Neuronal and Non-Neuronal GABA in COVID-19: Relevance for Psychiatry
Abstract
1. Introduction
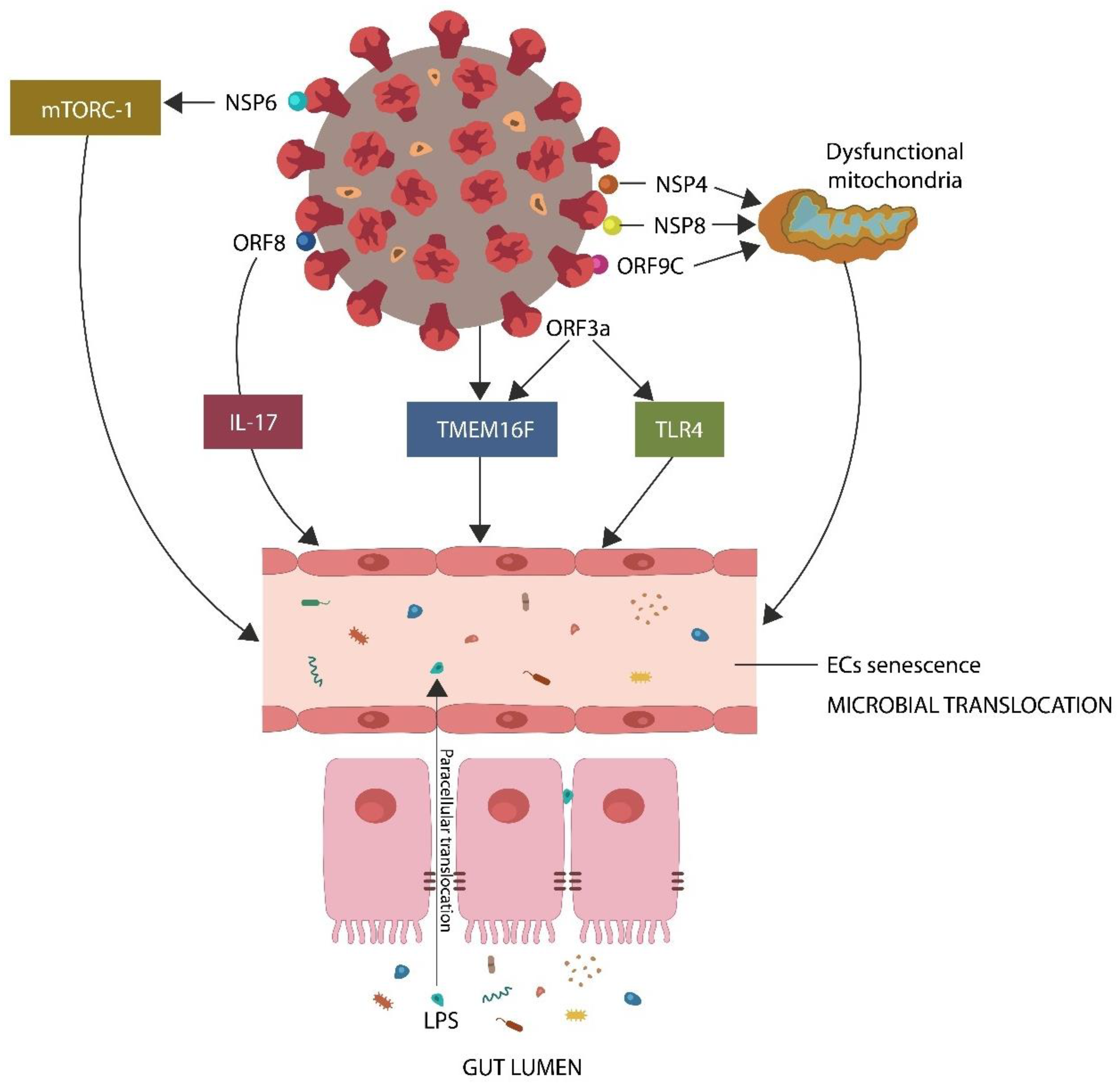
- The SARS-CoV-2 proteins nonstructural protein 6 (NSP6), open reading frame 8 (ORF8), and open reading frame 3 (ORF3a) interact directly with host mammalian target of rapamycin complex 1 (mTORC-1), interleukin 17 (IL-17), and transmembrane protein 16F (TMEM16F), inducing premature EC senescence, a phenotype characterized by low GABA [24,25,26,27,28,29] (Figure 1).
- The viral protein ORF3a interacts with toll-like receptor 4(TLR4), triggering EC senescence and lowering GABA [31].
2. Two Senescence Mechanisms in SARS-CoV-2 Infection
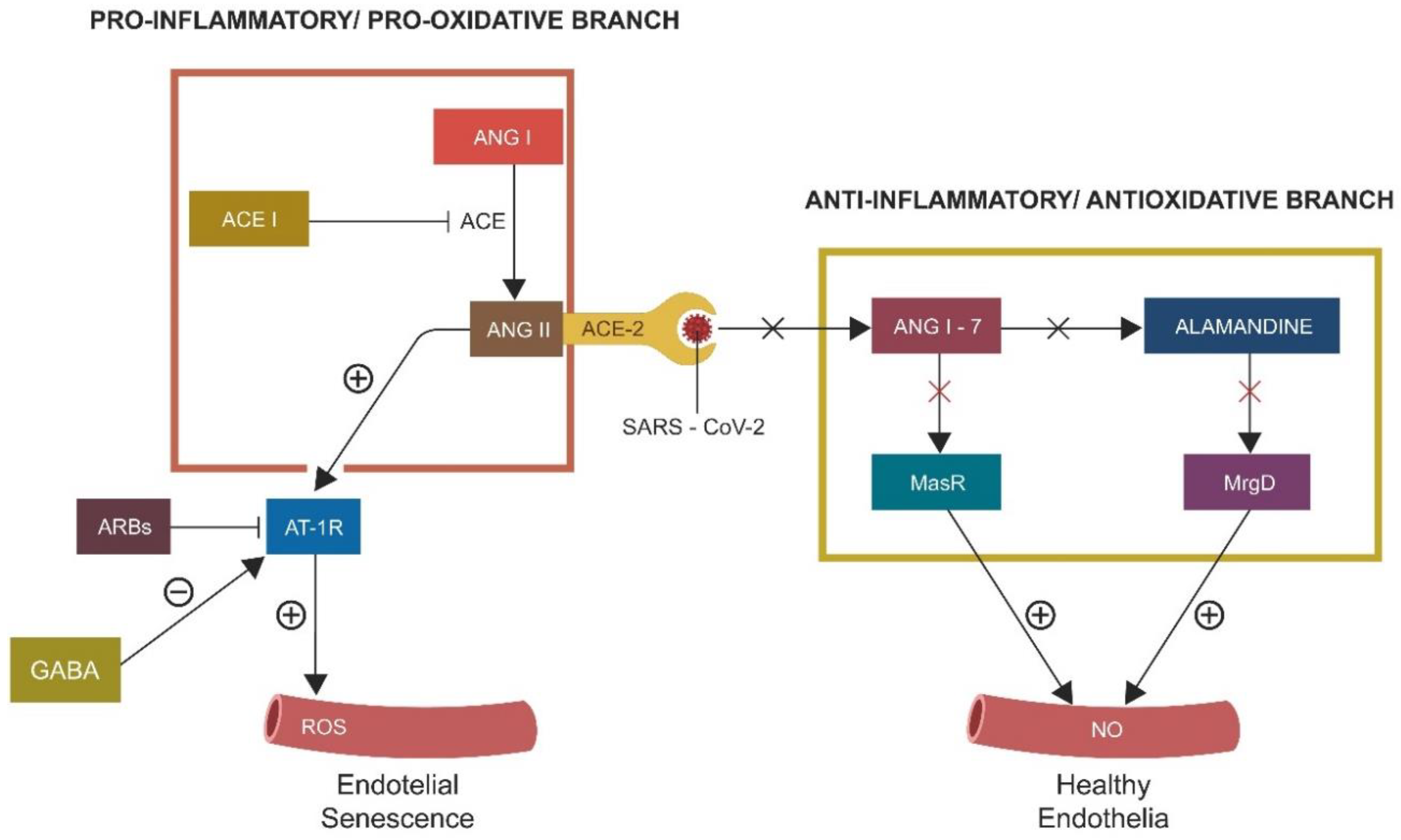
2.1. S1/ACE-2 Attachment and ANG II-Induced Senescence
2.2. S2/Furin Attachment and Syncytia-Induced Senescence
2.3. Molecular Mechanisms of Syncytia Formation
2.4. Biological Barrier Dysfunction
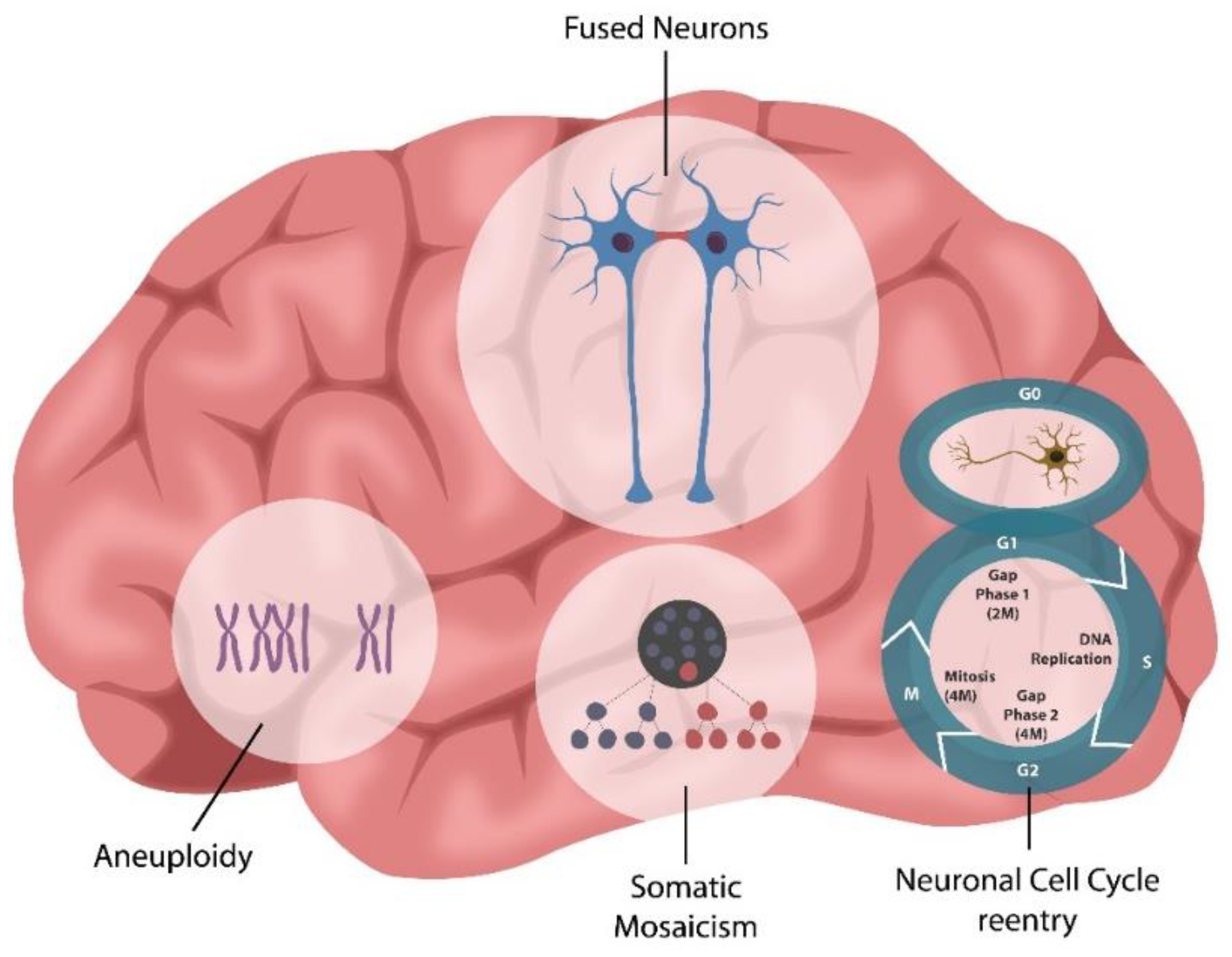
3. Cellular Senescence in Psychopathology
Adult Neurogenesis and SARS-CoV-2 Infection
4. GABA, Neuronal and Non-Neuronal Information Processing
4.1. Non-Neuronal Information Processing
4.2. eGABA
4.3. pGABA
4.4. mGABA
5. Ancient and Modern Viruses Disrupt GABAergic Signaling
5.1. Syncytia Inhibitors
5.2. GABA, Autophagy, and Blood Pressure
| Drug | Mechanism | References |
|---|---|---|
| Arginine mimetics | Furin inhibition | [195,196] |
| Niclosamide | TMEM16F inhibition | [202] |
| Ivermectin | GABA upregulation | [36,37] |
| ARBs/ACEi | GABA upregulation | [14,15,16] |
| Benzodiazepines | GABA upregulation | [213] |
6. Conclusions
- ACE-2 is protective for the GABAergic signaling in both neuronal and non-neuronal pathways.
