad estructural de un Andisol (Typic Durudand) bajo bosque nativo y pradera en el Sur de Chile. Agro Sur 2014, 42, 55–66. [Google Scholar] [CrossRef]
- Zúñiga, F.; Dec, D.; Valle, S.R.; Dörner, J.; MacDonald, R. Temporal dynamics of the physical quality of an Andisol under a grazing system subjected to different pasture improvement strategies. Soil Tillage Res. 2015, 145, 233–245. [Google Scholar] [CrossRef]
- Williams, P.H.; Haynes, R.J. Transformations and plant uptake of urine-sulphate in urine-affected areas of pasture soil. Plant Soil. 1992, 145, 167–175. [Google Scholar] [CrossRef]
- Ledgard, S.F.; Saunders, W.M.H. Effects of nitrogen fertilizer and urine on pasture performance and the influence of soil phosphorus and potassium status. N. Z. J. Agric. Res. 1982, 25, 541–547. [Google Scholar] [CrossRef]
- Safley, L.M.; Barker, J.C.; Westermann, P.W. Characteristics of fresh dairy manure. Trans. ASAE 1984, 27, 1150–1153. [Google Scholar] [CrossRef]
- Saarijärvi, K.; Virkajärvi, P. Nitrogen dynamics of cattle dung y urine patches on intensively managed boreal pasture. J. Agric. Sci. 2009, 147, 479–491. [Google Scholar] [CrossRef]
- Davidson, E.A.; Hart, S.C.; Shanks, C.A.; Firestone, M.K. Measuring gross nitrogen mineralization, immobilization, and nitrification by N-15 isotopic pool dilution in intact soil cores. J. Soil Sci. 1991, 42, 335–349. [Google Scholar] [CrossRef]
- Ryden, J.C. Denitrification loss from a grassland soil in the field receiving different rates of nitrogen as ammonium-nitrate. J. Soil Sci. 1983, 34, 355–365. [Google Scholar] [CrossRef]
- Singh, J.; Saggar, S.; Bolan, N.S. Influence of dicyandiamide on nitrogen transformation and losses in cow-urine-amended soil cores from grazed pasture. Anim. Prod. Sci. 2009, 49, 253–261. [Google Scholar] [CrossRef]
- Velthof, G.L.; Oenema, O.; Postma, R.; Beusichem, M.L.V.; Van Beusichem, M.L. Effects of type and amount of applied nitrogen fertilizer on nitrous oxide fluxes from intensively managed grassland. Nutr. Cycl. Agroecosyst. 1997, 46, 257–267. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).