Abstract
Vitamin D is necessary for normal bone development and conservation. Moreover, it has extraskeletal effects, which play a pivotal role as a modulator of innate and adaptive immune responses. Many studies have highlighted the beneficial effect of vitamin D in protecting against acute respiratory viral infection, including COVID-19. Within this context, we described the effect of vitamin D supplementation in the immunological response to SARS-CoV-2 infection. Long-term IgG SARS-CoV-2 antibody responses were assessed in a cohort of twenty-two subjects diagnosed with COVID-19 by chemiluminescence assay (CLIA). Among them, a 61-year-old nurse undergoing vitamin D therapy showed a positive IgG response against SARS-CoV-2 nucleocapsid over nine months after infection, suggesting vitamin D played a role in modulating early antibody response against SARS-CoV-2. This result provides evidence of a positive effect of vitamin D on the decrease of functional humoral immunity.
1. Introduction
Vitamin D is a fat-soluble vitamin necessary for normal bone development and conservation, as it increases calcium, magnesium, and phosphate absorption. The biochemical mechanism of vitamin D is through its active metabolite, the 1,25-dihydroxyvitamin D, which binds the vitamin D receptor (VDR) [1,2]. VDR is coupled to the retinoid X receptor alpha (RXRα) [3,4,5]. The VDR complex is located in the nucleus associated with corepressors when the ligand is missing [6,7]. Instead, when 1,25-dihydroxyvitamin D binds VDR, it forms the coactivator complex leading to gene transactivation [7]. During menopause, the decline of oestrogens results in increased bone turnover, a decrease in bone mineral density, and an elevated fracture risk. Therefore, calcium and vitamin D must be integrated since sun exposure is not enough and its presence in food is low [8,9]. Vitamin D has extraskeletal effects, including metabolic properties, regulation of cell proliferation, skin differentiation, reproduction, and vascular, muscle, and immune system function [1]. The presence of the vitamin D receptor on almost all cells of the immune system [2,10] has been clearly demonstrated, suggesting a pivotal role of the vitamin as a modulator of both innate and adaptive immune responses. Herein we report a case of a 61-year-old nurse, who underwent calcium and vitamin D therapy since age 52 to prevent osteoporosis [11], showing a positive IgG response against SARS-CoV-2 nucleocapsid (NAbs) over nine months after infection.
2. Presentation Section
Specimens were analyzed on the Abbott Architect platform (Abbott Park, IL, USA) for SARS-CoV-2 IgG with chemiluminescent immunoassay (CLIA) with an indexed limit of detection of 1.4 [12]. This analytical system was used to monitor response against NAbs [12,13] in twenty-two subjects working in a nursing home and diagnosed with COVID-19 infection in April 2020 (cluster infection) with mild symptoms. Seven were male and fifteen were female, with a mean age of 39.41 ± 9.32. Fever (37.8 ± 0.7 °C) and anosmia were present in 95% of cases. Only one was hospitalized for displaying dyspnea, in which neuron-specific enolase was high [14]. The antibody detection index was measured on serum samples at three, six, and nine months after infection. The tests showed that the humoral immune response declined at three and six months in fifteen (Figure 1A) and six subjects (Figure 1B) out of twenty-two, respectively. While in the case of a 61-year-old female nurse, her NAbs level remained positive for over six months (Figure 1B), keeping her positivity on the threshold limit at 12 months after infection diagnosis (Figure 2). A retrospective clinical analysis revealed she had a medical history of depression and hypertension, which was treated with the following therapeutic regimen: 10 mg/day escitalopram and ramipril 5 mg/day. In addition, she underwent vitamin D (50,000 IU) treatment monthly (from October to June) after beginning menopause at 52 years of age (Table 1). Treatment with vitamin D at the usual dosage was not discontinued when COVID-19 therapy (i.e., nadroparin 4UI/twice daily, azithromycin 500 mg/day, and acetaminophen 1000 mg, when needed, for six days) was undertaken. No clinical condition impairment was observed until 10 June 2020, when she tested negative through a nasopharyngeal swab. The assessment of her 25-hydroxyvitamin D serum levels, performed from July 2020 to April 2021 through CLIA method, revealed a concentration of the vitamin ranging from 20 to 31 pg/mL (Table 2).
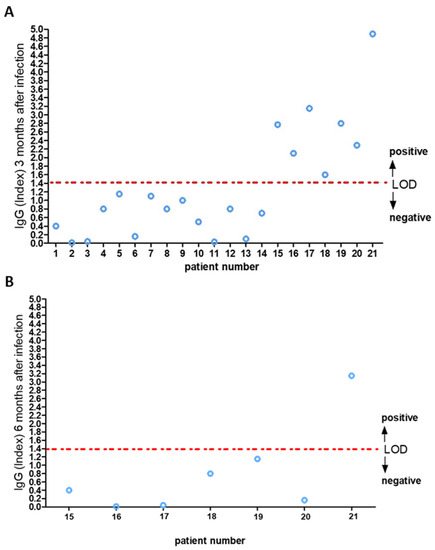
Figure 1.