- Inhibition of protective RAS promotes cellular senescence, lowering neuronal and non-neuronal GABA.
- Virus-induced syncytia formation is a major trigger of premature cellular senescence and related pathology.
- Aside from functioning as a neurotransmitter, GABA displays anti-hypertension, anti-senescence, anti-diabetes, antioxidant, and anti-inflammatory properties.
- ARBs and ACEi upregulate GABA, promoting adult neurogenesis that prevents senescence-mediated psychopathologies.
- The S2 protein of SARS-CoV-2 contains a triple-arginine insert that activates HERVs, promoting hyperinflammatory pathologies.
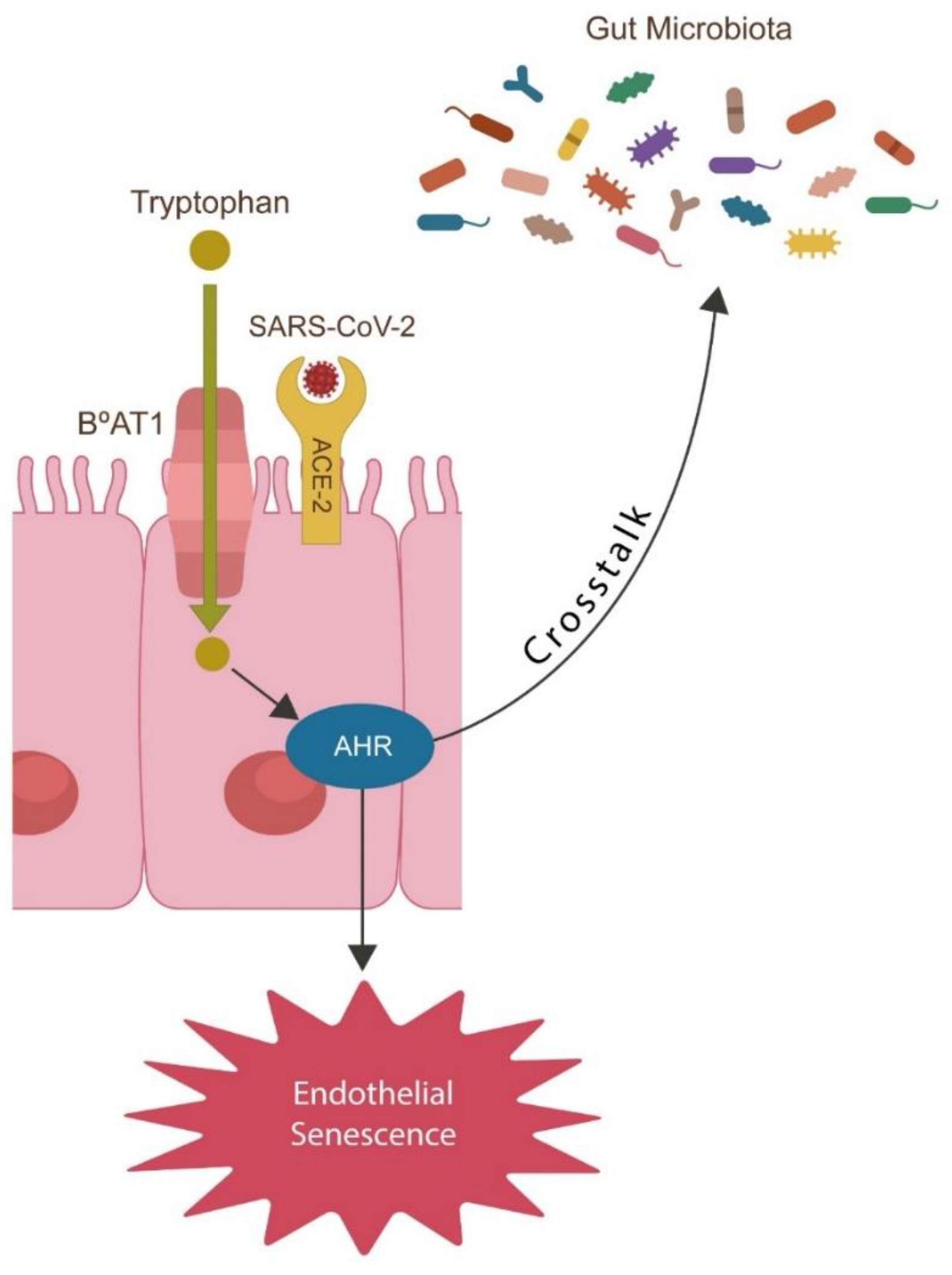
- SARS-CoV-2 alters Trp catabolism and the GABA-producing gut flora, facilitating microbial translocation from the GI tract into various tissues and organs, including the brain.
- Furin and TMEM16F inhibitors suppress syncytia formation, while ARBs and ACEi upregulate GABA, lowering ANG II-induced senescence.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Disclaimer
Abbreviations
| eGABA | endothelial GABA |
| mGABA | microbial GABA |
| pGABA | pancreatic GABA |
| nGABA | neuronal GABA |
| TLR4 | toll-like receptor 4 |
| ANG II | angiotensin converting enzyme 2 |
References
- Benavente, L.; Morís, G. Neurologic disorders associated with inflammatory bowel disease. Eur. J. Neurol. 2010, 18, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Abautret-Daly, Á.; Dempsey, E.; Parra-Blanco, A.; Medina, C.; Harkin, A. Gut–brain actions underlying comorbid anxiety and depression associated with inflammatory bowel disease. Acta Neuropsychiatr. 2017, 30, 275–296. [Google Scholar] [CrossRef] [PubMed]
- Nowakowski, J.; Chrobak, A.; Dudek, D. Psychiatry Illness in Inflammatory Bowel Diseases-Psychiatric Comorbidity and Biological Underpinnings. Psychiatr. Polska 2016, 50, 1157–1166. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Rodríguez, E.; Camarero-González, E. Paciente con enfermedad de Crohn y convulsiones por hipomagnesemia [Patient with Crohn’s disease and seizures due to hypomagnesemia]. Nutr. Hosp. 2007, 22, 720–722. [Google Scholar]
- Ma, P.; Li, T.; Ji, F.; Wang, H.; Pang, J. Effect of GABA on blood pressure and blood dynamics of anesthetic rats. Int. J. Clin. Exp. Med. 2015, 8, 14296–14302. [Google Scholar] [PubMed]
- Buzhdygan, T.; Lisinicchia, J.; Patel, V.; Johnson, K.; Neugebauer, V.; Paessler, S.; Jennings, K.; Gelman, B. Neuropsychological, Neurovirological and Neuroimmune Aspects of Abnormal GABAergic Transmission in HIV Infection. J. Neuroimmune Pharmacol. 2016, 11, 279–293. [Google Scholar] [CrossRef]
- Jehmlich, U.; Ritzer, J.; Grosche, J.; Härtig, W.; Liebert, U.G. Experimental measles encephalitis in Lewis rats: Dissemination of infected neuronal cell subtypes. J. Neurovirol. 2013, 19, 461–470. [Google Scholar] [CrossRef]
- Tian, J.; Middleton, B.; Kaufman, D.L. GABA administration prevents severe illness and death following coronavirus infection in mice. Preprint. bioRxiv 2020. [CrossRef]
- Bhat, R.; Axtell, R.; Mitra, A.; Miranda, M.; Lock, C.; Tsien, R.W.; Steinman, L. Inhibitory role for GABA in autoimmune inflammation. Proc. Natl. Acad. Sci. USA 2010, 107, 2580–2585. [Google Scholar] [CrossRef]
- Prud’Homme, G.J.; Glinka, Y.; Wang, Q. Immunological GABAergic interactions and therapeutic applications in autoimmune diseases. Autoimmun. Rev. 2015, 14, 1048–1056. [Google Scholar] [CrossRef]
- Li, Y.; Gao, J.; Xue, L.; Shang, Y.; Cai, W.; Xie, X.; Jiang, T.; Chen, H.; Zhang, J.; Wang, J.; et al. Determination of Antiviral Mechanism of Centenarian Gut-Derived Limosilactobacillus fermentum Against Norovirus. Front. Nutr. 2022, 9, 812623. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Kim, Y.S.; Lee, H.-M.; Jin, H.S.; Neupane, C.; Kim, S.; Lee, S.-H.; Min, J.-J.; Sasai, M.; Jeong, J.-H.; et al. GABAergic signaling linked to autophagy enhances host protection against intracellular bacterial infections. Nat. Commun. 2018, 9, 4184. [Google Scholar] [CrossRef] [PubMed]
- Otaru, N.; Ye, K.; Mujezinovic, D.; Berchtold, L.; Constancias, F.; Cornejo, F.A.; Krzystek, A.; de Wouters, T.; Braegger, C.; Lacroix, C.; et al. GABA Production by Human Intestinal Bacteroides spp.: Prevalence, Regulation, and Role in Acid Stress Tolerance. Front. Microbiol. 2021, 12, 656895. [Google Scholar] [CrossRef]
- Krasniqi, S.; Daci, A. Role of the Angiotensin Pathway and its Target Therapy in Epilepsy Management. Int. J. Mol. Sci. 2019, 20, 726. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Havens, J.; Yu, Q.; Wang, G.; Davisson, R.L.; Pickel, V.M.; Iadecola, C. The link between angiotensin II-mediated anxiety and mood disorders with NADPH oxi-dase-induced oxidative stress. Int. J. Physiol. Pathophysiol. Pharmacol. 2012, 4, 28–35. [Google Scholar] [PubMed]
- Pereira, M.G.; Becari, C.; Oliveira, J.A.; Salgado, M.C.O.; Garcia-Cairasco, N.; Costa-Neto, C.M. Inhibition of the renin–angiotensin system prevents seizures in a rat model of epilepsy. Clin. Sci. 2010, 119, 477–482. [Google Scholar] [CrossRef]
- Jo, Y.; Kim, S.; Ye, B.S.; Lee, E.; Yu, Y.M. Protective Effect of Renin-Angiotensin System Inhibitors on Parkinson’s Disease: A Nationwide Cohort Study. Front. Pharmacol. 2022, 13, 837890. [Google Scholar] [CrossRef] [PubMed]
- Saavedra, J.M. Angiotensin II AT1 receptor blockers as treatments for inflammatory brain disorders. Clin. Sci. 2012, 123, 567–590. [Google Scholar] [CrossRef]
- Chang, C.-H.; Chang, Y.-C.; Wu, L.-C.; Lin, J.-W.; Chuang, L.-M.; Lai, M.-S. Different angiotensin receptor blockers and incidence of diabetes: A nationwide population-based cohort study. Cardiovasc. Diabetol. 2014, 13, 91. [Google Scholar] [CrossRef]
- Ma, X.; Gao, F.; Chen, Q.; Xuan, X.; Wang, Y.; Deng, H.; Yang, F.; Yuan, L. ACE2 modulates glucose homeostasis through GABA signaling during metabolic stress. J. Endocrinol. 2020, 246, 223–236. [Google Scholar] [CrossRef]
- Sánchez-Lemus, E.; Honda, M.; Saavedra, J.M. Angiotensin II AT1 receptor blocker candesartan prevents the fast up-regulation of cerebrocortical benzodiazepine-1 receptors induced by acute inflammatory and restraint stress. Behav. Brain Res. 2012, 232, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Beaudoin, C.A.; Jamasb, A.R.; Alsulami, A.F.; Copoiu, L.; van Tonder, A.J.; Hala, S.; Bannerman, B.P.; Thomas, S.E.; Vedithi, S.C.; Torres, P.H.; et al. Predicted structural mimicry of spike receptor-binding motifs from highly pathogenic human coronaviruses. Comput. Struct. Biotechnol. J. 2021, 19, 3938–3953. [Google Scholar] [CrossRef] [PubMed]
- Yapici-Eser, H.; Koroglu, Y.E.; Oztop-Cakmak, O.; Keskin, O.; Gursoy, A.; Gursoy-Ozdemir, Y. Neuropsychiatric Symptoms of COVID-19 Explained by SARS-CoV-2 Proteins’ Mimicry of Human Protein Interactions. Front. Hum. Neurosci. 2021, 15, 656313. [Google Scholar] [CrossRef]
- Porges, E.C.; Jensen, G.; Foster, B.; Edden, R.A.; Puts, N.A. The trajectory of cortical GABA across the lifespan, an individual participant data meta-analysis of edited MRS studies. eLife 2021, 10, e62575. [Google Scholar] [CrossRef] [PubMed]
- Ethiraj, J.; Palpagama, T.H.; Turner, C.; van der Werf, B.; Waldvogel, H.J.; Faull, R.L.M.; Kwakowsky, A. The effect of age and sex on the expression of GABA signaling components in the human hippocampus and entorhinal cortex. Sci. Rep. 2021, 11, 21470. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Li, Q.; Wang, Y.; Shi, Y. Syncytia formation during SARS-CoV-2 lung infection: A disastrous unity to eliminate lymphocytes. Cell Death Differ. 2021, 28, 2019–2021. [Google Scholar] [CrossRef] [PubMed]
- Gordon, D.E.; Jang, G.M.; Bouhaddou, M.; Xu, J.; Obernier, K.; White, K.M.; O’Meara, M.J.; Rezelj, V.V.; Guo, J.Z.; Swaney, D.L.; et al. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature 2020, 583, 459–468. [Google Scholar] [CrossRef]
- Mao, L.-Y.; Ding, J.; Peng, W.-F.; Ma, Y.; Zhang, Y.-H.; Fan, W.; Wang, X. Interictal interleukin-17A levels are elevated and correlate with seizure severity of epilepsy patients. Epilepsia 2013, 54, e142–e145. [Google Scholar] [CrossRef]