IgG anti-nucleocapsid antibodies three (A) and six (B) months after infection. Limit of detection (LOD) index equal to 1.4.
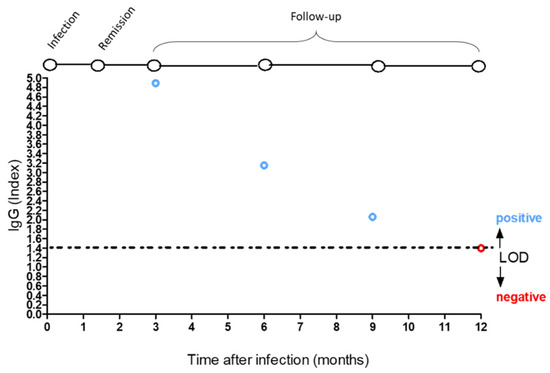
Figure 2.
Positive IgG index (blue circles) and negative IgG index value (red circles). Limit of detection (LOD) index equal to 1.4.

Table 1.
Clinical characteristics.

Table 2.
Serum level of 25-hydroxyvitamin D.
She received the first dose of the Pfizer-BioNtech COVID-19 vaccine in March 2021, showing a robust serum IgG titer (13,961.3 AU/mL) to spike proteins at 21 days after vaccination.
3. Discussion
Seven of the twenty-two participants had two serial measurements of IgG levels, and the remaining participant had three serial measurements. The first measurement was obtained three months after the onset of symptoms, and the last measurement was obtained six months after the onset of symptoms. Similar results were achieved in other studies in which rapid decline of anti–SARS-CoV-2 antibodies was highlighted in persons with mild COVID-19 symptoms [15]. To date, the duration of the SARS-CoV-2 immune response is not well characterized [16]. The duration and the strength of immunity after infection of SARS-CoV-2 are key issues for shield immunity, and the impacts of the combined interventions of shielding and social distancing in the high-transmission situation are the only defense we have today [17]. In this scenario, the duration of circulating IgG in a 61-year-old nurse, who underwent calcium and vitamin D therapy since age 52 to prevent osteoporosis, showed a positive IgG response against SARS-CoV-2 NAbs over twelve months after infection. The treatment with vitamin D at the usual dosage was not discontinued under COVID-19 therapy, suggesting a positive role of this fat-soluble secosteroid in modulating early antibody response against SARS-CoV-2. Of note, vitamin D supplementation correlated in this subject with sufficient vitamin D serum levels. It is also worth noting that multiple cross-sectional studies, interventional studies, and in vitro observations have highlighted the beneficial role of vitamin D in protecting against the risk of acquiring acute respiratory viral infection and outcome improvement in critically ill patients [18,19]. The mechanisms through which these antiviral effects occur are traditionally based on the ability of vitamin D to upregulate antimicrobial peptides and induce antiviral cytokines, which interfere with the viral replicative cycle [20,21]. However, more complex immunomodulatory effects cannot be ruled out. In this frame, the pervasive actions of vitamin D on both innate and adaptive immune response have raised the possibility of an interplay between it and the mechanisms by which the SARS-CoV-2 virus infects human beings, suggesting a potential benefit of vitamin D supplementation in primary prevention or as an adjunctive treatment of COVID-19 [22].
Furthermore, recently, a systematic review and meta-analysis indicated that low vitamin D status might be associated with an increased risk of COVID-19 infection [23]. Low vitamin D was also reported in critically ill COVID-19 ARDS patients, in which even the active form 1,25-dihydroxyvitamin D was found to be low [24]. This biochemical form mediates most of the endocrine effects of vitamin D, including immune-modulatory functions [25,26]. In particular, the 1,25-dihydroxyvitamin D is known to shift Th1 and Th17 responses towards Th2 responses [25]. This biochemical form should be administered in COVID-19 patients with chronic renal failure [26]. In this regard, a recent study highlighted that none of the vitamin D forms altered cytokine production in COVID-19 patients, demonstrating the presence of a high level of circulating plasmablasts in patients with 25-hydroxyvitamin D levels ≥ 20 ng/mL [22]. The latter observation is of particular interest as plasmablasts were implicated in the establishment of immune memory and the buildup of specific antibody titers during acute viral infections [27]. In this view, the first dose of the Pfizer-BioNtech COVID-19 vaccine received by the nurse showed a robust plasmatic IgG titer.