- Griffith, J.L.; Wong, M. The mTOR pathway in treatment of epilepsy: A clinical update. Futur. Neurol. 2018, 13, 49–58. [Google Scholar] [CrossRef]
- Wang, L.; de Kloet, A.; Pati, D.; Hiller, H.; Smith, J.A.; Pioquinto, D.J.; Ludin, J.A.; Oh, S.P.; Katovich, M.J.; Frazier, C.J.; et al. Increasing brain angiotensin converting enzyme 2 activity decreases anxiety-like behavior in male mice by activating central Mas receptors. Neuropharmacology 2016, 105, 114–123. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Q.; Cosme, R.S.C.; Gerzanich, V.; Tang, Q.; Simard, J.M.; Zhao, R.Y. Genome-Wide Characterization of SARS-CoV-2 Cytopathogenic Proteins in the Search of Antiviral Targets. mBio 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Targhetta, V.P.; Amaral, M.A.; Camara, N.O.S. Through DNA sensors and hidden mitochondrial effects of SARS-CoV-2. J. Venom. Anim. Toxins Incl. Trop. Dis. 2021, 27, e20200183. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Yuan, K.; Wang, Z.; Liu, W.-J.; Lu, Z.-A.; Liu, L.; Shi, L.; Yan, W.; Yuan, J.-L.; Li, J.-L.; et al. Neuropsychiatric manifestations of COVID-19, potential neurotropic mechanisms, and therapeutic interventions. Transl. Psychiatry 2021, 11, 499. [Google Scholar] [CrossRef] [PubMed]
- Emami, A.; Fadakar, N.; Akbari, A.; Lotfi, M.; Farazdaghi, M.; Javanmardi, F.; Rezaei, T.; Asadi-Pooya, A.A. Seizure in patients with COVID-19. Neurol. Sci. 2020, 41, 3057–3061. [Google Scholar] [CrossRef] [PubMed]
- Sfera, A.; Osorio, C.; Rahman, L.; del Campo, C.M.Z.-M.; Maldonado, J.C.; Jafri, N.; Cummings, M.A.; Maurer, S.; Kozlakidis, Z. PTSD as an Endothelial Disease: Insights from COVID-19. Front. Cell. Neurosci. 2021, 15, 770387. [Google Scholar] [CrossRef] [PubMed]
- Krusek, J.; Zemkova´, H. Effect of ivermectin on γ-aminobutyric acid-induced chloride currents in mouse hippocampal embryonic neurones. Eur. J. Pharmacol. 1994, 259, 121–128. [Google Scholar] [CrossRef]
- Li, M.P.; Eaton, M.M.M.; Steinbach, J.H.; Akk, G. The Benzodiazepine Diazepam Potentiates Responses of α1β2γ2L γ-Aminobutyric Acid Type A Receptors Activated by either γ-Aminobutyric Acid or Allosteric Agonists. Anesthesiology 2013, 118, 1417–1425. [Google Scholar] [CrossRef]
- Chan, Y.A.; Zhan, S.H. The Emergence of the Spike Furin Cleavage Site in SARS-CoV-2. Mol. Biol. Evol. 2021, 39, msab327. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, L.; Lu, X. Regulation of Angiotensin-Converting Enzyme 2: A Potential Target to Prevent COVID-19? Front. Endocrinol. 2021, 12, 725967. [Google Scholar] [CrossRef]
- Doughan, A.K.; Harrison, D.G.; Dikalov, S.I. Molecular Mechanisms of Angiotensin II–Mediated Mitochondrial Dysfunction. Circ. Res. 2008, 102, 488–496. [Google Scholar] [CrossRef]
- Bobkova, N.V. The Balance between Two Branches of RAS Can Protect from Severe COVID-19 Course. Biochem. (Moscow) Suppl. Ser. A Membr. Cell Biol. 2021, 15, 36–51. [Google Scholar] [CrossRef] [PubMed]
- Mahmudpour, M.; Roozbeh, J.; Keshavarz, M.; Farrokhi, S.; Nabipour, I. COVID-19 cytokine storm: The anger of inflammation. Cytokine 2020, 133, 155151. [Google Scholar] [CrossRef] [PubMed]
- Romero, E.; Guaza, C.; Castellano, B.; I Borrell, J. Ontogeny of sensorimotor gating and immune impairment induced by prenatal immune challenge in rats: Implications for the etiopathology of schizophrenia. Mol. Psychiatry 2008, 15, 372–383. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.; Roy, S.; Bandyopadhyay, G.; Scott, B.; Xiao, D.; Ramadoss, S.; Mahata, S.K.; Chaudhuri, G. γ-Aminobutyric Acid Is Synthesized and Released by the Endothelium. Circ. Res. 2016, 119, 621–634. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, J.; Liu, C.; Su, L.; Zhang, D.; Fan, J.; Yang, Y.; Xiao, M.; Xie, J.; Xu, Y.; et al. IP-10 and MCP-1 as biomarkers associated with disease severity of COVID-19. Mol. Med. 2020, 26, 97. [Google Scholar] [CrossRef] [PubMed]
- Stragier, B.; Hristova, I.; Sarre, S.; Ebinger, G.; Michotte, Y. In vivo characterization of the angiotensin-(1-7)-induced dopamine and γ-aminobutyric acid release in the striatum of the rat. Eur. J. Neurosci. 2005, 22, 658–664. [Google Scholar] [CrossRef]
- Brukman, N.G.; Uygur, B.; Podbilewicz, B.; Chernomordik, L.V. How cells fuse. J. Cell Biol. 2019, 218, 1436–1451. [Google Scholar] [CrossRef]
- Zhang, Y.; Le, T.; Grabau, R.; Mohseni, Z.; Kim, H.; Natale, D.R.; Feng, L.; Pan, H.; Yang, H. TMEM16F phospholipid scramblase mediates trophoblast fusion and placental development. Sci. Adv. 2020, 6, eaba0310. [Google Scholar] [CrossRef]
- Peacock, T.P.; Goldhill, D.H.; Zhou, J.; Baillon, L.; Frise, R.; Swann, O.C.; Kugathasan, R.; Penn, R.; Brown, J.C.; Sanchez-David, R.Y.; et al. The furin cleavage site in the SARS-CoV-2 spike protein is required for transmission in ferrets. Nat. Microbiol. 2021, 6, 899–909. [Google Scholar] [CrossRef]
- Leroy, H.; Han, M.; Woottum, M.; Bracq, L.; Bouchet, J.; Xie, M.; Benichou, S. Virus-Mediated Cell-Cell Fusion. Int. J. Mol. Sci. 2020, 21, 9644. [Google Scholar] [CrossRef]
- Armstrong, C.T.; Mason, P.; Anderson, R.; Dempsey, C.E. Arginine side chain interactions and the role of arginine as a gating charge carrier in voltage sensitive ion channels. Sci. Rep. 2016, 6, 21759. [Google Scholar] [CrossRef] [PubMed]
- Osorio, C.; Sfera, A.; Anton, J.J.; Thomas, K.G.; Andronescu, C.V.; Li, E.; Yahia, R.W.; Avalos, A.G.; Kozlakidis, Z. Virus-Induced Membrane Fusion in Neurodegenerative Disorders. Front. Cell. Infect. Microbiol. 2022, 12, 845580. [Google Scholar] [CrossRef] [PubMed]
- Chuprin, A.; Gal, H.; Biron-Shental, T.; Biran, A.; Amiel, A.; Rozenblatt, S.; Krizhanovsky, V. Cell fusion induced by ERVWE1 or measles virus causes cellular senescence. Genes Dev. 2013, 27, 2356–2366. [Google Scholar] [CrossRef] [PubMed]
- Berndt, B.; Zanker, K.S.; Dittmar, T. Cell fusion is a potent inducer of aneuploidy and drug resistance in tumor cell/ normal cell hybrids. Crit. Rev. Oncog. 2013, 18, 97–113. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.R.; Muir, J.; Rao, Y.; Browarski, M.; Gruenig, M.C.; Sheehan, D.F.; Haucke, V.; Kittler, J.T. Stabilization of GABAA Receptors at Endocytic Zones Is Mediated by an AP2 Binding Motif within the GABAA Receptor β3 Subunit. J. Neurosci. 2012, 32, 2485–2498. [Google Scholar] [CrossRef] [PubMed]
- Hegyi, H. GABBR1 has a HERV-W LTR in its regulatory region—A possible implication for schizophrenia. Biol. Direct 2013, 8, 5. [Google Scholar] [CrossRef] [PubMed]
- Obrietan, K.; Pol, A.N.V.D. GABAB receptor-mediated inhibition of GABAA receptor calcium elevations in developing hypothalamic neurons. J. Neurophysiol. 1998, 79, 1360–1370. [Google Scholar] [CrossRef]
- Hallenberger, S.; Bosch, V.; Angliker, H.; Shaw, E.; Klenk, H.-D.; Garten, W. Inhibition of furin-mediated cleavage activation of HIV-1 glycoprotein gpl60. Nature 1992, 360, 358–361. [Google Scholar] [CrossRef]
- Charvet, B.; Brunel, J.; Pierquin, J.; Iampietro, M.; Decimo, D.; Queruel, N.; Lucas, A.; Encabo-Berzosa, M.d.M.; Arenaz, I.; Marmolejo, T.P.; et al. SARS-CoV-2 induces human endogenous retrovirus type W envelope protein expression in blood lym-phocytes and in tissues of COVID-19 patients. medRxiv 2022. Available online: https://www.medrxiv.org/content/10.1101/2022.01.18.21266111v2 (accessed on 15 May 2022). [CrossRef]
- Schleiss, M.R. Letermovir and HCT: Too much of a good thing? Blood 2021, 138, 1–2. [Google Scholar] [CrossRef]
- Sikora, E.; Bielak-Zmijewska, A.; Dudkowska, M.; Krzystyniak, A.; Mosieniak, G.; Wesierska, M.; Wlodarczyk, J. Cellular Senescence in Brain Aging. Front. Aging Neurosci. 2021, 13, 646924. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Shan, P.; Hwangbo, C.; Zhang, Y.; Min, J.; Zhang, X.; Ardito, T.; Li, A.; Peng, T.; Sauler, M.; et al. Endothelial toll-like receptor 4 maintains lung integrity via epigenetic suppression of p16 INK4a. Aging Cell 2019, 18, e12914. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Song, Z.-G.; Liu, C.; Tan, S.; Lin, S.; Zhu, J.; Dai, F.-H.; Gao, J.; She, J.-L.; Mei, Z.; et al. Gut microbiome alterations and gut barrier dysfunction are associated with host immune homeostasis in COVID-19 patients. BMC Med. 2022, 20, 24. [Google Scholar] [CrossRef] [PubMed]
- Peerapornratana, S.; Sirivongrangson, P.; Tungsanga, S.; Tiankanon, K.; Kulvichit, W.; Putcharoen, O.; Kellum, J.A.; Srisawat, N. Endotoxin Adsorbent Therapy in Severe COVID-19 Pneumonia. Blood Purif. 2021, 51, 47–54. [Google Scholar] [CrossRef]
- Petruk, G.; Puthia, M.; Petrlova, J.; Samsudin, F.; Strömdahl, A.-C.; Cerps, S.; Uller, L.; Kjellström, S.; Bond, P.J.; Schmidtchen, A. SARS-CoV-2 Spike protein binds to bacterial lipopolysaccharide and boosts proinflammatory activity. J. Mol. Cell Biol. 2020, 12, 916–932. [Google Scholar] [CrossRef]
- Wolf, G.; Bohlender, J.; Bondeva, T.; Roger, T.; Thaiss, F.; Wenzel, U.O. Angiotensin II Upregulates Toll-Like Receptor 4 on Mesangial Cells. J. Am. Soc. Nephrol. 2006, 17, 1585–1593. [Google Scholar] [CrossRef]
- Lu, Y.-C.; Yeh, W.-C.; Ohashi, P.S. LPS/TLR4 signal transduction pathway. Cytokine 2008, 42, 145–151. [Google Scholar] [CrossRef]
- O’Connor, J.C.; A Lawson, M.; André, C.; Moreau, M.; Lestage, J.; Castanon, N.; Kelley, K.W.; Dantzer, R. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol. Psychiatry 2008, 14, 511–522. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Sun, S.-P.; Zhu, H.-S.; Jiao, X.-Q.; Zhong, K.; Guo, Y.-J.; Zha, G.-M.; Han, L.-Q.; Yang, G.-Y.; Li, H.-P. GABA regulates the proliferation and apoptosis of MAC-T cells through the LPS-induced TLR4 signaling pathway. Res. Veter. Sci. 2018, 118, 395–402. [Google Scholar] [CrossRef]
- Zhao, Y.; Cong, L.; Lukiw, W.J. Lipopolysaccharide (LPS) Accumulates in Neocortical Neurons of Alzheimer’s Disease (AD) Brain and Impairs Transcription in Human Neuronal-Glial Primary Co-cultures. Front. Aging Neurosci. 2017, 9, 407. [Google Scholar] [CrossRef]
- Solas, M.; Puerta, E.; Ramirez, M. Treatment Options in Alzheimer´s Disease: The GABA Story. Curr. Pharm. Des. 2015, 21, 4960–4971. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi-Matsui, M.; Yano, S.; Matsumoto, N.; Futai, M. Lipopolysaccharide induces multinuclear cell from RAW264.7 line with increased phagocytosis activity. Biochem. Biophys. Res. Commun. 2012, 425, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Giordano-Santini, R.; Kaulich, E.; Galbraith, K.M.; Ritchie, F.K.; Wang, W.; Li, Z.; Hilliard, M.A. Fusogen-mediated neuron−neuron fusion disrupts neural circuit connectivity and alters animal behavior. Proc. Natl. Acad. Sci. USA 2020, 117, 23054–23065. [Google Scholar] [CrossRef] [PubMed]
- Kemp, K.; Wilkins, A.; Scolding, N. Cell fusion in the brain: Two cells forward, one cell back. Acta Neuropathol. 2014, 128, 629–638. [Google Scholar] [CrossRef]
- Arendt, T.; Mosch, B.; Morawski, M. Neuronal Aneuploidy in Health and Disease:A Cytomic Approach to Understand the Molecular Individuality of Neurons. Int. J. Mol. Sci. 2009, 10, 1609–1627. [Google Scholar] [CrossRef]
- Kemp, K.; Gray, E.; Wilkins, A.; Scolding, N. Purkinje cell fusion and binucleate heterokaryon formation in multiple sclerosis cerebellum. Brain 2012, 135, 2962–2972. [Google Scholar] [CrossRef]
- Potter, H.; Chial, H.J.; Caneus, J.; Elos, M.; Elder, N.; Borysov, S.; Granic, A. Chromosome Instability and Mosaic Aneuploidy in Neurodegenerative and Neurodevelopmental Disorders. Front. Genet. 2019, 10, 1092. [Google Scholar] [CrossRef]
- Paquola, A.C.; Erwin, J.; Gage, F.H. Insights into the role of somatic mosaicism in the brain. Curr. Opin. Syst. Biol. 2016, 1, 90–94. [Google Scholar] [CrossRef]
- Lindqvist, D.; Epel, E.S.; Mellon, S.H.; Penninx, B.W.; Révész, D.; Verhoeven, J.E.; Reus, V.I.; Lin, J.; Mahan, L.; Hough, C.M.; et al. Psychiatric disorders and leukocyte telomere length: Underlying mechanisms linking mental illness with cellular aging. Neurosci. Biobehav. Rev. 2015, 55, 333–364. [Google Scholar] [CrossRef]
- Pousa, P.; Souza, R.; Melo, P.; Correa, B.; Mendonça, T.; Simões-E-Silva, A.; Miranda, D. Telomere Shortening and Psychiatric Disorders: A Systematic Review. Cells 2021, 10, 1423. [Google Scholar] [CrossRef]
- E Verhoeven, J.; Révész, D.; Epel, E.S.; Lin, J.; Wolkowitz, O.M.; Penninx, B.W.J.H. Major depressive disorder and accelerated cellular aging: Results from a large psychiatric cohort study. Mol. Psychiatry 2013, 19, 895–901. [Google Scholar] [CrossRef] [PubMed]
- Papanastasiou, E.; Gaughran, F.; Smith, S. Schizophrenia as segmental progeria. J. R. Soc. Med. 2011, 104, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-Y.; Lai, Y.-L.; Liu, K.-H.; Lin, S.; Chen, H.-Y.; Liang, C.-H.; Wu, H.-M.; Hsu, K.-S. TNFα-mediated necroptosis in brain endothelial cells as a potential mechanism of increased seizure susceptibility in mice following systemic inflammation. J. Neuroinflammation 2022, 19, 29. [Google Scholar] [CrossRef] [PubMed]
- Ogrodnik, M.; Zhu, Y.; Langhi, L.G.; Tchkonia, T.; Krüger, P.; Fielder, E.; Victorelli, S.; Ruswhandi, R.A.; Giorgadze, N.; Pirtskhalava, T.; et al. Obesity-Induced Cellular Senescence Drives Anxiety and Impairs Neurogenesis. Cell Metab. 2019, 29, 1061–1077. [Google Scholar] [CrossRef] [PubMed]
- Sekiguchi, F.; Tsubota, M.; Kawabata, A. Involvement of voltage-gated calcium channels in inflammation and inflam-matory pain. Biol. Pharm. Bull. 2018, 41, 1127–1134. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, N. The science of tea’s mood-altering magic. Nature 2019, 566, S8. [Google Scholar] [CrossRef] [PubMed]
- Sanches, M.; Colpo, G.D.; Cuellar, V.A.; Bockmann, T.; Rogith, D.; Soares, J.C.; Teixeira, A.L. Decreased Plasma Levels of Angiotensin-Converting Enzyme Among Patients with Bipolar Disorder. Front. Neurosci. 2021, 15, 617888. [Google Scholar] [CrossRef]
- Colbourne, L.; Luciano, S.; Harrison, P.J. Onset and recurrence of psychiatric disorders associated with anti-hypertensive drug classes. Transl. Psychiatry 2021, 11, 319. [Google Scholar] [CrossRef]
- Vian, J.; Pereira, C.; Chavarria, V.; Köhler, C.; Stubbs, B.; Quevedo, J.; Kim, S.-W.; Carvalho, A.F.; Berk, M.; Fernandes, B.S. The renin–angiotensin system: A possible new target for depression. BMC Med. 2017, 15, 144. [Google Scholar] [CrossRef]
- Xie, Y.; Xu, E.; Al-Aly, Z. Risks of mental health outcomes in people with covid-19: Cohort study. BMJ 2022, 376, e068993. [Google Scholar] [CrossRef]
- Firouzabadi, N.; Farshadfar, P.; Haghnegahdar, M.; Alavi-Shoushtari, A.; Ghanbarinezhad, V. Impact of ACE2 genetic variant on antidepressant efficacy of SSRIs. Acta Neuropsychiatr. 2021, 34, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Apple, D.M.; Fonseca, R.S.; Kokovay, E. The role of adult neurogenesis in psychiatric and cognitive disorders. Brain Res. 2017, 1655, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.; Soung, A.; Sissoko, C.; Nordvig, A.; Canoll, P.; Mariani, M.; Jiang, X.; Bricker, T.; Goldman, J.; Rosoklija, G.; et al. COVID-19 induces neuroinflammation and loss of hippocampal neurogenesis. Preprint Res. Sq. 2021, 1, rs.3.rs-1031824. [Google Scholar] [CrossRef]
- Ge, S.; Pradhan, D.A.; Ming, G.-L.; Song, H. GABA sets the tempo for activity-dependent adult neurogenesis. Trends Neurosci. 2007, 30, 1–8. [Google Scholar] [CrossRef]
- Mu, Y.; Lee, S.W.; Gage, F.H. Signaling in adult neurogenesis. Curr. Opin. Neurobiol. 2010, 20, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Owens, D.F.; Kriegstein, A.R. Is there more to GABA than synaptic inhibition? Nat. Rev. Neurosci. 2002, 3, 715–727. [Google Scholar] [CrossRef]
- Grasselli, C.; Ferrari, D.; Zalfa, C.; Soncini, M.; Mazzoccoli, G.; Facchini, F.A.; Marongiu, L.; Granucci, F.; Copetti, M.; Vescovi, A.L.; et al. Toll-like receptor 4 modulation influences human neural stem cell proliferation and differentiation. Cell Death Dis. 2018, 9, 280. [Google Scholar] [CrossRef]
- Kase, Y.; Okano, H. Expression of ACE2 and a viral virulence-regulating factor CCN family member 1 in human iPSC-derived neural cells: Implications for COVID-19-related CNS disorders. Inflamm. Regen. 2020, 40, 32. [Google Scholar] [CrossRef]
- Yu, X.; Ye, Z.; Houston, C.M.; Zecharia, A.Y.; Ma, Y.; Zhang, Z.; Uygun, D.S.; Parker, S.; Vyssotski, A.L.; Yustos, R.; et al. Wakefulness Is Governed by GABA and Histamine Cotransmission. Neuron 2015, 87, 164–178. [Google Scholar] [CrossRef]