4. Conclusions
In conclusion, our results provide evidence of a positive effect of vitamin D on the decrease of functional humoral immunity. The maintenance of long-term IgG anti-N SARS-CoV-2 antibody response is essential, as it may limit the viral spread and consequent disease progression.
Author Contributions
F.L., have full access to the data; study concept and design: E.C., M.C.C. and F.L.; acquisition of data: L.G., D.M. and B.D.; analysis and interpretation of data: E.C., M.C.C. and F.L.; drafting of the manuscript: E.C., M.C.C. and R.C. All authors have read and agreed to the published version of the manuscript.
Funding
We did not receive funding for this study.
Institutional Review Board Statement
This study is part of the clinical trials recorded in clinicaltrial.gov (NCT04322513) and it was conducted in compliance with the Institutional Review Board/Human Subjects Research Committee requirements and with the Declaration of Helsinki and the Guidelines for Good Clinical Practice criteria.
Informed Consent Statement
Written informed consent was obtained from each subject enrolled, or their legal guardians.
Data Availability Statement
The study was approved by the Institutional Ethics Committee (approval code: 2020.68). Clinical data (age, gender, clinical characteristics, co-morbidity, drug used) were obtained at the time of enrolment and are available.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bouillon, R.; Marcocci, C.; Carmeliet, G.; Bikle, D.; White, J.H.; Dawson-Hughes, B.; Lips, P.; Munns, C.F.; Lazaretti-Castro, M.; Giustina, A.; et al. Skeletal and Extraskeletal Actions of Vitamin D: Current Evidence and Outstanding Questions. Endocr. Rev. 2019, 40, 1109–1151. [Google Scholar] [CrossRef]
- Chun, R.F.; Shieh, A.; Gottlieb, C.; Yacoubian, V.; Wang, J.; Hewison, M.; Adams, J.S. Vitamin D Binding Protein and the Biological Activity of Vitamin D. Front. Endocrinol. 2019, 10, 718. [Google Scholar] [CrossRef] [PubMed]
- Long, M.; Sucheston-Campbell, L.E.; Campbell, M.J. Vitamin D Receptor and RXR in the Post-Genomic Era. J. Cell. Physiol. 2015, 230, 758–766. [Google Scholar] [CrossRef]
- Perri, M.; Pingitore, A.; Cione, E.; Vilardi, E.; Perrone, V.; Genchi, G. Proliferative and anti-proliferative effects of retinoic acid at doses similar to endogenous levels in Leydig MLTC-1/R2C/TM-3 cells. Biochim. Biophys. Acta (BBA) Gen. Subj. 2010, 1800, 993–1001. [Google Scholar] [CrossRef]
- Cato, L.; Neeb, A.; Brown, M.; Cato, A.C.B. Control of Steroid Receptor Dynamics and Function by Genomic Actions of the Cochaperones p23 and Bag-1L. Nucl. Recept. Signal. 2014, 12, e005. [Google Scholar] [CrossRef] [PubMed]
- Doig, C.; Singh, P.K.; Dhiman, V.; Thorne, J.L.; Battaglia, S.; Sobolewski, M.; Maguire, O.; O’Neill, L.P.; Turner, B.M.; McCabe, C.J.; et al. Recruitment of NCOR1 to VDR target genes is enhanced in prostate cancer cells and associates with altered DNA methylation patterns. Carcinogen 2013, 34, 248–256. [Google Scholar] [CrossRef]
- Quack, M.; Carlberg, C. The impact of functional vitamin D(3) receptor conformations on DNA-dependent vitamin D(3) signaling. Mol. Pharmacol. 2000, 57. [Google Scholar]
- Lerchbaum, E. Vitamin D and menopause—A narrative review. Maturitas 2014, 79, 3–7. [Google Scholar] [CrossRef]
- Sunyecz, J. The use of calcium and vitamin D in the management of osteoporosis. Ther. Clin. Risk Manag. 2008, 4, 827–836. [Google Scholar] [CrossRef]
- Aranow, C. Vitamin D and the Immune System. J. Investig. Med. 2011, 59, 881–886. [Google Scholar] [CrossRef] [PubMed]
- Levis, S.; Theodore, G. Summary of AHRQ’s comparative effectiveness review of treatment to prevent fractures in men and women with low bone density or osteoporosis: Update of the 2007 report. J. Manag. Care Pharm. 2012, 18 (Suppl. B), S1–S13. [Google Scholar] [CrossRef]