- Möhler, H. Role of GABAA receptors in cognition. Biochem. Soc. Trans. 2009, 37, 1328–1333. [Google Scholar] [CrossRef]
- Lou, H.C.; Thomsen, K.R.; Changeux, J.-P. The Molecular Organization of Self-awareness: Paralimbic Dopamine-GABA Interaction. Front. Syst. Neurosci. 2020, 14, 3. [Google Scholar] [CrossRef] [PubMed]
- Lyu, C.; Zhao, W.; Peng, C.; Hu, S.; Fang, H.; Hua, Y.; Yao, S.; Huang, J.; Mei, L. Exploring the contributions of two glutamate decarboxylase isozymes in Lactobacillus brevis to acid resistance and γ-aminobutyric acid production. Microb. Cell Fact. 2018, 17, 180. [Google Scholar] [CrossRef] [PubMed]
- Bak, L.K.; Schousboe, A.; Waagepetersen, H.S. The glutamate/GABA-glutamine cycle: Aspects of transport, neurotransmitter homeostasis and ammonia transfer. J. Neurochem. 2006, 98, 641–653. [Google Scholar] [CrossRef] [PubMed]
- Omotosho, Y.B.; Ying, G.W.; Stolar, M.; Mallari, A.J.P. COVID-19-Induced Diabetic Ketoacidosis in an Adult with Latent Autoimmune Diabetes. Cureus 2021, 13, e12690. [Google Scholar] [CrossRef]
- Emekli, A.S.; Parlak, A.; Göcen, N.Y.; Kürtüncü, M. Anti-GAD associated post-infectious cerebellitis after COVID-19 infection. Neurol. Sci. 2021, 42, 3995–4002. [Google Scholar] [CrossRef]
- Jin, Z.; Mendu, S.K.; Birnir, B. GABA is an effective immunomodulatory molecule. Amino Acids 2013, 45, 87–94. [Google Scholar] [CrossRef]
- Qin, P.; Wu, X.; Duncan, N.W.; Bao, W.; Tang, W.; Zhang, Z.; Hu, J.; Jin, Y.; Wu, X.; Gao, L.; et al. GABAA receptor deficits predict recovery in patients with disorders of consciousness: A preliminary multimodal [11C]Flumazenil PET and fMRI study. Hum. Brain Mapp. 2015, 36, 3867–3877. [Google Scholar] [CrossRef]
- Fujimori, S.; Yoneda, Y. [Neuropsychiatric disorders and GABA]. Nihon Shinkei Seishin Yakurigaku Zasshi 2004, 24, 265–271. (In Japanese) [Google Scholar]
- Clauss, R. Disorders of consciousness and pharmaceuticals that act on oxygen based amino acid and monoamine neuro-transmitter pathways of the brain. Curr. Pharm. Des. 2014, 20, 4053–4140. [Google Scholar]
- Tsubomoto, M.; Kawabata, R.; Zhu, X.; Minabe, Y.; Chen, K.; A Lewis, D.; Hashimoto, T. Expression of Transcripts Selective for GABA Neuron Subpopulations across the Cortical Visuospatial Working Memory Network in the Healthy State and Schizophrenia. Cereb. Cortex 2018, 29, 3540–3550. [Google Scholar] [CrossRef]
- Sakimoto, Y.; Oo, P.M.-T.; Goshima, M.; Kanehisa, I.; Tsukada, Y.; Mitsushima, D. Significance of GABAA Receptor for Cognitive Function and Hippocampal Pathology. Int. J. Mol. Sci. 2021, 22, 12456. [Google Scholar] [CrossRef] [PubMed]
- Wyss, C.; Tse, D.H.Y.; Kometer, M.; Dammers, J.; Achermann, R.; Shah, N.J.; Kawohl, W.; Neuner, I. GABA metabolism and its role in gamma-band oscillatory activity during auditory processing: An MRS and EEG study. Hum. Brain Mapp. 2017, 38, 3975–3987. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, C.S.; Demiralp, T. Human EEG gamma oscillations in neuropsychiatric disorders. Clin. Neurophysiol. 2005, 116, 2719–2733. [Google Scholar] [CrossRef] [PubMed]
- Coghlan, S.; Horder, J.; Inkster, B.; Mendez, M.A.; Murphy, D.; Nutt, D. GABA system dysfunction in autism and related disorders: From synapse to symptoms. Neurosci. Biobehav. Rev. 2012, 36, 2044–2055. [Google Scholar] [CrossRef]
- Harris, S.; Ma, H.; Zhao, M.; Boorman, L.; Zheng, Y.; Kennerley, A.; Bruyns-Haylett, M.; Overton, P.G.; Berwick, J.; Schwartz, T.H. Coupling between gamma-band power and cerebral blood volume during recurrent acute neocortical seizures. NeuroImage 2014, 97, 62–70. [Google Scholar] [CrossRef][Green Version]
- Muthukumaraswamy, S.D.; Edden, R.A.; Jones, D.K.; Swettenham, J.B.; Singh, K.D. Resting GABA concentration predicts peak gamma frequency and fMRI amplitude in response to visual stimulation in humans. Proc. Natl. Acad. Sci. USA 2009, 106, 8356–8361. [Google Scholar] [CrossRef]
- Kilb, W. Development of the GABAergic System from Birth to Adolescence. Neuroscience 2011, 18, 613–630. [Google Scholar] [CrossRef]
- Li, S.; Kumar T, P.; Joshee, S.; Kirschstein, T.; Subburaju, S.; Khalili, J.S.; Kloepper, J.; Du, C.; Elkhal, A.; Szabó, G.; et al. Endothelial cell-derived GABA signaling modulates neuronal migration and postnatal behavior. Cell Res. 2017, 28, 221–248. [Google Scholar] [CrossRef]
- Wu, X.; Fu, Y.; Knott, G.W.; Lu, J.; Di Cristo, G.; Huang, Z.J. GABA Signaling Promotes Synapse Elimination and Axon Pruning in Developing Cortical Inhibitory Interneurons. J. Neurosci. 2012, 32, 331–343. [Google Scholar] [CrossRef]
- Favuzzi, E.; Huang, S.; Saldi, G.A.; Binan, L.; Ibrahim, L.A.; Fernández-Otero, M.; Cao, Y.; Zeine, A.; Sefah, A.; Zheng, K.; et al. GABA-receptive microglia selectively sculpt developing inhibitory circuits. Cell 2021, 184, 4048–4063.e32. [Google Scholar] [CrossRef]
- Whitelaw, B. Microglia-mediated synaptic elimination in neuronal development and disease. J. Neurophysiol. 2018, 119, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Fuks, J.; Arrighi, R.B.G.; Weidner, J.M.; Mendu, S.K.; Jin, Z.; Wallin, R.P.A.; Rethi, B.; Birnir, B.; Barragan, A. GABAergic Signaling Is Linked to a Hypermigratory Phenotype in Dendritic Cells Infected by Toxoplasma gondii. PLoS Pathog. 2012, 8, e1003051. [Google Scholar] [CrossRef] [PubMed]
- Fruntes, V.; Limosin, F. Schizophrenia and viral infection during neurodevelopment: A pathogenesis model? Med. Sci. Monit. 2008, 14, RA71–RA77. [Google Scholar] [PubMed]
- Scordel, C.; Huttin, A.; Cochet-Bernoin, M.; Szelechowski, M.; Poulet, A.; Richardson, J.; Benchoua, A.; Gonzalez-Dunia, D.; Eloit, M.; Coulpier, M. Borna Disease Virus Phosphoprotein Impairs the Developmental Program Controlling Neurogenesis and Reduces Human GABAergic Neurogenesis. PLoS Pathog. 2015, 11, e1004859. [Google Scholar] [CrossRef] [PubMed]
- McCarron, J.G.; Lee, M.D.; Wilson, C. The Endothelium Solves Problems That Endothelial Cells Do Not Know Exist. Trends Pharmacol. Sci. 2017, 38, 322–338. [Google Scholar] [CrossRef]
- Lee, M.D.; Buckley, C.; Zhang, X.; Louhivuori, L.; Uhlén, P.; Wilson, C.; McCarron, J.G. Small-world connectivity dictates collective endothelial cell signaling. Proc. Natl. Acad. Sci. USA 2022, 119, e2118927119. [Google Scholar] [CrossRef] [PubMed]
- Junior, A.P.; Dos Santos, R.P.; Barrros, R.F. The Calcium Wave Model of the Perception-Action Cycle: Evidence from Semantic Relevance in Memory Experiments. Front. Psychol. 2013, 4, 252. [Google Scholar] [CrossRef]
- Carafoli, E.; Krebs, J. Why Calcium? How Calcium Became the Best Communicator. J. Biol. Chem. 2016, 291, 20849–20857. [Google Scholar] [CrossRef]
- Knight, H.; Brandt, S.; Knight, M.R. A history of stress alters drought calcium signalling pathways in Arabidopsis. Plant J. 1998, 16, 681–687. [Google Scholar] [CrossRef]
- Kawamoto, E.M.; Vivar, C.; Camandola, S. Physiology and Pathology of Calcium Signaling in the Brain. Front. Pharmacol. 2012, 3, 61. [Google Scholar] [CrossRef]
- Adaikkan, C.; Taha, E.; Barrera, I.; David, O.; Rosenblum, K. Calcium/Calmodulin-Dependent Protein Kinase II and Eukaryotic Elongation Factor 2 Kinase Pathways Mediate the Antidepressant Action of Ketamine. Biol. Psychiatry 2018, 84, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Wenzhong, L.; Hualan, L. COVID-19: The CaMKII-like system of S protein drives membrane fusion and induces syncytial multinucleated giant cells. Immunol. Res. 2021, 69, 496–519. [Google Scholar] [CrossRef] [PubMed]
- Baluška, F.; Levin, M. On Having No Head: Cognition throughout Biological Systems. Front. Psychol. 2016, 7, 902. [Google Scholar] [CrossRef] [PubMed]
- Snijders, T.; Aussieker, T.; Holwerda, A.; Parise, G.; Van Loon, L.J.C.; Verdijk, L.B. The concept of skeletal muscle memory: Evidence from animal and human studies. Acta Physiol. 2020, 229, e13465. [Google Scholar] [CrossRef]
- Pearsall, P.; Schwartz, G.E.; Russek, L.G. Changes in heart transplant recipients that parallel the personalities of their donors. Integr. Med. 2000, 2, 65–72. [Google Scholar] [CrossRef]
- Bunzel, B.; Schmidl-Mohl, B.; Wollenek, G. Does changing the heart mean changing personality? A retrospective inquiry on 47 heart transplant patients. Qual. Life Res. 1992, 1, 251–256. [Google Scholar] [CrossRef]
- Liester, M.B. Personality changes following heart transplantation: The role of cellular memory. Med. Hypotheses 2019, 135, 109468. [Google Scholar] [CrossRef]
- Moore, C.I.; Cao, R. The Hemo-Neural Hypothesis: On The Role of Blood Flow in Information Processing. J. Neurophysiol. 2008, 99, 2035–2047. [Google Scholar] [CrossRef]
- Cines, D.B.; Pollak, E.S.; A Buck, C.; Loscalzo, J.; A Zimmerman, G.; McEver, R.P.; Pober, J.S.; Wick, T.; A Konkle, B.; Schwartz, B.S.; et al. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood 1998, 91, 3527–3561. [Google Scholar]
- Datta, D.; Subburaju, S.; Kaye, S.; Baruah, J.; Choi, Y.K.; Nian, Y.; Khalili, J.S.; Chung, S.; Elkhal, A.; Vasudevan, A. Human forebrain endothelial cell therapy for psychiatric disorders. Mol. Psychiatry 2020, 26, 4864–4883. [Google Scholar] [CrossRef]
- Choi, Y.K.; Vasudevan, A. Endothelial GABA signaling: A phoenix awakened. Aging 2018, 10, 859–860. [Google Scholar] [CrossRef] [PubMed]
- Do, D.P.; Dowd, J.; Ranjit, N.; House, J.S.; Kaplan, G.A. Hopelessness, Depression, and Early Markers of Endothelial Dysfunction in U.S. Adults. Psychosom. Med. 2010, 72, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Vetter, M.W.; Martin, B.-J.; Fung, M.; Pajevic, M.; Anderson, T.J.; Raedler, T.J. Microvascular dysfunction in schizophrenia: A case–control study. Schizophrenia 2015, 1, 15023. [Google Scholar] [CrossRef] [PubMed]
- Azmitia, E.C.; Saccomano, Z.T.; Alzoobaee, M.F.; Boldrini, M.; Whitakerazmitia, P.M. Persistent Angiogenesis in the Autism Brain: An Immunocytochemical Study of Postmortem Cortex, Brainstem and Cerebellum. J. Autism Dev. Disord. 2015, 46, 1307–1318. [Google Scholar] [CrossRef] [PubMed]
- Sara, J.D.S.; Ahmad, A.; Toya, T.; Pardo, L.S.; Lerman, L.O.; Lerman, A. Anxiety Disorders Are Associated With Coronary Endothelial Dysfunction in Women With Chest Pain and Nonobstructive Coronary Artery Disease. J. Am. Heart Assoc. 2021, 10, e021722. [Google Scholar] [CrossRef]
- Ogaki, A.; Ikegaya, Y.; Koyama, R. Vascular Abnormalities and the Role of Vascular Endothelial Growth Factor in the Epileptic Brain. Front. Pharmacol. 2020, 11, 20. [Google Scholar] [CrossRef]
- Mohite, S.; de Campos-Carli, S.M.; Rocha, N.P.; Sharma, S.; Miranda, A.S.; Barbosa, I.G.; Salgado, J.V.; Simoes-E-Silva, A.C.; Teixeira, A.L. Lower circulating levels of angiotensin-converting enzyme (ACE) in patients with schizophrenia. Schizophr. Res. 2018, 202, 50–54. [Google Scholar] [CrossRef]
- Braun, M.; Ramracheya, R.; Bengtsson, M.; Clark, A.; Walker, J.N.; Johnson, P.R.; Rorsman, P. γ-Aminobutyric Acid (GABA) Is an Autocrine Excitatory Transmitter in Human Pancreatic β-Cells. Diabetes 2010, 59, 1694–1701. [Google Scholar] [CrossRef]
- Wu, C.-T.; Lidsky, P.V.; Xiao, Y.; Lee, I.T.; Cheng, R.; Nakayama, T.; Jiang, S.; Demeter, J.; Bevacqua, R.J.; Chang, C.A.; et al. SARS-CoV-2 infects human pancreatic β cells and elicits β cell impairment. Cell Metab. 2021, 33, 1565–1576.e5. [Google Scholar] [CrossRef]
- Sohrabipour, S.; Sharifi, M.R.; Talebi, A.; Soltani, N. GABA dramatically improves glucose tolerance in streptozotocin-induced diabetic rats fed with high-fat diet. Eur. J. Pharmacol. 2018, 826, 75–84. [Google Scholar] [CrossRef]
- Soltani, N.; Qiu, H.; Aleksic, M.; Glinka, Y.; Zhao, F.; Liu, R.; Li, Y.; Zhang, N.; Chakrabarti, R.; Ng, T.; et al. GABA exerts protective and regenerative effects on islet beta cells and reverses diabetes. Proc. Natl. Acad. Sci. USA 2011, 108, 11692–11697. [Google Scholar] [CrossRef] [PubMed]
- Chu, K.Y.; Leung, P.S. Angiotensin II in Type 2 Diabetes Mellitus. Curr. Protein Pept. Sci. 2009, 10, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Gal, H.; Krizhanovsky, V. Cell fusion induced senescence. Aging 2014, 6, 353–354. [Google Scholar] [CrossRef] [PubMed]
- Menegaz, D.; Hagan, D.W.; Almaça, J.; Cianciaruso, C.; Rodriguez-Diaz, R.; Molina, J.; Dolan, R.M.; Becker, M.W.; Schwalie, P.C.; Nano, R.; et al. Mechanism and effects of pulsatile GABA secretion from cytosolic pools in the human beta cell. Nat. Metab. 2019, 1, 1110–1126. [Google Scholar] [CrossRef] [PubMed]
- Aguayo-Mazzucato, C.; Midha, A. β-cell senescence in type 2 diabetes. Aging 2019, 11, 9967–9968. [Google Scholar] [CrossRef] [PubMed]
- Sfera, A.; Osorio, C.; Inderias, L.A.; Parker, V.; Price, A.I.; Cummings, M. The Obesity–Impulsivity Axis: Potential Metabolic Interventions in Chronic Psychiatric Patients. Front. Psychiatry 2017, 8, 20. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Chu, M.; Zhong, F.; Tan, X.; Tang, G.; Mai, J.; Lai, N.; Guan, C.; Liang, Y.; Liao, G. Digestive symptoms of COVID-19 and expression of ACE2 in digestive tract organs. Cell Death Discov. 2020, 6, 76. [Google Scholar] [CrossRef]
- Yu, W.; Ou, X.; Liu, X.; Zhang, S.; Gao, X.; Cheng, H.; Zhu, B.; Yan, J. ACE2 contributes to the maintenance of mouse epithelial barrier function. Biochem. Biophys. Res. Commun. 2020, 533, 1276–1282. [Google Scholar] [CrossRef]
- Koester, S.T.; Li, N.; Lachance, D.M.; Morella, N.M.; Dey, N. Variability in digestive and respiratory tract Ace2 expression is associated with the microbiome. PLoS ONE 2021, 16, e0248730. [Google Scholar] [CrossRef]
- Mpekoulis, G.; Frakolaki, E.; Taka, S.; Ioannidis, A.; Vassiliou, A.G.; Kalliampakou, K.I.; Patas, K.; Karakasiliotis, I.; Aidinis, V.; Chatzipanagiotou, S.; et al. Alteration of L-Dopa decarboxylase expression in SARS-CoV-2 infection and its association with the interferon-inducible ACE2 isoform. PLoS ONE 2021, 16, e0253458. [Google Scholar] [CrossRef]
- Reith, J.; Benkelfat, C.; Sherwin, A.; Yasuhara, Y.; Kuwabara, H.; Andermann, F.; Bachneff, S.; Cumming, P.; Diksic, M.; E Dyve, S. Elevated dopa decarboxylase activity in living brain of patients with psychosis. Proc. Natl. Acad. Sci. USA 1994, 91, 11651–11654. [Google Scholar] [CrossRef] [PubMed]
- Stevens, B.R.; Ellory, J.C.; Preston, R.L. B0AT1 Amino Acid Transporter Complexed With SARS-CoV-2 Receptor ACE2 Forms a Heterodimer Functional Unit: In Situ Conformation Using Radiation Inactivation Analysis. Function 2021, 2, zqab027. [Google Scholar] [CrossRef] [PubMed]
- Eroğlu, I.; Eroğlu, B.; Güven, G.S. Altered tryptophan absorption and metabolism could underlie long-term symptoms in survivors of coronavirus disease 2019 (COVID-19). Nutrition 2021, 90, 111308. [Google Scholar] [CrossRef] [PubMed]
- Singer, D.; Camargo, S.; Ramadan, T.; Schäfer, M.; Mariotta, L.; Herzog, B.; Huggel, K.; Wolfer, D.; Werner, S.; Penninger, J.; et al. Defective intestinal amino acid absorption in Ace2 null mice. Am. J. Physiol. Liver Physiol. 2012, 303, G686–G695. [Google Scholar] [CrossRef]
- Deng, J.; Zhou, F.; Hou, W.; Silver, Z.; Wong, C.Y.; Chang, O.; Huang, E.; Zuo, Q.K. The prevalence of depression, anxiety, and sleep disturbances in COVID-19 patients: A meta-analysis. Ann. N. Y. Acad. Sci. 2020, 1486, 90–111. [Google Scholar] [CrossRef]
- Giovannoni, F.; Li, Z.; Remes-Lenicov, F.; Dávola, M.E.; Elizalde, M.; Paletta, A.; Ashkar, A.A.; Mossman, K.L.; Dugour, A.V.; Figueroa, J.M.; et al. AHR signaling is induced by infection with coronaviruses. Nat. Commun. 2021, 12, 5148. [Google Scholar] [CrossRef]
- Wei, G.Z.; Martin, K.A.; Xing, P.Y.; Agrawal, R.; Whiley, L.; Wood, T.K.; Hejndorf, S.; Ng, Y.Z.; Low, J.Z.Y.; Rossant, J.; et al. Tryptophan-metabolizing gut microbes regulate adult neurogenesis via the aryl hydrocarbon receptor. Proc. Natl. Acad. Sci. USA 2021, 118, e2021091118. [Google Scholar] [CrossRef]
- Ji, J.; Qu, H. Cross-regulatory Circuit Between AHR and Microbiota. Curr. Drug Metab. 2019, 20, 4–8. [Google Scholar] [CrossRef]
- Lindén, J.; Lensu, S.; Tuomisto, J.; Pohjanvirta, R. Dioxins, the aryl hydrocarbon receptor and the central regulation of energy balance. Front. Neuroendocr. 2010, 31, 452–478. [Google Scholar] [CrossRef]
- Sfera, A.; Osorio, C.; Diaz, E.L.; Maguire, G.; Cummings, M. The Other Obesity Epidemic—Of Drugs and Bugs. Front. Endocrinol. 2020, 11, 488. [Google Scholar] [CrossRef]