- Maine, G.N.; Lao, K.M.; Krishnan, S.M.; Afolayan-Oloye, O.; Fatemi, S.; Kumar, S.; VanHorn, L.; Hurand, A.; Sykes, E.; Sun, Q. Longitudinal characterization of the IgM and IgG humoral response in symptomatic COVID-19 patients using the Abbott Architect. J. Clin. Virol. 2020, 133, 104663. [Google Scholar] [CrossRef]
- To, K.K.-W.; Tsang, O.T.-Y.; Leung, W.-S.; Tam, A.R.; Wu, T.-C.; Lung, D.C.; Yip, C.C.-Y.; Cai, J.-P.; Chan, J.M.-C.; Chik, T.S.-H.; et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: An observational cohort study. Lancet Infect. Dis. 2020, 20, 565–574. [Google Scholar] [CrossRef]
- Cione, E.; Siniscalchi, A.; Gangemi, P.; Cosco, L.; Colosimo, M.; Longhini, F.; Luciani, F.; De Sarro, G.; Berrino, L.; D’Agostino, B.; et al. Neuron-specific enolase serum levels in COVID-19 are related to the severity of lung injury. PLoS ONE 2021, 16, e0251819. [Google Scholar] [CrossRef]
- Ibarrondo, F.J.; Fulcher, J.A.; Goodman-Meza, D.; Elliott, J.; Hofmann, C.; Hausner, M.A.; Ferbas, K.G.; Tobin, N.H.; Aldrovandi, G.M.; Yang, O.O. Rapid Decay of Anti–SARS-CoV-2 Antibodies in Persons with Mild Covid-19. N. Engl. J. Med. 2020, 383, 1085–1087. [Google Scholar] [CrossRef]
- Long, Q.-X.; Tang, X.-J.; Shi, Q.-L.; Li, Q.; Deng, H.-J.; Yuan, J.; Hu, J.-L.; Xu, W.; Zhang, Y.; Lv, F.-J.; et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat. Med. 2020, 26, 1200–1204. [Google Scholar] [CrossRef]
- Weitz, J.S.; Beckett, S.J.; Coenen, A.R.; Demory, D.; Dominguez-Mirazo, M.; Dushoff, J.; Leung, C.-Y.; Li, G.; Măgălie, A.; Park, S.W.; et al. Modeling shield immunity to reduce COVID-19 epidemic spread. Nat. Med. 2020, 26, 849–854. [Google Scholar] [CrossRef]
- Ali, N. Role of vitamin D in preventing of COVID-19 infection, progression and severity. J. Infect. Public Health 2020, 13, 1373–1380. [Google Scholar] [CrossRef]
- Vimaleswaran, K.S.; Forouhi, N.G.; Khunti, K. Vitamin D and covid-19. BMJ 2021, 372, n544. [Google Scholar] [CrossRef]
- Ahmed, A.; Siman-Tov, G.; Hall, G.; Bhalla, N.; Narayanan, A. Human Antimicrobial Peptides as Therapeutics for Viral Infections. Viruses 2019, 11, 704. [Google Scholar] [CrossRef]
- Fisher, S.A.; Rahimzadeh, M.; Brierley, C.; Gration, B.; Doree, C.; Kimber, C.E.; Cajide, A.P.; Lamikanra, A.A.; Roberts, D.J. The role of vitamin D in increasing circulating T regulatory cell numbers and modulating T regulatory cell phenotypes in patients with inflammatory disease or in healthy volunteers: A systematic review. PLoS ONE 2019, 14, e0222313. [Google Scholar] [CrossRef]
- Cutolo, M.; Paolino, S.; Smith, V. Evidences for a protective role of vitamin D in COVID-19. RMD Open 2020, 6, e001454. [Google Scholar] [CrossRef]
- Liu, N.; Sun, J.; Wang, X.; Zhang, T.; Zhao, M.; Li, H. Low vitamin D status is associated with coronavirus disease 2019 outcomes: A systematic review and meta-analysis. Int. J. Infect. Dis. 2021, 104, 58–64. [Google Scholar] [CrossRef]
- Notz, Q.; Herrmann, J.; Schlesinger, T.; Kranke, P.; Sitter, M.; Helmer, P.; Stumpner, J.; Roeder, D.; Amrein, K.; Stoppe, C.; et al. Vitamin D deficiency in critically ill COVID-19 ARDS patients. Clin. Nutr. 2021, 0261. [Google Scholar] [CrossRef]
- Sundaram, M.; Coleman, L.A. Vitamin D and Influenza. Adv. Nutr. 2012, 3, 517–525. [Google Scholar] [CrossRef]
- Luciani, F.; Cione, E.; Caroleo, M.; Colosimo, M.; Zanolini, A.; Barca, A.; Cosimo, S.; Pasqua, P.; Gallelli, L. SARS-CoV-2 Translocate from Nasopharyngeal to Bronchoalveolar Site: A Case Presentation. Reports 2020, 3, 23. [Google Scholar] [CrossRef]
- Fink, K. Origin and Function of Circulating Plasmablasts during Acute Viral Infections. Front. Immunol. 2012, 3, 78. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).