- Sabbatinelli, J.; Prattichizzo, F.; Olivieri, F.; Procopio, A.D.; Rippo, M.R.; Giuliani, A. Where Metabolism Meets Senescence: Focus on Endothelial Cells. Front. Physiol. 2019, 10, 1523. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, V.; Ale-Agha, N.; Haendeler, J.; Ventura, N. The Aryl Hydrocarbon Receptor (AhR) in the Aging Process: Another Puzzling Role for This Highly Conserved Transcription Factor. Front. Physiol. 2020, 10, 1561. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Liao, Y.; Ding, X.; Jiang, Y.; Yan, J.; Xia, Y.; Tan, B.; Lin, Z.; Duan, J.; Jia, X.; et al. Slc6a13 deficiency promotes Th17 responses during intestinal bacterial infection. Mucosal Immunol. 2018, 12, 531–544. [Google Scholar] [CrossRef] [PubMed]
- Brevi, A.; Cogrossi, L.L.; Grazia, G.; Masciovecchio, D.; Impellizzieri, D.; Lacanfora, L.; Grioni, M.; Bellone, M. Much More Than IL-17A: Cytokines of the IL-17 Family Between Microbiota and Cancer. Front. Immunol. 2020, 11, 565470. [Google Scholar] [CrossRef]
- Hamada, H.; Garcia-Hernandez, M.D.L.L.; Reome, J.B.; Misra, S.K.; Strutt, T.M.; McKinstry, K.K.; Cooper, A.; Swain, S.L.; Dutton, R.W. Tc17, a Unique Subset of CD8 T Cells That Can Protect against Lethal Influenza Challenge. J. Immunol. 2009, 182, 3469–3481. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, M.; Liu, W.; Hu, C.; Li, H.; Deng, J.; Cao, Q.; Wang, Y.; Hu, W.; Li, Q. Th17/IL-17 induces endothelial cell senescence via activation of NF-κB/p53/Rb signaling pathway. Lab. Investig. 2021, 101, 1418–1426. [Google Scholar] [CrossRef]
- Ming, X.-F.; Montani, J.-P.; Yang, Z. Perspectives of Targeting mTORC1–S6K1 in Cardiovascular Aging. Front. Physiol. 2012, 3, 5. [Google Scholar] [CrossRef]
- Fuentes-Prior, P. Priming of SARS-CoV-2 S protein by several membrane-bound serine proteinases could explain enhanced viral infectivity and systemic COVID-19 infection. J. Biol. Chem. 2021, 296, 100135. [Google Scholar] [CrossRef]
- Zhang, Z.; Zheng, Y.; Niu, Z.; Zhang, B.; Wang, C.; Yao, X.; Peng, H.; Franca, D.N.; Wang, Y.; Zhu, Y.; et al. SARS-CoV-2 spike protein dictates syncytium-mediated lymphocyte elimination. Cell Death Differ. 2021, 28, 2765–2777. [Google Scholar] [CrossRef]
- Winstone, H.; Lista, M.J.; Reid, A.C.; Bouton, C.; Pickering, S.; Galao, R.P.; Kerridge, C.; Doores, K.J.; Swanson, C.M.; Neil, S.J.D. The Polybasic Cleavage Site in SARS-CoV-2 Spike Modulates Viral Sensitivity to Type I Interferon and IFITM2. J. Virol. 2021, 95, e02422-20. [Google Scholar] [CrossRef]
- Johnson, B.A.; Xie, X.; Bailey, A.L.; Kalveram, B.; Lokugamage, K.G.; Muruato, A.; Zou, J.; Zhang, X.; Juelich, T.; Smith, J.K.; et al. Loss of furin cleavage site attenuates SARS-CoV-2 pathogenesis. Nature 2021, 591, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Balestrieri, E.; Minutolo, A.; Petrone, V.; Fanelli, M.; Iannetta, M.; Malagnino, V.; Zordan, M.; Vitale, P.; Charvet, B.; Horvat, B.; et al. Evidence of the pathogenic HERV-W envelope expression in T lymphocytes in association with the respiratory outcome of COVID-19 patients. EBioMedicine 2021, 66, 103341. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Chen, P.-T.; Chang, G.-D.; Huang, C.-J.; Chen, H. Functional Characterization of the Placental Fusogenic Membrane Protein Syncytin1. Biol. Reprod. 2004, 71, 1956–1962. [Google Scholar] [CrossRef] [PubMed]
- Huppertz, B. The Critical Role of Abnormal Trophoblast Development in the Etiology of Preeclampsia. Curr. Pharm. Biotechnol. 2018, 19, 771–780. [Google Scholar] [CrossRef]
- Zhang, L.; Richards, A.; Barrasa, M.I.; Hughes, S.H.; Young, R.A.; Jaenisch, R. Reverse-transcribed SARS-CoV-2 RNA can integrate into the genome of cultured human cells and can be expressed in patient-derived tissues. Proc. Natl. Acad. Sci. USA 2021, 118, e2105968118. [Google Scholar] [CrossRef]
- Mi, S.; Lee, X.; Li, X.-P.; Veldman, G.M.; Finnerty, H.; Racie, L.; LaVallie, E.; Tang, X.-Y.; Edouard, P.; Howes, S.; et al. Syncytin is a captive retroviral envelope protein involved in human placental morphogenesis. Nature 2000, 403, 785–789. [Google Scholar] [CrossRef]
- Wang, X.; Huang, J.; Zhu, F. Human Endogenous Retroviral Envelope Protein Syncytin-1 and Inflammatory Abnormalities in Neuropsychological Diseases. Front. Psychiatry 2018, 9, 422. [Google Scholar] [CrossRef]
- Chen, C.-P.; Chen, L.-F.; Yang, S.-R.; Chen, C.-Y.; Ko, C.-C.; Chang, G.-D.; Chen, H. Functional Characterization of the Human Placental Fusogenic Membrane Protein Syncytin 21. Biol. Reprod. 2008, 79, 815–823. [Google Scholar] [CrossRef]
- Conde-Agudelo, A.; Romero, R. SARS-CoV-2 infection during pregnancy and risk of preeclampsia: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. 2021, 226, 68–89.e3. [Google Scholar] [CrossRef]
- Terán, Y.; Ponce, O.; Betancourt, L.; Hernández, L.; Rada, P. Amino acid profile of plasma and cerebrospinal fluid in preeclampsia. Pregnancy Hypertens. Int. J. Women’s Cardiovasc. Health 2012, 2, 416–422. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, Q.; Tan, D.; Luo, W.; Zhao, H.; Ma, J.; Liang, H.; Tan, Y. GABA A receptor π subunit promotes apoptosis of HTR-8/SVneo trophoblastic cells: Implications in preeclampsia. Int. J. Mol. Med. 2016, 38, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Licht, P.; Harbarth, P.; E Merz, W. Evidence for a modulation of human chorionic gonadotropin (hCG) subunit messenger ribonucleic acid levels and hCG secretion by gamma-aminobutyric acid in human first trimester placenta in vitro. Endocrinology 1992, 130, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Vacher, C.-M.; Lacaille, H.; O’Reilly, J.J.; Salzbank, J.; Bakalar, D.; Sebaoui, S.; Liere, P.; Clarkson-Paredes, C.; Sasaki, T.; Sathyanesan, A.; et al. Placental endocrine function shapes cerebellar development and social behavior. Nat. Neurosci. 2021, 24, 1392–1401. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.-W.; Chao, T.-L.; Li, C.-L.; Chiu, M.-F.; Kao, H.-C.; Wang, S.-H.; Pang, Y.-H.; Lin, C.-H.; Tsai, Y.-M.; Lee, W.-H.; et al. Furin Inhibitors Block SARS-CoV-2 Spike Protein Cleavage to Suppress Virus Production and Cytopathic Effects. Cell Rep. 2020, 33, 108254. [Google Scholar] [CrossRef]
- Becker, G.L.; Sielaff, F.; Than, M.E.; Lindberg, I.; Routhier, S.; Day, R.; Lu, Y.; Garten, W.; Steinmetzer, T. Potent Inhibitors of Furin and Furin-like Proprotein Convertases Containing Decarboxylated P1 Arginine Mimetics. J. Med. Chem. 2009, 53, 1067–1075. [Google Scholar] [CrossRef]
- Devi, K.P.; Pourkarim, M.R.; Thijssen, M.; Sureda, A.; Khayatkashani, M.; Cismaru, C.A.; Neagoe, I.B.; Habtemariam, S.; Razmjouei, S.; Kashani, H.R.K. A perspective on the applications of furin inhibitors for the treatment of SARS-CoV-2. Pharmacol. Rep. 2022, 74, 425–430. [Google Scholar] [CrossRef]
- Yakala, G.K.; Cabrera-Fuentes, H.A.; Crespo-Avilan, G.E.; Rattanasopa, C.; Burlacu, A.; George, B.L.; Anand, K.; Mayan, D.C.; Corlianò, M.; Hernández-Reséndiz, S.; et al. FURIN Inhibition Reduces Vascular Remodeling and Atherosclerotic Lesion Progression in Mice. Arter. Thromb. Vasc. Biol. 2019, 39, 387–401. [Google Scholar] [CrossRef]
- AbdelMassih, A.F.; Ye, J.; Kamel, A.; Mishriky, F.; Ismail, H.-A.; Ragab, H.A.; El Qadi, L.; Malak, L.; Abdu, M.; El-Husseiny, M.; et al. A multicenter consensus: A role of furin in the endothelial tropism in obese patients with COVID-19 infection. Obes. Med. 2020, 19, 100281. [Google Scholar] [CrossRef]
- Pomorski, T.G.; Menon, A.K. Lipid somersaults: Uncovering the mechanisms of protein-mediated lipid flipping. Prog. Lipid Res. 2016, 64, 69–84. [Google Scholar] [CrossRef]
- Whitlock, J.M.; Chernomordik, L.V. Flagging fusion: Phosphatidylserine signaling in cell—Cell fusion. J. Biol. Chem. 2021, 296, 100411. [Google Scholar] [CrossRef]
- Braga, L.; Ali, H.; Secco, I.; Chiavacci, E.; Neves, G.; Goldhill, D.; Penn, R.; Jimenez-Guardeño, J.M.; Ortega-Prieto, A.M.; Bussani, R.; et al. Drugs that inhibit TMEM16 proteins block SARS-CoV-2 spike-induced syncytia. Nature 2021, 594, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Feng, S.; Puchades, C.; Ko, J.; Figueroa, E.; Chen, Y.; Wu, H.; Gu, S.; Han, T.; Li, J.; et al. Identification of a conserved drug binding pocket in TMEM16 proteins. Preprint Res. Sq. 2022, 1, rs.3.rs-1296933. [Google Scholar] [CrossRef]
- Cairns, D.M.; Dulko, D.; Griffiths, J.K.; Golan, Y.; Cohen, T.; Trinquart, L.; Price, L.L.; Beaulac, K.R.; Selker, H.P. Efficacy of Niclosamide vs Placebo in SARS-CoV-2 Respiratory Viral Clearance, Viral Shedding, and Duration of Symptoms Among Patients with Mild to Moderate COVID-19. JAMA Netw. Open 2022, 5, e2144942. [Google Scholar] [CrossRef] [PubMed]
- Pedemonte, N.; Galietta, L.J. Structure and Function of TMEM16 Proteins (Anoctamins). Physiol. Rev. 2014, 94, 419–459. [Google Scholar] [CrossRef]
- Slawecki, M.L.; Carlson, G.C.; Keller, A. Differential distribution of inositol 1,4,5-triphosphate receptors in the rat olfactory bulb. J. Comp. Neurol. 1997, 389, 224–234. [Google Scholar] [CrossRef]
- Egorova, P.A.; Bezprozvanny, I.B. Inositol 1,4,5-trisphosphate receptors and neurodegenerative disorders. FEBS J. 2018, 285, 3547–3565. [Google Scholar] [CrossRef]
- Heuser, K.; Nome, C.G.; Pettersen, K.H.; Åbjørsbråten, K.S.; Jensen, V.; Tang, W.; Sprengel, R.; Taubøll, E.; A Nagelhus, E.; Enger, R. Ca2+ Signals in Astrocytes Facilitate Spread of Epileptiform Activity. Cereb. Cortex 2018, 28, 4036–4048. [Google Scholar] [CrossRef]
- Park, S.J.; Jeong, J.; Park, Y.-U.; Park, K.-S.; Lee, H.; Lee, N.; Kim, S.-M.; Kuroda, K.; Nguyen, M.D.; Kaibuchi, K.; et al. Disrupted-in-schizophrenia-1 (DISC1) Regulates Endoplasmic Reticulum Calcium Dynamics. Sci. Rep. 2015, 5, 8694. [Google Scholar] [CrossRef]
- de Bartolomeis, A.; Tomasetti, C.; Cicale, M.; Yuan, P.-X.; Manji, H.K. Chronic treatment with lithium or valproate modulates the expression of Homer1b/c and its related genes Shank and Inositol 1,4,5-trisphosphate receptor. Eur. Neuropsychopharmacol. 2012, 22, 527–535. [Google Scholar] [CrossRef]
- Khanim, F.; Ferretti, L.; Raffles, S.; Giles, H.; Jankute, M.; Merrick, B.; Bunce, C.; Drayson, M. Epilepsy doses of valproate combined with the anti-helminthic, niclosamide, synergistically kill myeloma cells: A potent new anti-myeloma drug combination. Exp. Hematol. 2014, 42, S26. [Google Scholar] [CrossRef]
- Akgun, O.; Erkisa, M.; Ari, F. Effective and new potent drug combination: Histone deacetylase and Wnt/β-catenin pathway inhibitors in lung carcinoma cells. J. Cell. Biochem. 2019, 120, 15467–15482. [Google Scholar] [CrossRef] [PubMed]
- Batti, L.; Sundukova, M.; Murana, E.; Pimpinella, S.; Reis, F.D.C.; Pagani, F.; Wang, H.; Pellegrino, E.; Perlas, E.; Di Angelantonio, S.; et al. TMEM16F Regulates Spinal Microglial Function in Neuropathic Pain States. Cell Rep. 2016, 15, 2608–2615. [Google Scholar] [CrossRef] [PubMed]
- Bandman, E.; Walker, C.R.; Strohman, R.C. Diazepam Inhibits Myoblast Fusion and Expression of Muscle Specific Protein Synthesis. Science 1978, 200, 559–561. [Google Scholar] [CrossRef] [PubMed]
- Volke, V.; Soosaar, A.; Koks, S.; Vasar, E.; Männistö, P. l-Arginine abolishes the anxiolytic-like effect of diazepam in the elevated plus-maze test in rats. Eur. J. Pharmacol. 1998, 351, 287–290. [Google Scholar] [CrossRef]
- Gong, L.; Zhang, X.; Qiu, K.; He, L.; Wang, Y.; Yin, J. Arginine promotes myogenic differentiation and myotube formation through the elevation of cytoplasmic calcium concentration. Anim. Nutr. 2021, 7, 1115–1123. [Google Scholar] [CrossRef]
- Williams, M.; Risley, E.A. Ivermectin Interactions with Benzodiazepine Receptors in Rat Cortex and Cerebellum In Vitro. J. Neurochem. 1984, 42, 745–753. [Google Scholar] [CrossRef]
- Bhandage, A.K.; Olivera, G.C.; Kanatani, S.; Thompson, E.; Loré, K.; Varas-Godoy, M.; Barragan, A. A motogenic GABAergic system of mononuclear phagocytes facilitates dissemination of coccidian parasites. eLife 2020, 9, e60528. [Google Scholar] [CrossRef]
- Tian, J.; Middleton, B.; Kaufman, D. GABAA-Receptor Agonists Limit Pneumonitis and Death in Murine Coronavirus-Infected Mice. Viruses 2021, 13, 966. [Google Scholar] [CrossRef]
- Kittler, J.T.; Delmas, P.; Jovanovic, J.N.; Brown, D.A.; Smart, T.G.; Moss, S.J. Constitutive Endocytosis of GABAA Receptors by an Association with the Adaptin AP2 Complex Modulates Inhibitory Synaptic Currents in Hippocampal Neurons. J. Neurosci. 2000, 20, 7972–7977. [Google Scholar] [CrossRef]
- Ngo, D.-H.; Vo, T.S. An Updated Review on Pharmaceutical Properties of Gamma-Aminobutyric Acid. Molecules 2019, 24, 2678. [Google Scholar] [CrossRef]
- Shimada, M.; Hasegawa, T.; Nishimura, C.; Kan, H.; Kanno, T.; Nakamura, T.; Matsubayashi, T. Anti-Hypertensive Effect of γ-Aminobutyric Acid (GABA)-Rich Chlorella on High-Normal Blood Pressure and Borderline Hypertension in Placebo-Controlled Double Blind Study. Clin. Exp. Hypertens. 2009, 31, 342–354. [Google Scholar] [CrossRef]
- Shyamaladevi, N.; Jayakumar, A.; Sujatha, R.; Paul, V.; Subramanian, E. Evidence that nitric oxide production increases γ-amino butyric acid permeability of blood-brain barrier. Brain Res. Bull. 2002, 57, 231–236. [Google Scholar] [CrossRef]
- Yoto, A.; Murao, S.; Motoki, M.; Yokoyama, Y.; Horie, N.; Takeshima, K.; Masuda, K.; Kim, M.; Yokogoshi, H. Oral intake of γ-aminobutyric acid affects mood and activities of central nervous system during stressed condition induced by mental tasks. Amino Acids 2011, 43, 1331–1337. [Google Scholar] [CrossRef] [PubMed]
- Czuczwar, S.J.; Patsalos, P.N. The New Generation of GABA Enhancers. CNS Drugs 2001, 15, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Iaria, P.; Blacher, J.; Asplanato, M.; Edric, K.; Safar, M.; Girerd, X. Une nouvelle cause d’hypertension artérielle résistante: La co-prescription avec des traitements anticomitiaux [A new cause of resistant arterial hypertension: Coprescription with anti-convulsant treatments]. Arch. Mal. Coeur. Vaiss. 1999, 92, 1005–1008. [Google Scholar]
- Chen, H.-H.; Li, Y.-D.; Cheng, P.-W.; Fang, Y.-C.; Lai, C.-C.; Tseng, C.-J.; Pan, J.-Y.; Yeh, T.-C. Gabapentin Reduces Blood Pressure and Heart Rate through the Nucleus Tractus Solitarii. Acta Cardiol. Sin. 2019, 35, 627–633. [Google Scholar] [CrossRef]
- Kitajima, T.; Kanbayashi, T.; Saito, Y.; Takahashi, Y.; Ogawa, Y.; Sugiyama, T.; Kaneko, Y.; Aizawa, R.; Shimizu, T. Diazepam reduces both arterial blood pressure and muscle sympathetic nerve activity in human. Neurosci. Lett. 2004, 355, 77–80. [Google Scholar] [CrossRef]
- Lokensgard, J.R.; Gekker, G.; Hu, S.; Arthur, A.F.; Chao, C.C.; Peterson, P.K. Diazepam-mediated inhibition of human immunodeficiency virus type 1 expression in human brain cells. Antimicrob. Agents Chemother. 1997, 41, 2566–2569. [Google Scholar] [CrossRef][Green Version]
- Lin, A.; Elbezanti, W.O.; Schirling, A.; Ahmed, A.; Van Duyne, R.; Cocklin, S.; Klase, Z. Alprazolam Prompts HIV-1 Transcriptional Reactivation and Enhances CTL Response Through RUNX1 Inhibition and STAT5 Activation. Front. Neurol. 2021, 12, 663793. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sfera, A.; Thomas, K.G.; Sasannia, S.; Anton, J.J.; Andronescu, C.V.; Garcia, M.; Sfera, D.O.; Cummings, M.A.; Kozlakidis, Z. Neuronal and Non-Neuronal GABA in COVID-19: Relevance for Psychiatry. Reports 2022, 5, 22. https://doi.org/10.3390/reports5020022
Sfera A, Thomas KG, Sasannia S, Anton JJ, Andronescu CV, Garcia M, Sfera DO, Cummings MA, Kozlakidis Z. Neuronal and Non-Neuronal GABA in COVID-19: Relevance for Psychiatry. Reports. 2022; 5(2):22. https://doi.org/10.3390/reports5020022
Chicago/Turabian StyleSfera, Adonis, Karina G. Thomas, Sarvin Sasannia, Jonathan J. Anton, Christina V. Andronescu, Michael Garcia, Dan O. Sfera, Michael A. Cummings, and Zisis Kozlakidis. 2022. "Neuronal and Non-Neuronal GABA in COVID-19: Relevance for Psychiatry" Reports 5, no. 2: 22. https://doi.org/10.3390/reports5020022
APA StyleSfera, A., Thomas, K. G., Sasannia, S., Anton, J. J., Andronescu, C. V., Garcia, M., Sfera, D. O., Cummings, M. A., & Kozlakidis, Z. (2022). Neuronal and Non-Neuronal GABA in COVID-19: Relevance for Psychiatry. Reports, 5(2), 22. https://doi.org/10.3390/reports5020022






